Abstract
Background
There is scarce data about the long‐term mortality as well as the prognostic value of cardiovascular magnetic resonance and late gadolinium enhancement (LGE) in patients with biopsy‐proven viral myocarditis. We sought to investigate: (1) mortality and (2) prognostic value of LGEcardiovascular magnetic resonance (location, pattern, extent, and distribution) in a >10‐year follow‐up in patients with biopsy‐proven myocarditis.
Methods and Results
Two‐hundred three consecutive patients with biopsy‐proven viral myocarditis and cardiovascular magnetic resonance were enrolled; 183 patients were eligible for standardized follow‐up. The median follow‐up was 10.1 years. End points were all‐cause death, cardiac death, and sudden cardiac death (SCD). We found substantial long‐term mortality in patients with biopsy‐proven myocarditis (39.3% all cause, 27.3% cardiac, and 10.9% SCD); 101 patients (55.2%) demonstrated LGE. The presence of LGE was associated with a more than a doubled risk of death (hazard ratio [HR], 2.40; 95% CI], 1.30–4.43), escalating to a HR of 3.00 (95% CI, 1.41–6.42) for cardiac death, and a HR of 14.79 (95% CI, 1.95–112.00) for SCD; all P≤0.009. Specifically, midwall, (antero‐) septal LGE, and extent of LGE were highly associated with death, all P<0.001. Septal LGE was the best independent predictor for SCD (HR, 4.59; 95% CI, 1.38–15.24; P=0.01).
Conclusions
In patients with biopsy‐proven viral myocarditis, the presence of midwall LGE in the (antero‐) septal segments is associated with a higher rate of mortality (including SCD) compared with absent LGE or other LGE patterns, underlining the prognostic benefit of a distinct LGE analysis in these patients.
Keywords: biopsy, cardiovascular magnetic resonance, late gadolinium enhancement, mortality, myocarditis, viral
Subject Categories: Cardiomyopathy, Heart Failure, Magnetic Resonance Imaging (MRI), Prognosis
Nonstandard Abbreviations and Acronyms
- CMR
cardiovascular magnetic resonance
- EMB
endomyocardial biopsy
- HR
hazard ratio
- LGE
late gadolinium enhancement
- LV
left ventricle
- LVEF
left ventricular ejection fraction
- SCD
sudden cardiac death
Clinical Perspective
What Is New?
We investigated (1) mortality and (2) the prognostic value of late gadolinium enhancement (LGE) cardiovascular magnetic resonance (including location, pattern, extent, and distribution) in a >10‐year follow‐up period in patients with biopsy‐proven myocarditis.
We demonstrate that these patients suffer from substantial long‐term mortality (39.3%) underlining the unmet need for stratification of patients at highest risk for death.
What Are the Clinical Implications?
The extent and the presence of midwall LGE in the (antero‐) septal segments are associated with a higher rate of mortality (including sudden cardiac death) compared with absent LGE or other LGE patterns, underlining the prognostic benefit of a distinct LGE analysis in these patients.
Myocarditis is characterized by a great variation in patient symptoms and clinical course, ranging from (1) mild discomfort to cardiogenic shock, and (2) complete restoration to sudden cardiac death (SCD). 1 , 2 Endomyocardial biopsies (EMBs) are currently the gold standard for the diagnosis of inflammatory diseases, 3 as only EMBs allow to differentiate between noninfectious (eg, immunomediated) and infectious myocarditis. Specific RNA and DNA viruses are the most common pathogens in acute myocarditis. 4 As EMBs require invasive procedures, accurate noninvasive techniques providing incremental data to EMBs especially for follow‐up investigations are highly desirable.
Cardiovascular magnetic resonance (CMR) is such a noninvasive tool, demonstrating high diagnostic accuracy in patients with myocarditis, which is acknowledged by current guidelines. 5 , 6 To date, late gadolinium enhancement (LGE) is the most established technique for the detection of myocardial damage. 7 Beside the diagnosis of myocarditis itself, markers of prognosis are of paramount importance. The presence of LGE seems to be a good predictor of an adverse outcome in patients with biopsy‐proven myocarditis, superior to other variables, such as reduced left ventricular ejection fraction (LVEF) or clinical presentation. 8 Other analyses, in nonischemic and ischemic cardiomyopathies, could confirm that the presence of LGE is associated with adverse prognosis. 9 , 10 However, the negative predictive value of LGE is even higher than its positive predictive value. 11 Thus, the identification of additional LGE characteristics, indicating patients who are at highest risk for an adverse outcome, would be highly appreciated.
Recent studies have suggested that patients with suspected myocarditis can be further risk‐stratified not only by the presence of LGE, but also by its location, pattern, extent, and distribution 12 , 13 : Septal and midwall LGE showed strongest associations with major adverse cardiovascular events, such as all‐cause death, heart‐failure decompensation, heart transplantation, and ventricular arrhythmia. 12 Aquaro et al demonstrated that patients with anteroseptal LGE had a worse prognosis than LGE in other locations (eg, in the inferolateral wall). 13 Both latter studies have in common that: (1) combined end points (major adverse cardiovascular events) were chosen, (2) follow‐up time was <5 years, and (3) only a minority underwent EMB.
Hence, there is a lack of prognostic data in patients with definite, biopsy‐proven myocarditis regarding the single, hard end point all‐cause death over a follow‐up period of >10 years. Therefore, we sought to investigate: (1) mortality, and (2) the prognostic value of LGE‐CMR (including location, pattern, extent, and distribution) in a >10‐year follow‐up period in patients with biopsy‐proven viral myocarditis.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patient Population
Two‐hundred three consecutive patients presenting for workup of myocarditis between January 2002 and January 2008 were enrolled in this long‐term follow‐up if they fulfilled the following criteria: (1) biopsy‐proven viral myocarditis defined as presence of myocardial inflammation and viral genome, (2) exclusion of relevant coronary artery stenosis (>50%) by coronary angiography, and (3) dedicated CMR protocol performed within 5 days of initial presentation. Patients with valvular heart disease and known contraindications for CMR were excluded.
The cohort of this study was part of a previously reported trial, 8 which could demonstrate a high predictive value for presence of LGE and an adverse patient outcome. Follow‐up in the present study was performed between April 2017 and July 2018. The study complies with the Declaration of Helsinki; the local ethics committee approved the research protocol, and written informed consent was obtained from the subjects.
Cardiovascular Magnetic Resonance Protocol
Electrocardiography‐gated CMR imaging was performed in breath‐hold using a 1.5T Magnetom Siemens Sonata (Siemens Healthcare, Erlangen, Germany) in line with the Society of Cardiovascular Magnetic Resonance/European Cardiovascular Magnetic Resonance recommendations. 14 Both cine and LGE short‐axis CMR images were prescribed every 10 mm (slice thickness, 6 mm) from base to apex. Cine CMR was performed using a steady‐state free precession sequence. LGE images were acquired on average 5 to 10 minutes after contrast administration, using segmented inversion recovery gradient echo sequence constantly adjusting inversion time. 8 The contrast dose (gadodiamide or gadopentetate‐dimeglumine) was 0.15 mmol/kg.
