Abstract
Background
Peripheral artery disease (PAD) is a manifestation of atherosclerosis characterized by reduced blood flow to the lower extremities and mobility loss. Preliminary evidence suggests PAD damages skeletal muscle, resulting in muscle impairments that contribute to functional decline. We sought to determine whether PAD is associated with an altered macrophage profile in gastrocnemius muscles and whether muscle macrophage populations are associated with impaired muscle phenotype and walking performance in patients with PAD.
Methods and Results
Macrophages, satellite cells, and extracellular matrix in gastrocnemius muscles from 25 patients with PAD and 7 patients without PAD were quantified using immunohistochemistry. Among patients with PAD, both the absolute number and percentage of cluster of differentiation (CD) 11b+CD206+ M2‐like macrophages positively correlated to satellite cell number (r=0.461 [P=0.023] and r=0.416 [P=0.042], respectively) but not capillary density or extracellular matrix. The number of CD11b+CD206− macrophages negatively correlated to 4‐meter walk tests at normal (r=−0.447, P=0.036) and fast pace (r=−0.510, P=0.014). Extracellular matrix occupied more muscle area in PAD compared with non‐PAD (8.72±2.19% versus 5.30±1.03%, P<0.001) and positively correlated with capillary density (r=0.656, P<0.001).
Conclusions
Among people with PAD, higher CD206+ M2‐like macrophage abundance was associated with greater satellite cell numbers and muscle fiber size. Lower CD206− macrophage abundance was associated with better walking performance. Further study is needed to determine whether CD206+ macrophages are associated with ongoing reparative processes enabling skeletal muscle adaptation to damage with PAD.
Registration
URL: https://www.clinicaltrials.gov; Unique identifiers: NCT00693940, NCT01408901, NCT0224660.
Keywords: macrophage, peripheral artery disease, skeletal muscle, walking performance
Subject Categories: Peripheral Vascular Disease, Aging, Stem Cells, Inflammation, Physiology
Nonstandard Abbreviations and Acronyms
- ABI
ankle‐brachial index
- BRAVO
Biomarker Risk Assessment in Vulnerable Outpatients
- CD
cluster of differentiation
- ECM
extracellular matrix
- FCGR3B
Fc fragment of IgG, low affinity IIIb, receptor
- GOALS
Group Oriented Arterial Leg Study
- PAD
peripheral artery disease
- PROPEL
Progenitor Cell Release Plus Exercise Improve Functional Performance in PAD
- RESTORE
Resveratrol to Improve Outcomes in Older People With PAD
- SR
Sirius Red
- WALCS III
Walking and Leg Circulation Study III
- WGA
wheat germ agglutinin
Clinical Perspective
What Is New?
People with peripheral artery disease (PAD) had higher cluster of differentiation (CD) 206+ macrophage abundance and extracellular matrix within the gastrocnemius muscle compared with those without PAD.
In patients with PAD, CD206+ macrophage abundance was associated with gastrocnemius muscle stem cell content and fiber size, consistent with the hypothesis that CD206+ macrophages coordinate muscle maintenance and repair during chronic ischemia‐reperfusion injury with PAD.
In the gastrocnemius muscle of patients with PAD, higher extracellular matrix was associated with higher capillary density; higher numbers of CD206− macrophages negatively correlated with 4‐meter walking performance in patients with PAD.
What Are the Clinical Implications?
CD206+ macrophage‐mediated reparative processes may be ongoing in gastrocnemius muscles of people with PAD and targeting these processes could preserve walking performance.
Restoring blood flow in conjunction with promoting adaptive muscle responses may provide the most effective approach to improve walking performance in patients with PAD.
Peripheral artery disease (PAD) is characterized by lower extremity muscle discomfort, weakness and pain during walking and is frequently accompanied by a reduction in physical activity, walking speed, endurance, and quality of life.1, 2, 3, 4, 5, 6 Artery narrowing may occur over months or years and the consequent reduction of blood flow to the lower limbs results in ischemia during walking followed by reperfusion during rest, both contributing to muscle damage.7, 8 Muscle ischemia‐reperfusion injury in PAD is associated with mitochondrial disruption, production of free radicals, and inflammation.7, 9, 10 Although revascularization is effective at restoring blood flow, vascular stenting is invasive, is not appropriate for many patients with PAD, and does not have long‐term durability.11 Supervised exercise is an effective therapy for improving walking performance in PAD but does not alter lower extremity blood flow, suggesting that stimulating adaptive muscle responses in addition to restoring blood flow may provide an effective strategy to reverse muscle dysfunction with PAD.12, 13, 14, 15
Skeletal muscle injury and repair are associated with macrophage infiltration, studied primarily in the context of acute injury via toxin injection into muscle in rodents.16, 17, 18, 19, 20, 21 Macrophages have also been shown to infiltrate human muscle following acute injury induced by electrical stimulation.22 Macrophages are highly adaptive immune cells with a continuum of phenotypes and polarization states. Although conventional classification is oversimplified and inadequate, particularly with regard to tissue resident macrophages, they are commonly referred to as classically activated, inflammatory M1 or alternatively activated, anti‐inflammatory M2 within the context of muscle regeneration.21, 23 Muscle repair is a tightly regulated process with initial infiltration of neutrophils that exacerbate damage, followed by M1 macrophages that phagocytize debris and secrete inflammatory cytokines. M1 macrophages are then replaced by M2 macrophages that secrete anti‐inflammatory cytokines and factors that promote extracellular matrix (ECM) accumulation during wound healing.24, 25, 26, 27, 28 Furthermore, M2 macrophages produce growth factors that promote stem cell recruitment29 and function following skeletal muscle injury.30, 31, 32 Muscle stem cells, satellite cells, are directly responsible for muscle regeneration following injury33, 34 and in vitro studies have shown that macrophages directly affect satellite cell proliferation and differentiation.24, 35 The role of satellite cell‐dependent muscle repair and the interplay with macrophage populations in response to chronic muscle damage associated with PAD is unknown.
Inflammation is associated with PAD severity and progression.36, 37, 38 Systemically, increases in mRNA levels of inflammatory biomarkers occur within peripheral blood monocytes36 and it is well established that people with PAD have elevated circulating concentrations of inflammatory markers such as C‐reactive protein and interleukin 6 compared with people without PAD.38, 39, 40, 41, 42, 43, 44 Among people with PAD, higher circulating inflammatory markers are associated with greater calf (gastrocnemius) skeletal muscle damage45 and have been linked to poorer functional outcomes.46, 47 However, reports on the relative abundance of different macrophage populations in response to ischemia in muscle are inconsistent. Recent work showed a greater abundance of macrophages, particularly M1‐like cluster of differentiation (CD) 80+ macrophages, in muscles of PAD patients with intermittent claudication compared with people without PAD.48 On the other hand, M2‐like macrophages promote angiogenesis and are higher in ischemic muscle than in normoxic muscle from the same patient with critical limb ischemia.49 Studies in rodent models of hind limb ischemia have demonstrated the association of macrophage populations with muscle damage, inflammation and recovery following experimental blood flow restriction.48, 49, 50, 51, 52, 53, 54 Altering gene expression to disrupt macrophage M2 differentiation following arterial resection/ligation in mouse hind limb muscles resulted in increased M1 macrophages and decreased angiogenesis.50 Similarly, a greater abundance of inflammatory M1 macrophages was associated with impaired walking early following artery ligation in mice.51 Liu et al52 reported that treatment of mice with the anti‐inflammatory/antioxidant, curcumin, following hind limb ischemia decreased macrophage infiltration and was associated with improved treadmill running. Other studies have suggested that M2 macrophages are beneficial, promoting angiogenesis in muscle following ischemia‐induced limb injury in rodents.49, 55, 56, 57, 58
We recently characterized macrophages resident in healthy human skeletal muscle, independent of injury, using both fluorescence‐activated cell sorting and immunohistochemistry.59 These analyses showed that CD11b is present on all resident muscle macrophages and only a very small subpopulation lack the well‐accepted M2 cell surface marker CD206. In a separate study, the abundance of CD206‐expressing macrophages increased with exercise training, and a higher abundance of CD206+ macrophages was associated with increased muscle fiber size and satellite cell abundance, suggesting that CD206+ macrophages in humans promote muscle adaptation and are, therefore, M2‐like.60 The purpose of this study was to quantify macrophage content in gastrocnemius muscles from individuals with PAD, compared with those without PAD, and to relate macrophage abundance and subtypes with walking performance in people with PAD. First, we hypothesized that those with PAD would have a higher abundance of macrophages lacking M2 cell surface markers (CD206 and CD163) than those without PAD, reflecting a more inflammatory M1‐like phenotype,48 which would be related to disease severity. Second, we hypothesized that among people with PAD, a higher relative abundance of CD206+ M2‐like macrophages would be associated with greater capillary density and satellite cell content and better walking performance.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon request.
Participants
Five observational studies or clinical trials at Northwestern University Feinberg School of Medicine were accessed to identify patients with PAD and those without PAD. ClinicalTrials.gov identifiers are listed where applicable. The studies accessed included 2 observational studies (WALCS III [Walking and Leg Circulation Study III]61, 62, 63 and BRAVO [Biomarker Risk Assessment in Vulnerable Outpatients], NCT0227678161, 64, 65) and 3 randomized trials (GOALS [Group Oriented Arterial Leg Study], NCT00693940; PROPEL [Progenitor Cell Release Plus Exercise Improve Functional Performance in PAD], NCT0140890166, 67, 68; and RESTORE [Resveratrol to Improve Outcomes in Older People With PAD], NCT0224660). Data for participants randomized into clinical trials were collected at baseline, and participants did not receive any study interventions before muscle biopsies. All studies were performed in accordance with the Declaration of Helsinki and were approved by the institutional review board at Northwestern University. All participants provided written informed consent before enrollment in any study. All procedures were performed at baseline, before interventions. Medications were identified by asking participants to bring their medication bottles to the baseline study visit or compose a list of their current medications. A trained professional reviewed each medication (M.M.M.) and we previously reported the number of patients taking statins.69 Intermittent claudication was determined using the San Diego claudication questionnaire and according to published methods.70 Detailed comorbidities and medications of this cohort of PAD (n=25) and non‐PAD (n=7) are presented in Table 1.
Table 1.
