Abstract
Background
Identification of patients with stable coronary heart disease who are at significant residual risk could be helpful for targeted prevention. Our aim was to determine the prognostic value of the recently introduced ceramide‐ and phospholipid‐based risk score, the Cardiovascular Event Risk Test (CERT2), in patients with stable coronary heart disease on optimal medical therapy and to identify biological processes that contribute to the CERT2 score.
Methods and Results
Plasma samples (n=11 222) obtained from the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial were analyzed using a tandem liquid chromatography‐mass spectrometry method. STABILITY was a trial in patients with stable coronary heart disease randomized to the lipoprotein‐associated phospholipase A2 inhibitor darapladib or placebo on optimized medical therapy at baseline, with a median follow‐up of 3.7 years. Hazard ratios per SD for the CERT2 risk score were 1.32 (95% CI, 1.25–1.39) for major adverse cardiovascular event, 1.47 (95% CI, 1.35–1.59) for cardiovascular death, 1.32 (95% CI, 1.16–1.49) for stroke, 1.23 (95% CI, 1.14–1.33) for myocardial infarction, and 1.56 (95% CI, 1.39–1.76) for hospitalization due to heart failure, when adjusted for traditional cardiovascular risk factors. CERT2 showed correlation (P<0.001, r>0.2) with inflammatory markers high‐sensitivity C‐reactive protein, interleukin 6, the heart failure marker N‐terminal pro‐B‐type natriuretic peptide, and low‐density lipoprotein cholesterol. After also adjusting for levels of other prognostic biomarkers, the CERT2 score was still independently related to the risk of cardiovascular death but not to nonfatal events.
Conclusions
The CERT2 risk score can detect residual risk in patients with stable coronary heart disease and is associated with biomarkers indicating inflammation, myocardial necrosis, myocardial dysfunction, renal dysfunction, and dyslipidemia.
REGISTRATION
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00799903.
Keywords: biomarker, cardiovascular, CERT2, inflammation, lipid, risk
Subject Categories: Prognosis, Lipids and Cholesterol, Risk Factors, Secondary Prevention, Biomarkers
Nonstandard Abbreviations and Acronyms
- CAD
coronary artery disease
- CHD
coronary heart disease
- DM
diabetes mellitus
- HHF
hospitalization for heart failure
- HR
hazard ratio
- hs‐CRP
high‐sensitivity C‐reactive protein
- hs‐TnT
high‐sensitivity troponin T
- IL‐6
interleukin 6
- LDL‐C
low‐density lipoprotein cholesterol
- MI
myocardial infarction
- NI
negative ionization
- NT‐proBNP
N‐terminal pro‐B‐type natriuretic peptide
- PI
positive ionization
- STABILITY
Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy
Clinical Perspective
What Is New?
The Cardiovascular Event Risk Test (CERT2) risk score can detect residual risk in patients with stable coronary heart disease on optimized medical therapy and is associated with inflammation, myocardial necrosis, myocardial dysfunction, renal dysfunction, and dyslipidemia.
CERT2 showed robust performance in a study population covering various geographical locations worldwide.
What Are the Clinical Implications?
The CERT2 risk score can be considered as a risk indicator in patients with stable coronary heart disease and it can be used in evaluating residual risk in patients taking guideline‐recommended optimal medical therapy.
CERT2 can be used to detect both lipid and inflammatory residual risk in patients with stable coronary heart disease.
Distinct ceramide lipid species either alone or combined in a score have been shown to be associated with cardiovascular mortality both in primary and secondary prevention.1, 2 Recently, the ceramide score prognostic value was further improved by adding omega‐3 fatty acid containing phospholipids into the risk algorithm.3 It is not entirely clear why these lipid molecules, in several cohorts, have appeared to be significantly better prognostic markers than hs‐CRP (high‐sensitivity C‐reactive protein) and low‐density lipoprotein cholesterol (LDL‐C).1, 2, 3 It is known, however, that ceramides play a central role in controlling apoptosis.4 In addition, ceramides have a bioactive role in inflammatory signaling, where there seems to be a vicious cycle between several cytokines and ceramides that maintain each other's biosynthesis, leading potentially to harmful chronic inflammation.5, 6 Furthermore, there is much evidence that certain ceramide species associate tightly with insulin resistance and diabetes mellitus (DM) risk.7, 8 Recently, sphingolipids, including ceramides, have been shown to accelerate low‐density lipoprotein particle aggregation and infiltration into the arterial wall.9
All of the above mechanisms seem theoretically relevant and support the role of ceramides as potentially clinically important factors in patients with stable coronary heart disease (CHD). However, a better understanding of the associations between ceramides with cardiovascular outcomes and different biological processes would help in clinical interpretation and decision making. To this end, we analyzed the ceramide‐phospholipid score (Cardiovascular Event Risk Test [CERT2]) and its components in the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial, which is large‐scale global trial with a rich biobank, including not only carefully defined cardiovascular phenotypes but also information on a large variety of other risk factors and biomarkers. These new data allowed us to investigate the associations between CERT2 and conventional cardiovascular risk factors and other prognostic biomarkers and also to evaluate the associations between CERT2 and cardiovascular outcomes. The purpose was to increase our understanding on the mechanisms behind the associations between CERT2 and its components and residual risk in patients with CHD.
Methods
As a result of limitations in the informed consent obtained from the participants, the data of the study cannot be made publicly available. For research use, the data can be requested from the corresponding author Lars Wallentin, and the proposed collaborations will be handled in the STABILITY trial steering committee.
