Abstract
Background
Carotid artery intima/media thickness (IMT) is a hallmark trait associated with future cardiovascular events. The goal of this study was to map new genes that regulate carotid IMT by genome‐wide association.
Methods and Results
We induced IMT by ligation procedure of the left carotid artery in 30 inbred mouse strains. Histologic reconstruction revealed significant variation in left carotid artery intima, media, adventitia, external elastic lamina volumes, intima‐to‐media ratio, and (intima+media)/external elastic lamina percent ratio in inbred mice. The carotid remodeling trait was regulated by distinct genomic signatures with a dozen common single‐nucleotide polymorphisms associated with left carotid artery intima volume, intima‐to‐media ratio, and (intima+media)/external elastic lamina percent ratio. Among genetic loci on mouse chromosomes 1, 4, and 12, there was natriuretic peptide receptor 2 (Npr2), a strong candidate gene. We observed that only male, not female, mice heterozygous for a targeted Npr2 deletion (Npr2 +/−) exhibited defective carotid artery remodeling compared with Npr2 wild‐type (Npr2 +/+) littermates. Fibrosis in carotid IMT was significantly increased in Npr2 +/− males compared with Npr2 +/− females or Npr2 +/+ mice. We also detected decreased Npr2 expression in human atherosclerotic plaques, similar to that seen in studies in Npr2 +/− mice.
Conclusions
We found that components of carotid IMT were regulated by distinct genetic factors. We also showed a critical role for Npr2 in genetic regulation of vascular fibrosis associated with defective carotid remodeling.
Keywords: carotid artery, genome‐wide association, inbred mice, Npr2, vascular remodeling
Subject Categories: Functional Genomics, Animal Models of Human Disease, Vascular Biology, Remodeling
Nonstandard Abbreviations and Acronyms
- 3D
3‐dimensional
- BP
systolic blood pressure
- chr
human chromosome
- EEL
external elastic lamina
- eNOS
endothelial nitric oxide synthase
- IMT
intima/media thickness
- LCA
left carotid artery
- Npr2−/−
Npr2 knockout
- Npr2
natriuretic peptide receptor 2
- Npr2+/−
Npr2 heterozygous
- Npr2+/+
Npr2 wild‐type
- QTLs
quantitative traits loci
- SNP
single‐nucleotide polymorphism
Clinical Perspective
What Is New?
Genetic variation in natriuretic peptide receptor 2 (Npr2) associated with defective carotid artery remodeling in a panel of mice that represent a human population.
Male but not female mice with a targeted Npr2 depletion exhibited defective carotid artery remodeling with increased fibrosis.
Expression of the Npr2 was reduced in human atherosclerotic plaques.
What Are the Clinical Implications?
Npr2 is a plausible target for new diagnostic and therapeutic approaches to treat vascular disease.
Introduction
Atherosclerosis is a complex disorder regulated by multiple genetic and environmental factors.1 Recently, many candidate genetic mechanisms have been proposed but most do not have major effects on development of human atherosclerosis.2, 3 A reliable clinical measure of atherosclerosis progression is carotid intima/media thickness (IMT), which predicts cardiovascular complications.4 Genetic linkage studies in Framingham Heart and Dominican Family cohorts identified significant quantitative traits loci (QTLs) on human chromosome (chr)7q, chr12q, and chr14q that control carotid IMT.5, 6 Genome‐wide association (GWA) studies mapped a number of single‐nucleotide polymorphisms (SNPs) associated with variation in carotid IMT.2, 3, 7 Despite such advances, those studies were underpowered and there are technical limitations to assessing specific mechanisms of carotid IMT and atherosclerotic plaque progression and regression in humans.8, 9, 10
We developed a robust mouse model of carotid IMT and showed significant genetic effects in 5 inbred strains of mice.11, 12 A forward genetic approach followed by congenic mapping was effective in uncovering 3 carotid intima modifier QTLs on mouse chr2, chr11, and chr18.13, 14, 15 Additional QTLs were identified on mouse chr5, chr9, chr12, and chr13, that contribute to carotid atherosclerosis.16, 17, 18 A plausible approach to identification of atherosclerosis traits includes GWA studies in a panel of mouse inbred strains.19 In an earlier study, we utilized this approach and identified several causative genes that control the elevated heart rate trait.20 The primary goal of this study was to use GWA to identify new genes that regulate carotid remodeling. By studying 30 strains of mice, we characterized a genetic locus that houses a novel gene candidate—natriuretic peptide receptor 2 (Npr2)—that regulates carotid artery remodeling.
Methods
The authors declare that all supporting data are available within the article and its online supplementary material.
Animals
We studied flow‐induced carotid remodeling in 9‐ to 12‐week‐old male mice from 30 inbred strains (Table S1). We purchased 10 mice per strain, 4 sham and 6 ligated, from the Jackson Laboratory (Bar Harbor, Maine). However, some of the inbred mouse strains exhibited poor survival and required additional mice, as we recently reported for 129X1 in a comparison with C57BL/6J.21 We also used male and female Npr2 wild‐type (Npr2 +/+) and Npr2 heterozygous (Npr2 +/−) littermate mice from our recently established colony.22 Presence of Npr2 alleles was determined by genotyping, as described previously.23 We were unable to use Npr2 knockout (Npr2 −/−) mice for surgery because of low body weights.23 Experimental mice were housed individually under a 12‐hour light/12‐hour dark cycle with free access to water and chow. All animal procedures were approved by the animal care committee of the University of Rochester and were in accordance with the Guide for the Care and Use of Laboratory Animals.24
Tail‐Cuff Plethysmography
Systolic blood pressure (BP) and heart rate were collected with a BP‐2000 (Visitech Systems, Apex, North Carolina) system in Npr2 mice. We performed our experiments according to a method described previously by our group.11, 20, 25
Carotid Artery Ligation
We studied flow‐induced carotid remodeling 2 weeks after the ligation procedure in all mice, as described previously.11 Briefly, animals were anesthetized with a cocktail of ketamine and xylazine (130 and 9 mg/kg, respectively, intraperitoneally). The neck area was opened by a midline incision and the bifurcation of the left carotid artery (LCA) was isolated. The internal and external LCA branches were ligated with 6‐0 silk, leaving the occipital artery intact. The neck opening was closed with a 6‐0 coated Vicryl suture. An analgesic flunixin meglumine (120 mg/kg, intraperitoneally) was given immediately after and once per day for 3 days after the surgery. A plastic box with additional bedding material was given to mice housed individually after the surgery.
