Abstract
Background
Sugar‐sweetened beverage (SSB) consumption has been associated with cardiometabolic risk. However, the association between total and type of SSB intake and incident cardiovascular disease (CVD) end points such as myocardial infarction, stroke, and revascularization is limited.
Methods and Results
We examined the prospective association of baseline SSB consumption with incident CVD in 106 178 women free from CVD and diabetes mellitus in the CTS (California Teachers Study), a cohort of female teachers and administrators, followed since 1995. SSBs were defined as caloric soft drinks, sweetened bottled waters or teas, and fruit drinks, and derived from a self‐administered food frequency questionnaire. CVD end points were based on annual linkage with statewide inpatient hospitalization records. Cox proportional hazards models were used to assess the association between SSB consumption and incident CVD. A total of 8848 CVD incident cases were documented over 20 years of follow‐up. After adjusting for potential confounders, we observed higher hazard ratios (HRs) for CVD (HR, 1.19; 95% CI, 1.06–1.34), revascularization (HR, 1.26; 95% CI, 1.04–1.54]), and stroke (HR, 1.21; 95% CI, 1.04–1.41) in women who consumed ≥1 serving per day of SSBs compared with rare/never consumers. We also observed a higher risk of CVD in women who consumed ≥1 serving per day of fruit drinks (HR, 1.42; 95% CI, 1.00–2.01 [P trend=0.021]) and caloric soft drinks (HR, 1.23; 95% CI, 1.05–1.44 [P trend=0.0002]), compared with rare/never consumers.
Conclusions
Consuming ≥1 serving per day of SSB was associated with CVD, revascularization, and stroke. SSB intake might be a modifiable dietary target to reduce risk of CVD among women.
Keywords: cardiovascular disease, nutritional epidemiology, observational study, sugar‐sweetened beverages
Subject Categories: Diet and Nutrition, Cardiovascular Disease, Epidemiology
Nonstandard Abbreviations and Acronyms
- BMI
body mass index
- CABG
coronary artery bypass grafting
- CHD
coronary heart disease
- CVD
cardiovascular disease
- CTS
California Teachers Study
- FFQ
food frequency questionnaire
- HR
hazard ratio
- MI
myocardial infarction
- OSHPD
Office of Statewide Health Planning and Development
- PCI
percutaneous coronary intervention
- PTCA
percutaneous transluminal coronary angioplasty
- RR
relative risk
- SSB
sugar‐sweetened beverage
Clinical Perspective
What Is New?
Prospective studies have addressed the association between sugar‐sweetened beverage intake and cardiovascular disease; however, few trials have been adequately statistically powered to examine coronary heart disease or stroke outcomes, with even fewer assessing different types of sugar‐sweetened beverages.
In a large US cohort, intake of sugar‐sweetened beverages was positively associated with cardiovascular disease, revascularization, and stroke; and, more specifically, intake of fruit drinks and caloric soft drinks was positively associated with cardiovascular disease.
What Are the Clinical Implications?
Our findings support clinical and public health efforts to reduce sugar‐sweetened beverage intake as a means to promote cardiovascular health.
Sugar‐sweetened beverages (SSBs) account for nearly half of the added sugars in the American diet.1, 2 Among US adults, the mean adjusted intake of added sugars is high (308 kcal/d or 17% of total energy),3 making SSBs a substantial contributor of calories in this population.1, 2 SSBs are manufactured carbonated and noncarbonated beverages containing caloric sweeteners or syrups and include caloric soft drinks (ie, not sugar‐free), fruit drinks, sports and energy drinks, sweetened waters, and tea and coffee beverages with added sugars.1 Reducing intake of added sugars is currently recommended by the World Health Organization,4 Dietary Guidelines for Americans,1 and the American Heart Association,5 but intake levels for the majority of Americans exceed recommendations and almost 50% of adults report consuming at least 1 SSB per day.6
Consumption of SSBs has been associated with weight gain, visceral adiposity and obesity,7, 8, 9, 10, 11, 12, 13 cardiometabolic risk factors and/or metabolic syndrome and type 2 diabetes mellitus,9, 14, 15, 16, 17, 18, 19 hypertension,20, 21, 22 and cardiovascular disease (CVD) events such as coronary heart disease (CHD) and stroke23, 24, 25, 26, 27, 28 among a variety of populations. Several biological mechanisms for this association have been suggested including that sugars augment the levels of glucose and insulin concentrations in the bloodstream, contributing to a high dietary glycemic load.29 A high glycemic load promotes physiological responses such as appetite stimulation and weight gain/adiposity, inducing insulin resistance and glucose intolerance.30 Additionally, enhancing levels of oxidative stress and inflammation, altering lipid metabolism including triglyceride synthesis, leading to endothelial dysfunction and beta cell stress.30, 31, 32, 33, 34 These mechanisms influence insulin resistance and risk of type 2 diabetes mellitus,35 as well as the atherosclerotic process and risk of CVD.31, 32, 36
Although prospective studies have addressed the association between SSB intake and CVD,23, 24, 25, 26, 27, 28 few have been adequately statistically powered to examine CHD23, 24, 27 or stroke25, 26, 27 outcomes. This is of particular importance since CHD remains the leading cause of death in the United States, and stroke is fifth.37 Furthermore, there are a limited number of studies that assess a variety of SSBs with most studies examining one specific SSB. Addressing the impact different SSBs have on CVD risk is necessary and findings can further contribute to the literature on SSBs and inform future recommendations. We aimed to examine the association between SSB consumption and CVD risk, examining incidence of CVD events including myocardial infarction (MI), revascularization, and stroke in a large prospective cohort of adult women over a 20‐year period. We hypothesized that higher levels of SSB consumption are associated with incident CVD.
Methods
All of the data associated with this publication and in the CTS (California Teachers Study) are available for research use. The CTS welcomes all such inquiries and encourages individuals to visit https://www.calteachersstudy.org/for-researchers.
Study Population and Design
The CTS is an ongoing prospective cohort study of 133 477 active and retired female teachers and administrators who completed a mailed questionnaire at study enrollment in 1995–1996 and members of the California State Teachers Retirement System.38 Annual follow‐up, mailings, and participant communication capture change of residence. Linkage with the Office of Statewide Health Planning and Development (OSHPD) identifies inpatient hospitalization and—since 2010—ambulatory, surgery, and emergency department procedures and diagnoses performed in California. Dates and causes of death are determined via linkage with state and national mortality files and the National Death Index.