Study Population
STABILITY and its biomarker substudy have been previously described in detail.10, 11 In brief, the trial was performed in 39 countries to investigate the effect of darapladib, a selective inhibitor of lipoprotein‐associated phospholipase A2 for cardiovascular events in patients with stable CHD taking optimal secondary prevention treatment. The inclusion criteria were previous myocardial infarction (MI), percutaneous coronary intervention, coronary artery bypass grafting, or demonstrated multivessel coronary artery disease (CAD). In addition, patients had to be on statin treatment and have at least 1 of the following criteria: age ≥60 years, DM requiring pharmacotherapy, high‐density lipoprotein cholesterol <40 mg/dL, smoker (defined as at least 5 cigarettes per day on average) or a previous smoker (defined as at least 5 cigarettes per day on average when smoking) who discontinued within the past 3 months, moderate renal dysfunction, or concomitant cerebrovascular or peripheral arterial disease. STABILITY investigators were strongly encouraged to treat patients according to local guidelines for secondary prevention and to ensure that all patients were taking antiplatelet therapy and a statin. Investigators were also encouraged to address nonpharmacological preventive care measures to achieve risk factor targets for secondary prevention according to European Society of Cardiology, American Heart Association/American College of Cardiology, or national guidelines. The exclusion criteria were MI during the previous month, coronary revascularization during the previous 3 months, or planned coronary revascularization procedure. The median follow‐up time was 3.7 years (interquartile range, 3.5–3.8). In patients included in the biomarker substudy, blood samples were obtained at randomization. Plasma aliquots were stored at −70°C until later analysis. The biomarkers in the STABILITY biomarker substudy program were analyzed at the Uppsala Clinical Research Center Laboratory as previously decribed.11, 12 The trial was approved by the institutional review boards and performed in accordance with the Declaration of Helsinki. All patients provided written informed consent for their participation. The trial was funded by GlaxoSmithKline and has been registered at ClinicalTrials.gov (NCT00799903; https://clinicaltrials.gov/ct2/show/NCT00799903).
Analysis of CERT2 Score Lipids
The CERT2 score comprises 7 lipids and is calculated based on the quartiles of 3 lipid ratios [ceramide(d18:1/24:1)/ceramide(d18:1/24:0), ceramide(d18:1/18:0)/phosphatidylcholine 14:0/22:6, and ceramide(d18:1/16:0)/phosphatidylcholine 16:0/22:5] and a single lipid (phosphatidylcholine 16:0/16:0). The score range was 0 to 12 points and stratified into 4 risk categories (0–3, low; 4–6, moderate; 7–8, increased; and 9–12, high). Details of CERT2 and CERT1 score calculation have been previously described.3
For the measurement of CERT2 lipids, baseline plasma samples from 11 222 participants were analyzed on a hybrid triple quadrupole/linear ion trap mass spectrometer (QTRAP 5500, AB Sciex), equipped with an ultra‐high performance liquid chromatography (Nexera‐X2, Shimadzu). Ceramide lipids were analyzed with a validated method, as previously described.13 Phospholipids were analyzed from the same extract as ceramides and with a targeted phospholipid platform. The chromatography was performed on an ACQUITY UltraPerformance LCBEH C18, 2.1×50 mm id 1.7 μm column (Waters Corp). Mobile phases consisted of: (A) 10 mmol/L ammonium acetate in liquid chromatography‐mass spectrometry grade water with 0.1% formic acid, and (B) 10 mmol/L ammonium acetate in acetonitrile:2‐propanol (3:4, V/V) with 0.1% formic acid. The following liquid chromatography gradient was used: 0.5 min at 75% B, linear increase of B from 75% to 100% in 2.0 min, 1.0 min at 100% B, 100% to 75% B in 0.1 min, and 0.9 min equilibration at 75% before the next injection. The flow rate was 500 μL/min and column temperature was 60°C. The injection volume of all samples was 5 μL. Positive ionization (PI) was used for ceramide analysis and negative ionization (NI) for phospholipid analysis, and the data were collected using multiple reaction monitoring. Mass spectrometry settings were the same for all ions, except for ion spray voltage, declustering potential, entry potential, and collision exit potential. The conditions were as following: curtain gas (nitrogen) 25, collision‐activated dissociation (nitrogen) 6, temperature 300 C, gas 1: 50, gas2: 50, interface heater on, ion spray voltage (PI 5000, NI −4500 V), declustering potential (PI 30, NI −100 V), entry potential (PI 10, NI −10 V), and collision exit potential (PI 20, NI −20 V). A 10‐ms dwell time was applied to all analytes. Collision energy was applied specifically for each lipid (Table S1). Results were processed using Analyst 1.6 and MultiQuant 3.0 software (AB Sciex).
Statistical Analysis
Baseline characteristics and patient demographics were compared between CERT2 risk groups using Kruskall–Wallis test for continuous variables and chi‐square test for categorical variables. The association between the components of the CERT2 risk score and other biomarkers were assessed by Spearman rank correlation coefficient. Biomarkers were logarithmic transformed when appropriate. The unadjusted association between CERT2 risk groups and clinical outcomes were presented by Kaplan–Meier curves.
Definitions of all outcome events were prespecified, as previously described,10 and the events were adjudicated by an independent clinical events committee. We evaluated the models and the included biomarkers’ prognostic performance for the primary outcome of major adverse cardiovascular event (ie, the composite of cardiovascular death, stroke and MI, and cardiovascular death), as well as secondary outcomes: major cardiovascular events, major coronary events, ie, the composite of coronary death, MI, urgent revascularization, all‐cause death, MI, stroke, hospitalization for heart failure (HHF), the composite of cardiovascular death and HHF, and the composite of major adverse cardiovascular events and HHF.
Cox proportional hazards models were used to investigate the covariate‐adjusted association between CERT2 risk score and outcomes. Four models were used, with an incremental addition of covariates. Model 0 included CERT2 risk score and randomized treatment. Model 1 included CERT2 risk score, randomized treatment, age, sex, prior MI, coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting), multivessel CAD, DM, hypertension, history of smoking, polyvascular disease, race, geographic region, systolic blood pressure, and body mass index. Model 2 included hemoglobin, white blood cell count, estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation, LDL‐C, high‐density lipoprotein cholesterol, and triglycerides in addition to model 1. The final model (3) included the following covariates in addition to model 2: high‐sensitivity troponin T (hs‐TnT), NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), cystatin C, hs‐CRP, and interleukin 6 (IL‐6). All continuous variables were included as restricted cubic splines with 4 knots placed at the 5th, 35th, 65th, and 95th sample percentiles, as previously suggested.14 The results were presented as the relative hazard for a 1‐SD increase in CERT2 risk score. The proportional hazards assumption was assessed by visual inspection of Schoenfeld residual plots. The discriminative value of CERT2 risk score was assessed using the C‐index. The models with and without CERT2 risk score were compared using likelihood ratio tests.
A statement of statistical significance implies a 2‐sided P<0.05, and there were no adjustments for multiple comparisons. All statistical analyses were performed with SAS 9.4 (SAS Institute Inc). L.W. and T.G.L. had full access to all of the data in the study and take responsibility for the integrity and data analysis.
