Abstract
Background
Trimethylamine N‐oxide (TMAO) may have prothrombotic properties. We examined the association of TMAO quartiles with major adverse cardiovascular events (MACE) and the effect of TMAO on the efficacy of ticagrelor.
Methods and Results
PEGASUS‐TIMI 54 (Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin ‐ Thrombolysis in Myocardial Infarction 54) randomized patients with prior myocardial infarction to ticagrelor or placebo (median follow‐up 33 months). Baseline plasma concentrations of TMAO were measured in a nested case‐control study of 597 cases with cardiovascular death, myocardial infarction, or stroke (MACE) and 1206 controls matched for age, sex, and estimated glomerular filtration rate [eGFR]. Odds ratios (OR) were used for the association between TMAO quartiles and MACE, adjusting for baseline clinical characteristics (age, sex, eGFR, region, body mass index, hypertension, hypercholesterolemia, diabetes mellitus, smoking, peripheral artery disease, index event, aspirin dosage and treatment arm), and cardiovascular biomarkers (hs‐TnT [high‐sensitivity troponin T], hs‐CRP [high‐sensitivity C‐reactive protein], NT‐proBNP [N‐terminal‐pro‐B‐type natriuretic peptide]). Higher TMAO quartiles were associated with risk of MACE (OR for quartile 4 versus quartile 1, 1.43, 95% CI, 1.06–1.93, P trend=0.015). The association was driven by cardiovascular death (OR 2.25, 95% CI, 1.28–3.96, P trend=0.003) and stroke (OR 2.68, 95% CI, 1.39–5.17, P trend<0.001). After adjustment for clinical factors, the association persisted for cardiovascular death (OR adj 1.89, 95% CI, 1.03–3.45, P trend=0.027) and stroke (OR adj 2.01, 95% CI, 1.01–4.01, P trend=0.022), but was slightly attenuated after adjustment for cardiovascular biomarkers (cardiovascular death: OR adj 1.74, 95% CI, 0.88–3.45, P trend=0.079; and stroke: OR adj 1.82, 95% CI, 0.88–3.78, P trend=0.056). The reduction in MACE with ticagrelor was consistent across TMAO quartiles (P interaction=0.92).
Conclusions
Among patients with prior myocardial infarction, higher TMAO levels were associated with cardiovascular death and stroke but not with recurrent myocardial infarction. The efficacy of ticagrelor was consistent regardless of TMAO levels.
Registration
URL: https://www.clinicaltrials.gov; Unique identifiers: PEGASUS‐TIMI 54, NCT01225562.
Keywords: antiplatelet therapy, cardiovascular death, gut microbiota, myocardial infarction, stroke, ticagrelor, trimethylamine N‐oxide
Subject Categories: Secondary Prevention, Biomarkers, Platelets, Myocardial Infarction
Nonstandard Abbreviations and Acronyms
- ACS
acute coronary syndromes
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- hs‐CRP
high‐sensitivity C‐reactive protein
- hs‐TnT
high‐sensitivity troponin T
- IQR
interquartile ranges
- MACE
major adverse cardiovascular events
- MI
myocardial infarction
- NT‐proBNP
N‐terminal‐pro‐B‐type natriuretic peptide hormone
- OR
odds ratio
- PEGASUS‐TIMI 54 trial
Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin‐Thrombolysis in Myocardial Infarction 54
- SD
standard deviation
- TMAO
trimethylamine N‐oxide
Clinical Perspective
What Is New?
In patients with prior myocardial infraction, we observed an association between higher trimethylamine N‐oxide levels and incident cardiovascular death or stroke events.
The associations remained significant after adjustment for clinical factors but were slightly attenuated with further adjustment for established biomarkers.
What Are the Clinical Implications?
Reconciling previous and current findings, trimethylamine N‐oxide levels offer insight for risk assessment but, at least among patients with prior myocardial infarction, do not offer additional information for tailoring more intensive antiplatelet therapy.
Introduction
Intestinal microbiota have been associated with several pathways (eg, inflammation, obesity, and diabetes mellitus) that affect cardiovascular health.1, 2 Trimethylamine N‐oxide (TMAO) is a circulating metabolite produced by gut microbiota involving nutrient precursors abundant in the western diet (red meat, betaine, choline, carnitine, trimethyllysine),3, 4, 5 and has been associated with incident cardiovascular risk in stable cohorts and patients with acute coronary syndromes.6, 7, 8, 9 TMAO may have prothrombotic properties that are partially reversible with the use of aspirin.10 In contrast to the setting of an acute coronary syndrome,11 the prognostic value of TMAO in stable patients with prior myocardial infarction (MI) has not been extensively explored, nor has an interaction with long‐term use of more potent antiplatelet therapy. Currently, there is an unmet medical need to identify patients at higher ischemic risk who might derive greater benefit from more intensive preventive strategies after MI.12, 13 This prognostication is especially important to individualize long‐term therapies given the variation of residual risk in patients with known cardiovascular disease (CVD).14
In the PEGASUS‐TIMI 54 trial (Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin‐Thrombolysis in Myocardial Infarction 54), patients with a history of MI during the preceding 1 to 3 years before randomization were assigned to ticagrelor or placebo.15 Our primary aim was to determine if baseline plasma TMAO levels were independently associated with major adverse cardiovascular events (MACE) in stable patients with prior MI. Our secondary aim was to determine if plasma TMAO levels can identify patients who have a greater risk reduction in MACE with prolonged use of ticagrelor after an MI.
