Abstract
Background
A significant proportion of patients with spontaneous coronary artery dissection (SCAD) have ongoing chronic chest pain despite healing of their dissection. We sought to determine whether coronary microvascular dysfunction contributes to post‐SCAD chronic chest pain by performing coronary reactivity testing in the cardiac catheterization laboratory.
Methods and Results
Eighteen patients consented to coronary reactivity testing at least 3 months post‐SCAD. Coronary flow reserve (CFR) and index of microcirculatory resistance were measured in the previously affected SCAD artery and 1 non‐SCAD artery. CFR <2.5 was defined as diagnostic of coronary microvascular dysfunction. An abnormal index of microcirculatory resistance was defined as >25 units. Seventeen women underwent coronary reactivity testing (1 had chronic dissection and was excluded). All presented with myocardial infarction and 2 underwent coronary stenting during the initial SCAD event. Fibromuscular dysplasia was present in 70.6% upon screening renal, iliac, and cerebrovascular arteries. Twelve patients (70.6%) had CFR <2.5 and 13 (76.5%) had an index of microcirculatory resistance >25 in at least 1 artery. There was no difference in the frequency of a low CFR measurement between SCAD and non‐SCAD arteries.
Conclusions
Among patients with chronic chest pain after an SCAD event, >70% had coronary microvascular dysfunction as indicated by abnormal CFR or index of microcirculatory resistance in at least 1 coronary artery on invasive coronary reactivity testing. Presence of coronary microvascular dysfunction in both SCAD and non‐SCAD arteries suggests that underlying microvascular abnormalities from vasculopathies such as coronary fibromuscular dysplasia may be the underlying etiology.
Keywords: acute myocardial infarction, angina, coronary angiography, coronary microvascular dysfunction, spontaneous coronary artery dissection
Subject Categories: Ischemia, Angiography, Acute Coronary Syndromes, Angina
While atherosclerosis remains the most common cause of myocardial infarction (MI), non‐atherosclerotic coronary artery disease is prevalent in young women and may account for up to 40% of the etiology of MI. 1 Moreover, in women aged <50 years, spontaneous coronary artery dissection (SCAD) accounts for up to 25% of MI. 1 Multiple factors may predispose to weakening of the arterial wall and increasing vulnerability for dissection. In our previously reported SCAD cohort, fibromuscular dysplasia (FMD) was the most common predisposing arteriopathy in 62.7% of patients with SCAD. 2
Recurrent SCAD can occur in 15% to 27% of cases. 3 Furthermore, a significant proportion of patients with SCAD have persistent chronic chest pain despite healing of their dissections, the etiology of which is poorly understood. A recent study examined 10 patients with prior SCAD who were referred for coronary endothelial and microvascular testing in the left anterior descending artery with adenosine, acetylcholine, and nitroglycerin. 4 They found endothelial and microvascular responses similar to a reference group of patients and reported that coronary epicardial and microvascular vasomotor dysfunction was not common in their small SCAD cohort. Only 30% of their cohort had FMD, a significantly lower percentage than the Vancouver cohort of the Canadian SCAD study and Mayo Clinic. 2 , 5 In our SCAD population with a high percentage of vasculopathies, we sought to determine whether coronary microvascular dysfunction (CMD) contributes to post‐SCAD chronic chest pain by performing coronary reactivity testing (CRT) with adenosine in both the previously dissected SCAD artery and 1 non‐SCAD artery.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Vancouver General Hospital is a quaternary referral center for patients with SCAD in British Columbia and from out‐of‐province. Patients followed at our SCAD clinic were enrolled in the Canadian SCAD study or NACAD (non‐atherosclerotic coronary artery disease) registry, previously described. 2 Patients with persistent chronic chest pain ≥3 times a week, at least 3 months post‐SCAD who were already going for a clinically indicated angiogram, were eligible for our study with CRT (n=42). Eighteen patients consented to having CRT immediately following clinically indicated coronary angiography if their repeat angiogram did not provide an explanation for their ongoing chest pain (Figure 1). The study was approved by the University of British Columbia Research Ethics Board and subjects gave informed consent.