Results
Characterization of Patients in CERT2 Risk Groups
CERT2 risk score (0–12) was determined using the previously established ceramides and phosphatidylcholines3 that were measured from baseline plasma samples of 11 222 patients who participated in the STABILITY trial. Patients with renal dysfunction, polyvascular disease and multivessel CAD, current smokers, and those with high white blood cell count showed higher CERT2 risk score (Table 1). There were regional differences in the risk groups, as patients especially from Eastern Europe were enriched in the high‐risk category (9–12), whereas an opposite trend was observed for patients from the Asia/Pacific region and North America (Table 1). Regarding race, white patients and patients of Central/South/South East Asian origin had a tendency for a higher CERT2 score, while the opposite was evident for black and East Asian/Japanese patients (Table 1). There was no clear trend for DM, body mass index, systolic blood pressure, or history of hypertension (Table 1). In contrast, patients with prior percutaneous coronary intervention/coronary artery bypass grafting had a tendency for a lower CERT2 score. Patients with higher CERT2 score were less likely to be taking statins and anti‐inflammatory and antiplatelet therapy (aspirin, P2Y12) (Table 1). There were significant positive associations between CERT2 risk categories and increased concentrations of biomarkers associated with an increased cardiovascular risk, ie, LDL‐C, triglycerides, lipoprotein‐associated phospholipase A2, white blood cell count, hs‐CRP, IL‐6, hs‐TnT, NT‐proBNP, growth differentiation factor‐15, creatinine clearance, and cystatin C (Table 1).
Table 1.
Patient Characteristics at the Time of Randomization by CERT2 Risk Score Categories
| CERT2: 0 to 3 | CERT2: 4 to 6 | CERT2: 7 to 8 | CERT2: 9 to 12 | P Value | |
|---|---|---|---|---|---|
| No. | 1708 | 4943 | 2795 | 1776 | |
| Age, y | 64 (58–70) | 65 (59–71) | 65 (59–71) | 65 (58–72) | 0.0004 |
| Men, No. (%) | 1405 (82.3) | 4028 (81.5) | 2287 (81.8) | 1428 (80.4) | 0.520 |
| Geographic region, No. (%) | |||||
| Asia/Pacific | 327 (19.1) | 734 (14.8) | 321 (11.5) | 198 (11.1) | <0.0001 |
| Eastern Europe | 317 (18.6) | 1341 (27.1) | 974 (34.8) | 784 (44.1) | |
| North America | 524 (30.7) | 954 (19.3) | 384 (13.7) | 184 (10.4) | |
| South America | 49 (2.9) | 167 (3.4) | 121 (4.3) | 55 (3.1) | |
| Western Europe | 491 (28.7) | 1747 (35.3) | 995 (35.6) | 555 (31.3) | |
| Race, No. (%) | |||||
| Black | 66 (3.9) | 108 (2.2) | 50 (1.8) | 23 (1.3) | <0.0001 |
| Central/South/South East Asian | 93 (5.4) | 331 (6.7) | 202 (7.2) | 152 (8.6) | |
| East Asian/Japanese | 202 (11.8) | 317 (6.4) | 91 (3.3) | 40 (2.3) | |
| Other | 40 (2.3) | 114 (2.3) | 44 (1.6) | 31 (1.7) | |
| White | 1307 (76.5) | 4073 (82.4) | 2408 (86.2) | 1530 (86.1) | |
| Diabetes mellitus, No. (%) | 677 (39.6) | 1855 (37.5) | 1064 (38.1) | 736 (41.4) | 0.023 |
| Body mass index, kg/m2 | 28.4 (25.8–31.7) | 28.3 (25.6–31.5) | 28.5 (25.8–32.0) | 28.4 (25.3–32.0) | 0.154 |
| Systolic blood pressure, mm Hg | 130 (120–142) | 131 (121–143) | 131 (120–143) | 131 (120–144) | 0.029 |
| History of hypertension, No. (%) | 1217 (71.3) | 3513 (71.1) | 1962 (70.2) | 1270 (71.5) | 0.765 |
| Significant renal dysfunctiona | 416 (24.4) | 1416 (28.6) | 868 (31.1) | 615 (34.6) | <0.0001 |
| Prior MI, No. (%) | 1025 (60.0) | 3012 (60.9) | 1761 (63.0) | 1125 (63.3) | 0.062 |
| Prior percutaneous coronary intervention or coronary artery bypass grafting, No. (%) | 1309 (76.6) | 3606 (73.0) | 2025 (72.5) | 1230 (69.3) | <0.0001 |
| Family history of premature CHD, No. (%) | 456 (26.7) | 1256 (25.5) | 665 (23.9) | 415 (23.4) | 0.057 |
| Polyvascular disease, No. (%) | 191 (11.2) | 738 (14.9) | 478 (17.1) | 375 (21.1) | <0.0001 |
| Multivessel CAD, No. (%) | 205 (12.0) | 700 (14.2) | 440 (15.7) | 294 (16.6) | <0.0001 |
| Smoking status, No. (%) | |||||
| Never smoked | 570 (33.4) | 1540 (31.2) | 802 (28.7) | 488 (27.5) | <0.0001 |
| Current smoker | 255 (14.9) | 874 (17.7) | 590 (21.1) | 434 (24.4) | |
| Former smoker | 882 (51.7) | 2529 (51.2) | 1403 (50.2) | 854 (48.1) | |
| Missing | 8 (0.2) | 7 (0.3) | 4 (0.2) | ||
| Statin treatment, No. (%) | 1677 (98.2) | 4838 (97.9) | 2733 (97.8) | 1708 (96.2) | 0.0002 |
| High‐intensity statin treatment, No. (%) | 119 (7.0) | 326 (6.6) | 168 (6.0) | 101 (5.7) | 0.329 |