Characteristics of Participants Without and Participants With PAD
| Non‐PAD (N=7)b | PAD (N=25) | P Value | |
|---|---|---|---|
| Age, y | 72.1±6.1 | 68.1±10.1 | 0.205 |
| ABI | 1.13±0.11 | 0.62±0.13 | <0.001a |
| BMIb | 25.1±2.5 | 28.2±4.9 | 0.156 |
| Men, % | 57.1 | 60.0 | 1.000 |
| Black race, % | 28.6 | 76.0 | 0.032a |
| Diabetes mellitus, %b | 0.0 | 36.0 | 0.530 |
| Hypertension, %b | 33.3 | 88.0 | 0.073 |
| Heart failure, %b | 0.0 | 0.0 | NA |
| Pulmonary disease, %b | 0.0 | 20.0 | 1.000 |
| Angina, %b | 0.0 | 8.0 | 1.000 |
| Myocardial infarction, %b | 0.0 | 8.0 | 1.000 |
| Cancer, %b | 66.7 | 12.0 | 0.073 |
| Current smoker, %b | 0.0 | 52.0 | 0.226 |
| Intermittent claudication, %b | 0.0 | 24.0 | 1.000 |
| Walking exercise frequency past wkb | 1.33±1.53 | 1.28±2.70 | 0.962 |
| Medications, No. (%) | |||
| ACEIs or ARBs | 4 (57.1) | 14 (56.0) | 1.000 |
| Antiplatelets | 1 (14.3) | 18 (72.0) | 0.010a |
| Statins | 4 (57.1) | 20 (80.0) | 0.327 |
| Cilostazol or pentoxifylline | 0 (0.00) | 5 (20.0) | 0.560 |
Values are expressed as mean±SD. ABI indicates ankle‐brachial index; ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; and PAD, peripheral artery disease.
Statistically significant P<0.05.
Based on n=3 non‐PAD participants.
Inclusion and Exclusion Criteria
Participants with a baseline ankle‐brachial index (ABI) of <0.90 were classified as having PAD, while participants with baseline ABI values between 0.9 and 1.30 were considered non‐PAD. The inability to walk without aide (walker or wheelchair), occurrence of a recent major health incident (operation or cardiovascular event), presence of significantly impaired cognitive function, and/or terminal illness warranted exclusion according to reported criteria.61, 64, 65, 66, 67, 68 Questionnaires, administered in a standard fashion, were used to determine the presence of comorbidities, such as diabetes mellitus and smoking history. One patient with PAD included in our prior publication (n=26)69 did not have a sufficient muscle biopsy sample for these analyses and was not included in the findings reported here. Walking performance data were only collected for participants with PAD, and only those individuals in which the data were obtained within 6 months of the muscle biopsy were included in functional analyses (n=23 of the 25 participants with PAD).
Ankle‐Brachial Index
The ABI was measured as previously described.69 A handheld Doppler probe (Nicolet Vascular Pocket Dop II) was used to obtain systolic blood pressures at the following locations: right brachial, dorsalis pedis, and posterior tibial arteries; and left dorsalis pedis, posterior tibial, and brachial arteries following 5 minutes of rest in the supine position. Dividing the average of the dorsalis pedis and posterior tibial pressures in each leg by the average of the brachial pressures gives the calculated ABI for each leg.71, 72 Only the ABI measurement from the leg with the lower ABI was used in determining associations with muscle features; this was also the leg used to obtain muscle biopsies. Using only either the highest or lowest of the 2 leg pressures did not change the reported outcomes (data not shown).
Six‐Minute Walk
Using a standardized script, a certified research coordinator asked patients to complete as many laps as possible in 6 minutes, walking back and forth in a 100‐foot hallway.2, 5, 73, 74 Participants were asked to walk continuously, but, if rest was needed, participants were allowed to stop during the 6 minutes. Standardized encouragement was given to help participants complete the task and be aware of their progress. A research assistant walked with and slightly behind the participant, so that the research assistant did not pace the participant.
Four‐Meter Walk Tests
Patients were asked to walk a 4‐meter distance at both “normal” and “fast” speeds. For each speed, the task was performed twice and time to complete the 4‐meter distance was recorded.75, 76 The faster walk for each speed was used to calculate walking velocity.
Muscle Biopsies
Muscle biopsies were obtained at baseline, before any trial intervention, from the medial head of the gastrocnemius muscle as described in detail elsewhere.69 Approximately 100 mg of tissue was embedded in tragacanth gum on cork, frozen in liquid‐nitrogen cooled isopentane, and stored at −80°C. Biopsies were sectioned in a cryostat with a chamber temperature of −22 to −25°C at a thickness of 7 μm. Before immunohistochemical or histochemical analyses, slides were removed from storage at −20°C and air dried at room temperature for 10 to 15 minutes.
Immunohistochemistry
Macrophage identification and quantification were performed according to a detailed and validated method.59 Briefly, macrophages were identified by stepwise staining with antibodies against the pan macrophage marker CD11b (Cell Sciences) or the M2c macrophage marker CD163 (Hycult Biotech), followed by CD206, a marker of M2 macrophages (R&D Systems) (Table S1). For staining, slides were fixed in −20°C acetone and blocking steps were taken to prevent nonspecific background staining and cross‐reactivity between amplification reagents. CD11b, CD163, and CD206 labeling was amplified using a biotin, streptavidin system. For CD11b or CD163, an extra amplification step with fluorophore‐conjugated tyramide signal amplification reagent was performed. Sections were washed and nuclei were visualized using 4′,6‐diamidino‐2‐phenylindole (DAPI) (ThermoFisher). After staining, slides were coverslipped with Vectashield (Vector Laboratories, H‐1000) and stitched images of whole muscle cross‐sections were acquired with a 20× objective. The abundance of all CD11b‐expressing cells (total macrophages), CD11b+CD206−, and CD11b+CD206+ were manually quantified using the event count tool in Zen software (Zeiss). For samples with remaining tissue (n=6 non‐PAD, n=18 PAD), CD163+CD206+ macrophages were also quantified. Of note, CD163 and CD11b staining cannot be performed on the same samples because of amplification cross‐reactivity; however, the proportion of CD206+ macrophages co‐expressing CD163 was between 89% and 99.7%, supporting the idea that CD206+ macrophages are M2‐like.
A primary antibody against CD16B/FCGR3B (LifeSpan BioSciences, Inc), a neutrophil‐specific marker in human tissue, was used to assess the presence of neutrophils within muscle samples. For staining, sections were fixed for 30 minutes with Bouin fixative (Electron Microscopy Sciences, 15990), dehydrated with xylenes, and rehydrated with decreasing concentrations of ethanol. Heat‐induced epitope retrieval was performed by incubating slides in sodium citrate buffer within a heated water bath, beginning at 65°C and heating to 92°C, then incubating at 92°C for an additional 20 minutes. Slides were cooled to room temperature and washed with distilled water. Nuclei were counterstained with hematoxylin, washed in tap water, differentiated with acidified ethanol, blued in Scott water, and rinsed before blocking. Endogenous alkaline phosphatases were quenched by incubating sections for 10 minutes with Bloxall reagent (Vector Laboratories, SP‐6000). Sections were then washed, blocked for 1 hour with 2.5% normal horse serum (Vector Laboratories, S‐2012) and incubated with primary antibody for 2 hours at room temperature. CD16 antibody was amplified using ImmPRESS AP (alkaline phosphatase) anti‐rabbit polymer (Vector Laboratories, MP‐5401) and visualized by incubation with the alkaline phosphatase substrate, Vector Red (Vector Laboratories, MP‐7401), according to the manufacturer's instructions. After staining, slides were coverslipped with VectaMount AQ Aqueous Mounting Medium (Vector Laboratories, H‐5501) and representative images were acquired using transmitted light microscopy.
ECM was quantified using α‐wheat germ agglutinin (WGA), which binds to glycosaminoglycans.77 After fixation with 4% paraformaldehyde, sections were washed with PBS then incubated with Texas Red–conjugated WGA for 2 hours (ThermoFisher). Sections were washed and coverslipped with Vectashield. Stitched images of whole muscle cross‐sections were acquired with a 10× objective. Quantification of WGA was performed by setting a threshold for positive staining and determining the percent of total area in the region of interest occupied by this threshold. At least 3 regions of interest/section were analyzed using the interactive measurement wizard in AxioVision software version 4.2.8 (Zeiss). Total collagen content within the ECM was quantified with Sirius Red (SR) staining. Collagens within muscle sections were denatured by fixing for 15 minutes with Bouin fixative at 56°C in a sealed chamber within a heated water bath. Following fixation, the sealed chamber was transferred to a chemical fume hood, Bouin fixative was removed, and slides were washed with distilled water. Following the final wash, slides were incubated for 2 hours in 0.1% SR staining solution (Electron Microscopy Sciences, 26357‐02). Following staining, slides were washed in 0.5% acetic acid in distilled water, dehydrated in 95% ethanol followed by 100% ethanol, and equilibrated in xylenes. Slides were mounted with Cytoseal 60 (ThermoFisher, 8311), which was allowed to harden in a chemical fume hood for 24 hours. Whole muscle cross‐sections were imaged with a 10× objective and quantification of SR was performed similar to WGA quantification.
To visualize collagen surrounding capillaries, frozen muscle sections were dried and rehydrated for 5 minutes with 1x PBS, followed by two 5‐minute washes with PBS. Sections were blocked for 1 hour in 2.5% normal horse serum then incubated for 90 minutes with a mixture of 2 lectins: biotinylated Ulex europaeus (Vector Laboratories) and biotinylated Griffonia Simplicifolia (Vector Laboratories), and a pan‐collagen antibody (ThermoFisher). Sections were then washed and incubated for 1 hour with Streptavidin Alexa Fluor 594 (ThermoFisher) to visualize capillaries and anti‐Rabbit Alexa Fluor 488 (ThermoFisher) to visualize collagen. After 3 final washes, the slides were coverslipped with Vectashield and imaged.