The CTS has been approved by the institutional review boards at City of Hope, the University of Southern California, the University of California San Francisco, and the University of California at Irvine, and participants provided informed consent. This data analysis was approved by the institutional review boards of City of Hope and the University of California San Diego.
Dietary Assessment and SSB Intake
Dietary intake during the year preceding baseline was assessed using a validated 103‐item self‐administered food frequency questionnaire (FFQ), developed from an early version of the Block 95. This FFQ ascertained usual serving size (ie, small, medium, large, or extra‐large serving) and frequency of consumption (ie, never or <1 time per month, 1 time per month, 2 or 3 times per month, 1 time per week, 2 times per week, 3or 4 times per week, 5 or 6 times per week, every day, or ≥2 times per day) of 103 food and beverage items. The reproducibility and validity of this instrument in the cohort has been previously published.39 Estimation of SSB consumption was determined from 3 items on the FFQ, specifically asked as: “First: mark the column to show How Often, on the average, you ate the food during the past year; second: mark the column to show How Much you usually eat of each food for ‘regular soft drinks (not diet soda),’ ‘Snapple, Calistoga, sweetened bottled waters or iced teas,’ and ‘Kool‐Aid, Hi‐C, or other drinks with added Vitamin C.’” The use of brand names was included in the FFQ mailed to participants. These 3 beverage types will be referred to as “caloric soft drinks,” “sweetened bottled waters or teas,” and “fruit drinks,” respectively. Fruit drinks only included sweetened (with added sugar) fruit drinks and excluded fruit juices. From the 9 possible frequency categories, SSB consumption was collapsed into 4 categories: rare or never, >rare/never to <1 serving per week, ≥1 serving per week to <1 serving per day, and ≥1 serving per day. A serving of sweetened bottled waters or teas, and fruit drinks is equivalent to 8 fluid ounces, with an approximate weight of 237 grams. A serving of caloric soft drinks is equivalent to 12 fluid ounces, with an approximate weight of 355 grams.
Ascertainment of CVD Incidence
CVD incidence was defined as first occurrence of fatal or nonfatal MI, revascularization procedure (including coronary artery bypass grafting and percutaneous coronary intervention and/or percutaneous transluminal coronary angioplasty), or fatal or nonfatal stroke. This was ascertained after the return of the baseline questionnaire, with 1995–1996 designated as the study start date. Similarly, incidence of MI, revascularization, and stroke was defined as first occurrence of each event after baseline. Annual linkage with statewide OSHPD hospitalization records, derived medical diagnoses, and in‐patient procedures for California residents for incident CVD was completed through December 31, 2015. Participants were followed from study start date until diagnosis with a CVD event, death, moved out of California, or end of follow‐up (December 31, 2015), whichever came first. CVD definitions followed the International Statistical Classification of Diseases, Ninth Revision (ICD‐9), and International Statistical Classification of Diseases, Tenth Revision (ICD‐10), coding system.
Assessment of Covariates
Covariates for this analysis included age, race/ethnicity, socioeconomic status, smoking status, alcohol intake, family history of CVD in first‐degree relatives, moderate to vigorous physical activity, aspirin use, multivitamin use, menopausal status and menopausal hormone therapy use, oral contraceptive use, history of hypertension, body mass index (BMI), total energy intake, and fruit and vegetable intake. These data were collected at baseline, by self‐report, as part of the enrollment questionnaire.
Socioeconomic status was determined by combining three 1990 US block census data variables (occupation, education, and family income), where all block groups in the state were ranked by occupation (percentage of adults employed in managerial/professional occupation), level of education (percentage of adults older than 25 years completing at least a college degree), and median family income, corresponding to quartiles analogous with the statewide adult population. A summary score was developed for socioeconomic status with categories ranging from 1 (lowest) to 4 (highest). Smoking status was derived from 3 questionnaire items addressing cumulative (lifetime) smoking exposure, age when first and last smoked, and average number of cigarettes currently or previously smoked. Alcohol intake was determined from frequency and number of drinks per week of beer, champagne and/or wine, and cocktails and/or liquor. Physical activity, including moderate to vigorous physical activity, was estimated using questionnaire‐derived intensity, duration, and frequency of listed activities, on an average day. BMI (kg/m2) was calculated as weight (kg) divided by height squared (m2), from self‐reported weight and height measurements.
Analytic Sample
This analysis includes 106 178 female study participants. We excluded participants who specified their data only be used for breast cancer research (n=22); those who resided outside of California at baseline (n=8847); those who returned incomplete or incomprehensible questionnaires (n=4); those who had incomplete FFQ data at baseline including vitamin use (n=2); those who had extreme caloric intake values (<600 [n=10 889] or >5000 [n=558] kcal/d); those who were aged ≥85 years at baseline (n=1611); those with a history of CVD including heart attack, stroke, and revascularization procedures at or before baseline (n=2372); and those with a history of diabetes mellitus at or before baseline (n=2994) (Figure 1).
Figure 1. Flowchart showing enrollment, exclusions, and final analytic sample for sugar‐sweetened beverage consumption and cardiovascular disease (CVD) risk in the CTS (California Teachers Study).
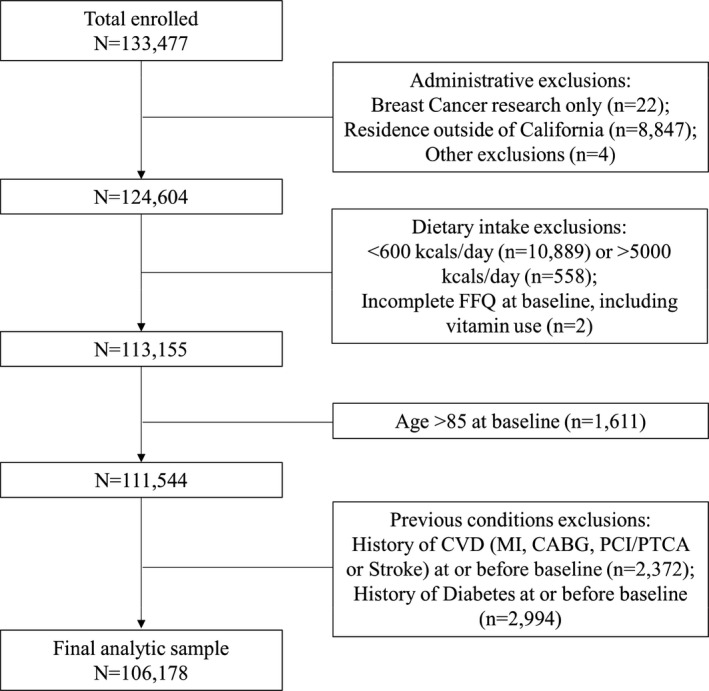
CABG indicates coronary artery bypass grafting; MI, myocardial infarction; PCI, percutaneous coronary intervention; and PTCA, percutaneous transluminal coronary angioplasty.