| Aspirin, No. (%) | 1622 (95) | 4591 (92.9) | 2557 (91.5) | 1579 (88.9) | <0.0001 |
| P2Y12, No. (%) | 609 (35.7) | 1662 (33.6) | 917 (32.8) | 556 (31.3) | 0.047 |
| ACEI or ARB, No. (%) | 1340 (78.5) | 3831 (77.5) | 2189 (78.3) | 1426 (80.3) | 0.111 |
| β‐Blocker, No. (%) | 1323 (77.5) | 3907 (79.0) | 2318 (82.9) | 1457 (82.0) | <0.0001 |
| Randomization to darapladib, No. (%) | 823 (48.2) | 2441 (49.4) | 1388 (49.7) | 933 (52.5) | 0.059 |
| White blood cell count, GI/L | 6.3 (5.4–7.3) | 6.5 (5.5–7.7) | 6.8 (5.7–8.0) | 7.1 (5.9–8.4) | <0.0001 |
| LDL‐C, mmol/L | 1.90 (1.53–2.33) | 2.10 (1.66–2.61) | 2.21 (1.76–2.82) | 2.42 (1.90–3.08) | <0.0001 |
| HDL‐C, mmol/L | 1.17 (1.00–1.36) | 1.19 (1.00–1.40) | 1.15 (0.98–1.37) | 1.14 (0.95–1.36) | <0.0001 |
| Triglycerides, mmol/L | 1.44 (1.06–1.95) | 1.52 (1.10–2.10) | 1.53 (1.12–2.19) | 1.57 (1.12–2.29) | <0.0001 |
| eGFR (Chronic Kidney Disease Epidemiology Collaboration), mL/min/1.73 m2 | 76.6 (64.4–88.1) | 75.1 (63.3–86.9) | 73.7 (61.0–86.2) | 73.3 (59.1–86.1) | <0.0001 |
| hs‐CRP, mgL | 0.9 (0.5–1.9) | 1.2 (0.6–2.6) | 1.8 (0.8–3.8) | 2.5 (1.1–5.8) | <0.0001 |
| hs‐TnT, ng/L | 8.4 (5.7–12.4) | 8.9 (6.1–13.5) | 10.0 (6.5–15.0) | 10.9 (6.9–17.3) | <0.0001 |
| NT‐proBNP, ng/L | 130 (64–262) | 163 (82–344) | 214 (103–464) | 283 (123–691) | <0.0001 |
| IL‐6, pg/mL | 1.7 (1.2–2.4) | 2.0 (1.4–2.9) | 2.3 (1.6–3.5) | 2.7 (1.8–4.5) | <0.0001 |
| Cystatin C, mg/L | 0.96 (0.85–1.11) | 0.99 (0.86–1.16) | 1.03 (0.89–1.21) | 1.06 (0.92–1.99) | <0.0001 |
| GDF15, pg/mL | 1138 (861–1654) | 1198 (887–1711) | 1283 (942–1852) | 1438 (1040–2170) | <0.0001 |
| Lp‐PLA2 activity, μmol/min per L | 161 (134–191) | 169 (141–200) | 178 (149–209) | 190 (156–224) | <0.0001 |
Chi‐square or Kruskal–Wallis test. CAD indicates coronary artery disease; CERT, Cardiovascular Event Risk Test; CHD, coronary heart disease; GDF15, growth differentiation factor‐15; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; hs‐TnT, high‐sensitivity troponin T; IL‐6, interleukin 6; LDL‐C, low‐density lipoprotein cholesterol; Lp‐PLA2, lipoprotein‐associated phospholipase A2; MI, myocardial infarction; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Significant renal dysfunction defined as estimated glomerular filtration rate [eGFR] ≥30 and ≤59 mL/min per 1.73 m2 OR urine albumin creatinine ratio ≥30 mg albumin/g creatinine.
Prediction of Cardiovascular Risk by CERT2 Risk Score
There were statistically significant associations between CERT2 and all cardiovascular outcomes. For CERT2, the highest unadjusted hazard ratios (HRs) per SD were observed for cardiovascular death (HR, 1.57; 95% CI, 1.45–1.69), all‐cause death (HR, 1.54; 95% CI, 1.45–1.64), and HHF (HR, 1.52; 95% CI, 1.35–1.70) (Table 2). Statistically significant associations were also observed for all other cardiovascular end points, including the primary composite end point for major adverse cardiovascular events or HHF and major coronary events, as well as MI and stroke separately (Table 2). The HRs were attenuated only slightly when the models were adjusted for multiple traditional cardiovascular risk factors and lipid biomarkers (Table 2). However, the HRs decreased more when additional biomarkers (hs‐TnT, NT‐proBNP, cystatin C, hs‐CRP, and IL‐6) were included in the models, after which only the end points including cardiovascular death remained significant (Table 2). In addition, the previous version of the ceramide score (CERT1) showed significant results for all of the end points (Table 3) but did not show as robust HRs as the improved CERT2 version. The unadjusted associations between CERT2 risk groups and the occurrence of major adverse cardiovascular events, HHF, and cardiovascular death are illustrated in Figure 1.
Table 2.
HRs Per 1‐SD (2.4) Increase of CERT2 for Different End Points and C‐Indices for Models With and Without CERT2
| End Point | HR (95% CI) | C‐Statistic −CERT2 | C‐Statistic +CERT2 | P Value |
|---|---|---|---|---|
| MACE or HHF | ||||
| Model 0 | 1.32 (1.26–1.39) | 0.510 (0.498–0.522) | 0.579 (0.565–0.594) | <0.0001 |
| Model 1 | 1.35 (1.28–1.42) | 0.665 (0.651–0.678) | 0.684 (0.671–0.697) | <0.0001 |
| Model 2 | 1.26 (1.20–1.33) | 0.693 (0.680–0.706) | 0.701 (0.688–0.715) | <0.0001 |
| Model 3 | 1.08 (1.02–1.15) | 0.750 (0.737–0.763) | 0.751 (0.738–0.764) | 0.009 |
| MACE | ||||
| Model 0 | 1.30 (1.24–1.37) | 0.508 (0.495–0.521) | 0.575 (0.559–0.590) | <0.0001 |
