Abstract
Background
The present study assessed the association between blood pressure (BP) and the risk of chronic kidney disease (CKD) according to gender and the use of antihypertensive drugs using data from a large‐scale health checkup.
Methods and Results
We conducted a retrospective cohort study using the JMDC database, which contains annual health checkup data of Japanese employees and their dependents aged <75 years. We included 154 692 participants (men, 69.68%; mean age, 44.74 years) without CKD. CKD was indicated by an estimated glomerular filtration rate <60 mL/min per 1.73 m2 or the presence of proteinuria. During the mean follow‐up period of 4.78 years, new‐onset CKD occurred in 14 888 participants. When the normal BP group (systolic/diastolic BP <120/<80 mm Hg) without treatment was used as a reference, the hazard ratios of the high BP (130–139/80–89 mm Hg) and grade 1 (140–159/90–99 mm Hg) and grade 2 or 3 hypertension (≥160/≥100 mm Hg) groups were 1.11 (95% CI, 1.06–1.17), 1.36 (95% CI, 1.28–1.45), and 1.76 (95% CI, 1.56–1.99) for untreated men, respectively. However, in treated men, even normal BP was associated with a 1.5‐fold higher risk of CKD. The association between BP and the risk of CKD was weaker in untreated women than in untreated men. The risk of CKD in treated women with normal BP was similar to that of untreated women with normal BP.
Conclusions
Gender differences were found in the association between BP and CKD risk. Kidney function in treated individuals should be followed carefully, especially in men.
Keywords: blood pressure, chronic kidney disease, cohort study, database
Subject Categories: Epidemiology, Hypertension
Nonstandard Abbreviations and Acronyms
- BP
blood pressure
- BMI
body mass index
- CKD
chronic kidney disease
- DM
diabetes mellitus
- eGFR
estimated glomerular filtration rate
- HR
hazard ratio
- JSH
Japanese Society of Hypertension
Clinical Perspective
What Is New?
Our study is the first of its kind to demonstrate the risk of chronic kidney disease in blood pressure groups according to current guidelines after stratification for sex and the use of antihypertensive drugs in a large‐scale Asian population.
What Are the Clinical Implications?
Blood pressure was significantly associated with an elevated risk of chronic kidney disease; however, the overall hazard ratio in treated individuals was higher versus that in those not taking antihypertensive drugs.
Kidney function in treated individuals should be monitored more carefully versus that in untreated people.
The association between blood pressure and the risk of chronic kidney disease was weaker in women versus that in men, and the risk of chronic kidney disease in women with controlled hypertension is similar to that in women with normal or high‐normal blood pressure.
Chronic kidney disease (CKD) has become a major global health burden in recent years. 1 Since damage sustained by kidneys cannot be reversed, priority should be given to the prevention of kidney disease by predicting CKD before the patient develops end‐stage renal disease.
Blood pressure (BP) is a strong risk factor for CKD. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 However, studies indicating this association have not involved stratification analysis by gender and the use of antihypertensive drugs. Individuals treated with antihypertensive drugs have a potentially higher cardiovascular disease risk compared with those not prescribed antihypertensive drugs, regardless of BP. 10 This is because treated individuals have a long history of elevated BP. However, the degree to which the risk of CKD is increased in treated individuals compared with untreated individuals with the same BP has yet to be assessed. The association between BP and the risk of CKD could also be different based on the use of antihypertensive drugs. Although one study 11 indicated a positive association between BP and kidney dysfunction after stratification by the use of antihypertensive drugs, this study was only based on 2 specific health checkup data points and only had a 2‐year follow‐up period. 11 Furthermore, the study 11 did not consider gender differences and defined BP by sextile of systolic BP and not by BP thresholds as recommended in hypertension guidelines. 12 , 13 , 14 Little information on gender differences in the association between BP and the risk of CKD is available. 6 Gender is an unmodifiable factor, unlike most other CKD risk factors. Also given gender differences in the progression of CKD 15 , gender must be considered as an important factor in studies of kidney disease.
To assess the association between BP and the risk of CKD after stratification by gender and the use of antihypertensive drugs, the present study conducted a longitudinal analysis based on multiple measurements of serum creatinine from a large‐scale health checkup.
Methods
To comply with our contract with JMDC Inc., data and study materials will not be made available to other researchers.
Study Design and Populations
We conducted a retrospective cohort study using the JMDC database. The JMDC database contains the annual health checkup data of Japanese employees and their dependents aged <75 years who are enrolled in the health insurance plans run primarily by large‐scale enterprises. 16 , 17 , 18 As it is mandatory for individuals aged ≥75 years to be enrolled in the health insurance system (Later Elders Insurance), the JMDC database does not have data related to elderly people.
The JMDC database has data of 937 240 individuals aged ≥30 years who underwent a minimum of 1 annual health checkup between April 2008 and March 2017. We received data from 388 973 of these participants who had an annual health checkup ≥5 times between April 2008 and March 2017. Since the measurement of serum creatinine is not mandatory in Japanese annual health checkups, serum creatinine was only measured in 246 043 (63.25%) participants. We also excluded 2296 participants whose data did not include information relating to BP or the use of antihypertensive drugs, and 9 with an estimated glomerular filtration rate (eGFR) >200 mL/min per 1.73 m2, as they were considered outliers. After exclusion, follow‐up data were available for 228,450 participants. Of those, 19,841 with CKD or kidney disease and 17,386 with a history of cardiovascular disease at baseline were excluded. Furthermore, we excluded 36,531 participants with an eGFR <70 mL/min per 1.73 m2 as they already had a high risk of CKD (indicated in Figure S1). Finally, 154 692 participants were included in the present analyses.
Based on Ethical Guidelines for Medical and Health Research Involving Human Subjects by the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare, Japan, this retrospective study was exempted from obtaining approval of the institutional review board or ethics committee and individual informed consent since the JMDC data for this study are unlinkable anonymized data.
Data Collection
The present data were collected at annual health checkups, which were conducted according to the guideline set out by the Japanese Ministry of Health, Labour and Welfare. Information regarding smoking status; alcohol consumption; use of antihypertensive, antidiabetic, and antidyslipidemia drugs; and history of cerebrovascular disease and ischemic heart disease was gathered by a self‐administered questionnaire. Diabetes mellitus (DM) was indicated by a fasting glucose level ≥7.00 mmol/L (≥126 mg/dL), a random glucose level ≥11.11 mmol/L (≥200 mg/dL), or the use of antidiabetic medications. Dyslipidemia was indicated by a low‐density lipoprotein cholesterol ≥3.62 mmol/L (≥140 mg/dL), high‐density lipoprotein cholesterol <1.03 mmol/L (40 mg/dL), triglycerides ≥1.69 mmol/L (150 mg/dL), or use of antidyslipidemia medications.
