Abstract
Background
The association between blood pressure (BP) control and incident diabetes mellitus remains unknown. We aim to investigate the association between degree of time‐averaged on‐treatment systolic blood pressure (SBP) control and incident diabetes mellitus in hypertensive adults.
Methods and Results
A total of 14 978 adults with hypertension without diabetes mellitus at baseline were included from the CSPPT (China Stroke Primary Prevention Trial). Participants were randomized double‐masked to daily enalapril 10 mg and folic acid 0.8 mg or enalapril 10 mg alone. BP measurements were taken every 3 months after randomization. The primary outcome was incident diabetes mellitus, defined as physician‐diagnosed diabetes mellitus, or use of glucose‐lowering drugs during follow‐up, or fasting glucose ≥126 mg/dL at the exit visit. Over a median of 4.5 years, a significantly higher risk of incident diabetes mellitus was found in participants with time‐averaged on‐treatment SBP 130 to <140 mm Hg (10.3% versus 7.4%; odds ratio [OR], 1.37; 95% CI, 1.15‒1.64), compared with those with SBP 120 to <130 mm Hg. Moreover, the risk of incident diabetes mellitus increased by 24% (OR, 1.24; 95% CI, 1.00‒1.53) and the incidence of regression to normal fasting glucose (<100 mg/dL) decreased by 29% (OR, 0.71; 95% CI, 0.57‒0.89) in participants with intermediate BP control (SBP/diastolic blood pressure, 130 to <140 and/or 80 to <90 mm Hg), compared with those with a tight BP control of <130/<80 mm Hg. Similar results were found when the time‐averaged BP were calculated using the BP measurements during the first 6‐ or 24‐month treatment period, or in the analysis using propensity scores.
Conclusions
In this non‐diabetic, hypertensive population, SBP control in the range of 120 to <130 mm Hg, compared with the 130 to <140 mm Hg, was associated with a lower risk of incident diabetes mellitus.
Keywords: degree of blood pressure control, hypertension, incident diabetes mellitus, regression to normal fasting glucose, systolic blood pressure
Subject Categories: Hypertension, Epidemiology
Nonstandard Abbreviations and Acronyms
- CSPPT
China Stroke Primary Prevention Trial
- FG
fasting glucose
- OR
odds ratio
Clinical Perspective
What Is New?
This is the first and largest study to investigate the association between degree of systolic blood pressure/diastolic blood pressure control and risk of incident diabetes mellitus in patients with hypertension without baseline diabetes mellitus.
It demonstrated that in hypertensive population, systolic blood pressure control in the range of 120 to <130 mm Hg, compared with the 130 to <140 mm Hg, was associated with a lower risk of incident diabetes mellitus.
What Are the Clinical Implications?
To date, what constitutes optimal blood pressure target with regards to future risk of metabolic diseases remains uncertain. This study represents one step towards filling these critical knowledge gaps.
If further confirmed, our findings would have important implications for clinical practice and guidelines. Our results support the adoption of a tight systolic blood pressure goal in general patients with hypertension for preventing incident diabetes mellitus.
According to data from the International Diabetes Federation, the global prevalence of diabetes mellitus in adults was estimated to be 8.8% in 2015 and is predicted to rise to 10.4% by 2040. 1 Diabetes mellitus can lead to several health complications, including cardiovascular diseases, renal dysfunction, amputation, and vision problems. 2 Therefore, primary prevention of diabetes mellitus is critically important to reduce population burden of diabetes mellitus and its serious consequences.
Hypertension is a well‐established major modifiable risk factor for cardiovascular diseases, peripheral artery disease, and renal diseases. 2 , 3 Although hypertension is common in patients with type 2 diabetes mellitus, 4 its role in the development of diabetes mellitus is uncertain. Several longitudinal studies showed that subjects with baseline hypertension or even prehypertension had a higher risk of developing diabetes mellitus than normotensive subjects. 5 , 6 , 7 , 8 , 9 However, only 2 previous small hospital‐based studies 10 , 11 in Italy and Japan, respectively, have evaluated the effect of blood pressure (BP) control (systolic BP [SBP]/diastolic BP [DBP] <140/90 mm Hg) on the risk of incident diabetes mellitus in treated hypertensive patients, and reported conflicting findings. Therefore, to date, what constitutes optimal SBP and DBP target with regards to future risk of diseases remains uncertain.
Our current report was motivated by the limited data about the degree of BP control and incident diabetes mellitus, and an exceptional opportunity to address this question in a large, randomized controlled trial with regular antihypertensive treatments, BP measurements and diabetes mellitus status reports. Specifically, using data from CSPPT (China Stroke Primary Prevention Trial), 12 we sought to determine whether degree of BP control was associated with the development of incident diabetes mellitus, among hypertensive patients without diabetes mellitus, myocardial infarction, and stroke at baseline.
Methods
Our article adheres to the American Heart Association Journals’ implementation of the Transparency and Openness Promotion Guidelines. The parent study (the CSPPT) and the current study were approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China (FWA assurance number: FWA00001263). All participants provided written informed consent. The data, analytic methods, and study materials that support the findings of this study will be available from the corresponding authors on request, after the request is submitted and formally reviewed and approved by the Ethics Committee of the Institute of Biomedicine, Anhui Medical University.
Study Participants and Design
All participants were part of the CSPPT (Clinicaltrials.gov identifier: NCT00794885). Detailed methods and primary results of the CSPPT have been reported previously. 12 , 13 , 14 , 15 Briefly, the CSPPT was a large, multi‐site, randomized, double‐masked, and actively‐controlled trial with a total of 20 702 participants, conducted from May 19, 2008 to August 24, 2013 in 32 communities in China. Eligible participants were men and women aged 45 to 75 years who had hypertension, defined as seated, resting SBP ≥140 mm Hg or DBP ≥90 mm Hg at both the screening and the recruitment visit, or who were on antihypertensive medications. The major exclusion criteria included history of physician‐diagnosed stroke, myocardial infarction, heart failure, post‐coronary revascularization, and/or congenital heart disease.