Vascular Ultrasound
We measured blood flow in the LCA in inbred mouse strains with a ultrasonic transit‐time volume flowmeter (Transonic Systems, Ithaca, New York) before termination, as described elsewhere.11 Carotid artery imaging in anesthetized Npr2 mice was done with a Vevo2100 machine (FUJIFILM VisualSonics, Toronto, Ontario, Canada) as described in our previous work.26
Histology and Morphometry
Two weeks after ligation, mice were perfusion fixed under anesthesia; carotids were collected, processed, and stained with hematoxylin and eosin; followed by morphometry analyses (MCID image software), as described elsewhere.11 We evaluated 10 area divisions of the LCA from the bifurcation every 200 μm through the 2‐mm length. Averaged area measurements of the LCA for each group were used to produce 3‐dimensional (3D) images with MATLAB programming (The Mathworks, Natick, Massachusetts), as described elsewhere.26 Unstained LCA sections of ligated Npr2 mice were processed with an Alcian Blue kit (ScienCell Research Laboratories, Carlsbad, California) or PicroSirius Red kit (Abcam, Cambridge, Massachusetts). We also stained consecutive cross‐sections of human endarterectomy with rabbit anti‐NPR2 (1:100; overnight at +4°C; Abcam), mouse anti‐human α1 smooth muscle actin (1:1 000; 60 minutes at room temperature; hematoxylin and eosin stain), or mouse anti‐human CD68 (1:1 000; 60 minutes at room temperature; eBiosciences, Thermo Fisher Scientific, Waltham, Massachusetts) antibodies followed by a secondary goat anti‐rabbit (1:400; Vector Laboratories, Burlingame, California) or horse anti‐mouse (1:200; Vector) antibodies with an ABC kit (Vector) and counterstained with hematoxylin, as described previously.21, 22 A percentage of positive staining was determined in stained mouse and human sections by the ImagePro analyzer version 6.2 (Media Cybernetics, Bethesda, Maryland) in a blinded manner as we have reported previously.27
Genome‐Wide Association of Carotid Remodeling in 30 Inbred Strains of Mice
GWA mapping for carotid remodeling traits was done using an efficient mixed‐model association method with a significance for P‐value thresholds (4.1×10−6) on the basis of power calculations in a similar number of mouse strains.28 The GWA results on variation for LCA intima, media, adventitia, and external elastic lamina (EEL) volumes, intima/media ratio, and (intima+media)/EEL×100% are listed in Tables S2 through S7. We used the publicly available Integrative Genomic Viewer version 2.4.17 (Broad Institute and Regents of the University of California) to visualize the significant peaks on the mouse genome.29
Human Samples
Cross‐sections of de‐identified human endarterectomy samples from 3 patients undergoing surgery on a carotid artery were collected with the approval from the subjects review board of the University of Rochester School of Medicine and Dentistry Research (RSRB00069961).
Statistical Analysis
Results are presented as mean±SEM. Statistical significance was determined using JMP version 13.0.0 software (SAS). Initial analyses of data sets across experiments showed a normal distribution. Two groups were compared using Student's t test. One‐way ANOVA was evaluated for each parameter with post‐hoc comparisons of means using the Tukey–Kramer honestly significant difference test. We performed multivariate and linear regression analyses between LCA intima volumes and LCA (intima+media)/EEL×100% to determine pairwise correlations in the experimental groups. P<0.05 was considered significant.
Results
Variation of the Remodeling Traits Across 30 Inbred Mouse Strains
A forward genetic approach followed by congenic mapping was effective in revealing the causes of increased LCA intima.13, 14, 15 To map the carotid intima trait, we investigated variation in the most common inbred strains (Figure 1A). We found that relative changes in LCA blood flow were similar among the studied strains compared with RCA blood flow after ligation (Table S1). However, the same reduction in blood flow resulted in significant variation in carotid arteries on the basis of 3D reconstruction of histologic measurements across the strains (Figures S1 through S3). There were 5 inbred strains that significantly differed in LCA intima volume from controls or other strains, whereas no intima was detected in controls (Figure 1A). Similar results were observed for intima/media ratio and (intima+media)/EEL×100% across 30 mouse strains (Figure 1B and 1C). We observed greater variation in LCA media, EEL, and adventitia volumes (Figures S3 through S6). There was disparity in the relationship between LCA intima volume and (intima+media)/EEL×100%. For example, below 40% LCA (intima+media)/EEL×100% was observed in mouse strains without (eg, BTBR) or with (eg, C3H) intima (Figure 1D). In contrast, mouse strains with a greater percentage of LCA (intima+media)/EELx100% had small (SM) vs large (SJL) LCA intima volume (Figure 1E). Thus, we characterized a significant variation in response to vascular injury across 30 inbred mouse strains permitting GWA studies for carotid remodeling traits.
Figure 1. Variation in carotid remodeling in 30 mouse strains.

A, Left carotid artery (LCA) intima volume, ×10−6 μm3. B, LCA intima/media ratio. C, LCA (intima+media)/EEL×100%. Open circles indicate controls. Black circles indicate ligated mice. Values are mean±SEM; *P<0.05 vs control or other mouse strains. n=4 to 6 per group. D, Representative 3‐dimensional (3D) reconstructions of the 2 mm‐length from the bifurcation of the ligated LCA in mice with low values of LCA (intima+media)/EEL×100%. E, Representative 3D reconstructions of mouse strains with a greater percentage of LCA (intima+media)/EEL×100%. Black color shows lumen, yellow indicates intima, red indicates media, and green indicates adventitia volume. EEL indicates external elastic lamina.
GWA Analysis of Carotid Remodeling Traits in 30 Inbred Strains of Mice
Most SNPs were associated with LCA intima/media ratio after ligation (Figure 2B and Table S6). Importantly, there were common SNPs regulating LCA intima volume, intima/media ratio, and (intima+media)/EEL×100% within genomic locations on mouse chr1, chr4, and chr12 (Figure 2, and Tables S2, S6, and S7). Among 12 common SNPs, we discovered 6 candidate genes (Slc24a4, solute carrier family 24 sodium/potassium/calcium exchanger, member 4; Tln1, talin 1; Npr2, natriuretic peptide receptor 2; Fam221b, family with sequence similarity 221, member B; Tmem8b, transmembrane protein 8B; Spaar, small regulatory polypeptide of amino acid response) known to regulate carotid intima and remodeling (Table). Our findings are supported by another mouse genetic study that proposed Npr2 with its ligand, C‐type natriuretic peptide, as candidate genes in high blood pressure.30, 31 Thus, we found that Npr2 is a plausible candidate for regulation of carotid remodeling traits in response to injury.
Figure 2. GWA of carotid remodeling traits in 30 mouse strains.
