Abstract
Background
The balance between ischemic and bleeding events and their association with platelet reactivity in patients receiving antiplatelet therapy after percutaneous coronary intervention (PCI), which differs among regions, is not fully evaluated for East Asians. We examined ischemic/bleeding events and platelet reactivity in Japanese patients undergoing PCI and determined associations between high/low platelet reactivity and clinical outcomes.
Methods and Results
PENDULUM (Platelet Reactivity in Patients with Drug Eluting Stent and Balancing Risk of Bleeding and Ischemic Event) is a prospective, multicenter registry of Japanese patients with PCI. Primary end points were incidence of first major adverse cardiac and cerebrovascular events (MACCE) and first major bleeding events at 12 months post‐PCI. Platelet reactivity (P2Y12 reaction unit [PRU] value) was measured at 12 to 48 hours post‐PCI; patients were grouped as having high PRU (>208), optimal PRU (>85 to ≤208), and low PRU (≤85). MACCE and major bleeding occurred in 4.4% and 2.8% of 6267 patients, respectively. The mean±SD PRU value was 182.1±77.1. MACCE was significantly higher in the high PRU (5.7%; n=2227) versus the optimal PRU group (3.6%; n=3002). The hazard ratio (HR) for high PRU versus optimal PRU level was significantly higher for MACCE (adjusted HR, 1.53; 95% CI, 1.14–2.06 [P=0.004]); stent thrombosis followed the same trend. Incidence of major bleeding did not differ significantly between groups. A high PRU level was significantly associated with MACCE in both patients with and patients without acute coronary syndrome.
Conclusions
These real‐world data suggest an association between high platelet reactivity and cardiovascular events in Japanese patients undergoing PCI. The trend was the same in both patients with and patients without acute coronary syndrome.
REGISTRATION
URL: https://www.umin.ac.jp/ctr. Unique identifier: UMIN 000020332.
Keywords: antiplatelet therapy, bleeding, ischemic, P2Y12, percutaneous coronary intervention
Subject Categories: Acute Coronary Syndromes, Treatment
Nonstandard Abbreviations and Acronyms
- ACS
acute coronary syndrome
- ADAPT‐DES
Assessment of Dual Antiplatelet Therapy With Drug Eluting Stents
- BARC
Bleeding Academic Research Consortium
- DES
drug‐eluting stent
- HPR
high P2Y12 reaction unit
- HR
hazard ratio
- LPR
low P2Y12 reaction unit
- MACCE
major adverse cardiac and cerebrovascular events
- MI
myocardial infarction
- OPR
optimal P2Y12 reaction unit
- PARIS
Patterns of Non‐adherence to Anti‐platelet Regimens in Stented Patients
- PCI
percutaneous coronary intervention
- PENDULUM
Platelet Reactivity in Patients with Drug Eluting Stent and Balancing Risk of Bleeding and Ischemic Event
- PHILO
Phase the International Study of Ticagrelor and Clinical Outcomes in Asian ACS Patients
- PRU
P2Y12 reaction unit
- PLATO
Study of Platelet Inhibition and Patient Outcomes
- SENIOR
Synergy II Everolimus Eluting Stent in Patients Older Than 75 Years Undergoing Coronary Revascularisation Associated With a Short Dual Antiplatelet Therapy
- ST
stent thrombosis
- UMIN
University hospital Medical Information Network
Clinical Perspective
What Is New?
Data regarding the relationship between the clinical events after percutaneous coronary intervention and platelet reactivity have been reported for non–East Asian patients but not for East Asian patients.
To date, this is the largest registry to elucidate the role of high platelet reactivity for patients with acute coronary syndrome and stable coronary artery disease.
What Are the Clinical Implications?
These real‐world data demonstrating the association of high platelet reactivity with ischemic events in Japanese patients undergoing percutaneous coronary intervention will aid in the management of antiplatelet treatment in the East Asian population.
