Abstract
Background
Childhood maltreatment remains a significant public health issue associated with a number of poor health outcomes. This study explores the association between childhood maltreatment and the subsequent development of cardiometabolic disease and all‐cause mortality.
Methods and Results
Using a UK primary care database between January 1, 1995 and December 31, 2018, we conducted a population‐based open retrospective cohort study. We matched 80 657 adult patients with a historic recording of childhood maltreatment or maltreatment‐related concerns (exposed group) to 161 314 unexposed patients. Outcomes of interest were the development of cardiovascular disease, hypertension, type 2 diabetes mellitus, and risk of all‐cause mortality. During the study period there were 243 new diagnoses of cardiovascular disease (incidence rate 8.3 per 10 000 person‐years) in the exposed group compared with 254 in the unexposed group (incidence rate 4.6 per 10 000 person‐years). Following adjustment for key covariates, this translated to an adjusted incidence rate ratio of 1.71 (95% CI 1.42–2.06). Additionally, the exposed group had an increased risk of hypertension (adjusted incidence rate ratio 1.42; 95% CI, 1.26–1.59), type 2 diabetes mellitus (adjusted incidence rate ratio 2.13; 95% CI, 1.86–2.45) and all‐cause mortality (adjusted incidence rate ratio 1.75; 95% CI, 1.52–2.02) during the study period compared with the unexposed group.
Conclusions
Considering the high prevalence of exposure to childhood maltreatment, we have demonstrated the substantial associated burden of preventable cardiometabolic disease. There is a clear need to ensure that public health approaches are implemented to prevent the adverse consequences following exposure to childhood maltreatment.
Keywords: cardiovascular diseases, childhood maltreatment, hypertension, type 2 diabetes mellitus
Subject Categories: Cardiovascular Disease; Diabetes, Type 2; Epidemiology
Nonstandard Abbreviations and Acronyms
- aIRR
adjusted incidence rate ratio
- CVD
cardiovascular disease
- IR
incidence rate
- THIN
The Health Improvement Network
Clinical Perspective
What Is New?
It is unclear whether those who are exposed to maltreatment in childhood have an increased risk of cardiometabolic abnormalities and death.
We assessed the risk of cardiometabolic disease and all‐cause mortality in a large primary care database, comprising 80 657 patients with a history of childhood maltreatment and 161 314 matched controls.
We found those with a history of maltreatment in childhood had a 71%, 42%, 113%, and 75% increased risk of subsequent cardiovascular disease, hypertension, type 2 diabetes mellitus, and all‐cause mortality, respectively.
What Are the Clinical Implications?
Considering the high prevalence of childhood maltreatment globally, these findings may translate into a substantial burden of cardiometabolic disease associated with childhood maltreatment.
Clinicians should be made aware of the disproportionally increased cardiovascular risk in this population, and thus are encouraged to consider early risk‐management interventions.
Childhood maltreatment is defined as any form of physical, sexual, or emotional abuse or neglect experienced by those under the age of 18 years.1 The prevalence is substantial, with 1 in 4 children thought to be affected within the United Kingdom 2 and 1 in 3 globally.1 Exposure to childhood maltreatment and wider household markers of dysfunction has been associated with negative health and social consequences in adulthood, which consequently creates a significant financial burden for the state.3, 4, 5, 6, 7, 8, 9, 10
Childhood maltreatment has been demonstrated to be associated with the subsequent development of cardiometabolic disease (cardiovascular disease [CVD], hypertension, and type 2 diabetes mellitus).11, 12, 13, 14 The currently hypothesized and accepted mechanism for this relationship includes 3 pathways that occur following childhood maltreatment: the adoption of poor lifestyle behaviors (physical inactivity, poor diet, disrupted sleep, substance misuse, and smoking), development of mental and biological ill health because of alteration of the immune, metabolic, neuroendocrine, and autonomic nervous system.15 A recent statement from the American Heart Association15 scientific consensus drew upon available observational data and gave recommendations for imminent research required to further understand this important relationship.
There were numerous key recommendations from the consensus meeting relating to global gaps in the literature on this topic. Some of the current evidence gaps that have yet to be addressed by preceding literature include the following: (1) heterogeneity of definitions concerning childhood adversity (with some studies including information on adverse childhood experiences, which are a broader class of adversity not usually included in the global or UK definition of childhood maltreatment)16, 17; (2) much of the evidence is derived from case–control and cross‐sectional studies, which were often small in size and susceptible to recall bias as information about exposure was noted during adulthood; (3) because of study design there was an inability to account for confounders; and (4) much of the available evidence is derived from populations outside of Europe, which may mean the results are not generalizable to the United Kingdom because of differences in child protection support and healthcare service infrastructure.18
Following this noted limitation in the consensus report relating to geographical scarcity of information, there are still very few cohort studies derived from UK populations. One such cohort study is the British Birth Cohort, which followed up all births in 1 week from March 1958 in England, Scotland, and Wales until the present.19, 20 Participants in this cohort appeared to experience higher levels of adiposity and biomarker inflammation following experiences of bullying and childhood adversity, which the authors stated put them at a high risk of type 2 diabetes mellitus.19, 20 However, this study did not provide risk estimates for the development of cardiometabolic outcomes in later life. Additionally, aside from the recording of neglect at age 7 years, questions relating to childhood adversity were introduced at the 45‐year point, which means that future cohort results are likely to be limited by recall bias.21 An alternative UK cohort study (Avon Longitudinal Study of Parents and Children) also explored the relationship between CVD risk factors and self‐reported abuse at cohort entry in 3612 women.22 However, again recording of childhood maltreatment occurs during adulthood, and the cohort data do not provide outcomes on cardiometabolic end points.