Analysis of satellite cell abundance was performed as follows. After drying at room temperature, sections were fixed in −20°C acetone, washed with PBS, incubated with 3% hydrogen peroxide, and blocked for 1 hour in 2.5% normal horse serum. Satellite cells were labelled by overnight incubation with anti–paired box 7 (Pax7) (Developmental Studies Hybridoma Bank, University of Iowa). Following PBS washes, sections were incubated with biotinylated anti‐mouse IgG1 (Jackson ImmunoResearch Laboratories) for 90 minutes. Washes were repeated followed by a 1‐hour incubation with streptavidin horseradish peroxidase (ThermoFisher). Fluorescent labelling was achieved by incubating with SuperBoost tyramide signal amplification Alexa Fluor 488 in PBS (ThermoFisher) for 20 minutes. Primary antibody against laminin (Millipore Sigma) was added followed by anti‐Rabbit Alexa Fluor 594 secondary antibody (ThermoFisher) to visualize and quantify muscle fibers. Sections were washed, incubated for 10 minutes with DAPI to label nuclei, and coverslipped. Whole cross‐section images were acquired with a 20× objective, satellite cells were identified as Pax7+/DAPI+, and quantification was expressed relative to laminin‐delineated fiber number. Laminin‐stained images were previously used to determine fiber size by minimum feret diameter.69
For a detailed list of all antibodies used for immunohistochemistry, see Table S1. An Axio Imager M1 (Zeiss) equipped with both ZEN software (blue edition, v2.3) and AxioVision (4.8.2) was used to capture stitched images of whole muscle cross‐sections for all image quantification.
Statistical Analysis
Continuous variables were summarized with mean and SD, except the abundance of macrophage populations between non‐PAD and PAD, which shows mean and standard error (SE). Categorical variables were compared using Fisher exact test. The distribution of continuous variables was assessed graphically and there were no statistically significant violations of normal distribution assumption for any of the variables of interest. Since the proportion of black patients was statistically significantly different between non‐PAD and PAD, we compared the muscle measures between participants with PAD and those without PAD (shown in Table 2 based on t tests (without assuming equal variance) stratified by race. Specifically, we compared PAD with non‐PAD in black and white participants, separately, and then combined the test results using Mantel–Haenszel weights. Partial correlation coefficients (r) were estimated to quantify relationships among muscle characteristics and walking performance within PAD after adjustment of race. The 95% CIs for partial correlation coefficients were determined using Fisher Z transformation. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc) and graphs were made using Prism 7 (GraphPad Software). All tests were 2‐tailed (α<0.05).
Table 2.
Race‐Adjusted Muscle Characteristics in Participants With and Without PAD
| Non‐PAD (N=7)a | PAD (N=25)b | P Value | |
|---|---|---|---|
| Total macrophages, per fibera | 0.44±0.16 | 0.63±0.36 | 0.139 |
| CD11b+CD206−, per fibera | 0.14±0.05 | 0.16±0.09 | 0.685 |
| CD11b+CD206+, per fibera | 0.30±0.11 | 0.48±0.29 | 0.094 |
| CD11b+CD206+, % of total macrophagesa | 67.69±2.97 | 74.03±8.74 | 0.036‡ |
| Satellite cells, per 100 fibers | 29.14±14.25 | 28.06±14.75 | 0.930 |
| ECM, % of total areab | 5.30±1.03 | 8.72±2.19 | <0.001‡ |
| SR staining, % of total areab | 5.07±1.19 | 7.74±2.88 | 0.041‡ |
Values are expressed as mean±SD. CD indicates cluster of differentiation; ECM, extracellular matrix; PAD, peripheral artery disease; and SR, Sirius Red.
Based on n=6 non‐PAD participants.
Based on n=24 patients with PAD.
P value is adjusted for race, ‡statistically significant P<0.05.
Results
Participant Characteristics
The demographic and clinical characteristics of the study population are shown in Table 1. Gastrocnemius muscle biopsies were analyzed from 25 participants with PAD and 7 participants without PAD. Muscle fiber size and fiber type distribution, mitochondrial markers, autophagy markers, and capillary density in muscle biopsies from these individuals were previously reported.69
Higher Ratio of CD206+ to CD206− Macrophages in Gastrocnemius Muscles From Patients With PAD Compared With Patients Without PAD
Macrophages were quantified on gastrocnemius muscle cross‐sections using immunohistochemistry with antibodies against the cell surface markers CD11b, a pan macrophage marker in muscle, and CD206, an accepted M2 macrophage marker, both previously validated in human muscle.59 Representative images in Figure 1 (Figure 1A and the higher magnification of the boxed region in Figure 1B) show total CD11b+ macrophages and coexpression of CD206 on the majority of CD11b+ macrophages. Quantification of the total number of CD11b+ macrophages showed that the average number of macrophages/fiber was higher in muscles from participants with PAD compared with participants without PAD, but the difference did not reach statistical significance after adjusting for race (0.63/fiber versus 0.44/fiber, P=0.139) (Figure 2A), likely attributable to the highly variable number of total macrophages in muscles from participants with PAD compared with patients without PAD (0.07–1.57/fiber versus 0.23–0.66/fiber, respectively). There was no difference in CD11b+CD206− macrophages/fiber (Figure 1B, arrows) between PAD and non‐PAD muscles (race‐adjusted) (Figure 2B). Immunohistochemical analysis of neutrophils using an antibody against the neutrophil specific marker CD16B showed few CD16B+ cells in muscle from either non‐PAD or PAD (data not shown). In PAD muscles, there was a trend for higher CD11b+CD206+ macrophages/fiber compared with non‐PAD muscles (P=0.094) (Figure 2C). As a result, the relative abundance of CD206+ macrophages, expressed as a percentage of total CD11b+ macrophages (% of total [race‐adjusted]) (Figure 2D), was significantly greater in PAD compared with non‐PAD muscles (P=0.036) (Figure 2D).
Figure 1. Representative images of total cluster of differentiation (CD) 11b+, CD206−, and CD206+ macrophage abundance in the gastrocnemius muscle of patients with peripheral artery disease (PAD) compared with patients without PAD.
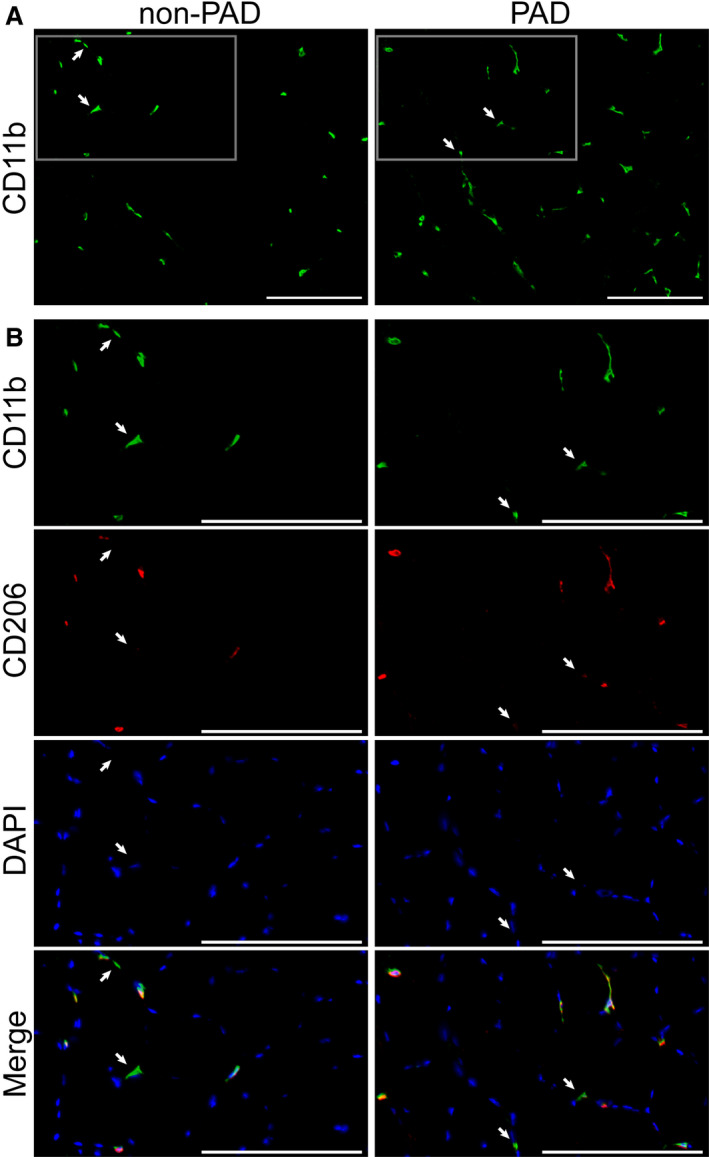
A, Total muscle macrophages in patients without PAD (left) and patients with PAD (right) visualized by immunohistochemistry with an antibody against the pan‐macrophage marker CD11b (green). Scale bar=200 µm. B, Higher magnification images of macrophages from boxed regions in panel A, showing CD11b+ (green) macrophages, CD206+ (red) macrophages, and the merged images, also overlaid with DAPI staining of nuclei (blue). Whereas the majority of macrophages are CD11b+CD206+, white arrows indicate CD11b+CD206− macrophages. Scale bar=200 µm.
Figure 2. Quantification of macrophage content in gastrocnemius muscle cross‐sections. A through D, Non‐peripheral artery disease (non‐PAD, n=6), peripheral artery disease (PAD) (n=25).
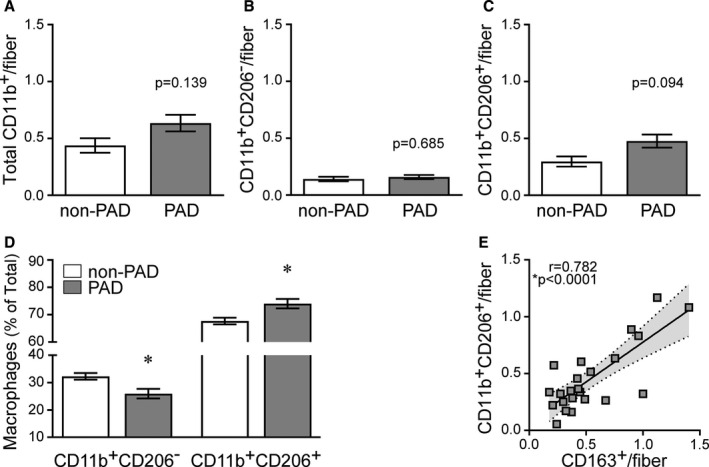
Data are expressed as mean±SE; P values adjusted for race, *significance P≤0.05. A, Total cluster of differentiation (CD) 11b+ macrophages in patients with PAD compared with patients without PAD. B, CD11b+CD206− macrophages in patients without PAD and patients with PAD. C, CD11b+CD206+ macrophages in patients without PAD and patients with PAD. D, Relative abundance (% of total) of CD206− and CD206+ macrophages in patients with PAD and patients without PAD. E, Association of the M2c macrophage marker CD163 with the number of CD206+ macrophages, supporting CD206+ macrophages as M2‐like. Non‐PAD (n=5), PAD (n=18). Association determined by Pearson product moment correlation, r=partial correlation coefficient.