The CSPPT consisted of 3 stages: screening and recruitment, a run‐in treatment period, and a randomized treatment period. During the screening period, community residents are screened by trained investigators for hypertension and medical history, disease diagnosis, and current treatment, according to the inclusion and exclusion criteria of the Run‐in Period. Before any particular study procedure is performed, each participant is asked to provide written informed consent.
The present study is a post‐hoc analysis of the CSPPT on 14 978 hypertensive participants with complete data on fasting glucose at baseline and at the exit visit (unless having physician‐diagnosed diabetes mellitus or use of glucose‐lowering drugs during the follow‐up), as well as who were free of diabetes mellitus (physician‐diagnosed diabetes mellitus or using glucose‐lowering drugs or fasting glucose (FG) was ≥126 mg/dL) at baseline. The flow of the participants is presented in Figure S1.
Intervention and Follow‐Up
During the run‐in period, all eligible participants were given an enalapril 10 mg tablet for 3 weeks. In the double‐masked treatment period, eligible participants are randomly assigned, in a 1:1 ratio, to one of two treatment groups using random permuted blocks stratified by methylenetetrahydrofolate reductase (MTHFR) C677T genotypes (CC, CT, or TT): a daily oral dose of one tablet containing 10 mg enalapril and 0.8 mg folic acid (the enalapril‐folic acid group), or a daily oral dose of one tablet containing 10 mg enalapril only (the enalapril group). Both types of tablets were concealed in a single‐capsule formulation and were identical in appearance, size, color, and taste. All study investigators and participants were masked to the randomization procedure and the treatment assignments.
The dosage of the study drugs is fixed during the trial. During the run‐in and double‐masked treatment period, if blood pressure was not adequately controlled, other classes of antihypertensive medications could be prescribed concomitantly, according to a pre‐specified algorithm: Step 1: (1) enalapril or enalapril–folic acid tablet daily+nitrendipine (10 mg, twice a day); or (2) enalapril or enalapril–folic acid tablet daily+hydrochlorothiazide (25 mg) daily; Step 2: enalapril or enalapril–folic acid tablet daily+nitrendipine (10 mg, twice a day)+hydrochlorothiazide (25 mg) daily. In Step 1, nitrendipine was the preferred choice. Alpha‐blockers, beta‐blockers, angiotensin receptor blockers, and other angiotensin‐converting enzyme inhibitors were not recommended. Blood pressure control within a normal range (SBP <140 mm Hg and DBP <90 mm Hg) was not mandatory.
Participants were followed up every 3 months. At each follow‐up visit, BP was measured; study drug compliance, concomitant medication use, adverse events and possible endpoint events were documented by trained research staff and physicians. At the exit visit, final blood samples were collected and assessed.
BP Measurements and Time‐Averaged On‐Treatment BP
After the patients had taken the antihypertensive drugs and breakfast, seated BP measurements were obtained by trained research staff after the patients had rested for 10 minutes using a mercury manometer (Yuwell‐Jiangsu Yuyue medical equipment & supply Co., Ltd), following the standard method and with appropriately sized cuffs (12‐cm wide and 23‐cm long; 15‐cm wide and 30‐cm long; or 18‐cm wide and 36‐cm long). Triplicate measurements on the same arm were taken, with at least 2 minutes between readings. The mean SBP and DBP of the 3 independent measures were used in analysis.
BP measurements were taken at baseline, randomization and every 3 months thereafter. Time‐averaged on‐treatment SBP or DBP was calculated for each participant using all post baseline results up to the last visit before the date of study outcome, or at the exit visit among those without study outcome (number of BP measurements during the treatment: median, 16; interquartile range, 12–18). We grouped participants into time‐averaged on‐treatment SBP and DBP categories, which indicate average BP control status during the treatment period. In the sensitivity analyses, we also calculated time‐averaged on‐treatment SBP or DBP using 2 alternative methods: (1) using the BP measurements from the randomization visit to the 6‐month visit (number of BP measurements during the period: median, 3; interquartile range, 2–3); (2) using the measurements from the randomization visit to the 24‐month visit (number of BP measurements during the period: median, 8; interquartile range, 7–9). In addition, SD of BP were used as visit‐to‐visit variability parameters and were determined using BP measurements from the randomization visit to the last visit before the date of study outcome or to the exit visit among those without study outcome.
Laboratory Assays
Fasting serum glucose, lipids and creatinine levels were measured using automatic clinical analyzers (Beckman Coulter) at the core laboratory of the National Clinical Research Center for Kidney Disease, Nanfang Hospital, Guangzhou, China.
Study Outcomes
The primary outcome was incident diabetes mellitus, defined as physician‐diagnosed diabetes mellitus or use of glucose‐lowering drugs during follow‐up, or new onset FG ≥126 mg/dL at the exit visit.
The secondary outcomes included: (1) incident impaired fasting glucose (IFG), defined as FG <100 mg/dL at baseline, while FG ≥100 and <126 mg/dL at the exit visit. The analysis of incident IFG included subjects with baseline FG <100 mg/dL and without incident diabetes mellitus during follow‐up; (2) physician‐diagnosed diabetes mellitus or use of glucose‐lowering drugs during follow‐up; and (3) regression to normal FG levels, defined as FG ≥100 and <126 mg/dL at baseline, FG <100 mg/dL at the exit visit. The analysis of regression to normal FG levels included subjects with baseline FG ≥100 and <126 mg/dL, and without physician‐diagnosed diabetes mellitus or use of glucose‐lowering drugs during follow‐up.