Considering the potential public health burden posed by cardiometabolic disease occurring following exposure to childhood maltreatment, it is important to document the cardiometabolic risk in a UK cohort, taking into consideration important confounding factors. Therefore, we conducted the first UK retrospective cohort study using “The Health Improvement Network” (THIN) data set exploring the association between officially confirmed childhood maltreatment and maltreatment‐related concerns (possible/suspected maltreatment) with the subsequent development of cardiometabolic outcomes and all‐cause mortality.
Methods
Transparency and Openness Statement
The anonymized data that support the findings of this study are available from the senior author (k.nirantharan@bham.ac.uk). However, this will be subject to approval from the data providers (Cegedim).
Study Design, Population, and Data Source
This study is a population‐based, retrospective open cohort study using the THIN database, which consists of 787 general practices. The study period was set between January 1, 1995 and December 31, 2018. An open cohort study allows for patients to enter and exit the study at different time points, with each individual patient only contributing person‐years of follow‐up from the time of cohort entry (index date) to the time they leave the cohort (exit date).
The database is representative of the UK population in terms of demographic structure and prevalence of key comorbidities.23 Symptoms, examinations, and diagnoses in THIN are recorded using a hierarchical clinical coding system called Read codes.24 As entry into the database relies on the use of specific electronic records software (Vision), the number of contributing practices can vary over time. In order to reduce under‐recording of events, general practices were included 12 months following their installment of electronic practice records or from the practice's acceptable mortality recording date.25
Exposure and Outcome Definition
The purpose of this study was to compare exposed (those with a code identifying officially confirmed childhood maltreatment or a maltreatment‐related concern code under the age of 18 years) adult (over the age of 18 years at index date) patients with unexposed patients (those without such codes) and then calculate their risk of developing cardiometabolic disease and all‐cause mortality.
Exposure codes relating to officially confirmed child maltreatment were selected with the assistance of public health clinicians and general practitioners who have expertise in Read code selection. Exposure codes used to define maltreatment‐related concern were adapted from previous research conducted using THIN and consist of codes designed to capture clinical concern relating to suspected or possible maltreatment.26 Patients were included in the exposed group if they had a maltreatment or maltreatment‐related code inserted before the age of 18 years but only were able to enter the cohort once they were the age of 18 years or older.
Outcome codes relating to cardiometabolic disease (defined as CVD [ischemic heart disease, heart failure, peripheral vascular disease and Stroke/Transient ischemic attack, hypertension, and type 2 diabetes mellitus]) are well coded in THIN as they form part of the Quality Outcomes Framework27 (performance indicators linked to general practice payments in the United Kingdom). Hence, Read code lists relating to cardiometabolic disease were largely based on Quality and Outcomes Framework recommended codes and expert opinion from general practitioners with expertise in Cardiometabolic Read code selection.
Read code lists relating to exposure terms and outcomes are provided (Data S1).
Selection of Unexposed Group
Each exposed patient was matched with up to 2 unexposed control patients, who had no documented Read code relating to the exposure. Controls were taken from other general practices within the database and were matched by age at index date (±1 year) and sex. Matching was conducted in the selection of the unexposed group only and not as part of any analytical approaches.
Follow‐Up Period
The index date for those in the exposed group was the date at which they reached 18 years of age, a year after registration with the general practice or the date the general practice was eligible to contribute to the database, whichever was the latest. To mitigate immortality time bias,28 the same index date was assigned to the corresponding unexposed patient. The follow‐up period for each patient was from the index date until the exit date. Exit date is defined as the earliest of the following dates: study end date, last date of data collection from a given general practice, date patient transferred from general practice, date of death, or date the outcome of interest occurred.
Covariates
Covariates relating to the development of the outcomes of interest such as body mass index, smoking status, the use of lipid‐lowering drugs, Charlson comorbidity index,29 and Townsend deprivation score30 were extracted in the baseline data.
Statistical Analysis
STATA version 15.1 MP/4 software (Statacorp 2017) was used to conduct all analysis.
