Abstract
Background
High sodium (Na+) intake is a widespread cardiovascular disease risk factor. High Na+ intake impairs endothelial function and exaggerates sympathetic reflexes, which may augment exercising blood pressure (BP) responses. Therefore, this study examined the influence of high dietary Na+ on BP responses during submaximal aerobic exercise.
Methods and Results
Twenty adults (8F/12M, age=24±4 years; body mass index 23.0±0.6 kg·m−2; VO2peak=39.7±9.8 mL·min−1·kg−1; systolic BP=111±10 mm Hg; diastolic BP=64±8 mm Hg) participated in this randomized, double‐blind, placebo‐controlled crossover study. Total Na+ intake was manipulated via ingestion of capsules containing either a placebo (dextrose) or table salt (3900 mg Na+/day) for 10 days each, separated by ≥2 weeks. On day 10 of each intervention, endothelial function was assessed via flow‐mediated dilation followed by BP measurement at rest and during 50 minutes of cycling at 60% VO2peak. Throughout exercise, BP was assessed continuously via finger photoplethysmography and every 5 minutes via auscultation. Venous blood samples were collected at rest and during the final 10 minutes of exercise for assessment of norepinephrine. High Na+ intake increased urinary Na+ excretion (placebo=140±68 versus Na+=282±70 mmol·24H−1; P<0.001) and reduced flow‐mediated dilation (placebo=7.2±2.4 versus Na+=4.2±1.7%; P<0.001). Average exercising systolic BP was augmented following high Na+ (placebo=Δ30.0±16.3 versus Na+=Δ38.3±16.2 mm Hg; P=0.03) and correlated to the reduction in flow‐mediated dilation (R=−0.71, P=0.002). Resting norepinephrine concentration was not different between conditions (P=0.82). Norepinephrine increased during exercise (P=0.002), but there was no Na+ effect (P=0.26).
Conclusions
High dietary Na+ augments BP responses during submaximal aerobic exercise, which may be mediated, in part, by impaired endothelial function.
Keywords: acute exercise, blood pressure, dietary sodium, exercise pressor reflex, flow‐mediated dilation
Subject Categories: Exercise, Diet and Nutrition, Blood Pressure
Nonstandard Abbreviations and Acronyms
- BP
blood pressure
- FMD
flow‐mediated dilation
- HR
heart rate
- Na+
sodium
- NaCl
salt
- PAR‐Q
physical activity readiness questionnaire
- TPR
total peripheral resistance
Clinical Perspective
What Is New?
Nine out of 10 Americans consume more sodium than is recommended, however it remains unclear how high sodium intake may affect future cardiovascular disease risk.
Using a double‐blind randomized clinical trial, we determined that high sodium intake augments blood pressure responses to submaximal aerobic exercise in otherwise healthy young adults.
Our data suggest that augmented blood pressure responses following high sodium intake may be linked to a reduction in endothelial‐dependent vasodilation, however future studies are required to fully elucidate these mechanisms.
What Are the Clinical Implications?
Recommendations for reduced sodium intake may be appropriate to maximize cardiovascular health even amongst individuals with normal blood pressure.
High dietary salt intake is a widespread cardiovascular disease risk factor, with more than 90% of Americans consuming more salt than the widely accepted recommendation of 2300 mg of sodium (Na+) per day.1 On average, US adults consume ≈3400 mg of Na+/day.2, 3 This is alarming, as high dietary salt has been linked with target organ damage,4, 5, 6 cardiovascular disease risk,2, 7 and future cardiovascular events.8, 9, 10 Additionally, published rodent data indicate that high dietary salt intake augments the exercise pressor reflex,11 the blood pressure (BP)‐raising reflex to exercise. Rodents fed high salt diets exhibited exaggerated sympathetic and BP responses to muscle contractions despite no change in resting BP. Importantly, an exaggerated BP response during aerobic exercise has prognostic value for future hypertension,12, 13, 14 coronary artery disease risk,15 and cardiovascular mortality,16 and these relations are stronger when evaluated using submaximal, rather than maximal, intensity aerobic exercise.17, 18, 19 However, the effects of high dietary sodium on BP responses during an acute bout of submaximal aerobic exercise have yet to be examined.
A potential mechanism by which high dietary salt may augment BP responses to exercise is via altered excitability and increased gain of central sympathetic neurons.20 At rest, increased extracellular sodium sensed by circumventricular organs in the brain results in increased sympathetic activity arising from the brainstem.21, 22 Data in rodents indicate that rats fed high salt diets have enhanced sympathetic responses to injection of glutamate (an excitatory amino acid) into the brainstem.23, 24, 25 Further, recently published human data indicate that infusion of hypertonic saline, which also elevates extracellular sodium, increases sympathetic and BP responses during isometric handgrip exercise.26 Additionally, high dietary salt intake has been demonstrated to impair endothelial function, as assessed via flow mediated dilation (FMD).27, 28, 29 Cross‐sectional data indicate that FMD is inversely related to BP responses during both submaximal30 and maximal31 exercise, such that those with lower FMD have larger increases in BP during aerobic exercise. Taken together, high salt diets may exaggerate BP responses to exercise through alterations in sympathetic outflow and vascular dysfunction.
Therefore, the purpose of this study was to determine the effects of high dietary salt intake on BP responses to submaximal aerobic exercise. Additionally, we sought to examine potential mechanisms that may affect BP responses to exercise. We hypothesized that excess dietary salt intake would augment the pressor response during aerobic exercise. Further, we hypothesized that impaired vascular function and greater increases in plasma norepinephrine concentrations following high dietary salt would be associated with the enhanced pressor response.
Methods
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Study Participants
All participants provided written and verbal consent prior to engaging in any study activities; study protocol and procedures were approved by the Institutional Review Board of the University of Delaware and conform to the provisions of the Declaration of Helsinki. The data reported herein are a part of a study registered on clinicaltrials.gov (NCT03565653).
Participants in this study include participants from a recently published study focused on high salt intake and postexercise hypotension,32 however the BP responses during submaximal aerobic exercise have not been published previously. After providing consent, study participants underwent a medical history screening including a report of habitual physical activity. Height (cm) and body mass (kg) were measured for calculation of body mass index (kg·m−2). Seated BP was measured via oscillometric assessment in triplicate following ≥5 minutes of seated rest (Dash 2000, GE Medical Systems). The average of the triplicate measures is reported here. Participants also completed the physical activity readiness questionnaire (PAR‐Q). Participant age ranged from 18 to 34 years and all participants were recreationally active (regularly participated in exercise ≥3 days/week). Exclusion criteria included a history of hypertension diagnosis, cardiovascular disease, cancer, diabetes mellitus, renal dysfunction, current pregnancy, obesity (body mass index >30 kg/m2), and use of nicotine products.