Figure 1.
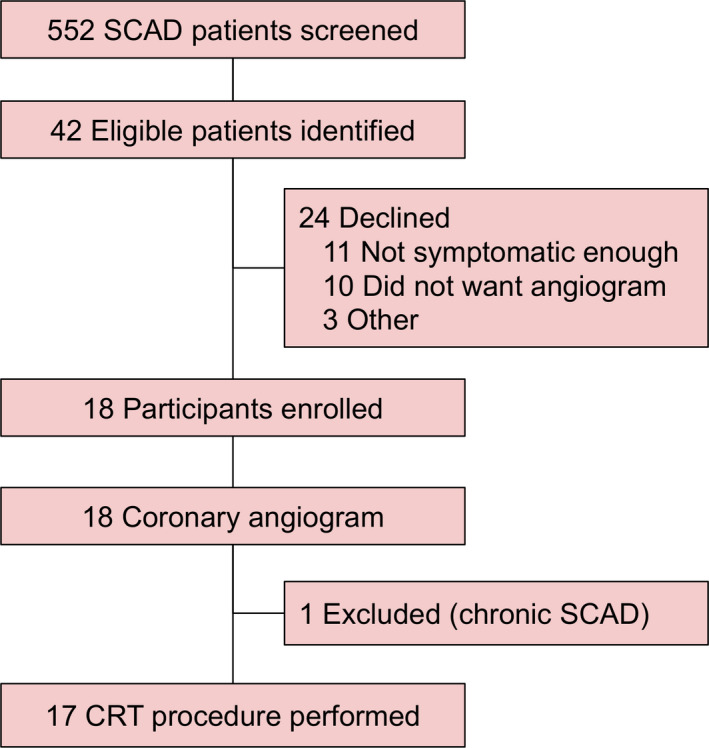
Study flow.
CRT indicates coronary reactivity testing; and SCAD, spontaneous coronary artery dissection.
Coronary Reactivity Testing
Fractional flow reserve, coronary flow reserve (CFR), and index of microcirculatory resistance (IMR) were measured in 2 coronary arteries: a previously affected SCAD artery and 1 non‐SCAD artery. CFR <2.5 was defined as diagnostic of CMD. Low CFR was defined as CFR <3.0 and an abnormal IMR was defined as >25 units. CRT was performed with the Radi wire (St Jude Medical) that has a distal dual pressure and temperature tipped sensor that acts as a distal thermistor and detects changes in temperature‐dependent electrical resistance. The shaft of the wire has a proximal thermistor that detects temperature. The transit time of room‐temperature saline injected down a coronary artery was determined by the thermodilution technique. At baseline, 3 injections of 3‐mL room‐temperature saline were injected into the coronary artery, and the resting mean transit time (Tmn) was measured. To induce steady‐state maximal hyperemia, intravenous infusion of adenosine (140 μg/kg per minute) was then administered, and 3 more injections of 3‐mL room‐temperature saline were performed to obtain the mean hyperemic Tmn. The mean aortic pressure (by guiding catheter) and the mean distal coronary pressure (by pressure wire) were measured simultaneously in the resting and maximal hyperemic states. CFR was calculated as the resting Tmn divided by the hyperemic Tmn. IMR was calculated as the distal coronary pressure (Pd) at maximal hyperemia divided by the inverse of the hyperemic Tmn. Fractional flow reserve was calculated by the ratio of mean distal coronary pressure/aortic pressure at maximal hyperemia.
Statistical Analysis
Descriptive statistics were used to summarize the patient baseline characteristics. Continuous variables were reported as mean±SD, or median and interquartile range. Categorical variables were summarized as frequency and percentage. Comparisons between groups was performed using standardized differences. Differences >20% were considered significant. Statistical analyses were performed with the SPSS software (IBM SPSS version 23, New York).