Statistical Analysis
Baseline characteristics are presented as mean±SDs or medians (25th percentile, 75th percentile) for continuous variables and proportions for categorical variables. Differences in baseline characteristics by time‐averaged on‐treatment SBP categories (<120, 120 to <130, 130 to <140, and ≥140 mm Hg) were compared using ANOVA tests, signed‐rank tests, or Chi‐square tests, accordingly.
In the CSPPT, at each follow‐up visit (every 3 months), physician‐diagnosed diabetes mellitus or use of glucose‐lowering drugs were documented by trained research staff and physicians. However, serum FG was only measured at baseline and the exit visit. Moreover, among the 1628 incident diabetes mellitus (primary outcome) in our current study, only 167 were those with physician‐diagnosed diabetes mellitus or the use of glucose‐lowering drugs during follow‐up (secondary outcome 2). Therefore, the relationship of time‐averaged on‐treatment SBP, DBP, or SBP/DBP levels with incident diabetes mellitus (primary outcome), incident IFG (secondary outcome 1), physician‐diagnosed diabetes mellitus or use of glucose‐lowering drugs during follow‐up (secondary outcome 2), and regression to normal FG levels (secondary outcome 3) were evaluated using multivariable logistic regression models (primary outcome, and secondary outcome 1 and 3), and Cox proportional hazard regression models (secondary outcome 2), respectively, without and with adjustment for age, sex, study center, body mass index (BMI), smoking, family history of diabetes mellitus, SBP, DBP, FG, total cholesterol, triglycerides, creatinine, folate, and the use of antihypertensive drugs at baseline, as well as use of calcium channel blockers or diuretics during the treatment period. In Cox proportional hazards regression models, time at risk was from the randomization of the study to the date of the secondary outcome 2, death, lost to follow‐up, or the exit visit among those without secondary outcome 2. The proportional hazards assumption was checked using statistical tests based on the scaled Schoenfeld residuals. Furthermore, possible modifications on the association between time‐averaged on‐treatment SBP and incident diabetes mellitus were also evaluated by stratified analyses and interaction testing on pertinent cardiovascular diseases risk factors. In addition, we explored the association between time‐averaged on‐treatment BP and incident diabetes mellitus using thin plate regression splines in generalized additive models implemented by the R package mgcv.
Propensity Score Analysis
As additional sensitivity analyses, we further evaluated our results in the analysis using propensity score matching method. A non‐parsimonious propensity score using variables that might affect BP control or incident diabetes mellitus was developed to predict the likelihood a participant would be in the different degree of time‐averaged on‐treatment SBP (130–140 or 120–130 mm Hg) control. Participants were matched 1:1 based on propensity scores. An automated balance optimization method using the function Match (in package Matching) in R and a caliper of 0.2 were used for matching. Standardized differences of post‐matched participant characteristics ≤10% between the 2 groups was considered to be balanced.
A 2‐tailed P<0.05 was considered to be statistically significant in all analyses. R software (version 3.5.2, http://www.R‐project.org) was used for all statistical analyses.
Results
Characteristics of Study Participants
As shown in the flowchart (Figure S1), a total of 14 978 participants without diabetes mellitus at baseline were included in the final analysis. The baseline characteristics were similar between participants included and those not‐included in the current study (Table S1).
Baseline characteristics of the study participants by time‐averaged on‐treatment SBP categories (SBP <120, 120 to <130, 130 to <140, and ≥140 mm Hg) are presented in Table 1. Participants with higher time‐averaged on‐treatment SBP were older, had higher BMI, total cholesterol, FG, and lower folate levels, and were more likely to smoke and consume alcohol at baseline. These characteristics were also more frequent among participants with incident diabetes mellitus (Table S2).
Table 1.
Baseline Characteristics of the Study Population by Time‐Averaged On‐Treatment SBP Categories*
| Variables | Time‐Averaged On‐Treatment SBP Categories, mm Hg | P Value | |||
|---|---|---|---|---|---|
| <120 | 120 to <130 | 130 to <140 | ≥140 | ||
| No. | 263 | 2658 | 5878 | 6179 | |
| Age, y | 58.1±7.4 | 58.8±7.2 | 59.7±7.4 | 60.9±7.4 | <0.001 |
| Men, n (%) | 99 (37.6) | 1042 (39.2) | 2500 (42.5) | 2469 (40.0) | 0.005 |
| Body mass index, kg/m2 | 24.6±3.5 | 24.6±3.5 | 24.8±3.6 | 25.1±3.7 | <0.001 |
| Current smoking, n (%) | 43 (16.3) | 543 (20.4) | 1432 (24.4) | 1517 (24.6) | <0.001 |
| Current alcohol drinking, n (%) | 39 (14.9) | 581 (21.9) | 1481 (25.2) | 1482 (24.0) | <0.001 |
| Family history of diabetes mellitus, n (%) | 13 (4.9) | 89 (3.4) | 244 (4.2) | 207 (3.4) | 0.056 |
| Enalapril group, n (%) | 139 (52.9) | 1332 (50.1) | 2907 (49.5%) | 3124 (50.6) | 0.514 |
| BP, mm Hg | |||||
| SBP at baseline | 147.6±16.1 | 156.0±16.3 | 163.6±17.1 | 175.6±21.2 | <0.001 |
| DBP at baseline | 90.2±10.5 | 92.1±10.7 | 93.5±11.2 | 96.0±12.7 | <0.001 |
| Time‐averaged on‐treatment SBP | 117.1±2.7 | 126.3±2.7 | 135.1±2.8 | 148.6±7.7 | <0.001 |
| Time‐averaged on‐treatment DBP | 76.4±4.8 | 79.4±5.6 | 81.9±6.2 | 85.4±7.9 | <0.001 |
| Laboratory results, mmol/L | |||||
| Total cholesterol | 5.3±1.1 | 5.4±1.1 | 5.5±1.1 | 5.5±1.2 | <0.001 |
| Triglycerides | 1.4 (1.0–1.2) | 1.4 (1.0–1.9) | 1.4 (1.0–1.9) | 1.4 (1.0–1.2) | <0.001 |
| Fasting glucose, mg/dL | 95.2±11.7 | 95.6±12.0 | 96.9±12.4 | 97.9±12.6 | <0.001 |
| Creatinine, μmol/L | 66.4±16.7 | 65.2±15.7 | 66.2±16.1 | 65.7±17.2 | 0.056 |
| Folate, ng/mL | 8.1 (5.8–10.4) | 8.2 (5.6–10.7) | 8.0 (5.6–10.3) | 7.9 (5.5–10.3) | 0.003 |
| Medication use, n (%) | |||||
| Antihypertensive drugs | 122 (46.4) | 1205 (45.3) | 2602 (44.3) | 2959 (47.9) | <0.001 |
| Lipid‐lowering drugs | 1 (0.4) | 28 (1.1) | 44 (0.7) | 40 (0.6) | 0.202 |
| Antiplatelet drugs | 10 (3.8) | 74 (2.8) | 174 (3.0) | 191 (3.1) | 0.749 |
BP indicates blood pressure; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
Continuous variables are presented as mean±SDs or medians (25th percentile–75th percentile). Categorical variables are presented as n (%).