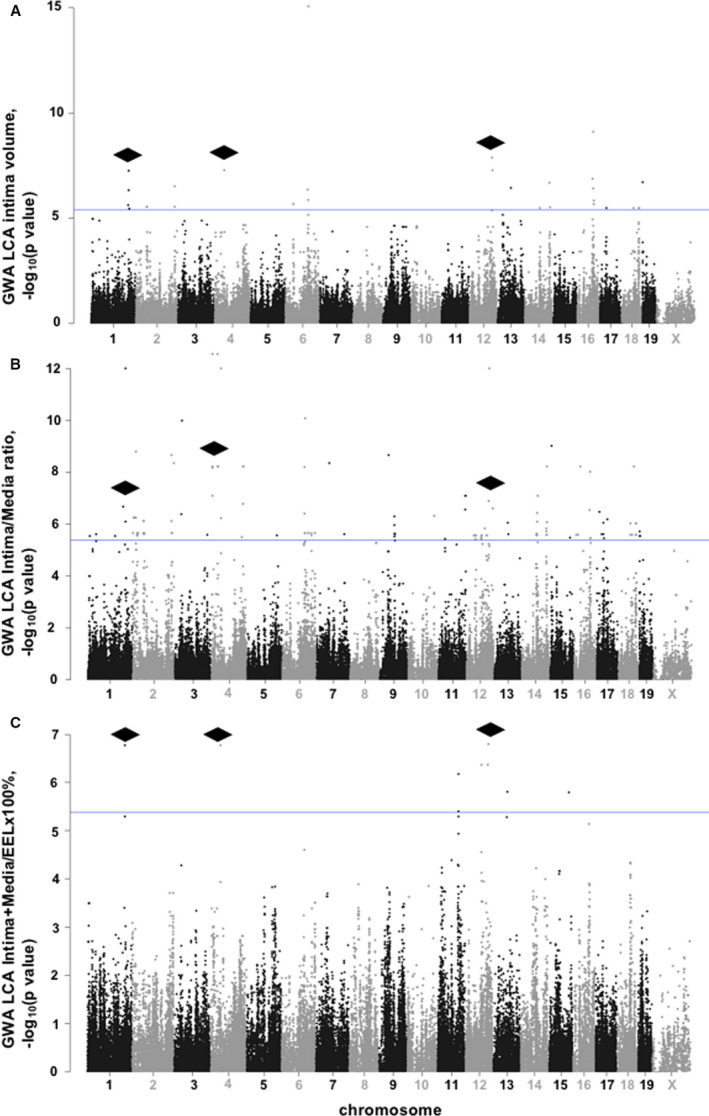
A, GWA of LCA intima volume, ×10−6 μm3. B, GWA of LCA intima/media ratio. C, GWA of LCA (intima+media)/EEL×100%. Each circle represents an SNP. Mouse chromosomes are presented on the X‐axis. Blue lines show significance threshold. Black rhombi point to common regions of significant SNPs associated with LCA intima, intima/media ratio, and (intima+media)/EEL×100% traits in 30 inbred mouse strains. EEL indicates external elastic lamina; GWA, genome‐wide association; LCA, left carotid artery; and SNP, single‐nucleotide polymorphism.
Table 1.
Common SNPs Associated With LCA Intima, Intima‐to‐Media Ratio and (Intima+Media)/EEL×100% Traits in 30 Inbred Mouse Strains
| dbSNP | Position, bp | P Value | Effect Size | Gene |
|---|---|---|---|---|
| rs36294984 | Chr12: 102175044 | 1.64E‐07 | 5.35241 | Slc24a4: intron |
| rs36281276 | Chr12: 102181524 | 1.64E‐07 | 5.35241 | Slc24a4: intron |
| rs36677986 | Chr1: 165121248 | 1.68E‐07 | 5.35456 | Intergenic variant |
| rs37370522 | Chr1: 165124350 | 1.68E‐07 | 5.35456 | Intergenic variant |
| rs38372684 | Chr1: 165124376 | 1.68E‐07 | 5.35456 | Intergenic variant |
| rs27831183 | Chr4: 43542462 | 1.72E‐07 | 5.35142 | Tln1: intron |
| rs28320686 | Chr4: 43620641 | 1.72E‐07 | 5.35142 | Intergenic variant |
| rs28320653 | Chr4: 43645470 | 1.72E‐07 | 5.35142 | Npr2: intron |
| rs28320604 | Chr4: 43650523 | 1.72E‐07 | 5.35142 | Npr2: intron |
| rs28320543 | Chr4: 43662649 | 1.72E‐07 | 5.35142 | Fam221b: intron |
| rs28320511 | Chr4: 43688855 | 1.72E‐07 | 5.35142 | Tmem8b: intron |
| rs28311534 | Chr4: 43732460 | 1.72E‐07 | 5.35142 | Spaar: noncoding RNA |
bp indicates base pair; EEL, external elastic lamina; Fam221b, family with sequence similarity 221, member B; LCA, left carotid artery; Npr2, natriuretic peptide receptor 2; Slc24a4, solute carrier family 24 (sodium/potassium/calcium exchanger), member 4; SNP, SNP single‐nucleotide polymorphism; Spaar, small regulatory polypeptide of amino acid response; Tln1, talin 1; and Tmem8b, transmembrane protein 8B.
Carotid Remodeling in Npr2‐Npr2 +/− Mice
We confirmed earlier genetic studies in mice on a causal role for the Npr2 gene in salt‐induced kidney injury.22 As we reported for direct BP measurements, tail‐cuff systolic BP (114–117 mm Hg) and heart rate (574–618 beats/min) profiles were similar between Npr2 +/+ and Npr2 +/− males and females (not shown). We found that a carotid ligation procedure resulted in a similar reduction of blood flow (Table S8) or estimated shear stress (not shown) between Npr2 +/+ and Npr2 +/− littermates compared with sham animals. However, there were significant differences in LCA remodeling on the basis of histologic evaluation across Npr2 genotypes (Figure 3). We observed a significant increase in LCA intima/media ratio, adventitia, and EEL volumes versus sham animals in male Npr2 +/+ mice (Figure 3A and 3C). In contrast, LCA compartmental volumes were significantly reduced in Npr2 +/− versus Npr2 +/+ male mice after ligation (Figure 3A and 3C). Histologic evaluation of the LCA revealed sex‐dependent differences in response to ligation in Npr2 genotypes (Figure 3A and 3B). LCA intima/media ratio, adventitia, and EEL volumes were similar among Npr2 +/+ and Npr2 +/− females (Figure 3B and 3D). In Npr2 +/+ females, we observed lower LCA intima, adventitia, and EEL volumes compared with Npr2 +/+ males (Figure 3C and 3D). Unlike Npr2 +/− males, we found that Npr2 +/− females exhibited significantly increased LCA EEL after ligation (Figure 3). Thus, we confirmed that the Npr2 gene plays a role in carotid artery response to injury, but only in males.
Figure 3. Flow‐dependent carotid remodeling in Npr2 mice.
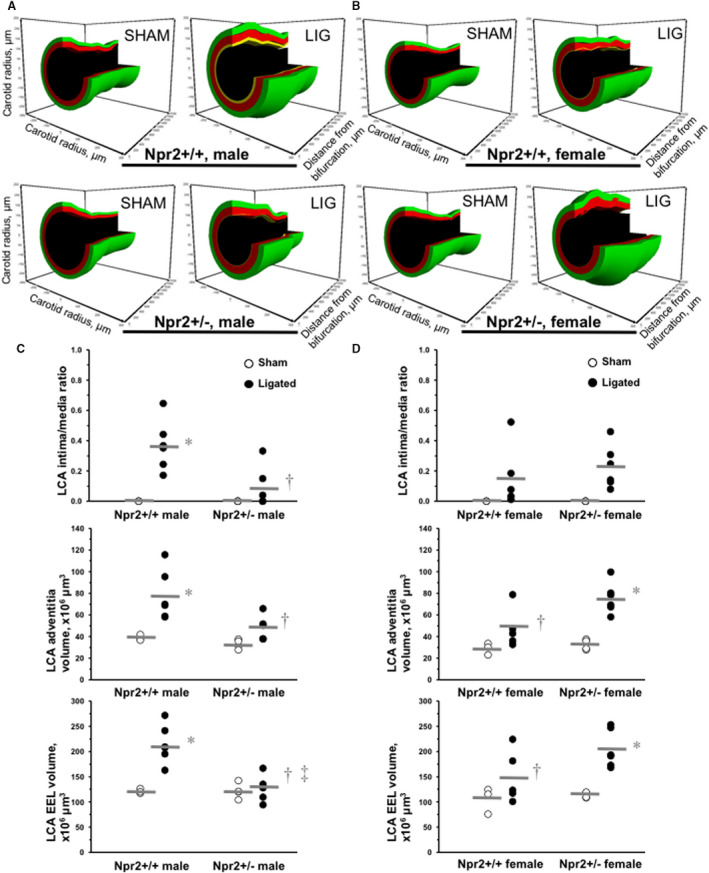
A, Representative 3‐dimensional (3D) reconstructions of the 2‐mm length from the bifurcation of the LCA after sham or ligation operation in males of Npr2 wild‐type (Npr2 +/+) and Npr2 heterozygous (Npr2 +/−) mice. B, Representative 3D reconstructions of LCA after sham or ligation operation in females of Npr2 +/+ and Npr2 +/− mice. Black indicates lumen, yellow indicates intima, red indicates media, and green indicates adventitia volume. C, Quantifications of LCA intima/media ratio, adventitia, and EEL volume in males of Npr2 +/+ and Npr2 +/− mice. D, Quantifications of LCA intima/media ratio, adventitia, and EEL volume in females of Npr2 +/+ and Npr2 +/− mice. Open circles indicate individual sham LCAs. Black circles indicate ligated LCAs. Gray lines indicate mean values. EEL indicates external elastic lamina; and LCA, left carotid artery. *P<0.05 vs sham; † P<0.05 vs Npr2 +/+ males; ‡ P<0.05 vs Npr2 +/− females. n=3 to 6 per group.