Definition of BP Category
The Japanese guidelines for annual health checks recommend measuring BP twice consecutively in a seated position.
As per the Japanese Society of Hypertension (JSH) 2019 guidelines, 14 we classified participants into 5 categories according to their systolic/diastolic BP: normal BP (<120/<80 mm Hg), high‐normal BP (120–129/<80 mm Hg), high BP (130–139/80–89 mm Hg), grade 1 hypertension (140–159/90–99 mm Hg), and grade 2 or 3 hypertension (≥160/≥100 mm Hg). The BP thresholds stated in the JSH 2019 guidelines 14 are consistent with the US hypertension management guidelines, 12 where systolic/diastolic BP ≥130/≥80 mm Hg is defined as hypertension.
Outcome and follow‐Up
Serum creatinine was assayed enzymatically. An eGFR was calculated from the serum creatinine using a modified version of the equation used most commonly in Japan: eGFR (mL/min per 1.73 m2)=194×(serum creatinine)−1.094 × age−0.287 (×0.739, for women). 19 The presence of proteinuria was confirmed by a dipstick test that showed 1+ or above, which corresponds to urinary protein levels >30 mg/dL. CKD was indicated by an eGFR <60 mL/min per 1.73 m2 and/or the presence of proteinuria.
Baseline was considered as the first annual health checkup in the present data. The primary outcome was the new onset of CKD confirmed during the annual checkups. 7 The date of CKD incidence was defined as the midpoint between the most recent date the individual presented without CKD and the date on which CKD was first confirmed. 7 If a participant had >1 CKD event during the follow‐up, only the first event contributed to the analysis. As for participants without CKD, the final follow‐up date was the date of the final annual health checkup included in the present data. Therefore, the follow‐up period was from baseline to the date of CKD incidence or final checkup.
Statistical Analysis
We calculated age‐standardized CKD rates by direct method using the population assumed to be the same proportion of individuals aged <45, 45–59, and ≥60 years. The body mass index (BMI)‐adjusted difference in eGFR at baseline between men and women was assessed by the ANCOVA separately by the BP category and the use of antihypertensive treatment. The Cox proportional hazard model was applied to assess the adjusted hazard ratios (HRs) for CKD incidence in BP groups. Then, dummy variables were created for the BP category and were included in the model at the same time. The untreated lowest BP group was set as a reference. The trend P values for the association between BP category and the risk for CKD were assessed in untreated or treated individuals separately. We also assessed the HR for antihypertensive treatment (untreated vs treated participants) in the model with systolic BP and covariates. Furthermore, the associations of BP category with the risk of an eGFR <60 mL/min per 1.73 m2 and the presence proteinuria were also assessed.
Interactions were assessed by the likelihood ratio test comparing a model, which included confounding factors and BP category, with a model further including appropriate interaction terms. For example, we defined 9 interaction terms (gender × each BP group other than untreated normal BP) to assess the interaction between gender and the 10 BP categories on the risk of CKD. The likelihood ratio chi‐square value was used to assess improvement in the goodness of fit.
We performed stratification analyses according to age, BMI, current smoking status, alcohol consumption, DM, dyslipidemia, eGFR, and the number of visits.
We also used restricted cubic splines with 3 knots to analyze the associations between systolic BP and the risk of CKD. Systolic BP of 110 mm Hg was treated as the reference value as it was the mean value in the normal BP group.
The covariates were age, BMI <18.5 kg/m2 and BMI ≥25 kg/m2 (to consider the U‐shaped association), current smoking status, alcohol consumption, DM, dyslipidemia, and eGFR at baseline. The association between BP and CKD adjusted by linear and quadratic terms of BMI instead of BMI category was also assessed. We interpolated the missing BMI values (n=8) from the regression slope on age. In participants with unknown smoking (n=10) and drinking status (n=1863), we set the design variable to the sex‐specific mean of the codes (0, 1). For all multivariate analyses in the present study, we used these interpolated or design variables for missing values.
We stratified all analyses by gender, except for the analysis of the interaction effect of gender. Continuous variables were expressed as mean±SD. All data were analyzed using SAS software version 9.4 (SAS Institute Inc.). A P value <.05 was considered statistically significant.
Results
Baseline Characteristics
In total, 107 792 (69.68%) participants were men. The mean values for age, BMI, systolic BP, and diastolic BP were 44.74±7.43 years, 22.86±3.44 kg/m2, 120.22±15.21 mm Hg, and 74.42±11.05 mm Hg, respectively. Of the 144 385 untreated patients, the number of participants with normal BP, high‐normal BP, high BP, grade 1 hypertension, and grade 2 or 3 hypertension was 69973 (48.46%), 22 826 (15.81%), 36 172 (25.05%), 12 887 (8.93%), and 2527 (1.75%), respectively. In treated participants, the number of participants with normal BP, high‐normal BP, high BP, grade 1 hypertension, and grade 2 or 3 hypertension was 1251 (12.14%), 1141 (11.07%), 3803 (36.90%), 3231 (31.35%), and 881 (8.55%), respectively. The baseline characteristics according to the BP categories in untreated men and women and treated men and women are shown in Table 1 and Table 2. After adjusting for BMI, eGFR was significantly higher in women than men in the normal BP, high‐normal BP, high BP, and grade 1 hypertension groups among untreated participants and in the treated normal BP group (P≤0.016) (Table S1).
Table 1.
