Abstract
Background
The association of depressive symptoms with health status in peripheral artery disease (PAD) is understudied. No reports of differential impact on women have been described.
Methods and Results
The PORTRAIT (Patient‐Centered Outcomes Related to Treatment Practices in Peripheral Artery Disease Investigating Trajectories) registry enrolled 1243 patients from vascular specialty clinics with new or worsening PAD symptoms. Depressive symptoms were assessed at baseline and 3 months using the 8‐Item Patient Health Questionnaire (score ≥10 indicating clinically relevant depressive symptoms). Disease‐specific and generic health status were measured by Peripheral Artery Questionnaire and EQ‐5D Visual Analogue Scale at baseline and 3, 6, and 12 months. An adjusted general linear model for repeated measures was constructed for baseline and 3‐, 6‐, and 12‐month health status outcomes by depressive symptoms at baseline. Differences by sex were tested with interaction effects. The mean age was 67.6±9.4 years with 38% (n=470) women. More women than men (21.1% versus 12.9%; P<0.001) presented with severe depressive symptoms. In the adjusted model, patients with depressive symptoms had worse health status at each time point (all P<0.0001). Results were similar for EQ‐5D Visual Analogue Scale scores. The magnitude in 1‐year change in health status scores did not differ by sex. Depressive symptoms explained 19% of the association between sex differences in 1‐year Peripheral Artery Questionnaire summary scores.
Conclusions
Women with PAD have a high burden of depressive symptoms. Depressive symptoms were associated with a strikingly worse disease‐specific health status recovery path over the year following PAD diagnosis in men and women. Developing and testing interventions to address depressive symptoms in PAD are urgently needed.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01419080.
Keywords: depressive symptoms, health status, peripheral artery disease, sex differences
Subject Categories: Peripheral Vascular Disease, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- ABI
ankle‐brachial index
- GLM
general linear model
- PAD
peripheral artery disease
- PAQ
Peripheral Artery Questionnaire
- PHQ‐8
8‐Item Patient Health Questionnaire
- PORTRAIT
Patient‐Centered Outcomes Related to Treatment Practices in Peripheral Artery Disease Investigating Trajectories
- VAS
Visual Analogue Scale
Clinical Perspective
What Is New?
Patients with peripheral artery disease, and especially women, who experience depressive symptoms have worse 1‐year peripheral artery disease–specific health status outcomes, with large differences compared with those who do not experience such symptoms.
What Are the Clinical Implications?
Depressive symptoms in chronic disease populations, such as peripheral artery disease, should be a continuous focus of multidisciplinary treatment so as to ensure quality peripheral artery disease care and optimize health status outcomes.
Peripheral artery disease (PAD) remains a significant health problem, 1 affecting >200 million people worldwide 2 and >8 million people >40 years of age in the United States alone. 3 , 4 In patients with multiple atherosclerotic risk factors, including smoking and diabetes mellitus, 5 the prevalence of PAD can be as high as 30%. 6 PAD is associated with a high risk of cardiovascular mortality and morbidity, 7 , 8 with a disproportionate high risk of cardiovascular events compared with coronary artery disease and cerebrovascular disease. 9 From a societal perspective, PAD is a costly disease. Total annual costs associated with PAD‐related hospitalizations in the United States are estimated to be in excess of $21 billion, 10 which will continue to increase as the population ages.
The disability associated with PAD is significant. PAD may present itself as excruciating pain while walking and may impact patients’ mobility and functioning, as well as their health status and quality of life. Although PAD presents itself at least as commonly in women as in men, 11 , 12 , 13 , 14 women’s experiences of the disease burden may be worse than that of men’s, as expressed by greater functional impairment, 15 worse health‐related quality of life, 16 and poorer outcomes after lower extremity revascularization procedures. 17 , 18 Women diagnosed with cardiovascular disease are also at a much higher risk of presenting with depressive symptoms compared with men. 19 The manifestation of depressive symptoms is independently associated with higher cardiac and all‐cause mortality, rehospitalization, and worse functional status, including angina and quality of life after myocardial infarction. 20 , 21 , 22 , 23 Depressive symptoms are also more common in women with PAD than in men with PAD. 24 , 25 Although depressive symptoms are known to be associated with a more dramatic annual decline in functional performance, 26 reduced walking distance, 27 less quality‐of‐life benefit after revascularization, 28 and an increased risk of death/major adverse cardiovascular events, coronary heart disease, and contralateral PAD events, 29 it is unclear as to what extent depressive symptoms may be associated with a worse health status recovery profile in patients with PAD, and whether women are affected differently than men.
To address this gap in knowledge, we sought to (1) determine the prevalence of depressive symptoms and their treatment; (2) compare the trajectory of 1‐year PAD‐specific health status following a new or worsening PAD diagnosis as a function of depressive symptoms; and (3) explore sex differences in both the prevalence and health status impact of depressive symptoms among patients with PAD. We hypothesized that women with PAD have a higher burden of depressive symptoms compared with men with PAD, both at baseline and in the year following a new or worsening PAD diagnosis, and that depressive symptoms would be associated with worse health status outcomes over time. Establishing PAD‐specific health status recovery profiles as a function of depressive symptoms can underscore the importance of this comorbidity, especially in women, and stimulate new research into ways to address this problem in a more holistic approach to PAD care.
Methods
Data requests for the PORTRAIT study can be submitted to the corresponding author. Because of the sensitive nature of the data collected for this study, requests to access a deidentified data set from qualified researchers trained in human subject confidentiality protocols may be considered on an individual basis by contacting the corresponding author or by contacting the PORTRAIT (Patient‐Centered Outcomes Related to Treatment Practices in Peripheral Artery Disease Investigating Trajectories) registry group on the website. 30
Patients included in this study were enrolled from the PORTRAIT registry, 30 for which the methods have previously been described. Briefly, it is an international, prospective, observational study designed to address gaps in knowledge about the quality of care and health status outcomes of patients with PAD. Between June 2011 and December 2015, 1275 patients with a new diagnosis of PAD or those with an exacerbation of symptoms presenting to 16 vascular specialty clinics were enrolled. Of these 16 vascular specialty clinics, 10 were from the United States, 5 were from The Netherlands, and 1 was from Australia. Patients from The Netherlands and Australia were included in this study as PAD has become a global health problem with high morbidity and mortality. 31 Integrating data from these countries allows us to determine country/region‐based variations in disease characteristics, treatment patterns, and outcomes and help improve practices. Patients with a Doppler resting ankle‐brachial index (ABI) ≤0.90 or a significant decrease in postexercise ankle pressure of ≥20 mmHg were enrolled in the study. Other inclusion criteria included (1) patients aged ≥18 years; (2) new‐onset or recent exacerbation of exertional leg symptoms, regardless of whether symptoms were typical (buttock, thigh, hip, or calf pain; numbness or discomfort inhibiting the patient’s ability to walk distances) or atypical. To classify patients’ symptoms as atypical or typical, the coordinators were instructed to abstract this from patients’ medical records and as described by their treating physician. Patients with a noncompressible ABI ≥1.30, those who underwent a lower‐limb revascularization procedure in the past year (angioplasty, bypass surgery, atherectomy, or endarterectomy) for the ipsilateral leg relative to where the patient was currently having symptoms, patients with a current episode of critical limb ischemia (ischemic rest pain, ulceration, or gangrene; Fontaine III or IV; or Rutherford category 4–6), patients who could not speak English, Spanish, or Dutch, and patients with hearing impairment or current imprisonment were excluded. Approval from the institutional review board of each participating site was obtained, and participants provided informed consent for all study procedures and interviews. All patients provided consent to have their medical data abstracted from their electronic medical records. A 2‐step process was designed to obtain medical record information from outside the enrolling facility: (1) during follow‐up, patients were asked whether they obtained care outside of the enrolling facility; and (2) if patients indicated they obtained outside care, their medical record information was requested per the patient’s consent that was obtained on enrollment.