VO2peak
Participants reported to the Metabolic Stress Testing Laboratory at the University of Delaware to undergo a maximal cardiopulmonary exercise test. Participants performed cycling exercise to volitional fatigue (Lode CPET, Lode, the Netherlands) using a ramped protocol that has been described previously.33 Briefly, participants began cycling at a constant power of 30 W for 3 minutes. After the 3‐minute warm up, power increased by 1 W every 2‐seconds until participants were unable to maintain a pedaling cadence of 60 revolutions per minute. Oxygen consumption and carbon dioxide production were measured and averaged in 15‐second intervals using indirect calorimetry via an automated open circuit system (Parvo Medics, Sandy, UT) throughout the exercise test. Gas analyzers were calibrated with standard gases (16% O2, 4.05% CO2) and the pneumotach was calibrated using a standard volume of air (3L) prior to each test. Heart rate (HR) was monitored via a chest‐worn HR monitor (Polar H7, Polar, USA).
Following the VO2peak test and a brief rest period (≈10–15 minutes), participants resumed cycling and the workload corresponding to 60% VO2peak was determined via VO2 measurement. This workload was used during the experimental visits for the 50‐minute exercise bouts (described below).
Sodium Intervention
After completing the VO2peak test, participants were randomized into a double‐blind, placebo‐controlled, crossover study. For 10 days, participants were asked to consume a recommended (2300 mg Na+/day) sodium diet. We provided participants with instructions for interpreting nutrition labels and meeting sodium intake requirements. Participants also consumed unmarked capsules each day containing either salt (Morton table salt (NaCl); 3900 mg Na+/day) or a placebo (NOW Foods dextrose). Total sodium intake during the high salt condition was designed to be 6200 mg/day and during the dextrose condition was 2300 mg/day. Each participant completed both conditions in random order separated by ≥2 weeks for male and 1 month for female participants. All female participants were using oral hormonal contraceptives and experimental visits occurred during the placebo week of oral contraceptives. Participants recorded their diet during the first intervention and were asked to match their diet during the second intervention. A copy of their diet log was provided to serve as a menu. On the tenth day of each intervention, participants reported to the Cardiovascular Physiology Laboratory at the University of Delaware for their experimental visit. For 24 hours prior to the experimental visit, participants collected their urine.
Twenty‐four‐Hour Urine Collection
Urine was collected during the final 24‐hours of both interventions in a light‐protected, sterile 3500 mL container. Participants returned the container upon arriving to the laboratory for the experimental visit. We measured total urine volume, urine specific gravity (Goldberg Brix Refractometer, Reichert Technologies), urinary electrolyte concentrations (EasyElectrolyte Analyzer, Medica), and urinary osmolality (Advanced 3D3 Osmometer, Advanced Instruments) from a mixed aliquot from the 24‐hour collection container. Urine flow rate was calculated and used to determine 24‐hour sodium excretion. Participants were instructed to abstain from alcohol, caffeine, and exercise for the 24‐hours prior to and during the 24‐hour urine collection.
Study Visit
On the tenth day of each diet, participants reported to the Cardiovascular Physiology Laboratory at the University of Delaware. Upon arrival, participants provided a spot urine sample for assessment of hydration status via urine specific gravity (Goldberg Brix Refractometer, Reichert Technologies). Body weight, composition, and total body water were measured via bioelectrical impedance (Tanita Body Composition Analyzer, Model TBF‐300A; Arlington Heights, IL). Samples from female participants were tested using hormonal pregnancy tests (Moore Medical) to ensure that female participants were not pregnant.
Participants then laid supine and rested for >10 minutes. FMD testing was then performed. Briefly, baseline measurements of the left brachial artery diameter and blood flow velocity were recorded for 1 minute. A rapid inflation cuff around the left forearm distal to the elbow was inflated to a suprasystolic pressure (250 mm Hg) for 5 minutes. The cuff was rapidly deflated and brachial artery diameter and blood velocity were measured continuously for 3 minutes. The first complete velocity envelope following deflation of the cuff was used to determine the velocity‐time integral as previously described.34, 35 The location of the ultrasound probe was recorded to ensure that the same region of the brachial artery was analyzed under each condition.
Next, participants were instrumented for single‐lead electrocardiography (ECG) and oscillometric BP measurements at the upper arm (right arm; Dash 2000, GE Medical Systems). Following ≥15 minutes of supine rest, a venous blood sample was collected (described below). In a subset of participants (n=9), a 20‐ or 22‐gauge intravenous catheter was placed in the antecubital fossa for collection of a venous blood sample during submaximal aerobic exercise.
Beat‐to‐beat BP was recorded via finger photoplethysmography (Finometer, Finapres Medical Systems) at the middle finger of the left hand, which was supported at heart level. Systolic BP and diastolic BP were defined as the maximum and minimum value from the arterial BP waveform during each cardiac cycle, respectively. Mean BP was calculated as the integral of the BP waveform. Total peripheral resistance (TPR) and cardiac output were estimated from the arterial BP waveform using the Modelflow method.36 Brachial BP was recorded from the right arm via an automated oscillometric sphygmomanometer (Dash 2000, GE Medical Systems).
Exercise Protocol
Many investigations regarding BP responses to exercise have utilized the early stages of graded exercise tests,12, 13, 14 however we utilized steady‐state submaximal exercise of similar intensity (60% of VO2peak) in order to induce postexercise hypotension to answer other questions about the effects of high dietary sodium intake on blood pressure regulation.32 Following instrumentation, participants rested quietly in a dimly lit room for 10 minutes while undergoing continuous recordings of beat‐to‐beat BP and HR. Following this period of supine rest, participants were moved to an upright cycle ergometer (Lode Excaliber, Lode). Seated brachial BP was measured in triplicate via auscultation and used to calibrate the beat‐to‐beat BP signal.