Statistical Analysis
Mean and SEM or proportion and frequency were calculated for baseline characteristics of study participants in each SSB consumption category. Cox proportional hazard modeling was used to estimate hazard ratios (HRs) and 95% CIs of CVD incidence according to SSB consumption. This approach was also used for first occurrence of MI, revascularization, and stroke, separate from the first CVD event. We also examined the independent association between type of SSB and incident CVD. In our analysis, a median method was used to examine the linear trend across intake categories. The median intake value of SSB in each category was designated to all individuals in that category. The statistical significance of the linear trend was tested by Cox proportional hazard model using the median intake value as a continuous independent variable in the multivariable model. The proportional hazards assumption was tested by inspecting the survival curves according to SSB consumption categories as well as testing time‐varying covariates in the model.
In multivariable analysis, we adjusted for the following potential confounders: age; race/ethnicity (white, Asian/Pacific Islander, black, Hispanic, Native American, or mixed/other; further categorized as white versus all other before including it in the model); socioeconomic status (quartiles: first, second, third, fourth, or unknown); smoking status (never, past, current cigarette use [1–12, 13–24, ≥25 per day], or unknown use); alcohol intake (0, <20, or ≥20 g/d); family history of CVD (yes or no), moderate to vigorous physical activity (quintiles minutes per week: 0–30, 30–105, 105–210, 210–360, >360, or unknown); aspirin use (1–3 times per week, 4–6 times per week, daily, undetermined frequency, or unknown); multivitamin use (never, 1–3 times per week, 4–6 times per week, daily, undetermined frequency); menopausal status and menopausal hormone therapy use (premenopausal or perimenopausal/postmenopausal with never, past, or current hormone therapy use of estrogen, estrogen and progesterone, or other hormone combinations); oral contraceptive use (never, past, or current); and history of hypertension (yes or no). In separate models, we further adjusted for BMI, total energy intake, and fruit and vegetable intake as a measure of diet quality as possible mediators. Fruit and vegetable intake was adjusted for total energy by using the residual method,40 before including it in the model. Three progressively adjusted multivariable Cox regression models after the age‐adjusted model were fitted. Model 1 included all of the above‐mentioned covariates except for BMI, total energy intake, and fruit and vegetable intake. Model 2 additionally adjusted for BMI, total energy intake, and fruit and vegetable intake. The final model, model 3, includes covariates that were known and tested (if ≥10% change in HR) confounders in this exposure and outcome association. Variables with a P<0.05 remained in model 3. Additionally, the models examining the association between type of SSB and risk of CVD were adjusted for the other beverage types, ie, the sweetened water or tea analysis was adjusted for fruit drink and caloric soft drink, and vice versa.
A sensitivity analysis was conducted to further assess the amount of SSB and determine whether key information about the association was lost with our semiquantitative categorization. For this analysis, we categorized SSB intake in cups per day (1 cup=8 fl oz) as: rarely/never, up to .5 cup per day, up to 1 cup per day, up to 1.5 cups per day, and ≥1.5 cups per day. Another sensitivity analysis was conducted to examine the possibility of reverse causality. We excluded CVD events that occurred within the first 2 and 4 years of follow‐up. All P values presented are from 2‐tailed analyses; P<0.05 was considered statistically significant. Analyses were conducted with SAS version 9.4 (SAS Institute Inc).
Results
CTS participants were, on average (mean±SD), aged 52.1±13.4 years, and followed for 1 807 182 person‐years to first CVD event. During 20 years of follow‐up, we ascertained 8848 incident cases of CVD, 2677 incident cases of MI, 2889 incident cases of revascularization, and 5258 incident cases of stroke. Among all participants, 4.2% were daily consumers of SSBs, whereas 40.9% of participants reported rarely/never consuming SSBs. This included consumption of any type of SSB measured: sweetened bottled waters or teas, fruit drinks, and caloric soft drinks. Regarding consumption by type of SSB among all participants, 4.3% were daily consumers of sweetened bottled waters or teas, 0.4% were daily consumers of fruit drinks, and 3.1% were daily consumers of caloric soft drinks (Table S1). Participants with the highest SSB intake tended to be younger, current smokers (7.6%), and past or current oral contraceptive users (72.6%), and averaged (mean±SEM) 220.1±3.68 minutes per week of moderate to vigorous physical activity (Table 1). With respect to dietary intake and clinical factors, participants with the highest SSB intake had a daily higher intake of total energy and carbohydrate; had a lower intake of protein, fat, and fruit and vegetables; had the highest obesity rates (17.5%); and 15% had hypertension. Comprehensive participant characteristics are reported in Table S2.
Table 1.