In addition, participants with higher time‐averaged on‐treatment SBP had a higher frequency use of CCBs or diuretics during the treatment period (Table S3). Moreover, participants with incident diabetes mellitus had higher prevalence of taking CCBs or diuretics during treatment period, as compared with those without incident diabetes mellitus (Table S4).
Time‐Averaged On‐Treatment SBP and Incident Diabetes Mellitus
During a median follow‐up duration of 4.5 years (interquartile range, 4.2–4.7), incident diabetes mellitus occurred in 1628 (10.9%) participants.
The relationship of time‐averaged on‐treatment SBP with the risk of incident diabetes mellitus was presented in Figure 1A. Compared with participants with time‐averaged on‐treatment SBP in the range of 120 to <130 mm Hg (tight SBP control), the risk of incident diabetes mellitus was significantly increased in participants with SBP in the range of 130 to <140 mm Hg (intermediate SBP control) (10.3% versus 7.4%; odds ratio [OR], 1.37; 95% CI, 1.15‒1.64); and SBP ≥140 mm Hg (13.0% versus 7.4%; OR, 1.67; 95% CI, 1.39‒2.00). Participants with time‐averaged on‐treatment SBP <120 mm Hg also had a small increased risk of incident diabetes mellitus but not statistically significant (8.7% versus 7.4%; OR, 1.25; 95% CI, 0.78‒1.99) (Table 2).
Figure 1. Association between time‐averaged on‐treatment systolic blood pressure (A) or diastolic blood pressure (B) and incident diabetes mellitus in patients with hypertension.* .
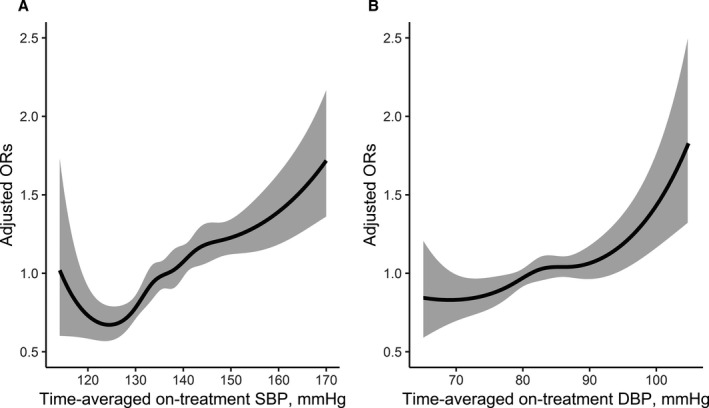
DBP indicates diastolic blood pressure; OR, odds ratio; and SBP, systolic blood pressure. *Adjusted for age, sex, study center, body mass index , smoking, family history of diabetes mellitus, systolic blood pressure, diastolic blood pressure, fasting glucose, total cholesterol, triglycerides, creatinine, folate, and the use of antihypertensive drugs at baseline, as well as use of calcium channel blockers or diuretics during the treatment period.
Table 2.