Relationship Between Carotid Intima Volume and Stenosis in Npr2 +/− Mice
In a previous study we reported a strong correlation between increase in LCA intima volume and LCA (intima+media)/EEL×100% in 5 inbred mouse strains, as also seen in human atherosclerosis.12 In the present work we found a significant correlation (R=0.6959, P<0.001) between LCA intima volume and (intima+media)/EEL×100% among 30 inbred strains of mice (Figure 4A). However, this correlation was not significant in Npr2 +/− (R=0.3872) animals when compared with Npr2 +/+ (R=0.7928, P<0.01) littermates (Figure 4B). Furthermore, there was essentially no correlation between LCA intima volume and (intima+media)/EEL×100% in the Npr2 +/− male (R=0.2362) versus Npr2 +/− female (R=0.9142, P<0.05) mice (Figure 4C). These results show that even partial depletion of Npr2 resulted in a defective carotid artery remodeling response, but only in males.
Figure 4. Relationships between carotid remodeling traits in mice.
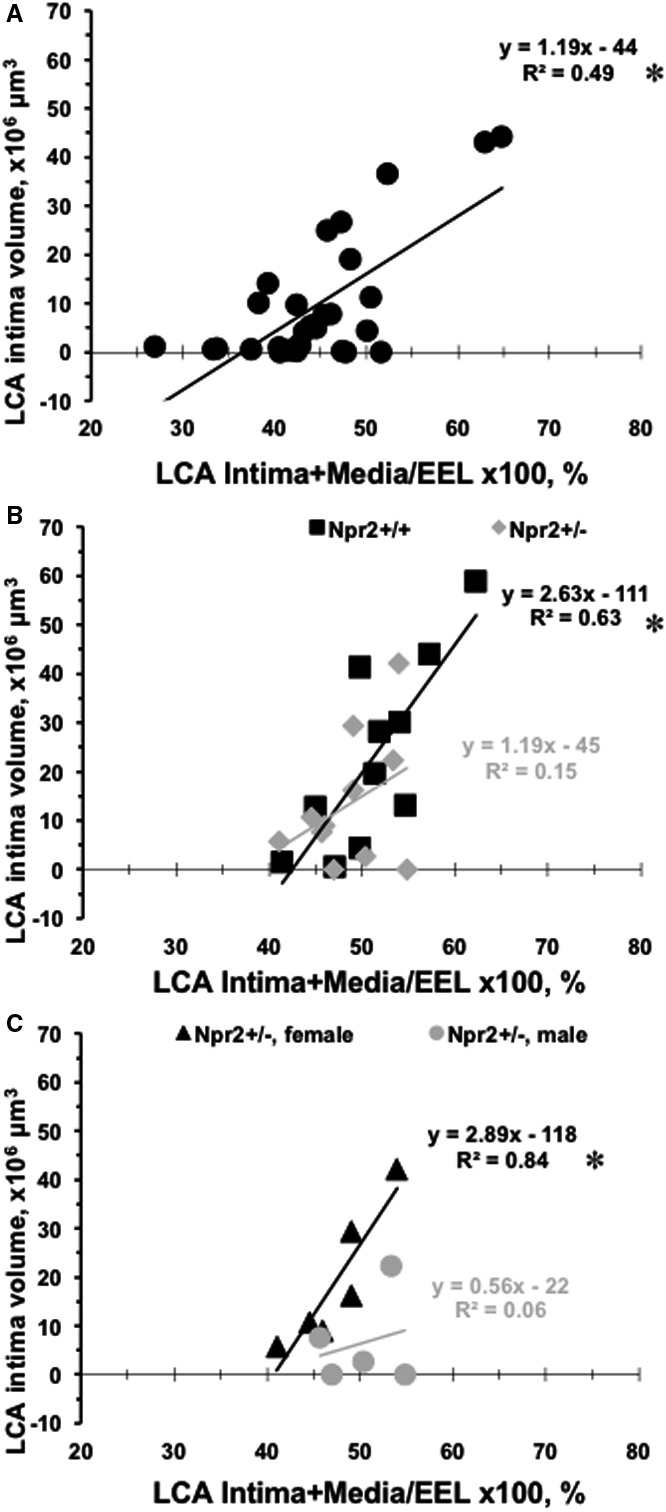
A, Ligated LCA (intima+media)/EEL×100% on X‐axis are plotted vs LCA intima volumes on Y‐axis for each of 30 inbred mouse strains (black circles). B, Ligated LCA (intima+media)/EEL×100% on the X‐axis are plotted vs LCA intima volumes on Y‐axis for Npr2 wild‐type (black squares: Npr2 +/+) and Npr2 heterozygous (gray diamonds: Npr2 +/−) mice. C, Ligated LCA (intima+media)/EEL×100% on X‐axis are plotted vs LCA intima volumes on the Y‐axis for Npr2 +/− females (black triangles) and Npr2 +/− males (gray circles). *P<0.001, † P<0.01, and ‡ P<0.05 indicate level of significance of correlation between LCA (intima+media)/EEL×100% and LCA intima volume. EEL indicates external elastic lamina; and LCA, left carotid artery.
Differences in Carotid Fibrosis in Npr2 Mice
We recently showed that pharmacologic intervention could improve carotid remodeling by inhibiting fibrosis after injury.21 Representative images of LCA cross‐sections stained with Alcian Blue show remodeling differences between male Npr2 +/− versus male Npr2 +/+ (Npr2 females are shown in insets in Figure 5A and 5B). The relative staining expression within the LCA intima/media (black brackets) was significantly greater in Npr2 +/− versus male Npr2 +/+ and Npr2 +/‐ females (Figure 5C). An increase in fibrosis in Npr2 +/− males was also confirmed by PicroSirius Red staining of carotid arteries after ligation (Figure S7). Our data suggest that decreased carotid remodeling in male Npr2 +/− mice is, in part, due to an increase in vascular fibrosis.