Results
Eighteen patients consented to participate in the study, however, at the time of the repeat angiogram, 1 was excluded because of chronic dissection, thus, 17 underwent CRT. Compared with the 535 patients with SCAD in the Vancouver cohort of the Canadian SCAD registry who did not undergo CRT, the 17 patients who underwent CRT had a significantly higher frequency of a family history of cardiovascular disease (68.8% versus 35.8%) and a trend towards a higher incidence of FMD upon screening other vascular beds (76.5% versus 57.5%) (Table 1). They also had a higher incidence of chest pain 1‐year post‐SCAD (50% versus 16% had chest pain ≥3 times a week) and more emergency room visits for chest pain at 1 year (68.8% versus 29.5%). Further, their baseline Seattle Angina Questionnaire was more unfavorable in all domains (Table 1).
Table 1.
Baseline Characteristics of 535 Patients With SCAD Who Did Not Undergo CRT Compared With 17 Patients With SCAD WHO Did Undergo CRT
|
SCAD Cohort With CRT n=17 |
SCAD Cohort Without CRT n=535 |
Standardized Difference | |
|---|---|---|---|
| Age, y | 47.6±8.6 | 52.6±9.7 | −0.55 |
| Women | 16 (100) | 400 (90.1) | 0.55 |
| BMI >30 | 5 (31.3) | 73 (17.3) | 0.39 |
| Family history of CVD | 11 (68.8) | 159 (35.8) | 0.75 |
| Hypertension | 5 (29.4) | 194 (36.8) | −0.15 |
| Diabetes mellitus | 1 (5.9) | 21 (4.0) | 0.09 |
| Hyperlipidemia | 4 (23.5) | 134 (25.5) | −0.04 |
| Current smoker | 3 (17.6) | 52 (9.9) | 0.23 |
| Depression | 5 (29.4) | 105 (19.9) | 0.23 |
| Hypothyroidism | 5 (29.4) | 66 (12.5) | 0.43 |
| Presentation | |||
| STEMI | 3 (17.6) | 136 (25.4) | −0.19 |
| NSTEMI | 14 (82.4) | 398 (74.4) | 0.19 |
| EF <50% | 3 (17.6) | 118 (22.8) | −0.11 |
| Any WMA | 15 (94.1) | 437 (84.2) | 0.18 |
| Revascularized during initial event | 2 (11.8) | 86 (16.1) | −0.12 |
| FMD present | 13 (76.5) | 295 (57.5) | 0.46 |
| Baseline SAQ (values are mean±SD or n [%]) | |||
| Physical limitation | 68.1±18.4 | 82.9±18.5 | −0.80 |
| Angina stability | 45.6±20.2 | 57.5±22.1 | −0.56 |
| Angina frequency | 52.9±18.6 | 86.9±16.4 | −1.94 |
| Treatment satisfaction | 69.4±30.6 | 81.9±19.0 | −0.49 |
| Quality of life | 44.6±21.2 | 66.6±21.7 | −1.03 |
CVD indicates cardiovascular disease; EF, ejection fraction; FMD, fibromuscular dysplasia; NSTEMI, non‒ST‐segment‒elevation myocardial infarction; SAQ, Seattle Angina Questionnaire; SCAD, spontaneous coronary artery dissection; STEMI, ST‐segment‒elevation myocardial infarction; and WMA, wall motion abnormality.
In the SCAD cohort that underwent CRT, the average age was 48.3±8.9 years. These patients had a low frequency of cardiac risk factors including hypertension (29.4%), diabetes mellitus (5.9%), hyperlipidemia (23.5%), and current smoking (17.6%) (Table 1). The mean baseline Seattle Angina Questionnaire (frequency domain) was 52.9±18.6 demonstrating that they have a high burden of chest pain. All presented with an MI, with ST‐segment‒elevation MI in 3 women, and 2 underwent coronary stenting during the initial SCAD event. FMD was present in 70.6% upon screening renal, iliac, and cerebrovascular arteries.
