Abstract
Background
In calcific aortic valve disease on tricuspid aortic valves (TAVs), men have higher aortic valve calcification and less fibrosis than women. However, little is known in bicuspid aortic valves (BAV). We thus aimed to investigate the impact of age, sex, and valve phenotype (TAVs versus BAVs) on fibro‐calcific remodeling in calcific aortic valve disease.
Methods and Results
We included 2 cohorts: 411 patients who underwent multidetector computed tomography (37% women) for aortic valve calcification density assessment and 138 explanted aortic valves (histological cohort; 50% women). The cohorts were divided in younger (<60 years old) or older patients with BAV (≥60 years old), and TAV patients. In each group, women and men were matched. Women presented less aortic valve calcification density than men in each group of the multidetector computed tomography cohort (all P≤0.01). Moreover, in women, younger patients with BAV had the lowest aortic valve calcification density (both P=0.02). In multivariate analysis, aortic valve calcification density correlated with age (β estimate±standard error: 6.5±1.8; P=0.0004) and male sex (109.2±18.4; P<0.0001), and there was a trend with TAVs (41.5±23.0; P=0.07). Women presented a higher collagen content than men (77.8±10.8 versus 69.9±12.9%; P<0.001) in the entire cohort. In women, younger patients with BAV had denser connective tissue than TAV and older patients with BAV (both P≤0.05), while no difference was observed between men.
Conclusions
In calcific aortic valve disease, women had less calcification and more fibrotic remodeling than men, regardless of the phenotype of the valve or age of the patient. Moreover, younger women with BAVs had less valve calcification. Thus, mineralization/fibrosis of the aortic valve is likely to have sex/age‐specific mechanisms and be influenced by the valve morphology.
Keywords: aortic stenosis, bicuspid, calcification, fibrosis, sex, tricuspid
Subject Categories: Valvular Heart Disease, Fibrosis, Women, Computerized Tomography (CT), Basic Science Research
Nonstandard Abbreviations and Acronyms
- AVAi
indexed aortic valve area
- AVC
aortic valve calcification
- BAV
bicuspid aortic valve
- CAVD
calcific aortic valve disease
- MDCT
multidetector computed tomography
- MG
mean transvalvular gradient
- OBAV
older patients with bicuspid aortic valve
- TAV
tricuspid aortic valve
- YBAV
younger patients with bicuspid aortic valve
Clinical Perspective
What Is New?
Hemodynamic severity of aortic stenosis may not be correlated to the degree of aortic valve calcification in young women with bicuspid aortic valve; that is, young women may have a hemodynamically severe aortic stenosis with no or minimal aortic valve calcification.
Women have a more fibrotic remodeling of the aortic valve, while men have a more calcific remodeling, irrespective of valve phenotype or age of the patients.
What Are the Clinical Implications?
Quantification of aortic valve calcification should thus be interpreted with caution in young women with bicuspid aortic valve.
The calcific/fibrotic patterns will be important to understand to develop pharmacological therapies, and especially to design studies to test these therapies.
In women, the menopausal status may need to be taken into consideration to individualize strategy.
Calcific aortic valve disease (CAVD) is the most frequent valvular heart disease in high‐income countries and the second most common indication for cardiac surgery after coronary artery bypass grafting.1 CAVD is characterized by a complex pathophysiology, which involves a fibro‐calcific remodeling of the leaflets.2 The incidence of CAVD increases markedly with age and affects 3% of the population over 65 years of age and 4.6% of the population over 75 years old.3, 4 Male sex has been associated with a 2‐fold increased risk to develop CAVD.5, 6
Bicuspid aortic valve (BAV) is the most frequent cardiac congenital abnormality affecting 1% to 2% of the general population, with a ratio of 3 men to 1 woman.7 BAV is characterized by an aortic valve presenting 2 leaflets instead of 3. CAVD is the most common complication of BAV and develops 10 to 15 years earlier in BAV compared with patients with tricuspid aortic valve (TAV). Patients with BAV are at higher risk for an aortic valve intervention during their lifetime, with nearly 50% of them needing an aortic valve replacement for CAVD.1, 8
Mineralization and extensive valvular fibrosis are the 2 main culprit lesions of CAVD, leading to the thickening and stiffening of aortic valve leaflets and obstruction of cardiac outflow.9, 10, 11 Aortic valve calcification (AVC) load, measured by multidetector computed tomography (MDCT), and hemodynamic severity of aortic stenosis, assessed by Doppler echocardiography, are well correlated.12, 13, 14 We recently showed that AVC is a predictor of the severity of CAVD in patients with TAV independently of the age, whereas in patients with BAV a major interaction was found between AVC and age on determining the severity of the disease. Indeed, AVC was a predictor of stenosis severity in older BAV but not in younger patients with BAV.15
Moreover, women reach similar hemodynamic stenosis severity than men for a lower AVC load.16, 17 We have previously shown in TAV that this sex‐related discrepancy was linked to higher valvular fibrosis in women compared with men.18 Whether these results are observed in patients with BAV have not been investigated yet. However, such differences in calcification/fibrosis ratio in men versus women, TAV versus BAV, and younger versus older patients could be of major importance to better understand CAVD pathophysiology and, ultimately, develop medical therapies. Thus, the aim of this study was to determine sex‐specific CAVD lesions according to age and aortic valve phenotype (TAV versus BAV).
Methods
The authors declare that all supporting data and methods are available within the article.