Baseline Characteristics of Untreated Participants
| Strata | Variables | Category | ||||
|---|---|---|---|---|---|---|
| Normal BP | high‐Normal BP | High BP | Grade 1 Hypertension | Grade 2 or 3 Hypertension | ||
| Men | No. | 39 877 | 16 968 | 29 644 | 11 008 | 2019 |
| Age, y | 43.8±6.9 | 43.5±7.1 | 44.7±7.0 | 46.0±7.4 | 47.3±7.3 | |
| BMI, kg/m2 | 22.3±2.7 | 23.3±3.0 | 23.9±3.2 | 24.7±3.7 | 25.3±4.1 | |
| BMI <18.5 kg/m2, % | 5.7 | 3.0 | 2.1 | 1.8 | 1.5 | |
| BMI ≥25.0 kg/m2, % | 15.3 | 25.1 | 32.3 | 41.2 | 48.2 | |
| Current smoking, % | 46.0 | 46.1 | 43.9 | 43.8 | 40.0 | |
| Alcohol consumption, % | 31.9 | 35.2 | 42.0 | 46.9 | 48.4 | |
| Dyslipidemia, % | 39.3 | 45.7 | 51.6 | 57.3 | 59.7 | |
| DM, % | 3.1 | 4.3 | 5.4 | 8.1 | 11.1 | |
| eGFR, mL/min per 1.73 m2 | 84.7±10.9 | 85.4±11.2 | 84.6±11.0 | 84.8±11.4 | 84.8±11.8 | |
| Systolic BP, mm Hg | 109.2±7.1 | 123.8±2.8 | 128.1±7.2 | 141.8±7.9 | 157.4±13.6 | |
| Diastolic BP, mm Hg | 68.0±6.5 | 73.2±4.8 | 81.9±4.5 | 89.2±6.1 | 102.5±7.9 | |
| Women | No. | 30 096 | 5858 | 6528 | 1879 | 508 |
| Age, y | 42.9±6.7 | 45.2±7.7 | 46.2±7.9 | 49.0±8.3 | 49.2±7.7 | |
| BMI, kg/m2 | 20.7±2.7 | 22.1±3.4 | 22.7±3.8 | 23.7±4.5 | 24.4±4.8 | |
| BMI <18.5 kg/m2, % | 18.6 | 9.9 | 9.7 | 6.7 | 5.9 | |
| BMI ≥25.0 kg/m2, % | 6.9 | 17.7 | 22.9 | 32.0 | 36.8 | |
| Current smoking, % | 8.4 | 7.9 | 7.8 | 6.5 | 7.3 | |
| Alcohol consumption, % | 8.5 | 9.2 | 11.0 | 13.0 | 14.2 | |
| Dyslipidemia, % | 19.3 | 30.0 | 35.0 | 43.5 | 49.2 | |
| DM, % | 0.8 | 2.9 | 3.6 | 6.2 | 8.9 | |
| eGFR, mL/min per 1.73 m2 | 86.2±12.5 | 86.1±12.5 | 85.7±12.1 | 85.7±12.2 | 85.3±12.5 | |
| Systolic BP, mm Hg | 105.3±8.3 | 123.8±2.8 | 129.0±7.4 | 144.1±8.0 | 164.6±13.6 | |
| Diastolic BP, mm Hg | 63.8±7.1 | 71.3±5.5 | 80.5±5.6 | 87.6±7.0 | 99.4±9.9 | |
Data on body mass index (BMI), smoking status, and alcohol consumption were unavailable for 8, 9, and 1750 participants, respectively. BP indicates blood pressure; DM, diabetes mellitus; and eGFR, estimated glomerular filtration rate.
Table 2.
Baseline Characteristics of Treated Participants
| Strata | Variables | Category | ||||
|---|---|---|---|---|---|---|
| Normal BP | high‐Normal BP | High BP | Grade 1 Hypertension | Grade 2 or 3 Hypertension | ||
| Men | No. | 960 | 845 | 3061 | 2679 | 731 |
| Age, y | 52.1±7.5 | 52.8±8.2 | 51.1±7.3 | 50.7±7.5 | 49.9±7.3 | |
| BMI, kg/m2 | 24.5±3.6 | 25±3.7 | 25.5±3.8 | 25.8±4.1 | 26.2±4.5 | |
| BMI <18.5 kg/m2, % | 2.1 | 1.4 | 0.8 | 0.9 | 1.1 | |
| BMI ≥25.0 kg/m2, % | 40.2 | 44.7 | 50.5 | 53.0 | 56.0 | |
| Current smoking, % | 42.0 | 38.6 | 37.1 | 36.8 | 39.2 | |
| Alcohol consumption, % | 44.9 | 51.0 | 49.6 | 52.5 | 50.3 | |
| Dyslipidemia, % | 59.1 | 58.8 | 60.9 | 63.3 | 65.5 | |
| DM, % | 18.6 | 21.5 | 19.3 | 19.1 | 20.5 | |
| eGFR, mL/min per 1.73 m2 | 82.4±10.2 | 83.4±11.6 | 83.4±11.2 | 83.7±11.3 | 84.0±11.2 | |
| Systolic BP, mm Hg | 111.5±6.1 | 124.3±2.9 | 129.2±6.7 | 142.9±8.1 | 158.7±14.1 | |
| Diastolic BP, mm Hg | 70.6±5.9 | 73.6±4.6 | 82.8±4.6 | 89.9±6.0 | 101.4±8.5 | |
| Women | No. | 291 | 296 | 742 | 552 | 150 |
| Age, y | 53.7±7.7 | 55.5±7.7 | 53.2±8 | 54.0±8.7 | 54.3±8.4 | |
| BMI, kg/m2 | 23.3±3.7 | 24.2±4.5 | 24.4±4.2 | 24.9±4.8 | 25.7±5.2 | |
| BMI <18.5 kg/m2, % | 3.8 | 2.7 | 3.4 | 4.5 | 3.3 | |
| BMI ≥25.0 kg/m2, % | 25.8 | 33.4 | 38.3 | 42.0 | 45.3 | |
| Current smoking, % | 12.7 | 9.5 | 5.8 | 4.5 | 6.0 | |
| Alcohol consumption, % | 11.8 | 10.6 | 10.2 | 10.3 | 9.4 | |
| Dyslipidemia, % | 60.5 | 60.1 | 53.1 | 55.8 | 58.7 | |
| DM, % | 12.0 | 14.5 | 11.6 | 17.0 | 14.0 | |
| eGFR, mL/min per 1.73 m2 | 83.9±10.2 | 83.6±10.3 | 84.2±11.2 | 84.3±12.0 | 83.4±12.0 | |
| Systolic BP, mm Hg | 110.8±6.2 | 124.3±3.0 | 130.7±6.5 | 144.6±7.2 | 165.0±12.6 | |
| Diastolic BP, mm Hg | 69.2±6.3 | 71.7±5.9 | 81.0±5.5 | 86.5±7.9 | 99.0±9.3 | |
Data on smoking status and alcohol consumption were unavailable for 1 and 113 participants, respectively. BMI indicates body mass index; BP, blood pressure; DM, diabetes mellitus; and eGFR, estimated glomerular filtration rate.