Cardiovascular Magnetic Resonance Analysis
Cine images were evaluated as described previously. 8 In brief, endocardial and epicardial borders were outlined on the short‐axis cine images. Volumes and ejection fraction were derived by summation of epicardial and endocardial contours. The left ventricular mass was calculated by subtracting endocardial from epicardial volume at end‐diastole and multiplying by 1.05 g/cm3. For post‐processing and quantification of the LGE images dedicated software (QMass, Medis Medical Imaging, Leiden, The Netherlands) was used.
All images were analyzed by consensus of 2 experienced readers (S.G., A.S.) blinded to the results of clinical data or EMB. Epicardial and endocardial contours were placed manually on all LGE images. LGE was defined at an image intensity level ≥2 SD above the mean of remote myocardium 15 the results were expressed as percentage of myocardial left ventricular mass. LGE was further divided by its localization (anterior, inferior, septal, lateral; 17‐segment model), its distribution (linear, patchy, diffuse), and its pattern (epicardial, midwall), as previously described. 12 , 13 , 16
Endomyocardial Biopsy Protocol
Endomyocardial biopsies were performed in all patients. At least 5 biopsies were taken (mainly in both left and right ventricle) and were stained with Masson's trichrome, as well as Giemsa stain, and examined by light microscopy. For immunohistology, tissue sections were treated with an avidin‐biotin‐immunoperoxidase method (Vectastain Elite Kit; Vector, Burlingame, CA), applying the following monoclonal antibodies: CD3 (T cells; Novocastra Laboratories, Newcastle, United Kingdom), CD68 (macrophages; DAKO, Hamburg, Germany), and human leukocyte antigen‐DR (DAKO) as described previously. The detection of >14 infiltrating leukocytes/mm2 (CD3+ T lymphocytes and/or CD68+ macrophages) in the presence of myocyte damage and/or fibrosis, in addition to enhanced human leukocyte antigen class II expression in professional antigen‐presenting immune cells and endothelium was used for the diagnosis of myocarditis. 8
Detection of Viral Genomes
DNA and RNA were extracted with the use of proteinase‐K digestion, followed by extraction with phenol/chloroform. Nested polymerase chain reaction/reverse transcriptase–polymerase chain reaction was performed for the detection of enteroviruses (including coxsackievirus groups A and B, and echoviruses), parvovirus B19, adenoviruses, human cytomegalovirus, Epstein‐Barr virus, and human herpes virus type 6. As control for successful extraction of DNA and RNA, oligonucleotide sequences were chosen from the glyceraldehyde‐3‐phosphate‐dehydrogenase gene. Specificity of all viral amplification products was confirmed by automatic DNA sequencing. 8
Clinical Follow‐Up and End Points
Clinical follow‐up was performed using a standardized questionnaire. In case of a suspected event, all necessary medical records were obtained and reviewed by the authors acting as an end point committee. There were three primary end points: (1) all‐cause death, defined as death from any cause, including aborted SCD; (2) cardiac death, defined as death from all cardiac causes, including SCD, heart failure, and aborted SCD; and (3) SCD, defined as unexpected arrest of presumed cardiac origin within 1 hour after onset of any symptoms that could be interpreted as being cardiac in origin. Aborted SCD was considered as resuscitation after cardiac arrest defined as performance of the physical act of cardioversion, appropriate implantable cardioverter‐defibrillator shocks, or cardiopulmonary resuscitation in a patient who remained alive 28 days later. For appropriate implantable cardioverter‐defibrillator shocks, defibrillator discharges were considered appropriate; these included automatic defibrillation shocks triggered by ventricular tachycardia or fibrillation and documented by stored intracardiac electrocardiographic data. 8
Statistical Analysis
Absolute numbers and percentages were computed to describe the patient population. Medians (with interquartile range) or mean±SD were computed as appropriate. Categorical values were compared by chi‐square test or Fisher's exact test as appropriate. Kaplan–Meier curves were calculated for visualizing the cumulative survival of patients with and without LGE (and the combination with LVEF dichotomized by a 40% cut‐off). Time to event was measured from the date of CMR examination. A log‐rank test was performed to compare both survival curves. A multivariable Cox proportional hazards model was used for analyzing independent associations with all‐cause death and cardiac death. All values of P<0.05 were considered significant. All P values are the results of 2‐tailed tests. We adjusted for multiple comparisons by using Bonferroni analysis and chose significance levels accordingly. A P value of P<0.0167 was defined to be significant. All statistical analyses were performed using GraphPad software (GraphPad Software, San Diego, CA) or SPSS 22.0 (IBM Corp., Armonk, NY).
RESULTS
Patient Characteristics
There were 183 of the 203 patients available for clinical follow‐up at a median of 10.1 years, yielding a follow‐up rate of 90.1%. The remaining 20 patients were lost because of no contact. Baseline characteristics are displayed in Table 1. At time of CMR, patients were at median 53‐years‐old (interquartile range, 40–67 years), symptoms of acute coronary syndrome were the most common initial presentation, 166 patients (90.7%) demonstrated ST‐segment abnormalities. Biventricular EMB was performed in 106 (57.9%) patients, revealing parvovirus B19 as the most frequent virus (n=105; 57.4%). None of the patients received antiviral medication. All patients with heart failure were treated with state‐of‐the‐art heart‐failure medication according to guidelines. When indicated, an implantable cardioverter‐defibrillator was offered and inserted in 24 patients.
Table 1.