Following seated BP measures, participants began submaximal aerobic exercise with a 5‐minute warm‐up period at self‐selected resistance (matched between conditions). The resistance was then increased to the power output determined during the VO2peak test (described above) for 50 minutes. After 50 minutes of exercise at 60% VO2peak, participants completed 5 additional minutes of aerobic exercise at self‐selected resistance as a cool down. Exercise workloads for warm up, exercise, and cool down were matched between conditions. Participants pedaled at their preferred cadence and resistance was continuously adjusted to maintain a constant power output. Participants were permitted to drink water ad libitum during the first exercise trial and water intake during exercise was matched for the second exercise trial.
Throughout exercise, beat‐to‐beat BP and HR were monitored continuously. Additionally, brachial BP (via auscultation) and rating of perceived exertion (Borg scale) were recorded every 5 minutes. In the subset of participants that had an intravenous catheter inserted, venous blood samples were collected in the final 10 minutes of exercise prior to cool‐down.
Biochemical Analysis
Serum electrolytes (EasyElectrolyte Analyzer, Medica), plasma osmolality (Advanced 3D3 Osmometer, Advanced Instruments), hemoglobin (Hb 201+, HemoCue), and hematocrit (Sure prep™ capillary tubes, Clay Adams spun in a microcentrifuge at 1950g for 5 minutes, Legend Micro 17, Thermo Sorvall) were measured. Remaining venous blood samples were spun at 750 g for 15 minutes at 4°C (Allegra X‐22R, Beckman Coulter). Plasma norepinephrine concentrations were measured in triplicate using enzyme‐linked immunosorbent assay (Norepinephrine ELISA, Alpco). The average intra‐assay coefficient of variation was 11.9%. The minimum detectable activity (sensitivity) for the assay is 36 pg/mL. All samples were above the assay's sensitivity.
Data and Statistical Analysis
Beat‐to‐beat BP and ECG signals were recorded continuously (LabChart Pro 8, AD Instruments) at 1000 Hz and stored for offline analysis. During exercise, beat‐to‐beat and brachial BP were averaged over 10‐minute intervals. Because baseline diameter can influence FMD results, FMDs were allometrically scaled in accordance with previous recommendations.37
The statistical approaches reported here were informed by recent guidelines for statistical reporting of cardiovascular research.38 The differences in Δsystolic BP, Δmean BP, and Δdiastolic BP following submaximal cycling exercise were examined using a generalized linear mixed‐model analysis with repeated measures for diet and time. In the case of a significant effect (time, diet, or interaction) Tukey's post hoc analysis was performed to determine where differences exist (ie at which time point, in the case of a significant diet effect). On a preliminary basis, BP responses during exercise were examined for any sex differences using a generalized linear mixed‐model analysis with repeated measures for diet and time. The effects of salt capsules on blood, urine, and FMD measures were tested using 2‐tailed paired t tests. Because high salt intake may expand plasma volume,39, 40 plasma NE concentrations were also indexed to hemoglobin. The average changes in systolic, diastolic and mean BP were also tested using 2‐tailed paired t tests. In the case of non‐normally distributed data, a Wilcoxon matched‐pairs signed rank test was used. The relation between the reduction in FMD and the change in exercising systolic BP was assessed using Pearson's correlation coefficient. Non‐allometrically scaled FMD data were used in the correlation analysis. Statistics were completed using IBM SPSS version 26.0 and GraphPad Prism version 8.3
Results
Participant characteristics are reported in Table 1. Participants were recreationally active, non‐obese, and had normal‐to‐elevated BP.41 Table 2 demonstrates the effects of the capsule intervention. Urinary sodium excretion during the placebo condition was ≈140 mmol/day (3220 mg/day) suggesting that sodium intake was above the 2300 mg/day that participants were asked to consume. Ten days of salt capsules significantly increased urinary sodium excretion (Table 2, P<0.001); however, serum sodium concentration, plasma osmolality, and resting plasma norepinephrine concentrations (n=15) were not different between conditions. Adequate paired FMD assessments were obtained in 16 individuals. FMD was significantly reduced following 10 days of salt capsules compared with placebo (Figure 1) and remained significantly different when FMD was allometrically scaled (Table 3). Velocity‐time integral was not different between conditions (Table 3).
Table 1.
Participant Characteristics
| n (F/M) | 20 (8/12) |
| Age, y | 24±4 |
| Height, cm | 172±10 |
| Body mass, kg | 69±13 |
| BMI, kg·m−2 | 23±3 |
| VO2peak, mL·min−1·kg−1 | 39.7±9.8 |
| Systolic BP, mm Hg | 111±10 |
| Diastolic BP, mm Hg | 64±8 |
| Mean BP, mm Hg | 80±8 |
Data are mean±SD. BMI indicates body mass index; BP, blood pressure; and VO2peak, peak oxygen uptake.
Table 2.
Effect of Capsule Intervention
| Measure | Dextrose | Sodium | P Value |
|---|---|---|---|
| Systolic BP, mm Hg | 108±11 | 108±8 | 0.90 |
| Diastolic BP, mm Hg | 61±7 | 61±6 | 0.83 |
| Mean BP, mm Hg | 77±7 | 77±6 | 0.83 |
| Urinary Na+ excretion, mmol·24 h−1 a | 140±68a | 282±70a | <0.001a |
| Plasma Osmolality, mOsm·kg·H2O−1 | 294±5 | 294±5 | 0.69 |
| Serum Na+, mmol/L | 141.0±1.8 | 141.4±2.1 | 0.32 |
| Serum K+, mmol/L | 4.12±0.49 | 4.06±0.51 | 0.50 |
| Serum Cl−, mmol/La | 104.8±2.0a | 106.2±1.8a | 0.02a |
| Hematocrit, % | 42.3±2.8 | 41.5±3.8 | 0.32 |
| Hemoglobin, mg/dLa | 13.5±1.0a | 12.8±1.3a | <0.01a |
| Plasma NE, pg/mL | 285.9±206.7 | 277.6±196.7 | 0.82 |
| Plasma NE Indexed to Hemoglobin | 21.3±15.5 | 21.4±14.9 | 0.97 |
Data are presented as mean±SD. BP indicates blood pressure; Cl−, chloride; K+, potassium; Na+, sodium; and NE, norepinephrine.
Significant difference.
Figure 1. Following 10 days of high sodium intake (filled bar), flow‐mediated dilation is significantly reduced compared with 10 day of habitual of sodium intake (open bar).