Patient Populations
A total of 537 patients with CAVD who underwent a comprehensive Doppler transthoracic echocardiography and an MDCT (n=434; MDCT cohort) or an aortic valve replacement were recruited for the study. From the 537 selected patients, 138 surgically explanted aortic valves were collected from the Quebec Heart and Lung Institute's tissue bank for histological studies (histological cohort). Patients with rheumatic aortic stenosis, infective endocarditis, cervical or thoracic radiotherapy‐induced valve lesions, severe renal disease, reduced left ventricular ejection fraction (<50%), more than mild aortic/mitral regurgitation, mitral stenosis, or prior aortic valve procedure were excluded. Patients were classified into 3 groups according to age and valve phenotype: (1) younger patients with bicuspid aortic valve (YBAV, <60 years old); (2) older patients with bicuspid aortic valve (OBAV, ≥60 years old); and (3) patients with tricuspid aortic valves (TAVs). In each group, men and women were frequency (MDCT cohort) or 1:1 (histological cohort) matched for (in order of importance) age (within 3 years), hemodynamic severity of CAVD determined by peak aortic jet velocity (within 0.25 m/s) mean transvalvular gradient (MG; within 5 mm Hg), indexed aortic valve area (AVAi; 0.1 cm2/m2), stroke volume index (within 3 mL/m2), left ventricular ejection fraction (within 5%), estimated glomerular filtration rate, systolic blood pressure (within 5 mm Hg), body mass index (within 5 kg/m2; Figure 1). The study protocol was approved by the research ethics committee of the Quebec Heart and Lung Institute, and the participants gave written informed consent.
Figure 1. Flowchart.
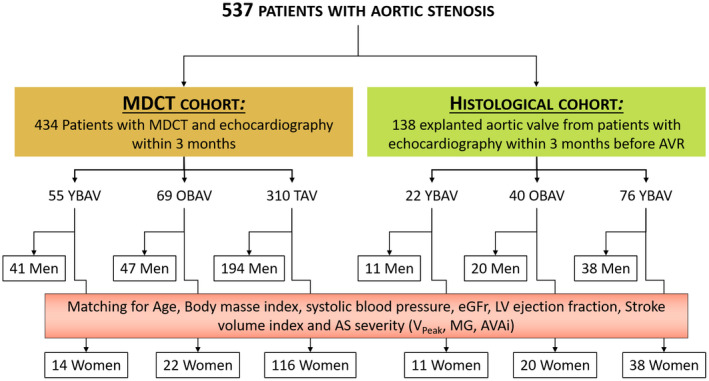
The flow chart of the study describes the multidetector computed tomography cohort and the histological cohort as well as the match between men and women in each age‐valve phenotype group. AVAi indicates indexed aortic valve area; AVR, aortic valve replacement; eGFR, estimated glomerular filtration rate; LV, left ventricular; MDCT, multidetector computed tomography; MG, mean transvalvular gradient; and Vpeak, peak aortic jet velocity.
Clinical Data
The clinical data included age, sex, body surface area, body mass index, documented diagnoses of hypertension, diabetes mellitus, coronary artery disease, renal disease, and estimated glomerular filtration rate, calculated using the formula of Modification of Diet in Renal Disease study.
MDCT Scans and AVC Assessment
MDCT scans without contrast were performed using a 64 slices helical scanner (Somatom Definition, Siemens AG Medical Solution, Germany) with a tube potential at 120 kV and a tube current‐time product at 60 to 80 mAs. Operators blinded to patient data performed all MDCT examinations and analyses. The protocol for the MDCT image acquisition and interpretation was previously published.17, 18 Image analyses were performed offline on dedicated workstations with validated software (Aquarius iNtuition, TeraRecon, Foster City, CA) for the measurement of AVC. AVC scores were quantified with the Agatston scoring method, and all AVC data were expressed in Agatston units.19 To account for smaller heart size in women, we calculated the AVC density by dividing the AVC score by the cross‐sectional aortic annulus area (π×[Aortic annulus diameter/2]2) measured by echocardiography.17
Doppler Echocardiography
Comprehensive Doppler echocardiography was performed using commercially available ultrasound system. All echocardiographic exams were performed by the same team of sonographers and cardiologists, and all images were analyzed in the same laboratory by experienced readers according to the current recommendations of the American Society of Echocardiography.20 Doppler echocardiographic indices of aortic stenosis severity included peak aortic jet velocity, MG obtained by the Bernoulli formula, and aortic valve area calculated by the continuity equation and indexed to body surface area (AVAi).20, 21, 22 Left ventricular systolic function was assessed using left ventricular ejection fraction as measured by the biplane Simpson method. Stroke volume was calculated by multiplying the left ventricular outflow tract area by the flow velocity‐time integral obtained by pulsed wave Doppler and was indexed to body surface area.
Aortic Valve Histology
Each valve excised at the time of surgery was placed in a container filled with HEPES solution and analyzed in the department of pathology. One of the cusps was decalcified in Cal‐Ex (Fisher, Nepean, Ontario, Canada) for 24 hours and fixed in formaldehyde 10% for histological processing. Aortic valve leaflets were embedded in optimal cutting temperature compound and 6 μm sections of the 138 matched YBAV, OBAV, and TAV men and women were obtained from a skilled operator using a cryotome. Histological sections were analyzed with Masson's trichrome and Picrosirius red staining. Masson's trichrome staining allowed us to differentiate loose (light blue sections) and dense connective tissue (dark blue sections; Figure 2A). Picrosirius red staining was used to study, under polarized light, collagen fibers (red/orange/green; Figure 2B). All sections were fixed in acetone‐methanol (60:40) at −20°C for 10 minutes and washed with running tap water for 5 minutes. All staining kits were obtained from Sigma‐Aldrich Corporation (Ontario, Canada).
Figure 2. Masson's trichrome and Picrosirius red staining of aortic valves according to the sex‐age‐phenotype group.