Patient Characteristics
| All Patients | LGE Present | No LGE | P Value | |
|---|---|---|---|---|
| (n=183) | (n=101) | (n=82) | ||
| Age, y | 53 (40–67) | 56 (39–68) | 51 (40–60) | 0.14 |
| Time to follow‐up, y | 10.1 (5.8–11.6) | 9.4 (3.7–13.2) | 10.4 (9.2–11.4) | 0.07 |
| Sex (female) | 57 (31) | 22 (22) | 35 (43) | 0.002* |
| BMI, kg/m2 | 26.3 (24.1–29.1) | 26.8 (24.6–29.4) | 25.9 (23.6–28.4) | 0.17 |
| BSA, m2 | 2.0 (1.8–2.1) | 2.0 (1.8–2.2) | 1.9 (1.8–2.1) | 0.011* |
| Primary clinical presentation | ||||
| Symptoms of ACS | 68 (37.2) | 34 (33.7) | 34 (41.5) | 0.29 |
| Subacute new‐onset HF | 56 (30.6) | 36 (35.6) | 20 (24.4) | 0.11 |
| Recurring episodes of overt HF | 13 (7.1) | 8 (7.9) | 5 (6.1) | 0.78 |
| Combination of palpitations, fatigue, dyspnea on exertion | 46 (25.1) | 23 (22.8) | 23 (28.0) | 0.49 |
| Presence of ICD | 24 (13.1) | 18 (17.8) | 6 (7.3) | 0.037* |
| Presence of cardiovascular risk factors | ||||
| Arterial hypertension | 41 (22.4) | 19 (18.8) | 22 (26.8) | 0.66 |
| Diabetes mellitus | 13 (7.1) | 9 (8.9) | 4 (4.9) | 0.05 |
| Hypercholesterinemia | 24 (13.1) | 8 (7.9) | 16 (19.5) | 0.24 |
| Smoking | 14 (7.7) | 3 (3.0) | 11 (13.4) | 0.07 |
| Family history | 26 (14.2) | 8 (7.9) | 18 (22.0) | 0.12 |
| Medical treatment | ||||
| β blockers | 101 (55.2) | 60 (59.4) | 41 (50.0) | 0.003* |
| ACE‐I/Sartans | 98 (53.6) | 59 (58.4) | 39 (47.6) | 0.002* |
| Initial NYHA functional class | ||||
| NYHA I | 43 (23.5) | 25 (24.8) | 18 (22.0) | 0.79 |
| NYHA II | 56 (30.6) | 28 (27.7) | 28 (34.2) | |
| NYHA III | 67 (36.6) | 39 (38.6) | 28 (34.2) | |
| NYHA IV | 17 (9.3) | 9 (8.9) | 8 (9.8) | |
| Virus type by EMB | ||||
| PVB19 | 105 (57.4) | 61 (60.4) | 44 (53.7) | 0.26 |
| HHV6 | 43 (23.5) | 18 (17.8) | 25 (30.5) | |
| PVB19/HHV6 | 29 (15.9) | 18 (17.8) | 11 (13.4) | |
| EBV | 2 (1.1) | 1 (1.0) | 1 (1.2) | |
| PVB19/HHV6/EBV | 1 (0.5) | 1 (1.0) | 0 | |
| PVB19/EBV | 2 (1.1) | 2 (2.0) | 0 | |
| HHV6/EBV | 1 (0.5) | 0 | 1 (1.2) | |
| Blood testing | ||||
| Troponin positive | 43 (23.5) | 30 (29.7) | 13 (15.9) | 0.028* |
| BNP, pg/mL | 209 (46–674) | 300 (79–979) | 125 (32–476) | 0.004* |
| NT‐proBNP, pg/mL | 1840 (156–7714) | 2359 (384–19 357) | 1535 (36–4057) | 0.28 |
| CMR imaging parameters | ||||
| LV function | ||||
| LVEF, % | 44 (31–60) | 38 (25–56) | 53 (37–65) | <0.001* |
| LVEDV, mL | 172 (135–212) | 190 (148–263) | 161 (129–195) | 0.002* |
| LVEDV indexed, mL/m2 | 85.8 (67.3–113.1) | 94.5 (69.9–128.9) | 80.5 (66.4–99.4) | 0.011* |
| LVESV, mL | 93 (49–147) | 119 (59–175) | 80 (44–120) | <0.001* |
| LVESV indexed, mL/m2 | 45.4 (25.1–79.1) | 59.6 (27.8–91.6) | 37.7 (21.7–61.4) | 0.001* |
| LVEF >40% | 105 (57.4) | 48 (47.5) | 54 (65.9) | 0.013* |
| LGE mass | ||||
| LGE mass, g | … | 7.2 (3.1–14.2) | … | |
| LGE, % of LV mass | … | 4.5 (2.9–10.9) | … | |
| Segments of LGE | … | 4 (2–6) | … | |
| LGE location | ||||
| Anterior | … | 39 (38.6) | … | |
| Lateral | … | 55 (54.4) | … | |
| Inferior | … | 50 (49.5) | … | |
| Septal | … | 45 (44.6) | … | |
| Anteroseptal cluster | … | 36 (35.6) | … | |
| Inferolateral cluster | … | 45 (44.6) | … | |
| Anteroseptal+inferolateral | … | 8 (7.9) | … | |
| LGE distribution | ||||
| Linear | … | 53 (52.5) | … | |
| Patchy | … | 27 (26.7) | … | |
| Diffuse | … | 16 (15.8) | … | |
| LGE pattern | ||||
| Epicardial | … | 39 (38.6) | … | |
| Midwall | … | 47 (46.5) | … | |
| Other | … | 15 (14.9) | … | |
Values are n (%) or median (interquartile range). ACE‐I indicates ACE inhibitors; ACS, acute coronary syndrome; BMI, body mass index; BNP, brain natriuretic peptide; BSA, body surface area; CMR, cardiovascular magnetic resonance; EBV, Epstein‐Barr virus; EMB, endomyocardial biopsy; HF, heart failure; HHV6, human herpes virus type 6; ICD, implantable cardioverter‐defibrillator; LGE, late gadolinium enhancement; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PVB19, parvovirus B19; and SCD, sudden cardiac death.
P<0.05.
Cardiovascular Magnetic Resonance Characteristics
Overall LVEF was reduced (median 44%, interquartile range, 31%–60%), LGE was present in 101 patients (55.2%; Table 1). Dividing patients into groups by the presence of LGE revealed that patients with LGE demonstrated lower LVEF, larger ventricles, higher brain natriuretic peptide, and increased troponin levels (all P<0.05). Beside a lower rate of human herpes virus type 6 in patients who were LGE positive, type of viruses did not differ significantly between both groups. LGE showed preponderance for: (1) inferolateral wall location (44.6%), (2) a linear distribution (52.5%), and (3) a midwall pattern (46.5%; Table 1).
Follow‐Up Results and Predictors of Mortality
During follow‐up, 72 of 183 patients (39.3%) died. Most of these patients (n=50; 27.3%) suffered from cardiac death, including SCD (n=20; 10.9%) and aborted SCD (n=12; 6.6%). Twenty‐two deaths (12.0%) related to cancer, fatal infections, or accidents. For prediction of mortality, patients were divided into patients with no event versus patients with all‐cause death (Table 2), cardiac death (Table 3), and SCD (Table 4).
Table 2.