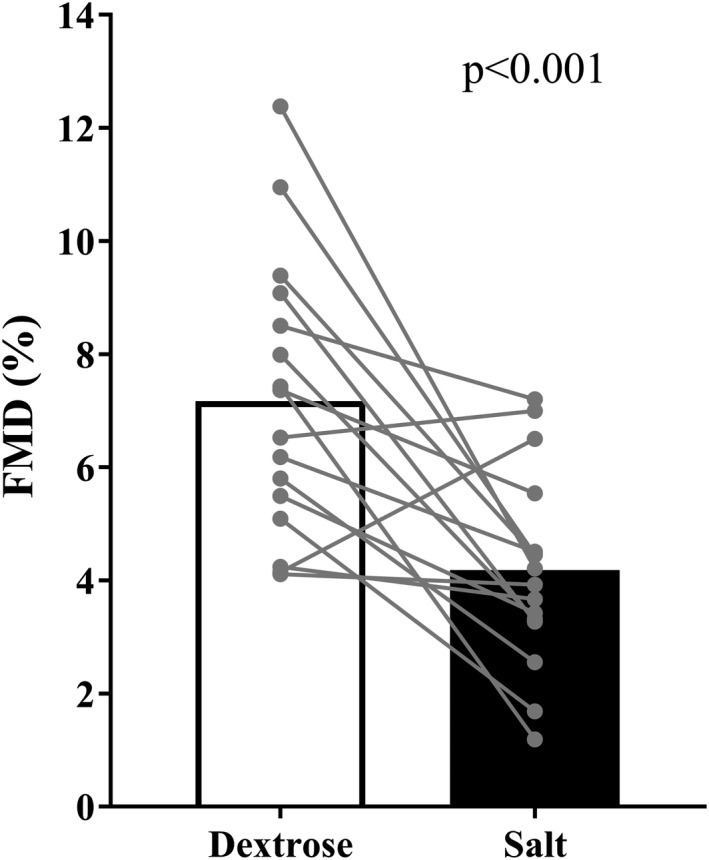
Data are presented as mean and individual data points.
Table 3.
Brachial Artery Flow‐Mediated Dilation Results
| Measure | Dextrose | Sodium | P Value |
|---|---|---|---|
| Baseline diameter, mm | 3.7±0.6 | 3.8±0.6 | 0.31 |
| Peak diameter, mm | 3.9±0.6 | 3.9±0.5 | 0.66 |
| Allometrically scaled FMD, %a | 6.8±2.1a | 4.2±2.1a | 0.003a |
| Shear rate (AUC) | 32 205±12 964 | 31 932±14 597 | 0.94 |
| VTI, cm | 91.8±32.5 | 87.9±37.7 | 0.73 |
Data are presented as mean±SD. AUC indicates area under the curve; FMD, flow‐mediated dilation; and VTI, velocity‐time integral.
Significant difference. N=16.
Average exercise workload was 122±8 W. Baseline HR was similar between conditions (placebo=57±2, sodium=55±2 beats·min−1, main effect of diet; P=0.42) and increased similarly during exercise (placebo=143±5, sodium=141±4 beats·min−1, main effect of diet; P=0.57) during exercise. This increase represented, on average, 61±1% of HR reserve, consistent with the target workload. As expected, metabolites (serum potassium, hydrogen ions, and lactate) and plasma norepinephrine were increased during exercise in the subset of participants from whom exercising blood samples were collected (Δplasma norepinephrine=1635±1184 pg/mL; Δserum potassium=0.54±0.34 mmol/L; ΔpH=−0.12±0.05; Δlactate=1.5±0.7 mmol/L). However, neither metabolites nor plasma norepinephrine concentrations were different between conditions (Table 4).
Table 4.
Hematologic Parameters During Exercise
| Measure | Dextrose | Sodium | P Value |
|---|---|---|---|
| Serum Na+, mmol/L | 143.1±1.0 | 143.1±1.6 | 0.94 |
| Serum K+, mmol/L | 4.69±0.25 | 4.60±0.35 | 0.56 |
| Serum Cl−, mmol/L | 106.7±1.9 | 106.9±2.0 | 0.87 |
| pH | 7.6±0.1 | 7.6±0.1 | 0.46 |
| Lactate, mmol/L | 2.3±2.2 | 2.7±1.8 | 0.47 |
| Hemoglobin, mg/dL | 15.1±0.3 | 14.9±0.3 | 0.28 |
| Hematocrit, % | 45.8±1.2 | 45.4±1.2 | 0.66 |
| Plasma NE, pg/mL | 1694±690 | 2186±830 | 0.25 |
| Plasma NE indexed to hemoglobin | 116.4±16.4 | 149.5±37.5 | 0.22 |
Data are presented as mean±SD. Cl− indicates chloride; K+, potassium; Na+, sodium; NE, norepinephrine; and Osm, osmolality. N=9.
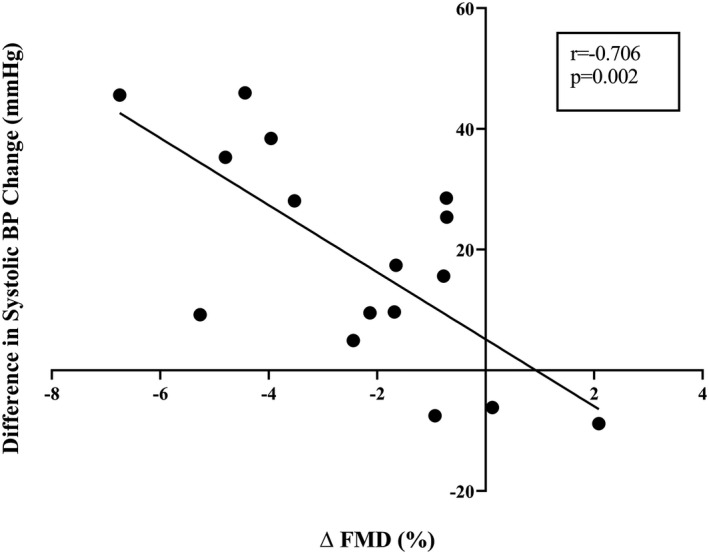
Figure 2 demonstrates BP responses to submaximal aerobic exercise. Systolic and mean, but not diastolic BP responses were augmented following 10 days of high, compared with habitual, sodium intake. When considered as the average change in BP, systolic (Figure 2B), but not mean or diastolic (Figure 2D and 2F), BP responses were augmented following 10 days of high salt. Pulse pressure increased during submaximal aerobic exercise (main effect of time: P<0.001) and this increase was augmented following 10 days of high salt intake (main effect of diet: P=0.023). No sex differences were observed in the effect of high sodium intake on BP responses to submaximal aerobic exercise (main effect of sex: P>0.60 for systolic, mean, and diastolic BP). The difference in the change in systolic BP during exercise was significantly correlated to the reduction in FMD (Figure 3). The increase in HR during aerobic exercise was not different following salt capsules compared to placebo (main effect of diet; P=0.35). Similarly, Modelflow‐derived estimates of cardiac output increased and TPR decreased (main effect of time; P<0.001 for both), however these changes were not different between conditions (main effect of diet; P=0.96 and 0.77, respectively).