The mean fractional flow reserve in 34 arteries (17 SCAD and 17 non‐SCAD arteries) was 0.89±0.13, mean CFR was 3.0±1.7, and mean IMR was 26.2±11.4. Twelve patients (70.6%) had CFR <2.5 in ≥1 artery; 13 (76.5%) had CFR <3.0 in ≥1 artery, and 13 (76.5%) had an IMR >25 in ≥1 artery. In 10 patients (58.8%) both CFR <2.5 and IMR >25 were present. Sixteen patients experienced chest pain during the adenosine infusion. There was no difference in the frequency of a low CFR measurement between SCAD and non‐SCAD arteries (Table 2 and Figure 2). One patient developed severe spasm and a catheter‐related dissection requiring coronary stenting.
Table 2.
Coronary Reactivity Testing Results: Mean Fractional Flow Reserve, Coronary Flow Reserve, and Index of Microcirculatory Resistance in SCAD and Non‐SCAD Arteries
| Overall (n=34 arteries) | SCAD (n=17 arteries) | Non‐SCAD (n=17 arteries) | P Value | |
|---|---|---|---|---|
| FFR, mean±SD | 0.89±0.13 | 0.91±0.08 | 0.86±0.16 | 0.33 |
| CFR, mean±SD | 3.0±1.7 | 3.1±1.6 | 2.9±1.8 | 0.74 |
| IMR, mean±SD | 26.2±11.4 | 26.1±12.3 | 26.2±10.7 | 0.98 |
| CFR <3.0, n (%) | 20 (58.8) | 10 (58.8) | 10 (58.8) | >0.90 |
| CFR <2.5, n (%) | 16 (47.1) | 6 (35.3) | 10 (58.8) | 0.30 |
| IMR >25 U, n (%) | 18 (52.9) | 9 (52.9) | 9 (52.9) | >0.90 |
CFR indicates coronary flow reserve; CRT, coronary reactivity testing; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; and SCAD, spontaneous coronary artery dissection.
Figure 2.
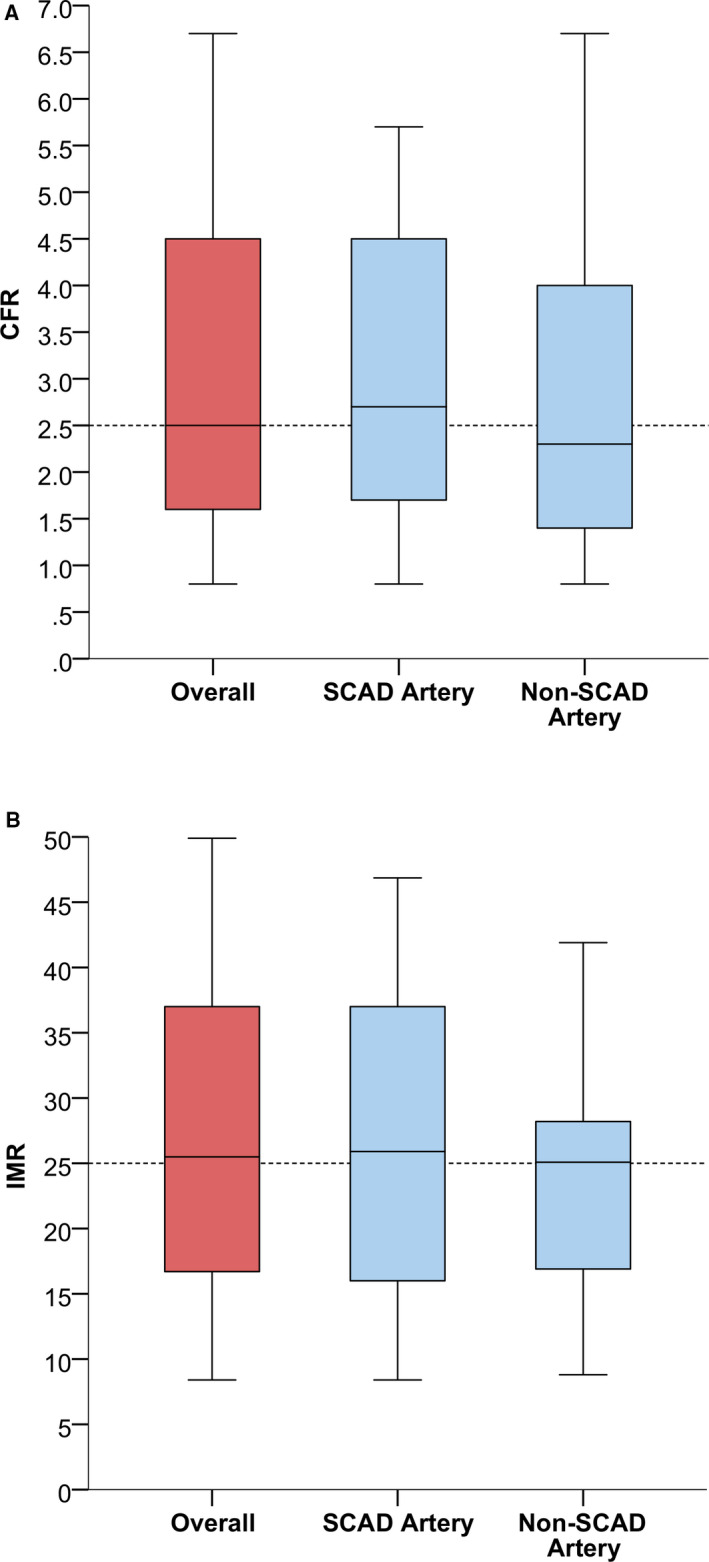
Box and whisker plot of CFR (A) and IMR (B) in the overall cohort and in SCAD and non‐SCAD arteries.
CFR indicates coronary flow reserve; IMR, index of microcirculatory resistance; and SCAD, spontaneous coronary artery dissection.
We reviewed all 17 angiograms for signs of coronary FMD including irregular stenosis, smooth stenosis, arterial enlargement (such as dilation or ectasia), and arterial tortuosity as per Saw et al. 6 All 17 patients had possible signs of coronary FMD. Specifically, 4 patients had diffuse irregular stenosis (3 of the LAD and 1 of the RCA), 1 patient had focal tubular smooth stenosis of the LAD, 5 patients had arterial enlargement (consisting of dilation) all in the LAD, and all 17 patients had arterial tortuosity (3 mild, 8 moderate, 4 severe, and 2 with 360 degree loops). As noted in Saw et al, these findings are suggestive of coronary FMD but not specific and without optical coherence tomography imaging, definitive conclusions cannot be made.