Univariable Predictors of All‐Cause Death
| No Event | All‐Cause Death | P Value | |
|---|---|---|---|
| (n=111) | (n=72) | ||
| Age, y | 49 (38–59) | 62 (47–69) | <0.001* |
| Sex (female) | 35 (31.5) | 22 (30.6) | 0.89 |
| BMI, kg/m2 | 26.6 (24.4–29.7) | 26.1 (23.6–28.7) | 0.47 |
| BSA, m2 | 2.0 (1.8–2.1) | 2.0 (1.8–2.2) | 0.85 |
| Primary clinical presentation | |||
| Symptoms of ACS | 42 (37.8) | 26 (36.1) | 0.88 |
| Subacute new‐onset HF | 37 (33.3) | 19 (26.4) | 0.33 |
| Recurring episodes of overt HF | 5 (4.5) | 8 (11.1) | 0.14 |
| Combination of palpitations, fatigue, dyspnea on exertion | 27 (24.3) | 19 (26.4) | 0.86 |
| Presence of cardiovascular risk factors | |||
| Arterial hypertension | 29 (26.1) | 12 (16.7) | 0.11 |
| Diabetes mellitus | 8 (7.2) | 5 (6.9) | 0.11 |
| Hypercholesterinemia | 19 (17.1) | 5 (6.9) | 0.94 |
| Smoking | 13 (11.7) | 1 (1.4) | 0.16 |
| Family history | 24 (21.6) | 2 (2.8) | 0.05 |
| Medical treatment | |||
| β blockers | 49 (44.1) | 52 (72.2) | <0.001* |
| ACE‐I/Sartans | 47 (42.3) | 51 (70.8) | <0.001* |
| Initial NYHA functional class | |||
| NYHA I | 36 (32.4) | 7 (9.7) | 0.003* |
| NYHA II | 33 (29.7) | 23 (31.9) | |
| NYHA III | 33 (29.7) | 34 (47.2) | |
| NYHA IV | 9 (8.1) | 8 (11.1) | |
| Virus type by EMB | |||
| PVB19 | 67 (60.4) | 38 (52.8) | 0.39 |
| HHV6 | 24 (21.6) | 19 (26.4) | |
| PVB19/HHV6 | 18 (16.2) | 11 (15.3) | |
| EBV | 1 (0.9) | 1 (1.4) | |
| PVB19/HHV6/EBV | … | 1 (1.4) | |
| PVB19/EBV | … | 2 (2.8) | |
| HHV6/EBV | 1 (0.9) | … | |
| Blood testing | |||
| Troponin positive | 26 (23.4) | 17 (23.6) | 0.98 |
| BNP, pg/mL | 111 (30–457) | 478 (133–1202) | <0.001* |
| NT‐proBNP, pg/mL | 135 (37–1535) | 4391 (1742–20 621) | 0.002* |
| CMR | |||
| LVEF, % | 55 (37–65) | 36 (25–46) | <0.001* |
| LVEDV, mL | 155 (125–194) | 199 (163–274) | <0.001* |
| LVEDV indexed, mL/m2 | 77 (64–95) | 101 (85–127) | <0.001* |
| LVESV, mL | 65 (44–121) | 133 (94–172) | <0.001* |
| LVESV indexed, mL/m2 | 33 (22–62) | 69 (49–90) | <0.001* |
| LVEF >40% | 74 (66.7) | 28 (38.9) | <0.001* |
| LGE present | 46 (41.4) | 55 (76.4) | <0.001* |
Values are n (%) or median (interquartile range). ACS indicates acute coronary syndrome; BMI, body mass index; BNP, brain natriuretic peptide; BSA, body surface area; CMR, cardiovascular magnetic resonance; EBV, Epstein‐Barr virus; EMB, endomyocardial biopsy; HF, heart failure; HHV6, human herpes virus type 6; ICD, implantable cardioverter‐defibrillator; LGE, late gadolinium enhancement; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PVB19, parvovirus B19; and SCD, sudden cardiac death.
P<0.05.
Table 3.
Univariable Predictors of Cardiac Death
| No Event | Cardiac Death | P Value | |
|---|---|---|---|
| (n=111) | (n=50) | ||
| Age, y | 49 (38–59) | 62 (47–69) | 0.001* |
| Sex (female) | 35 (31.5) | 10 (20) | 0.13 |
| BMI, kg/m2 | 26.6 (24.4–29.7) | 26.0 (23.7–28.7) | 0.49 |
| BSA, m2 | 2.0 (1.8–2.1) | 2.0 (1.8–2.2) | 0.60 |
| Primary clinical presentation | |||
| Symptoms of ACS | 42 (37.8) | 18 (36) | 0.82 |
| Subacute new‐onset HF | 37 (33.3) | 11 (22) | 0.15 |
| Recurring episodes of overt HF | 5 (4.5) | 5 (10) | 0.13 |
| Combination of palpitations, fatigue, dyspnea on exertion | 27 (24.3) | 16 (32) | 0.27 |
| Presence of cardiovascular risk factors | |||
| Arterial hypertension | 29 (26.1) | 10 (20.0) | 0.15 |
| Diabetes mellitus | 8 (7.2) | 5 (10.0) | 0.06 |
| Hypercholesterinemia | 19 (17.1) | 3 (6.0) | 0.52 |
| Smoking | 13 (11.7) | 1 (2.0) | 0.22 |
| Family history | 24 (21.6) | 2 (4.0) | 0.08 |
| Medical treatment | |||
| β blockers | 49 (44.1) | 36 (72.0) | <0.001* |
| ACE‐I/Sartans | 47 (42.3) | 35 (70.0) | <0.001* |
| Initial NYHA functional class | |||
| NYHA I | 36 (32.4) | 6 (12) |
0.11 |
| NYHA II | 33 (29.7) | 15 (30) | |
| NYHA III | 33 (29.7) | 23 (46) | |
| NYHA IV | 9 (8.1) | 6 (12) | |
| Virus type by EMB | |||
| PVB19 | 67 (60.4) | 26 (52) |
0.16 |
| HHV6 | 24 (21.6) | 12 (24) | |
| PVB19/HHV6 | 18 (16.2) | 8 (13) | |
| EBV | 1 (0.9) | 1 (2) | |
| PVB19/HHV6/EBV | … | 1 (2) | |
| PVB19/EBV | … | 2 (4) | |
| HHV6/EBV | 1 (0.9) | … | |
| Blood testing | |||
| Troponin positive | 26 (23.4) | 13 (26.0) | 0.72 |
| BNP, pg/mL | 111 (30–457) | 448 (105–970) | 0.001* |
| NT‐proBNP, pg/mL | 135 (37–1535) | 2990 (1295–12 770) | 0.009* |
| CMR LV function | |||
| LVEF, % | 55 (37–65) | 36 (24–46) | <0.001* |
| LVEDV, mL | 155 (125–194) | 199 (163–261) | <0.001* |
| LVEDV indexed, mL/m2 | 77 (64–95) | 100 (85–122) | <0.001* |
| LVESV, mL | 65 (44–121) | 137 (95–169) | <0.001* |
| LVESV indexed, mL/m2 | 33 (22–62) | 69 (51–87) | <0.001* |
| LVEF >40% | 74 (66.7) | 19 (38%) | 0.003* |
| LGE present | 46 (41.4) | 40 (80.0) | <0.001* |
Values are n (%) or median (interquartile range). ACS indicates acute coronary syndrome; BMI, body mass index; BNP, brain natriuretic peptide; BSA, body surface area; CMR, cardiovascular magnetic resonance; EBV, Epstein‐Barr virus; EMB, endomyocardial biopsy; HF, heart failure; HHV6, human herpes virus type 6; ICD, implantable cardioverter‐defibrillator; LGE, late gadolinium enhancement; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PVB19, parvovirus B19; and SCD, sudden cardiac death.
P<0.05.
Table 4.