Figure 2. Changes in (A and B) systolic (C and D) mean, and (E and F) diastolic blood pressure from baseline during submaximal aerobic exercise.
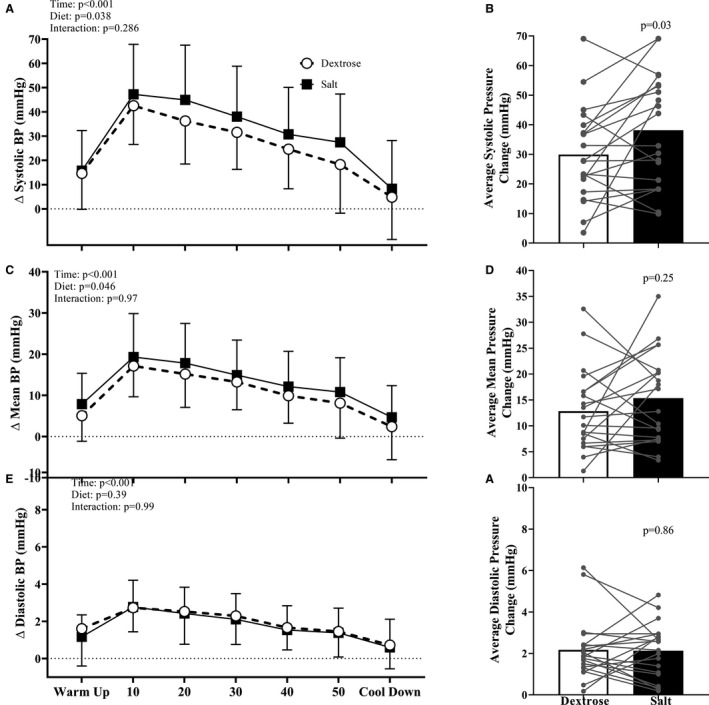
Following 10 days of habitual sodium intake (open circles) BP increase during aerobic exercise. However, following 10 days of high sodium intake (closed squares), systolic and mean blood pressure responses are augmented. Data are presented as mean±SD (A, C, and E) or mean and individual data points (B, D, and F).
Figure 3. The reductions in flow‐mediated dilation were negatively and significantly correlated to the increases in systolic blood pressure during submaximal aerobic exercise, such that individuals with the greatest reductions in flow‐mediated dilation had the largest increases in exercising systolic blood pressure.
Discussion
The novel findings of the present study are that high dietary salt intake augments BP responses to submaximal aerobic exercise. Similar to the existing animal literature,11 we found that BP responses were augmented during submaximal exercise following high sodium intake. Further, previous data in individuals with borderline hypertension indicated that modest reductions in sodium intake attenuate pressor responses to exercise.42 In contrast to the present findings, as well as previous human42 and animal11 literature, a recent study in participants with hypertension indicated no effect of dietary sodium intake on the change in BP during a single bout of exercise.43 However, mean BP during acute exercise was still reduced following sodium restriction, in agreement with our findings and others. Together, these findings suggest a role of sodium in BP dysregulation that may increase the risk of new‐onset hypertension14, 44 and future cardiovascular disease and mortality.13, 16, 45 Considering the widespread over‐consumption of sodium in the United States,3, 46 these data indicating that high dietary sodium intake augments BP responses to submaximal exercise are cause for concern.
We also sought to examine potential mechanisms contributing to the increase in exercising BP, including endothelial function and an index sympathetic activity. The findings of this study confirm the findings of several previous studies demonstrating impaired endothelial function following high sodium intake.27, 28, 29 Further, we applied allometric scaling to our data to account for potential differences in baseline brachial artery diameter.37 Similar to previously published data,28, 39 reductions in hemoglobin and mild, non‐significant, reductions in hematocrit in the present study suggest an expansion of the plasma volume following high sodium intake, which could possibly contribute to the statistically insignificant increases in baseline brachial artery diameter observed in this study. Even after accounting for these differences in baseline diameter, FMD was significantly reduced following high sodium intake.
Similar to previous, cross‐sectional, observations,30, 31 our findings indicate that impaired endothelial function induced by 10 days of high sodium intake may have contributed to the augmented systolic BP responses. Interestingly, in a large community sample with more than 2000 participants, FMD was negatively correlated to submaximal exercise systolic BP, however worse FMD was also associated with higher resting systolic BP.30 In contrast, the participants in the present study did not demonstrate elevated BP following reductions in FMD resulting from short‐term high sodium intake. While a thorough explanation for these discrepancies remains unknown, it should be noted that our participants were young, healthy, non‐smokers with no history of diabetes mellitus, hypercholesterolemia, or hypertension, while participants in the previous study were older, 7% had diabetes mellitus 12% were smokers, 14% were receiving lipid treatment, and 24% were medicated for hypertension. Therefore, direct comparisons are difficult to make.
In young adults, exaggerated systolic BP responses during exercise result from a failure to reduce TPR during exercise.47 The endothelium‐dependent dilation that is reflected by FMD occurs in response to increased wall shear stress during hyperemia,48 therefore, high sodium intake may have impaired the conduit artery vasodilator response to exercise hyperemia. While exercising Modelflow‐derived TPR was not different between conditions, these estimates are limited and exercise‐induced increases in body temperature may have confounded these estimates.49 However, velocity‐time integral, which reflects microvascular function,34, 35 was not different between conditions during the FMD test, suggesting that high salt intake may have impaired conduit, but not microvascular blood vessel function. Therefore, we speculate that the reduced FMD reflects an impaired conduit artery vasodilator response to exercise and contributed to the augmented systolic BP response to submaximal aerobic exercise observed in the present study. However, the lack of a difference in TPR and velocity‐time integral may explain why exercising diastolic BP was not different between conditions. Of note, the change in FMD explains ≈50% of the variance in the change in exercising systolic BP between conditions, therefore it is likely that some other mechanism (or mechanisms) contribute to these differences.