Methods
We encourage parties interested in collaboration and data sharing to contact the corresponding author directly for further discussions.
Study Population
The PEGASUS‐TIMI 54 trial was a randomized double‐blind, placebo‐controlled trial that tested the cardiovascular benefit of prolonged dual antiplatelet therapy with the addition of ticagrelor (a P2Y12 receptor antagonist) to low‐dose aspirin among stable patients with a history of MI.16 The trial tested two doses of ticagrelor, 90 mg twice daily, a well‐established dosage regimen in patients with acute coronary syndromes, and 60 mg twice daily versus placebo in a randomly assigned ratio 1:1:1.16 All patients were on a background therapy of aspirin 75 to 150 mg daily. The protocol, including the biomarker substudy, was approved by the relevant ethics committee at each participating site. Eligible patients were defined by a history of spontaneous MI 1 to 3 years before the enrollment, were at least 50 years of age, and had one of the following additional high‐risk features: age of 65 years or older, diabetes mellitus requiring medication, a second prior spontaneous MI, multivessel coronary artery disease, or chronic renal disease, defined as an estimated glomerular filtration rate (eGFR) <60 mL per minute per 1.73 m2 body surface area.15 Briefly, patients were ineligible if they required the use of P2Y12 receptor antagonist or anticoagulant therapy during the study period; had a history of bleeding disorder, ischemic stroke, or intracranial bleeding; or had gastrointestinal bleeding within the previous 6 months or a major surgery within the previous 30 days.15 The study protocol was approved by local institutional review committees and written informed consent was obtained from all participants. Among a total of 21 162 patients who underwent randomization from October 2010 through May 2013, a biomarker substudy was conducted in 8635 participants.15
Study Design
We conducted a nested case‐control study evaluating the association between TMAO levels and risk of MACE among participants in the PEGASUS‐TIMI 54 trial who had a collection of blood sample at the baseline regardless of the randomization arm.15 Within this study, eligible cases were men or women who experienced the prespecified primary composite end point of cardiovascular death, MI, or stroke. Eligible controls were men or women who remained free from cardiovascular death, MI, or stroke through the end of follow‐up. Eligible controls were matched for age (range ±3 years), sex, and eGFR (range ±5 mL/min per 1.73 m2) with a prespecified ratio of 2 controls for each case. The definitions for cardiovascular death, MI, and stroke have been previously described.15, 16 We initially defined 603 cases and 1206 matched controls (ratio 2:1), but the final sample with measurable TMAO concentrations yielded 597 cases and 1206 controls (see Table S1 for baseline characteristics in the entire PEGASUS‐TIMI 54 trial versus the TMAO nested case‐control cohort).
Biomarker Analysis
TMAO concentrations in plasma were quantified using stable isotope dilution high‐performance liquid chromatography with online tandem electrospray ionization mass spectrometry (LC/MS/MS) using d9‐(trimethyl)‐labeled internal standard as described previously using a Shimadzu Nexera Ultra High Performance Liquid Chromatograph (UHPLC) system interfaced with Shimadzu 8050 triple quadrupole mass spectrometer.7, 11, 17 Exploratory measurements were also done for plasma choline and betaine as potential precursors of TMAO.4, 8, 18
Statistical Analysis
For the baseline characteristics according to TMAO quartiles, continuous variables are presented as medians with interquartile ranges (IQR) and were compared with Kruskal–Wallis test; categorical variables are presented as absolute counts and percentages and were compared with chi‐square test. We compared values of TMAO between cases and controls with P values calculated using Wilcoxon Rank Sum tests. We evaluated the correlation between TMAO concentrations and the following continuous variables: age, body mass index (BMI), eGFR, hs‐CRP (high‐sensitivity C‐reactive protein), hs‐TnT (high‐sensitivity troponin T), and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide hormone). For our primary aim, we performed conditional logistic regression to explore the association between the exposure (TMAO quartiles) and the binary outcome in 597 cases and 1194 controls matching for age, sex, and eGFR regardless of the randomization arm. We categorized TMAO in quartiles and compared the odds ratio (OR) for the highest quartile versus the lowest quartile and assessed the P value for trend across categories.11 The trend P value was calculated by testing the coefficient of an ordinal TMAO variable from the logistic regression model. We also used TMAO concentrations as a continuous variable (standardized by subtracting the mean and dividing by the standard deviation) and presented the estimates per increase of one standard deviation (1 SD). To evaluate potential confounding factors, we examined the differences of baseline characteristics across TMAO quartiles. We adjusted our conditional logistic regression model (primary outcome versus not) for baseline CVD clinical risk factors (age, sex, eGFR, diabetes mellitus, BMI, hypertension, hypercholesterolemia, smoking status, peripheral artery disease), region of enrollment, severity of the qualifying index events (type of MI), aspirin dosage, and treatment arm and made further adjustment for the baseline traditional prognostic cardiovascular biomarkers, hs‐CRP, hs‐TnT, and NT‐proBNP. These biomarkers have been previously shown to predict future MACE in patients with coronary artery disease.13, 19, 20 Because renal function is a major confounding factor,21 we added a subgroup analysis comparing baseline eGFR ≥60 mL/min per 1.73 m2 versus eGFR <60 mL/min per 1.73 m2. We repeated this same process for each individual end point of cardiovascular death, MI, and stroke using an unmatched logistic regression model, including this time the total population of 597 cases and 1206 controls. For our secondary aim, we investigated whether the effect of ticagrelor on the primary end point varied by quartiles of TMAO. The interaction P value associated with this test is computed using a likelihood ratio test that compares a full model with the main effect terms for TMAO quartiles, treatment, and an interaction effect of TMAO by treatment, with a reduce model that had main effect terms for TMAO and treatment only. The same analysis plans were applied for choline and betaine. A P<0.05 was considered significant for all analyses.