Univariable Predictors of SCD
| No Event | SCD | P Value | |
|---|---|---|---|
| (n=111) | (n=20) | ||
| Age, y | 49 (38–59) | 61 (45–66) | 0.06 |
| Sex (female) | 35 (31.5) | 3 (15.0) | 0.13 |
| BMI, kg/m2 | 26.6 (24.4–29.7) | 27.8 (25.0–31.1) | 0.34 |
| BSA, m2 | 2.0 (1.8–2.1) | 2.0 (1.8–2.2) | 0.26 |
| Primary clinical presentation | |||
| Symptoms of ACS | 42 (37.8) | 7 (35.0) | 0.81 |
| Subacute new‐onset HF | 37 (33.3) | 6 (30.0) | 0.77 |
| Recurring episodes of overt HF | 5 (4.5) | 3 (15.0) | 0.33 |
| Combination of palpitations, fatigue, dyspnea on exertion | 27 (24.3) | 4 (20.0) | 0.91 |
| Presence of cardiovascular risk factors | |||
| Arterial hypertension | 29 (26.1) | 6 (30.0) | 0.14 |
| Diabetes mellitus | 8 (7.2) | 3 (15.0) | 0.09 |
| Hypercholesterinemia | 19 (17.1) | 2 (10.0) | 0.92 |
| Smoking | 13 (11.7) | 1 (5.0) | 0.69 |
| Family history | 24 (21.6) | 1 (5.0) | 0.22 |
| Medical treatment | |||
| β blockers | 49 (44.1) | 16 (80.0) | 0.009* |
| ACE‐I/Sartans | 47 (42.3) | 15 (75.0) | 0.024* |
| Initial NYHA functional class | |||
| NYHA I | 36 (32.4) | 1 (5.0) |
0.07 |
| NYHA II | 33 (29.7) | 4 (20.0) | |
| NYHA III | 33 (29.7) | 12 (60.0) | |
| NYHA IV | 9 (8.1) | 3 (15.0) | |
| Virus type by EMB | |||
| PVB19 | 67 (60.4) | 10 (50.0) |
0.34 |
| HHV6 | 24 (21.6) | 4 (20.0) | |
| PVB19/HHV6 | 18 (16.2) | 4 (20.0) | |
| EBV | 1 (0.9) | 1 (5.0) | |
| PVB19/HHV6/EBV | … | … | |
| PVB19/EBV | … | 1 (5.0) | |
| HHV6/EBV | 1 (0.9) | … | |
| Blood testing | |||
| Troponin positive | 26 (23.4) | 6 ( 30.0) | 0.53 |
| BNP, pg/mL | 111 (30–457) | 217 (98–890) | 0.10 |
| NT‐proBNP, pg/mL | 135 (37–1535) | 2976 (1010–9283) | 0.039* |
| CMR | |||
| LVEF, % | 55 (37–65) | 34 (23–48) | <0.001* |
| LVEDV, mL | 155 (125–194) | 200 (156–280) | 0.002* |
| LVEDV indexed, mL/m2 | 77 (64–95) | 103 (76–125) | 0.006* |
| LVESV, mL | 65 (44–121) | 142 (86–204) | <0.001* |
| LVESV indexed, mL/m2 | 33 (22–62) | 73 (39–97) | <0.001* |
| LGE present | 46 (41.4) | 19 (95.0) | <0.001* |
Values are n (%) or median (interquartile range). ACS indicates acute coronary syndrome; BMI, body mass index; BNP, brain natriuretic peptide; BSA, body surface area; CMR, cardiovascular magnetic resonance; EBV, Epstein‐Barr virus; EMB, endomyocardial biopsy; HF, heart failure; HHV6, human herpes virus type 6; ICD, implantable cardioverter‐defibrillator; LGE, late gadolinium enhancement; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PVB19, parvovirus B19; and SCD, sudden cardiac death.
P<0.05.
Beside a higher prevalence of death in patients with parvovirus B19/Epstein‐Barr virus virus (n=2), type of viruses, primary clinical presentation, and troponin status did not differ significantly between the groups. In contrast, older age, dyspnea New York Heart Association grade III, and increased N‐terminal brain natriuretic peptide levels were associated with increased mortality (all P<0.05). Furthermore, patients suffering from death demonstrated significantly (1) reduced LVEF (36% versus 55%), (2) increased LV volumes, and (3) higher prevalence of LGE (76.4% versus 41.4%) (all P<0.01). Multivariable Cox proportional hazards regression revealed as independent predictors for: (1) all‐cause death: age (HR, 1.03; 95% CI, 1.00–1.05; P=0.004), indexed left ventricular end‐diastolic volume (HR, 1.01; 95% CI, 1.00–1.02; P=0.029), presence of LGE (HR, 2.40; 95% CI, 1.30–4.43; P=0.005), and LVEF (HR, 0.98; 95% CI, 0.95–1.00; P=0.029), (2) cardiac death: age (HR, 1.03; 95% CI, 1.00–1.06; P=0.015), presence of LGE (HR, 3.00; 95% CI, 1.41–6.42; P=0.005), and LVEF (HR, 0.96; 95% CI, 0.94–0.99; P=0.001), and (3) SCD: presence of LGE (HR, 14.79; 95% CI, 1.95–112.00; P=0.009) and LVEF (HR, 0.97; 95% CI, 0.95–0.99; P=0.012) (Tables 5, 6, 7). Kaplan–Meier survival curves comparing patients with LGE versus no LGE are displayed in Figure 1 for (1) all‐cause death, (2) cardiac death, and (3) SCD. Besides LGE, reduced LVEF seems to be a strong independent predictor for cardiac mortality. Stratifying patients by LGE status and LVEF (dichotomized by a 40% cut‐off), Kaplan–Meier survival curves suggest superior prognostic value of LGE versus LVEF, even for occurrence of SCD (Figure 2): LGE‐positive patients with LVEF ≤40% were at higher risk of suffering from SCD than LGE‐negative patients with LVEF ≤40% (P<0.0001).
Table 5.
Cox Regression Analysis: All‐Cause Death—LGE‐Positive Patients versus LGE‐Negative Patients
| HR (95% CI) | P Value | |
|---|---|---|
| Age | 1.03 (1.0–1.05) | 0.004* |
| NYHA >2 | 0.93 (0.51–1.69) | 0.806 |
| BNP, pg/mL | 1.00 (0.99–1.00) | 0.313 |
| LVEDVi, mL/m2 | 1.01 (1.00–1.02) | 0.029 |
| LGE presence | 2.40 (1.30–4.43) | 0.005* |
| Troponin positive | 0.72 (0.35–1.47) | 0.368 |
| LVEF | 0.98 (0.95–1.00) | 0.029 |
A P<0.0167 was considered significant after Bonferroni adjustment for multiple testing. BNP indicates brain natriuretic peptide; HR, hazard ratio; LGE, late gadolinium enhancement; LVEDVi, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; and NYHA, New York Heart Association.
Significant P values.
Table 6.
Cox Regression Analysis: Cardiac Death—LGE‐Positive Patients versus LGE‐Negative Patients
| HR (95% CI) | P Value | |
|---|---|---|
| Age | 1.03 (1.0–1.06) | 0.015* |
| NYHA >2 | 1.04 (0.50–2.14) | 0.921 |
| BNP, pg/mL | 1.00 (0.99–1.00) | 0.335 |
| LGE presence | 3.00 (1.41–6.42) | 0.005* |
| LVEF | 0.96 (0.94–0.99) | 0.001* |
A P<0.0167 was considered significant after Bonferroni adjustment for multiple testing. BNP indicates brain natriuretic peptide; HR, hazard ratio; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; and NYHA, New York Heart Association.
Significant P values.
Table 7.
Cox Regression Analysis: SCD—LGE‐Positive Patients versus LGE‐Negative Patients
A P<0.0167 was considered significant after Bonferroni adjustment for multiple testing. HR indicates hazard ratio; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; and SCD, sudden cardiac death.
Significant P values.
Figure 1. Kaplan–Meier survival curves for all‐cause, cardiac, and sudden cardiac death.