Data Collection and Study Definitions
Information on patients’ baseline characteristics was obtained by trained personnel using medical chart abstraction as well as in‐person interviews during the first visit and before initiation of treatment. On enrollment, detailed information was obtained through chart abstraction on demographics, cardiac risk factors, comorbidities, disease severity from diagnostic tests, and baseline treatment (medications, cardiovascular risk management strategies, and referrals). Follow‐up assessments were conducted by a centralized call center at 3, 6, and 12 months using standardized interviews. Information on the primary PAD treatment strategy was determined at 3 months as either noninvasive (medical therapy, including pharmacologic treatment and smoking cessation counseling) or invasive treatment (including either surgical or endovascular intervention). Primary PAD treatment information from all US sites was adjudicated by a central committee; from non‐US sites, patient‐reported information was used.
Assessment of Depressive Symptoms
Depressive symptoms were assessed using the 8‐Item Patient Health Questionnaire (PHQ‐8), 32 an 8‐item depression scale that has been established as a valid screening tool to screen for a major depressive disorder, as well as to quantify the frequency of depressive symptoms experienced in the past 2 weeks. 33 Scores range from 0 to 27, with a higher score indicating a higher level of depressive symptoms, and a score ≥10 has 88% sensitivity and specificity to detect major depression. The severity of depressive symptoms has been described using the following categories: “no” depressive symptoms if PHQ‐8 scores are <5, mild depressive symptoms for PHQ‐8 scores ≥5 and ≤9, and moderate/severe depressive symptoms for scores ≥10. 34
Assessment of Outcomes
Health status was measured using the disease‐specific Peripheral Artery Questionnaire (PAQ) and the generic EQ‐5D Visual Analogue Scale (VAS) instrument. The PAQ 35 is a 20‐item, validated, PAD‐specific, multidimensional health status instrument that measures 6 health status domains relevant to patients with PAD: physical function, symptoms, symptom stability, social limitations, treatment satisfaction, and quality of life. A summary score is calculated as the average of the physical limitation, symptoms, quality of life, and social functioning scores. Scores range from 0 to 100 points, with higher scores indicating better functioning.
The EQ‐5D 36 is a standardized generic measure of health status that provides a simple measure of health status for clinical assessment. The questionnaire consists of 2 parts: a descriptive section (EQ‐5D index score) and a VAS (EQ‐5D VAS score). We used the EQ‐5D VAS score to assess patients’ overall health. The 20‐cm VAS ranges from the worst (a score of 0) to the best (a score of 100) imaginable health state, with higher scores indicating better health status.
Other Variables
Depression treatment information was derived from patients’ medical records. Information about counseling and/or pharmacologic treatment was abstracted from patients’ medical records at the time of their enrollment (up to 1 month after enrollment).
Statistical Analysis
Patients’ baseline characteristics were compared by sex using χ2 tests or the Fisher exact test for categorical variables and Student t tests for continuous variables. Categorical variables were organized as frequencies and percentages, and continuous variables were summarized as means and SDs or medians and interquartile ranges. For the covariates considered in the model, missingness was minimal, with only 1.5% of patients who had missing information for 1 covariate and only 1 patient who had missing information for 3 covariates. The highest number of covariate missingness was for “high school education” (n=10) and “avoiding care because of cost” (n=8); 98% of the total cohort had no missing covariate information. Given the minimal level of missingness, complete case analysis was used for our analyses.
Mean PHQ‐8 depressive symptoms, as well as the dichotomous prevalence of clinically relevant depressive symptoms (PHQ‐8 score ≥10), were summarized by sex at each time point.
General linear models (GLMs) for repeated measures, with a random effect for site, were used to study baseline and 3‐, 6‐, and 12‐month health status outcomes by the presence of clinically relevant depressive symptoms (PHQ‐8 ≥10 at baseline versus PHQ‐8 score <10). We tested the 2‐way interactions between time×sex, time×depressive symptoms, and depressive symptoms×sex as well as the 3‐way interaction between sex×depressive symptoms×time. GLMs were created both for the PAQ summary and EQ‐5D VAS scores over time. We selected the following covariates for multivariable modeling: age, country, White race, avoiding care because of cost, high school education, ABI, exacerbation of symptoms, bilateral disease, smoking, history of diabetes mellitus, coronary artery disease, and sleep apnea. Consistent with prior work, these covariates were chosen as these were previously identified as most explanatory for patients’ health status outcomes (PAQ summary scores) in the PORTRAIT registry. 37 Estimates for health status differences by depressive symptom groups were presented as mean estimates, and depressive symptom group differences over time were summarized as least square means.
For descriptive purposes, and by ways of sensitivity analysis, we compared health status outcomes by severity of depression (no, mild, or moderate‐severe), and performed post hoc testing. In addition, for the modeling, we performed 2 sensitivity analyses. First, we examined the degree to which depressive symptoms explained sex differences in disease‐specific health status (PAQ summary score). We ran the fully adjusted GLM without depressive symptoms or interaction terms, containing only the main effect for sex. We then replicated the analysis while adding depressive symptoms to the model to examine the difference in estimates for the main sex effect. Second, we explored the potential effect of nonresponse bias. Baseline characteristics of those with and without complete follow‐up were compared. Characteristics with a >10% standardized difference were added as covariates to the original GLM to determine if the estimated effects were different.
A 2‐sided P<0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
In terms of participation rate, of a total of 1636 patients screened, 1243 were enrolled in the study. Follow‐up rates were 92% at 3 months, 88% at 6 months, and 87% at 12 months. With respect to those enrolled versus not enrolled in the study, there were no significant differences between the 2 groups in terms of age, sex, race, and insurance. Baseline characteristics of the total cohort (n=1243) stratified by sex and depressive symptoms (PHQ‐8 ≥10) are shown in Tables 1 and 2. The mean age was 67.6±9.4 years, and 38% (n=470) were women. A total of 52.6% (n=654) had new‐onset PAD symptoms, whereas 47.4% (n=589) had an exacerbation of symptoms at the time of enrollment.
Table 1.