CKD Incidence Risk and BP Levels and the Use of Antihypertensive Drugs
During the mean follow‐up period of 4.78 years (median, 4.82 years; interquartile range, 3.10–6.37 years), new‐onset CKD occurred in 14 888 participants. The age‐standardized CKD rate increased as BP increased (Table S2). The CKD rate ranged from 16.98 to 34.96 per 1000 patient‐years in untreated men, 17.63 to 28.30 per 1000 patient‐years in untreated women, 34.39 to 47.50 per 1000 patient‐years in treated men, and 18.62–37.93 per 1000 patient‐years in treated women (Table S2).
We calculated the HRs for BP categories, which was defined by BP and the use of hypertension treatment, with the untreated normal BP group as the reference group and adjusting for covariates including eGFR at baseline. The BP category was significantly associated with the risk of CKD in untreated and treated men (trend P≤0.0023) (Figure 1). However, the HRs in untreated men were lower than in the treated men (range of HR, 1.00–1.76 for untreated men vs 1.41–2.05 for treated men) (Figure 1). Although BP was significantly associated with the risk of CKD in women (trend P≤0.0011), the association between BP and the risk of CKD was weaker in untreated women than in untreated men (Figure 2). Furthermore, in treated women, the HR of normal and high‐normal BP was 0.98 and 0.95, respectively (P≥0.80) (Figure 2). A significant interaction between gender and the 10 BP category with the risk of CKD was observed (interaction P<0.0001; likelihood ratio chi‐square and degrees of freedom, 39.81 and 9, respectively]). In the model used in Figure 1 and Figure 2, DM was also significantly associated with CKD (HR, 1.91 [95% CI, 1.79–2.03] for men and 1.35 [95% CI, 1.13–1.61] for women).
Figure 1. Blood pressure (BP) and the risk of chronic kidney disease (CKD) incidence in men stratified by the use of antihypertensive treatment. HR indicates hazard ratio.
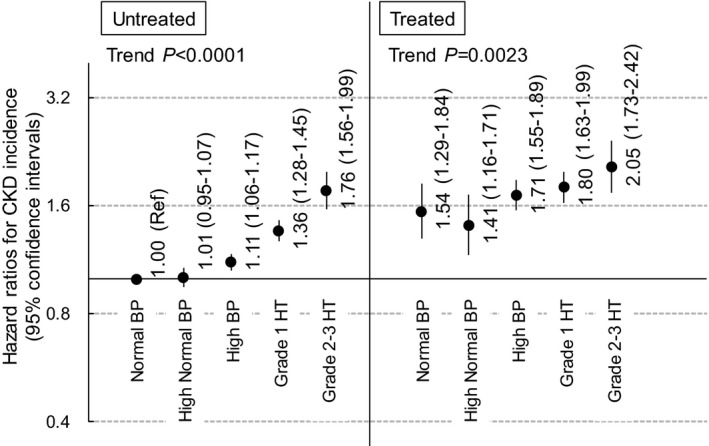
Covariates were age, body mass index <18.5 kg/m2, body mass index ≥25 kg/m2, current smoking status, alcohol consumption, diabetes mellitus, dyslipidemia, and estimated glomerular filtration rate at baseline.
Figure 2. Blood pressure (BP) and the risk of chronic kidney disease (CKD) incidence in women stratified by the use of antihypertensive treatment.
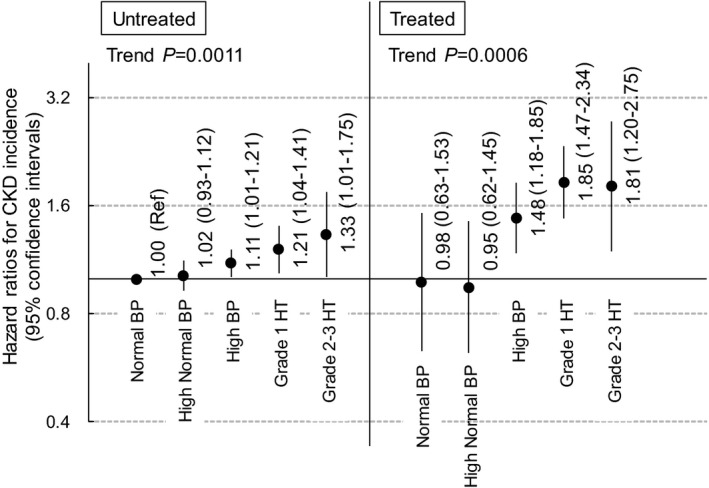
HR indicates hazard ratio.
The covariates, systolic BP, and use of antihypertensive drugs were included in the same Cox model. When the untreated status was set as a reference, the HRs for the use of antihypertensive drugs were 1.44 (95% CI, 1.36–1.54) for men and 1.33 (95% CI, 1.15–1.54) for women. The interaction between gender and the use of antihypertensive drugs on the risk of CKD was also significant (P<0.0001).
Sensitivity Analysis
We performed stratified analyses according to age (<50/≥50 years), BMI (<25/≥25 kg/m2), current smoking status, alcohol consumption, DM, and dyslipidemia (Table 3 and Table 4). The BP category was strongly associated with the risk of CKD in men without DM (Table 3) and women with a BMI of ≥25 kg/m2 (Table 4) (interaction P≥0.051). When women were stratified by an age of 50 years, the interaction between BMI of 25 kg/m2 and BP category on the risk of CKD was observed in women aged <50 years (P for interaction=0.061) but not in women aged ≥50 years (P for interaction=0.77).
Table 3.