Association Between Time‐Averaged On‐Treatment Blood Pressure and Incident Diabetes Mellitus
| BP, mm Hg | n | No. of Events (%) | Crude Model | Adjusted Model* | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| Time‐averaged on‐treatment SBP | ||||||
| <120 | 263 | 23 (8.7) | 1.20 (0.76‒1.88) | 0.435 | 1.25 (0.78‒1.99) | 0.358 |
| 120 to <130 | 2658 | 197 (7.4) | Ref. | Ref. | ||
| 130 to <140 | 5878 | 606 (10.3) | 1.44 (1.21‒1.70) | <0.001 | 1.37 (1.15‒1.64) | <0.001 |
| ≥140 | 6179 | 802 (13.0) | 1.86 (1.58‒2.19) | <0.001 | 1.67 (1.39‒2.00) | <0.001 |
| Time‐averaged on‐treatment DBP | ||||||
| <70 | 491 | 42 (8.6) | 0.80 (0.57‒1.11) | 0.172 | 0.76 (0.53‒1.07) | 0.117 |
| 70 to <80 | 4788 | 504 (10.5) | Ref. | Ref. | ||
| 80 to <90 | 7434 | 814 (10.9) | 1.05 (0.93‒1.18) | 0.461 | 1.13 (0.98‒1.30) | 0.090 |
| ≥90 | 2265 | 268 (11.8) | 1.14 (0.97‒1.34) | 0.101 | 1.30 (1.06‒1.61) | 0.012 |
| P for trend | 0.031 | 0.003 | ||||
| Time‐averaged on‐treatment SBP/DBP | ||||||
| <130 and <80 | 1603 | 125 (7.8) | Ref. | Ref. | ||
| 130 to <140 and/or 80 to <90 | 6618 | 651 (9.8) | 1.29 (1.06‒1.58) | 0.012 | 1.24 (1.00‒1.53) | 0.047 |
| ≥140 and/or ≥90 | 6757 | 852 (12.6) | 1.71 (1.40‒2.08) | <0.001 | 1.54 (1.24‒1.92) | <0.001 |
BP indicates blood pressure; DBP, diastolic blood pressure; OR, odds ratio; and SBP, systolic blood pressure.
Adjusted for age, sex, study center, body mass index, smoking, family history of diabetes mellitus, systolic blood pressure, diastolic blood pressure, fasting glucose, total cholesterol, triglycerides, creatinine, folate, and the use of antihypertensive drugs at baseline, as well as use of calcium channel blockers or diuretics during the treatment period.
Moreover, further adjustment for the concomitant use of beta‐blockers, lipid‐lowering drugs, and antiplatelet drugs during the treatment period (Table S5); the treatment (enalapril or enalapril‐folic acid) compliance during the treatment period (Table S6); change in BMI (BMI at the exit minus that at baseline) (Table S7); averaged BMI (BMI at the exit plus that at baseline divided by 2) (Table S8); or visit‐to‐visit variability in BP during the treatment period (Table S9) did not substantially change the results.
Time‐Averaged On‐Treatment SBP During the First 6‐ or 24‐Month Treatment Period and the Subsequent Incident Diabetes Mellitus
When the time‐averaged on‐treatment BP were calculated using the BP measurements from the randomization visit to the 6‐ or 24‐month visit, compared with participants with time‐averaged on‐treatment SBP 120 to <130 mm Hg, the risk of subsequent incident diabetes mellitus in those with SBP 130 to <140 mm Hg was increased by 23% (OR, 1.23; 95% CI, 1.01‒1.49) (Table S10) and 28% (OR, 1.28; 95% CI, 1.05‒1.56) (Table S11), respectively.
Time‐Averaged On‐Treatment DBP and Incident Diabetes Mellitus
We observed an approximately linear relationship of time‐averaged on‐treatment DBP and the risk of incident diabetes mellitus (P for trend=0.003; Figure 1B, Table 2).
Combined Time‐Averaged On‐Treatment SBP and DBP and Incident Diabetes Mellitus
Compared with those with time‐averaged on‐treatment SBP <130 and DBP <80 mm Hg (tight BP control), the risk of incident diabetes mellitus was increased by 24% in participants with time‐averaged on‐treatment SBP 130 to <140 and/or DBP 80 to <90 mm Hg (9.8% versus 7.8%; intermediate BP control; OR, 1.24; 95% CI, 1.00‒1.53) (Table 2).
Subgroup Analyses by Potential Effect Modifiers
The similar results were found in participants without (Table S12) or with (Table S13) the concomitant use of diuretics during the treatment period.
Moreover, none of the other variables, including sex, age (<65 versus ≥65 years), BMI (<20, 20 to <25, ≥25 kg/m2), waist circumference (<90 versus ≥90 cm); SBP (<160 versus ≥160 mm Hg), FG (<100 versus ≥100 mg/mL), total cholesterol (<6.2 versus ≥6.2 mmol/L); triglycerides (<1.7 versus ≥1.7 mmol/L), current smoking (no versus yes), current alcohol drinking (no versus yes), and family history of diabetes mellitus (yes versus no) at baseline, treatment group (enalapril versus enalapril+folic acid), as well as the CCBs usage (no versus yes) during the treatment period, significantly modified the association between time‐averaged on‐treatment SBP (130 to <140 versus 120 to <130 mm Hg) and the risk of incident diabetes mellitus (all P‐interactions>0.05) (Figure 2).
Figure 2. Association between time‐averaged on‐treatment systolic blood pressure (120 to <130 vs 130 to <140 mm Hg) and incident diabetes mellitus in various subgroups.*† .

BMI indicates body mass index; CCB, calcium channel blockers; FG, fasting glucose; OR, odds ratio; and SBP, systolic blood pressure. *Adjusted for age, sex, study center, body mass index, smoking, family history of diabetes mellitus, systolic blood pressure, diastolic blood pressure, fasting glucose, total cholesterol, triglycerides, creatinine, folate, and the use of antihypertensive drugs at baseline, as well as use of calcium channel blockers or diuretics during the treatment period, if not be stratified. †The reference was time‐averaged on‐treatment systolic blood pressure 120 to <130 mm Hg.
Propensity Score Analysis
After propensity score matching, 4966 participants (2483 in each group) were included in the analysis for time‐averaged on‐treatment SBP (120 to <130 mm Hg versus 130 to <140 mm Hg) and incident diabetes mellitus association. Candidate variables used in the development of the propensity score have been listed in Table S14. All the post‐matched participant characteristics were highly balanced (Table S14, Figure S2). Consistently, a significantly higher risk of incident diabetes mellitus was found in participants with time‐averaged on‐treatment SBP 130 to <140 mm Hg (versus <120 to <130 mm Hg; 10.2% versus 7.4%; OR, 1.43; 95% CI, 1.17‒1.75). Further adjustments for the matching variables did not substantially change the results (Table 3).