Baseline Patient Characteristics, Stratified by Sex
| Characteristics |
Women (n=470 [38%]) |
Men (n=773 [62%]) |
Total (n=1243) |
P Value |
|---|---|---|---|---|
| Demographics and socioeconomic status | ||||
| Age, y | ||||
| Mean±SD | 68.0±9.9 | 67.3±9.2 | 67.6±9.4 | 0.23 |
| Median (IQR) | 68.0 (61.0–75.0) | 67.0 (61.0–73.0) | 68.0 (61.0–74.0) | |
| Race | <0.001 | |||
| White | 206 (63.0) | 353 (78.8) | 559 (72.1) | |
| Black | 106 (32.4) | 75 (16.7) | 181 (23.4) | |
| Other | 15 (4.6) | 20 (4.5) | 35 (4.5) | |
| Country | <0.001 | |||
| United States | 327 (69.6) | 448 (58.0) | 775 (62.3) | |
| The Netherlands | 117 (24.9) | 257 (33.2) | 374 (30.1) | |
| Australia | 26 (5.5) | 68 (8.8) | 94 (7.6) | |
| Health insurance | 466 (99.1) | 770 (99.6) | 1236 (99.4) | 0.44 |
| Education, high school or above | 312 (66.8) | 539 (70.4) | 851 (69.0) | 0.19 |
| Married | 208 (44.4) | 524 (68.1) | 732 (59.2) | <0.001 |
| Working for pay | 82 (17.5) | 211 (27.4) | 293 (23.6) | <0.001 |
| Avoid care because of cost | 78 (16.8) | 96 (12.5) | 174 (14.1) | 0.035 |
| Activity during leisure time | ||||
| Sedentary | 240 (51.6) | 258 (34.2) | 498 (40.8) | <0.001 |
| Mild | 146 (31.4) | 257 (34.0) | 403 (33.0) | |
| Moderate | 79 (17.0) | 240 (31.8) | 319 (26.1) | |
| Risk factors and comorbidities | ||||
| Smoking status | ||||
| Never | 79 (16.8) | 53 (6.9) | 132 (10.6) | <0.001 |
| Former | 213 (45.4) | 435 (56.3) | 648 (52.2) | |
| Current | 177 (37.7) | 284 (36.8) | 461 (37.1) | |
| CAD | 162 (34.5) | 324 (41.9) | 486 (39.1) | 0.009 |
| Dyslipidemia | 381 (81.1) | 606 (78.4) | 987 (79.4) | 0.26 |
| Hypertension | 404 (86.0) | 589 (76.2) | 993 (79.9) | <0.001 |
| Diabetes mellitus | 168 (35.7) | 247 (32.0) | 415 (33.4) | 0.17 |
| Congestive heart failure | 42 (8.9) | 81 (10.5) | 123 (9.9) | 0.38 |
| Chronic kidney disease | 59 (12.6) | 79 (10.2) | 138 (11.1) | 0.20 |
| Chronic back pain | 72 (15.3) | 97 (12.5) | 169 (13.6) | 0.17 |
| Sleep apnea | 32 (6.8) | 68 (8.8) | 100 (8.0) | 0.21 |
| PAD treatment history | ||||
| Amputation | 3 (0.6) | 13 (1.7) | 16 (1.3) | 0.11 |
| PAD bypass | 27 (5.7) | 72 (9.3) | 99 (8.0) | 0.024 |
| PAD endarterectomy | 13 (2.8) | 24 (3.1) | 37 (3.0) | 0.73 |
| PAD atherectomy | 11 (2.3) | 20 (2.6) | 31 (2.5) | 0.79 |
| PAD angioplasty | 94 (20.0) | 153 (19.8) | 247 (19.9) | 0.93 |
| Cilostazol | 28 (6.0) | 48 (6.2) | 76 (6.1) | 0.85 |
| Antiplatelet therapy | 323 (69.0) | 518 (67.4) | 841 (68.0) | 0.54 |
| Statin | 320 (68.4) | 546 (71.0) | 866 (70.0) | 0.33 |
| PAD characteristics on presentation | ||||
| Symptoms | ||||
| New onset | 244 (51.9) | 410 (53.0) | 654 (52.6) | 0.70 |
| Exacerbation of symptoms | 226 (48.1) | 363 (47.0) | 589 (47.4) | |
| ABI | ||||
| Mean±SD | 0.65±0.18 | 0.67±0.19 | 0.67±0.19 | 0.045 |
| Rutherford category | ||||
| Mild claudication | 95 (20.7) | 187 (24.5) | 282 (23.1) | 0.21 |
| Moderate claudication | 239 (52.1) | 362 (47.4) | 601 (49.1) | |
| Severe claudication | 125 (27.2) | 215 (28.1) | 340 (27.8) | |
| Duration of pain, mo | ||||
| <1 | 10 (2.5) | 20 (3.0) | 30 (2.8) | 0.38 |
| 1–6 | 129 (31.7) | 191 (28.9) | 320 (29.9) | |
| 7–12 | 78 (19.2) | 110 (16.6) | 188 (17.6) | |
| >12 | 190 (46.7) | 341 (51.5) | 531 (49.7) | |
| Atypical PAD symptoms | 75 (17.2) | 88 (12.3) | 163 (14.2) | 0.020 |
| Bilateral disease | 264 (56.2) | 364 (47.1) | 628 (50.5) | 0.001 |
| Lesion site | ||||
| Proximal | 130 (27.8) | 217 (28.3) | 347 (28.1) | 0.55 |
| Distal | 149 (31.9) | 223 (29.1) | 372 (30.1) | |
| Both | 188 (40.3) | 327 (42.6) | 515 (41.7) | |
| PAD treatment after enrollment | ||||
| Cilostazol | 62 (13.2) | 85 (11.1) | 147 (11.9) | 0.25 |
| Antiplatelet therapy | 368 (78.6) | 583 (75.8) | 951 (76.9) | 0.25 |
| Statin | 370 (79.1) | 629 (81.8) | 999 (80.8) | 0.24 |
| Smoking cessation physician advice | 153 (71.2) | 231 (64.9) | 384 (67.3) | 0.12 |
| Unsupervised PAD exercise therapy | 200 (42.6) | 249 (32.2) | 449 (36.1) | <0.001 |
| Supervised PAD exercise therapy | 95 (20.2) | 177 (22.9) | 272 (21.9) | 0.27 |
| Invasive treatment | 82 (19.9) | 141 (20.1) | 223 (20.0) | 0.94 |
| Surgical treatment | 6 (1.5) | 23 (3.3) | 29 (2.6) | 0.07 |
| Endovascular treatment | 78 (18.9) | 122 (17.4) | 200 (18.0) | 0.51 |
| Depression severity and treatment at baseline | ||||
| PHQ‐8 score severity (baseline) | ||||
| None (PHQ‐8 <5) | 257 (54.7) | 510 (66.0) | 767 (61.7) | |
| Mild (5 ≥ PHQ‐8 ≤ 9) | 114 (24.3) | 163 (21.1) | 277 (22.3) | <0.001 |
| Moderate/severe (PHQ‐8 ≥10) | 99 (21.1) | 100 (12.9) | 199 (16.0) | |
| PHQ‐8 depression score, mean±SD | 5.6±5.3 | 4.2±4.8 | 4.7±5.0 | <0.001 |
| Baseline depression treatments | ||||
| None | 331 (70.4) | 660 (85.4) | 991 (79.7) | 0.001 |
| Pharmacologic treatment | 79 (16.8) | 71 (9.2) | 150 (12.1) | |
| Counseling | 13 (2.8) | 12 (1.6) | 25 (2.0) | |
| Both | 47 (10.0) | 30 (3.9) | 77 (6.2) | |
| Antidepressant use at baseline | ||||
| SSRI | 72 | 46 | 118 | <0.001 |
| SNRI | 25 | 12 | 37 | <0.001 |
| TCA | 17 | 14 | 31 | 0.06 |
| Atypical agents | 31 | 29 | 60 | 0.03 |
| Miscellaneous | ||||
| Perphenazine | 0 | 1 | 1 | 1.00 |
Continuous variables were compared using the Student t test. Categorical variables were compared using the χ2 or the Fisher exact test (health insurance). Values are listed as number (percentage), unless otherwise described. ABI indicates ankle‐brachial index; CAD, coronary artery disease; IQR, interquartile range; PAD, peripheral artery disease; PHQ‐8, 8‐Item Patient Health Questionnaire; SNRI, serotonin‐norepinephrine reuptake inhibitor; SSRI, selective serotonin receptor inhibitor; and TCA, tricyclic antidepressant.
Table 2.