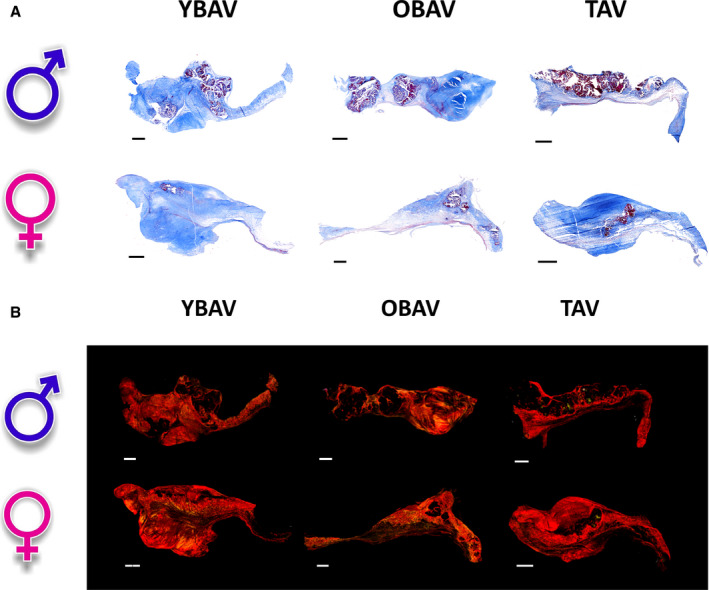
A, Valve histology at 20× original magnification with Masson's trichrome staining. Dark blue sections represent collagen fibers (dense connective tissue); light blue sections represent extracellular matrix fibers (loose connective tissue); red and purple nodules represent calcium nodules; and red fibers represent myofibroblast‐like cells. B, Valve histology at 20× under polarized light with Picrosirius red staining. Red‐orange‐green fibers represent collagen fibers. ♂ indicates men; ♀, women; OBAV, older patients with a bicuspid aortic valve; YBAV, younger patients with a bicuspid aortic valve; and TAV, tricuspid aortic valve. Scale bar=1 mm.
They were later analyzed with a homemade algorithm developed with MathWorks's MATLAB software detecting pixel RGB values for color differentiation. Masson's trichrome staining data were expressed as the ratio of dense and loose connective tissue area on the global tissue area in brightfield. Picrosirius red amount of collagen fibers were expressed as the ratio of polarized pixels on the global brightfield tissue pixels.
Statistical Analysis
Continuous variables were tested for normality by the Shapiro‐Wilk test. Results were expressed as mean ±SD, median (percentile 25–75), or percentage as appropriate.
Differences between groups and sexes were evaluated by a 2‐way ANOVA followed by Tukey post hoc tests for continuous normally distributed variables; Wilcoxon rank sum test, followed by Steel‐Dwass post hoc tests for continuous non‐normally distributed; and chi‐square or Fisher exact tests as appropriate for categorical variables. In the MDCT cohort, correlates of AVC density were identified by univariate and multivariate linear regressions and presented with β estimate±standard error.
A P<0.05 was considered statistically significant. All statistical analyses were performed with JMP 14.0 (SAS Institute) and SigmaPlot 11.0 (Systat Software) softwares.
Results
MDCT Cohort
Patients Characteristics
Among the 434 patients of the MDCT cohort, 310 (71%) patients had TAV (116 [37%] women, 76±7 years old), 55 (13%) were YBAV patients (14 [25%] women, 50±9 years old), and 69 (16%) were OBAV patients (22 [32%] women, 71±9 years old). Patient demographics and clinical characteristics are summarized in Table 1. The matched variables were not different between men and women in each group (all P>0.25). In each group, as expected, women had a lower body surface area and a lower prevalence of coronary artery disease. Given that YBAV patients were younger, they had fewer comorbidities than older TAV and OBAV patients. However, stenosis severity was comparable between sexes in the 3 groups (all P>0.47).
Table 1.
Characteristics of the MDCT Cohort
| YBAV (n=55) | OBAV (n=69) | TAV (n=310) | P Value for Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men (n=41) | Women (n=14) | P Value | Men (n=47) | Women (n=22) | P Value | Men (n=194) | Women (n=116) | P Value | ||
| Clinical data | ||||||||||
| Age, y | 50.3±7.8 | 47.2±11.3 | 0.82 | 70.3±6.6 | 73.4±7.2 | 0.67 | 75.4±8.3 | 76.9±9.1 | 0.66 | <0.001* , † , ‡ |
| Body mass index, kg/m² | 29.5±4.9 | 27.4±7.6 | 0.75 | 27.4±3.8 | 26.1±3.9 | 0.90 | 27.4±4.1 | 28.3±6.4 | 0.55 | 0.06 |
| Body surface area, m² | 2.08±0.15 | 1.72±0.19 | <0.001 | 1.98±0.15 | 1.61±0.14 | <0.001 | 1.94±0.17 | 1.70±0.18 | <0.001 | <0.001* , † |
| Systolic blood pressure, mm Hg | 124±14 | 118±13 | 0.90 | 127±21 | 128±17 | 1.00 | 131±18 | 133±19 | 0.82 | 0.006† |
| Diastolic blood pressure, mm Hg | 77±8 | 73±9 | 0.76 | 73±9 | 71±9 | 0.98 | 70±10 | 70±11 | 1.00 | 0.004† |
| eGFR, mL/min | 77.3±17.9 | 77.4±19.2 | 1.00 | 66.9±16.8 | 61.2±14.7 | 0.88 | 63.2±16.5 | 59.9±19.8 | 0.76 | <0.001* , † |
| Coronary artery disease, n (%) | 10 (25) | 0 (0) | 0.009 | 16 (34) | 5 (23) | 0.43 | 101 (52) | 37 (32) | 0.001 | <0.001† , ‡ |
| Diabetes mellitus, n (%) | 8 (20) | 1 (7) | 0.25 | 7 (15) | 3 (14) | 0.92 | 55 (28) | 25 (22) | 0.23 | 0.16 |
| Hypertension, n (%) | 18 (44) | 4 (29) | 0.30 | 28 (59) | 13 (59) | 0.97 | 144 (74) | 88 (76) | 0.81 | <0.001* , †‡ |
| Doppler echocardiographic data | ||||||||||
| Peak aortic jet velocity, m/s | 3.94±0.89 | 3.79±0.84 | 0.99 | 4.27±0.70 | 4.25±0.98 | 1.00 | 3.64±0.73 | 3.75±0.87 | 0.87 | <0.001‡ |
| Mean gradient, mm Hg | 39±18 | 34±15 | 0.89 | 45±16 | 48±24 | 0.98 | 32±14 | 35±16 | 0.78 | <0.001‡ |
| Aortic valve area index, cm²/m² | 0.54±0.15 | 0.56±0.16 | 1.00 | 0.48±0.12 | 0.45±0.18 | 0.94 | 0.58±0.11 | 0.59±0.13 | 1.00 | <0.001‡ |
| Stroke volume index, mL/m² | 45±10 | 44±7 | 1.00 | 44±9 | 40±8 | 0.47 | 44±9 | 45±8 | 0.97 | 0.45 |
| Left ventricular ejection fraction, % | 63±5 | 65±4 | 0.84 | 63±6 | 63±7 | 1.00 | 64±6 | 66±6 | 0.19 | 0.02‡ |
eGFR indicates estimated glomerular filtration rate; OBAV, older patients with a bicuspid aortic valve; TAV, patients with a tricuspid aortic valve; and YBAV, younger patients with a bicuspid aortic valve.