All values are displayed in Table 2 and collectively show that CD206+ macrophages are the predominant macrophage population in skeletal muscles from all participants. These are likely M2‐like macrophages, as the majority of CD206+ macrophages (89.3–99.7%) also expressed CD163, another M2 cell surface marker, and the number of CD163+ macrophages significantly correlated to the number of CD11b+CD206+ macrophages on adjacent sections (r=0.782, P<0.0001) (Figure 2E). Macrophage content was not correlated to ABI (Table S2), a widely accepted measure of PAD severity.3, 5, 78
CD206+ Macrophages are Positively Associated With Satellite Cell Number and Fiber Size But Not Capillary Density in PAD Gastrocnemius Muscle
Immunohistochemistry for satellite cell content (Figure 3A through 3C), expressed as the number of Pax7+ nuclei/100 fibers, showed no difference in gastrocnemius muscle cross‐sections between PAD and non‐PAD (Table 2, all values race‐adjusted). Scatter plots shown in Figure 4 illustrate positive correlations between the relative abundance of CD206+ macrophages and satellite cell content (r=0.416, P=0.042) (Figure 4A), as well as CD206+ macrophages and mean fiber size (r=0.397, P=0.054) (Figure 4B) within PAD muscles. In PAD muscles, satellite cell content also positively correlated with mean fiber size (r=0.557, P=0.004) (Figure 4C). In addition to the relative abundance, the absolute number of CD206+ macrophages/fiber positively correlated to satellite cell content (r=0.461, P=0.023) (Table S2). Double immunohistochemistry with CD206 and Pax7 antibodies showed that CD206+ macrophages were frequently found in close proximity to satellite cells (Figure 4D and Figure S1).
Figure 3. Representative images of muscle satellite cells with laminin‐demarcated muscle fibers in gastrocnemius from peripheral artery disease (PAD).
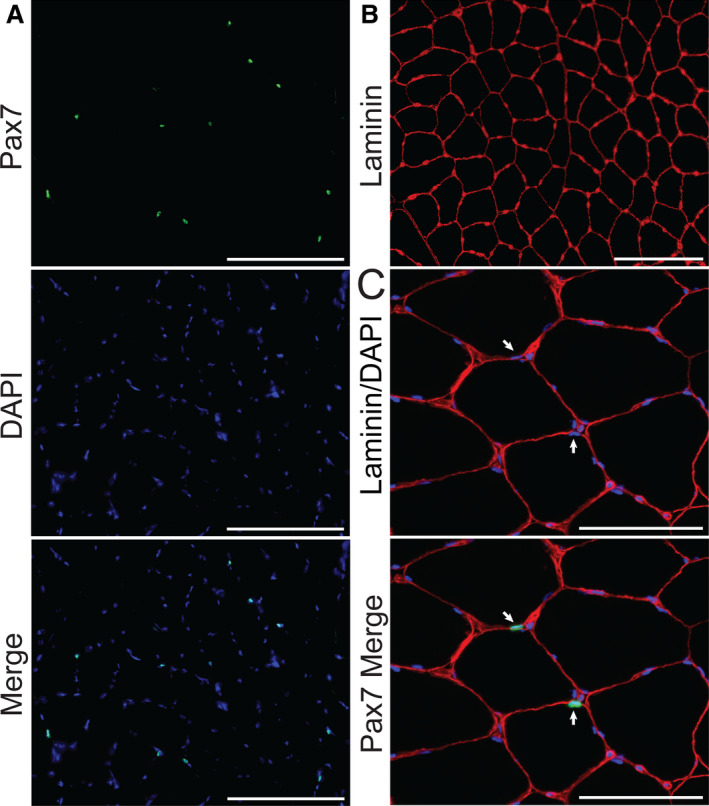
A, Paired box 7+ (Pax7+) (green) staining overlapping cell nuclei (DAPI, blue) identifies satellite cells. Scale bar=200 µm. B, Laminin demarcating muscle fiber borders (red). Scale bar=200 µm. C, Higher magnification showing satellite cells located beneath the laminin‐stained basal lamina. White arrows point to satellite cell nuclei; Pax7 (green), nuclei (DAPI, blue), laminin (red). Scale bar=100 µm.
Figure 4. Relative abundance of cluster of differentiation (CD) 206+ macrophages is positively associated with satellite cell number and fiber size in the gastrocnemius muscle from patients with peripheral artery disease (PAD).
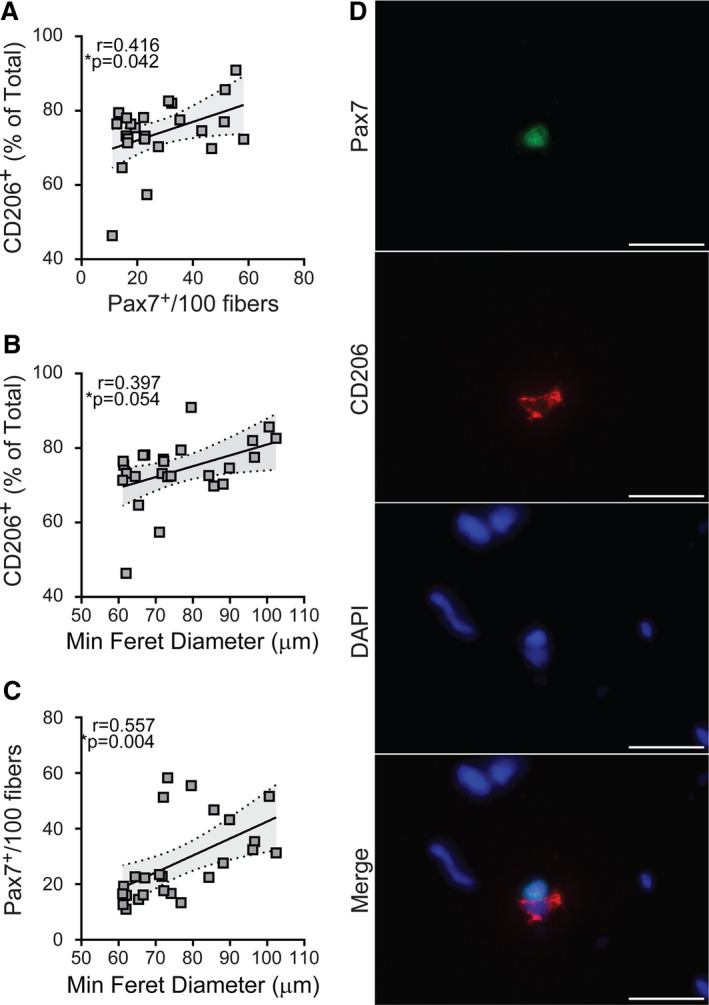
Scatterplots showing correlations between the relative abundance of CD206+ macrophages and (A) satellite cell content and (B) fiber size, measured by minimum feret diameter. C, Correlation between satellite cells and fiber size. For all measures, relationships determined by Pearson product moment correlation adjusting for race, r=partial correlation coefficient, PAD (n=25). D, Representative images showing a satellite cell (paired box 7 [Pax7]+, green) and a CD206+ macrophage (red) in close proximity. DAPI (blue) stains nuclei. Scale bar=20 µm.
We previously measured capillary density within gastrocnemius muscle from these volunteers and reported higher capillary density within muscles from patients with PAD compared with those without PAD.69 Here we report that among people with PAD, capillary density was not correlated to CD206+ macrophage number or relative abundance (r=0.027 [P=0.905] and r=0.179 [P=0.417], respectively) (Table S2).
ECM and Collagen Content are Higher in PAD Gastrocnemius Muscle Compared With Non‐PAD and Correlate to Capillary Density
ECM surrounding muscle fibers, measured by fluorescent WGA staining (representative images, Figure 5A), was greater in gastrocnemius muscles from patients with PAD compared with those without PAD (P<0.001) (Figure 5B and Table 2; all values race‐adjusted). In PAD muscle, WGA staining was positively correlated to capillary density (r=0.656, P<0.001) (Figure 5C). To further characterize ECM abundance, we analyzed total fibrous collagen content using Sirius Red (SR) (Figure 6A). Similar to WGA, collagen (SR+ area) surrounding muscle fibers was greater in PAD muscle compared with non‐PAD (P=0.041) (Figure 6B and Table 2), and the 2 ECM measures were significantly correlated to each other (r=0.443, P=0.013) (Figure 6C). SR staining appeared particularly prominent surrounding capillaries in PAD muscle (Figure S2A). Immunohistochemistry with a pan‐collagen antibody combined with lectins to specifically stain capillaries79 showed collagen deposition surrounding capillaries (Figure S2B). In addition, some cross‐sectional capillaries appeared filled with collagen in PAD muscle (Figure S2B, blue arrows). No associations were observed between CD206+ macrophages and ECM or collagen content (data not shown), and none of these muscle properties were correlated to ABI in patients with PAD (Table S2).
Figure 5. Peripheral artery disease (PAD) gastrocnemius muscles show higher extracellular matrix (ECM) content compared with non‐PAD.