Categorical baseline data were described using proportions, and continuous data were described using means or median with standard deviations or interquartile range. Missing data are highlighted in relevant baseline characteristic tables. Where there were missing data in our covariates, they were treated as a separate missing category and included in the final analysis.
In order to calculate an incidence rate ([IR] per 10 000 person‐years) for each of the outcomes of interest, patients with the same pre‐existing illness (defined as a Cardiometabolic Read code) were excluded to ensure the IR reflected outcomes that occurred following cohort entry. Poisson regression offsetting for log(person‐years) of follow‐up specified as an exposure was then used to calculate an incidence rate ratio (IRR) for each outcome of interest during the study period. Alternative models such as the negative binominal Poisson model were used to examine the possible effects of dispersion. However, the results were identical, suggesting absence of overdispersion. Therefore, a Poisson model was utilized. The mathematical model used for the Poisson model was the following:31, 32
where y is dependent variable; E(y), Expected count value; x, independent variable; b0, b1 etc., Regression coefficients; and t, exposure time.
Following adjustment for the covariates, we calculated and present an adjusted IRR (aIRR). All CVD outcomes were adjusted for body mass index, age, sex, smoking, diabetes mellitus status, lipid‐lowering drug use, hypertension, and Townsend deprivation score at baseline. The hypertension outcome was adjusted for these factors excluding hypertension and likewise for type 2 diabetes mellitus outcome, we excluded diabetes mellitus status from the covariates. Mortality was adjusted for the same factors as CVD in addition to Charlson comorbidity index.29 In all of the Poisson models, the year of registration was also included in the multivariable model to account for any changes in recording practice over time.26 In all models relating to the main analyses, the adjusted model showed a better fit than the unadjusted model (higher pseudo R2 and likelihood ratios). Further details relating to the covariate significance and fit criterion can be seen in Table S1.
Decisions regarding covariate adjustment were influenced by previous research examining cardiometabolic outcomes.14, 33, 34, 35 Previous literature has stated the importance of taking into consideration the confounding role of age, sex, and deprivation when exploring the relationship between childhood maltreatment and cardiometabolic disease, hence their inclusion in our model.14 Other covariates included in our model have been previously suggested as mediators in this relationship.36 However, because of the young age of this cohort at cohort entry, it is clear that many of these covariates are likely to occur after index date. We, however, adjusted for the presence of such covariates at baseline because it was not possible to determine whether the exposure of interest or the covariates occurred first. IRRs are presented with 95% CIs where statistical significance was set at P<0.05.
A sensitivity analysis was conducted to explore whether findings differed when only looking at officially confirmed maltreatment codes.
Ethical Approval
Anonymized data provided by the data provider to the University of Birmingham were used throughout the study. Studies using the THIN database have had initial ethical approval from the National Health Service South‐East Multicentre Research Ethics Committee, subject to prior independent scientific review. The Scientific Review Committee (IQVIA) approved the study protocol (Reference Number: SRC18THIN034) before its undertaking.
Results
We identified 80 657 exposed patients who were matched to 161 314 unexposed patients during the study period, who on average were followed up for 2.3 years. The median follow‐up was similar in the exposed group (2.3 years) compared with the unexposed group (2.2 years). Mean age (23 years) and sex proportions (42% males) were similar in the cohort because of matching. There were substantial missing data for body mass index (49%). There was a higher proportion of smokers in the exposed cohort (38%) compared with the unexposed cohort (20%). There was also a greater proportion of socio‐economic deprivation, comorbidity, and pre‐existing cardiometabolic disease at baseline in the exposed population compared with the unexposed. Baseline characteristics are described in Table 1.
Table 1.