Stratification Analysis in Men
| Strata | No. | Events | HRs (95% CIs) | Interaction P | |||
|---|---|---|---|---|---|---|---|
| Untreated | Treated | ||||||
|
Normal and high‐ Normal BP |
High BP and Grade 1–3 Hypertension |
Normal and high‐Normal BP |
High BP and Grade 1–3 Hypertension |
||||
| Age <50 y | 80 678 | 7690 | 1.00 (reference) | 1.16 (1.10–1.22) | 1.50 (1.22–1.84) | 1.66 (1.51–1.83) | 0.19 |
| Age ≥50 y | 27 114 | 2956 | 1.00 (reference) | 1.30 (1.19–1.42) | 1.52 (1.27–1.82) | 1.94 (1.74–2.16) | |
| BMI <25 kg/m2 | 78 218 | 6612 | 1.00 (reference) | 1.18 (1.12–1.24) | 1.59 (1.32–1.91) | 1.77 (1.59–1.97) | 0.44 |
| BMI ≥25 kg/m2 | 29 572 | 4034 | 1.00 (reference) | 1.22 (1.13–1.31) | 1.35 (1.11–1.64) | 1.77 (1.60–1.95) | |
| Nonsmoking | 59 832 | 5745 | 1.00 (reference) | 1.21 (1.14–1.28) | 1.43 (1.19–1.70) | 1.70 (1.55–1.87) | 0.37 |
| Smoking | 47 953 | 4901 | 1.00 (reference) | 1.18 (1.11–1.26) | 1.48 (1.21–1.81) | 1.85 (1.66–2.06) | |
| Nondrinking | 65 514 | 6635 | 1.00 (reference) | 1.18 (1.12–1.25) | 1.46 (1.22–1.74) | 1.78 (1.62–1.95) | 0.92 |
| Drinking | 40 946 | 3886 | 1.00 (reference) | 1.21 (1.12–1.29) | 1.44 (1.17–1.77) | 1.72 (1.54–1.92) | |
| Non‐DM | 101 510 | 9416 | 1.00 (reference) | 1.20 (1.15–1.25) | 1.61 (1.39–1.88) | 1.82 (1.69–1.97) | 0.0017 |
| DM | 6282 | 1230 | 1.00 (reference) | 1.11 (0.97–1.29) | 1.06 (0.80–1.41) | 1.50 (1.27–1.77) | |
| Nondyslipidemia | 56 453 | 4826 | 1.00 (reference) | 1.16 (1.09–1.24) | 1.42 (1.13–1.77) | 1.87 (1.66–2.09) | 0.24 |
| Dyslipidemia | 51 339 | 5820 | 1.00 (reference) | 1.22 (1.15–1.29) | 1.49 (1.26–1.76) | 1.72 (1.57–1.88) | |
| eGFR <90 mL/min per 1.73 m2 | 79 261 | 8217 | 1.00 (reference) | 1.19 (1.14–1.25) | 1.52 (1.31–1.76) | 1.77 (1.64–1.92) | 0.80 |
| eGFR ≥90 mL/min per 1.73 m2 | 28 531 | 2429 | 1.00 (reference) | 1.19 (1.09–1.30) | 1.22 (0.90–1.66) | 1.71 (1.47–1.99) | |
| Visits <5 times | 50 004 | 4161 | 1.00 (reference) | 1.26 (1.17–1.35) | 1.45 (1.21–1.73) | 1.74 (1.56–1.94) | 0.11 |
| Visits ≥5 times | 57 788 | 6485 | 1.00 (reference) | 1.20 (1.14–1.27) | 1.46 (1.19–1.79) | 1.84 (1.68–2.01) | |
The data indicate the hazard ratios (HRs) for chronic kidney disease incidence when the normal/high‐normal blood pressure (BP) group is treated as a reference. The model was adjusted by covariates indicated in the legend of Figure 1 (other than the factor used for stratification). Data on body mass index (BMI), smoking status, and alcohol consumption (drinking) were unavailable for 2, 7, and 1332 participants, respectively. DM indicates diabetes mellitus; and GFR, estimate glomerular filtration rate.
Table 4.
Stratification Analysis in Women
| Strata | No. | Events | HRs (95% CIs) | Interaction P | |||
|---|---|---|---|---|---|---|---|
| Untreated | Treated | ||||||
| Normal and high‐Normal BP | High BP and Grade 1–3 Hypertension | Normal and high‐Normal BP | High BP and Grade 1–3 Hypertension | ||||
| Age <50 y | 36 413 | 3594 | 1.00 (reference) | 1.09 (1.00–1.19) | 0.86 (0.50–1.48) | 1.75 (1.41–2.16) | 0.44 |
| Age ≥50 y | 10 487 | 648 | 1.00 (reference) | 1.30 (1.09–1.56) | 1.19 (0.80–1.75) | 1.79 (1.40–2.29) | |
| BMI <25 kg/m2 | 40 740 | 3479 | 1.00 (reference) | 1.06 (0.97–1.16) | 1.01 (0.69–1.48) | 1.61 (1.30–1.99) | 0.051 |
| BMI ≥25 kg/m2 | 6154 | 761 | 1.00 (reference) | 1.36 (1.16–1.59) | 0.93 (0.54–1.61) | 1.81 (1.41–2.31) | |
| Nonsmoking | 43 094 | 3858 | 1.00 (reference) | 1.14 (1.05–1.24) | 0.93 (0.66–1.30) | 1.68 (1.43–1.97) | 0.60 |
| Smoking | 3803 | 383 | 1.00 (reference) | 1.03 (0.78–1.36) | 1.05 (0.48–2.28) | 1.19 (0.60–2.37) | |
| Nondrinking | 42 087 | 3871 | 1.00 (reference) | 1.15 (1.06–1.25) | 0.93 (0.66–1.30) | 1.70 (1.45–2.01) | 0.37 |
| Drinking | 4282 | 326 | 1.00 (reference) | 0.94 (0.72–1.23) | 1.07 (0.43–2.64) | 1.08 (0.59–1.99) | |
| Non‐DM | 45 807 | 4104 | 1.00 (reference) | 1.13 (1.04–1.22) | 0.90 (0.64–1.28) | 1.55 (1.30–1.84) | 0.44 |
| DM | 1093 | 138 | 1.00 (reference) | 1.21 (0.80–1.86) | 1.49 (0.70–3.18) | 2.47 (1.54–3.96) | |
| Nondyslipidemia | 34 844 | 3085 | 1.00 (reference) | 1.09 (0.98–1.20) | 0.97 (0.60–1.58) | 1.24 (0.96–1.59) | 0.092 |
| Dyslipidemia | 12 056 | 1157 | 1.00 (reference) | 1.23 (1.08–1.41) | 1.05 (0.69–1.57) | 2.15 (1.74–2.65) | |
| eGFR <90 mL/min per 1.73 m2 | 34 339 | 3447 | 1.00 (reference) | 1.12 (1.03–1.23) | 0.97 (0.70–1.36) | 1.51 (1.27–1.80) | 0.41 |
| eGFR ≥90 mL/min per 1.73 m2 | 12 561 | 795 | 1.00 (reference) | 1.19 (0.99–1.43) | 0.95 (0.42–2.16) | 2.42 (1.70–3.46) | |
| Visits <5 times | 23 322 | 1302 | 1.00 (reference) | 1.29 (1.13–1.48) | 1.21 (0.80–1.83) | 1.67 (1.29–2.14) | 0.13 |
| Visits ≥5 times | 23 578 | 2940 | 1.00 (reference) | 1.05 (0.96–1.16) | 0.78 (0.49–1.24) | 1.65 (1.35–2.02) | |
The data indicate the hazard ratios (HRs) for chronic kidney disease incidence when the normal/high‐normal blood pressure (BP) group is treated as a reference. The model was adjusted by covariates indicated in the legend of Figure 1 (other than the factor used for stratification). Data on body mass index (BMI), smoking status, and alcohol consumption (drinking) were unavailable for 6, 3, and 531 participants, respectively. DM indicates diabetes mellitus; and eGFR, estimate glomerular filtration rate.