Results
Baseline Characteristics
Baseline characteristics of the patients, overall and stratified by the quartiles of plasma TMAO quartiles, are shown in Table 1. Patients with higher TMAO quartiles compared to those with lower TMAO quartiles were more likely to be older, female, and had higher BMI, and more frequently had comorbidities including a history of hypertension, diabetes mellitus, impaired renal function, and peripheral artery disease but were less likely to be current smokers (Table 1). TMAO concentrations were moderately negatively correlated with eGFR (r=−0.41, P<0.001) and weakly positively correlated with hs‐TNT (r=0.29, P<0.001) and NT‐proBNP (r=0.20, P<0.001) (Table S2).
Table 1.
Baseline Characteristics Stratified by TMAO Quartile (N=1803)
| Variable | 1st Quartile N=452 (0.12–3.24 μmol/L) | 2nd Quartile N=450 (3.25–4.75 μmol/L) | 3rd Quartile N=450 (4.76–7.20 μmol/L) | 4th Quartile N=451 (7.22–157.37 μmol/L) | P Value |
|---|---|---|---|---|---|
| Matched variables | |||||
| Age, median (IQR) | 64 (58, 70) | 66 (59, 73) | 69 (62, 75) | 69 (62, 76) | <0.001 |
| Female, n (%) | 95 (21.0) | 100 (22.2) | 115 (25.6) | 152 (33.7) | <0.001 |
| eGFR, median (IQR) | 75.9 (65.8, 88.0) | 71.7 (59.4, 81.8) | 64.4 (53.5, 75.6) | 55.2 (40.6, 70.6) | <0.001 |
| Other variables | |||||
| BMI, median (IQR) | 28.3 (25.5, 31.1) | 28.2 (25.3, 31.2) | 28.4 (25.6, 31.6) | 29.9 (26.6, 33.7) | <0.001 |
| White race, n (%) | 430 (95.1) | 434 (96.4) | 440 (97.8) | 432 (95.8) | 0.185 |
| Region | 0.049 | ||||
| Eastern Europe, n (%) | 160 (35.4) | 151 (33.6) | 139 (30.9) | 134 (29.7) | |
| North America, n (%) | 143 (31.6) | 151 (33.6) | 171 (38.0) | 189 (41.9) | |
| Western Europe, n (%) | 149 (33.0) | 148 (32.9) | 140 (31.1) | 128 (28.4) | |
| Smoking status, n (%) | 0.002 | ||||
| Never | 114 (25.2) | 141 (31.3) | 156 (34.7) | 153 (33.9) | |
| Former | 226 (50.0) | 216 (48.0) | 212 (47.2) | 231 (51.2) | |
| Current | 112 (24.8) | 93 (20.7) | 81 (18.0) | 67 (14.9) | |
| Hypertension, n (%) | 339 (75.0) | 357 (79.3) | 362 (80.4) | 388 (86.0) | 0.001 |
| Dyslipidemia, n (%) | 356 (78.8) | 377 (83.8) | 375 (83.3) | 372 (82.5) | 0.19 |
| Diabetes mellitus, n (%) | 100 (22.1) | 133 (29.6) | 155 (34.4) | 224 (49.7) | <0.001 |
| Multivessel CAD, n (%) | 306 (67.7) | 283 (62.9) | 273 (60.7) | 292 (64.7) | 0.157 |
| >1 prior MI, n (%) | 93 (20.6) | 81 (18.0) | 92 (20.4) | 100 (22.2) | 0.48 |
| History of PAD, n (%) | 33 (7.3) | 42 (9.3) | 38 (8.4) | 57 (12.6) | 0.039 |
| Qualifying event | |||||
| STEMI, n (%) | 229 (50.8) | 220 (48.9) | 225 (50.0) | 172 (38.2) | <0.001 |
| NSTEMI, n (%) | 207 (45.9) | 220 (48.9) | 215 (47.8) | 257 (57.1) | 0.004 |
| Years from event, median (IQR) | 1.7 (1.2, 2.3) | 1.6 (1.2, 2.2) | 1.7 (1.3, 2.3) | 1.6 (1.2, 2.2) | 0.132 |
| Medications | |||||
| Aspirin, n (%) | 450 (99.6) | 450 (100) | 449 (99.8) | 451 (100) | 0.30 |
| Aspirin dosage, median (IQR) | 81 (80, 100) | 81 (80, 100) | 81 (80, 100) | 81 (80, 100) | 0.008 |
| Aspirin ≥100 mg, n (%) | 144 (31.9) | 138 (30.8) | 171 (38.3) | 153 (34.0) | 0.090 |
| Statin, n (%) | 429 (94.9) | 424 (94.2) | 426 (94.7) | 406 (90.0) | 0.008 |
| Beta‐blocker, n (%) | 385 (85.2) | 383 (85.1) | 384 (85.3) | 395 (87.6) | 0.67 |
| ACEI or ARB, n (%) | 363 (80.3) | 359 (79.8) | 358 (79.6) | 344 (76.3) | 0.44 |
| Placebo, n (%) | 159 (35.2) | 163 (36.2) | 152 (33.8) | 140 (31.0) | 0.26 |
| Ticagrelor 60 mg bid, n (%) | 158 (35.0) | 130 (28.9) | 153 (34.0) | 151 (33.5) | |
| Ticagrelor 90 mg bid, n (%) | 135 (29.9) | 157 (34.9) | 145 (32.2) | 160 (35.5) | |
| Biomarker measurement | |||||