Baseline Characteristics of Those Exposed and Unexposed to Childhood Maltreatment
| Baseline Characteristics (SD, IQR, or Percentage) | ||
|---|---|---|
| Exposed Group | Unexposed Group | |
| Number of patients | 80 657 | 161 314 |
| Median follow‐up period (person y) | 2.3 (IQR 0.9–5.1) | 2.2 (IQR 0.7–4.9) |
| Age at cohort entry (y) | 23.3 (SD 7.3) | 23.4 (SD 7.2) |
| Age when maltreatment occurred (y) | 9.6 (SD 5.2) | … |
| Sex; Male (%) | 33 614 (41.7%) | 67 228 (41.7%) |
| Body mass index | ||
| <25 kg/m2 | 24 091 (29.9%) | 56 694 (35.2%) |
| 25–30 kg/m2 | 7642 (9.5%) | 18 659 (11.6%) |
| >30 kg/m2 | 6322 (7.8%) | 11 132 (6.9%) |
| Not available | 42 602 (52.9%) | 74 829 (46.4%) |
| Smoking status | ||
| Current smoker | 30 462 (37.8%) | 31 517 (19.5%) |
| Noncurrent/not available | 50 195 (62.2%) | 129 797 (80.5%) |
| Townsend index | ||
| (Least deprived) 1 | 6296 (7.8%) | 27 027 (16.8%) |
| 2 | 7295 (9.8%) | 25 086 (15.6%) |
| 3 | 13 469 (16.7%) | 29 771 (18.5%) |
| 4 | 19 116 (23.7%) | 29 857 (18.5%) |
| 5 | 19 860 (24.6%) | 22 849 (14.2%) |
| Not available | 13 991 (17.4%) | 26 725 (16.6%) |
| Charlson comorbidity index | ||
| (Least comorbid) 0 | 59 352 (73.6%) | 130 956 (81.2%) |
| 1 | 19 975 (24.8%) | 28 735 (17.8%) |
| 2 | 888 (1.1%) | 1178 (0.7%) |
| 3 | 276 (0.3%) | 289 (0.2%) |
| 4 and above | 166 (0.2%) | 156 (0.1%) |
| Lipid‐lowering drug use | 309 (0.4%) | 777 (0.5%) |
| Pre‐existing cardiometabolic disease | ||
| All cardiovascular disease | 395 (0.5%) | 345 (0.2%) |
| Ischemic heart disease | 115 (0.1%) | 115 (0.1%) |
| Heart failure | 43 (0.1%) | 50 (0.0%) |
| Stroke/transient ischemic attack | 214 (0.3%) | 160 (0.1%) |
| Peripheral vascular disease | 56 (0.1%) | 51 (0.0%) |
| Hypertension | 633 (0.8%) | 1111 (0.7%) |
| Diabetes mellitus | 556 (0.7%) | 631 (0.4%) |
IQR indicates interquartile range.
There were 243 new CVD events in the exposed group (0.3%, IR 8.3 per 10 000 person‐years) compared with 254 (0.2%, IR 4.6 per 10 000 person‐years) in the unexposed group. This translated to an increased aIRR of 1.71 (95% CI, 1.42–2.06). When broken down by type of CVD event, this risk persisted significantly for ischemic heart disease (aIRR 1.57; 95% CI, 1.16–2.13) and Stroke/transient ischemic attack (aIRR 2.15; 95% CI, 1.66–2.81). Though the risk was raised for heart failure and peripheral vascular disease, this did not reach statistical significance (heart failure: aIRR 1.51; 95% CI, 0.92–2.51; peripheral vascular disease: aIRR 1.54; 95% CI, 0.87–2.73).
There were 537 (0.7%, IR 18.6 per 10 000 person‐years) and 504 (0.6%, IR 17.3 per 10 000 person‐years) new diagnoses of hypertension and type 2 diabetes mellitus in the exposed group compared with 780 (0.5%, IR 14.3 per 10 000 person‐years) and 414 (0.3%, 7.6 per 10 000 person‐years), respectively, in the unexposed group. This translated into an increased risk of developing hypertension (aIRR 1.42; 95% CI, 1.26–1.59) and type 2 diabetes mellitus (aIRR 2.13; 95% CI, 1.86–2.45) in the exposed group when compared with the unexposed group. Further details can be seen in Table 2 and Figure.
Table 2.
Risk of Developing Cardiometabolic Disease in Those Exposed and Unexposed to Childhood Maltreatment
| All Cardiovascular Disease | Ischemic Heart Disease | Stroke/Transient Ischemic Attack | Heart Failure | Peripheral Vascular Disease | Hypertension | Type 2 Diabetes Mellitus | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | |
| Number of patients | 80 262 | 160 969 | 80 542 | 161 199 | 80 443 | 161 154 | 60 614 | 161 264 | 80 601 | 161 263 | 80 024 | 160 203 | 80 101 | 160 683 |
| Numbers of outcomes | 243 | 254 | 94 | 100 | 139 | 117 | 30 | 40 | 30 | 27 | 537 | 780 | 504 | 414 |
| Person‐y | 291928 | 549 812 | 293 452 | 551 136 | 293 030 | 551 051 | 294 004 | 551 687 | 293 954 | 551 707 | 289 125 | 543 743 | 290 782 | 548 301 |
| Incidence rate (per 10 000 person‐ y) | 8.3 | 4.6 | 3.2 | 1.8 | 4.7 | 2.1 | 1.0 | 0.7 | 1.0 | 0.5 | 18.6 | 14.3 | 17.3 | 7.6 |
| Incidence rate ratio (95% CI)a | 1.80 (1.51–2.15) | 1.77 (1.33–2.34) | 2.23 (1.75–2.86) | 1.41 (0.88–2.26) | 2.09 (1.24–3.51) | 1.29 (1.16–1.45) | 2.30 (2.02–2.61) | |||||||
| P‐value | <0.001 | <0.001 | <0.001 | 0.157 | 0.006 | <0.001 | <0.001 | |||||||
| Adjusted incidence rate ratio (95% CIs)b , c | 1.71 (1.42–2.06) | 1.57 (1.16–2.13) | 2.15 (1.66–2.81) | 1.51 (0.92–2.51) | 1.54 (0.87–2.73) | 1.42 (1.26–1.59) | 2.13 (1.86–2.45) | |||||||
| P‐value | <0.001 | 0.003 | <0.001 | 0.106 | 0.135 | <0.001 | <0.001 | |||||||
Unadjusted incidence rate ratio.