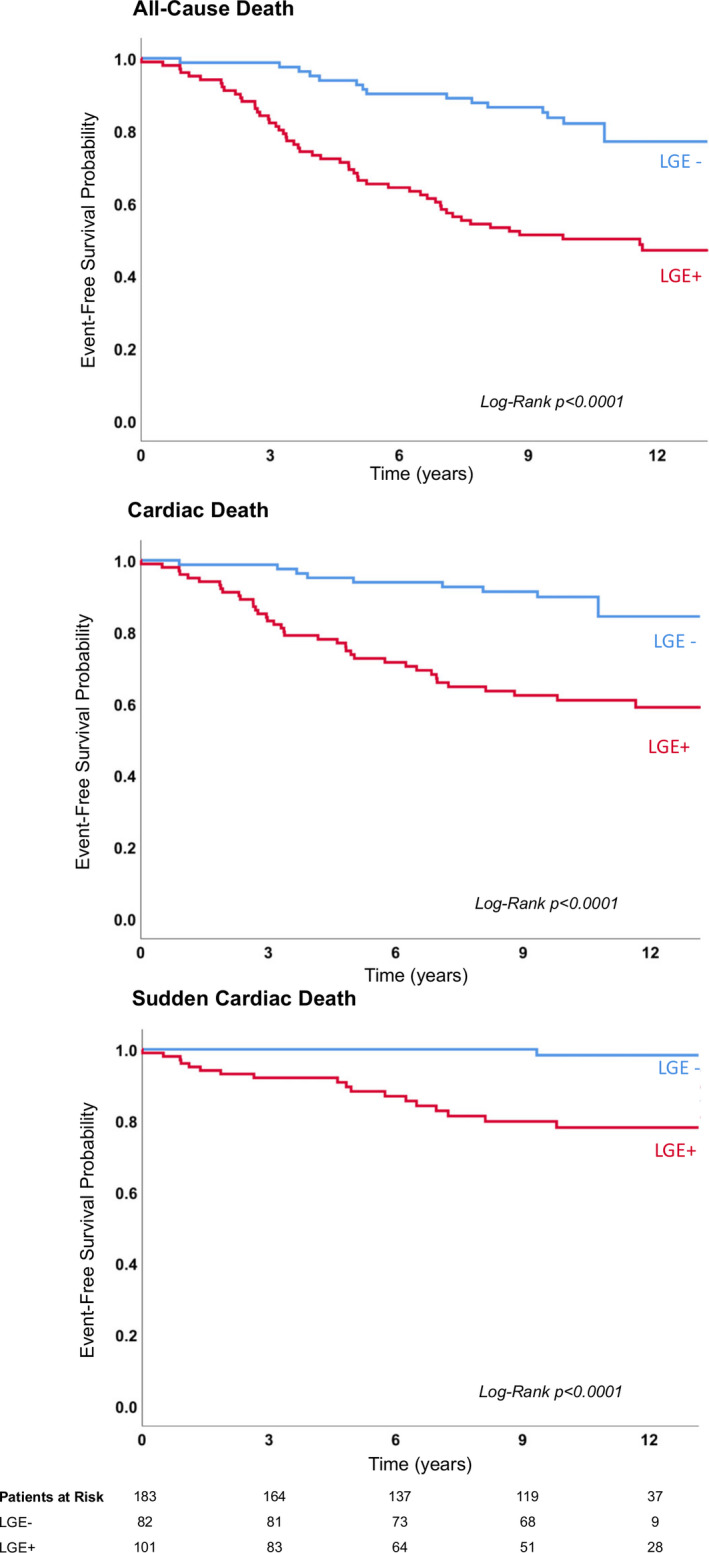
Kaplan–Meier survival curves of patients with biopsy‐proven viral myocarditis divided in all‐cause death (top), cardiac death (middle), and sudden cardiac death (bottom). Note that only a single late gadolinium enhancement‐ (LGE‐) negative patients suffered from sudden cardiac death during this >10‐year follow‐up.
Figure 2. Kaplan–Meier survival curves for all‐cause, cardiac, and sudden cardiac death by presence of LGE and LVEF.
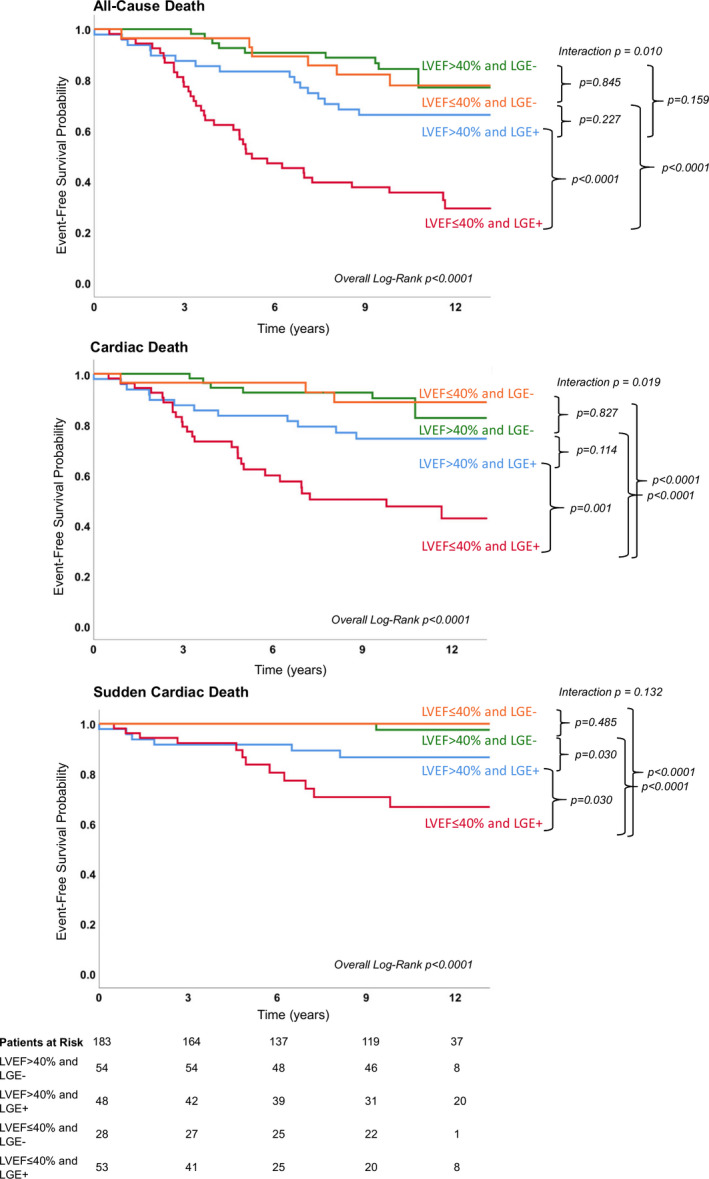
Patients with LVEF ≤40% and LGE absence have a significantly better prognosis than patients with LVEF ≤40% and LGE presence. LGE indicates late gadolinium enhancement; and LVEF, left ventricular ejection fraction.
Detection of Patients at Highest Risk by LGE Parameters
For further risk stratification, we performed a subanalysis exclusively focused on patients who were LGE positive reaching the end point versus patients who were LGE positive with no event (Tables S1–S3). LGE‐CMR predictors for mortality were: (1) higher amount of LGE, (2) (antero‐) septal location, and (3) midwall pattern (all P<0.001). Beside these LGE‐parameters, other parameters such as increased age, New York Heart Association class >II, higher left ventricular end‐diastolic volume, and lower LVEF were predictors of an adverse outcome in patients who were LGE postive. At multivariable analysis, septal LGE was the strongest predictive parameter for all‐cause death (HR, 3.56; 95% CI, 1.18–10.69; P=0.024), cardiac death (HR, 5.38; 95% CI, 1.54–18.85; P=0.009), and SCD (HR, 4.59; 95% CI, 1.38–15.24; P=0.013) (Tables 8, 9, 10). Figure 3 shows a patient with septal LGE and LVEF >40% suffering from SCD during follow‐up.