| hs‐TnT (ng/L) (median, IQR) | 6.9 (4.3, 11.1) | 8.0 (5.3, 12.8) | 9.3 (5.8, 15.6) | 12.3 (7.3, 22.5) | <0.001 |
| NT‐proBNP (pg/mL) (median, IQR) | 131.3 (69.3, 291.2) | 163.3 (77.0, 352.3) | 181.0 (81.1, 463.0) | 276.9 (116.1, 619.1) | <0.001 |
| hs‐CRP (mg/dL) (median, IQR) | 1.5 (0.7, 3.2) | 1.6 (0.7, 3.5) | 1.5 (0.8, 3.3) | 2.0 (0.9, 4.2) | 0.003 |
Continuous variables are presented as medians with interquartile ranges (IQR) and were compared with Kruskal–Wallis test. Categorical variables are presented as absolute counts and percentages and were compared with chi‐square test. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; hs‐TnT, high‐sensitivity troponin T; IQR, interquartile range; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; NT‐proBNP, N‐terminal‐proB‐type natriuretic peptide; PAD, peripheral artery disease; STEMI, ST‐segment–elevation myocardial infarction; and TMAO, trimethylamine N‐oxide.
The Association of TMAO Quartiles With Major Adverse Cardiovascular Events
The TMAO concentrations were borderline statistically significantly different between MACE cases and controls, with significant differences between cardiovascular death cases and controls, as well as between stroke cases and controls, whereas no significant differences were found for recurrent MI cases and controls (Figure 1). When analyzed as quartiles, as compared with patients in the first quartile (Q1) of TMAO, the risk for the primary end point of cardiovascular death, MI, or stroke was significantly higher in patients in the highest quartile (Q4) of TMAO using conditional logistic regression (OR for Q4 versus Q1 1.43, 95% CI, 1.06–1.93, P for trend across quartiles=0.015, Table 2). The association was primarily driven by cardiovascular death (Q4 OR 2.25, 95% CI, 1.28–3.96, P trend=0.003) and stroke (Q4 OR 2.68, 95% CI, 1.39–5.17, P trend<0.001). The association with MACE was attenuated after adjustment for clinical factors including age, sex, eGFR, hypertension, hypercholesterolemia, diabetes mellitus, peripheral artery disease, qualifying index event, smoking, region, BMI, aspirin dosage, and treatment arm (adjusted OR 1.27, 95% 0.92–1.75, P for trend=0.10) and further adjustment for other biomarkers (hs‐TnT, NT‐proBNP and hs‐CRP) (adjusted OR 1.22, 95% CI, 0.86–1.74, P for trend=0.20). After adjustment for clinical factors, the association remained significant for cardiovascular death (adjusted OR 1.89, 95% CI, 1.03–3.45, P trend=0.027) and stroke (adjusted OR 2.01, 95% CI<1.01–4.01, P trend=0.022, Figure 2) but was slightly attenuated after further adjustment for biomarkers (adjusted OR fourth versus lowest quartiles for cardiovascular death 1.74, 95% CI, 0.88–3.45, P for trend=0.079; adjusted OR for stroke 1.82, 95% CI, 0.88–3.78, P for trend=0.056). We did not find an association between TMAO quartiles and risk of recurrent MI events, even when modeled as a continuous variable (Table S3). We did not find a significant interaction by baseline renal function, although the associations tended to be stronger for those with eGFR ≥60 mL/min per 1.73 m² compared with those who had an eGFR <60 mL/min per 1.73 m² after adjustment for clinical variables (Table S4). The increased risk association with TMAO quartiles was consistent regardless the randomization arm (pooled ticagrelor versus placebo, Table S5). Neither plasma choline nor betaine was significantly associated with MACE after adjustment for clinical factors (Table S6).
Figure 1. TMAO concentrations comparing cases vs controls for MACE, cardiovascular death, recurrent MI, and stroke events.