| Characteristic | Total | Rare/Never | >Rare/Never to <1 Serving Per wk | ≥1 Serving Per wk to <1 Serving Per d | ≥1 Serving Per d |
|---|---|---|---|---|---|
| No. | 106 178 | 43 425 | 35 422 | 22 825 | 4506 |
| SSB intake, fl oz/d | 2.6±0.0 | 0±0.0 | 2.6±0.0 | 5.5±0.0 | 13.5±0.1 |
| Dietary intake | |||||
| Energy, kcal/d | 1902.1±2.1 | 1753.2±3.2 | 1949.9±3.6 | 2042.6±4.5 | 2248.6±10.1 |
| Carbohydrate, g/d | 255.1±0.3 | 251.4±0.2 | 253.1±0.2 | 259.8±0.2 | 282.3±0.5 |
| Protein, g/d | 77.2±0.1 | 80.1±0.1 | 76.7±0.1 | 74.2±0.1 | 67.7±0.2 |
| Total fat, g/d | 59.9±0.1 | 59.6±0.1 | 61.4±0.1 | 59.6±0.1 | 53.6±0.2 |
| Fruit and vegetable, g/d | 321.2±0.6 | 361.2±0.8 | 301.4±0.9 | 286.7±1.2 | 265.0±2.6 |
| Age, y | 52.1±0.0 | 56.0±0.1 | 49.5±0.1 | 49.3±0.1 | 49.0±0.2 |
| Race/ethnicity | |||||
| White | 92 654 (87.3) | 39 208 (90.3) | 29 989 (84.7) | 19 500 (85.4) | 3957 (87.8) |
| All other | 13 524 (12.7) | 4217 (9.7) | 5433 (15.3) | 3325 (14.6) | 549 (12.2) |
| Educationc | |||||
| Academic/professional doctorate | 2501 (2.4) | 1079 (2.5) | 770 (2.2) | 522 (2.3) | 130 (2.9) |
| Master's degree | 27 802 (26.2) | 11 130 (25.6) | 9444 (26.7) | 6018 (26.4) | 1210 (26.9) |
| Bachelor's degree | 23 654 (22.3) | 9677 (22.3) | 8269 (23.3) | 4804 (21.1) | 904 (20.1) |
| Associate's degree or less | 416 (0.4) | 141 (0.3) | 147 (0.4) | 106 (0.5) | 22 (0.5) |
| Unknown | 51 805 (48.8) | 21 398 (49.3) | 16 792 (47.4) | 11 375 (49.8) | 2240 (49.7) |
| Occupation | |||||
| Teacher, any kind | 61 940 (58.3) | 21 846 (50.3) | 22 358 (63.1) | 14 708 (64.4) | 3028 (67.2) |
| Pupil services | 3235 (3.1) | 1213 (2.8) | 1155 (3.3) | 723 (3.2) | 144 (3.2) |
| Administration | 3834 (3.6) | 1401 (3.2) | 1297 (3.7) | 926 (4.1) | 210 (4.7) |
| Any other combination | 1751 (1.7) | 623 (1.4) | 648 (1.8) | 402 (1.8) | 78 (1.7) |
| Unknown | 35 418 (33.4) | 18 342 (42.2) | 9964 (28.1) | 6066 (26.6) | 1046 (23.2) |
| Socioeconomic status | |||||
| First quartile, low | 4393 (4.1) | 1627 (3.8) | 1565 (4.4) | 1012 (4.4) | 189 (4.2) |
| Second quartile, low‐medium | 17 953 (16.9) | 7005 (16.1) | 6147 (17.4) | 4046 (17.7) | 755 (16.8) |
| Third quartile, medium‐high | 34 326 (32.3) | 13 724 (31.6) | 11 737 (33.1) | 7354 (32.2) | 1511 (33.5) |
| Fourth quartile, high | 48 109 (45.3) | 20 524 (47.3) | 15 479 (43.7) | 10 109 (44.3) | 1997 (44.3) |
| Unknown | 1397 (1.3) | 559 (1.3) | 504 (1.4) | 309 (1.3) | 54 (1.2) |
| Marital status | |||||
| Married | 49 355 (46.5) | 19 500 (44.9) | 17 219 (48.6) | 10 581 (46.4) | 2055 (45.6) |
| Separated or divorced | 9670 (9.1) | 4099 (9.4) | 3198 (9.0) | 1958 (8.6) | 415 (9.2) |
| Widowed | 6758 (6.4) | 3694 (8.5) | 1742 (4.9) | 1123 (4.9) | 199 (4.4) |
| All other | 40 395 (38.0) | 16 132 (37.2) | 13 263 (37.4) | 9163 (40.1) | 1837 (40.8) |
| Moderate to vigorous physical activity, min/wk | 225.9±0.8 | 238.3±1.2 | 214.4±1.3 | 221.0±1.6 | 220.1±3.9 |
| Smoking, current | 5352 (5.0) | 2222 (5.1) | 1584 (4.5) | 1202 (5.3) | 344 (7.6) |
| Alcohol consumption, ≥20 g/d | 9114 (8.6) | 4388 (10.1) | 2615 (7.4) | 1767 (7.7) | 344 (7.6) |
| Obese, BMI ≥30 kg/m2 | 13 683 (12.9) | 5343 (12.3) | 4369 (12.3) | 3181 (13.9) | 8790 (17.5) |
| Hypertension | 16 196 (15.3) | 7849 (18.1) | 4545 (12.8) | 3130 (13.7) | 672 (14.9) |
| Daily aspirin use | 6904 (6.5) | 3576 (8.2) | 1821 (5.1) | 1222 (5.4) | 285 (6.3) |
| Daily antihypertensive medication use | 14 432 (13.6) | 7183 (16.5) | 3915 (11.0) | 2730 (12.0) | 604 (13.4) |
| Daily multivitamin use | 38 307 (36.1) | 17 723 (40.8) | 11 485 (32.4) | 7515 (32.9) | 1584 (35.2) |
| CVS family historyd | 50 805 (47.9) | 22 417 (51.6) | 15 956 (45.1) | 10 346 (45.3) | 2086 (46.3) |
| Menopausal status and menopausal HT use | |||||
| Premenopausal | 43 404 (40.9) | 13 143 (30.3) | 17 130 (48.4) | 10 978 (48.1) | 2151 (47.8) |
| Perimenopausal or postmenopausal, no HT use | 12 469 (11.7) | 6349 (14.6) | 3398 (9.6) | 2301 (10.1) | 421 (9.3) |
| Perimenopausal or postmenopausal, past HT use | 7899 (7.4) | 4129 (9.5) | 2151 (6.1) | 1359 (6.0) | 260 (5.8) |
| Perimenopausal or postmenopausal, current HT use, estrogen | 13 375 (12.6) | 6620 (15.2) | 3864 (10.9) | 2399 (10.5) | 492 (10.9) |
| Perimenopausal or postmenopausal, current HT use, estrogen, and progesterone | 15 063 (14.2) | 7203 (16.6) | 4503 (12.7) | 2832 (12.4) | 525 (11.7) |
| Perimenopausal or postmenopausal, all other HT combinations | 13 968 (13.2) | 5981 (13.8) | 4376 (12.4) | 2956 (13.0) | 655 (14.5) |
| Oral contraceptive use, past and current | 70 188 (66.1) | 25 715 (61.5) | 24 968 (70.5) | 16 235 (71.1) | 3270 (72.6) |
BMI indicates body mass index; CTS, California Teachers Study; CVD, cardiovascular disease; HT, hormone therapy; and SSB, sugar‐sweetened beverage.
Values are expressed as mean±SEM or number (percentage).
1 serving of caloric soft drink is 12 fluid ounces, 1 serving of sweetened bottled water or tea, or fruit drink is 8 fluid ounces.
Education was obtained after baseline, during fourth mail‐in questionnaire follow‐up (2005–2006).
Cardiovascular disease family history includes first‐degree relatives’ (parent, sibling, offspring) heart attack/myocardial infarction and stroke.