High platelet reactivity after percutaneous coronary intervention (PCI) has been reported to be significantly associated with ischemic complications. In addition, low platelet reactivity has been reported to be associated with bleeding complications. It has been shown that the balance between the incidence of ischemic and bleeding complications after PCI is a critical factor in deciding optimal antiplatelet treatment.1, 2, 3, 4
In recently published consensus documents, the possibility of a differential ischemic/bleeding tradeoff in East Asians and non–East Asians was highlighted.5, 6 However, there are not enough data from East Asian patients regarding ischemic/bleeding events and their association with platelet reactivity after PCI in daily practice. This study aimed to examine the most recent available data of ischemic/bleeding events and platelet reactivity in Japanese patients undergoing PCI, and to elucidate the association between high and low platelet reactivity, compared with optimal platelet reactivity, and clinical outcomes.
Methods
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design, Setting, and Participants
The PENDULUM (Platelet Reactivity in Patients with Drug Eluting Stent and Balancing Risk of Bleeding and Ischemic Event) registry was a prospective, multicenter study of Japanese patients who underwent PCI. The enrollment of patients was conducted in 67 institutions nationwide between December 2015 and June 2017. Patients were aged 20 years and older, indicated for PCI with drug‐eluting stents (DES), and administered antiplatelet drugs. Full inclusion and exclusion criteria are listed in Table S1.
The protocol was approved by the institutional review board or independent ethics committee at each participating center, and the study was performed in accordance with the principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects. Written informed consent was given by all patients before participation. This trial was registered in the University hospital Medical Information Network (UMIN) Clinical Trial Registry (UMIN 000020332).
Procedures
Dual antiplatelet therapy (DAPT) was based on the standard of care. Drug type, dosage, and treatment duration were selected at the discretion of the attending physician. The approved dosages of aspirin, clopidogrel, and prasugrel in Japan are as follows: aspirin, 100 mg is administered once daily and the dosage can be increased up to 300 mg once daily; clopidogrel, 300 mg is administered once as a loading dose on the treatment start day, followed by 75 mg once daily as a maintenance dosage; and prasugrel, 20 mg is administered once as a loading dose, followed by 3.75 mg once daily as a maintenance dosage. In Japan, both clopidogrel and prasugrel have been approved for the treatment of acute coronary syndrome (ACS) and stable angina, and in patients who previously experienced old myocardial infarction (MI) and were planning to undergo PCI. The standard duration of DAPT according to the Japanese treatment guidelines is a minimum of 6 months for patients without ACS and a minimum of 12 months for patients with ACS.7, 8
Data were aggregated for a period up to 12 months after the index PCI procedure. Study outcomes were assessed at 30 days, 12 months, 24 months, and 30 months after the index PCI. Patients underwent follow‐up as part of routine clinical practice. Patients were expected to visit the hospital whenever possible but could be questioned by telephone or letter if visits were difficult. The following data were collected: drug administration status (type [eg, antiplatelet, anticoagulant], dosage, administration period, and interruption period), thrombotic events, hemorrhagic events, and other adverse events. Reasons for treatment interruption or discontinuation were confirmed. Thrombotic and hemorrhagic events were evaluated by independent assessment committees.
End Points
Primary end points were the incidence of first major adverse cardiac and cerebrovascular events (MACCE; all‐cause death, nonfatal MI, nonfatal stroke, and stent thrombosis [ST]) and first major bleeding (Bleeding Academic Research Consortium [BARC]9 type 3 and 5) 12 months after index PCI. Nonfatal MI was defined as a new acute MI or reinfarction after a diagnosis of index PCI following ischemic chest pain and the presence of a myocardial injury marker (myocardial prolapse enzyme: creatine phosphokinase, creatine kinase‐MB, troponin T, or troponin I) or an ECG. Nonfatal stroke was defined as a new neurological sign or symptom with a responsible lesion confirmed by computed tomography or magnetic resonance imaging examination. Stroke was classified into ischemic stroke (cerebral infarction) and nonischemic stroke (eg, cerebral hemorrhage and subarachnoid hemorrhage). Ischemic stroke was defined as a new neurological sign or symptom with a new associated infarct that was confirmed by computed tomography or magnetic resonance imaging examination, regardless of whether neurological signs or symptoms persisted for more than 24 hours. ST was classified as definite, probable, or possible according to Academic Research Consortium definitions. Detailed definitions of efficacy events are listed in Data S1.