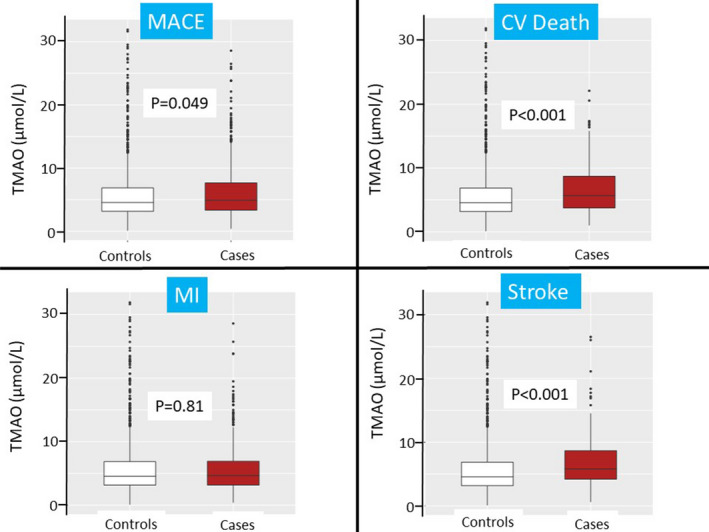
The P values were obtained using Wilcoxon ranking test. Values beyond the 99% percentile are not graphed to avoid an excessive y axis. CV indicates cardiovascular; MACE, major adverse cardiovascular events; MI, myocardial infarction; and TMAO, trimethylamine N‐oxide.
Table 2.
Risk of Cardiovascular Events by TMAO Quartiles
| TMAO Quartiles Definition | 1st Quartile (N=452) (Range 0.12–3.24 μmol/L) | 2nd Quartile (N=450) (Range 3.25–4.75 μmol/L) | 3rd Quartile (N=450) (Range 4.76–7.20 μmol/L) | 4th Quartile (N=451) (Range 7.22–157.37 μmol/L) | P for Trend |
|---|---|---|---|---|---|
| Odds ratios for primary end point (cardiovascular death, MI, and stroke) | |||||
| Number of cases (%) | 138 (30.5) | 141 (31.3) | 152 (33.8) | 166 (36.8) | |
| Matcheda | Ref | 1.03 (0.78–1.37, P=0.81) | 1.19 (0.89–1.59, P=0.24) | 1.43 (1.06–1.93, P=0.02) | 0.015 |
| Odds ratios for cardiovascular death | |||||
| Number of cases (%) | 23 (5.1) | 30 (6.7) | 38 (8.4) | 48 (10.6) | |
| Adjustment for matching variableb | Ref | 1.32 (0.75–2.34, P=0.34) | 1.68 (0.97–2.93, P=0.067) | 2.25 (1.28–3.96, P=0.005) | 0.003 |
| Odds ratios for recurrent MI | |||||
| Number of cases (%) | 108 (23.9) | 101 (22.4) | 101 (22.4) | 102 (22.6) | |
| Adjustment for matching variableb | Ref | 0.94 (0.68–1.30, P=0.70) | 1.09 (0.79–1.51, P=0.60) | 1.10 (0.79–1.55, P=0.57) | 0.42 |
| Odds ratios for stroke | |||||
| Number of cases (%) | 15 (3.3) | 19 (4.2) | 31 (6.9) | 41 (9.1) | |
| Adjustment for matching variableb | Ref | 1.25 (0.62–2.52, P=0.52) | 2.05 (1.07–3.93, P=0.031) | 2.68 (1.39–5.17, P=0.003) | <0.001 |
The trend P value was calculated by testing the coefficient of an ordinal TMAO variable from the logistic regression model. eGFR indicates estimated glomerular filtration rate; MI, myocardial infarction; and TMAO, trimethylamine N‐oxide.
Conditional (matched) logistic regression was used for the primary end point with matching variables (age, sex, eGFR).
Unconditional (unmatched) logistic regression was used for each individual component cardiovascular death, MI, and stroke events adjusting for matching variables (age, sex, eGFR).
Figure 2. Risks of cardiovascular death, myocardial infarction, and stroke by TMAO quartiles adjusted for clinical variables (black) and biomarkers (red).
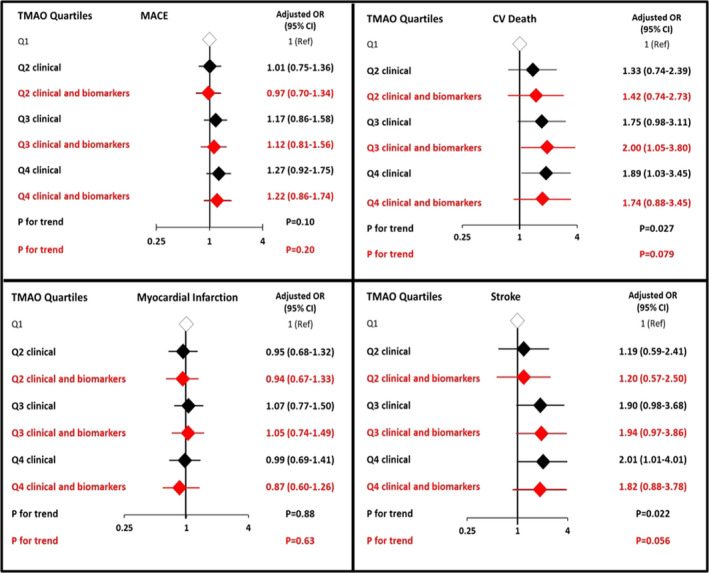
MACE was defined as a composite of cardiovascular death, myocardial infarction, and stroke. In the clinical variable model (black), the ORs were adjusted for age, sex, estimated glomerular filtration rate, hypertension, hypercholesterolemia, diabetes mellitus, peripheral artery disease, qualifying index event, smoking, region, BMI, aspirin dosage, and treatment arm. In the clinical and biomarkers mode (red), further adjustment was done for hs‐CRP (high‐sensitive C‐reactive protein), hs‐TnT (high‐sensitivity troponin T); and NT‐proBNP (N‐terminal‐pro hormone BNP). The trend P value was calculated by testing the coefficient of an ordinal TMAO variable from the logistic regression model. MACE indicates major adverse cardiovascular events; OR, odds ratio; and TMAO, trimethylamine N‐oxide.