| Model 1 | 1.32 (1.25–1.39) | 0.654 (0.640–0.669) | 0.672 (0.658–0.686) | <0.0001 |
| Model 2 | 1.23 (1.16–1.31) | 0.682 (0.667–0.696) | 0.689 (0.675–0.704) | <0.0001 |
| Model 3 | 1.08 (1.02–1.15) | 0.731 (0.717–0.745) | 0.732 (0.718–0.746) | 0.012 |
| Major coronary event | ||||
| Model 0 | 1.26 (1.19–1.33) | 0.515 (0.502–0.529) | 0.566 (0.550–0.582) | <0.0001 |
| Model 1 | 1.28 (1.21–1.36) | 0.642 (0.627–0.657) | 0.656 (0.641–0.671) | <0.0001 |
| Model 2 | 1.20 (1.13–1.27) | 0.671 (0.656–0.687) | 0.676 (0.661–0.692) | <0.0001 |
| Model 3 | 1.07 (1.00–1.14) | 0.717 (0.702–0.732) | 0.717 (0.702–0.732) | 0.053 |
| Cardiovascular death | ||||
| Model 0 | 1.57 (1.45–1.69) | 0.510 (0.490–0.529) | 0.625 (0.602–0.648) | <0.0001 |
| Model 1 | 1.47 (1.35–1.59) | 0.729 (0.710–0.749) | 0.752 (0.733–0.770) | <0.0001 |
| Model 2 | 1.38 (1.26–1.50) | 0.760 (0.741–0.779) | 0.771 (0.752–0.789) | <0.0001 |
| Model 3 | 1.14 (1.04–1.26) | 0.831 (0.815–0.847) | 0.831 (0.815–0.847) | 0.005 |
| MI | ||||
| Model 0 | 1.16 (1.08–1.25) | 0.512 (0.493–0.531) | 0.542 (0.521–0.564) | 0.0001 |
| Model 1 | 1.23 (1.14–1.33) | 0.649 (0.628–0.670) | 0.657 (0.636–0.678) | <0.0001 |
| Model 2 | 1.15 (1.06–1.25) | 0.675 (0.655–0.696) | 0.680 (0.659–0.700) | 0.0009 |
| Model 3 | 1.06 (0.97–1.16) | 0.707 (0.687–0.728) | 0.708 (0.688–0.729) | 0.176 |
| HHF | ||||
| Model 0 | 1.52 (1.35–1.70) | 0.523 (0.495–0.551) | 0.619 (0.587–0.652) | <0.0001 |
| Model 1 | 1.56 (1.39–1.76) | 0.761 (0.736–0.787) | 0.787 (0.763–0.811) | <0.0001 |
| Model 2 | 1.45 (1.28–1.64) | 0.800 (0.777–0.822) | 0.811 (0.789–0.833) | <0.0001 |
| Model 3 | 1.02 (0.89–1.17) | 0.892 (0.875–0.909) | 0.892 (0.875–0.909) | 0.763 |
| Stroke | ||||
| Model 0 | 1.29 (1.15–1.46) | 0.499 (0.470–0.529) | 0.575 (0.541–0.610) | <0.0001 |
| Model 1 | 1.32 (1.16–1.49) | 0.655 (0.623–0.687) | 0.672 (0.640–0.703) | <0.0001 |
| Model 2 | 1.29 (1.14–1.48) | 0.678 (0.644–0.711) | 0.687 (0.655–0.720) | 0.0001 |
| Model 3 | 1.15 (0.99–1.33) | 0.719 (0.689–0.750) | 0.722 (0.692–0.752) | 0.059 |
| All‐cause death | ||||
| Model 0 | 1.54 (1.45–1.64) | 0.502 (0.486–0.518) | 0.624 (0.605–0.643) | <0.0001 |
| Model 1 | 1.44 (1.35–1.54) | 0.711 (0.695–0.727) | 0.736 (0.721–0.752) | <0.0001 |
| Model 2 | 1.34 (1.25–1.44) | 0.743 (0.727–0.758) | 0.754 (0.738–0.770) | <0.0001 |
| Model 3 | 1.12 (1.04–1.21) | 0.800 (0.785–0.815) | 0.801 (0.786–0.815) | 0.003 |
Model 0 includes the Cardiovascular Event Risk Test (CERT2) score and randomized treatment. Model 1 includes CERT2 score, randomized treatment, age, sex, prior myocardial infarction (MI), coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting), multivessel coronary artery disease), diabetes mellitus, hypertension, history of smoking, polyvascular disease, geographic region, systolic blood pressure, and body mass index. Model 2 includes the following covariates in addition to model 1: hemoglobin, white blood cell count, Chronic Kidney Disease Epidemiology Collaboration, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and triglycerides. Model 3 includes the following covariates in addition to model 2: high‐sensitivity troponin T, pro‐B‐type natriuretic peptide, cystatin C, high‐sensitivity C‐reactive protein, and interleukin 6. P value relates to the significance of the hazard ratio (HR). HHF indicates hospitalization for heart failure; and MACE, major adverse cardiovascular event.
Table 3.
HRs Per 1‐SD (3.4) Increase of CERT1 for Different End Points and C‐Indices for Models With and Without CERT1
| End Point | HR (95% CI) | C‐Statistic −CERT | C‐Statistic +CERT | P Value |
|---|---|---|---|---|
| MACE or HHF | ||||
| Model 0 | 1.20 (1.14–1.26) | 0.510 (0.498–0.522) | 0.554 (0.540–0.569) | <0.0001 |
| Model 1 | 1.24 (1.18–1.31) | 0.664 (0.650–0.677) | 0.674 (0.661–0.688) | <0.0001 |
| Model 2 | 1.21 (1.14–1.27) | 0.693 (0.680–0.706) | 0.697 (0.684–0.711) | <0.0001 |
| Model 3 | 1.09 (1.03–1.15) | 0.750 (0.737–0.763) | 0.751 (0.738–0.763) | 0.0031 |
| MACE | ||||
| Model 0 | 1.19 (1.13–1.25) | 0.508 (0.495–0.522) | 0.551 (0.536–0.566) | <0.0001 |
| Model 1 | 1.22 (1.16–1.29) | 0.653 (0.639–0.668) | 0.663 (0.649–0.677) | <0.0001 |
| Model 2 | 1.18 (1.11–1.24) | 0.682 (0.667–0.696) | 0.685 (0.671–0.699) | <0.0001 |
| Model 3 | 1.08 (1.02–1.15) | 0.731 (0.717–0.745) | 0.732 (0.718–0.746) | 0.0126 |
| Major coronary event | ||||
| Model 0 | 1.17 (1.11–1.23) | 0.515 (0.502–0.529) | 0.549 (0.533–0.565) | <0.0001 |