All cardiovascular disease, ischemic heart disease, stroke/transient ischemic attack, heart failure, and peripheral vascular disease outcomes were adjusted for body mass index, age, sex, smoking, diabetes mellitus status, lipid‐lowering drug use, hypertension, and Townsend deprivation score at baseline as well as year of registration into the database. The hypertension outcome was adjusted for these factors excluding hypertension. The type 2 diabetes mellitus outcome was adjusted for these factors excluding hypertension and diabetes mellitus status.
The estimate associated with year of registration in the multivariable regression (adjusted incidence rate ratio; 95% CI, P‐value): All cardiovascular disease (0.99; 0.99–1.00, 0.014), ischemic heart disease (0.99; 0.98–1.00, 0.136), stroke/transient ischemic attack (0.99; .98–1.00, 0.197), heart failure (0.99; 0.98–1.01, 0.320), peripheral vascular disease (1.00; 0.98–1.02, 0.996), hypertension (0.99;0.99–0.99, <0.001), and type 2 diabetes mellitus (1.00; 1.00–1.01, 0.499).
Figure 1. The risk of developing cardiometabolic disease and all‐cause mortality in those exposed and unexposed to childhood maltreatment.
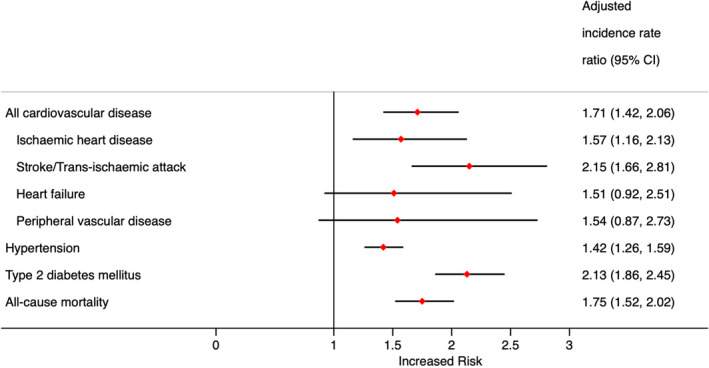
When exploring the risk of all‐cause mortality, during the study period 501 patients in the exposed group had died (0.6%, IR 17.4 per 10 000 person‐years) compared with 452 in the unexposed cohort (0.3%, IR 8.3 per 10 000 person‐years). This translated into 75% increased risk for mortality (aIRR 1.75; 95% CI, 1.52–2.02). Further details can be seen in Table 3.
Table 3.
Risk of Mortality in Those Exposed and Unexposed to Childhood Maltreatment
| Exposed | Unexposed | |
|---|---|---|
| Number of patients | 80 657 | 161 314 |
| Numbers of outcomes | 501 | 452 |
| Person‐y | 288 757 | 545 808 |
| Incidence rate (per 10 000 person‐y) | 17.4 | 8.3 |
| Incidence rate ratio (95% CIs)a | 2.10 (1.84–2.48) | |
| P‐value | <0.001 | |
| Adjusted incidence rate ratio (95% CIs)b , c | 1.75 (1.52–2.02) | |
| P‐value | <0.001 | |
Unadjusted incidence rate ratio.
Adjusted for body mass index, age, sex, smoking status, diabetes mellitus status, lipid‐lowering drug use, hypertension, Charlson comorbidity score, and Townsend deprivation score, as well as year of registration into the database.
The estimate associated with year of registration in the multivariable regression (adjusted incidence rate ratio; 95% CI, P‐value): (0.99; 0.99–1.00, 0.006).
We identified within the total cohort that 22 078 (27.3% of total exposed cohort) patients had confirmatory codes relating to childhood maltreatment. These patients were matched with 44 156 unexposed patients (27.3% of the total unexposed cohort). The cohort details are described in Table S2. The average age at index date was higher than the combined cohort (27 years old). When comparing confirmed exposed cases only with their unexposed controls, the risk of developing the outcomes of interest (Tables S3 and S4) persisted; CVD aIRR 1.77 (95% CI, 1.36–2.30), hypertension aIRR 1.60 (95% CI, 1.36–1.87), type 2 diabetes mellitus aIRR 2.07 (1.68–2.56) and all‐cause mortality (aIRR 1.58; 95% CI, 1.27–1.96).