Figure 5. Differences in carotid fibrosis in Npr2 mice.
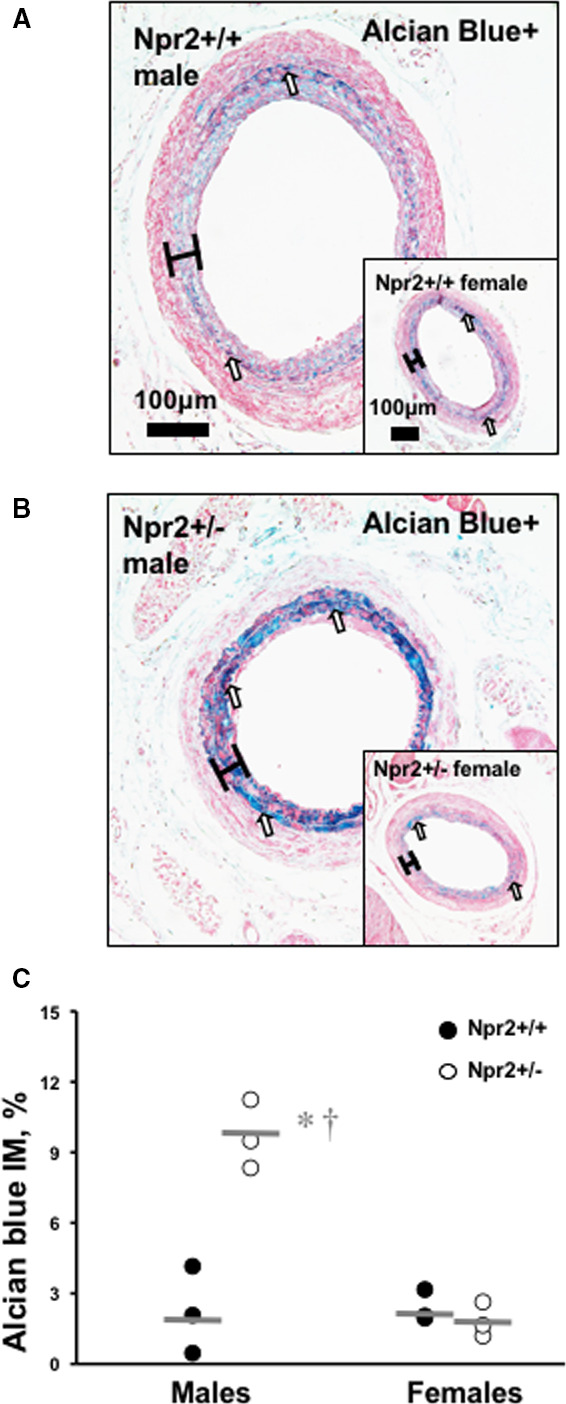
A, Representative image of PicroSirius Red–stained ligated LCA in male Npr2 wild‐type (Npr2 +/+) mouse. B, Representative image of Alcian Blue–stained ligated LCA in male Npr2 heterozygous (Npr2 +/−) mouse. Insets show corresponding females. Scale bar=100 μm. Black brackets indicate intima/media area. C, Quantification of fibrosis (blue color) in intima/media area of the LCA (%). Black circles indicate individual Npr2 +/+ mice. Open circles indicate Npr2 +/− mice. Gray lines indicate mean values. *P<0.001 vs Npr2 +/+ males; † P<0.001 vs Npr2 +/− females. n=3 animals per group. LCA indicates left carotid artery.
Decreased NPR2 Expression in Human Atherosclerotic Plaque
We identified a significant 4‐fold reduction in NPR2+ immunoreactivity within the atherosclerotic lesions (dark bar) compared with the medial compartment (white brackets) of human carotid artery (Figure 6A). The NPR2 protein expression profile showed the same decline as a smooth muscle–specific staining (smooth muscle actin+) in human lesions (Figure 6B). In contrast, macrophage‐specific staining (CD68+) was increased in lesions compared with media in human endarterectomy samples (Figure 6C).
Figure 6. Decreased NPR2 expression in smooth muscle cells in human carotid lesions.
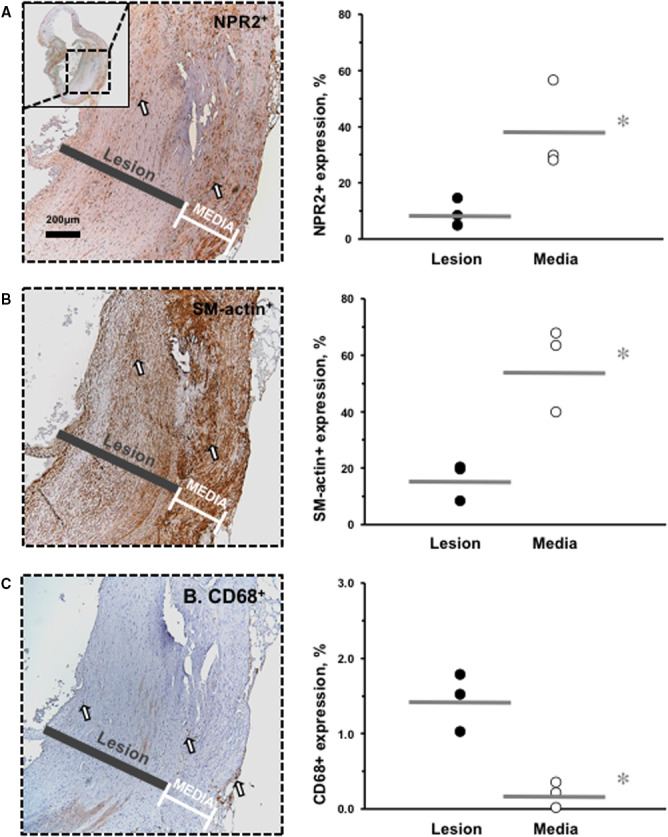
A, NPR2 immunostaining. Inset shows lower power of the carotid endarterectomy sample. B, α1‐SM‐actin immunostaining. C, CD68 immunostaining. Positive cells are stained brown (open arrows). Black bars indicate lesion area. White brackets indicate media area. Magnification bar=200 μm. For quantification, black circles show relative expression in the lesion of the carotid endarterectomy samples and open circles indicate media of the carotid endarterectomy samples. Gray lines indicate mean values. *P<0.05 vs media; † P<0.01 vs media. n=3 animals per group. SM indicates smooth muscle.
Discussion
The significant variation in response to vascular injury across 30 inbred mouse strains allowed us to identify 12 common SNPs associated with variation in LCA intima volume, intima/media ratio, and (intima+media)/EEL×100% traits. Our findings suggest that the gene discovered, Npr2, is a plausible candidate for regulation of carotid remodeling traits in response to vascular injury. Indeed, Npr2 +/− mice had significantly less intima and EEL volumes than Npr2 +/+ mice. We also found greater fibrosis in carotid IMT in Npr2 +/− mice, but both of these changes were significant only in Npr2 +/− males.