| Model 1 | 1.21 (1.15–1.28) | 0.641 (0.626–0.656) | 0.649 (0.634–0.664) | <0.0001 |
| Model 2 | 1.16 (1.10–1.24) | 0.671 (0.656–0.686) | 0.674 (0.658–0.689) | <0.0001 |
| Model 3 | 1.08 (1.02–1.15) | 0.716 (0.701–0.731) | 0.717 (0.702–0.732) | 0.0107 |
| Cardiovascular death | ||||
| Model 0 | 1.26 (1.17–1.36) | 0.511 (0.491–0.530) | 0.566 (0.543–0.589) | <0.0001 |
| Model 1 | 1.25 (1.16–1.35) | 0.727 (0.707–0.747) | 0.734 (0.715–0.754) | <0.0001 |
| Model 2 | 1.22 (1.12–1.33) | 0.758 (0.739–0.778) | 0.761 (0.742–0.781) | <0.0001 |
| Model 3 | 1.07 (0.98–1.17) | 0.831 (0.815–0.847) | 0.830 (0.814–0.847) | 0.1462 |
| MI | ||||
| Model 0 | 1.15 (1.07–1.24) | 0.512 (0.493–0.531) | 0.543 (0.522–0.565) | 0.0001 |
| Model 1 | 1.22 (1.13–1.32) | 0.648 (0.627–0.669) | 0.657 (0.636–0.678) | <0.0001 |
| Model 2 | 1.15 (1.06–1.25) | 0.675 (0.655–0.696) | 0.678 (0.658–0.699) | 0.0008 |
| Model 3 | 1.10 (1.01–1.20) | 0.706 (0.686–0.727) | 0.708 (0.688–0.729) | 0.0242 |
| HHF | ||||
| Model 0 | 1.33 (1.20–1.49) | 0.523 (0.495–0.551) | 0.590 (0.558–0.621) | <0.0001 |
| Model 1 | 1.39 (1.25–1.56) | 0.762 (0.736–0.787) | 0.779 (0.754–0.803) | <0.0001 |
| Model 2 | 1.39 (1.23–1.56) | 0.800 (0.777–0.822) | 0.808 (0.786–0.830) | <0.0001 |
| Model 3 | 1.09 (0.96–1.23) | 0.892 (0.875–0.909) | 0.892 (0.875–0.909) | 0.1892 |
| Stroke | ||||
| Model 0 | 1.24 (1.10–1.39) | 0.499 (0.470–0.529) | 0.567 (0.534–0.600) | 0.0002 |
| Model 1 | 1.28 (1.13–1.44) | 0.655 (0.623–0.687) | 0.669 (0.638–0.701) | <0.0001 |
| Model 2 | 1.27 (1.12–1.44) | 0.678 (0.645–0.712) | 0.688 (0.655–0.720) | 0.0003 |
| Model 3 | 1.16 (1.02–1.33) | 0.720 (0.689–0.750) | 0.723 (0.692–0.753) | 0.028 |
| All‐cause death | ||||
| Model 0 | 1.29 (1.21–1.37) | 0.503 (0.487–0.519) | 0.574 (0.555–0.593) | <0.0001 |
| Model 1 | 1.25 (1.17–1.33) | 0.710 (0.693–0.726) | 0.720 (0.704–0.736) | <0.0001 |
| Model 2 | 1.21 (1.13–1.29) | 0.742 (0.726–0.758) | 0.746 (0.730–0.762) | <0.0001 |
| Model 3 | 1.07 (1.00–1.15) | 0.800 (0.785–0.815) | 0.800 (0.785–0.815) | 0.0663 |
Model 0 includes the Cardiovascular Event Risk Test (CERT) score and randomized treatment. Model 1 includes CERT score, randomized treatment, age, sex, prior myocardial infarction (MI), coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting), multivessel coronary artery disease, diabetes mellitus, hypertension, history of smoking, polyvascular disease, geographic region, systolic blood pressure, and body mass index. Model 2 includes the following covariates in addition to model 1: hemoglobin, white blood cell count, Chronic Kidney Disease Epidemiology Collaboration, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and triglycerides. Model 3 includes the following covariates in addition to model 2: high‐sensitivity troponin T, pro‐B‐type natriuretic peptide, cystatin C, high‐sensitivity C‐reactive protein, and interleukin 6. HHF indicates hospitalization for heart failure; and MACE, major adverse cardiovascular event.
Figure 1. CERT2 is associated with the risk of future cardiovascular events.
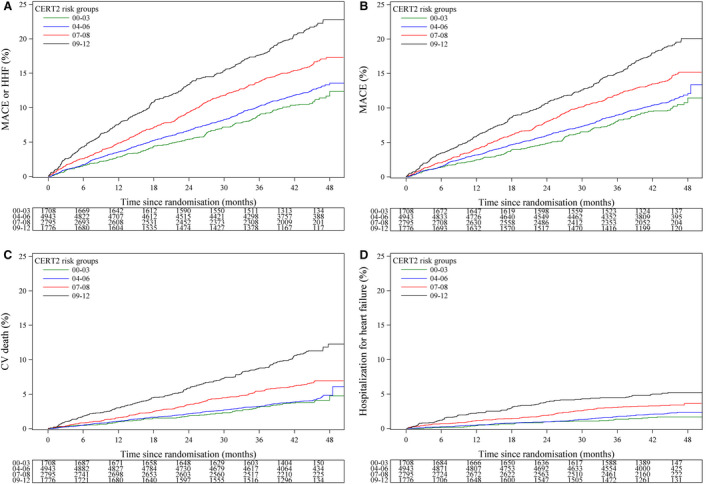
Kaplan–Meier curves of the cumulative event rate by the Cardiovascular Event Risk Test (CERT2) risk groups for (A) major adverse cardiovascular event (MACE)/hospitalization for heart failure (HHF), (B) MACE, (C) cardiovascular (CV) death, and (D) HHF.
Addition of CERT2 on top of traditional risk factors improved the C index for all investigated end points. However, the CERT2 score provided very limited if any incremental prognostic information when added to a model of 21 risk factors including also hs‐TnT, NT‐proBNP, cystatin C, hs‐CRP, and IL‐6 (Table 2).
Correlation of CERT2 Components With Other Cardiovascular Risk Biomarkers
Association of CERT2 and its components with other biomarkers was investigated, and the highest correlations for the score were observed for hs‐CRP, IL‐6, NT‐proBNP, and LDL‐C (Figure 2). Of the CERT2 components, ceramide(d18:1/24:1)/ceramide(d18:1/24:0) showed only modest correlation with any other biomarker, whereas ceramide(d18:1/18:0)/phosphatidylcholine (14:0/22:6) and ceramide(d18:1/16:0)/phosphatidylcholine (16:0/22:5) lipid ratios were especially correlated with the inflammatory markers hs‐CRP and IL‐6. The fourth component, phosphatidylcholine 16:0/16:0 showed a strong correlation with LDL‐C, as did individual ceramide molecules that were also associated with triglycerides (Figure 2).