Ticagrelor Efficacy in the Relation to TMAO Quartiles
The efficacy signal of ticagrelor was similar across plasma TMAO quartiles (Q1 to Q4) (adjusted P interaction=0.92, Figure 3), and the effect of ticagrelor was generally consistent for each individual end point (cardiovascular death, MI, or stroke) regardless of baseline TMAO quartiles (Figure S1).
Figure 3. Treatment effect of ticagrelor vs placebo on the primary end point of cardiovascular death, MI, and stroke by TMAO quartiles.
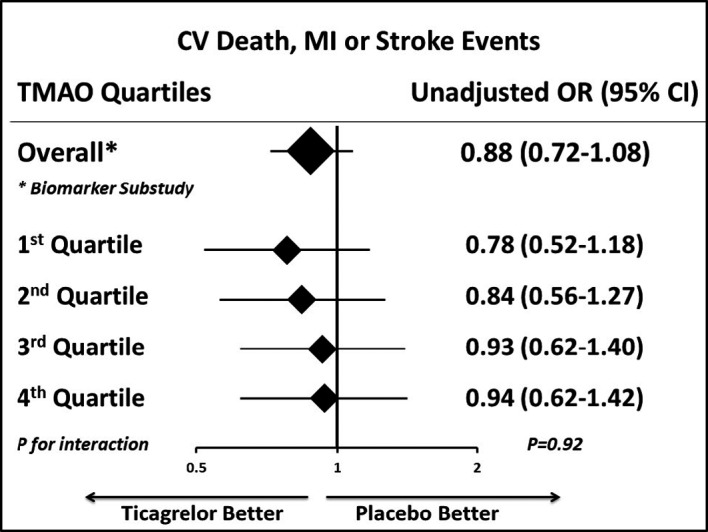
CV indicates cardiovascular disease; MI, myocardial infarction; OR, odds ratio; and TMAO, trimethylamine N‐oxide.
Discussion
In this analysis from the PEGASUS‐TIMI 54 trial, patients with prior MI who had higher plasma TMAO were more likely to experience subsequent cardiovascular death or stroke. The associations between TMAO and cardiovascular death or stroke remained significant after adjustment for clinical factors but were slightly attenuated with further adjustment for established biomarkers, with the ORs remaining large but the lower bound of the 95% CIs crossing 1. The associations tended to be stronger for patients with normal renal function compared with those who had an eGFR <60 mL/min per 1.73 m2. Nonsignificant association was noted for TMAO quartiles and recurrent MI events. The efficacy of ticagrelor on the primary efficacy end point was consistent regardless of baseline TMAO quartile. We did not observe any statistically significant association between plasma choline or betaine and risks of MACE.18
Few studies reported an association between TMAO levels and the mechanisms of stroke events. Among 1244 hypertensive patients in China, a nested‐case control study suggested an association between higher TMAO levels and first stroke event, especially with respect to hemorrhagic stroke.22 In another cohort study of 268 patients who underwent carotid stenting, increased TMAO levels were associated with an increased risk of new ischemic brain lesion on cerebral imaging after stenting.23 To explore the potential etiology of stroke, two large observational studies, together including more than 6900 elderly participants, showed an increased risk of incident atrial fibrillation among those with higher TMAO levels even after adjustment for traditional confounding clinical factors.24 The available findings rather support a possible risk modulation of stroke with TMAO levels, but the evidence is low and more studies are needed to clarify the mechanisms.
As expected, patients with higher TMAO concentrations were more likely to have renal dysfunction. There is existing evidence that TMAO may be involved in the process of chronic kidney disease and that renal dysfunction may lead to higher circulating plasma concentrations of TMAO.6 In subgroup analyses by baseline eGFR function, we found that the association of TMAO tended to be greater in those with normal renal function.
The prothrombotic properties described with TMAO in previous studies and the possible response to aspirin led us to investigate specifically this microbiota marker in the PEGASUS‐TIMI 54 trial.10, 25 Our data show that the clinical efficacy of ticagrelor is preserved regardless of TMAO levels. Reconciling previous and current findings, TMAO levels offer insight for risk assessment but, at least among patients with prior MI, do not offer additional information for tailoring more intensive antiplatelet therapy. The absence of an association with MI in our analysis does not support the role of TMAO as a pure specific marker of atherothrombotic risks. Some of the prior associations between TMAO and MI might have reflected TMAO's association with multiple cardiovascular comorbidities including age, hypertension, dyslipidemia, diabetes mellitus, and renal dysfunction.