GWA identified several QTLs on human chr7q, chr12q, and chr14q that influence carotid IMT variation in the Framingham Heart and Dominican Family studies.5, 6 A follow‐up GWA mapping of the maximum common carotid IMT revealed 11 SNPs, including a proprotein convertase subtilisin/kexin type 2 in the Framingham Heart Study.2 A similar number of SNPs were associated with carotid IMT in a cohort from China.3 Despite advances in uncovering genetic causes of the carotid IMT, human genetics studies suffer from lack of power and technical limitations. An alternative for identifying new regulatory elements for human carotid IMT are experiments in animal models of carotid artery injury or atherosclerosis.13, 16, 32, 33 A primary QTL on mouse chr12, named Cath1, in addition to loci on chr5, chr9, and chr13, contribute to carotid atherosclerosis based on meta‐analysis from 3 genetic crosses between BALB/cJ, C57BL/6J, C3H/HeJ, and SM/J inbred mouse strains on the apolipoprotein E−/− background.16, 17, 18 A carotid neointima hyperplasia locus in response to injury was also detected on mouse chr12 in the genetic cross between C57BL/6H.ApoE −/− and C3H/HeJ.ApoE −/− mice, which was independent from systemic inflammation.33 In addition to carotid atherosclerosis mouse models, we developed a mouse model of low‐flow–induced carotid IMT.11, 12 We believe that this model resembles early events in vascular remodeling that are associated with progression of human carotid IMT.34 Our group discovered 3 significant carotid intima modifier loci on mouse chr2, chr11, and chr18 in response to low blood flow in a genetic cross between C3HeB/FeJ and SJL/J mice.13 We confirmed that the Im2 locus on mouse chr11 contributes to carotid intima inflammation using the congenic mapping approach.14, 15 Similar to the linkage and GWA for carotid IMT in humans, our GWA results in mice reveal new candidates on chr1, chr4, and chr12 that control carotid remodeling in response to low blood flow. The comprehensive analyses of genetic regulation of the compartments of the carotid IMT allowed us to find common SNPs that associate with intima volume, intima/media ratio, and (intima+media)/EEL×100% traits. Among the candidates, Npr2 was previously identified as a BP candidate gene on chr4 and linked to essential hypertension in humans.30, 31 Our findings further support polygenic control of carotid artery remodeling and suggest Npr2 as a plausible candidate.
A targeted deletion of exons 3 through 7, which encode the carboxyl‐terminal half of the extracellular domain and transmembrane segment of the Npr2, resulted in dwarfism and female sterility in Npr2 −/− mice.23 A rare genetic disorder, acromesomelic dysplasia, type Maroteaux, presents with short‐limbed dwarfism after homozygous loss‐of‐function mutations in human NPR2.35 The highest association of the NPR2 was found with body height and fibrinogen levels in a large‐scale GWA, which is relevant to earlier findings in a small genetic study in humans and after Npr2 perturbation in mice.23, 35 Npr2, also known as Npr‐B, belongs to a family of natriuretic peptide–binding proteins and represents 1 of the 5 transmembrane guanylyl cyclases found in humans.36 A primary ligand for Npr2, C‐type natriuretic peptide, can relax aortic rings, probably by binding to Npr2 and increasing cyclic guanosine monophosphate production that activates protein kinase GI, which phosphorylates target proteins.37 A downstream target of Npr2‐dependent signaling, protein kinase GI phosphorylates and activates a myosin light‐chain phosphatase that increases the calcium levels necessary for cell contraction, which lowers calcium sensitivity. Activation of the Npr2/cyclic guanosine monophosphate axis in pericytes is responsible for relaxation of precapillary arterioles and capillaries.38 Genetic studies in mice suggested that C‐type natriuretic peptide and Npr2 are candidate genes in salt‐induced BP.30 We and others showed that Npr2 has no significant role in BP homeostasis in mice.22, 23 However, experiments in genetically manipulated mice, hypertensive rats, and in a large human GWA study showed that C‐type natriuretic peptide production by endothelial cells is most important for reduction of BP.23, 39, 40, 41, 42 Herein, we found that Npr2 is critical for adaptation of the carotid artery in response to vascular injury. A striking difference in carotid sizes in Npr2 +/− mice was also related to a significant decrease in intima volume. A moderate increase in carotid fibrosis in Npr2 +/− mice resulted in a constrictive carotid phenotype in a nonfibrotic C57BL/6 background, as we recently reported.21 These data support our idea of genetic regulation of the unique cellular and biochemical processes in carotid artery disease.43 Our GWA findings are supported by the alteration in carotid remodeling in Npr2 +/− mice and significant reduction of NPR2 expression in human atherosclerotic plaque. We believe that future clinical studies will uncover Npr2‐mediated mechanisms of carotid IMT in humans.
Another significant finding in our study is that male, but not female, Npr2 +/− mice exhibited constrictive carotid artery remodeling in response to low blood flow. The molecular basis for sex bias in carotid IMT development may be because of direct vasoprotective properties of estrogen on endothelial cells44, 45, 46 by antagonizing inflammatory responses such as tumor necrosis factor‐α signaling.47 For example, in a surgical injury model, female mice had a >90% reduction in carotid intima formation relative to males, which was attenuated by ovariectomy.48 Functional genetic studies in rodents have identified several sexual dimorphic factors contributing to carotid remodeling that were not apparent from human data, including lower endothelial nitric oxide synthase messenger RNA levels in female aorta versus male, and less oxidized phospholipid levels in females.49, 50 Intriguingly, greater carotid pathologic remodeling in male mice mirrors sexual dimorphism in vascular injury response and disease in humans. Data from the AXA study, the Gutenberg Heart study, and an Okinawa–Nagano study revealed that men have higher carotid IMT levels relative to women.51, 52, 53 Furthermore, several risk factors for carotid IMT display sexual dimorphism: In the Tromso study, fibrinogen levels and amount of physical activity were associated with carotid IMT in men only, whereas triglyceride levels were associated with carotid IMT in women only.54, 55 Other risk factors for carotid IMT, such as age, systolic BP, HDL, total cholesterol, body mass index, and smoking, did not correlate strongly with carotid IMT in a sex‐specific manner.55 When combined, both human and mouse carotid artery data strongly suggest a protective role for estrogen in regulating vascular intima growth responses, which contribute to progression of carotid atherosclerosis and artery occlusion.
In conclusion, we have demonstrated the power of using mouse genetic analyses to identify candidate genes, and provide novel evidence for the role of Npr2 in the genetic regulation of vascular fibrosis associated with increased flow‐dependent carotid remodeling. Future studies will explore the underlying mechanisms by which Npr2 regulates fibrosis toward the goal of developing new clinical therapeutic approaches to treating vascular disease.
Sources of Funding
This study was supported in part by funds from the University of Rochester Award 2016 (to V.A.K. and M.M.D.), R01 HL134910 (to C.Y.), HL42488 (to A.J.L.), and HL140958 (to B.B.B.) the National Institutes of Health.
Disclosures
V.A.K. has received a research support from Novartis Pharmaceuticals Corp. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S8
Figures S1–S7
Acknowledgments
Ther authors thank Janice Gerloff and Kathy Donlon for help with animal handling and histologic evaluation of mouse arteries.
(J Am Heart Assoc. 2020;9:e014257 DOI: 10.1161/JAHA.119.014257.)