Figure 2. CERT2 is associated with lipid and inflammatory biomarkers.
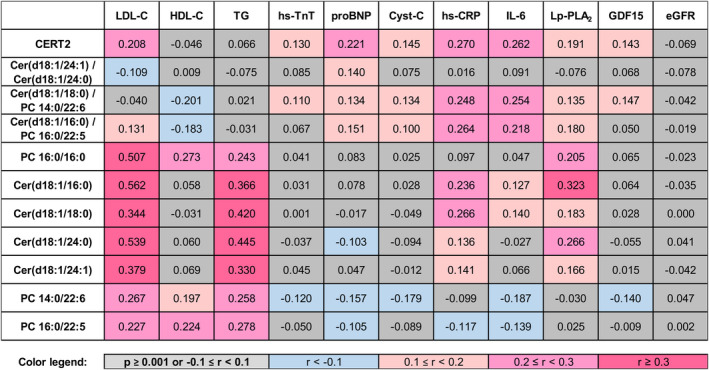
The figure presents Spearman correlations of the Cardiovascular Event Risk Test (CERT2) and its components with other biomarkers. eGFR indicates estimated glomerular filtration rate; GDF15, growth differentiation factor‐15; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; hs‐TnT, high‐sensitivity troponin T; IL‐6, interleukin 6; LDL‐C, low‐density lipoprotein cholesterol; Lp‐PLA2, lipoprotein‐associated phospholipase A2; and proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Discussion
The present results confirmed an earlier association between CERT2 and cardiovascular events, especially all‐cause and cardiovascular death.3 In addition, for the first time we report an association between CERT2 and HHF. CERT2 was associated with clinical characteristics, cardiovascular risk factors and contemporary biomarkers, but, despite this, showed independent prognostic value after adjustment with numerous clinical variables including randomized treatment, age, sex, prior MI, prior percutaneous coronary intervention or coronary artery bypass grafting, presence of multivessel CAD, DM, history of hypertension, history of smoking, peripheral vascular disease, geographic region, systolic blood pressure, and body mass index. Furthermore, addition of standard laboratory information such as hemoglobin, white blood cell count, estimated glomerular filtration rate, LDL‐C, high‐density lipoprotein cholesterol, and triglycerides on top of the above‐listed clinical parameters did not attenuate the predictive value of CERT2. However, after adding hs‐TnT, NT‐proBNP, cystatin C, hs‐CRP, and IL‐6 as adjusting factors, CERT2 was significantly associated only with fatal outcomes.
A clear strength of the present study was the validation of the performance of the CERT2 score in a large (N=11 222) clinical trial, which was conducted across different geographic regions and has a rich database of cardiovascular phenotypes and biomarkers. A limitation of the study was that the mass spectrometry method did not include standard compounds for all CERT2 components.
Future research will show whether patients with high CERT2 score will benefit from more aggressive lipid lowering by highest statin doses or PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors. In the present study, patients with high CERT2 score were significantly less frequently treated with aspirin. It remains to be evaluated whether aspirin treatment truly affects CERT2 score and serum concentrations of its components and whether the association is attributable to anti‐inflammatory or antithrombotic processes, or both. In contrast, patients with high CERT2 risk score were more frequently treated with β‐blockers. This observation, together with the other results, warrants systematic clinical and mechanistic investigations on how different medications affect ceramides and phosphatidylcholines. Moreover, systematic lifestyle intervention studies are needed to evaluate their usefulness in risk reduction in patients with high CERT2 score. Importantly, a ceramide synthesis inhibitor has been shown to significantly reduce lipid‐induced insulin resistance. 15 Thus, it would be interesting to perform studies evaluating the effect of ceramide inhibition on the occurrence of complications in patients with stable CHD. CERT2 score contains omega‐3 fatty acid–containing phosphatidylcholine molecules. Thus, the protective effect of fish oils17 might be associated with these particular phosphatidylcholines and they may serve as a proxy of supplementation efficacy.
Evaluation of associations between CERT2 score and clinical characteristics as well as other biomarkers revealed no significant relationship between CERT2 score and age or sex. For the first time, CERT2 was tested and showed robust performance in a study population covering various geographical locations worldwide. Nevertheless, there were differences in the score distribution in different locations, and further investigations are needed to investigate the factors behind this phenomenon. Here, we showed that the CERT2 score was associated with smoking as well as polyvascular disease and multivessel CAD. Patients with renal dysfunction had higher CERT2 scores, while the association with high blood pressure and DM was much weaker. CERT2 was strongly associated with the levels of lipid biomarkers (LDL‐C and triglyceride), which is in line with recently published lipidomic data, 17 and suggests that ceramides, constituents of the circulating lipoproteins, are associated with hyperlipidemia. However, it is noteworthy that the CERT2 score was prognostic even after adjustment for LDL‐C and triglyceride levels. This indicates that the sphingolipids might have additional pathophysiologic importance for cardiovascular disease beyond conventional lipids. The CERT2 score was also significantly associated with inflammatory markers (hs‐CRP and IL‐6), suggesting that variations in ceramide levels might be a reflection of vascular inflammation. Thus, these findings imply that CERT2 could reflect both lipid and inflammatory residual risk in patients with stable CHD. Higher CERT2 score was also associated with higher hs‐TnT and NT‐proBNP concentrations. These findings indicate that alterations of ceramides might be associated with, and possibly contribute to, myocardial necrosis and myocardial dysfunction. Thus, CERT2 score could potentially be useful as a tool to determine residual risk in patients with stable CHD as it is associated with all cardiovascular events and reflects disturbances of several key mechanisms for cardiovascular disease such as dyslipidemia, inflammation, myocardial necrosis, and myocardial and renal dysfunction.
Conclusions
The CERT2 risk score is associated with all cardiovascular outcomes and with biomarkers related to inflammation, myocardial necrosis, myocardial dysfunction, dyslipidemia, and renal dysfunction. The CERT2 risk score can be considered as a risk indicator in patients with stable CHD and it could be useful in evaluating residual risk in patients on guideline‐recommended evidence‐based optimal medical therapy.
Sources of Funding
None.