Baseline Patient Characteristics, Stratified by Sex and Clinically Relevant Depressive Symptoms (PHQ‐8 ≥10)
| Characteristics | Women | P Value | Men | P Value | ||
|---|---|---|---|---|---|---|
| PHQ‐8 ≥10 | PHQ‐8 <10 | PHQ‐8 ≥10 | PHQ‐8 <10 | |||
| (n=99) | (n=371) | (n=100) | (n=673) | |||
| Demographics and socioeconomic status | ||||||
| Age, y | ||||||
| Mean±SD | 63.0±9.9 | 69.3±9.5 | <0.001 | 63.3±8.9 | 67.9±9.0 | <0.001 |
| Race* | ||||||
| White | 47 (63.5) | 159 (62.8) | 50 (76.9) | 303 (79.1) | ||
| Black | 19 (25.7) | 87 (34.4) | 0.013 | 8 (12.3) | 67 (17.5) | 0.031 |
| Other | 8 (10.8) | 7 (2.8) | 7 (10.8) | 13 (3.4) | ||
| Country | ||||||
| United States | 74 (74.7) | 253 (68.2) | 65 (65.0) | 383 (56.9) | ||
| The Netherlands | 15 (15.2) | 102 (27.5) | 0.006 | 23 (23.0) | 234 (34.8) | 0.05 |
| Australia | 10 (10.1) | 16 (4.3) | 12 (12.0) | 56 (8.3) | ||
| Health insurance* | 95 (96.0) | 371 (100.0) | 0.001 | 100 (100.0) | 670 (99.6) | 1.000 |
| Education, high school or above | 59 (60.8) | 253 (68.4) | 0.16 | 67 (67.7) | 472 (70.8) | 0.53 |
| Married | 34 (34.7) | 174 (47.0) | 0.028 | 56 (56.6) | 468 (69.9) | 0.008 |
| Working for pay | 14 (14.3) | 68 (18.4) | 0.34 | 20 (20.0) | 191 (28.5) | 0.08 |
| Avoid care because of cost | 31 (32.0) | 47 (12.8) | <0.001 | 23 (23.0) | 73 (10.9) | <0.001 |
| Activity during leisure time | ||||||
| Sedentary | 64 (65.3) | 176 (48.0) | 62 (63.3) | 196 (29.8) | ||
| Mild | 23 (23.5) | 123 (33.5) | 0.008 | 19 (19.4) | 238 (36.2) | <0.001 |
| Moderate | 11 (11.2) | 68 (18.5) | 17 (17.3) | 223 (33.9) | ||
| Risk factors and comorbidities | ||||||
| Smoking status* | ||||||
| Never | 13 (13.3) | 66 (17.8) | 0.004 | 7 (7.0) | 46 (6.8) | 0.11 |
| Former | 34 (34.7) | 179 (48.2) | 47 (47.0) | 388 (57.7) | ||
| Current | 51 (52.0) | 126 (34.0) | 46 (46.0) | 238 (35.4) | ||
| CAD | 43 (43.4) | 119 (32.1) | 0.034 | 48 (48.0) | 276 (41.0) | 0.19 |
| Dyslipidemia | 82 (82.8) | 299 (80.6) | 0.61 | 74 (74.0) | 532 (79.0) | 0.25 |
| Hypertension | 85 (85.9) | 319 (86.0) | 0.97 | 77 (77.0) | 512 (76.1) | 0.84 |
| Diabetes mellitus | 47 (47.5) | 121 (32.6) | 0.006 | 34 (34.0) | 213 (31.6) | 0.64 |
| Congestive heart failure | 9 (9.1) | 33 (8.9) | 0.95 | 16 (16.0) | 65 (9.7) | 0.05 |
| Chronic kidney disease | 14 (14.1) | 45 (12.1) | 0.59 | 8 (8.0) | 71 (10.5) | 0.43 |
| Chronic back pain | 17 (17.2) | 55 (14.8) | 0.56 | 20 (20.0) | 77 (11.4) | 0.015 |
| Sleep apnea | 11 (11.1) | 21 (5.7) | 0.05 | 13 (13.0) | 55 (8.2) | 0.11 |
| PAD treatment history | ||||||
| Amputation* | 1 (1.0) | 2 (0.5) | 0.51 | 3 (3.0) | 10 (1.5) | 0.23 |
| PAD bypass | 10 (10.1) | 17 (4.6) | 0.036 | 12 (12.0) | 60 (8.9) | 0.32 |
| PAD endarterectomy* | 2 (2.0) | 11 (3.0) | 1.00 | 3 (3.0) | 21 (3.1) | 1.00 |
| PAD angioplasty | 24 (24.2) | 70 (18.9) | 0.23 | 19 (19.0) | 134 (19.9) | 0.83 |
| Cilostazol | 5 (5.1) | 23 (6.2) | 0.66 | 6 (6.1) | 42 (6.3) | 0.94 |
| Antiplatelet therapy | 75 (75.8) | 248 (67.2) | 0.10 | 70 (70.7) | 448 (66.9) | 0.45 |
| Statin | 67 (67.7) | 253 (68.6) | 0.87 | 65 (65.7) | 481 (71.8) | 0.21 |
| PAD characteristics on presentation | ||||||
| Symptoms | ||||||
| New onset | 53 (53.5) | 191 (51.5) | 50 (50.0) | 360 (53.5) | ||
| Exacerbation | 46 (46.5) | 180 (48.5) | 0.72 | 50 (50.0) | 313 (46.5) | 0.51 |
| ABI | ||||||
| Mean±SD | 0.68±0.20 | 0.64±0.18 | 0.06 | 0.68±0.20 | 0.67±0.19 | 0.88 |
| Rutherford category | 0.57 | 0.001 | ||||
| Mild claudication | 20 (20.8) | 75 (20.7) | 18 (18.0) | 169 (25.5) | ||
| Moderate claudication | 46 (47.9) | 193 (53.2) | 39 (39.0) | 323 (48.6) | ||
| Severe claudication | 30 (31.3) | 95 (26.2) | 43 (43.0) | 172 (25.9) | ||
| Duration of pain, mo* | ||||||
| <1 | 3 (3.7) | 7 (2.1) | 1 (1.2) | 19 (3.3) | ||
| 1–6 | 21 (25.9) | 108 (33.1) | 15 (17.9) | 176 (30.4) | ||
| 7–12 | 18 (22.2) | 60 (18.4) | 0.47 | 21 (25.0) | 89 (15.4) | 0.023 |
| >12 | 39 (48.1) | 151 (46.3) | 47 (56.0) | 294 (50.9) | ||
| Symptoms | ||||||
| Typical | 69 (77.5) | 291 (84.1) | 83 (84.7) | 543 (88.1) | ||
| Atypical | 20 (22.5) | 55 (15.9) | 0.14 | 15 (15.3) | 73 (11.9) | 0.33 |
| Bilateral disease | 48 (48.5) | 216 (58.2) | 0.08 | 55 (55.0) | 309 (45.9) | 0.09 |
| Lesion site | ||||||
| Proximal | 29 (29.6) | 101 (27.4) | 32 (32.3) | 185 (27.7) | ||
| Distal | 19 (19.4) | 130 (35.2) | 25 (25.3) | 198 (29.6) | ||
| Both | 50 (51.0) | 138 (37.4) | 0.007 | 42 (42.4) | 285 (42.7) | 0.54 |
| PAD treatment after enrollment | ||||||
| Cilostazol | 6 (6.1) | 56 (15.2) | 0.017 | 8 (8.1) | 77 (11.5) | 0.31 |
| Antiplatelet therapy | 80 (80.8) | 288 (78.0) | 0.55 | 79 (79.8) | 504 (75.2) | 0.32 |
| Statin | 74 (74.7) | 296 (80.2) | 0.23 | 75 (75.8) | 554 (82.7) | 0.10 |
| Smoking cessation advice (among smokers) | 47 (88.7) | 106 (65.4) | 0.001 | 40 (80.0) | 191 (62.4) | 0.015 |
| Unsupervised PAD exercise therapy | 31 (31.3) | 169 (45.6) | 0.010 | 31 (31.0) | 218 (32.4) | 0.78 |
| Supervised PAD exercise therapy | 20 (20.2) | 75 (20.2) | 1.00 | 19 (19.0) | 158 (23.5) | 0.32 |
| Invasive treatment | 16 (19.8) | 66 (19.9) | 0.97 | 22 (25.3) | 119 (19.3) | 0.20 |
| Surgical treatment | 0 (0.0) | 6 (1.8) | 0.60 | 5 (5.7) | 18 (2.9) | 0.19 |
| Endovascular treatment | 16 (19.8) | 62 (18.7) | 0.83 | 18 (20.7) | 104 (16.9) | 0.38 |
| Depression severity and treatment at baseline | ||||||
| PHQ‐8 depression score, mean±SD | 14.2±3.7 | 3.4±2.7 | <0.001 | 14.1±3.7 | 2.7±2.7 | <0.001 |
| Baseline antidepression treatments | ||||||
| None | 54 (54.5) | 277 (74.7) | 0.001 | 66 (66.0) | 594 (88.3) | <0.001 |
| Pharmacologic | 24 (24.2) | 55 (58.5) | 17 (17.0) | 54 (8.0) | ||
| Counseling | 4 (4.0) | 9 (9.6) | 3 (3.0) | 9 (1.3) | ||
| Both | 17 (17.2) | 30 (31.9) | 14 (14.0) | 16 (2.4) | ||
Values are listed as number (percentage), unless otherwise described. ABI indicates ankle‐brachial index; CAD, coronary artery disease; PAD, peripheral artery disease; and PHQ‐8, 8‐Item Patient Health Questionnaire.