After adjusting for CVD risk factors and potential confounders, we observed a positive, significant association between SSB intake and risk of CVD (Table 2). Women who consumed SSBs daily had an 18% higher risk of CVD (HR, 1.18; 95% CI, 1.05–1.32 [P trend=0.019]) compared with women who rarely/never consumed SSBs (model 1, Table 2). Further adjustment for BMI, total energy intake, and fruit and vegetable intake (diet quality marker), as potential mediators, attenuated the effect size (HR, 1.16; 95% CI, 1.03–1.31 [P trend=0.052]) (model 2, Table 2 and model 4, Table S3), yet the final model showed a 19% higher risk of CVD (HR, 1.19; 95% CI, 1.06–1.34 [P trend=0.016]) (model 3), among SSB daily consumers compared with those participants who rarely/never consumed SSBs. Further adjustment for specific dietary intake covariates is provided in Table S3.
Table 2.
CVDa Risk According to SSB Consumption in Semiquantitative Frequency Categories
| SSB Consumptionb | P Trend | ||||
|---|---|---|---|---|---|
| Rare/Never | >Rare/Never to <1 Serving Per wk | ≥1 Serving Per wk to <1 Serving Per d | ≥1 Serving Per d | ||
| CVD | |||||
| No. of cases | 4648 | 2382 | 1494 | 324 | |
| Rate per 10 000 person‐y | 64.8 | 38.7 | 37.8 | 41.4 | |
| Age‐adjusted HR (95% CI) | 1.0 | 0.99 (0.95–1.05) | 1.02 (0.96–1.08) | 1.26 (1.13–1.42) | 0.0006 |
| Multivariable‐adjusted HR (95% CI) | |||||
| Model 1 | 1.0 | 1.00 (0.95–1.06) | 1.01 (0.95–1.07) | 1.18 (1.05–1.32) | 0.019 |
| Model 2 | 1.0 | 1.00 (0.95–1.05) | 1.00 (0.94–1.07) | 1.16 (1.03–1.31) | 0.052 |
| Model 3 | 1.0 | 1.01 (0.96–1.07) | 1.02 (0.96–1.09) | 1.19 (1.06–1.34) | 0.016 |
| MIc | |||||
| No. of cases | 1441 | 681 | 460 | 95 | |
| Rate per 10 000 person‐y | 19.6 | 10.9 | 11.5 | 12.0 | |
| Age‐adjusted HR (95% CI) | 1.0 | 0.95 (0.87–1.04) | 1.06 (0.95–1.18) | 1.26 (1.02–1.55) | 0.022 |
| Multivariable‐adjusted HR (95% CI) | |||||
| Model 1 | 1.0 | 0.96 (0.87–1.05) | 1.05 (0.94–1.16) | 1.14 (0.92–1.40) | 0.148 |
| Model 2 | 1.0 | 0.95 (0.87–1.06) | 1.04 (0.93–1.16) | 1.15 (0.92–1.43) | 0.154 |
| Model 3 | 1.0 | 0.98 (0.89–1.07) | 1.07 (0.96–1.19) | 1.18 (0.95–1.47) | 0.060 |
| Revascularizationd | |||||
| No. of cases | 1468 | 798 | 505 | 118 | |
| Rate per 10 000 person‐y | 20.0 | 12.8 | 12.6 | 14.9 | |
| Age‐adjusted HR (95% CI) | 1.0 | 1.01 (0.93–1.10) | 1.03 (0.93–1.15) | 1.35 (1.12–1.64) | 0.006 |
| Multivariable‐adjusted HR (95% CI) | |||||
| Model 1 | 1.0 | 1.03 (0.94–1.12) | 1.03 (0.93–1.15) | 1.24 (1.02–1.50) | 0.044 |
| Model 2 | 1.0 | 1.04 (0.95–1.14) | 1.02 (0.92–1.14) | 1.23 (1.01–1.50) | 0.082 |
| Model 3 | 1.0 | 1.05 (0.96–1.15) | 1.04 (0.94–1.16) | 1.26 (1.04–1.54) | 0.037 |
| Strokee | |||||
| No. of cases | 2787 | 1415 | 867 | 189 | |
| Rate per 10 000 person‐y | 38.2 | 22.7 | 21.7 | 23.9 | |
| Age‐adjusted HR (95% CI) | 1.0 | 1.01 (0.94–1.08) | 1.01 (0.93–1.09) | 1.26 (1.09–1.46) | 0.017 |
| Multivariable‐adjusted HR (95% CI) | |||||
| Model 1 | 1.0 | 1.02 (0.95–1.08) | 1.00 (0.93–1.08) | 1.19 (1.03–1.39) | 0.076 |
| Model 2 | 1.0 | 1.00 (0.94–1.07) | 0.99 (0.92–1.08) | 1.18 (1.01–1.37) | 0.146 |
| Model 3 | 1.0 | 1.01 (0.95–1.08) | 1.01 (0.93–1.09) | 1.21 (1.04–1.41) | 0.056 |
Model 1 adjusted for age, race/ethnicity, socioeconomic status, smoking status, alcohol intake, cardiovascular disease family history, physical activity, aspirin use, multivitamin use, menopausal status, menopausal hormone therapy use, oral contraceptive use, and history of hypertension. Model 2 adjusted for variables in model 1 and body mass index, total energy intake, and fruit and vegetable intake. Model 3 adjusted for age, race/ethnicity, socioeconomic status, smoking status, alcohol intake, cardiovascular disease (CVD) family history, physical activity, aspirin use, menopausal status, menopausal hormone therapy use, history of hypertension, body mass index, and total energy intake. HR indicates hazard ratio; and SSB, sugar‐sweetened beverage.
Incident CVD event was defined as the first noted myocardial infarction (MI), revascularization (including coronary artery bypass grafting or percutaneous transluminal coronary angioplasty) or stroke, total person‐time 1 807 182 years.
One serving of caloric soft drink is 12 fluid ounces and 1 serving of sweetened bottled water or tea or fruit drink is 8 fluid ounces.
Total person‐time 1 843 233 years.
Revascularization includes coronary artery bypass grafting and percutaneous transluminal coronary angioplasty, total person‐time 1 835 429 years.
Total person‐time 1 831 462 years.
The risk of first revascularization event and risk of stroke, was 26% (HR, 1.26; 95% CI, 1.04–1.54 [P trend=0.037]) and 21% (HR, 1.21 [95% CI, 1.04–1.41 [P trend=0.056]) higher, respectively, in daily versus rare/never consumers of SSBs (model 3, Table 2).