Table 8.
Cox Regression Analysis for All‐Cause Death—LGE‐Positive Patients Only
| HR (95% CI) | P Value | |
|---|---|---|
| Age | 1.03 (1.0–1.06) | 0.077 |
| NYHA >2 | 0.75 (0.34–1.64) | 0.467 |
| LGE/Myo percentage | 1.06 (1.00–1.11) | 0.045 |
| Septal LGE | 3.56 (1.18–10.69) | 0.024 |
| LVEF | 0.98 (0.96–1.00) | 0.121 |
A P<0.0167 was considered significant after Bonferroni adjustment for multiple testing. HR indicates hazard ratio; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; Myo, myocardium; and NYHA, New York Heart Association.
Table 9.
Cox Regression Analysis for Cardiac Death—LGE‐Positive Patients Only
| HR (95% CI) | P Value | |
|---|---|---|
| NYHA >2 | 1.08 (0.44–2.66) | 0.867 |
| LGE/Myo percentage | 1.03 (0.98–1.08) | 0.283 |
| Septal LGE | 5.38 (1.54–18.85) | 0.009* |
| LVEF | 0.98 (0.96–1.01) | 0.177 |
A P<0.0167 was considered significant after Bonferroni adjustment for multiple testing. HR indicates hazard ratio; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; Myo, myocardium; and NYHA, New York Heart Association.
Significant P values.
Table 10.
Cox Regression Analysis for SCD—LGE‐Positive Patients Only
| HR (95% CI) | P Value | |
|---|---|---|
| Septal LGE | 4.59 (1.38–15.24) | 0.013* |
| LVEF | 0.99 (0.96–1.02) | 0.478 |
A P<0.0167 was considered significant after Bonferroni adjustment for multiple testing. HR indicates hazard ratio; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; and SCD, sudden cardiac death.
Significant P values.
Figure 3. Patient with septal LGE and LVEF >40% who suffered from SCD.
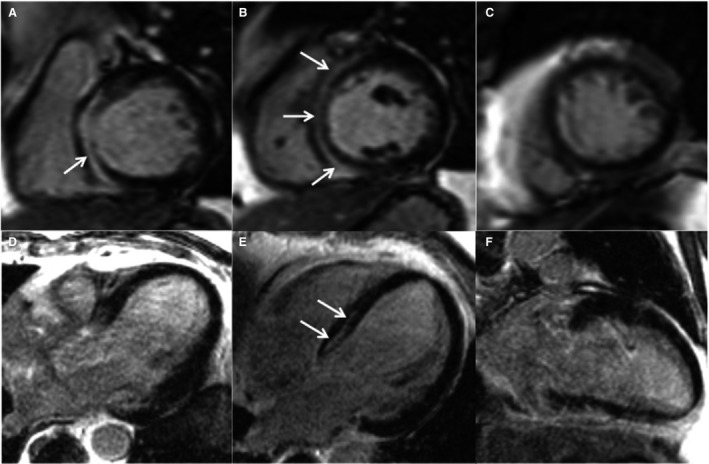
This is a case of a 62‐year‐old man without prior cardiac history who underwent CMR for workup of myocarditis. The patient presented with dyspnea under exertion and atypical angina. CMR revealed a LVEF of 43%. LGE images showed a midwall pattern in the septum (arrows) with an extent of 7.9% (of left ventricular mass). A through C, Short axis views, (D) 3‐chamber view, (E) 4‐chamber view, (F) 2‐chamber view. Biventricular EMB specimen revealed PVB19‐myocarditis. Six years later, this patient suffered from SCD. CMR indicates cardiac magnetic resonance; EMB, endomyocardial biopsy; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; PVB19, parvovirus B19; and SCD, sudden cardiac death.
DISCUSSION
This is the first study evaluating mortality and predictive value of LGE‐CMR parameters in patients with biopsy‐proven viral myocarditis at 10‐year follow‐up. We found a high 10‐year mortality rate (39.3%), mainly attributable to cardiac reasons (27.3%).
These findings underline the unmet need of noninvasive predictors of outcome to identify patients at highest risk for mortality because the type of virus determined by EMB seems not to be sufficient to predict patients' outcomes. We could demonstrate that not only the pure presence or extent of LGE, but specifically a septal or midwall LGE pattern is associated with the highest risk of mortality.
Patient and CMR Characteristics
There were 183 of the 203 patients with biopsy‐proven viral myocarditis available for clinical follow‐up at a median of 10.1 years, yielding a follow‐up rate of 90.1%. Baseline characteristics are displayed in Table 1.
At time of CMR, patients were at median 53‐years‐old, and median LVEF was 44%, comparable to another study. 12 Similar to current literature, parvovirus B19 was the most frequent virus found on EMB (57.4%). 17 LGE showed preponderance for the inferolateral wall location (44.6%) and a linear distribution (52.5%), as previously described. 5 , 12 , 13
Follow‐Up Results and Predictors of Mortality
A high rate of mortality could be observed in this 10‐year long‐term follow‐up study, yielding a mortality rate of 39.3%, corresponding to a doubling of the mortality rate of 19.2% in the 5‐year outcome data. 8 Similar to the 5‐year outcome data, 8 most deaths occurred for cardiac reasons (27.3%), such as SCD (10.9%). Furthermore, the presence of viral genomes was not a sufficient predictor for outcome as previously shown. 18 , 19
Besides LGE, which seems to be the most reliable predictor of outcome, other parameters such as older age, dyspnea New York Heart Association grade III, and increased N‐terminal brain natriuretic peptide levels were also associated with increased mortality, in line with the current literature. 8 , 20 , 21
Stratifying patients by LVEF (dichotomized by a 40% cut‐off) and LGE status, the Kaplan–Meier survival curves show that LGE status can further risk‐stratify patients in the ≤40% LVEF group with regard to all end points, whereas in the >40% LVEF group the effect of LGE is only seen with regard to sudden cardiac death as an end point (Figure 2).
Our findings support results from other studies in large populations of ischemic and nonischemic cardiomyopathies, which have proven additional prognostic value of LGE to the predictive value of a depressed LVEF alone. 9 , 10
Detection of Patients at Highest Risk by LGE Parameters
Despite the strong association of LGE with mortality, it is important to keep in mind that not all patients with LGE suffer adverse events. In fact, recent data suggest that the negative predictive value of a normal CMR is much stronger than the positive predictive value of a LGE‐positive CMR. 11 Thus, additional risk stratification in patients who are LGE positive is desirable. Mahrholdt et al were the first to describe different LGE patterns in patients with viral myocarditis. 22 They described: (1) a subepicardial layer of the lateral wall associated with a more favorable outcome, and (2) a midwall layer of the anteroseptal wall associated with an adverse outcome. Recent studies have confirmed these results in large populations. 12 , 13 Gräni et al demonstrated that a septal and midwall LGE showed strongest associations with adverse events. 12 Aquaro et al concluded that midwall LGE in the anteroseptal region was the best independent CMR predictor of the combined end point of cardiac death, appropriate implantable cardioverter‐defibrillator firing, resuscitated cardiac arrest, and hospitalization for heart failure. 13
We also performed a subanalysis focusing exclusively on patients who were LGE positive suffering from death versus patients who were LGE positive with no event (Tables S1–S3). LGE‐CMR predictors for mortality were: (1) higher amount of LGE, (2) (antero‐) septal location, and (3) midwall pattern (Tables 8, 9, 10, Figure 3). This is not only in line with previous data, 12 , 13 , 22 but expands current knowledge because we (1) included patients with definite biopsy‐proven viral myocarditis, (2) performed an >10‐year long‐term outcome, and (3) did not use a combined but the single hard end point all‐cause death.