Includes all covariates used in modeling by PHQ‐8 ≥10. Covariates include age, country, White race, avoiding care because of cost, high school education, exacerbation of symptoms, bilateral disease, smoking, history of diabetes mellitus, coronary artery disease, sleep apnea, and ABI. Continuous variables were compared using the Student t test. Categorical variables were compared using the χ 2 or the Fisher exact test (race, health insurance, smoking status, amputation, PAD endarterectomy, and duration of pain).
At enrollment, there was no significant difference in age between women and men for the overall cohort (68.0±9.9 versus 67.3±9.2 years; P=0.23). Women were less likely to be married, less likely to be employed, and more likely than men to avoid care because of cost. Women were more likely to be sedentary during leisure time and were less likely to engage in moderate to strenuous exercise compared with their male counterparts. Compared with men, women had lower mean ABI values and were more likely to present with atypical symptoms and to present with bilateral symptoms. Cardiovascular risk management strategies were not different by sex, except for higher rates of unsupervised exercise therapy among women. There were no differences in the rates of invasive treatment at 3 months (either surgical or endovascular) by sex (Table 1).
In the overall cohort (n=1243), a total of 199 patients (16.0%) had moderate‐severe depressive (PHQ‐8 ≥10) symptoms on presentation, with rates being much higher in women than in men (21.1% versus 12.9%; P<0.001) at presentation. Mean PHQ‐8 scores were 5.6±5.3 in women versus 4.2±4.8 in men at baseline (Table 1).
After stratification by sex and clinically relevant depressive symptoms (PHQ‐8 ≥10), mean PHQ‐8 scores among those with depressive symptoms were similar between men and women (14.2±3.7 versus 14.1±3.7). Of those with clinically relevant depressive symptoms, almost half of women and one third of men were on some form of depression treatment. A complete breakdown of the types of depression treatment by sex and depressive symptom status for this cohort is provided in Table 2. In both men and women, patients with depressive symptoms were more likely to be younger, avoid care because of cost, and be sedentary compared with their respective counterparts without depressive symptoms; both men and women with depressive symptoms were less likely to be married compared with those without such symptoms (Table 2).
Unadjusted mean PAQ health status scores by baseline depressive symptoms and by sex are presented in Table 3. Both men and women with depressive symptoms (PHQ‐8 ≥10 at baseline) had consistently lower unadjusted PAQ health status scores than those without significant depressive symptoms (mean differences ranging between 17.8 and 26.9) for the overall group. In women with and without depressive symptoms, mean differences ranged between 17.8 and 24.2, which were similar to the differences in men (mean differences ranged between 18.9 and 26.9). Women with depressive symptoms had lower PAQ summary scores compared with men with depressive symptoms, albeit only reaching statistical significance at 12 months (P=0.038). For the EQ‐5D VAS scores, mean differences between patients with and without depressive symptoms ranged between 12.3 and 13.1 for women and between 14.3 and 19.5 for men and were also similar, except for the baseline levels (difference in scores for men with and without depressive symptoms was greater than for women with and without depressive symptoms [Table 3]).
Table 3.
Unadjusted Mean PAQ and EQ‐5D VAS Scores in Patients With and Without Depressive Symptoms at Baseline and 3, 6, and 12 Months, Stratified by Sex
| Variable | PHQ‐8 Score ≥10 (Baseline) | P Value* | |||||
|---|---|---|---|---|---|---|---|
| Women | Men | ||||||
|
Yes (n=99) |
No (n=371) |
Mean Differences |
Yes (n=100) |
No (n=673) |
Mean Differences | ||
| PAQ summary score, mean±SD | |||||||
| Baseline | 24.7±16.8 | 48.9±19.7 | 24.2 | 29.2±19.5 | 56.1±19.3 | 26.9 | 0.09 |
| 3 mo | 45.9±27.5 | 66.6±23.9 | 20.7 | 51.2±28.6 | 71.7±21.7 | 20.5 | 0.22 |
| 6 mo | 52.7±26.8 | 70.5±24.4 | 17.8 | 53.1±25.7 | 73.7±22.1 | 20.6 | 0.93 |
| 12 mo | 47.0±28.2 | 68.7±24.8 | 21.7 | 56.6±28.0 | 75.5±22.7 | 18.9 | 0.038 |
| EQ‐5D VAS, mean±SD | |||||||
| Baseline | 55.7±21.9 | 68.0±18.7 | 12.3 | 49.7±19.8 | 69.2±17.3 | 19.5 | 0.047 |
| 3 mo | 58.5±19.9 | 70.8±18.9 | 12.3 | 53.8±23.1 | 72.8±15.6 | 19 | 0.16 |
| 6 mo | 60.4±21.3 | 73.2±17.2 | 12.8 | 57.2±22.2 | 71.5±16.4 | 14.3 | 0.37 |
| 12 mo | 58.5±19.5 | 71.6±17.9 | 13.1 | 58.1±18.9 | 72.5±16.1 | 14.4 | 0.89 |
Continuous variables were compared using Student t test. PAQ indicates Peripheral Artery Questionnaire; PHQ‐8, 8‐Item Patient Health Questionnaire; and VAS, Visual Analogue Scale.
P values for differences in PAQ summary and EQ‐5D VAS scores between women and men with depressive symptoms (PHQ‐8 ≥10).
For the repeated measures model, we first tested the 3‐way interaction between sex×depressive symptoms×time, evaluating whether the magnitude of health status changes over time differed by sex. This interaction term was nonsignificant (P=0.39), suggesting no differences in the sex‐associated disparities over time. In the final adjusted repeated measures model, the 2‐way interaction between time and sex as well as time and depressive symptoms remained significant. The 2‐way interaction between sex×depression was not significant (P=0.58). Patients with depressive symptoms at the time of their PAD presentation consistently had lower PAQ health status scores at each time point compared with scores at baseline (adjusted mean score, 25.4 [Interquartile range: 21.3–29.4] versus 46.7 [Interquartile range: 43.6–49.9]; P<0.0001) and at 12 months of follow‐up (adjusted mean score, 50.0 [Interquartile range: 45.3–54.6] versus 66.1 [62.8–69.5]; P<0.0001), amounting to differences between groups ranging from 16.3 to 21.4 (Figure). In this model, the interaction between depressive symptoms and time was also significant (P<0.0001), indicating that improvement in health status score trajectories over time lagged behind in those with depressive symptoms versus those who did not experience such symptoms. For the model with EQ‐5D VAS scores over time, results largely mirrored the PAQ results (Figure).