The main secondary end points were the incidence of each component of MACCE, cardiovascular death, target vessel revascularization, and bleeding events based on all categories of BARC criteria, and Thrombolysis in Myocardial Infarction criteria.9, 10
Platelet reactivity was measured as P2Y12 reaction unit (PRU) values using the VerifyNow system (Instrumentation Laboratory) and results were reported in PRU. The measurement between 12 and 48 hours after the index PCI was mandatory. Measurements immediately after PCI, 12 months after the index, and the earliest visit after ischemic/bleeding events were optional and collected whenever possible.
The relationship between platelet reactivity and each primary end point was examined. Patients were stratified into 3 groups, high PRU (HPR [high P2Y12 reaction unit]: >208), optimal PRU (OPR [optimal P2Y12 reaction unit]: >85 to ≤208), and low PRU (LPR [low P2Y12 reaction unit]: ≤85) based on the PRU values, according to the recent consensus document for platelet function and genetic testing to guide the use of P2Y12 antagonists.6
Statistical Analysis
The required sample size for the registry was calculated based on both the incidence of MACCE and major bleeding at 12 months after index PCI. From the literature review, we found that the incidences of MACCE and major bleeding were 3%11, 12, 13, 14, 15, 16, 17, 18 and 4%,19, 20 respectively, in the Japanese population. Using this information, we set the incidence of the primary end points at 3%. We applied precision‐based sample size calculation and the precision was set as ±0.5% within the range of the 95% CI. Allowing for a withdrawal rate of 10% during the first 12 months of the study, the required number of patients was calculated as 4969 (rounded up to 5000 patients).
The frequencies of patients experiencing any of the primary outcome events (each first event of MACCE and major bleeding events) for 12 months after the index PCI were calculated. To compare the 2 groups, the chi‐square test or Fisher’s test was used for binary variables, and the Student’s t test was used for continuous variables. Kaplan–Meier curves were used to describe the incidences of events through to 12 months after the index PCI. If a patient had multiple events of the same outcome, the first event was selected as an end point. Patients who discontinued the study and those alive at the end of the observation period were handled as censored data. The 3 levels, HPR, OPR, and LPR, were used in Cox regression models to calculate the hazard ratios (HRs), 95% CIs, and P values for clinical events; OPR was used as the reference level. For adjustment of covariates, the following clinically relevant factors were selected: sex, age, body weight, smoking, ACS/non‐ACS, and a composite of prior MI, prior PCI, and prior coronary artery bypass graft surgery for MACCE, all‐cause death, nonfatal MI, and nonfatal stroke; age, smoking, ACS/non‐ACS, and a composite of prior MI, prior PCI, and prior coronary artery bypass graft surgery for ST; and sex, age, body weight, smoking, ACS/non‐ACS, and a composite of history of cerebral hemorrhage and gastrointestinal hemorrhage for bleeding events. For the primary outcomes, summary statistics for PRU values at 12 to 48 hours after index PCI were calculated for patients with or without the events. Subgroup analyses for ACS and non‐ACS, and P2Y12 inhibitors were performed by analyzing each category separately. Statistical analyses were conducted using SAS release 9.4 (SAS Institute Inc). All tests were 2‐sided with a 5% level of significance.
Results
Between December 2015 and June 2017, 6422 patients were registered from 67 institutions. A total of 6267 patients were included in the full analysis set; 155 patients were excluded. Of the patients in the full analysis set, 6147 (98.1%) patients were evaluated for 1‐year follow‐up analysis (Figure 1).
Figure 1. Patient flow diagram at 1‐year follow‐up.