*P<0.05 for YBAV vs OBAV; † P<0.05 for YBAV vs TAV; ‡ P<0.05 for OBAV vs TAV.
Differences in Aortic Valve Calcification Evaluated by MDCT
In YBAV, OBAV, and TAV groups, women had lower AVC density (YBAV: 202 [0–341] versus 476 [265–701] Agatston units/cm², P=0.001; OBAV: 429 [227–929] versus 889 [477–1080] Agatston units /cm², P=0.01; TAV: 360 [157–544] versus 428 [279–731] Agatston units/cm², P=0.009) compared with men (Figure 3A). In women, YBAV patients had significantly lower AVC density than OBAV and TAV patients (both P=0.02). In men, OBAV patients presented a higher AVC density compared with YBAV and TAV patients (P=0.003 and P<0.0001, respectively), which may be related to a slightly more severe stenosis in OBAV patients (Figure 3B).
Figure 3. Aortic valve gradient assessed by echocardiography and calcification assessed by MDCT according to the sex‐age‐phenotype group.
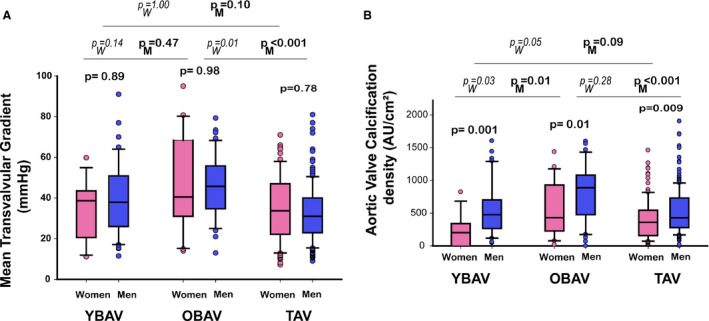
Box plot graphs showing (A) mean transvalvular gradient and (B) aortic calcification density (ie, aortic valve calcification divided by cross‐sectional aortic annulus). In each age‐phenotype group, women and men had equivalent hemodynamic severity aortic stenosis; however, women had lower calcification than men. In men, OBAV patients show higher aortic valve calcification than TAV patients linked to slightly more severe aortic stenosis, and YBAV despite equivalent aortic stenosis severity. In women, YBAV shows the lowest aortic valve calcification density despite similar aortic stenosis severity. Box plot format: the box indicates the 25th to 75th percentiles; the line within the box indicates the median; and the whiskers indicate the 10th to 90th percentiles. OBAV indicates old bicuspid aortic valve; PM, P‐value between men groups; PW, P‐value between women groups; TAV, tricuspid aortic valve; and YBAV, young bicuspid aortic valve.
In univariate analysis, AVC density was correlated with stenosis severity (ie, AVAi and MG; all P≤0.0001) in the whole cohort. After adjustment for body surface area, coronary artery disease, systolic blood pressure, diabetes mellitus, estimated glomerular filtration rate, stenosis severity (MG and AVAi), and stroke volume index, AVC density was significantly correlated to age (6.5±1.8; P=0.0004) and male sex (109.2±18.4; P<0.0001), and there was a trend toward association with tricuspid valve morphology (41.5±23.0; P=0.07). In univariate analysis, AVC density was correlated with stenosis severity (ie, AVAi and MG; all P≤0.0003) in all groups, except in YBAV women (P=0.45). After adjusting for systolic blood pressure, coronary artery disease, estimated glomerular filtration rate, and stroke volume index, AVC density was correlated with stenosis severity (ie, AVAi and MG; all P<0.004) in all groups, except in OBAV women, where there was only a trend toward association (P≥0.06), and in YBAV women where the association was absent (both P≥0.58). The interaction between phenotype and sex to predict AVC density was statistically significant (P=0.02).
Figure 4. Correlation between aortic valve calcification density and hemodynamic severity of aortic stenosis (mean gradient) according to the sex‐age‐phenotype group.
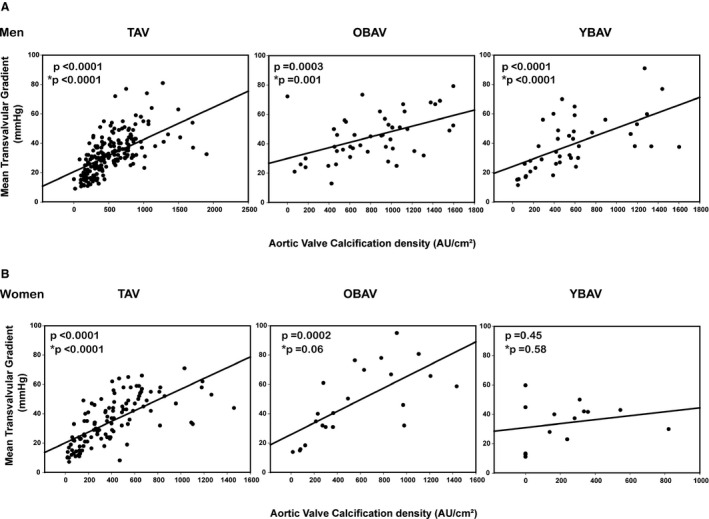
Correlation in men (A) and in women (B). *P value adjusted for body surface area, systolic blood pressure, coronary artery disease, estimated glomerular filtration rate, and stroke volume index.