With regards to type of SSB, a significant positive association was observed for fruit drinks and caloric soft drinks with incident CVD risk. Women who consumed ≥1 serving per day of fruit drinks had greater CVD (HR, 1.42; 95% CI, 1.00–2.01 [P trend=0.021]) risk, versus those who were rare/never consumers of fruit drinks (Figure 2). Similarly, compared with the rare/never consumers of caloric soft drinks, women who consumed ≥1 serving per day of caloric soft drinks had a 23% (HR, 1.23; 95% CI, 1.05–1.44 [P trend=0.0002]) higher risk of CVD. We observed a nonsignificant, positive association for sweetened bottled water or tea consumption and CVD risk. Details on the progressively adjusted models for these beverage‐specific associations are provided in Table S4. Beverage‐specific associations for MI, revascularization, and stroke are presented in Table S5.
Figure 2. Association of specific sugar‐sweetened beverage consumption and incident cardiovascular disease (CVD).
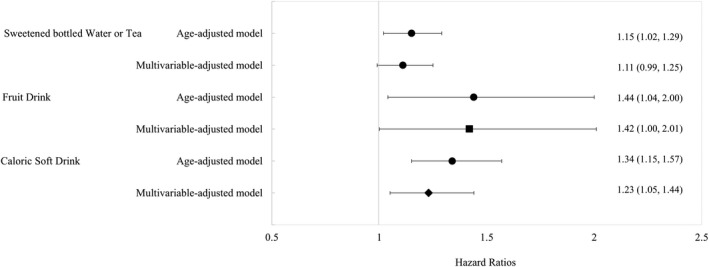
Hazard ratios comparing ≥1 sugar‐sweetened beverage serving per day vs rare/never (reference) categories. Multivariable‐adjusted model adjusted age, race/ethnicity, socioeconomic status, smoking status, alcohol intake, CVD family history, physical activity, aspirin use, menopausal status, menopausal hormone therapy use, history of hypertension, body mass index, total energy intake, and consumption of sugar‐sweetened bottled waters or teas, fruit drinks, and caloric soft drinks (other than the main exposure, depending on model). ▪ P for trend statistical significance at P<0.05. ♦ P for trend statistical significance at P<0.001.
Our sensitivity analysis addressing SSB intake in cups per day showed findings similar to those of the main analysis. The HR of CVD for participants consuming up to 1.5 cups per day was 1.19 (95% CI, 1.07–1.34] and for >1.5 cups per day was 1.22 (95% CI, 1.09–1.37] (P trend<0.0001) compared with rare/never consumers of SSBs (model 3, Table S4). The risk of MI and the risk of stroke were 25% (HR, 1.25; 95% CI, 1.02–1.54 [P trend=0.063]) and 26% (HR, 1.26; 95% CI, 1.09–1.46 [P trend=0.001]) higher, respectively, among women who consumed >1.5 cups per day of SSBs versus rare/never consumers. Revascularization risk was equivalent to the main analysis results (model 3, Table S6). Sensitivity analyses excluding events that occurred during the first 2 and 4 years of follow‐up did not alter the association found between SSB consumption and risk of CVD (Tables S7 and S8).
Discussion
We observed a significant positive association between daily consumption of SSBs and risk of CVD event among adult women over a period of 20 years. We also found a higher risk of revascularization and stroke with daily consumption of SSBs. We observed a significant, positive association between caloric soft drink and fruit drink consumption and risk of CVD.
The positive association found between daily SSB intake and risk of CVD is consistent with results from another longitudinal analysis of SSB consumption and CHD in an all‐female cohort,23 where Fung et al23 observed a 23% higher risk (relative risk [RR], 1.23; 95% CI, 1.06–1.43) in CHD among middle‐aged women who consumed 1 or 2 SSB servings per day. We did not observe a statistically significant association between SSB consumption and incident MI when using a semiquantitative exposure categorization, as Fung et al23 did, but we did see an association when SSBs were classified by cups per day (HR, 1.25; 95% CI, 1.02–1.54) comparing >1.5 cups per day versus rare/never. This SSB intake is equal to consuming >1 can of 12 fluid ounces of caloric soft drink or .75 of a 16 fluid ounce bottle of sweetened water and/or tea or fruit drink per day. Our findings for fruit drinks and soft drinks are also in line with findings from the NHS (Nurses’ Health Study), where researchers observed a positive association with a 2‐serving increase per day in fruit drinks and cola‐type carbonated beverages and incident CHD.23
Our documentation of a 26% greater risk (HR, 1.26; 95% CI, 1.04–1.54) of a revascularization procedure in women who consumed ≥1 serving per day of SSB versus those who rarely/never consumed SSBs, with identical risk by cups per day SSB classification, is a novel contribution. Since coronary artery bypass grafting and percutaneous coronary intervention/percutaneous transluminal coronary angioplasty revascularization intervention procedures are representative of a degree of coronary artery disease that leads to MI, we consider them in the context of our MI findings. Our HR findings for revascularization and MI risk with SSB intake as cups per day were nearly identical. Nonetheless, further research on SSB intake and incident revascularization is warranted.