Discussion
To our knowledge this was the first study using UK primary care data to explore the relationship between childhood maltreatment and the subsequent development of cardiometabolic disease and all‐cause mortality. The main analysis found an increased risk of developing combined types of CVD and hypertension as well as a doubling of risk of developing type 2 diabetes mellitus. Additionally, we found an increased risk of all‐cause mortality in this cohort. When isolating to only patients who had a confirmed code of maltreatment, this described risk persisted across all outcomes.
Our study adds to the global literature describing a positive relationship between the development of cardiometabolic disease following exposure to childhood maltreatment,14, 15 particularly expanding on these findings with data taken from a large UK cohort. As our exposure and outcome definition is different from previously reported data,11, 12, 13, 14 it is difficult to make direct comparisons of our demonstrated IR with other data sets. However, of particular note where there was a previous discrepancy in literature describing the effect size between exposure to childhood maltreatment and subsequent hypertension diagnosis, we have demonstrated a positive association.14, 37, 38, 39
Interestingly, although there were missing data at baseline for covariates, we did notice some significant differences between the exposed and unexposed groups. When considering smoking rates at baseline, the unexposed group current smoking prevalence is considerably higher than the exposed group. This is in line with current literature, which suggests that individuals take on certain harmful coping mechanisms such as smoking in response to high levels of distress, and this may be a contributing but preventable factor mediating the relationship between childhood maltreatment and cardiometabolic disease.40
These findings are of particular note in the United Kingdom, where there is an increasing burden of morbidity relating to cardiometabolic disease.41, 42 Considering the estimated prevalence of childhood maltreatment within the United Kingdom (potentially 1 in 4 children being affected),2 this could suggest a significant proportion of the cardiometabolic disease burden may be attributable to maltreatment. Therefore, there is a clear public health message that requires a population‐based approach to not only prevent childhood maltreatment but also the negative consequences as a result of it. There is a push by academic bodies and the UK Government to improve evidence in the area of early‐years intervention to prevent these negative consequences.43, 44 Specifically, focusing on cardiometabolic disease prevention using holistic and family‐oriented‐based programs has shown great promise in the reduction of risk factors that may mediate the pathway between maltreatment and disease.45, 46 Additionally, considering the increased smoking risk in this group that may mediate the relationship, evidence‐based school‐based or family‐based smoking cessation programs may need to be targeted at this high‐risk group.47
Additionally, our results play an important role in the global context of the literature. Current global evidence has been limited by factors relating to the retrospective nature of case ascertainment, limited sample size, and inability to account for confounders, which we have taken into consideration in the design of our study.14, 15 We hope these findings provide further evidence for the association and the need for public health approaches to tackle the burden of cardiometabolic disease associated with childhood maltreatment.
However, the results of this study must be considered in light of its limitations. The use of electronic care records relies upon the accuracy of imputation of codes by the healthcare professionals contributing to the data set. Although we believe that recording of cardiometabolic outcomes may be largely accurate, there have yet to be any studies validating recording of childhood maltreatment that may introduce a misclassification bias into our exposure selection.48 This may mean that the unexposed group may include patients who have experienced childhood maltreatment, which may in fact underestimate the effect size seen in this study.
It has been shown from previous literature that the recording of childhood maltreatment in primary care records has improved over time.26 This may mean that the potential for misclassification bias is greater in the earlier years of the study. However, in our study we have tried to mitigate for the change in documentation rates over time by adjusting for year of registration. Interestingly, effect of year of registration played little role in the overall effect size (Tables 2 and 3 footnotes).
Another similar important consideration is that if maltreatment was recorded by the general practitioner, it could mean that the maltreatment was particularly severe. In order to mitigate this, we have utilized the maltreatment‐related code lists to identify other factors relating to maltreatment that could identify children who may be at risk of childhood maltreatment. Also, in this study, because of nongranularity of the exposure Read codes, we were unable to examine outcomes in the subtypes of abuse or of differing levels of severity.
In conclusion, our study demonstrated an increased risk of developing cardiometabolic disease and all‐cause mortality following childhood maltreatment. This highlights the need for public health approaches to both encourage the prevention of childhood maltreatment and for reduction of risk factors responsible for cardiometabolic disease, which may increase as a result of maltreatment.
Sources of Funding
None.
Disclosures
None.
Supporting information
Data S1 Tables S1–S4
Acknowledgments
This study contributed to the PhD thesis for the main author JSC. JSC, TT, KG, JT, SB, and KN were responsible for initial conception of the study. JSC, KG, and TT were responsible for data extraction, analysis, and first draft of the manuscript. The final manuscript was authorized by all the authors with JT providing expert knowledge on childhood maltreatment and SB with KN providing methodological expertise.
(J Am Heart Assoc. 2020;9:e015855 DOI: 10.1161/JAHA.119.015855.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.015855
For Sources of Funding and Disclosures, see page 8.
References
- 1. World Health Organization . Violence info—child maltreatment. 2017. Available at: http://apps.who.int/violence-info/child-maltreatment/. Accessed July 29, 2019.