Disclosures
Significant: Zora Biosciences Oy holds patent disclosures related to the diagnostic and prognostic use of ceramides and phospholipids in cardiovascular disease. M.H., D.K., A.J., and R.L. are employees and R.L. is a shareholder of Zora Biosciences Oy. Modest: L.W. discloses institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb/Pfizer, GlaxoSmithKline, Roche Diagnostics, and Merck & Co, and consulting fees from Abbott. T.G.L. and J.L. report institutional research grants from GlaxoSmithKline. C.H. discloses an institutional research grant from GlaxoSmithKline; honoraria from Pfizer; and consultant/advisory board fees from AstraZeneca, Bayer, Boehringer Ingelheim, and Coala Life. A.S. discloses institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb/Pfizer, GlaxoSmithKline, and Roche Diagnostics; and consultancy fees from Olink Proteomics. C.B.G. reports research grants and consulting/speaker fees from Boehringer Ingelheim, Bristol‐Myers Squibb, Janssen Pharmaceuticals, Pfizer, AstraZeneca, and Novartis; research grants from Daichii‐Sankyo, AKROS, Apple, GlaxoSmithKline, and the US Food and Drug Administration; and consulting/speaker fees from Bayer Corp, Boston Scientific Corp, Abbvie, Espero BioPharma, Medscape, Merck, National Institutes of Health, NovoNordisk, Roche Diagnostics, Rho Diagnostics, Sirtex, and Verseon. W.K. discloses research grants from Roche Diagnostics, Beckmann, Singulex, and Abbott; other research support from European Research Agency (ERA‐CVD); honoraria from The Medicines Company, GlaxoSmithKline, Novartis, Pfizer, Sanofi, AstraZeneca, Amgen; expert witness from Novartis; and is a consultant/on an advisory board for Novartis, Pfizer, DalCor, Sanofi, Kowa, and Amgen. R.A.H.S. reports a research grant from GlaxoSmithKline; and other from GlaxoSmithKline. H.D.W. discloses grants and nonfinancial support from GlaxoSmithKline; grants from Sanofi‐Aventis, Eli Lilly, the National Institutes of Health, Omthera Pharmaceuticals, Pfizer New Zealand, Elsai Inc., and Dalcor Pharma UK; honoraria and nonfinancial support from AstraZeneca; and is on advisory boards for Sirtex and Acetelion and received personal fees from CSL Behring and American Regent.
Supporting information
Table S1
Appendix S1
(J Am Heart Assoc. 2020;9:e015258 DOI: 10.1161/JAHA.119.015258.)
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Lars Wallentin, Email: lars.wallentin@ucr.uu.se.
Reijo Laaksonen, Email: reijo.laaksonen@zora.fi.
References
- 1. Laaksonen R, Ekroos K, Sysi‐Aho M, Hilvo M, Vihervaara T, Kauhanen D, Suoniemi M, Hurme R, März W, Scharnagl H, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL‐cholesterol. Eur Heart J. 2016;37:1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Havulinna AS, Sysi‐Aho M, Hilvo M, Kauhanen D, Hurme R, Ekroos K, Salomaa V, Laaksonen R. Circulating ceramides predict cardiovascular outcomes in the population‐based FINRISK 2002 cohort. Arterioscler Thromb Vasc Biol. 2016;36:2424–2430. [DOI] [PubMed] [Google Scholar]
- 3. Hilvo M, Meikle PJ, Pedersen ER, Tell GS, Dhar I, Brenner H, Schöttker B, Lääperi M, Kauhanen D, Koistinen KM, et al. Development and validation of a ceramide‐ and phospholipid‐based cardiovascular risk estimation score for coronary artery disease patients. Eur Heart J. 2020;41:371–380. [DOI] [PubMed] [Google Scholar]
- 4. Mullen TD, Obeid LM. Ceramide and apoptosis: exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anticancer Agents Med Chem. 2012;12:340–363. [DOI] [PubMed] [Google Scholar]
- 5. Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nixon GF. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol. 2009;158:982–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chavez JA, Summers SA. A ceramide‐centric view of insulin resistance. Cell Metab. 2012;15:585–594. [DOI] [PubMed] [Google Scholar]
- 8. Hilvo M, Salonurmi T, Havulinna AS, Kauhanen D, Pedersen ER, Tell GS, Meyer K, Teeriniemi A‐M, Laatikainen T, Jousilahti P, et al. Ceramide stearic to palmitic acid ratio predicts incident diabetes. Diabetologia. 2018;61:1424–1434. [DOI] [PubMed] [Google Scholar]
- 9. Ruuth M, Nguyen SD, Vihervaara T, Hilvo M, Laajala TD, Kondadi PK, Gisterå A, Lähteenmäki H, Kittilä T, Huusko J, et al. Susceptibility of low‐density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur Heart J. 2018;39:2562–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. STABILITY Investigators, White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370:1702–1711. [DOI] [PubMed] [Google Scholar]
- 11. Lindholm D, Lindbäck J, Armstrong PW, Budaj A, Cannon CP, Granger CB, Hagström E, Held C, Koenig W, Östlund O, et al. Biomarker‐based risk model to predict cardiovascular mortality in patients with stable coronary disease. J Am Coll Cardiol. 2017;70:813–826. [DOI] [PubMed] [Google Scholar]
- 12. Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, Koenig W, Siegbahn A, Steg PG, Soffer J, et al. Inflammatory Biomarkers Interleukin‐6 and C‐reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial. J Am Heart Assoc. 2017;6:e005077 DOI: 10.1161/JAHA.116.005077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kauhanen D, Sysi‐Aho M, Koistinen KM, Laaksonen R, Sinisalo J, Ekroos K. Development and validation of a high‐throughput LC‐MS/MS assay for routine measurement of molecular ceramides. Anal Bioanal Chem. 2016;408:3475–3483. [DOI] [PubMed] [Google Scholar]
- 14. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York, NY: Springer; 2015:1–26–1–27. [Google Scholar]
- 15. Bikman BT, Guan Y, Shui G, Siddique MM, Holland WL, Kim JY, Fabriàs G, Wenk MR, Summers SA. Fenretinide prevents lipid‐induced insulin resistance by blocking ceramide biosynthesis. J Biol Chem. 2012;287:17426–17437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 17. Rämö JT, Ripatti P, Tabassum R, Söderlund S, Matikainen N, Gerl MJ, Klose C, Surma MA, Stitziel NO, Havulinna AS, et al. Coronary artery disease risk and lipidomic profiles are similar in hyperlipidemias with family history and population‐ascertained hyperlipidemias. J Am Heart Assoc. 2019;8:e012415 DOI: 10.1161/JAHA.119.012415 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Appendix S1