Data on the association between SSB consumption and stroke are rare. The associations we observed in our primary analysis (semiquantitative categorization) and our sensitivity analysis (cups per day) are similar to those of Bernstein et al25 using data from the NHS. In the NHS, women who consumed ≥1 serving per day of sugar‐sweetened soda, had a 19% greater risk of total stroke (HR, 1.19; 95% CI, 1.05–1.48) in comparison to women who reported no SSB intake.25 Additionally, in a Swedish cohort of adult men and women followed for 10.3 years, Larsson et al26 observed a 19% greater risk of total stroke (RR, 1.19; 95% CI, 1.04–1.36) among adults consuming the highest (>2 servings per day [200 mL per serving)] versus the lowest (0.1 to <0.5 servings per day) intake of SSBs. In contrast to our findings, the association in their female‐only model was statistically insignificant (RR, 1.14; 95% CI, 0.92–1.41). The findings from a cohort of Japanese women followed for 18 years comparing almost every day versus rare/never consumers reported an HR of 1.21 (95% CI, 0.88–1.68).27
Our results are partially consistent with recently published meta‐analyses assessing the relationship between SSB consumption and CVD risk.22, 41 Xi et al22 pooled data from 4 prospective cohort studies including adult men and women and found a positive association between intake of SSBs and risk of CHD where those in the highest SSB consumption group had a 16% greater risk (RR, 1.16; 95% CI, 1.06–1.27]) of CHD than those in the lowest SSB consumption group.22 The CHD definition included other end points including MI. The same meta‐analysis found a marginal association between the highest SSB intake and risk of total stroke (RR, 1.10; 95% CI, 1.00–1.20) compared with the lowest SSB intake, with no significant association between SSB consumption and the risk of stroke in dose‐response analysis (summary RR, 1.06; 95% CI, 0.97–1.15 [P trend >0.05]). Narain et al41 reported that a high SSB intake was associated with a 19% greater risk of MI (RR, 1.19; 95% CI, 1.09–1.31) compared with low SSB intake, yet found no effect on risk of stroke (RR, 1.10; 95% CI, 0.97–1.25).41 When stratified by sex, SSB consumption was only highly associated with ischemic stroke in women (RR, 1.33; 95% CI, 1.07–1.66).41
As previously mentioned, there are several plausible biological mechanisms by which SSBs might impact CVD risk, including an increase in bloodstream concentrations of both glucose and insulin inducing a high glycemic load,29 appetite stimulation, and weight gain, contributing to impaired glucose tolerance and insulin resistance.30 Furthermore, this chain of events alters lipid metabolism and promotes synthesis of triglycerides, contributing to endothelial dysfunction and β‐cell stress30, 31, 32, 33, 34 and influencing metabolic35 and CVD risk.31, 32, 36 It is also possible that SSB consumption may serve as an indicator of a suboptimal diet and unfavorable lifestyle. Individuals who frequently consume SSBs are more likely to follow suboptimal diets.42, 43, 44 In our sample, we observed unfavorable dietary intake and behaviors among women who frequently consumed SSBs and adjusted for these lifestyle factors.
Study Strengths
Our study has several strengths including a large sample size, extensive follow‐up time, and prospective data collection on SSBs, diet, and lifestyle characteristics. Our sensitivity analysis addressed the possibility of reverse causality, and our analyses adjusted for potential confounders. Additional study strengths include our ability to annually link with statewide hospitalization and procedure records, which allowed for well‐defined and characterized end points, minimizing participant burden, and reducing bias caused by loss to follow‐up.
Study Limitations
Limitations of this study include being restricted to a single dietary assessment in which SSB consumption was measured, and we recognize the possibility of random measurement error. Additionally, assessment of other beverages, such as artificially sweetened beverages including low‐calorie sweet carbonated beverages (diet soft drinks) and other diet carbonated beverages, as well as sweetened hot beverages, were not included in the FFQ version used and could not be evaluated. Although dietary data were collected prospectively, social desirability bias cannot be disregarded, nor the potential for residual and unmeasured confounding. In addition, we cannot rule out change in beverage consumption intake over time, which we could not measure. We also acknowledge that the proportion of daily SSB consumption in our study population is small and relatively sparse when compared with the other SSB categories, leading to possible inflated measures of association as an implication of our findings. SSB consumption trends among US adults has declined in recent years.45, 46 Thus, considering our findings, we would expect an attenuation in the magnitude of the measure of association with current consumption shifts. In addition, our analyses could have benefited from further adjustment of cardiometabolic risk factors such as measured systolic and diastolic blood pressure and blood assay values for total and high‐density lipoprotein cholesterol and particularly triglycerides, which are shown to be an independent risk factor for CVD in women.47, 48
Conclusions
We found that daily consumption of at least 1 serving of a SSB is associated with a higher risk of CVD, revascularization, and stroke in women after accounting for CVD risk factors, suboptimal lifestyle behaviors, and dietary intake. Daily caloric soft drink consumption was also found to be associated with a higher risk of first CVD event. In sensitivity analysis, a higher risk of MI was observed among women with a daily intake of >1.5 cups of SSBs. Our results expand the literature on unfavorable effects of SSB intake, highlighting the importance of efforts to reduce SSB intake and changes to support healthier beverage consumption.
Sources of Funding
The CTS and the research reported in this article were supported by the National Cancer Institute of the National Institutes of Health under award numbers U01‐CA199277, P30‐CA033572, P30‐CA023100, UM1‐CA164917, and R01‐CA077398. Additionally, research described in this article was supported by grant CA023100‐29 from the University of California San Diego Moores Cancer Center (principal investigator: Maria Elena Martinez), and grant T32 HL079891‐11 from the National Heart, Lung, and Blood Institute (principal investigator: Matthew Allison).
Disclosures
None.
Supporting information
Tables S1–S8
Acknowledgments
The authors would like to thank the participants and staff of the CTS for their continuous involvement and valuable contributions. We also acknowledge the workforce of the California's Office of Statewide Health Planning and Development and California Cancer Registry. The authors would like to thank the CTS Steering Committee that is responsible for the formation and maintenance of the study within which this research was conducted. A full list of CTS team members is available at https://www.calteachersstudy.org/team. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The opinions, findings, and conclusions expressed herein are those of the author and do not necessarily reflect the official views of the State of California, Department of Public Health, National Cancer Institute, National Institutes of Health, Centers for Disease Control and Prevention or their Contractors and Subcontractors, or the Regents of the University of California or any of its programs.
(J Am Heart Assoc. 2020;9:e014883 DOI: 10.1161/JAHA.119.014883.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. U.S. Department of Health and Human Services and U.S. Department of Agriculture . 2015–2020 Dietary Guidelines for Americans. 2015. Available at: http://health.gov/dietaryguidelines/2015/guidelines/. Accessed February 7, 2019.