Discussion
In our study, among patients with chronic chest pain after an SCAD event, >70% had CMD as indicated by abnormal CFR or IMR in at least 1 coronary artery on invasive CRT suggesting that CMD may be the dominant cause of persistent chest pain post‐SCAD.
Since we did not find a difference in the presence of CMD in SCAD and non‐SCAD arteries, this suggests that underlying microvascular abnormalities are responsible for CMD, rather than from the prior dissection itself. Such underlying microvascular abnormalities may include vasculopathies from coronary FMD. In this cohort, 70.6% had underlying FMD, similar to the prevalence we previously reported in our 327‐patient SCAD series. 2 Other potential vasculopathies causing CMD include systemic inflammatory disease and hereditary connective tissue disorders. Compared with Waterbury et al. who reported low rates of coronary epicardial and microvascular vasomotor dysfunction in 10 patients with prior SCAD with ongoing angina, our patients had similar baseline characteristics including age, sex, and risk factor profile aside from rates of FMD which were 70.6% in our cohort and only 30% in their cohort. 4
In patients without SCAD, CMD as indicated by CFR <2.5 accounts for the diagnosis in over half of patients with signs and symptoms of ischemia but no obstructive coronary artery disease on coronary angiography. 7 , 8 Furthermore, a low CFR is associated with a 26.7% event‐rate (death, non‐fatal MI, non‐fatal stroke, or congestive heart failure) at 5.4 years compared with 12.2% event‐rate with a normal CFR. 7 Moreover, medical therapy such as statins and angiotensin‐converting enzyme inhibitors improve CFR and symptoms in CMD patients. 9 , 10 The use of these agents in SCAD patients with persistent chest pain and CMD will be further explored in studies such as the SAFER‐SCAD (Statin and Angiotensin‐Cconverting Enzyme Inhibitor on Symptoms in Patients With SCAD) study (NCT02008786).