Figure 1. Adjusted mean Peripheral Artery Questionnaire (PAQ) summary and EQ‐5D Visual Analogue Scale (VAS) scores at baseline and 3, 6, and 12 months for patients with (8‐Item Patient Health Questionnaire [PHQ‐8] score ≥10) and without (PHQ‐8 score <10) baseline depressive symptoms.
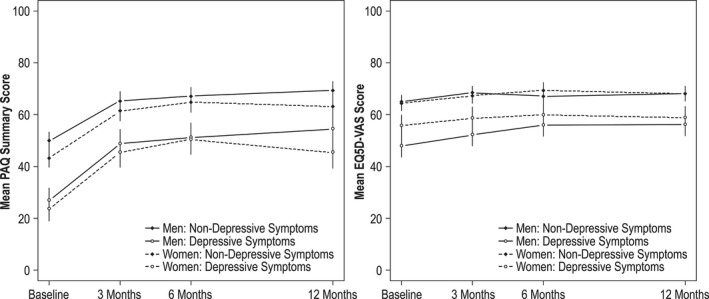
Covariates in the model included age, country, race, avoiding care because of cost, high school education, ankle‐brachial index, exacerbation of symptoms, bilateral disease, smoking, history of diabetes mellitus, coronary artery disease, and sleep apnea.
As part of the descriptive sensitivity analyses, we looked at both PAQ summary scores and EQ‐5D VAS over time by severity of depressive symptoms over time (no depressive symptoms, PHQ‐8 score <5; mild depressive symptoms, PHQ‐8 score ≤5–≤9; moderate‐severe depressive symptoms, PHQ‐8 score ≥10) (Tables S1 through S3). In a post hoc analysis, women with moderate‐severe depressive symptoms had statistically significant lower PAQ summary scores compared with all other groups, except for men whose depressive symptoms were also moderate‐severe (P=0.088). This was similar at 3 and 6 months. At 1 year, women with moderate‐severe depressive symptoms had statistically lower PAQ summary scores at all time points. For the mean EQ‐5D VAS levels, women with moderate‐severe depressive symptoms had lower scores compared with all other groups, but no differences were noted for men whose depressive symptoms were moderate‐severe at 3 and 12 months or for men with mild symptoms at 6 months (Table S4).
For the sensitivity analysis as it relates to our GLM modeling, we examined the association between sex and health status outcomes over time, without including depressive symptoms in the fully adjusted GLM. In that model, women scored an average of 6.4 points lower than men (women versus men, −6.4; 95% CI, −8.5 to −4.3; P<0.0001). After adding depressive symptoms to the adjusted model, this difference decreased to 5.2 points in the adjusted model (women versus men, −5.2; 95% CI, −7.2 to −3.3; P<0.0001). Depressive symptoms explained 19% ([6.4–5.2]/6.4) of the difference in outcomes between the 2 sexes.
To assess for nonresponse bias, we performed a second sensitivity analysis, comparing those with complete follow‐up with those without complete follow‐up. Large differences (standardized difference >10%) between the 2 groups, which were not already adjusted for in the main analyses, were duration of pain, lesion site, Rutherford category, history of chronic kidney disease, and history of amputation (Table S5). Addition of these covariates to the main analyses yielded similar results for both PAQ summary scores and EQ‐5D VAS at baseline and 3, 6, and 12 months (Figure and Figure S1).
Discussion
Our study demonstrates that a disproportionately higher percentage of women are affected by depressive symptoms when they seek specialty care for new or worsening PAD symptoms, with rates almost twice that observed in men. Experiencing clinically significant depressive symptoms is associated with enormous gaps in their 1‐year health status recovery patterns compared with health status levels that are typically seen in their nondepressed counterparts, up to twice the magnitude that is defined as a minimally clinically important difference (Poghni, Peri‐Okonny, MD, unpublished data, 2019), leaving patients with depressive symptoms at a disadvantaged position to optimally benefit from the PAD treatments that are offered to them. Although the effect size for the association between depressive symptoms and health status changes over time did not differ by sex, women are disproportionately affected given the increased prevalence of depressive symptoms observed in women. Depressive symptoms also explained about a fifth of the variation seen in health status differences by sex documented in the year following a PAD diagnosis.
Our study provides a unique perspective; it prospectively followed up patients who were dealing with new or recurrent symptoms of PAD and were actively seeking specialty care. This similar time point of identification in the clinical pathway for PAD allowed us to prospectively reconstruct the 1‐year health status trajectory in men and women by patients’ depressive symptoms at the time of seeking PAD care. Also notable is that our patients were included before they were assigned to treatment, and regardless of whether they underwent invasive versus noninvasive management of their disease, which is different from most other available PAD databases. 38 , 39
Despite the enormous patient and economic burden associated with both depression 40 and PAD, 10 , 41 we are just starting to understand the potential impact of how mental health concerns may complicate PAD treatment and outcomes. Although it has been demonstrated in coronary artery disease that depressive symptoms are disproportionately present in women compared with men, and that these symptoms are linked with adverse clinical and health status outcomes, 19 , 42 , 43 , 44 the association between depressive symptoms and PAD‐specific health status outcomes and how this may differ by sex have not been studied in PAD.
Various reasons for an increased depressive symptom burden in women with cardiovascular disease have been studied. Of the factors studied, socioeconomic factors may partially explain as to why women may experience this increased vulnerability. 45 , 46 In support of this, we found that less than half of the overall female cohort as well as only one third of those with clinically relevant depressive symptoms were married, and more women than men avoided care because of cost. It is unknown whether any biological sex differences specific to PAD explain some of the differences observed. We did demonstrate in our study that women had lower ABIs, indicating more advanced disease as well as bilateral disease. We did not find any evidence for differential PAD treatment patterns or quality of PAD care by sex. A bias toward undertreatment of depressive symptoms in the context of PAD specialty care was present as only a third of patients were receiving care and/or follow‐up for their depressive symptoms, with the lowest treatment rates for men.
Having a depressed mood has potentially major implications for the success of patients’ PAD rehabilitation process and their PAD functioning over time. Depressive symptoms marked a suboptimal PAD recovery pathway, with differences as large as 16 to 21 points on the PAQ summary scale 1 year following active PAD treatment, differences that are almost twice the minimally clinically important difference, as defined from the patients’ perspective. To put these findings further in perspective: in the CLEVER (Supervised Exercise, Stent Revascularization, or Medical Therapy for Claudication Due to Aortoiliac Peripheral Artery Disease: A Randomized Clinical Trial), 47 differences at 18 months between patients treated with optimal medical therapy only versus those who underwent peripheral stenting were 24 points on the PAQ summary scale; and for optimal medical therapy versus supervised exercise therapy, 13 points. Such large discrepancies in PAD health status outcomes related to patients’ depressive mood demand for more proactive PAD care that can detect and support patients in need, so as to allow them to be successful in managing their PAD.
Although the magnitude of the effect on health status changes over time for depressive symptoms in men and women was not different, women had a higher prevalence of symptoms and higher mean depressive symptom scores at all time periods. Sex differences in PAD‐specific health status were also partially explained by depressive symptoms (19%). Whether this has implications for patients’ long‐term PAD outcomes needs to be further established, including intervention studies that would include and test depression interventions as a way to maximize PAD rehabilitation outcomes. Preliminary studies have shown that depression is associated with an adverse PAD prognosis as well as with an increased risk of experiencing adverse cardiovascular events. 29 , 48 , 49 In a recent study, depression was independently associated with an elevated risk of amputation, with an even higher risk in those who were not treated with antidepressents. 50 Whether the higher rates of depressive symptoms contribute to more advanced disease, 51 lower physical functioning, 52 and poorer outcomes after lower‐extremity revascularization 17 , 18 and higher in‐hospital mortality 51 in women remains to be seen. Multidirectional relationships between depressive symptoms and cardiovascular outcomes have been described before in cardiovascular disease, but to a much lesser extent in PAD. 53 Previous studies were cross‐sectional, were small sampled, or did not focus on sex differences. In coronary artery disease, sex differences in quality of care, biological differences, and differences in disease manifestations, as well as interrelatedness with women’s psychosocial profiles, and subsequent clinical outcomes have been well described, 54 but these associations have yet to be fully studied and understood in PAD.