To consider the outcomes caused by the onset of proteinuria and an eGFR <60 mL/min per 1.73 m2 separately, we assessed the HR for these outcomes instead of the CKD incidence. The untreated normal BP or high‐normal BP group was used as the reference. The HR for proteinuria among men/women was 1.19/1.21 in the untreated high BP and hypertension group, 1.44/1.16 in the treated high BP and hypertension group, and 1.76/2.15 in the treated high BP and hypertension group (Table 5). The HRs for an eGFR <60 mL/min per 1.73 m2 in men were 1.22 in untreated participants with high BP and hypertension, 1.83 in treated participants with normal and high‐normal BP, and 1.90 for treated participants with high BP and hypertension. However, BP was not significantly associated with the risk of an eGFR <60 mL/min per 1.73 m2 in women (P≥0.19) (Table 5).
Table 5.
HRs for the Incidence of Proteinuria or eGFR <60 mL/min per 1.73 m2
| Strata | Outcome | Variable | HRs (95% CIs) | |||
|---|---|---|---|---|---|---|
| Untreated | Treated | |||||
| Normal and high‐Normal BP | High BP and Grade 1–3 Hypertension | Normal and high‐Normal BP | High BP and Grade 1–3 Hypertension | |||
| Men | Proteinuria | No. of events | 3397 | 3432 | 167 | 814 |
| Incidence*, per 1000 patient‐y | 12.58 | 16.31 | 22.01 | 30.20 | ||
| HR† (95% CI) | 1.00 (reference) | 1.19 (1.14–1.25) | 1.44 (1.23–1.69) | 1.76 (1.62–1.92) | ||
| Men | eGFR <60 mL/min per 1.73 m2 | No. of events | 1348 | 1504 | 102 | 420 |
| Incidence*, per 1000 patient‐y | 5.43 | 7.19 | 11.99 | 13.97 | ||
| HR† (95% CI) | 1.00 (reference) | 1.22 (1.13–1.32) | 1.83 (1.49–2.25) | 1.90 (1.69–2.14) | ||
| Women | Proteinuria | No. of events | 2060 | 574 | 25 | 128 |
| Incidence*, per 1000 patient‐y | 10.90 | 14.39 | 13.56 | 32.56 | ||
| HR† (95% CI) | 1.00 (reference) | 1.21 (1.10–1.33) | 1.16 (0.77–1.73) | 2.15 (1.77–2.61) | ||
| Women | eGFR <60 mL/min per 1.73 m2 | No. of events | 1172 | 345 | 20 | 74 |
| Incidence*, per 1000 patient‐y | 7.35 | 7.88 | 3.97 | 13.59 | ||
| HR† (95% CI) | 1.00 (reference) | 1.06 (0.94–1.21) | 0.84 (0.53–1.32) | 1.18 (0.92–1.52) | ||
BP indicates blood pressure; and eGFR, estimated glomerular filtration rate.
The incidence rates were calculated after age standardization (<45/45–59/≥60 years) by the direct method.
The hazard ratio (HR) was adjusted by covariates indicated in the legend of Figure 1.
The significant association between BP and the risk of CKD remained after adjusting for linear and quadratic terms of BMI instead of BMI category (trend P≤0.0052, Figure S2, and Figure S3). However, the HR in untreated women with grade 2 or 3 hypertension was not significant (HR, 1.29; 95% CI, 0.98–1.69) (Figure S3). In untreated participants, antihypertensive treatment was initiated in 3155 of 36 172 (8.72%) with high BP, 3233 of 12 887 (25.09%) with grade 1 hypertension, and 1339 of 2527 (52.99%) with grade 2 or 3 hypertension during the follow‐up period. After excluding the 7727 participants who were treated during the follow‐up, the HR of grade 2 or 3 hypertension in untreated men and untreated women increased to 2.34 and 1.47, respectively, when compared with the untreated normal BP group (Figure S4 and Figure S5).
The association between systolic BP and CKD risk was analyzed using restricted cubic splines. The risk of CKD was significant for SBP ≥130 mm Hg in untreated men, untreated women, and treated women, and for systolic BP ≥140 mm Hg in treated men (Figure S6).
Discussion
BP was significantly associated with an elevated risk of CKD in men, regardless of the use of antihypertensive drugs. This was also proven by the analysis using the cubic spline curve (Figure S6). The overall HR was higher in treated men than in untreated men. The association between BP and the risk of CKD in untreated women was weaker than in untreated men. The risk of CKD in treated women with normal or high‐normal BP was almost the same as that in untreated women with the corresponding BP level.
The use of antihypertensive drugs was associated with an elevated risk of CKD in men, even in the normal and high‐normal BP groups. It was found that treated men had a 1.44‐fold higher risk of CKD than untreated men after adjusting for systolic BP. A meta‐analysis based on 39 705 Japanese patients demonstrated that treated men had a 1.56‐fold higher risk for total cardiovascular disease mortality than those who did not undergo antihypertensive treatment. 10 Longer duration of hypertension could contribute to the higher CKD risk in treated individuals. 20 Therefore, we should consider following treated men more carefully than untreated men, even when BP is well controlled.
In the untreated group, the association between BP and the risk of CKD in women was weaker than in men (Figure 2 and Figure S6). This may be caused by gender‐related differences, such as estrogen production, which has been suggested to prevent atherosclerosis. 21 Our findings may raise the hypothesis that the deleterious effect of elevated BP on the kidneys may be weaker in women. However, no study has clarified the gender difference and the effect of intensive treatment on kidney protection. A recent meta‐analysis based on randomized controlled trials in adults without DM who had CKD did not find intensive antihypertensive treatment effective for the reduction of systolic/diastolic BP to <130/80 mm Hg for the prevention of kidney dysfunction. 22 In the present study, normal or high‐normal BP with treatment that is well controlled, hypertension did not elevate the risk of CKD in women. Thus, appropriate antihypertensive treatment could be more effective for preventing kidney dysfunction in women with hypertension than in men with hypertension.