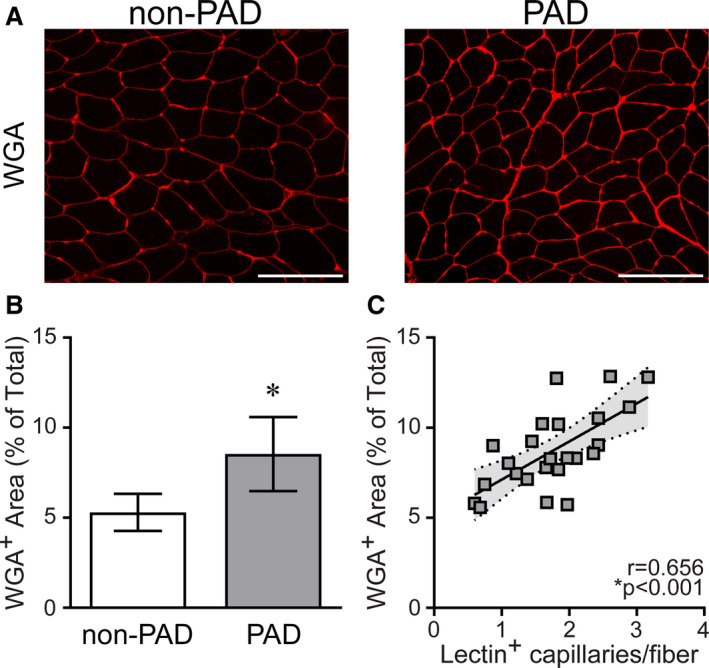
A, Representative images of gastrocnemius muscle showing fluorescently labeled α‐wheat germ agglutinin (WGA) staining of glycosaminoglycans in the ECM between muscle fibers in patients without PAD and patients with PAD, quantified in (B). Scale bar=200 µm. Non‐PAD (n=7), PAD (n=24). Data are expressed as mean±SD; P values adjusted for race, *significance P≤0.05. C, Correlation of ECM content with capillary density (the number of lectin+ capillaries/fiber) in patients with PAD (n=24), determined by Pearson product moment correlation adjusting for race, r=partial correlation coefficient. No relationship between ECM content and capillary density was observed in non‐PAD samples (data not shown).
Figure 6. Peripheral artery disease (PAD) gastrocnemius muscles show higher collagen content compared with non‐PAD.
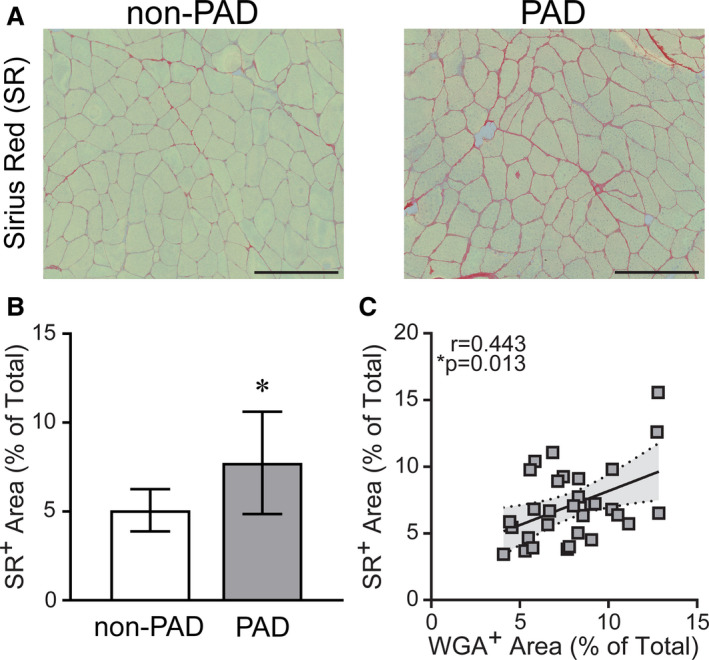
A, Representative images of gastrocnemius muscle showing Sirius Red (SR) staining of collagens between muscle fibers in patients without PAD and patients with PAD, quantified in (B). Scale bar=200 μm. Non‐PAD (n=7), PAD (n=24). Data are expressed as mean±SD; P values adjusted for race, *significance P≤0.05. C, Correlation of collagen content (SR+ area) with extracellular matrix (ECM) content (α‐wheat germ agglutinin [WGA]+ area) in all patients (n=31), determined by Pearson product moment correlation, r=partial correlation coefficient.
CD206− Macrophages are Negatively Associated With Walking Performance in PAD
The abundance of CD206− macrophages/fiber negatively correlated to 4‐meter walk tests both at normal (r=−0.447, P=0.036) (Figure 7A) and fast pace (r=−0.510, P=0.014) (Figure 7B) in participants with PAD. CD206+ macrophages did not correlate to 4‐meter walking performance and there were no statistically significant correlations between any muscle measures and 6‐minute walk distance (data not shown).
Figure 7. Cluster of differentiation (CD) 206− macrophages in gastrocnemius muscle are associated with poor walking performance in patients with peripheral artery disease (PAD).
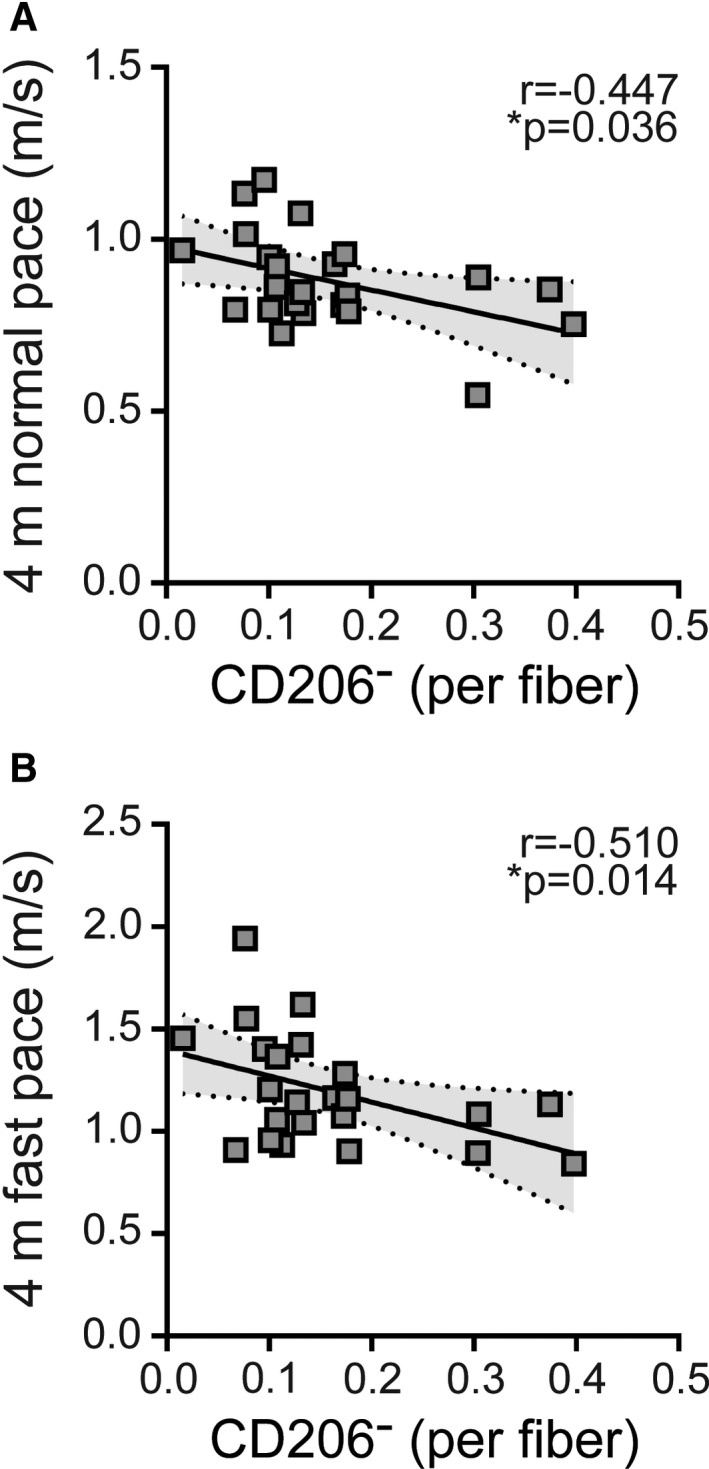
Scatterplots showing correlations between the abundance of CD206− macrophages and functional performance measured with (A) 4‐meter normal pace test (95% CI of r=−0.726 to −0.021), (B) 4‐meter fast pace test (95% CI of r=−0.762 to −0.101) For all measures, relationships determined by Pearson product moment correlation adjusting for race, r=partial correlation coefficient, PAD (n=23).
Discussion
In this cross‐sectional study of calf muscle biopsies, we observed higher average macrophages/fiber in people with PAD compared with those without PAD, which was attributable to a higher abundance of CD11b+CD206+ macrophages. The majority of CD206+ macrophages coexpressed CD163, supporting the idea that they are anti‐inflammatory and M2‐like. Among people with PAD, the greater relative abundance of CD206+ macrophages was associated with greater satellite cell content and larger mean fiber size, further supporting the idea that CD206+ macrophages are M2‐like and may promote reparative processes in lower extremity muscles of people with PAD. In response to skeletal muscle damage, satellite cells proliferate, differentiate, and fuse into muscle fibers to repair and maintain fiber number and size.33, 34 M2 macrophages facilitate satellite cell function through the production of growth and differentiation factors, such as insulin‐like growth factor 1.30, 31, 32 Adjusting for race attenuated a positive relationship between the relative abundance of CD206+ macrophages and 4‐meter walking performance, suggesting that the association of CD206+ macrophage abundance with walking performance among participants with PAD reported here may be confounded by race.80, 81 Further study with a larger sample size is needed.
To our knowledge, this is the first study to report an association between satellite cells and CD206+, M2‐like macrophage abundance in PAD, although several studies have shown satellite cell‐macrophage interactions during acute muscle injury and regeneration in rodent models.24, 35, 82, 83 Recent work in the mdx mouse model of Duchenne muscular dystrophy has highlighted macrophage contributions to regenerative muscle responses in this degenerative disease.84, 85, 86 In particular, promoting M2 macrophage polarization in mdx mouse muscle increased proregenerative muscle responses, resulting in increased muscle mass, fiber size, strength, and function.84 Direct communication between CD206+ macrophages and satellite cells in mdx mouse muscle may be mediated by the protein Klotho and contributes to muscle fiber size.86 Additionally, macrophage depletion in mdx mice increases collagen and fat deposition and converts satellite cell pools to adipogenic, intensifying the dystrophic phenotype.85 Results reported here, in people with PAD, are consistent with the idea that M2‐like macrophages may directly interact with satellite cells and may facilitate satellite cell–mediated maintenance of muscle fibers in the face of chronic ischemia‐reperfusion injury occurring with PAD.