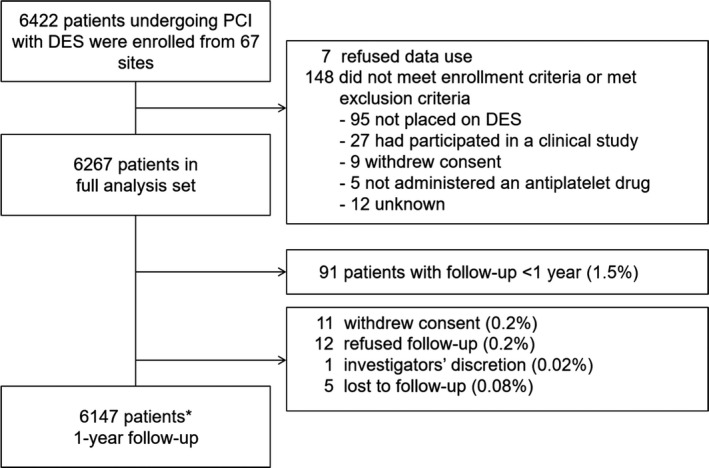
*Includes 156 patients who died. DES indicates drug‐eluting stent, and PCI, percutaneous coronary intervention.
Study Population
The mean age of patients was 70.0 years; 4909 (78.3%) were men and 2015 (32.2%) had ACS. Image‐guided PCI was performed in 5918 patients (94.4%), a transradial approach was used in 4516 patients (72.1%), and a proton pump inhibitor was used at discharge in 5295 patients (84.5%) (Table 1). Antiplatelet therapy at discharge was aspirin in 6143 patients (98.0%), clopidogrel in 2213 patients (35.3%), and prasugrel in 3921 patients (62.6%). Baseline characteristics stratified by PRU level are also shown in Table 1.
Table 1.
Baseline Patient Characteristics
| Total (N=6267) | LPR (n=677) | OPR (n=3002) | HPR (n=2227) | |
|---|---|---|---|---|
| Age, y | 70.0 (10.7) | 68.5 (11.8) | 69.1 (10.7) | 71.8 (10.2) |
| ≥75 | 2324 (37.1%) | 227 (33.5%) | 979 (32.6%) | 989 (44.4%) |
| Men | 4909 (78.3%) | 528 (78.0%) | 2451 (81.6%) | 1650 (74.1%) |
| Body weight, kg | 64.0 (12.6) | 62.4 (12.7) | 65.2 (12.7) | 62.9 (12.3) |
| ≤50 kg | 794 (12.7%) | 111 (16.4%) | 314 (10.5%) | 320 (14.4%) |
| Body mass index, kg/m² | 24.2 (3.6) | 23.5 (3.5) | 24.5 (3.6) | 24.1 (3.6) |
| Hypertension | 5186 (82.8%) | 525 (77.5%) | 2462 (82.0%) | 1905 (85.5%) |
| Hyperlipidemia | 4919 (78.5%) | 503 (74.3%) | 2340 (77.9%) | 1795 (80.6%) |
| Diabetes mellitus | 2767 (44.2%) | 232 (34.3%) | 1306 (43.5%) | 1075 (48.3%) |
| Cigarette smoking, current | 1327 (21.2%) | 159 (23.5%) | 667 (22.2%) | 409 (18.4%) |
| Anemia | 1161 (18.5%) | 84 (12.4%) | 360 (12.0%) | 638 (28.6%) |
| Heart failure | 850 (13.6%) | 79 (11.7%) | 353 (11.8%) | 371 (16.7%) |
| Peripheral arterial disease | 421 (6.7%) | 35 (5.2%) | 152 (5.1%) | 212 (9.5%) |
| Atrial fibrillation | 538 (8.6%) | 55 (8.1%) | 233 (7.8%) | 227 (10.2%) |
| Malignancy | 367 (5.9%) | 39 (5.8%) | 151 (5.0%) | 160 (7.2%) |
| Previous MI | 1575 (25.1%) | 129 (19.1%) | 750 (25.0%) | 619 (27.8%) |
| Previous PCI | 2567 (41.0%) | 177 (26.1%) | 1214 (40.4%) | 1043 (46.8%) |
| Previous CABG | 265 (4.2%) | 33 (4.9%) | 121 (4.0%) | 100 (4.5%) |
| History of ischemic stroke | 655 (10.5%) | 51 (7.5%) | 273 (9.1%) | 302 (13.6%) |
| History of cerebral hemorrhage | 124 (2.0%) | 16 (2.4%) | 48 (1.6%) | 54 (2.4%) |
| History of renal insufficiency | 1103 (17.6%) | 78 (11.5%) | 454 (15.1%) | 500 (22.5%) |
| Clinical presentation | ||||
| Non‐ACS | 4252 (67.8%) | 380 (56.1%) | 2060 (68.6%) | 1607 (72.2%) |
| ACS | 2015 (32.2%) | 297 (43.9%) | 942 (31.4%) | 620 (27.8%) |