- 2. Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie‐Rosett J. Dietary sugars intake and cardiovascular health. Circulation. 2009;120:1011–1020. [DOI] [PubMed] [Google Scholar]
- 3. Powell ES, Smith‐Taillie LP, Popkin BM. added sugars intake across the distribution of US children and adult consumers: 1977–2012. J Acad Nutr Diet. 2016;116:1543–1550.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Guideline: Sugars Intake for Adults and Children. Geneva: Switzerland; 2015. [PubMed] [Google Scholar]
- 5. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 6. Rosinger A, Herrick K, Gahche J, Park S. Sugar‐sweetened Beverage Consumption Among U.S. adults, 2011–2014. NCHS Data Brief. 2017;1–8. [PubMed] [Google Scholar]
- 7. Malik VS, Schulze MB, Hu FB. Intake of sugar‐sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olsen NJ, Heitmann BL. Intake of calorically sweetened beverages and obesity. Obes Rev. 2009;10:68–75. [DOI] [PubMed] [Google Scholar]
- 9. Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar‐Sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle‐aged women. JAMA. 2004;292:927–934. [DOI] [PubMed] [Google Scholar]
- 10. Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta‐analysis. Am J Public Health. 2007;97:667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta‐analyses of randomised controlled trials and cohort studies. BMJ. 2012;346:e7492. [DOI] [PubMed] [Google Scholar]
- 12. Ma J, McKeown NM, Hwang SJ, Hoffman U, Jacques PF, Fox CS. Sugar‐sweetened beverage consumption is associated with change of visceral adipose tissue over 6 years of follow‐up. Circulation. 2016;133:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long‐term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malik VS, Popkin BM, Bray GA, Després JP, Hu FB. Sugar‐sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar‐sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168:1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar‐sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta‐analysis. Diabetes Care. 2010;33:2477–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barrio‐Lopez MT, Martinez‐Gonzalez MA, Fernandez‐Montero A, Beunza JJ, Zazpe I, Bes‐Rastrollo M. Prospective study of changes in sugar‐sweetened beverage consumption and the incidence of the metabolic syndrome and its components: the SUN cohort. Br J Nutr. 2019;110:1722–1731. [DOI] [PubMed] [Google Scholar]
- 18. Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle‐aged adults in the community. Circulation. 2007; 116:480–488. [DOI] [PubMed] [Google Scholar]
- 19. Duffey KJ, Gordon‐Larsen P, Steffen LM, Jacobs DR, Popkin BM. Drinking caloric beverages increases the risk of adverse cardiometabolic outcomes in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2010;92:954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen L, Caballero B, Mitchell DC, Loria C, Lin PH, Champagne CM, Elmer PJ, Ard JD, Batch BC, Anderson CAM, et al. Reducing consumption of sugar‐sweetened beverages is associated with reduced blood pressure: a prospective study among United States adults. Circulation. 2010;121:2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen L, Curhan G, Forman J. Association of sweetened beverage intake with incident hypertension. J Gen Intern Med. 2012;27:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xi B, Huang Y, Reilly KH, Li S, Zheng R, Barrio‐Lopez MT, Martinez‐Gonzalez MA, Zhou D. Sugar‐sweetened beverages and risk of hypertension and CVD: a dose‐response meta‐analysis. Br J Nutr. 2015;113:709–717. [DOI] [PubMed] [Google Scholar]
- 23. Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation. 2012;125:1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernstein AM, de Koning L, Flint AJ, Rexrode KM, Willett WC. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr. 2012;95:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Larsson SC, Akesson A, Wolk A. Sweetened beverage consumption is associated with increased risk of stroke in women and men. J Nutr. 2014;144:856–860. [DOI] [PubMed] [Google Scholar]
- 27. Eshak ES, Iso H, Kokubo Y, Saito I, Yamagishi K, Inoue M, Tsugane S. Soft drink intake in relation to incident ischemic heart disease, stroke, and stroke subtypes in Japanese men and women: the Japan Public Health Centre–based study cohort I. Am J Clin Nutr. 2012;96:1390–1397. [DOI] [PubMed] [Google Scholar]
- 28. Gardener H, Rundek T, Markert M, Wright CB, Elkind MSV, Sacco RL. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Intern Med. 2012;27:1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Janssens JP, Shapira N, Debeuf P, Michiels L, Putman R, Bruckers L, Renard D, Molenberghs G. Effects of soft drink and table beer consumption on insulin response in normal teenagers and carbohydrate drink in youngsters. Eur J Cancer Prev. 1999;8:289–295. [DOI] [PubMed] [Google Scholar]
- 30. Ludwig DS. The Glycemic Index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–2423. [DOI] [PubMed] [Google Scholar]
- 31. Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. [DOI] [PubMed] [Google Scholar]
- 32. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- 33. Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high‐sensitivity C‐reactive protein in middle‐aged women. Am J Clin Nutr. 2002;75:492–498. [DOI] [PubMed] [Google Scholar]
- 34. Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris‐Etherton PM, et al. Triglycerides and cardiovascular disease. Circulation. 2011;123:2292–2333. [DOI] [PubMed] [Google Scholar]
- 35. Bhupathiraju SN, Tobias DK, Malik VS, Pan A, Hruby A, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta‐analysis. Am J Clin Nutr. 2014;100:218–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–II10. [DOI] [PubMed] [Google Scholar]
- 37. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 38. Bernstein L, Allen M, Anton‐Culver H, Deapen D, Horn‐Ross PL, Peel D, Pinder R, Reynolds P, Sullivan‐Halley J, West D, et al. High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States). Cancer Causes Control. 2002;13:625–635. [DOI] [PubMed] [Google Scholar]
- 39. Horn‐Ross PL, Lee VS, Collins CN, Stewart SL, Canchola AJ, Lee MM, Reynolds P, Clarke CA, Bernstein L, Stram DO. Dietary assessment in the California Teachers Study: reproducibility and validity. Cancer Causes Control. 2008;19:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 41. Narain A, Kwok CS, Mamas MA. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: a systematic review and meta‐analysis. Int J Clin Pract. 2016;70:791–805. [DOI] [PubMed] [Google Scholar]
- 42. Rodríguez‐Monforte M, Flores‐Mateo G, Sánchez E. Dietary patterns and CVD: a systematic review and meta‐analysis of observational studies. Br J Nutr. 2015;114:1341–1359. [DOI] [PubMed] [Google Scholar]
- 43. Anand SS, Hawkes C, De Souza RJ, Mente A, Dehghan M, Nugent R, Zulyniak MA, Weis T, Bernstein AM, Krauss RM, et al. Food consumption and its impact on cardiovascular disease: importance of solutions focused on the globalized food system a report from the workshop convened by the World Heart Federation. J Am Coll Cardiol. 2015;6:1590–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heidemann C, Schulze MB, Franco OH, Van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation. 2008;118:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kit BK, Fakhouri TH, Park S, Nielsen SJ, Ogden CL. Trends in sugar‐sweetened beverage consumption among youth and adults in the United States: 1999–2010. Am J Clin Nutr. 2013;98:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bleich SN, Vercammen KA, Koma JW, Li Z. Trends in beverage consumption among children and adults, 2003–2014. Obesity. 2018;26:432–441. [DOI] [PubMed] [Google Scholar]
- 47. Bass KM, Newschaffer CJ, Klag MJ, Bush TL. Plasma lipoprotein levels as predictors of cardiovascular death in women. Arch Intern Med. 1993;153:2209–2216. [PubMed] [Google Scholar]
- 48. Castelli WP. The triglyceride issue: a view from Framingham. Am Heart J. 1986;112:432–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S8