Animal models of hind limb ischemia have primarily focused on acute ischemic injury, which is characterized by the induction of inflammatory M1 macrophages.25, 48, 51, 52, 53, 54 However, acute injury models are unlikely to represent the pathology, nor potential skeletal muscle adaptations, associated with gradual onset and long‐term exposure of lower extremity human skeletal muscle to repeated bouts of ischemia‐reperfusion, characteristic of PAD.87, 88, 89 In fact, a study by Tang et al90 showed that acute arterial occlusion in rats resulted in inflammation, inflammatory cell infiltration, and muscle necrosis, which was absent following gradual arterial occlusion. Thus, macrophage phenotypes in ischemic muscle may differ depending on the rate of blood flow restriction during PAD progression,90, 91 which may explain the variability we observed in total macrophage abundance across PAD muscles. However, macrophage population abundance was not associated with ABI, an indicator of disease severity, and we did not find a difference in CD11b+CD206− macrophage abundance between PAD and non‐PAD muscle, contrary to findings showing greater inflammatory macrophage abundance in PAD muscles.48 Within individuals with PAD, there was an association between higher CD206− macrophage abundance and reduced walking performance, suggesting that they are inflammatory. However, we have not definitively identified this population as M1‐like as we have been unable to successfully detect other M1 cell surface markers, such as CD80 and CD86, immunohistochemically in human skeletal muscle. In addition, the small mass of frozen muscle tissue available for analysis does not permit isolation and characterization of macrophage functional properties.
In this same cohort of patients with PAD, we previously reported a positive correlation between fiber size and walking performance, measured with both the normal and fast paced 4‐meter walk tests.69 We show here that total ECM, as well as collagen content, is higher in gastrocnemius muscles from patients with PAD compared with those without PAD. Thus, in addition to the association of smaller overall calf muscle size with poor walking performance in PAD,92 a greater area of PAD muscle is occupied by noncontractile ECM/collagen compared with muscle in people without PAD. Additionally, we report a positive association between muscle ECM content and capillary density with PAD. M2 macrophages are a source of transforming growth factor β, a cytokine that promotes ECM production and, if left unchecked, leads to fibrosis26, 27, 28; however, neither ECM nor capillary density in PAD correlated to CD206+ macrophage number or relative abundance. SR analysis of PAD muscles revealed some samples with excess collagen in and around capillaries, which may impair function so that higher capillary density in PAD may not necessarily improve tissue oxygenation.69, 93 Capillary density in muscle from patients with PAD is controversial, with some studies reporting higher capillary density with PAD69, 94, 95, 96 and others reporting lower capillary density with PAD.93, 97, 98, 99 We found a higher capillary density in PAD muscle compared with non‐PAD muscle,69 which may represent a compensatory mechanism in patients with PAD who have reduced oxygen delivery, but higher capillary density is associated with elevated ECM and collagen. These findings are consistent with previous reports of greater collagen density in PAD gastrocnemius samples, particularly surrounding microvessels, and thickening of the capillary basement membrane with PAD.93, 94, 100 Further, it has been shown in PAD that smooth muscle cells within the vascular wall promote collagen deposition within skeletal muscles.100, 101 Taken together, these findings support the hypothesis that in addition to limiting muscle contractile function, increased collagen deposition may limit capillary function, potentially serving as an additional stimulus for compensatory capillary proliferation.
Although the walking performance of patients with PAD is associated with ABI,3, 5, 71 the fact that muscle properties are correlated with walking performance independent of ABI suggests that reduced blood flow is not the only determinant of walking performance in patients with PAD. To our knowledge, no prior studies have assessed the associations of calf muscle macrophages with walking performance in people with PAD. The uncoupling of hemodynamics and skeletal muscle cellular properties could reveal beneficial muscle adaptations to repeated ischemia‐reperfusion that may be promoted with exercise, which is effective for improving walking performance but does not effectively increase lower extremity blood flow in patients with PAD.12, 13, 14, 15 Restoring blood flow in conjunction with promoting adaptive muscle responses may provide the most effective approach to improve walking performance in patients with PAD.
Study Limitations
There are several limitations to the present study. First, the study is cross‐sectional so no causal inferences can be made. Second, the relatively small sample size limits the extrapolation of our findings to the larger population of patients with PAD. Third, because of the small sample size statistical power is low and future study with a larger population is needed. Analysis of a greater number of patients with PAD and a larger number of healthy gastrocnemius muscle samples for comparison are needed to confirm the associations observed. Fourth, there was a higher proportion of blacks in the PAD group compared with the non‐PAD group, and statistical adjustment cannot completely overcome this confounding. Further study is needed to determine the influence of race on muscle characteristics in patients with and patients without PAD. Fifth, differences between patients with PAD and patients without PAD may be influenced by other confounding variables, since the prevalence of diabetes mellitus, smoking, and other characteristics were substantially higher in participants with PAD compared with those without PAD. However, the small sample size does not allow adequate adjustment for potential confounders and thus is a limitation of the study. Finally, isolation and single‐cell analyses of resident muscle macrophage populations in PAD are required to characterize macrophage phenotypes. Although CD206 expression likely distinguishes inflammatory and anti‐inflammatory macrophages, the function of macrophage subtypes could not be determined with these analyses.
Conclusions
Gastrocnemius muscles from people with PAD have a higher relative abundance of CD11b+CD206+ macrophages and higher ECM accumulation compared with people without PAD. Among people with PAD, a higher relative abundance of CD206+ macrophages is associated with a greater number of satellite cells and larger muscle fiber size. Higher numbers of CD11b+CD206− macrophages, consistent with greater inflammation, are associated with poorer walking performance, suggesting that possessing a greater proportion of CD206+ macrophages in lower extremity muscle may contribute to functional preservation with PAD. Given that studies in animal models of hind limb ischemia show beneficial outcomes in muscle directly related to M2 macrophage abundance,50, 102, 103, 104, 105, 106 our results suggest that CD206+ macrophage‐mediated reparative processes may be ongoing in gastrocnemius muscles of people with PAD and that these processes could be targeted to preserve walking performance.
Sources of Funding
The work was supported by National Institutes of Health grants R01HL088589, R01HL083064, R01HL089619, R01HL107510, R21AG047510, R01HL109244, and R01HL126117 (McDermott).
Disclosures
None.
Supporting information
Tables S1–S2 Figures S1–S2
(J Am Heart Assoc. 2020;9:e015929 DOI: 10.1161/JAHA.118.015929.)
Sarah H. White, PhD, is currently located at the Department of Animal Science, Texas A&M University, College Station, TX
For Sources of Funding and Disclosures, see page 12.
Contributor Information
Mary M. McDermott, Email: mdm608@northwestern.edu.
Charlotte A. Peterson, Email: cpete4@uky.edu.
References
- 1. Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14:156–170. [DOI] [PubMed] [Google Scholar]
- 2. McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. [DOI] [PubMed] [Google Scholar]
- 3. McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. [DOI] [PubMed] [Google Scholar]
- 4. McDermott MM. Functional impairment in peripheral artery disease and how to improve it in 2013. Curr Cardiol Rep. 2013;15:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. [DOI] [PubMed] [Google Scholar]
- 6. McDermott MM, Kibbe MR. Improving lower extremity functioning in peripheral artery disease: exercise, endovascular revascularization, or both? JAMA. 2017;317:689–690. [DOI] [PubMed] [Google Scholar]
- 7. Hamburg NM, Creager MA. Pathophysiology of intermittent claudication in peripheral artery disease. Circ J. 2017;81:281–289. [DOI] [PubMed] [Google Scholar]
- 8. Gillani S, Cao J, Suzuki T, Hak DJ. The effect of ischemia reperfusion injury on skeletal muscle. Injury. 2012;43:670–675. [DOI] [PubMed] [Google Scholar]
- 9. Koutakis P, Ismaeel A, Farmer P, Purcell S, Smith RS, Eidson JL, Bohannon WT. Oxidative stress and antioxidant treatment in patients with peripheral artery disease. Physiol Rep. 2018;6:e13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paradis S, Charles AL, Meyer A, Lejay A, Scholey JW, Chakfe N, Zoll J, Geny B. Chronology of mitochondrial and cellular events during skeletal muscle ischemia‐reperfusion. Am J Physiol Cell Physiol. 2016;310:C968–C982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bunte MC, Shishehbor MH. Next generation endovascular therapies in peripheral artery disease. Prog Cardiovasc Dis. 2018;60:593–599. [DOI] [PubMed] [Google Scholar]
- 12. Pandey A, Banerjee S, Ngo C, Mody P, Marso SP, Brilakis ES, Armstrong EJ, Giri J, Bonaca MP, Pradhan A, et al. Comparative efficacy of endovascular revascularization versus supervised exercise training in patients with intermittent claudication: meta‐analysis of randomized controlled trials. JACC Cardiovasc Interv. 2017;10:712–724. [DOI] [PubMed] [Google Scholar]
- 13. Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six‐month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER III, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: the CLEVER study. J Am Coll Cardiol. 2015;65:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olin JW, White CJ, Armstrong EJ, Kadian‐Dodov D, Hiatt WR. Peripheral artery disease: evolving role of exercise, medical therapy, and endovascular options. J Am Coll Cardiol. 2016;67:1338–1357. [DOI] [PubMed] [Google Scholar]