| Unstable angina | 790 (12.6%) | 156 (23.0%) | 354 (11.8%) | 225 (10.1%) |
| Non‐STEMI | 323 (5.2%) | 54 (8.0%) | 146 (4.9%) | 98 (4.4%) |
| STEMI | 908 (14.5%) | 90 (13.3%) | 443 (14.8%) | 298 (13.4%) |
| Baseline laboratory parameters | ||||
| Hemoglobin, g/dL | 13.3 (2.0) | 13.9 (2.5) | 13.7 (1.8) | 12.5 (1.9) |
| Creatinine clearance, mL/min | 68.2 (35.5) | 72.9 (42.6) | 72.3 (34.7) | 61.3 (33.0) |
| White blood cell count, ×103/μL | 6.94 (2.82) | 7.20 (4.21) | 6.94 (2.52) | 6.80 (2.59) |
| Angiographic features | ||||
| No. of diseased vessels | ||||
| 1 | 3165 (50.5%) | 345 (51.0%) | 1539 (51.3%) | 1097 (49.3%) |
| 2 | 1865 (29.8%) | 201 (29.7%) | 889 (29.6%) | 666 (29.9%) |
| 3 | 1151 (18.4%) | 122 (18.0%) | 535 (17.8%) | 429 (19.3%) |
| Left main disease | 349 (5.6%) | 36 (5.3%) | 155 (5.2%) | 143 (6.4%) |
| LVEF, % | 56.7 (12.9) | 55.7 (13.7) | 56.6 (12.6) | 57.3 (13.3) |
| Procedural data | ||||
| Puncture site | ||||
| Femoral access | 1632 (26.0%) | 142 (21.0%) | 720 (24.0%) | 659 (29.6%) |
| Brachial access | 270 (4.3%) | 25 (3.7%) | 119 (4.0%) | 109 (4.9%) |
| Radial access | 4516 (72.1%) | 525 (77.5%) | 2233 (74.4%) | 1514 (68.0%) |
| Imaging guided | 5918 (94.4%) | 639 (94.4%) | 2848 (94.9%) | 2095 (94.1%) |
| PCI for chronic total occlusion | 429 (6.8%) | 32 (4.7%) | 221 (7.4%) | 151 (6.8%) |
| Second‐generation DES | 6267 (100%) | 677 (100%) | 3002 (100%) | 2227 (100%) |
| Medication status at discharge | ||||
| Aspirin | 6143 (98.0%) | 664 (98.1%) | 2946 (98.1%) | 2181 (97.9%) |
| P2Y12 inhibitor | 6195 (98.9%) | 673 (99.4%) | 2984 (99.4%) | 2183 (98.0%) |
| PRUa | 182.1 (77.1) | |||
| Clopidogrel | 2213 (35.3%) | 95 (14.0%) | 855 (28.5%) | 1141 (51.2%) |
| PRUa | 212.9 (71.1) | |||
| Prasugrel | 3921 (62.6%) | 578 (85.4%) | 2100 (70.0%) | 1012 (45.4%) |
| PRUa | 163.5 (74.5) | |||
| DOAC | 610 (9.7%) | 59 (8.7%) | 283 (9.4%) | 250 (11.2%) |
| Proton pump inhibitor | 5295 (84.5%) | 574 (84.8%) | 2504 (83.4%) | 1910 (85.8%) |
| NSAIDs except aspirin | 334 (5.3%) | 38 (5.6%) | 147 (4.9%) | 131 (5.9%) |
| Steroids | 250 (4.0%) | 29 (4.3%) | 117 (3.9%) | 91 (4.1%) |
Data are expressed as number of patients (percentage) or mean (SD). ACS indicates acute coronary syndrome; CABG, coronary artery bypass graft surgery; DES, drug‐eluting stent; DOAC, direct oral anticoagulant; HPR, high P2Y12 reaction unit; LPR, low P2Y12 reaction unit; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OPR, optimal P2Y12 reaction unit; and STEMI, ST‐segment–elevation myocardial infarction.