Histological Cohort
Patients Characteristics
In the histological cohort, 76 patients were classified in the TAV (38 women, 72.4±8.1 years old), 22 in the YBAV group (11 women, 57.0±3.1 years old) and 40 in the OBAV group (20 women, 72.4±8.2 years old).
There were no significant differences between men and women inside each group except for body surface area (Table 2); ie, women had lower body surface area when compared with men in each group (all P<0.001). By design, YBAV patients were younger than OBAV and TAV patients (both P<0.001). As expected, they had a lower prevalence of systemic arterial hypertension when compared with TAV (P=0.01); however, they had a higher prevalence of coronary artery disease compared with OBAV.
Table 2.
Characteristics of the Histological Study Population
| YBAV (n=22) | OBAV (n=40) | TAV (n=76) | P Value for Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men (n=11) | Women (n=11) | P Value | Men (n=20) | Women (n=20) | P Value | Men (n=38) | Women (n=38) | P Value | ||
| Clinical data | ||||||||||
| Age, y | 56.5±3.6 | 57.5±2.5 | 0.58 | 69.1±6.2 | 69.5±5.5 | 0.80 | 72.7±7.6 | 72.2±8.8 | 0.65 | 0.75 * , † |
| Body mass index, kg/m² | 28.7±6.2 | 27.1±4.1 | 0.45 | 28.6±4.1 | 27.0±3.4 | 0.28 | 29.2±5.3 | 30.6±6.4 | 0.19 | 0.18 |
| Body surface area, m² | 1.98±0.2 | 1.70±0.13 | <0.001 | 1.93±0.19 | 1.70±0.16 | <0.001 | 1.97±0.21 | 1.81±0.21 | <0.001 | 0.26 |
| Systolic blood pressure, mm Hg | 118±13 | 128±18 | 0.14 | 130±14 | 130±11 | 0.37 | 128±17 | 135±17 | 0.61 | 0.72 |
| Diastolic blood pressure, mm Hg | 76±9 | 76±12 | 0.92 | 79±9 | 77±7 | 0.40 | 71±9 | 74±11 | 0.75 | 0.33 |
| eGFR, mL/min | 83.7±13.1 | 82.2±17.3 | 0.87 | 79.3±14.4 | 79.1±15.1 | 0.83 | 80.6±20.5 | 76.8±17.1 | 0.27 | 0.75 |
| Coronary artery disease, n (%) | 5 (45) | 4 (36) | 0.66 | 2 (10) | 3 (15) | 0.63 | 24 (63) | 20 (53) | 0.35 | <0.001* , ‡ |
| Diabetes mellitus, n (%) | 4 (36) | 1 (9) | 0.13 | 6 (30) | 4 (16) | 0.46 | 14 (37) | 15 (39) | 0.81 | 0.29 |
| Hypertension, n (%) | 7 (63) | 7 (63) | 1.00 | 17 (85) | 16 (80) | 0.67 | 32 (84) | 35 (92) | 0.15 | 0.09† |
| Echocardiographic data | ||||||||||
| Peak aortic jet velocity, m/s | 4.24±0.45 | 4.31±0.56 | 0.89 | 3.67±0.99 | 3.78±0.80 | 0.19 | 3.83±0.75 | 3.78±0.81 | 0.65 | 0.42 |
| Mean gradient, mm Hg | 45±10 | 45±9 | 0.84 | 45±13 | 45±15 | 0.86 | 40±14 | 39±12 | 0.28 | 0.73 |
| Aortic valve area index, cm²/m² | 0.41±0.05 | 0.39±0.09 | 0.43 | 0.38±0.09 | 0.38±0.08 | 0.99 | 0.41±0.07 | 0.40±0.09 | 0.97 | 0.81 |
| Stroke volume index, mL/m² | 41±7 | 41±8 | 0.98 | 41±13 | 39±9 | 0.44 | 39±6 | 40±8 | 0.17 | 0.21 |
| Left ventricular ejection fraction, % | 63±6 | 62±9 | 0.57 | 61±4 | 63±5 | 0.36 | 60±8 | 61±7 | 0.51 | 0.59 |
eGFR indicates estimated glomerular filtration rate; OBAV, older patients with a bicuspid aortic valve; TAV, patients with a tricuspid aortic valve; and YBAV, younger patients with a bicuspid aortic valve.
*P<0.05 for YBAV vs OBAV; † P<0.05 for YBAV vs TAV; ‡ P<0.05 for OBAV vs TAV.
Semiquantitative Assessment of Fibrous Tissue With Masson's Trichrome and Picrosirius Staining
As previously shown,18 in our histological cohort, we confirmed by a semiquantification of Masson's trichrome staining that women had a higher content of dense fibrosis compared with men (reaching statistical significance in the whole cohort, YBAV and TAV patients, all P<0.05; Figure 5A). Interestingly, YBAV women seem to have more dense connective tissue than OBAV and TAV women (P=0.05). This difference in the amount of dense connective tissue was not seen in men (all P>0.07).
Figure 5. Histological assessment of matched stenotic valves.
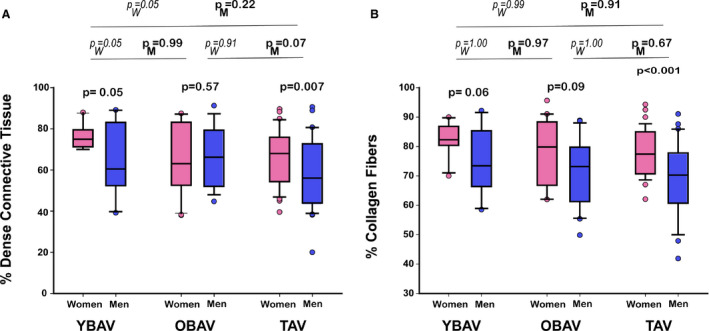
Box plot graphs showing (A) dense connective tissue proportion by Masson's trichrome staining and (B) collagen fibers proportion by Picrosirius red staining. In each age group, percentage of dense connective tissue and percentage of collagen fibers are higher in women than in men, while significance is reach only in TAV patients and YBAV patients for dense connective tissue. The percentage of dense connective tissue appear to be higher in YBAV, especially in women, while the percentage of collagen was equivalent in each group according to sex of the patient. Box plot format: the box indicates the 25th to 75th percentiles; the line within the box indicates the median; and the whiskers indicate the 10th to 90th percentiles. OBAV indicates older patients with bicuspid aortic valve; PM, P‐value between men groups; PW, P value between women groups; TAV, tricuspid aortic valve; and YBAV, younger patients with bicuspid aortic valve.