- 16. Chazaud B, Brigitte M, Yacoub‐Youssef H, Arnold L, Gherardi R, Sonnet C, Lafuste P, Chretien F. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev. 2009;37:18–22. [DOI] [PubMed] [Google Scholar]
- 17. Kharraz Y, Guerra J, Mann CJ, Serrano AL, Munoz‐Canoves P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediators Inflamm. 2013;2013:491497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saclier M, Cuvellier S, Magnan M, Mounier R, Chazaud B. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. 2013;280:4118–4130. [DOI] [PubMed] [Google Scholar]
- 19. Sciorati C, Rigamonti E, Manfredi AA, Rovere‐Querini P. Cell death, clearance and immunity in the skeletal muscle. Cell Death Differ. 2016;23:927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tidball JG, Dorshkind K, Wehling‐Henricks M. Shared signaling systems in myeloid cell‐mediated muscle regeneration. Development. 2014;141:1184–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–R1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mackey AL, Kjaer M. The breaking and making of healthy adult human skeletal muscle in vivo. Skelet Muscle. 2017;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–R353. [DOI] [PubMed] [Google Scholar]
- 24. Arnold L, Henry A, Poron F, Baba‐Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beneke A, Guentsch A, Hillemann A, Zieseniss A, Swain L, Katschinski DM. Loss of PHD3 in myeloid cells dampens the inflammatory response and fibrosis after hind‐limb ischemia. Cell Death Dis. 2017;8:e2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Braga TT, Agudelo JS, Camara NO. Macrophages during the fibrotic process: M2 as friend and foe. Front Immunol. 2015;6:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz‐Canoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lolmede K, Campana L, Vezzoli M, Bosurgi L, Tonlorenzi R, Clementi E, Bianchi ME, Cossu G, Manfredi AA, Brunelli S, et al. Inflammatory and alternatively activated human macrophages attract vessel‐associated stem cells, relying on separate HMGB1‐ and MMP‐9‐dependent pathways. J Leukoc Biol. 2009;85:779–787. [DOI] [PubMed] [Google Scholar]
- 30. Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. Macrophages recruited via CCR2 produce insulin‐like growth factor‐1 to repair acute skeletal muscle injury. FASEB J. 2011;25:358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tonkin J, Temmerman L, Sampson RD, Gallego‐Colon E, Barberi L, Bilbao D, Schneider MD, Musaro A, Rosenthal N. Monocyte/macrophage‐derived IGF‐1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol Ther. 2015;23:1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dumont N, Frenette J. Macrophages protect against muscle atrophy and promote muscle recovery in vivo and in vitro: a mechanism partly dependent on the insulin‐like growth factor‐1 signaling molecule. Am J Pathol. 2010;176:2228–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brack AS, Rando TA. Tissue‐specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell. 2012;10:504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7‐positive satellite cells in acute injury‐induced skeletal muscle regeneration. Development. 2011;138:3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saclier M, Yacoub‐Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31:384–396. [DOI] [PubMed] [Google Scholar]
- 36. Pande RL, Brown J, Buck S, Redline W, Doyle J, Plutzky J, Creager MA. Association of monocyte tumor necrosis factor alpha expression and serum inflammatory biomarkers with walking impairment in peripheral artery disease. J Vasc Surg. 2015;61:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brevetti G, Giugliano G, Brevetti L, Hiatt WR. Inflammation in peripheral artery disease. Circulation. 2010;122:1862–1875. [DOI] [PubMed] [Google Scholar]
- 38. Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C‐reactive protein, interleukin‐6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005;112:976–983. [DOI] [PubMed] [Google Scholar]
- 39. Wildman RP, Muntner P, Chen J, Sutton‐Tyrrell K, He J. Relation of inflammation to peripheral arterial disease in the National Health and Nutrition Examination Survey, 1999–2002. Am J Cardiol. 2005;96:1579–1583. [DOI] [PubMed] [Google Scholar]
- 40. Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur Heart J. 2007;28:354–362. [DOI] [PubMed] [Google Scholar]
- 41. McDermott MM, Guralnik JM, Corsi A, Albay M, Macchi C, Bandinelli S, Ferrucci L. Patterns of inflammation associated with peripheral arterial disease: the INCHIANTI study. Am Heart J. 2005;150:276–281. [DOI] [PubMed] [Google Scholar]
- 42. McDermott MM, Green D, Greenland P, Liu K, Criqui MH, Chan C, Guralnik JM, Pearce WH, Ridker PM, Taylor L, et al. Relation of levels of hemostatic factors and inflammatory markers to the ankle brachial index. Am J Cardiol. 2003;92:194–199. [DOI] [PubMed] [Google Scholar]
- 43. McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, Tao H, Ridker PM, Criqui MH. Relation of interleukin‐6 and vascular cellular adhesion molecule‐1 levels to functional decline in patients with lower extremity peripheral arterial disease. Am J Cardiol. 2011;107:1392–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ozkaramanli Gur D, Guzel S, Akyuz A, Alpsoy S, Guler N. The role of novel cytokines in inflammation: defining peripheral artery disease among patients with coronary artery disease. Vasc Med. 2018;23:428–436. DOI: 10.1177/1358863X18763096 [DOI] [PubMed] [Google Scholar]
- 45. McDermott MM, Ferrucci L, Guralnik JM, Tian L, Green D, Liu K, Tan J, Liao Y, Pearce WH, Schneider JR, et al. Elevated levels of inflammation, d‐dimer, and homocysteine are associated with adverse calf muscle characteristics and reduced calf strength in peripheral arterial disease. J Am Coll Cardiol. 2007;50:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McDermott MM, Ferrucci L, Liu K, Criqui MH, Greenland P, Green D, Guralnik JM, Ridker PM, Taylor LM, Rifai N, et al. D‐dimer and inflammatory markers as predictors of functional decline in men and women with and without peripheral arterial disease. J Am Geriatr Soc. 2005;53:1688–1696. [DOI] [PubMed] [Google Scholar]
- 47. McDermott MM, Greenland P, Green D, Guralnik JM, Criqui MH, Liu K, Chan C, Pearce WH, Taylor L, Ridker PM, et al. D‐dimer, inflammatory markers, and lower extremity functioning in patients with and without peripheral arterial disease. Circulation. 2003;107:3191–3198. [DOI] [PubMed] [Google Scholar]
- 48. Ganta VC, Choi M, Farber CR, Annex BH. Antiangiogenic VEGF165b regulates macrophage polarization via S100A8/S100A9 in peripheral artery disease. Circulation. 2019;139:226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Patel AS, Smith A, Nucera S, Biziato D, Saha P, Attia RQ, Humphries J, Mattock K, Grover SP, Lyons OT, et al. TIE2‐expressing monocytes/macrophages regulate revascularization of the ischemic limb. EMBO Mol Med. 2013;5:858–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ganta VC, Choi MH, Kutateladze A, Fox TE, Farber CR, Annex BH. A microRNA93‐interferon regulatory factor‐9‐immunoresponsive gene‐1‐itaconic acid pathway modulates M2‐like macrophage polarization to revascularize ischemic muscle. Circulation. 2017;135:2403–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pellegrin M, Bouzourene K, Poitry‐Yamate C, Mlynarik V, Feihl F, Aubert JF, Gruetter R, Mazzolai L. Experimental peripheral arterial disease: new insights into muscle glucose uptake, macrophage, and T‐cell polarization during early and late stages. Physiol Rep. 2014;2:e00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Y, Chen L, Shen Y, Tan T, Xie N, Luo M, Li Z, Xie X. Curcumin ameliorates ischemia‐induced limb injury through immunomodulation. Med Sci Monit. 2016;22:2035–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hsieh PL, Rybalko V, Baker AB, Suggs LJ, Farrar RP. Recruitment and therapeutic application of macrophages in skeletal muscles after hind limb ischemia. J Vasc Surg. 2018;67:1908–1920.e1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shireman PK. The chemokine system in arteriogenesis and hind limb ischemia. J Vasc Surg. 2007;45(suppl A):A48–A56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jetten N, Donners MM, Wagenaar A, Cleutjens JP, van Rooijen N, de Winther MP, Post MJ. Local delivery of polarized macrophages improves reperfusion recovery in a mouse hind limb ischemia model. PLoS One. 2013;8:e68811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu H, Zhang M, Liu Z, Xing J, Moriasi C, Dai X, Zou MH. AMP‐activated protein kinase alpha1 in macrophages promotes collateral remodeling and arteriogenesis in mice in vivo. Arterioscler Thromb Vasc Biol. 2016;36:1868–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Corona BT, Rathbone CR. Accelerated functional recovery after skeletal muscle ischemia‐reperfusion injury using freshly isolated bone marrow cells. J Surg Res. 2014;188:100–109. [DOI] [PubMed] [Google Scholar]
- 58. Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti‐inflammatory M2, but not pro‐inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–118. [DOI] [PubMed] [Google Scholar]
- 59. Kosmac K, Peck BD, Walton RG, Mula J, Kern PA, Bamman MM, Dennis RA, Jacobs CA, Lattermann C, Johnson DL, et al. Immunohistochemical identification of human skeletal muscle macrophages. Bio Protoc. 2018;8:e2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Walton RG, Kosmac K, Mula J, Fry CS, Peck BD, Groshong JS, Finlin BS, Zhu B, Kern PA, Peterson CA. Human skeletal muscle macrophages increase following cycle training and are associated with adaptations that may facilitate growth. Sci Rep. 2019;9:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McDermott MM, Liu K, Carr J, Criqui MH, Tian L, Li D, Ferrucci L, Guralnik JM, Kramer CM, Yuan C, et al. Superficial femoral artery plaque, the ankle‐brachial index, and leg symptoms in peripheral arterial disease: the Walking and Leg Circulation Study (WALCS) III. Circ Cardiovasc Imaging. 2011;4:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McDermott MM, Liu K, Carroll TJ, Kibbe M, Ferrucci L, Guralnik JM, Morasch M, Pearce W, Carr J, Yuan C, et al. Plaque characteristics in the superficial femoral artery correlate with walking impairment questionnaire scores in peripheral arterial disease: the Walking and Leg Circulation Study (WALCS) III. J Surg Radiol. 2012;3:148–157. [PMC free article] [PubMed] [Google Scholar]