George J. Dugbartey is currently affiliated with the Department of Pharmacology and Toxicology, School of Pharmacy, College of Health Sciences, University of Ghana, Legon, Accra, Ghana.
For Sources of Funding and Disclosures, see page 11.
References
- 1. Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Donnell CJ, Cupples LA, D'Agostino RB, Fox CS, Hoffmann U, Hwang SJ, Ingellson E, Liu C, Murabito JM, Polak JF, et al. Genome‐wide association study for subclinical atherosclerosis in major arterial territories in the NHLBI's Framingham Heart Study. BMC Med Genet. 2007;8(suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie G, Myint PK, Voora D, Laskowitz DT, Shi P, Ren F, Wang H, Yang Y, Huo Y, Gao W, et al. Genome‐wide association study on progression of carotid artery intima media thickness over 10 years in a Chinese cohort. Atherosclerosis. 2015;243:30–37. [DOI] [PubMed] [Google Scholar]
- 4. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. [DOI] [PubMed] [Google Scholar]
- 5. Fox CS, Cupples LA, Chazaro I, Polak JF, Wolf PA, D'Agostino RB, Ordovas JM, O'Donnell CJ. Genomewide linkage analysis for internal carotid artery intimal medial thickness: evidence for linkage to chromosome 12. Am J Hum Genet. 2004;74:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sacco RL, Blanton SH, Slifer S, Beecham A, Glover K, Gardener H, Wang L, Sabala E, Juo SH, Rundek T. Heritability and linkage analysis for carotid intima‐media thickness: the family study of stroke risk and carotid atherosclerosis. Stroke. 2009;40:2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shendre A, Wiener HW, Irvin MR, Aouizerat BE, Overton ET, Lazar J, Liu C, Hodis HN, Limdi NA, Weber KM, et al. Genome‐wide admixture and association study of subclinical atherosclerosis in the Women's Interagency HIV Study (WIHS). PLoS One. 2017;12:e0188725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters SA, den Ruijter HM, Palmer MK, Grobbee DE, Crouse JR III, O'Leary DH, Evans GW, Raichlen JS, Bots ML. Extensive or restricted ultrasound protocols to measure carotid intima‐media thickness: analysis of completeness rates and impact on observed rates of change over time. J Am Soc Echocardiogr. 2012;25:91–100. [DOI] [PubMed] [Google Scholar]
- 9. Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Volzke H, Tuomainen TP, Sander D, Plichart M, Catapano AL, Robertson CM, et al. Carotid intima‐media thickness progression to predict cardiovascular events in the general population (the PROG‐IMT collaborative project): a meta‐analysis of individual participant data. Lancet. 2012;379:2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y, Guallar E, Qiao Y, Wasserman BA. Is carotid intima‐media thickness as predictive as other noninvasive techniques for the detection of coronary artery disease? Arterioscler Thromb Vasc Biol. 2014;34:1341–1345. [DOI] [PubMed] [Google Scholar]
- 11. Korshunov VA, Berk BC. Flow‐induced vascular remodeling in the mouse: a model for carotid intima‐media thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–2191. [DOI] [PubMed] [Google Scholar]
- 12. Korshunov VA, Berk BC. Strain‐dependent vascular remodeling: the “Glagov phenomenon” is genetically determined. Circulation. 2004;110:220–226. [DOI] [PubMed] [Google Scholar]
- 13. Korshunov VA, Berk BC. Genetic modifier loci linked to intima formation induced by low flow in the mouse carotid. Arterioscler Thromb Vasc Biol. 2009;29:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smolock EM, Burke RM, Wang C, Thomas T, Batchu SN, Qiu X, Zettel M, Fujiwara K, Berk BC, Korshunov VA. Intima modifier locus 2 controls endothelial cell activation and vascular permeability. Physiol Genomics. 2014;46:624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smolock EM, Machleder DE, Korshunov VA, Berk BC. Identification of a genetic locus on chromosome 11 that regulates leukocyte infiltration in mouse carotid artery. Arterioscler Thromb Vasc Biol. 2013;33:1014–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Q, Li Y, Zhang Z, Gilbert TR, Matsumoto AH, Dobrin SE, Shi W. Quantitative trait locus analysis of carotid atherosclerosis in an intercross between C57BL/6 and C3H apolipoprotein E‐deficient mice. Stroke. 2008;39:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rowlan JS, Zhang Z, Wang Q, Fang Y, Shi W. New quantitative trait loci for carotid atherosclerosis identified in an intercross derived from apolipoprotein E‐deficient mouse strains. Physiol Genomics. 2013;45:332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grainger AT, Jones MB, Chen MH, Shi W. Polygenic control of carotid atherosclerosis in a BALB/cJ x SM/J intercross and a combined cross involving multiple mouse strains. G3 (Bethesda). 2017;7:731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bennett BJ, Farber CR, Orozco L, Kang HM, Ghazalpour A, Siemers N, Neubauer M, Neuhaus I, Yordanova R, Guan B, et al. A high‐resolution association mapping panel for the dissection of complex traits in mice. Genome Res. 2010;20:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smolock EM, Ilyushkina IA, Ghazalpour A, Gerloff J, Murashev AN, Lusis AJ, Korshunov VA. A genetic locus on mouse chromosome 7 controls elevated heart rate. Physiol Genomics. 2012;44:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Korshunov VA, Quinn B, Faiyaz A, Ahmed R, Sowden MP, Doyley MM, Berk BC. Strain‐selective efficacy of sacubitril/valsartan on carotid fibrosis in response to injury in two inbred mouse strains. Br J Pharmacol. 2019;176:2795–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dugbartey GJ, Quinn B, Luo L, Mickelsen DM, Ture SK, Morrell CN, Czyzyk J, Doyley MM, Yan C, Berk BC, et al. The protective role of natriuretic peptide receptor 2 against high salt injury in the renal papilla. Am J Pathol. 2019;189:1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tamura N, Doolittle LK, Hammer RE, Shelton JM, Richardson JA, Garbers DL. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci USA. 2004;101:17300–17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Animals . National Research Council: guide for the care and use of laboratory animals. 2011.