Picrosirius red staining showed that women had more collagen fibers than men in the entire cohort (77.8±10.8 versus 69.9±12.9%; P<0.001) and in TAV patients (77.7±8.4 versus 68.8±12.1%; P<0.001), while there was only a trend in YBAV (78.0±15.7 versus 70.5±18.9%; P=0.06) and in OBAV (77.8±12.2 versus 71.6±10.8%; P=0.09) patients (Figure 5B). No difference was seen between groups nor interaction between sex and group (P=0.79). Interestingly, the percentage of collagen fibers was correlated with hemodynamic severity of the stenosis in YBAV women (P=0.001), while there was no significant association in other groups (all P≥0.14).
Discussion
Our study demonstrates for the first time that (1) women with stenosed BAV have less calcification than men for the same hemodynamic severity of CAVD and confirm that women with stenosed TAV have less calcification than men; (2) in young (<60 years of age) women with BAV, the degree of aortic valve calcification is not related to hemodynamic severity of aortic stenosis, while the quantity of collagen fibers is; (3) the quantity of dense connective tissue was higher in young BAV women than in other female groups. Interestingly, this variation of dense connective tissue was not seen in men according to phenotype of the valve and age; and (4) collagen fibers are more abundant in women than in men but without differences between the valve morphology (BAV versus TAV) or the age of the patients.
Several studies have recently shown that women, for similar aortic stenosis severity, exhibit lower AVC than men after taking into account the effect of smaller body size, heart, and aortic annulus size.16, 17, 18, 23, 24 However, in these studies TAV and BAV patients were analyzed together, with a large predominance of TAV patients and/or comorbidities that were highly different between men and women. In our cohort, men and women were well matched for all clinical data and hemodynamic severity of CAVD, and we have demonstrated that BAVs from women also have lower AVC density compared with BAVs from male patients with similar CAVD severity and comorbidities.
Intrinsic differences between valves from men and women have been demonstrated at cellular and genetic levels.25, 26 Apart from genetic mutations related to BAV and CAVD development, mechanical stress has been pointed out as an important feature leading to calcification and fibrosis of bileaflet valves. Interestingly, electron microscopy established that the ventricular side of the BAV is massively stripped from the protective endothelium layer compared with TAV, suggesting a much higher mechanical stress imposed on BAVs.27 In our study, the level of calcification was indeed higher in younger and older men with BAVs compared with men with TAVs, supporting these results. However, the degree of fibrosis was not affected by the morphology of the valve in all the different age groups in men, but was higher in women with TAVs. In the literature, numerous in vitro studies using Flexcell technology to reproduce the different forces imposed on valvular interstitial cells have been published.28, 29, 30, 31, 32 Proteins implicated in the development of the valvular mineralization (ie, bone morphogenetic protein 2, osteoprotegerin, receptor activator of nuclear factor kappa‐Β ligand, osteocalcin, osteopontin) have been highlighted as well as many markers of fibrosis and extracellular matrix reorganization like matrix metalloproteinases. Unfortunately, none of these studies investigated the sexual differences in response to these stimuli. However, one could speculate that, as we obtained in our study, the calcification markers are upregulated in men with BAVs as well as TAVs but to a lesser extent, while in women, actors of the fibrotic processes could be activated independently of valvular morphology and age of the patients, and result in enhanced remodeling.
Sex hormones may also have an impact on valve calcification. Recently, androgen receptors have been found to be expressed in aortic valves and their expression was more important in calcified valves.33 In this study, in vitro testosterone supplementation increased calcification of vascular smooth muscle cells, while in other studies the opposite was found.34 In postmenopausal women, PvuII polymorphism in estrogen receptor α gene is related to the development of CAVD.35 Also, β‐estradiol treatment of rat/porcine valvular interstitial cells cultured in osteogenic media decreased cell proliferation in female cells.36 However, the used osteogenic media contained a high concentration of dexamethasone, which may have an antiestrogen effect. In women, the calcification process seemed to be influenced by the phenotype of the valve and, more importantly, by the age of the patient, while fibrosis is not regulated by these factors. Especially in young BAV women, calcification is less important, while collagen content is equivalent to that of older BAV and TAV patients, and connective tissue appears to be more dense. These age‐related differences were not seen in men. This could be linked to the hormonal status that is different in women before and after menopause.
Finally, response to other stimuli could be differently regulated in men and women, such as the higher impact of interferon‐α in men compared with women. Interferon‐α is a weak proinflammatory cytokine that cooperates with lipopolysaccharide in aortic valve interstitial cells. In cell culture, inflammation, apoptosis, and osteoblastic differentiation were more important after 24 hours of a treatment with interferon‐α in men compared with women,37 which may explain the increase in aortic valve calcification in men compared with women.
A recent study investigated the sex differences in the pathology of aortic valves in rheumatic heart disease, an autoimmune disease caused by group A streptococci infection and frequently affecting the aortic valve. Aortic valves from female patients had increased collagen, monocytes/macrophages, neovascularization, and inflammatory genes involved in the nuclear factor kappa‐light‐chain enhancer of activated B cells pathway. They also found reduced apoptotic signals in women, which is consistent with reduced mineralization that we observed in our specimens from female patients.38
Interestingly, it seems like the disease is more similar between OBAV and TAV than in YBAV patients. These findings suggest that the pathophysiological mechanisms leading to the development of CAVD might be, at least in part, different between the older and the younger middle‐aged patients. In the younger patients, it is believed that lipid‐mediated inflammation, and insulin resistance might play a predominant role in the pathogenesis of CAVD, whereas altered phosphocalcic metabolism and activation of the renin‐angiotensin system might be the cause in older patients.39, 40, 41, 42, 43 Further studies are needed to identify the age‐related differences in the pathogenesis of CAVD.