- 63. McDermott MM, Liu K, Carroll TJ, Tian L, Ferrucci L, Li D, Carr J, Guralnik JM, Kibbe M, Pearce WH, et al. Superficial femoral artery plaque and functional performance in peripheral arterial disease: Walking and Leg Circulation Study (WALCS III). JACC Cardiovasc Imaging. 2011;4:730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McDermott MM, Greenland P, Liu K, Tian L, Green D, Shah SJ, Huffman M, Wilkins J, Kibbe M, Liao Y, et al. Vulnerable blood in high risk vascular patients: study design and methods. Contemp Clin Trials. 2014;38:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McDermott MM, Liu K, Green D, Greenland P, Tian L, Kibbe M, Tracy R, Shah SJ, Wilkins J, Huffman M, et al. Changes in D‐dimer and inflammatory biomarkers before ischemic events in patients with peripheral artery disease: the BRAVO study. Vasc Med. 2016;21:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, Domanchuk K, Ferrucci L, Lloyd‐Jones D, Kibbe M, et al. Home‐based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013;310:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McDermott MM, Domanchuk K, Liu K, Guralnik JM, Tian L, Criqui MH, Ferrucci L, Kibbe M, Jones DL, Pearce W, et al. The Group Oriented Arterial Leg Study (GOALS) to improve walking performance in patients with peripheral artery disease. Contemp Clin Trials. 2012;33:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Domanchuk K, Ferrucci L, Guralnik JM, Criqui MH, Tian L, Liu K, Losordo D, Stein J, Green D, Kibbe M, et al. Progenitor cell release plus exercise to improve functional performance in peripheral artery disease: the PROPEL Study. Contemp Clin Trials. 2013;36:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. White SH, McDermott MM, Sufit RL, Kosmac K, Bugg AW, Gonzalez‐Freire M, Ferrucci L, Tian L, Zhao L, Gao Y, et al. Walking performance is positively correlated to calf muscle fiber size in peripheral artery disease subjects, but fibers show aberrant mitophagy: an observational study. J Transl Med. 2016;14:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non‐invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. [DOI] [PubMed] [Google Scholar]
- 71. McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, Pearce W. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. [DOI] [PubMed] [Google Scholar]
- 72. Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC, McDermott MM. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. [DOI] [PubMed] [Google Scholar]
- 73. McDermott MM, Tian L, Liu K, Guralnik JM, Ferrucci L, Tan J, Pearce WH, Schneider JR, Criqui MH. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2008;51:1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McDermott MM, Guralnik JM, Tian L, Ferrucci L, Liu K, Liao Y, Criqui MH. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol. 2007;50:974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Emde B, Heinen A, Godecke A, Bottermann K. Wheat germ agglutinin staining as a suitable method for detection and quantification of fibrosis in cardiac tissue after myocardial infarction. Eur J Histochem. 2014;58:2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Crawford F, Welch K, Andras A, Chappell FM. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst Rev. 2016;9:CD010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kirkeby S, Mandel U, Vedtofte P. Identification of capillaries in sections from skeletal muscle by use of lectins and monoclonal antibodies reacting with histo‐blood group ABH antigens. Glycoconj J. 1993;10:181–188. [DOI] [PubMed] [Google Scholar]
- 80. McDermott MM, Polonsky TS, Kibbe MR, Tian L, Zhao L, Pearce WH, Gao Y, Guralnik JM. Racial differences in functional decline in peripheral artery disease and associations with socioeconomic status and education. J Vasc Surg. 2017;66:826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. McDermott MM, Polonsky TS, Guralnik JM, Ferrucci L, Tian L, Zhao L, Stein J, Domanchuk K, Criqui MH, Taylor DA, et al. Racial differences in the effect of granulocyte macrophage colony‐stimulating factor on improved walking distance in peripheral artery disease: the PROPEL randomized clinical trial. J Am Heart Assoc. 2019;8:e011001 DOI: 10.1161/JAHA.118.011001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ceafalan LC, Fertig TE, Popescu AC, Popescu BO, Hinescu ME, Gherghiceanu M. Skeletal muscle regeneration involves macrophage‐myoblast bonding. Cell Adh Migr. 2018;12:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Du H, Shih CH, Wosczyna MN, Mueller AA, Cho J, Aggarwal A, Rando TA, Feldman BJ. Macrophage‐released ADAMTS1 promotes muscle stem cell activation. Nat Commun. 2017;8:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Capote J, Kramerova I, Martinez L, Vetrone S, Barton ER, Sweeney HL, Miceli MC, Spencer MJ. Osteopontin ablation ameliorates muscular dystrophy by shifting macrophages to a pro‐regenerative phenotype. J Cell Biol. 2016;213:275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Madaro L, Torcinaro A, De Bardi M, Contino FF, Pelizzola M, Diaferia GR, Imeneo G, Bouche M, Puri PL, De Santa F. Macrophages fine tune satellite cell fate in dystrophic skeletal muscle of mdx mice. PLoS Genet. 2019;15:e1008408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wehling‐Henricks M, Welc SS, Samengo G, Rinaldi C, Lindsey C, Wang Y, Lee J, Kuro OM, Tidball JG. Macrophages escape Klotho gene silencing in the mdx mouse model of Duchenne muscular dystrophy and promote muscle growth and increase satellite cell numbers through a Klotho‐mediated pathway. Hum Mol Genet. 2018;27:14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mansour Z, Bouitbir J, Charles AL, Talha S, Kindo M, Pottecher J, Zoll J, Geny B. Remote and local ischemic preconditioning equivalently protects rat skeletal muscle mitochondrial function during experimental aortic cross‐clamping. J Vasc Surg. 2012;55:497–505.e491. [DOI] [PubMed] [Google Scholar]
- 88. Kocman EA, Ozatik O, Sahin A, Guney T, Kose AA, Dag I, Alatas O, Cetin C. Effects of ischemic preconditioning protocols on skeletal muscle ischemia‐reperfusion injury. J Surg Res. 2015;193:942–952. [DOI] [PubMed] [Google Scholar]
- 89. Thaveau F, Zoll J, Rouyer O, Chafke N, Kretz JG, Piquard F, Geny B. Ischemic preconditioning specifically restores complexes I and II activities of the mitochondrial respiratory chain in ischemic skeletal muscle. J Vasc Surg. 2007;46:541–547; discussion 547. [DOI] [PubMed] [Google Scholar]
- 90. Tang GL, Chang DS, Sarkar R, Wang R, Messina LM. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J Vasc Surg. 2005;41:312–320. [DOI] [PubMed] [Google Scholar]
- 91. Yang Y, Tang G, Yan J, Park B, Hoffman A, Tie G, Wang R, Messina LM. Cellular and molecular mechanism regulating blood flow recovery in acute versus gradual femoral artery occlusion are distinct in the mouse. J Vasc Surg. 2008;48:1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. McDermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L, Liu K, Schneider JR, Sharma L, Tan J, et al. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Baum O, Torchetti E, Malik C, Hoier B, Walker M, Walker PJ, Odriozola A, Graber F, Tschanz SA, Bangsbo J, et al. Capillary ultrastructure and mitochondrial volume density in skeletal muscle in relation to reduced exercise capacity of patients with intermittent claudication. Am J Physiol Regul Integr Comp Physiol. 2016;310:R943–R951. [DOI] [PubMed] [Google Scholar]
- 94. Ho TK, Rajkumar V, Black CM, Abraham DJ, Baker DM. Increased angiogenic response but deficient arteriolization and abnormal microvessel ultrastructure in critical leg ischaemia. Br J Surg. 2006;93:1368–1376. [DOI] [PubMed] [Google Scholar]
- 95. Keeling AN, Carroll TJ, McDermott MM, Liu K, Liao Y, Farrelly CT, Pearce WH, Carr J. Clinical correlates of size and number of collateral vessels in peripheral artery disease. Vasc Med. 2012;17:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. McGuigan MR, Bronks R, Newton RU, Sharman MJ, Graham JC, Cody DV, Kraemer WJ. Muscle fiber characteristics in patients with peripheral arterial disease. Med Sci Sports Exerc. 2001;33:2016–2021. [DOI] [PubMed] [Google Scholar]
- 97. Askew CD, Green S, Walker PJ, Kerr GK, Green AA, Williams AD, Febbraio MA. Skeletal muscle phenotype is associated with exercise tolerance in patients with peripheral arterial disease. J Vasc Surg. 2005;41:802–807. [DOI] [PubMed] [Google Scholar]
- 98. Jones WS, Duscha BD, Robbins JL, Duggan NN, Regensteiner JG, Kraus WE, Hiatt WR, Dokun AO, Annex BH. Alteration in angiogenic and anti‐angiogenic forms of vascular endothelial growth factor‐A in skeletal muscle of patients with intermittent claudication following exercise training. Vasc Med. 2012;17:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Robbins JL, Jones WS, Duscha BD, Allen JD, Kraus WE, Regensteiner JG, Hiatt WR, Annex BH. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. J Appl Physiol (1985). 2011;111:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ha DM, Carpenter LC, Koutakis P, Swanson SA, Zhu Z, Hanna M, DeSpiegelaere HK, Pipinos II, Casale GP. Transforming growth factor‐beta 1 produced by vascular smooth muscle cells predicts fibrosis in the gastrocnemius of patients with peripheral artery disease. J Transl Med. 2016;14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Baum O, Djonov V, Ganster M, Widmer M, Baumgartner I. Arteriolization of capillaries and FGF‐2 upregulation in skeletal muscles of patients with chronic peripheral arterial disease. Microcirculation. 2005;12:527–537. [DOI] [PubMed] [Google Scholar]
- 102. He S, Gleason J, Fik‐Rymarkiewicz E, DiFiglia A, Bharathan M, Morschauser A, Djuretic I, Xu Y, Krakovsky M, Jankovic V, et al. Human placenta‐derived mesenchymal stromal‐like cells enhance angiogenesis via T cell‐dependent reprogramming of macrophage differentiation. Stem Cells. 2017;35:1603–1613. [DOI] [PubMed] [Google Scholar]
- 103. Tepekoylu C, Lobenwein D, Urbschat A, Graber M, Pechriggl EJ, Fritsch H, Paulus P, Grimm M, Holfeld J. Shock wave treatment after hindlimb ischaemia results in increased perfusion and M2 macrophage presence. J Tissue Eng Regen Med. 2018;12:e486–e494. [DOI] [PubMed] [Google Scholar]
- 104. Takeda Y, Costa S, Delamarre E, Roncal C, Leite de Oliveira R, Squadrito ML, Finisguerra V, Deschoemaeker S, Bruyere F, Wenes M, et al. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479:122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Brechot N, Gomez E, Bignon M, Khallou‐Laschet J, Dussiot M, Cazes A, Alanio‐Brechot C, Durand M, Philippe J, Silvestre JS, et al. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin‐1‐deficient mice. PLoS One. 2008;3:e3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Krishnasamy K, Limbourg A, Kapanadze T, Gamrekelashvili J, Beger C, Hager C, Lozanovski VJ, Falk CS, Napp LC, Bauersachs J, et al. Blood vessel control of macrophage maturation promotes arteriogenesis in ischemia. Nat Commun. 2017;8:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2 Figures S1–S2