- 2. Radford L, Corral S, Bradley C, Fisher H, Bassett C, Howat N, Collishaw S. Child abuse and neglect in the UK today. 2011.
- 3. Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton Jones L, Dunne M. The effect of multiple adverse childhood experiences on health: a systematic review and meta‐analysis. Lancet Public Health. 2017;e356–e366. [DOI] [PubMed] [Google Scholar]
- 4. Gilbert R, Kemp A, Thoburn J, Sidebotham P, Radford L, Glaser D, Macmillan HL. Recognising and responding to child maltreatment. Lancet. 2009;167–180. [DOI] [PubMed] [Google Scholar]
- 5. Bellis MA, Hughes K, Ford K, Ramos Rodriguez G, Sethi D, Passmore J. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta‐analysis. Lancet Public Health. 2019;e517–e528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chandan JS, Thomas T, Gokhale KM, Bandyopadhyay S, Taylor J, Nirantharakumar K. The burden of mental ill health associated with childhood maltreatment in the UK, using The Health Improvement Network database: a population‐based retrospective cohort study. Lancet Psychiatry. 2019;926–934. [DOI] [PubMed] [Google Scholar]
- 7. Chandan JS, Thomas T, Bradbury‐Jones C, Taylor J, Bandyopadhyay S, Nirantharakumar K. Risk of cardiometabolic disease and all‐cause mortality in female survivors of domestic abuse. J Am Heart Assoc. 2020;e014580 DOI: 10.1161/JAHA.119.014580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chandan JS, Thomas T, Bradbury‐Jones C, Russell R, Bandyopadhyay S, Nirantharakumar K, Taylor J. Female survivors of intimate partner violence and risk of depression, anxiety and serious mental illness. Br J Psychiatry. 2019;1–6. [DOI] [PubMed] [Google Scholar]
- 9. Chandan JS, Thomas T, Bradbury‐Jones C, Taylor J, Bandyopadhyay S, Nirantharakumar K. Intimate partner violence and temporomandibular joint disorder. J Dent. 2019;98–100. [DOI] [PubMed] [Google Scholar]
- 10. Chandan JS, Thomas T, Raza K, Bradbury‐Jones C, Taylor J, Bandyopadhyay S, Nirantharakumar K. Intimate partner violence and the risk of developing fibromyalgia and chronic fatigue syndrome. J Interpers Violence. 2019;88. [DOI] [PubMed] [Google Scholar]
- 11. Su S, Jimenez MP, Roberts CTF, Loucks EB. The role of adverse childhood experiences in cardiovascular disease risk: a review with emphasis on plausible mechanisms. Curr Cardiol Rep. 2015;17:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huffhines L, Noser A, Patton SR. The link between adverse childhood experiences and diabetes. Curr Diab Rep. 2016;54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rich‐Edwards JW, Spiegelman D, Lividoti Hibert EN, Jun H‐J, Todd TJ, Kawachi I, Wright RJ. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am J Prev Med. 2010;529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Basu A, McLaughlin KA, Misra S, Koenen KC. Childhood maltreatment and health impact: the examples of cardiovascular disease and type 2 diabetes mellitus in adults. Clin Psychol Sci Pract. 2017;125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suglia SF, Koenen KC, Boynton‐Jarrett R, Chan PS, Clark CJ, Danese A, Faith MS, Goldstein BI, Hayman LL, Isasi CR, et al. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American Heart Association. Circulation. 2018;e15–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization (WHO) . Child maltreatment. 2017.
- 17. HM Government . Working Together to Safeguard Children: a guide to inter‐agency working to safeguard and promote the welfare of children. 2018. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/722305/Working_Together_to_Safeguard_Children_‐_Guide.pdf. Accessed July 11, 2018.
- 18. Munro ER, Manful E. Safeguarding children: a comparison of England's data with that of Australia, Norway and the United States. 2012. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/183946/DFE-RR198.pdf. Accessed August 2, 2019.
- 19. Takizawa R, Danese A, Maughan B, Arseneault L. Bullying victimization in childhood predicts inflammation and obesity at mid‐life: a five‐decade birth cohort study. Psychol Med. 2015;2705–2715. [DOI] [PubMed] [Google Scholar]
- 20. Thomas C, Hyppönen E, Power C. Obesity and type 2 diabetes risk in midadult life: the role of childhood adversity. Pediatrics. 2008;e1240–e1249. [DOI] [PubMed] [Google Scholar]
- 21. Power C, Pinto Pereira SM, Li L. Childhood maltreatment and BMI trajectories to mid‐adult life: follow‐up to age 50 y in a british birth. Cohort. 2015;e0119985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson EL, Fraser A, Caleyachetty R, Hardy R, Lawlor DA, Howe LD. Associations of adversity in childhood and risk factors for cardiovascular disease in mid‐adulthood. Child Abuse Negl. 2018;138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;251–255. [DOI] [PubMed] [Google Scholar]
- 24. Booth N. What are the Read Codes? Health Libr Rev. 1994;177–182. [DOI] [PubMed] [Google Scholar]
- 25. Maguire A, Blak BT, Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf. 2009;76–83. [DOI] [PubMed] [Google Scholar]
- 26. Woodman J, Freemantle N, Allister J, de Lusignan S, Gilbert R, Petersen I. Variation in recorded child maltreatment concerns in UK primary care records: a cohort study using The Health Improvement Network (THIN) database. PLoS One. 2012;e49808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. NHS Digital . Quality and Outcomes Framework (QOF) business rules v42 2019‐2020 baseline release—NHS Digital. 2019. Available at: https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-collections/quality-and-outcomes-framework-qof/quality-and-outcome-framework-qof-business-rules/quality-and-outcomes-framework-qof-business-rules-v42-2019-2020-baseline-releas. Accessed July 31, 2019.