Although previous animal investigations have identified an increase in sympathetic reflexes following high sodium intake,20, 23, 24, 50 plasma norepinephrine concentrations during exercise were not affected by sodium intake in the present study. These data indicate that sympathetic outflow during dynamic exercise may not have been affected by high sodium intake in the present study. However, plasma norepinephrine concentrations are less sensitive to differences in sympathetic outflow than other sympathetic assessments (ie microneurography).51 Therefore, future mechanistic studies of the effects of high dietary sodium intake on the exercise pressor reflexes utilizing microneurography may yet reveal an influence of the sympathetic nervous system.
One such study utilizing microneurography does indicate an increase in sympathetic activity during isometric handgrip exercise following acute sodium loading achieved via hypertonic saline infusion.26 It should be noted, however, that the acute sodium load in this previous study was sufficient to raise serum sodium concentrations and plasma osmolality, whereas these variables were not different between conditions in the present study. The prolonged time course of the present study may have allowed for counter‐regulatory mechanisms to buffer changes in serum sodium concentrations. For example, one previous study demonstrated that 1 week of high sodium intake increased skin sodium accumulation,52 which may have buffered changes in serum sodium. An acute infusion of hypertonic saline may not have provided enough time to deposit sodium in the skin. Interestingly, we have also demonstrated that a single high‐salt meal does not alter BP responses to maximal intensity aerobic exercise,53 suggesting that longer‐term exposure to high sodium intake may be required to induce these changes in exercise BP responses. It remains unclear, therefore, if the disagreements between these studies reflect differences in sodium loading (ie acute versus chronic), differences in exercise modality (ie static versus dynamic exercise), that the prior study raised serum sodium/plasma osmolality, whereas this was not the case in the present study, or some combination of these factors.
Limitations
There are several limitations of the present study that must be addressed. The study population for this study was limited to recreationally active young adults with normal‐to‐elevated BP, a population that tends to have “salt resistant” BP (ie BP that is not affected by sodium intake). Individuals with hypertension tend to be more predisposed to have salt‐sensitive BP,54 and therefore the observations from this study may not be true for adults with hypertension. Further, our sample size was relatively small and future studies utilizing larger cohorts are needed to confirm the observations from this study.
Additionally, while we observed robust increases in plasma norepinephrine concentrations during exercise, we did not detect differences in exercising plasma norepinephrine concentrations between conditions. However, we only collected blood samples from a subset of participants during exercise (n=9) and therefore may have been underpowered to observe differences in plasma norepinephrine during exercise. We also did not measure urinary norepinephrine; however, we think that it is unlikely that we would have observed differences in urinary catecholamines that were not detected in the plasma, as urinary and plasma catecholamines tend to be closely correlated.55 Ideally, studies examining the sympathetic component of BP regulation should utilize more sensitive measures of sympathetic outflow (eg microneurography), however, our exercise modality precluded the use of peroneal microneurography.
Further, while our data indicate that reductions in FMD were associated with a larger increase in exercising systolic BP, the use of correlation analysis precludes a definitive conclusion that reduced vasodilator capacity augments exercising BP following high salt intake. It is possible that high salt intake had another effect that contributed to both reduced FMD and augmented exercising BP (eg increased oxidative stress). Similarly, the use of paired t tests assumes no carryover, or residual, effects of the intervention, therefore we did not assess carryover in this study. However, our study design (double‐blind, randomized, placebo‐controlled trial) makes it unlikely that carryover effects influenced our findings.
On a preliminary basis, we examined sex differences in the effect of high dietary sodium on BP responses during exercise. While we did not observe any significant effect of sex on these responses, it is likely that we were not adequately powered to appropriately examine the influence of sex. Future studies should address this important question.
Finally, participants were asked to consume a recommended (ie 2300 mg/day) sodium diet for each condition. However, urinary sodium excretion indicates that sodium intake on the placebo condition was closer to habitual (ie 3400 mg/day) sodium intake among Americans. Despite the lack of adherence to a recommended sodium diet, participants reported no difference in sodium intake via diet logs and we still established a significant difference in sodium intake between conditions, as indicated by urinary sodium excretion.
Conclusions
In conclusion, the current data indicate that excess dietary sodium intake augments BP responses to an acute bout of submaximal aerobic exercise. These data indicate that impaired endothelial function induced by high sodium intake may be a contributing mechanism to augmented BP responses during exercise. Because of the widespread overconsumption of dietary sodium and the relation between augmented BP responses to exercise and cardiovascular disease, the results have important clinical implications. Efforts to reduce sodium intake should be encouraged to maximize the effects of exercise on cardiovascular health.
Sources of Funding
This research was supported by American College of Sports Medicine Foundation Doctoral Student Research Grant 17‐00521 (Babcock), NIH R01HL128388 (Farquhar), and American Heart Association 18POST34060020 (Robinson). This publication was made possible by the Delaware Center of Biomedical Research Excellence in Cardiovascular Health, supported by a grant from the National Institute of General Medical Sciences – NIGMS (5 P20 GM113125) from the NIH.
Disclosures
None.
Acknowledgments
The authors would like to thank Sofia Sanchez, MBA, RDN, LDN and Wendy Nichols, BSN. We would also like to thank the individuals who participated in this study.
Author contributions: All authors participated in the conception and design of experiments; Babcock, Robinson, Watso, Migdal and Farquhar performed experiments; Babcock and Robinson analyzed data; Babcock, Robinson, Martens, Edwards, Pescatello, and Farquhar interpreted results of experiments; Babcock prepared figures; Babcock and Farquhar drafted manuscript; all authors edited, revised, and approved the final version of the manuscript.
(J Am Heart Assoc. 2020;9:e015633 DOI: 10.1161/JAHA.120.015633.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Jackson SL, King SM, Zhao L, Cogswell ME. Prevalence of excess sodium intake in the United States—NHANES, 2009–2012. MMWR Morb Mortal Wkly Rep. 2016;64:1393–1397. [DOI] [PubMed] [Google Scholar]
- 2. Cook NR, Appel LJ, Whelton PK. Sodium intake and all‐cause mortality over 20 years in the trials of hypertension prevention. J Am Coll Cardiol. 2016;68:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. U S Department of Health and Human Services . Dietary guidelines for Americans 2015–2020. 2015.