As a potential mechanism for adverse outcome, septal LGE might involve the conduction system yielding a substrate for malignant arrhythmias. Assomull et al 23 demonstrated that midwall fibrosis determined by CMR is a predictor of the combined end point of all‐cause death and cardiovascular hospitalization, which is independent of ventricular remodeling. Furthermore, midwall fibrosis by CMR predicts SCD/ventricular tachycardia, and at least some of these patients might present as dilated cardiomyopathy as the end‐stage of a preceding myocarditis. 23
Limitations
Newer CMR mapping techniques (T1 and T2 mapping) were not performed because they were not available at the time of patient enrollment. In addition, one must keep in mind that their value depends on sequence and vendor, and that they still have to prove its prognostic value in larger multicenter studies.
Isolated use of LGE for the diagnosis of myocarditis is not recommended because positivity may indicate necrosis, fibrosis, or edema, but is not specific for inflammation. 24 However, our study aimed to investigate the prognostic value of LGE in patients with biopsy‐proven viral myocarditis. We could demonstrate that LGE as a single imaging component is not only a powerful predictor of adverse events, but also has a good negative predictive value, which is very helpful for risk stratification in the clinical routine.
T2‐weighted MRI was not consistently performed because this technique is problematic on many levels: Susceptibility to arrhythmia and motion, low signal‐to‐noise ratio, impaired image quality, and inconsistent results limit its widespread use. 7 , 24
Unfortunately, follow‐up data on imaging studies (e.g. echo) were available for only a limited number of patients, not allowing robust statistical analysis.
CONCLUSIONS
In patients with biopsy‐proven viral myocarditis, LGE‐CMR allows effective detection of patients at highest risk for death: Midwall and septal LGE patterns were highly associated with death, whereas patients with no LGE (or other LGE patterns) showed a more favorable outcome. Therefore, patients with biopsy‐proven myocarditis demonstrating a midwall and septal LGE pattern should be thoroughly monitored in clinical practice.
Sources of Funding
This work was funded in part by the Robert Bosch Foundation: KKF 13‐2, KKF 15‐5, and KKF 770. This project was supported by the Deutsche Forschungsgemeinschaft Klinische Forschungsgruppe‐KFO‐274 (German Research Foundation; Platelets‐Molecular Mechanisms and Translational Implications) and by the Deutsche Forschungsgemeinschaft Projektnummer 374031971–TRR 240.
Disclosures
None.
Supporting information
Tables S1–S3
Acknowledgment
Open access funding enabled and organized by Projekt DEAL.
(J Am Heart Assoc. 2020;9:e015351 DOI: 10.1161/JAHA.119.015351.)
For Sources of Funding and Disclosures, see page 14.
References
- 1. Cooper LT, Fairweather D. We see only what we look for: imaging cardiac inflammation. Circ Cardiovasc Imaging. 2013;6:165–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fabre A, Sheppard MN. Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart. 2006;92:316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, et al.; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 4. Heymans S, Eriksson U, Lehtonen J, Cooper LT Jr. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol. 2016;68:2348–2364. [DOI] [PubMed] [Google Scholar]
- 5. Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–1258. [DOI] [PubMed] [Google Scholar]
- 6. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, et al.; ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 7. Greulich S, Ferreira VM, Dall'Armellina E, Mahrholdt H. Myocardial inflammation-are we there yet? Curr Cardiovasc Imaging Rep. 2015;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grün S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, Kispert EM, Hill S, Ong P, Klingel K, et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604–1615. [DOI] [PubMed] [Google Scholar]
- 9. Wong TC, Piehler KM, Zareba KM, Lin K, Phrampus A, Patel A, Moon JC, Ugander M, Valeti U, Holtz JE, et al. Myocardial damage detected by late gadolinium enhancement cardiovascular magnetic resonance is associated with subsequent hospitalization for heart failure. J Am Heart Assoc. 2013;2:e000416 DOI: 10.1161/JAHA.113.000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheong BY, Muthupillai R, Wilson JM, Sung A, Huber S, Amin S, Elayda MA, Lee VV, Flamm SD. Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120:2069–2076. [DOI] [PubMed] [Google Scholar]
- 11. Schumm J, Greulich S, Wagner A, Grün S, Ong P, Bentz K, Klingel K, Kandolf R, Bruder O, Schneider S, et al. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J Cardiovasc Magn Reson. 2014;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gräni C, Eichhorn C, Bière L, Murthy VL, Agarwal V, Kaneko K, Cuddy S, Aghayev A, Steigner M, Blankstein R, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70:1964–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, Pepe A, Todiere G, Lanzillo C, Scatteia A, et al.; Cardiac Magnetic Resonance Working Group of the Italian Society of Cardiology . Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J Am Coll Cardiol. 2017;70:1977–1987. [DOI] [PubMed] [Google Scholar]
- 14. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols . Standardized cardiovascular magnetic resonance imaging (CMR) protocols, Society for Cardiovascular Magnetic Resonance: board of trustees Task Force on standardized protocols. J Cardiovasc Magn Reson. 2008;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. [DOI] [PubMed] [Google Scholar]
- 16. American College of Cardiology Foundation Task Force on Expert Consensus Documents , Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-Herold M, Kramer CM, Manning WJ, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55:2614–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper LT, et al. Update on myocarditis. J Am Coll Cardiol. 2012;59:779–792. [DOI] [PubMed] [Google Scholar]
- 18. Greulich S, Kindermann I, Schumm J, Perne A, Birkmeier S, Grün S, Ong P, Schäufele T, Klingel K, Schneider S, et al. Predictors of outcome in patients with parvovirus B19 positive endomyocardial biopsy. Clin Res Cardiol. 2016;105:37–52. [DOI] [PubMed] [Google Scholar]
- 19. Kindermann I, Kindermann M, Kandolf R, Klingel K, Bültmann B, Müller T, Lindinger A, Böhm M. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–648. [DOI] [PubMed] [Google Scholar]
- 20. Ammirati E, Cipriani M, Moro C, Raineri C, Pini D, Sormani P, Mantovani R, Varrenti M, Pedrotti P, Conca C, et al.; Registro Lombardo delle Miocarditi . Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis. Circulation. 2018;138:1088–1099. [DOI] [PubMed] [Google Scholar]
- 21. Caforio AL, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S, Ramondo A, Carturan E, Iliceto S, Thiene G, et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007;28:1326–1333. [DOI] [PubMed] [Google Scholar]
- 22. Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–1590. [DOI] [PubMed] [Google Scholar]
- 23. Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. [DOI] [PubMed] [Google Scholar]
- 24. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