Table 3.
Association Between Time‐Averaged On‐Treatment Systolic Blood Pressure (120 to <130 vs 130 to <140 mm Hg) and Incident Diabetes Mellitus in the Propensity Score Matching Analysis
| Time‐Averaged On‐Treatment SBP, mm Hg | n | No. of Events (%) | Crude Model | Adjusted Model* | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| 120 to <130 | 2483 | 183 (7.4) | Ref. | Ref. | ||
| 130 to <140 | 2483 | 254 (10.2) | 1.43 (1.17‒1.75) | <0.001 | 1.45 (1.19‒1.78) | <0.001 |
OR indicates odds ratio; and SBP, systolic blood pressure.
Adjusted for age, sex, study center, body mass index, smoking, family history of diabetes mellitus, systolic blood pressure, diastolic blood pressure, fasting glucose, total cholesterol, triglycerides, creatinine, folate, and the use of antihypertensive drugs at baseline, as well as use of calcium channel blockers or diuretics during the treatment period.
Secondary Outcomes
We found the similar trends for the incident IFG (secondary outcome 1) (Table S15), and physician‐diagnosed diabetes mellitus or use of glucose‐lowering drugs during follow‐up (secondary outcome 2) (Table S16).
Moreover, compared with those with time‐averaged on‐treatment SBP <130 and DBP <80 mm Hg (tight BP control), the incidence of regression to normal FG levels (<100 mg/dL) among those with IFG at baseline was decreased by 29% in participants with time‐averaged on‐treatment SBP 130 to <140 and/or DBP 80 to <90 mm Hg (intermediate BP control; 24.7% versus 33.6%; OR, 0.71; 95% CI, 0.57‒0.89) (Table S17).
Discussion
Our study has yielded novel findings. It demonstrated that in a hypertensive population, SBP control in the range of 120 to <130 mm Hg, compared with the 130 to <140 mm Hg, was associated with a lower risk of incident diabetes mellitus. This association remained after adjusting for comprehensive covariables, including age, sex, BP, BMI, smoking, family diabetes mellitus history, lipids, FG, creatinine at baseline, and use of CCBs and diuretics during treatment period. The results were consistent when the time‐averaged on‐treatment BP were calculated using the BP measurements across the treatment period, during the first 6‐ or 24‐month treatment period, or in the propensity scores analysis. The results were also robust in subgroup analyses.
Moreover, we found that achieving the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline, 16 and the 2018 European Society of Cardiology and European Society of Hypertension Guidelines‐recommended BP control goal of <130/<80 mm Hg (for most well‐tolerated patients) 17 was associated with a significant, decreased risk of incident diabetes mellitus (2.0% absolute risk reduction); and a significant, increased incidence of regression to normal FG levels among those with IFG at baseline (8.9% absolute incidence increase) than the prior US guideline goals of <140/<90 mm Hg. 18 Diabetes mellitus is a serious, costly disease, which has become a worldwide public health problem. 19 , 20 Treatment of diabetes mellitus usually could not restore normoglycemia, and prevent all the adverse. 21 , 22 Therefore, more effective strategy for primary prevention of diabetes mellitus is of great clinical and public health importance. However, to date, the optimal SBP and DBP control levels for future risk of diabetes mellitus remains uncertain. This study represents one step towards filling these critical knowledge gaps.
Previous prospective studies 10 , 11 on the association of BP control and the risk of incident diabetes mellitus yielded conflicting findings because of relatively small sample size and methodology limitations. In a community hospital‐based study of 1754 treated hypertensive patients, Izzo et al 10 found that uncontrolled blood pressure (SBP >140 and/or DBP >90 mm Hg) was associated with a 2‐fold increased risk of incident diabetes mellitus. However, this study was limited in that only the last available blood pressure measurement was used to classify controlled and uncontrolled patients. A single occasion blood pressure measurement may be influenced by prior conditions, such as the timing of medication administration. Furthermore, the study lacked information on important baseline characteristics, such as waist circumference and family history of diabetes mellitus. Another hospital‐based study of 694 treated hypertensive patients 11 reported that neither SBP (≥140 versus <140 mm Hg; HR, 0.99; 95% CI, 0.56‒1.76) nor DBP (≥90 versus <90 mm Hg; HR, 1.75; 95% CI, 0.90‒3.39) were associated with the development of type 2 diabetes mellitus. Of note, this study only had baseline treated BP levels.
Our study has multiple strengths. It includes a large sample size, a long‐term follow‐up with regular BP measurements every 3 months using standard methods, incident diabetes mellitus based on both fasting serum glucose at the baseline and the exit visit, and physician diagnosis/glucose lowering medication use during the treatment period, adjustments for a comprehensive range of covariables/confounders, and multiple sensitivity analyses and subgroup analyses to ensure the robustness of the study findings. In contrast to the 2 previous studies, our study first showed that SBP control in the range of 120 to <130 mm Hg, compared with the 130 to <140 mm Hg, were associated with the lowest risk of incident diabetes mellitus. Our findings are clinically meaningful in term of the magnitude of risk reduction of incident diabetes mellitus (≈25%).