- 4. Frohlich ED, Chien Y, Sesoko S, Pegram BL. Relationship between dietary sodium intake, hemodynamics, and cardiac mass in SHR and WKY rats. Am J Physiol. 1993;264:R30–R34. [DOI] [PubMed] [Google Scholar]
- 5. Matavelli LC, Zhou X, Varagic J, Susic D, Frohlich ED. Salt loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H814–H819. [DOI] [PubMed] [Google Scholar]
- 6. Sanders PW. Vascular consequences of dietary salt intake. Am J Physiol Renal Physiol. 2009;297:F237–F243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whelton PK, Appel LJ, Sacco RL, Anderson CAM, Antman EM, Campbell N, Dunbar SB, Frohlich ED, Hall JE, Jessup M, et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126:2880–2889. [DOI] [PubMed] [Google Scholar]
- 8. Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow‐up of the trials of hypertension prevention (TOHP). BMJ. 2007;334:885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mente A, O'Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601–611. [DOI] [PubMed] [Google Scholar]
- 10. Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634. [DOI] [PubMed] [Google Scholar]
- 11. Yamauchi K, Tsuchimochi H, Stone AJ, Stocker SD, Kaufman MP. Increased dietary salt intake enhances the exercise pressor reflex. Am J Physiol Heart Circ Physiol. 2014;306:H450–H454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matthews CE, Pate RR, Jackson KL, Ward DS, Macera CA, Kohl HW, Blair SN. Exaggerated blood pressure response to dynamic exercise and risk of future hypertension. J Clin Epidemiol. 1998;51:29–35. [DOI] [PubMed] [Google Scholar]
- 13. Miyai N, Arita M, Morioka I, Miyashita K, Nishio I, Takeda S. Exercise BP response in subjects with high‐normal BP: exaggerated blood pressure response to exercise and risk of future hypertension in subjects with high‐normal blood pressure. J Am Coll Cardiol. 2000;36:1626–1631. [DOI] [PubMed] [Google Scholar]
- 14. Miyai N, Arita M, Miyashita K, Morioka I, Shiraishi T, Nishio I. Blood pressure response to heart rate during exercise test and risk of future hypertension. Hypertension. 2002;39:761–766. [DOI] [PubMed] [Google Scholar]
- 15. Mariampillai JE, Liestol K, Kjeldsen SE, Prestgaard EE, Engeseth K, Bodegard J, Berge E, Gjesdal K, Erikssen J, Grundvold I, et al. Exercise systolic blood pressure at moderate workload is linearly associated with coronary disease risk in healthy men. Hypertension. 2020;75:44–50. [DOI] [PubMed] [Google Scholar]
- 16. Weiss SA, Blumenthal RS, Richey Sharrett A, Redberg RF, Mora S. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation. 2010;121:2109–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lim PO, Donnan PT, MacDonald TM. Blood pressure determinants of left ventricular wall thickness and mass index in hypertension: comparing office, ambulatory and exercise blood pressures. J Hum Hypertens. 2001;15:627–633. [DOI] [PubMed] [Google Scholar]
- 18. Kokkinos P, Pittaras A, Narayan P, Faselis C, Singh S, Manolis A. Exercise capacity and blood pressure associations with left ventricular mass in prehypertensive individuals. Hypertension. 2007;49:55–61. [DOI] [PubMed] [Google Scholar]
- 19. Kokkinos PF, Andreas PE, Coutoulakis E, Colleran JA, Narayan P, Dotson CO, Choucair W, Farmer C, Fernhall B. Determinants of exercise blood pressure response in normotensive and hypertensive women: role of cardiorespiratory fitness. J Cardiopulm Rehabil. 2002;22:178–183. [DOI] [PubMed] [Google Scholar]
- 20. Stocker SD, Madden CJ, Sved AF. Excess dietary salt intake alters the excitability of central sympathetic networks. Physiol Behav. 2010;100:519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kinsman BJ, Simmonds SS, Browning KN, Stocker SD. Organum vasculosum of the lamina terminalis detects NaCl to elevate sympathetic nerve activity and blood pressure nervous system. Hypertension. 2017;69:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nomura K, Hiyama TY, Sakuta H, Matsuda T, Lin CH, Kobayashi K, Kuwaki T, Takahashi K, Matsui S, Noda M. [Na(+)] increases in body fluids sensed by central Nax induce sympathetically mediated blood pressure elevations via H(+)‐dependent activation of ASIC1A. Neuron. 2019;101:60–75.e66. [DOI] [PubMed] [Google Scholar]
- 23. Ito S, Gordon FJ, Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol. 1999;276:R1600–R1607. [DOI] [PubMed] [Google Scholar]
- 24. Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension. 2007;50:354–359. [DOI] [PubMed] [Google Scholar]
- 25. Pawloski‐Dahm CM, Gordon FJ. Increased dietary salt sensitizes vasomotor neurons of the rostral ventrolateral medulla. Hypertension. 1993;22:929–933. [DOI] [PubMed] [Google Scholar]
- 26. Brian MS, Matthews EL, Watso JC, Babcock MC, Wenner MM, Rose WC, Stocker SD, Farquhar WB. The influence of acute elevations in plasma osmolality and serum sodium on sympathetic outflow and blood pressure responses to exercise. J Neurophysiol. 2017;119:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. DuPont JJ, Greaney JL, Wenner MM, Lennon‐Edwards SL, Sanders PW, Farquhar WB, Edwards DG. High dietary sodium intake impairs endothelium‐dependent dilation in healthy salt‐resistant humans. J Hypertens. 2013;31:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lennon‐Edwards SL, Ramick MG, Matthews EL, Brian MS, Farquhar WB, Edwards DG. Salt loading has a more deleterious effect on flow‐mediated dilation in salt‐resistant men than women. Nutr Metab Cardiovasc Dis. 2014;24:990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matthews EL, Brian MS, Ramick MG, Lennon‐Edwards SL, Edwards DG, Farquhar WB. High dietary sodium reduces brachial artery flow‐mediated dilation in humans with salt‐sensitive and salt‐resistant blood pressure. J Appl Physiol (1985). 2015;118:1510–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thanassoulis G, Lyass A, Benjamin EJ, Larson MG, Vita JA, Levy D, Hamburg NM, Widlansky ME, O'Donnell CJ, Mitchell GF, et al. Relations of exercise blood pressure response to cardiovascular risk factors and vascular function in the Framingham Heart Study. Circulation. 2012;125:2836–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stewart KJ, Sung J, Silber HA, Fleg JL, Kelemen MD, Turner KL, Bacher AC, Dobrosielski DA, Deregis JR, Shapiro EP, et al. Exaggerated exercise blood pressure is related to impaired endothelial vasodilator function. Am J Hypertens. 2004;17:314–320. [DOI] [PubMed] [Google Scholar]