Several observational studies reported no significant gender differences in the association between BP and the risk of CKD. 4 , 8 , 23 However, an Iranian study with 3313 participants indicated a weaker association between BP and the risk of CKD in women than in men; this study did not consider the use of antihypertensive drugs. 6 The inconsistent results could be the result of the different age of the participants in the above studies 4 , 8 , 23 and those in the Iranian study 6 (≈60 years and 39.7 years, respectively). The mean age of the participants in our study was 44.74 years.
The association between BP and the increased risk of CKD was more remarkable in men without DM than in men with DM (Table 3). The deleterious effect of BP on the kidney could be more explicit in men without DM. BP was clearly associated with an elevated risk of CKD in women with a BMI of ≥25 kg/m2, implying the synergistic effect of BP and being overweight on the risk of CKD (Table 4). One reason for being overweight in women could be reduced estrogens or increased testosterone, which increases the appetite and causes weight gain in women. 24 Estrogens have a renoprotective effect but testosterone adversely affects kidney function. 15 Estrogen levels are higher in nonobese women than in obese women, especially in the premenopausal group. 25 In the present study, the interaction between BMI and BP on the risk of CKD appears to remain only among women aged <50 years. Among overweight women in the present study, reduced estrogens or increased testosterone may have accelerated the deleterious effect of BP on the kidney. This hypothesis should be clarified by studies with data on sex hormones and menopause.
When CKD outcomes were classified as eGFR <60 mL/min per 1.73 m2 and the presence of proteinuria, the BP category was significantly associated with both these suboutcomes in men. Meanwhile, in women, BP was associated with an elevated risk of proteinuria but not an eGFR <60 mL/min per 1.73 m2 (Table 5). The present study was conducted based on multiple examinations after excluding participants with an eGFR <70 mL/min per 1.73 m2, which may have influenced the results. However, none of the previous studies have considered this point. These exclusion criteria may have influenced the association between BP and the presence of proteinuria.
Approximately 50% of patients with grade 2 or 3 hypertension untreated at baseline had not been treated for 5 years in the present study. These findings may link to issues regarding the insufficient treatment rate in Japanese patients with hypertension. 14 The HRs of grade 2 or 3 hypertension in untreated participants slightly increased after excluding those who were treated during the follow‐up (Figure S4 and Figure S5), supporting the consensus that patients with hypertension who remain untreated have an increased risk of CKD.
The present study must be interpreted within the context of its potential limitations. First, the JMDC database predominantly includes young or middle‐aged individuals who work in large enterprises. We received the data from adults who visited health checkups ≥5 times, whereas the original JMDC database has a larger sample size. The participants were more likely to have a stable income and could afford better health care than those in low‐income areas. The present findings are not generalizable, especially to individuals aged ≥75 years, those in low‐income areas, and unhealthy or patient populations. However, similar gender differences in the association between BP and CKD have been reported in an Iranian study while it did not consider the use of antihypertensive drugs. 6 Second, since the present study is observational, our findings cannot interpret the effect of intensive antihypertensive treatment on kidney protection. Third, the use of creatinine levels or the presence of proteinuria measured by only 1 measurement in each health checkup as an indication of CKD in the present study is considered a limitation. The current guidelines 26 define CKD as the presence of kidney damage or an eGFR <60 mL/min per 1.73 m2 for ≥3 months. Therefore, acute kidney injury or dehydration might have been partly responsible for the incidence of CKD in the present study. The CKD outcomes in previous studies were defined in a similar manner. 4 , 5 , 7 , 23 , 27 , 28 CKD indicated by an eGFR <60 mL/min per 1.73 m2 or the presence of proteinuria has been reported to be strongly associated with an elevated risk of cardiovascular disease. Fourth, we did not exclude type 1 DM since the present database does not have information on the type of DM. However, since the prevalence of type 1 DM in Asia is only 6.9 per 10 000 people, 29 the effect of type 1 DM on our findings is limited. Finally, the data from the annual health checkups did not include information regarding the type of antihypertensive treatment. For instance, dihydropyridine calcium channel blockers can decrease the tone of the afferent arteriole, increasing intraglomerular pressure and thus potentially increasing proteinuria. The difference in the antihypertensive drug type might have affected associations based on gender differences found in the present study. The BP change could also affect CKD risk. 8 Considering antihypertensive treatment and BP change can improve the predictive value for CKD.
Conclusions
BP was significantly associated with an elevated risk for CKD incidence, but the overall HR was higher in treated individuals than in those who did not undergo antihypertensive treatment. However, the risk of CKD in women with controlled hypertension was similar to untreated women with normal or high‐normal BP, inconsistent with the trend in men. Kidney function in treated individuals should be followed carefully, especially in men. Future intervention studies assessing antihypertensive treatment on kidney dysfunction should focus on gender differences.
Sources of Funding
This study was supported by Grants for Scientific Research (17K15853 and 17K19930) from Ministry of Education, Culture, Sports, Science and Technology, Japan; academic contributions from Pfizer Japan Inc.; scholarship donations from Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Research Support from Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., and Bayer Yakuhin Co., Ltd.; a Health Care Science Institute Research grant; a grant from the Health Science Center; and a grant from the Foundation for Total Health Promotion.
Disclosures
K.A., Y.I., T.O., and H.M. concurrently held the position of director of the Tohoku Institute for Management of Blood Pressure, which was supported by Omron Healthcare Co., Ltd., Division of Public Health, Hygiene, and Epidemiology, Faculty of Medicine, Tohoku Medical and Pharmaceutical University received scholarship donations for education from Baxter Co., Ltd. and Otsuka Pharmaceutical Co., Ltd. K.A. received grants Omron Healthcare Co., Ltd and personal fees from Takeda Pharmaceutical Co., Ltd. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2Figures S1-S6
Acknowledgments
We thank Ms Makiko Kaneko and Ms Nauta Yamanaka and all of the staff at JMDC Inc. Author contributions: M.S, T.H., T. Murakami., and H.M. designed the experiments. M.S. analyzed the data and wrote the first draft of the article. S.N., K.T., Y.I., T.O., and T. Mori. interpreted the data. All authors contributed to the writing of the article, critically read and revised the article for important intellectual content, and agreed with the article results and conclusions, matching the ICMJE criteria for authorship.