The biological mechanisms underlying BP control and diabetes mellitus is not completely clear. One possibility is that hypertension and diabetes mellitus may share some common pathogenic pathways. For example, hypertension is a major determinant of endothelial dysfunction. 23 Several studies have shown that the reduced endothelium dependent vasodilatation may lead to diminished capillary recruitment, and therefore limit insulin delivery to metabolically active, insulin‐sensitive muscle tissue. 24 , 25 , 26 Also, the altered endothelial permeability could impair the insulin delivery to interstitial space. 24 The interstitial insulin levels have been reported to be a rate‐limiting step for insulin effectiveness. 27 Moreover, previous studies have found that antihypertensive treatment significantly improved the endothelial function. 28 Therefore, we hypothesize that optimal BP control may result in a stronger improvement in endothelial function, which may increase capillary dilation and microvascular perfusion, and lead to a reduction in diabetes mellitus risk (Figure S3). More studies are needed to confirm our results and further examine the underlying mechanisms.
Our study has some limitations. First, although this study adjusted a broad array of covariates in the regression models, it cannot exclude residual confounding from unmeasured factors. Second, subjects of this study were participants of the CSPPT. In the CSPPT, there was a strict inclusion and exclusion criteria. Therefore, findings of the current study may possibly be affected by selection bias. Moreover, this study was conducted in a Chinese hypertensive population without baseline diabetes mellitus. Caution is needed to generalize to other populations with different characteristics. Third, although the self‐reported diabetes mellitus was documented based on the physicians’ diagnoses and the use of glucose‐lowering drugs during the treatment period every 3 months in the CSPPT, serum FG was only assessed at the baseline and the exit visits. Moreover, although our definition of diabetes mellitus was similar to that of previous studies, 29 , 30 we did not measure glycated hemoglobin A1c, postprandial glucose, or perform glucose tolerance tests. Fourth, besides the current alcohol drinking status, cumulative dosage was not collected in the CSPPT. Therefore, the possible effect of excessive drinking could not be evaluated in our current study. Moreover, in the CSPPT, BMI was only measured at baseline and the exit visit. As such, we could not evaluate the possibly modifying of time‐averaged BMI on the study outcomes. Fifth, this study observed a non‐significant higher risk of incident diabetes mellitus in those with on‐treatment SBP <120 mm Hg, compared with those with SBP 120 to <130 mm Hg. Because of small sample size (diabetes mellitus events=23) in this group with treatment SBP <120 mm Hg, the analysis was under‐powered. Finally, this was just a post hoc analysis of a randomized trial, the ability to make causal inferences was limited. Moreover, although similar findings were found for all the secondary outcomes (incident IFG; physician‐diagnosed diabetes mellitus or the use of glucose‐lowering drugs during follow‐up; and regression to normal FG levels) and the sensitivity analyses using BP measurement during the first 6 months or during the first 24 months, actual onset date of the most diabetes mellitus was difficult to define, time‐averaged on‐treatment BP values after actual onset of diabetes mellitus may have been used as a predictor in the analysis for some participants. Therefore, the causal relationship is not clearly. In addition, considering the relatively attenuated ORs in the sensitivity analyses with shorter time windows (using BP measurement during the first 6 months or during the first 24 months), maybe our current results were pulled by those with longer follow‐up. Overall, our study served as hypothesis‐generating; all findings need to be further investigated and confirmed in future related randomized trials.
In summary, this is the first and largest study to investigate the association between degree of SBP/DBP control and the risk of incident diabetes mellitus in patients with hypertension without baseline diabetes mellitus. It revealed that in a hypertensive population, SBP control in the range of 120 to <130 mm Hg, compared with the 130 to <140 mm Hg, was associated with a lower risk of incident diabetes mellitus. If further confirmed, our findings would have important implications for clinical practice and guidelines. Our results support the adoption of a tight SBP goal in general hypertensive patients for preventing incident diabetes mellitus.
Sources of Funding
This work was supported by funding from the following: the National Key Research and Development Program (2016YFE0205400, 2018ZX09739010, 2018ZX09301034003), the Science and Technology Planning Project of Guangzhou, China (201707020010), the Science, Technology and Innovation Committee of Shenzhen (JSGG20170412155639040, GJHS20170314114526143, JSGG20180703155802047), the Economic, Trade and Information Commission of Shenzhen Municipality (20170505161556110, 201705051609263 90), the National Natural Science Foundation of China (81730019, 81973133), and Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2017J009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosures
Dr Xiping Xu reports grants from the National Key Research and Development Program (2016YFE0205400, 2018ZX09739010, 2018ZX09301034003), the Science and Technology Planning Project of Guangzhou, China (201707020010), the Science, Technology and Innovation Committee of Shenzhen (JSGG20170412155639040, GJHS20170314114526143, JSGG20180703155802047), the Economic, Trade and Information Commission of Shenzhen Municipality (20170505161556110, 20170505160926390). Dr Xianhui Qin reports grants from the National Natural Science Foundation of China (81730019, 81973133), Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2017J009). The remaining authors have no disclosures to report.
Supporting information
Tables S1–S17
Figures S1–S3
Acknowledgments
Author contributions: Y.Z., F.F.H., X.X., and X.Q. contributed to the study design. Y.Z., X.X., X.Q., Y.Z., J.L., J.T., B.W., Y.C., and Y.H. contributed to data collection. Y.Z., J.N., Y.Z., J.L., M.L., G.W., J.T., C.L., B.W., Y.C., X.W., Y.H., X.X., F.F.H., and X.Q. contributed to acquisition, analysis, or interpretation of draft. Y.Z., C.L. and X.Q. contributed to statistical analysis. Y.Z. and X.Q. contributed in drafting the article. All authors contributed to critical revision of the article for important intellectual content.
(J Am Heart Assoc. 2020;9:e017015 DOI: 10.1161/JAHA.120.017015.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017015
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Fan Fan Hou, Email: ffhouguangzhou@163.com.
Xianhui Qin, Email: pharmaqin@126.com.