Future work needs to explore whether these same mechanisms explain women’s increased vulnerability to depressive symptoms in PAD. It is also important to increase the knowledge and awareness of vascular specialists who treat patients with PAD, such that they know that women have a higher prevalence of depressive symptoms from an epidemiological standpoint such that they could be referred for further depression evaluation and treatment as part of an integrated care vision that maximized patients’ outcomes.
Our study must be interpreted in the light of several potential limitations. Our study cohort included patients seen at vascular specialty clinics, and our findings may not be representative of the general PAD population who may not have access to specialty clinics. For this study, we focused on depressive symptoms, and we acknowledge that there are a myriad of mental health concerns, as well as other unmeasured psychosocial and clinical factors, that may be intertwined with depressive symptoms, or that may have also impacted patients’ health status.
In summary, our findings indicate that the burden of depressive symptoms in PAD is substantial, and patients affected by them, especially women, have distinctly worse PAD‐specific health status after receiving PAD specialty care. Effect sizes were large and carried over at each follow‐up point in the year after seeking PAD treatment. There is a need to explore mechanisms of this increased vulnerability in women. These have been extensively described in other atherosclerotic diseases (eg, exposure to psychosocial stressors and socioeconomic factors), like acute myocardial infarction. 54 The problem is not limited to women alone; among men, too, patients who are dealing with depressive symptoms have worse PAD health outcomes over time. Depressive symptoms in older, chronic disease populations, such as PAD, should be a continuous focus of its multidisciplinary treatment so as to ensure quality PAD care and optimize outcomes. In conclusion, depression warrants screening and treatment in its own right, but especially so in PAD, it is imperative to pay attention to this problem.
Sources of Funding
Research reported in this article was partially funded through the Patient‐Centered Outcomes Research Institute (PCORI) Award (IP2 PI000573‐01; CE‐1304‐6677) and an unrestricted grant by Gore. The statements in this article are solely the responsibility of the authors and do not necessarily represent the views of the PCORI, its Board of Governors, or its Methodology Committee. All articles for the PORTRAIT (Patient‐Centered Outcomes Related to Treatment Practices in Peripheral Artery Disease Investigating Trajectories) registry are prepared by independent authors who are not governed by the funding sponsors and are reviewed by an academic publications committee before submission. The funding organizations and sponsors of the study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
Dr Mena‐Hurtado is a consultant for Bard, Cook, Medtronic, Abbott, Boston Scientific, Optum Labs LLC and Cardinal Health. Dr Spertus is supported by grants from Gilead, Genentech, Lilly, Amorcyte, and EvaHeart; and has a patent Seattle Angina Questionnaire with royalties paid. Dr Smolderen is supported by an unrestricted research grant from Terumo and is a consultant for Optum Labs LLC. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S5
Figure S1
Acknowledgments
We thank all patients who participated in this study, allowing us to conduct multifaceted research in peripheral artery disease.
(J Am Heart Assoc. 2020;9:e014583 DOI: 10.1161/JAHA.119.014583.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.014583
For Sources of Funding and Disclosures, see page 12.
References
- 1. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. [DOI] [PubMed] [Google Scholar]
- 2. Sampson UK, Fowkes FG, McDermott MM, Criqui MH, Aboyans V, Norman PE, Forouzanfar MH, Naghavi M, Song Y, Harrell FE Jr, et al. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Glob Heart. 2014;9:145–158.e21. [DOI] [PubMed] [Google Scholar]
- 3. Lloyd‐Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. [DOI] [PubMed] [Google Scholar]
- 4. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, et al; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention . Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. [DOI] [PubMed] [Google Scholar]
- 5. Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA, Mukamal KJ. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirsch AT, Criqui MH, Treat‐Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. [DOI] [PubMed] [Google Scholar]
- 7. Grenon SM, Vittinghoff E, Owens CD, Conte MS, Whooley M, Cohen BE. Peripheral artery disease and risk of cardiovascular events in patients with coronary artery disease: insights from the Heart and Soul Study. Vasc Med. 2013;18:176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–699. [DOI] [PubMed] [Google Scholar]
- 9. Steg PG, Bhatt DL, Wilson PW, D'Agostino R Sr, Ohman EM, Rother J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, et al. One‐year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. [DOI] [PubMed] [Google Scholar]
- 10. Mahoney EM, Wang K, Cohen DJ, Hirsch AT, Alberts MJ, Eagle K, Mosse F, Jackson JD, Steg PG, Bhatt DL, et al. One‐year costs in patients with a history of or at risk for atherothrombosis in the United States. Circ Cardiovasc Qual Outcomes. 2008;1:38–45. [DOI] [PubMed] [Google Scholar]
- 11. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. [DOI] [PubMed] [Google Scholar]
- 12. Kullo IJ, Bailey KR, Kardia SL, Mosley TH Jr, Boerwinkle E, Turner ST. Ethnic differences in peripheral arterial disease in the NHLBI Genetic Epidemiology Network of Arteriopathy (GENOA) study. Vasc Med. 2003;8:237–242. [DOI] [PubMed] [Google Scholar]
- 13. Zheng ZJ, Rosamond WD, Chambless LE, Nieto FJ, Barnes RW, Hutchinson RG, Tyroler HA, Heiss G; ARIC Investigators . Lower extremity arterial disease assessed by ankle‐brachial index in a middle‐aged population of African Americans and whites: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Prev Med. 2005;29:42–49. [DOI] [PubMed] [Google Scholar]
- 14. Gofin R, Kark JD, Friedlander Y, Lewis BS, Witt H, Stein Y, Gotsman MS. Peripheral vascular disease in a middle‐aged population sample: the Jerusalem Lipid Research Clinic Prevalence Study. Isr J Med Sci. 1987;23:157–167. [PubMed] [Google Scholar]
- 15. McDermott MM, Greenland P, Liu K, Criqui MH, Guralnik JM, Celic L, Chan C. Sex differences in peripheral arterial disease: leg symptoms and physical functioning. J Am Geriatr Soc. 2003;51:222–228. [DOI] [PubMed] [Google Scholar]
- 16. Collins TC, Suarez‐Almazor M, Bush RL, Petersen NJ. Gender and peripheral arterial disease. J Am Board Fam Med. 2006;19:132–140. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen LL, Brahmanandam S, Bandyk DF, Belkin M, Clowes AW, Moneta GL, Conte MS. Female gender and oral anticoagulants are associated with wound complications in lower extremity vein bypass: an analysis of 1404 operations for critical limb ischemia. J Vasc Surg. 2007;46:1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Enzler MA, Ruoss M, Seifert B, Berger M. The influence of gender on the outcome of arterial procedures in the lower extremity. Eur J Vasc Endovasc Surg. 1996;11:446–452. [DOI] [PubMed] [Google Scholar]
- 19. Parashar S, Rumsfeld JS, Reid KJ, Buchanan D, Dawood N, Khizer S, Lichtman J, Vaccarino V; PREMIER Registry Investigators . Impact of depression on sex differences in outcome after myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frasure‐Smith N, Lesperance F, Talajic M. Depression following myocardial infarction: impact on 6‐month survival. JAMA. 1993;270:1819–1825. [PubMed] [Google Scholar]
- 21. Parashar S, Rumsfeld JS, Spertus JA, Reid KJ, Wenger NK, Krumholz HM, Amin A, Weintraub WS, Lichtman J, Dawood N, et al. Time course of depression and outcome of myocardial infarction. Arch Intern Med. 2006;166:2035–2043. [DOI] [PubMed] [Google Scholar]