Limitations
Our study findings are applicable just to a secondary prevention population with previous MI and treated with aspirin. However, a previous cohort of patients undergoing elective coronary angiography reported a value of 6.2 μmol/L corresponding approximately to the 75th percentile,1 which is not dissimilar to our 75th percentile (7.22 μmol/L). Finally, we did not collect data on nutritional habits or use of antibiotics prior to blood sampling (as a potential unmeasured confounding factor associated with TMAO levels).
Conclusions
Among patients with prior MI, higher systemic TMAO levels were associated with higher risk of cardiovascular death and stroke after adjusting for clinical factors but not with recurrent MI. The efficacy of ticagrelor on cardiovascular outcomes was consistent regardless of baseline TMAO levels.
Sources of Funding
The PEGASUS‐TIMI 54 trial was supported by a grant from AstraZeneca. This work was supported in part by the National Institutes of Health and the Office of Dietary Supplements (P01HL147823, HL103866, and HL126827 to Hazen; R01 HL130819 to Wang) and the Leducq Foundation (to Hazen). Mass spectrometry studies were performed on instruments housed in a facility supported in part by a Center of Excellence Award by Shimadzu Scientific Instruments.
Disclosures
The TIMI Study Group has received significant research grant support from Accumetrics, Amgen, AstraZeneca, Bayer, Beckman Coulter, Bristol‐Myers Squibb, CV Therapeutics, Daiichi Sankyo Co Ltd, Eisai, Eli Lilly and Co, GlaxoSmithKline, Intarcia, Integrated Therapeutics, Janssen Research and Development, Jazz Pharmaceuticals, Medicines Company, MedImmune, Merck and Co, Nanosphere, Novartis Pharmaceuticals, Nuvelo, Ortho‐Clinical Diagnostics, Pfizer, Quark Pharmaceuticals, Roche Diagnostics, Sanofi‐Aventis, Sanofi‐Synthelabo, Siemens Medical Solutions, Singulex and Takeda. Gencer's activities in the TIMI Group, Harvard Medical School, are supported by grants from the Geneva University Hospitals, Eugenio Litta and Arthemis Foundations. Morrow reports research grants: Abbott Laboratories, Amgen, AstraZeneca, Daiichi Sankyo, Eisai, GlaxoSmithKline, Merck, Novartis, Roche Diagnostics, Takeda. Consultant/Advisory Board; Abbott Laboratories, Aralez, Bayer, Merck, Peloton, Roche Diagnostics, and Verseon. Steg reports significant research grants from Merck, Sanofi, and Servier; other personal fees and nonfinancial support from AstraZeneca, Sanofi, Servier; personal fees from Amarin, Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Merck, Janssen, Novartis, Pfizer, GSK, and Regeneron. Dr Deepak L. Bhatt discloses the following relationships—Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS‐II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co‐leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. Storey reports research grants, consultancy fees and honoraria from AstraZeneca; consultancy fees and honoraria from Bayer and Bristol Myers Squibb/Pfizer alliance; consultancy fees and research grants from GlyCardial Diagnotics and Thromboserin; and consultancy fees from Amgen, Haemonetics and Portola. Cohen reports grants and personal fees from AstraZeneca, during the conduct of the study; personal fees from Merck, personal fees from Janssen, personal fees from Maquet, personal fees from malpractice attorneys, grants from Janssen, grants from Edwards, personal fees from Merck, personal fees from BMS/Pfizer, personal fees from Janssen, personal fees from BI, personal fees from Lilly, outside the submitted work. Bonaca reports consulting for AstraZeneca, Merck, Aralez, Bayer. Sabatine reports research grant support through Brigham and Women's Hospital from: Abbott Laboratories; Amgen; AstraZeneca; Bayer; Critical Diagnostics; Daiichi‐Sankyo; Eisai; Genzyme; Gilead; GlaxoSmithKline; Intarcia; Janssen Research and Development; Jazz Pharmaceuticals; Medicines Company; MedImmune; Merck; Novartis; Poxel; Pfizer; Quark Pharmaceuticals; Roche Diagnostics; Takeda. Consulting for: Alnylam; Amgen; Anthos Therapeutics; AstraZeneca; Bristol‐Myers Squibb; Cubist; CVS Caremark; DalCor; Dr. Reddy’s Laboratories; Dyrnamix; Esperion; IFM Therapeutics; Intarcia; Ionis; Janssen Research and Development; Medicines Company; MedImmune; Merck; MyoKardia; Novartis; Zeus Scientific. Dr Johanson is an employee of AstraZeneca. Drs Hazen and Wang are named as co‐inventors on patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics, and have the right to receive royalty payment for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab, Quest Diagnostics, and Procter & Gamble (P&G). Dr Hazen is a paid consultant for P&G; has received research funds from P&G and Roche Diagnostics. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S6 Figure S1
(J Am Heart Assoc. 2020;9:e015331 DOI: 10.1161/JAHA.119.015331.)
For Sources of Funding and Disclosures, see page 8.