(J Am Heart Assoc. 2020;9:e015592 DOI: 10.1161/JAHA.119.015592.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, Maddukuri G, Tsai CY, Floyd T, Al-Aly Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. [DOI] [PubMed] [Google Scholar]
- 2. Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003;14:2934–2941. [DOI] [PubMed] [Google Scholar]
- 3. Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. [DOI] [PubMed] [Google Scholar]
- 4. Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, Shiigai T, Narita M, Koyama A. Risk factors for chronic kidney disease in a community-based population: a 10-year follow‐up study. Kidney Int. 2007;71:159–166. [DOI] [PubMed] [Google Scholar]
- 5. Obermayr RP, Temml C, Knechtelsdorfer M, Gutjahr G, Kletzmayr J, Heiss S, Ponholzer A, Madersbacher S, Oberbauer R, Klauser-Braun R. Predictors of new-onset decline in kidney function in a general middle-european population. Nephrol Dial Transplant. 2008;23:1265–1273. [DOI] [PubMed] [Google Scholar]
- 6. Tohidi M, Hasheminia M, Mohebi R, Khalili D, Hosseinpanah F, Yazdani B, Nasiri AA, Azizi F, Hadaegh F. Incidence of chronic kidney disease and its risk factors, results of over 10 year follow up in an Iranian cohort. PLoS One. 2012;7:e45304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kanno A, Kikuya M, Ohkubo T, Hashimoto T, Satoh M, Hirose T, Obara T, Metoki H, Inoue R, Asayama K, et al. Pre-hypertension as a significant predictor of chronic kidney disease in a general population: the Ohasama Study. Nephrol Dial Transplant. 2012;27:3218–3223. [DOI] [PubMed] [Google Scholar]
- 8. Yano Y, Fujimoto S, Sato Y, Konta T, Iseki K, Iseki C, Moriyama T, Yamagata K, Tsuruya K, Narita I, et al. New-onset hypertension and risk for chronic kidney disease in the Japanese general population. J Hypertens. 2014;32:2371–2377; discussion 2377. [DOI] [PubMed] [Google Scholar]
- 9. Wan EY, Yu EYT, Chin WY, Fong DY, Choi EP, Lam CL. Association of blood pressure and risk of cardiovascular and chronic kidney disease in Hong Kong hypertensive patients. Hypertension. 2019;74:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Asayama K, Satoh M, Murakami Y, Ohkubo T, Nagasawa SY, Tsuji I, Nakayama T, Okayama A, Miura K, Imai Y, et al. Cardiovascular risk with and without antihypertensive drug treatment in the Japanese general population: participant-level meta-analysis. Hypertension. 2014;63:1189–1197. [DOI] [PubMed] [Google Scholar]
- 11. Hirayama A, Konta T, Kamei K, Suzuki K, Ichikawa K, Fujimoto S, Iseki K, Moriyama T, Yamagata K, Tsuruya K, et al. Blood pressure, proteinuria, and renal function decline: associations in a large community-based population. Am J Hypertens. 2015;28:1150–1156. [DOI] [PubMed] [Google Scholar]
- 12. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 13. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 14. Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235–1481. [DOI] [PubMed] [Google Scholar]
- 15. Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14:151–164. [DOI] [PubMed] [Google Scholar]
- 16. Harada M, Fujihara K, Osawa T, Yamamoto M, Kaneko M, Kitazawa M, Matsubayashi Y, Yamada T, Yamanaka N, Seida H, et al. Relationship between number of multiple risk factors and coronary artery disease risk with and without diabetes mellitus. J Clin Endocrinol Metab. 2019;104:5084–5090. [DOI] [PubMed] [Google Scholar]
- 17. Takeuchi M, Kawakami K. Association between Hemoglobin and Hemoglobin A1c: a data-driven analysis of health checkup data in Japan. J Clin Med. 2018;7:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fukushima A, Khabtheni W, Guelfucci F, Onishi Y, Sugiyama D, Okamura T, Toumi M. Impact of hypertension on hospitalizations for cardiovascular diseases in a worksite population: an epidemiologic study using claims data for workers. Am J Hypertens. 2019;32:298–307. [DOI] [PubMed] [Google Scholar]
- 19. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. [DOI] [PubMed] [Google Scholar]
- 20. Perneger TV, Whelton PK, Klag MJ. History of hypertension in patients treated for end-stage renal disease. J Hypertens. 1997;15:451–456. [DOI] [PubMed] [Google Scholar]
- 21. Arnal JF, Gourdy P, Elhage R, Garmy-Susini B, Delmas E, Brouchet L, Castano C, Barreira Y, Couloumiers JC, Prats H, et al. Estrogens and atherosclerosis. Eur J Endocrinol. 2004;150:113–117. [DOI] [PubMed] [Google Scholar]
- 22. Tsai WC, Wu HY, Peng YS, Yang JY, Chen HY, Chiu YL, Hsu SP, Ko MJ, Pai MF, Tu YK, et al. Association of intensive blood pressure control and kidney disease progression in nondiabetic patients with chronic kidney disease: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maeda T, Yoshimura C, Takahashi K, Ito K, Yasuno T, Abe Y, Masutani K, Nakashima H, Mukoubara S, Arima H. Usefulness of the blood pressure classification in the new 2017 ACC/AHA hypertension guidelines for the prediction of new-onset chronic kidney disease. J Hum Hypertens. 2019;33:873–878. [DOI] [PubMed] [Google Scholar]
- 24. Hirschberg AL. Sex hormones, appetite and eating behaviour in women. Maturitas. 2012;71:248–256. [DOI] [PubMed] [Google Scholar]
- 25. Leeners B, Geary N, Tobler PN, Asarian L. Ovarian hormones and obesity. Hum Reprod Update. 2017;23:300–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. [DOI] [PubMed] [Google Scholar]
- 27. Kanno A, Kikuya M, Asayama K, Satoh M, Inoue R, Hosaka M, Metoki H, Obara T, Hoshi H, Totsune K, et al. Night-time blood pressure is associated with the development of chronic kidney disease in a general population: the Ohasama Study. J Hypertens. 2013;31:2410–2417. [DOI] [PubMed] [Google Scholar]
- 28. Chonchol M, Gnahn H, Sander D. Impact of subclinical carotid atherosclerosis on incident chronic kidney disease in the elderly. Nephrol Dial Transplant. 2008;23:2593–2598. [DOI] [PubMed] [Google Scholar]
- 29. Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Hosseini Fard H, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot Perspect. 2020;10:98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2Figures S1-S6