However, sex‐specific pathophysiology of CAVD explaining the differences observed between men and women and in women between YBAV, OBAV, and TAV patients are far from being understood. The understanding of these processes may help develop targeted medical therapies in aortic stenosis.
Limitations
The principal limitation of this study is the limited number of stenosed BAVs. The smaller BAV group translates into lower power to detect differences.
Conclusions
In stenosed aortic valves, the pathophysiology appears to be sex‐specific and probably age‐specific in women. Aortic valves explanted from women present with less calcification and denser connective tissue, with more fibrosis than men regardless of age and phenotype (ie, bicuspid versus tricuspid). Moreover, YBAV women had the lowest valve calcification for the same hemodynamic stenosis severity compared with OBAV and TAV women. This age‐related difference was not seen in men. Further studies are required to better understand aortic valve remodeling in aortic stenosis according to the sex and age of the patient.
Sources of Funding
This study was funded by the Heart and Stroke Foundation of Canada (Grant‐in‐aid #G‐18‐0022132).
Disclosures
None.
Acknowledgments
We thank Véronic Tremblay, Geneviève Guèvremont, Marie‐Chloé Boulanger, and the IUCPQ Biobank (Christine Racine and Caroline Gagnon) for valve collection and their technical assistance.
(J Am Heart Assoc. 2020;9:e015610 DOI: 10.1161/JAHA.119.015610.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–925. [DOI] [PubMed] [Google Scholar]
- 2. Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, Pibarot P. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 4. Carabello BA. Aortic stenosis. N Engl J Med. 2002;346:677–682. [DOI] [PubMed] [Google Scholar]
- 5. Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular health study. J Am Coll Cardiol. 1997;29:630–634. [DOI] [PubMed] [Google Scholar]
- 6. Mohler ER, Sheridan MJ, Nichols R, Harvey WP, Waller BF. Development and progression of aortic valve stenosis: atherosclerosis risk factors–a causal relationship? A clinical morphologic study. Clin Cardiol. 1991;14:995–999. [DOI] [PubMed] [Google Scholar]
- 7. Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55:2789–2800. [DOI] [PubMed] [Google Scholar]
- 8. Braverman AC, Guven H, Beardslee MA, Makan M, Kates AM, Moon MR. The bicuspid aortic valve. Curr Probl Cardiol. 2005;30:470–522. [DOI] [PubMed] [Google Scholar]
- 9. Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. [DOI] [PubMed] [Google Scholar]
- 10. Yetkin E, Waltenberger J. Molecular and cellular mechanisms of aortic stenosis. Int J Cardiol. 2009;135:4–13. [DOI] [PubMed] [Google Scholar]
- 11. Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373:956–966. [DOI] [PubMed] [Google Scholar]
- 12. Messika‐Zeitoun D, Aubry MC, Detaint D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Breen JF, Scott C, Tajik AJ, et al. Evaluation and clinical implications of aortic valve calcification measured by electron‐beam computed tomography. Circulation. 2004;110:356–362. [DOI] [PubMed] [Google Scholar]
- 13. Kaden JJ, Freyer S, Weisser G, Willingstorfer W, Bilbal A, Pfleger S, Suselbeck T, Haase KK, Dempfle CE, Borggrefe M. Correlation of degree of aortic valve stenosis by doppler echocardiogram to quantity of calcium in the valve by electron beam tomography. Am J Cardiol. 2002;90:554–557. [DOI] [PubMed] [Google Scholar]
- 14. Cueff C, Serfaty JM, Cimadevilla C, Laissy JP, Himbert D, Tubach F, Duval X, Iung B, Enriquez‐Sarano M, Vahanian A, et al. Measurement of aortic valve calcification using multislice computed tomography: correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart. 2011;97:721–726. [DOI] [PubMed] [Google Scholar]
- 15. Shen M, Tastet L, Capoulade R, Larose É, Bédard É, Arsenault M, Chetaille P, Dumesnil JG, Mathieu P, Clavel MA, et al. Effect of age and aortic valve anatomy on calcification and haemodynamic severity of aortic stenosis. Heart. 2017;103:32–39. [DOI] [PubMed] [Google Scholar]
- 16. Aggarwal SR, Clavel MA, Messika‐Zeitoun D, Cueff C, Malouf J, Araoz PA, Mankad R, Michelena H, Vahanian A, Enriquez‐Sarano M. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ Cardiovasc Imaging. 2013;6:40–47. [DOI] [PubMed] [Google Scholar]
- 17. Clavel MA, Messika‐Zeitoun D, Pibarot P, Aggarwal S, Malouf J, Araoz P, Michelena H, Cueff C, Larose É, Capoulade R, et al. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler‐echocardiographic and computed tomographic study. J Am Coll Cardiol. 2013;62:2329–2338. [DOI] [PubMed] [Google Scholar]
- 18. Simard L, Côté N, Dagenais F, Mathieu P, Couture C, Trahan S, Bossé Y, Mohammadi S, Pagé S, Joubert P, et al. Sex‐related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis: is valvular fibrosis the explanation? Circ Res. 2017;120:681–691. [DOI] [PubMed] [Google Scholar]
- 19. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 20. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. [DOI] [PubMed] [Google Scholar]
- 21. Vahanian A, Iung B. The new ESC/EACTS guidelines on the management of valvular heart disease. Arch Cardiovasc Dis. 2012;105:465–467. [DOI] [PubMed] [Google Scholar]