- 22. Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health‐related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Melle JP, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ, van den Brink RH, van den Berg MP. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta‐analysis. Psychosom Med. 2004;66:814–822. [DOI] [PubMed] [Google Scholar]
- 24. Smolderen KG, Spertus JA, Vriens PW, Kranendonk S, Nooren M, Denollet J. Younger women with symptomatic peripheral arterial disease are at increased risk of depressive symptoms. J Vasc Surg. 2010;52:637–644. [DOI] [PubMed] [Google Scholar]
- 25. Brostow DP, Petrik ML, Starosta AJ, Waldo SW. Depression in patients with peripheral arterial disease: a systematic review. Eur J Cardiovasc Nurs. 2017;16:181–193. [DOI] [PubMed] [Google Scholar]
- 26. Ruo B, Liu K, Tian L, Tan J, Ferrucci L, Guralnik JM, McDermott MM. Persistent depressive symptoms and functional decline among patients with peripheral arterial disease. Psychosom Med. 2007;69:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smolderen KG, Aquarius AE, de Vries J, Smith OR, Hamming JF, Denollet J. Depressive symptoms in peripheral arterial disease: a follow‐up study on prevalence, stability, and risk factors. J Affect Disord. 2008;110:27–35. [DOI] [PubMed] [Google Scholar]
- 28. Smolderen KG, Safley DM, House JA, Spertus JA, Marso SP. Percutaneous transluminal angioplasty: association between depressive symptoms and diminished health status benefits. Vasc Med. 2011;16:260–266. [DOI] [PubMed] [Google Scholar]
- 29. Cherr GS, Zimmerman PM, Wang J, Dosluoglu HH. Patients with depression are at increased risk for secondary cardiovascular events after lower extremity revascularization. J Gen Intern Med. 2008;23:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smolderen KG, Gosch K, Patel M, Jones WS, Hirsch AT, Beltrame J, Fitridge R, Shishehbor MH, Denollet J, Vriens P, et al. PORTRAIT (Patient‐Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories): overview of design and rationale of an international prospective peripheral arterial disease study. Circ Cardiovasc Qual Outcomes. 2018;11:e003860. [DOI] [PubMed] [Google Scholar]
- 31. Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–e1030. [DOI] [PubMed] [Google Scholar]
- 32. Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ‐8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–173. [DOI] [PubMed] [Google Scholar]
- 33. American Pyschiatric Association . Diagnostic and Statistical Manual of Mental Disorders, DSM‐IV. 4th ed Washington, DC: American Psychiatry Publishing; 1994. [Google Scholar]
- 34. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spertus J, Jones P, Poler S, Rocha‐Singh K. The peripheral artery questionnaire: a new disease‐specific health status measure for patients with peripheral arterial disease. Am Heart J. 2004;147:301–308. [DOI] [PubMed] [Google Scholar]
- 36. EuroQol Group . EuroQol—a new facility for the measurement of health‐related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 37. Smolderen KG, Wang J, Jones P, Labrosciano C, Spertus J. Abstract 19221: developing a clinical predication model for 1‐year health status outcomes in peripheral arterial disease: insights from the PORTRAIT registry. Circulation. 2017;136:A19221. [Google Scholar]
- 38. Jones WS, Kennedy KF, Hawkins BM, Attaran RR, Secemsky EA, Latif F, Shammas NW, Feldman DN, Aronow HD, Gray B, et al. Expanding opportunities to understand quality and outcomes of peripheral vascular interventions: the ACC NCDR PVI Registry. Am Heart J. 2019;216:74–81. [DOI] [PubMed] [Google Scholar]
- 39. Cronenwett JL, Kraiss LW, Cambria RP. The Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2012;55:1529–1537. [DOI] [PubMed] [Google Scholar]
- 40. Roehrig C. Mental disorders top the list of the most costly conditions in the United States: $201 billion. Health Aff (Millwood). 2016;35:1130–1135. [DOI] [PubMed] [Google Scholar]
- 41. Mahoney EM, Wang K, Keo HH, Duval S, Smolderen KG, Cohen DJ, Steg G, Bhatt DL, Hirsch AT; Reduction of Atherothrombosis for Continued Health Registry Investigators . Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes. 2010;3:642–651. [DOI] [PubMed] [Google Scholar]
- 42. Smolderen KG, Spertus JA, Gosch K, Dreyer RP, D'Onofrio G, Lichtman JH, Geda M, Beltrame J, Safdar B, Bueno H, et al. Depression treatment and health status outcomes in young patients with acute myocardial infarction: insights from the VIRGO Study (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients). Circulation. 2017;135:1762–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smolderen KG, Buchanan DM, Gosch K, Whooley M, Chan PS, Vaccarino V, Parashar S, Shah AJ, Ho PM, Spertus JA. Depression treatment and 1‐year mortality after acute myocardial infarction: insights from the TRIUMPH Registry (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status). Circulation. 2017;135:1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rutledge T, Vaccarino V, Johnson BD, Bittner V, Olson MB, Linke SE, Cornell CE, Eteiba W, Sheps DS, Francis J, et al. Depression and cardiovascular health care costs among women with suspected myocardial ischemia: prospective results from the WISE (Women's Ischemia Syndrome Evaluation) Study. J Am Coll Cardiol. 2009;53:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jo SJ, Yim HW, Bang MH, Lee MO, Jun TY, Choi JS, Lee MS, Lee WC, Park YM. The association between economic status and depressive symptoms: an individual and community level approach. Psychiatry Investig. 2011;8:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aseltine RH Jr, Kessler RC. Marital disruption and depression in a community sample. J Health Soc Behav. 1993;34:237–251. [PubMed] [Google Scholar]
- 47. Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER III, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: the CLEVER study. J Am Coll Cardiol. 2015;65:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cherr GS, Wang J, Zimmerman PM, Dosluoglu HH. Depression is associated with worse patency and recurrent leg symptoms after lower extremity revascularization. J Vasc Surg. 2007;45:744–750. [DOI] [PubMed] [Google Scholar]
- 49. Smolderen KG, Plomondon ME, Armstrong EJ, Hess E, Waldo S, Tsai TT, Maddox TM. Depression and long‐term prognostic outcomes following peripheral endovascular interventions in the VA Healthcare System. Vasc Med. 2018;23:454–460. [DOI] [PubMed] [Google Scholar]
- 50. Arya S, Lee S, Zahner GJ, Cohen BE, Hiramoto J, Wolkowitz OM, Khakharia A, Binney ZO, Grenon SM. The association of comorbid depression with mortality and amputation in veterans with peripheral artery disease. J Vasc Surg. 2018;68:536–545.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lo RC, Bensley RP, Dahlberg SE, Matyal R, Hamdan AD, Wyers M, Chaikof EL, Schermerhorn ML. Presentation, treatment, and outcome differences between men and women undergoing revascularization or amputation for lower extremity peripheral arterial disease. J Vasc Surg. 2014;59:409–418.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bloemenkamp DG, Mali WP, Tanis BC, van den Bosch MA, Kemmeren JM, Algra A, van der Graaf Y. Functional health and well‐being of relatively young women with peripheral arterial disease is decreased but stable after diagnosis. J Vasc Surg. 2003;38:104–110. [DOI] [PubMed] [Google Scholar]
- 53. Mastenbroek MH, Hoeks SE, Pedersen SS, Scholte Op Reimer WJ, Voute MT, Verhagen HJ. Gender disparities in disease‐specific health status in postoperative patients with peripheral arterial disease. Eur J Vasc Endovasc Surg. 2012;43:433–440. [DOI] [PubMed] [Google Scholar]
- 54. Smolderen KG, Brush A, Dreyer RP. Psychosocial factors and recovery after acute myocardial infarction in younger women. Curr Cardiol Rep. 2019;21:50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figure S1