- 28. Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;b5087. [DOI] [PubMed] [Google Scholar]
- 29. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;373–383. [DOI] [PubMed] [Google Scholar]
- 30. Townsend P, Phillimore P, Beattie A. Health and deprivation: inequality and the North. Routledge; 1988.
- 31. MBAskool.com . Offset definition|Statistics Dictionary|MBA Skool‐Study.Learn.Share. Available at: https://www.mbaskool.com/business-concepts/statistics/7554-offset.html. Accessed March 21, 2020.
- 32. Casella G, Berger RL. Statistical Inference, 2nd ed. Pacific Grove, CA: Duxbury; 2002. [Google Scholar]
- 33. Tracy A, Subramanian A, Adderley NJ, Cockwell P, Ferro C, Ball S, Harper L, Nirantharakumar K. Cardiovascular, thromboembolic and renal outcomes in IgA vasculitis (Henoch‐Schönlein purpura): a retrospective cohort study using routinely collected primary care data. Ann Rheum Dis. 2019;261–269. [DOI] [PubMed] [Google Scholar]
- 34. Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, Nirantharakumar K. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol. 2017;1429–1437. [DOI] [PubMed] [Google Scholar]
- 35. Chandan JS, Thomas T, Lee S, Marshall T, Willis B, Nirantharakumar K, Gill P. The association between idiopathic thrombocytopenic purpura and cardiovascular disease: a retrospective cohort study. J Thromb Haemost. 2018;474–480. [DOI] [PubMed] [Google Scholar]
- 36. Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease. Circulation. 2004;1761–1766. [DOI] [PubMed] [Google Scholar]
- 37. Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long‐term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta‐analysis. PLoS Med. 2012;e1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suglia SF, Clark CJ, Boynton‐Jarrett R, Kressin NR, Koenen KC. Child maltreatment and hypertension in young adulthood. BMC Public Health. 2014;1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stein DJ, Scott K, Haro Abad JM, Aguilar‐Gaxiola S, Alonso J, Angermeyer M, Deytteneare K, De Girolamo G, Iwata N, Posada‐Villa J, et al. Early childhood adversity and later hypertension: data from the World Mental Health Survey. Ann Clin Psychiatry 2010;19–28. [PMC free article] [PubMed] [Google Scholar]
- 40. Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, Giovino GA. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;1652. [DOI] [PubMed] [Google Scholar]
- 41. Sharma M, Nazareth I, Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open. 2016;e010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bhatnagar P, Wickramasinghe K, Wilkins E, Townsend N. Trends in the epidemiology of cardiovascular disease in the UK. Heart. 2016;1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. UK Government . Evidence‐based early years intervention—Science and Technology Committee—House of Commons. 2018. Available at: https://publications.parliament.uk/pa/cm201719/cmselect/cmsctech/506/50602.htm. Accessed March 9, 2019.
- 44. NICE . Recommendations for research|Child abuse and neglect|Guidance. 2017. Available at: https://www.nice.org.uk/guidance/ng76/chapter/Recommendations-for-research. Accessed April 8, 2019. [Google Scholar]
- 45. Miller GE, Brody GH, Yu T, Chen E. A family‐oriented psychosocial intervention reduces inflammation in low‐SES African American youth. Proc Natl Acad Sci USA. 2014;11287–11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Campbell F, Conti G, Heckman JJ, Moon SH, Pinto R, Pungello E, Pan Y. Early childhood investments substantially boost adult health. Science. 2014;1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Das JK, Salam RA, Arshad A, Finkelstein Y, Bhutta ZA. Interventions for adolescent substance abuse: an overview of systematic reviews. J Adolesc Health. 2016;S61–S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McBrien KA, Souri S, Symonds NE, Rouhi A, Lethebe BC, Williamson TS, Garies S, Birtwhistle R, Quan H, Fabreau GE, et al. Identification of validated case definitions for medical conditions used in primary care electronic medical record databases: a systematic review. J Am Med Informatics Assoc. 2018;1567–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Tables S1–S4