- 25. Batchu SN, Hughson A, Gerloff J, Fowell DJ, Korshunov VA. Role of Axl in early kidney inflammation and progression of salt‐dependent hypertension. Hypertension. 2013;62:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Korshunov VA, Wang H, Ahmed R, Mickelsen DM, Zhou Q, Yan C, Doyley MM. Model‐based vascular elastography improves the detection of flow‐induced carotid artery remodeling in mice. Sci Rep. 2017;7:12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gerloff J, Korshunov VA. Immune modulation of vascular resident cells by Axl orchestrates carotid intima‐media thickening. Am J Pathol. 2012;180:2134–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kang HM, Zaitlen NA, Wade CM, Kirby A, Heckerman D, Daly MJ, Eskin E. Efficient control of population structure in model organism association mapping. Genetics. 2008;178:1709–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sugiyama F, Churchill GA, Higgins DC, Johns C, Makaritsis KP, Gavras H, Paigen B. Concordance of murine quantitative trait loci for salt‐induced hypertension with rat and human loci. Genomics. 2001;71:70–77. [DOI] [PubMed] [Google Scholar]
- 31. Rehemudula D, Nakayama T, Soma M, Takahashi Y, Uwabo J, Sato M, Izumi Y, Kanmatsuse K, Ozawa Y. Structure of the type B human natriuretic peptide receptor gene and association of a novel microsatellite polymorphism with essential hypertension. Circ Res. 1999;84:605–610. [DOI] [PubMed] [Google Scholar]
- 32. Nestor AL, Cicila GT, Karol SE, Langenderfer KM, Hollopeter SL, Allison DC. Linkage analysis of neointimal hyperplasia and vascular wall transformation after balloon angioplasty. Physiol Genomics. 2006;25:286–293. [DOI] [PubMed] [Google Scholar]
- 33. Yuan Z, Pei H, Roberts DJ, Zhang Z, Rowlan JS, Matsumoto AH, Shi W. Quantitative trait locus analysis of neointimal formation in an intercross between C57BL/6 and C3H/HeJ apolipoprotein E‐deficient mice. Circ Cardiovasc Genet. 2009;2:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wentzel JJ, Krams R, Slager CJ. Letter regarding article by Korshunov and Berk, “strain‐dependent vascular remodeling: the ‘Glagov phenomenon’ is genetically determined”. Circulation. 2005;111:e119; author reply e119 [DOI] [PubMed] [Google Scholar]
- 35. Bartels CF, Bukulmez H, Padayatti P, Rhee DK, van Ravenswaaij‐Arts C, Pauli RM, Mundlos S, Chitayat D, Shih LY, Al‐Gazali LI, et al. Mutations in the transmembrane natriuretic peptide receptor NPR‐B impair skeletal growth and cause acromesomelic dysplasia, type Maroteaux. Am J Hum Genet. 2004;75:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Potter LR, Abbey‐Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate‐dependent signaling functions. Endocr Rev. 2006;27:47–72. [DOI] [PubMed] [Google Scholar]
- 37. Drewett JG, Fendly BM, Garbers DL, Lowe DG. Natriuretic peptide receptor‐B (guanylyl cyclase‐B) mediates C‐type natriuretic peptide relaxation of precontracted rat aorta. J Biol Chem. 1995;270:4668–4674. [DOI] [PubMed] [Google Scholar]
- 38. Spiranec K, Chen W, Werner F, Nikolaev VO, Naruke T, Koch F, Werner A, Eder‐Negrin P, Dieguez‐Hurtado R, Adams RH, et al. Endothelial C‐type natriuretic peptide acts on pericytes to regulate microcirculatory flow and blood pressure. Circulation. 2018;138:494–508. [DOI] [PubMed] [Google Scholar]
- 39. Moyes AJ, Khambata RS, Villar I, Bubb KJ, Baliga RS, Lumsden NG, Xiao F, Gane PJ, Rebstock AS, Worthington RJ, et al. Endothelial C‐type natriuretic peptide maintains vascular homeostasis. J Clin Invest. 2014;124:4039–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nakao K, Kuwahara K, Nishikimi T, Nakagawa Y, Kinoshita H, Minami T, Kuwabara Y, Yamada C, Yamada Y, Tokudome T, et al. Endothelium‐derived C‐type natriuretic peptide contributes to blood pressure regulation by maintaining endothelial integrity. Hypertension. 2017;69:286–296. [DOI] [PubMed] [Google Scholar]
- 41. Caniffi C, Cerniello FM, Gobetto MN, Sueiro ML, Costa MA, Arranz C. Vascular tone regulation induced by C‐type natriuretic peptide: differences in endothelium‐dependent and ‐independent mechanisms involved in normotensive and spontaneously hypertensive rats. PLoS One. 2016;11:e0167817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. International Consortium for Blood Pressure Genome‐Wide Association S , Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov's phenomenon. Arterioscler Thromb Vasc Biol. 2007;27:1722–1728. [DOI] [PubMed] [Google Scholar]
- 44. Wang D, Oparil S, Chen YF, McCrory MA, Skibinski GA, Feng W, Szalai AJ. Estrogen treatment abrogates neointima formation in human C‐reactive protein transgenic mice. Arterioscler Thromb Vasc Biol. 2005;25:2094–2099. [DOI] [PubMed] [Google Scholar]
- 45. Arnal JF, Fontaine C, Billon‐Gales A, Favre J, Laurell H, Lenfant F, Gourdy P. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. 2010;30:1506–1512. [DOI] [PubMed] [Google Scholar]
- 46. Dworatzek E, Mahmoodzadeh S. Targeted basic research to highlight the role of estrogen and estrogen receptors in the cardiovascular system. Pharmacol Res. 2017;119:27–35. [DOI] [PubMed] [Google Scholar]
- 47. Xing D, Feng W, Miller AP, Weathington NM, Chen YF, Novak L, Blalock JE, Oparil S. Estrogen modulates TNF‐alpha‐induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor‐beta activation. Am J Physiol Heart Circ Physiol. 2007;292:H2607–H2612. [DOI] [PubMed] [Google Scholar]
- 48. Tolbert T, Thompson JA, Bouchard P, Oparil S. Estrogen‐induced vasoprotection is independent of inducible nitric oxide synthase expression: evidence from the mouse carotid artery ligation model. Circulation. 2001;104:2740–2745. [DOI] [PubMed] [Google Scholar]
- 49. Chu Y, Heistad DD, Knudtson KL, Lamping KG, Faraci FM. Quantification of mRNA for endothelial NO synthase in mouse blood vessels by real‐time polymerase chain reaction. Arterioscler Thromb Vasc Biol. 2002;22:611–616. [DOI] [PubMed] [Google Scholar]
- 50. Liu J, Li W, Chen R, McIntyre TM. Circulating biologically active oxidized phospholipids show on‐going and increased oxidative stress in older male mice. Redox Biol. 2013;1:110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gariepy J, Salomon J, Denarie N, Laskri F, Megnien JL, Levenson J, Simon A. Sex and topographic differences in associations between large‐artery wall thickness and coronary risk profile in a French working cohort: the AXA Study. Arterioscler Thromb Vasc Biol. 1998;18:584–590. [DOI] [PubMed] [Google Scholar]
- 52. Sinning C, Wild PS, Echevarria FM, Wilde S, Schnabel R, Lubos E, Herkenhoff S, Bickel C, Klimpe S, Gori T, et al. Sex differences in early carotid atherosclerosis (from the community‐based Gutenberg‐Heart Study). Am J Cardiol. 2011;107:1841–1847. [DOI] [PubMed] [Google Scholar]
- 53. Shimabukuro M, Hasegawa Y, Higa M, Amano R, Yamada H, Mizushima S, Masuzaki H, Sata M. Subclinical carotid atherosclerosis burden in the Japanese: comparison between Okinawa and Nagano residents. J Atheroscler Thromb. 2015;22:854–868. [DOI] [PubMed] [Google Scholar]
- 54. Stensland‐Bugge E, Bonaa KH, Joakimsen O, Njolstad I. Sex differences in the relationship of risk factors to subclinical carotid atherosclerosis measured 15 years later: the Tromso study. Stroke. 2000;31:574–581. [DOI] [PubMed] [Google Scholar]
- 55. Stensland‐Bugge E, Bonaa KH, Joakimsen O. Age and sex differences in the relationship between inherited and lifestyle risk factors and subclinical carotid atherosclerosis: the Tromso study. Atherosclerosis. 2001;154:437–448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S8
Figures S1–S7