- 22. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 23. Pawade T, Clavel MA, Tribouilloy C, Dreyfus J, Mathieu T, Tastet L, Renard C, Gun M, Jenkins WSA, Macron L, et al. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ Cardiovasc Imaging. 2018;11:e007146. [DOI] [PubMed] [Google Scholar]
- 24. Choi BH, Ko SM, Shin JK, Chee HK, Kim JS, Kim J. Association between aortic valvular calcification and characteristics of the aortic valve in patients with bicuspid aortic valve stenosis. Acta Radiol. 2019;60:468–477. [DOI] [PubMed] [Google Scholar]
- 25. McCoy CM, Nicholas DQ, Masters KS. Sex‐related differences in gene expression by porcine aortic valvular interstitial cells. PLoS ONE. 2012;7:e39980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dargis N, Lamontagne M, Gaudreault N, Sbarra L, Henry C, Pibarot P, Mathieu P, Bossé Y. Identification of gender‐specific genetic variants in patients with bicuspid aortic valve. Am J Cardiol. 2016;117:420–426. [DOI] [PubMed] [Google Scholar]
- 27. Bouchareb R, Boulanger M, Pépin A, Pibarot P, Mathieu P. Mechanical stress enhances aortic valve calcification: implication for bicuspid aortic valve mineralization. Can J Cardiol. 2012;28:S214; Abstract #317. [Google Scholar]
- 28. Fondard O, Detaint D, Iung B, Choqueux C, dle‐Biassette H, Jarraya M, Hvass U, Couetil JP, Henin D, Michel JB, et al. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J. 2005;26:1333–1341. [DOI] [PubMed] [Google Scholar]
- 29. Smith KE, Metzler SA, Warnock JN. Cyclic strain inhibits acute pro‐inflammatory gene expression in aortic valve interstitial cells. Biomech Model Mechanobiol. 2010;9:117–125. [DOI] [PubMed] [Google Scholar]
- 30. Ketelhuth DF, Bäck M. The role of matrix metalloproteinases in atherothrombosis. Curr Atheroscler Rep. 2011;13:162–169. [DOI] [PubMed] [Google Scholar]
- 31. Ferdous Z, Jo H, Nerem RM. Differences in valvular and vascular cell responses to strain in osteogenic media. Biomaterials. 2011;32:2885–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lehmann S, Walther T, Kempfert J, Rastan A, Garbade J, Dhein S, Mohr FW. Mechanical strain and the aortic valve: influence on fibroblasts, extracellular matrix, and potential stenosis. Ann Thorac Surg. 2009;88:1476–1483. [DOI] [PubMed] [Google Scholar]
- 33. Zhu D, Hadoke PW, Wu J, Vesey AT, Lerman DA, Dweck MR, Newby DE, Smith LB, MacRae VE. Ablation of the androgen receptor from vascular smooth muscle cells demonstrates a role for testosterone in vascular calcification. Sci Rep. 2016;6:24807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Son BK, Akishita M, Iijima K, Ogawa S, Maemura K, Yu J, Takeyama K, Kato S, Eto M, Ouchi Y. Androgen receptor‐dependent transactivation of growth arrest‐specific gene 6 mediates inhibitory effects of testosterone on vascular calcification. J Biol Chem. 2010;285:7537–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nordstrom P, Glader CA, Dahlen G, Birgander LS, Lorentzon R, Waldenstrom A, Lorentzon M. Oestrogen receptor alpha gene polymorphism is related to aortic valve sclerosis in postmenopausal women. J Intern Med. 2003;254:140–146. [DOI] [PubMed] [Google Scholar]
- 36. Masjedi S, Lei Y, Patel J, Ferdous Z. Sex‐related differences in matrix remodeling and early osteogenic markers in aortic valvular interstitial cells. Heart Vessels. 2017;32:217–228. [DOI] [PubMed] [Google Scholar]
- 37. Parra‐Izquierdo I, Castanos‐Mollor I, Lopez J, Gomez C, San Roman JA, Sanchez Crespo M, Garcia‐Rodriguez C. Calcification induced by type I interferon in human aortic valve interstitial cells is larger in males and blunted by a Janus kinase inhibitor. Arterioscler Thromb Vasc Biol. 2018;38:2148–2159. [DOI] [PubMed] [Google Scholar]
- 38. Xiao F, Zheng R, Yang D, Cao K, Zhang S, Wu B, Shao Y, Zhou B. Sex‐dependent aortic valve pathology in patients with rheumatic heart disease. PLoS ONE. 2017;12:e0180230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Capoulade R, Clavel MA, Dumesnil JG, Chan KL, Teo KK, Tam JW, Côté N, Mathieu P, Després JP, Pibarot P. Impact of metabolic syndrome on progression of aortic stenosis: influence of age and statin therapy. J Am Coll Cardiol. 2012;60:216–223. [DOI] [PubMed] [Google Scholar]
- 40. Mohty D, Pibarot P, Després JP, Cartier A, Arsenault B, Picard F, Mathieu P. Age‐related differences in the pathogenesis of calcific aortic stenosis: the potential role of resistin. Int J Cardiol. 2010;142:126–132. [DOI] [PubMed] [Google Scholar]
- 41. Côté N, Couture C, Pibarot P, Després JP, Mathieu P. Angiotensin receptor blockers are associated with lower remodelling of stenotic aortic valves. Eur J Clin Invest. 2011;41:1172–1179. [DOI] [PubMed] [Google Scholar]
- 42. Capoulade R, Clavel MA, Mathieu P, Côté N, Dumesnil JG, Arsenault M, Bédard E, Pibarot P. Impact of hypertension and renin‐angiotensin system inhibitors in aortic stenosis. Eur J Clin Invest. 2013;43:1262–1272. [DOI] [PubMed] [Google Scholar]
- 43. Côté N, Mahmut A, Fournier D, Pépin A, Couture C, Després JP, Trahan S, Pagé S, Bossé Y, Pibarot P, et al. Angiotensin receptor blockers are associated with reduced fibrosis and interleukin‐6 expression in calcific aortic valve disease. Pathobiology. 2014;81:15–24. [DOI] [PubMed] [Google Scholar]