See Editorial by Kolluru and Kevil
References
- 1. Tang WHW, Backhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC State‐of‐the‐Art Review. J Am Coll Cardiol. 2019;73:2089–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kasselman LJ, Vernice NA, DeLeon J, Reiss AB. The gut microbiome and elevated cardiovascular risk in obesity and autoimmunity. Atherosclerosis. 2018;271:203–213. [DOI] [PubMed] [Google Scholar]
- 3. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. Intestinal microbiota metabolism of L‐carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota‐generated metabolite trimethylamine‐N‐oxide. Eur Heart J. 2014;35:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li XS, Wang Z, Cajka T, Buffa JA, Nemet I, Hurd AG, Gu X, Skye SM, Roberts AB, Wu Y, et al. Untargeted metabolomics identifies trimethyllysine, a TMAO‐producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight. 2018;3:99096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, et al. Impact of chronic dietary red meat, white meat, or non‐meat protein on trimethylamine N‐oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li XS, Obeid S, Wang Z, Hazen BJ, Li L, Wu Y, Hurd AG, Gu X, Pratt A, Levison BS, et al. Trimethyllysine, a trimethylamine N‐oxide precursor, provides near‐ and long‐term prognostic value in patients presenting with acute coronary syndromes. Eur Heart J. 2019;40:2700–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O, et al. Gut microbiota‐dependent trimethylamine N‐oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bohula EA, Bonaca MP, Braunwald E, Aylward PE, Corbalan R, De Ferrari GM, He P, Lewis BS, Merlini PA, Murphy SA, et al. Atherothrombotic risk stratification and the efficacy and safety of vorapaxar in patients with stable ischemic heart disease and previous myocardial infarction. Circulation. 2016;134:304–313. [DOI] [PubMed] [Google Scholar]
- 13. Qamar A, Giugliano RP, Bohula EA, Park JG, Jarolim P, Murphy SA, Blazing MA, Califf RM, Cannon CP, Braunwald E, et al. Biomarkers and clinical cardiovascular outcomes with ezetimibe in the IMPROVE‐IT Trial. J Am Coll Cardiol. 2019;74:1057–1068. [DOI] [PubMed] [Google Scholar]
- 14. Kaasenbrood L, Boekholdt SM, van der Graaf Y, Ray KK, Peters RJ, Kastelein JJ, Amarenco P, LaRosa JC, Cramer MJ, Westerink J, et al. Distribution of estimated 10‐year risk of recurrent vascular events and residual risk in a secondary prevention population. Circulation. 2016;134:1419–1429. [DOI] [PubMed] [Google Scholar]
- 15. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, et al.; Committee P‐TS and Investigators . Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. [DOI] [PubMed] [Google Scholar]
- 16. Bonaca MP, Bhatt DL, Braunwald E, Cohen M, Steg PG, Storey RF, Held P, Jensen EC, Sabatine MS. Design and rationale for the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin‐Thrombolysis in Myocardial Infarction 54 (PEGASUS‐TIMI 54) trial. Am Heart J. 2014;167:437–444.e5. [DOI] [PubMed] [Google Scholar]
- 17. Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine‐N‐oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meyer KA, Shea JW. Dietary choline and betaine and risk of CVD: a systematic review and meta‐analysis of prospective studies. Nutrients. 2017;9:E711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eisen A, Bonaca MP, Jarolim P, Scirica BM, White HD, Tendera M, Dellborg M, Nicolau JC, Morais J, Fox KA, et al. High‐sensitivity troponin I in stable patients with atherosclerotic disease in the TRA 2 degrees P ‐ TIMI 50 trial. Clin Chem. 2017;63:307–315. [DOI] [PubMed] [Google Scholar]
- 20. Bonaca MP, O'Malley RG, Jarolim P, Scirica BM, Murphy SA, Conrad MJ, Cannon CP, White HD, Braunwald E, Morrow DA, et al. Serial cardiac troponin measured using a high‐sensitivity assay in stable patients with ischemic heart disease. J Am Coll Cardiol. 2016;68:322–323. [DOI] [PubMed] [Google Scholar]
- 21. Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. Gut microbe‐generated metabolite trimethylamine‐N‐oxide as cardiovascular risk biomarker: a systematic review and dose‐response meta‐analysis. Eur Heart J. 2017;38:2948–2956. [DOI] [PubMed] [Google Scholar]
- 22. Nie J, Xie L, Zhao BX, Li Y, Qiu B, Zhu F, Li GF, He M, Wang Y, Wang B, et al. Serum trimethylamine N‐oxide concentration is positively associated with first stroke in hypertensive patients. Stroke. 2018;49:2021–2028. [DOI] [PubMed] [Google Scholar]
- 23. Wu C, Li C, Zhao W, Xie N, Yan F, Lian Y, Zhou L, Xu X, Liang Y, Wang L, et al. Elevated trimethylamine N‐oxide related to ischemic brain lesions after carotid artery stenting. Neurology. 2018;90:e1283–e1290. [DOI] [PubMed] [Google Scholar]
- 24. Svingen GFT, Zuo H, Ueland PM, Seifert R, Loland KH, Pedersen ER, Schuster PM, Karlsson T, Tell GS, Schartum‐Hansen H, et al. Increased plasma trimethylamine‐N‐oxide is associated with incident atrial fibrillation. Int J Cardiol. 2018;267:100–106. [DOI] [PubMed] [Google Scholar]
- 25. Zhu W, Wang Z, Tang WHW, Hazen SL. Gut microbe‐generated trimethylamine N‐oxide from dietary choline is prothrombotic in subjects. Circulation. 2017;135:1671–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6 Figure S1