- 32. Babcock MC, Robinson AT, Watso JC, Migdal KU, Martens CR, Edwards DG, Pescatello LS, Farquhar WB. Salt loading blunts central and peripheral postexercise hypotension. Med Sci Sports Exerc. 2020;52:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davis JA, Whipp BJ, Lamarra N, Huntsman DJ, Frank MH, Wasserman K. Effect of ramp slope on determination of aerobic parameters from the ramp exercise test. Med Sci Sports Exerc. 1982;14:339–343. [PubMed] [Google Scholar]
- 34. Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long‐term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–169. [DOI] [PubMed] [Google Scholar]
- 35. Lee V, Martin BJ, Fung M, Anderson TJ. The optimal measure of microvascular function with velocity time integral for cardiovascular risk prediction. Vasc Med. 2012;17:287–293. [DOI] [PubMed] [Google Scholar]
- 36. Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three‐element model. J Appl Physiol. 1993;74:2566–2573. [DOI] [PubMed] [Google Scholar]
- 37. Atkinson G, Batterham AM, Thijssen DH, Green DJ. A new approach to improve the specificity of flow‐mediated dilation for indicating endothelial function in cardiovascular research. J Hypertens. 2013;31:287–291. [DOI] [PubMed] [Google Scholar]
- 38. Lindsey ML, Gray GA, Wood SK, Curran‐Everett D. Statistical considerations in reporting cardiovascular research. Am J Physiol Heart Circ Physiol. 2018;315:H303–H313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Babcock MC, Brian MS, Watso JC, Edwards DG, Stocker SD, Wenner MM, Farquhar WB. Alterations in dietary sodium intake affect cardiovagal baroreflex sensitivity. Am J Physiol Regul Integr Comp Physiol. 2018;315:R688–R695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Babcock MC, Robinson AT, Migdal KU, Watso JC, Wenner MM, Stocker SD, Farquhar WB. Reducing dietary sodium to 1000 mg per day reduces neurovascular transduction without stimulating sympathetic outflow. Hypertension. 2019;73:587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483. [DOI] [PubMed] [Google Scholar]
- 42. Ambrosioni E, Costa FV, Borghi C, Montebugnoli L, Giordani MF, Magnani B. Effects of moderate salt restriction on intralymphocytic sodium and pressor response to stress in borderline hypertension. Hypertension. 1982;4:789–794. [DOI] [PubMed] [Google Scholar]
- 43. Ratchford SM, Broxterman RM, La Salle DT, Kwon OS, Hopkins PN, Richardson RS, Trinity JD. Salt restriction lowers blood pressure at rest and during exercise without altering peripheral hemodynamics in hypertensive individuals. Am J Physiol Heart Circ Physiol. 2019;317:H1194–H1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matthews KA, Woodall KL, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension. 1993;22:479–485. [DOI] [PubMed] [Google Scholar]
- 45. Singh JP, Larson MG, Manolio TA, O'Donnell CJ, Lauer MS, Evans JC, Levy D. Blood pressure response during treadmill testing as a risk factor for new‐onset hypertension: the Framingham Heart Study. Circulation. 1999;99:1831–1836. [DOI] [PubMed] [Google Scholar]
- 46. Bailey RL, Parker EA, Rhodes DG, Goldman JD, Clemens JC, Moshfegh AJ, Thuppal SV, Weaver CM. Estimating sodium and potassium intakes and their ratio in the American diet: data from the 2011–2012 NHANES. J Nutr. 2016;146:745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilson MF, Sung BH, Pincomb GA, Lovallo WR. Exaggerated pressure response to exercise in men at risk for systemic hypertension. Am J Cardiol. 1990;66:731–736. [DOI] [PubMed] [Google Scholar]
- 48. Silber HA, Bluemke DA, Ouyang P, Du YP, Post WS, Lima JA. The relationship between vascular wall shear stress and flow‐mediated dilation: endothelial function assessed by phase‐contrast magnetic resonance angiography. J Am Coll Cardiol. 2001;38:1859–1865. [DOI] [PubMed] [Google Scholar]
- 49. Shibasaki M, Wilson TE, Bundgaard‐Nielsen M, Seifert T, Secher NH, Crandall CG. Modelflow underestimates cardiac output in heat‐stressed individuals. Am J Physiol Regul Integr Comp Physiol. 2011;300:R486–R491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Simmonds SS, Lay J, Stocker SD. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension. 2014;64:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khan MH, Sinoway LI, MacLean DA. Effects of graded LBNP on MSNA and interstitial norepinephrine. Am J Physiol Heart Circ Physiol. 2002;283:H2038–H2044. [DOI] [PubMed] [Google Scholar]
- 52. Selvarajah V, Maki‐Petaja KM, Pedro L, Bruggraber SFA, Burling K, Goodhart AK, Brown MJ, McEniery CM, Wilkinson IB. Novel mechanism for buffering dietary salt in humans: effects of salt loading on skin sodium, vascular endothelial growth factor C, and blood pressure. Hypertension. 2017;70:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Migdal KU, Robinson AT, Watso JC, Babcock MC, Serrador JM, Farquhar WB. A high‐salt meal does not augment blood pressure responses during maximal exercise. Appl Physiol Nutr Metab. 2020;45:123–128. [DOI] [PubMed] [Google Scholar]
- 54. Farquhar WB, Edwards DG, Jurkovitz CT, Weintraub WS. Dietary sodium and health: more than just blood pressure. J Am Coll Cardiol. 2015;65:1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saxena AR, Chamarthi B, Williams GH, Hopkins PN, Seely EW. Predictors of plasma and urinary catecholamine levels in normotensive and hypertensive men and women. J Hum Hypertens. 2014;28:292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]