References
- 1. Ogurtsova K, Da RFJ, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. [DOI] [PubMed] [Google Scholar]
- 2. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He M, Qin X, Cui Y, Cai Y, Sun L, Xu X, Wang B, Tang G, Xing H, Wang X, et al. Prevalence of unrecognized lower extremity peripheral arterial disease and the associated factors in Chinese hypertensive adults. Am J Cardiol. 2012;110:1692–1698. [DOI] [PubMed] [Google Scholar]
- 4. Qin X, Li J, Zhang Y, Ma W, Fan F, Wang B, Xing H, Tang G, Wang X, Xu X, et al. Prevalence and associated factors of diabetes and impaired fasting glucose in Chinese hypertensive adults aged 45 to 75 years. PLoS One. 2012;7:e42538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wei GS, Coady SA, Goff JDC, Brancati FL, Levy D, Selvin E, Vasan RS, Fox CS. Blood pressure and the risk of developing diabetes in african americans and whites: ARIC, CARDIA, and the Framingham Heart Study. Diabetes Care. 2011;34:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conen D, Ridker PM, Mora S, Buring JE, Glynn RJ. Blood pressure and risk of developing type 2 diabetes mellitus: the Women's Health Study. Eur Heart J. 2007;28:2937–2943. [DOI] [PubMed] [Google Scholar]
- 7. Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis Risk in Communities Study. N Engl J Med. 2000;342:905–912. [DOI] [PubMed] [Google Scholar]
- 8. Hayashi T, Tsumura K, Suematsu C, Endo G, Fujii S, Okada K. High normal blood pressure, hypertension, and the risk of type 2 diabetes in Japanese men. The Osaka Health Survey. Diabetes Care. 1999;22:1683–1687. [DOI] [PubMed] [Google Scholar]
- 9. Mullican DR, Lorenzo C, Haffner SM. Is prehypertension a risk factor for the development of type 2 diabetes? Diabetes Care. 2009;32:1870–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Izzo R, Simone GD, Chinali M, Iaccarino G, Trimarco V, Rozza F, Giudice R, Trimarco B, Luca ND. Insufficient control of blood pressure and incident diabetes. Diabetes Care. 2009;32:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tatsumi Y, Morimoto A, Asayama K, Sonoda N, Miyamatsu N, Ohno Y, Miyamoto Y, Izawa S, Ohkubo T. Risk of developing type 2 diabetes according to blood pressure levels and presence or absence of hypertensive treatment: the Saku study. Hypertens Res. 2019;42:105–113. [DOI] [PubMed] [Google Scholar]
- 12. CSPPT Investigators . Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325–1335. [DOI] [PubMed] [Google Scholar]
- 13. Fan F, Yuan Z, Qin X, Li J, Zhang Y, Li Y, Yu T, Ji M, Ge J, Zheng M, et al. Optimal systolic blood pressure levels for primary prevention of stroke in general hypertensive adults: findings from the CSPPT (China Stroke Primary Prevention Trial). Hypertension. 2017;69:697–704. [DOI] [PubMed] [Google Scholar]
- 14. Huang X, Li Y, Li P, Li J, Bao H, Zhang Y, Wang B, Sun N, Wang J, He M, et al. Association between percent decline in serum total homocysteine and risk of first stroke. Neurology. 2017;89:2101–2107. [DOI] [PubMed] [Google Scholar]
- 15. Qin X, Li J, Zhang Y, Chen D, Wang B, He M, Fu J, Tang G, Cai Y, Shi X, et al. Effect of folic acid supplementation on risk of new‐onset diabetes in adults with hypertension in China: findings from the China Stroke Primary Prevention Trial (CSPPT). J Diabetes. 2016;8:286–294. [DOI] [PubMed] [Google Scholar]
- 16. Whelton PK, Carey RM, Aronow WS, Casey DJ, Collins KJ, Dennison HC, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/ APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483. [DOI] [PubMed] [Google Scholar]
- 17. ESC Scientific Document Group . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 18. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 19. Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd‐Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. [DOI] [PubMed] [Google Scholar]
- 20. Qin X, Huo Y. H‐type hypertension, stroke and diabetes in China: opportunities for primary prevention. J Diabetes. 2016;8:38–40. [DOI] [PubMed] [Google Scholar]
- 21. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 22. UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 23. Ghiadoni L, Taddei S, Virdis A. Hypertension and endothelial dysfunction: therapeutic approach. Curr Vasc Pharmacol. 2012;10:42–60. [DOI] [PubMed] [Google Scholar]
- 24. Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291:1978–1986. [DOI] [PubMed] [Google Scholar]
- 25. Serné EH, Stehouwer CD, ter Maaten JC, ter Wee PM, Rauwerda JA, Donker AJ, Gans RO. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation. 1999;99:896–902. [DOI] [PubMed] [Google Scholar]
- 26. Bonadonna RC, Saccomani MP, Del Prato S, Bonora E, DeFronzo RA, Cobelli C. Role of tissue‐specific blood flow and tissue recruitment in insulin‐mediated glucose uptake of human skeletal muscle. Circulation. 1998;98:234–241. [DOI] [PubMed] [Google Scholar]
- 27. Miles PD, Levisetti M, Reichart D, Khoursheed M, Moossa AR, Olefsky JM. Kinetics of insulin action in vivo. Identification of rate‐limiting steps. Diabetes. 1995;44:947–953. [DOI] [PubMed] [Google Scholar]
- 28. Shahin Y, Khan JA, Samuel N, Chetter I. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: a meta‐analysis of randomised controlled trials. Atherosclerosis. 2011;216:7–16. [DOI] [PubMed] [Google Scholar]
- 29. Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bleys J, Navas‐Acien A, Guallar E. Serum selenium and diabetes in U.S. adults. Diabetes Care. 2007;30:829–834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S17
Figures S1–S3