Study limitations: this is a small observational cohort study and larger studies are needed to make definitive conclusions. We did not perform CRT in asymptomatic post‐SCAD patients and therefore cannot compare the frequency of CMD to the rest of our SCAD cohort. CMD is frequent in both SCAD and non‐SCAD patients and although rates of CMD were high in our cohort, a causal relationship cannot be definitively concluded in this study.
Conclusions
Among patients with chronic chest pain after SCAD, >70% had CMD as indicated by abnormal CFR and/or IMR in at least 1 coronary artery on invasive CRT. This is hypothesis generating and future studies should be performed to corroborate this finding in other data sets. The presence of CMD in both SCAD and non‐SCAD arteries suggests that underlying microvascular abnormalities from vasculopathies such as coronary FMD may be the underlying etiology. Future research should examine etiologies of CMD and targeted therapies for these post‐SCAD symptomatic patients.
Sources of Funding
This work was supported by the Vancouver General Hospital Division of Cardiology Research Fund.
Disclosures
Dr Saw has received unrestricted research grant supports (from the Canadian Institutes of Health Research, Heart & Stroke Foundation of Canada, National Institutes of Health, Michael Smith Foundation of Health Research, University of British Columbia Division of Cardiology, AstraZeneca, Abbott Vascular, Boston Scientific, and Servier), speaker honoraria (AstraZeneca, Abbott Vascular, Boston Scientific, and Sunovion), consultancy and advisory board honoraria (AstraZeneca, Boston Scientific, and Abbott Vascular, Gore, Baylis, FEops), and proctorship honoraria (Abbott Vascular and Boston Scientific). Dr Sedlak has advisory board and speaker honoraria (BI‐Lilly, Bayer, Sanofi, Novartis, Novo Nordisk, and Amgen). The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2020;9:e015834 DOI: 10.1161/JAHA.120.015834.)
For Sources of Funding and Disclosures, see page 5.
REFERENCES
- 1. Saw J, Aymong E, Mancini GBJ, Sedlak T, Starovoytov A, Ricci D. Non‐atherosclerotic coronary artery disease in young women. Can J Cardiol. 2014;30:814–819. [DOI] [PubMed] [Google Scholar]
- 2. Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, Mancini GBJ. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70:1148–1158. [DOI] [PubMed] [Google Scholar]
- 3. Saw J, Mancini GB, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. 2016;68:297–312. [DOI] [PubMed] [Google Scholar]
- 4. Waterbury TM, Tweet MS, Hayes SN, Prasad A, Lerman A, Gulati R. Coronary endothelial function and spontaneous coronary artery dissection. Eur Heart J Acute Cardiovasc Care. 2018;1:1–6. [DOI] [PubMed] [Google Scholar]
- 5. Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJ, Rihal CS, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–588. [DOI] [PubMed] [Google Scholar]
- 6. Saw J, Bezerra H, Gornik HL, Machan L, Mancini GB. Angiographic and intracoronary manifestations of coronary fibromuscular dysplasia. Circulation. 2016;133:1548–1559. [DOI] [PubMed] [Google Scholar]
- 7. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pizzi C, Manfrini O, Fontana F, Bugiardini R. Angiotensin‐converting enzyme inhibitors and 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase in cardiac Syndrome X: role of superoxide dismutase activity. Circulation. 2004;109:53–58. [DOI] [PubMed] [Google Scholar]
- 10. Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper‐DeHoff RM, Sopko G, Sharaf BM, Kelsey SF, Merz CN, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin‐converting enzyme inhibition is associated with improved microvascular function: a double‐blind randomized study from the National Heart, Lung and Blood Institute Women's Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011;162:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]