The P2Y12 reaction unit (PRU) of P2Y12 inhibitor (n=5906), clopidogrel (n=2091), and prasugrel (n=3690) was measured at 12 to 48 hours after percutaneous coronary intervention (PCI).
Clinical Outcomes
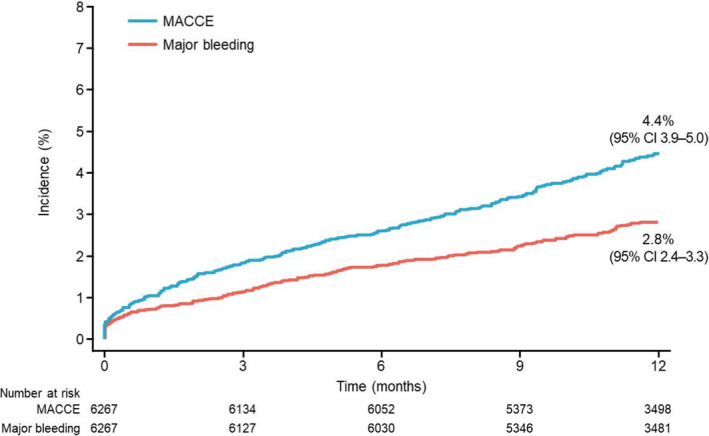
At 1 year, the cumulative incidence of MACCE was 4.4% (95% CI, 3.9–5.0) and that of major bleeding was 2.8% (95% CI, 2.4–3.3) (Figure 2). The cumulative incidence of all‐cause death, nonfatal MI, nonfatal stoke, and ST at 1 year was 2.7% (95% CI, 2.3–3.1), 1.0% (95% CI, 0.8–1.3), 0.9% (95% CI, 0.7–1.1), and 0.3% (95% CI, 0.2–0.5), respectively, and all bleeding was observed in 7.1% (95% CI, 6.5–7.8) of patients (Table S2).
Figure 2. Time‐to‐event curves of major adverse cardiac and cerebrovascular events (MACCE) (all‐cause death, nonfatal myocardial infarction [MI], nonfatal stroke, and stent thrombosis) and major bleeding from baseline to year 1.
Relationship Between Primary Outcomes and PRU
We obtained valid PRU measurements in 5906 (94.2%) patients at a mean time of 21.6±5.5 hours after index PCI (the distribution of PRU is shown in Figure 3 and measurement time is shown in Figure S1). The mean±SD PRU was 182.1±77.1, and the mean±SD PRU with clopidogrel and prasugrel treatment were 212.9±71.1 and 163.5±74.5, respectively. The numbers of patients in the HPR, OPR, and LPR groups were 2227 (37.7%), 3002 (50.8%), and 677 (11.5%), respectively (Table 1). The PRU value at 12 to 48 hours after index PCI was strongly associated with the incidence of MACCE at 1 year (3.0% for LPR, 3.6% for OPR, and 5.7% for HPR), with the incidence of MACCE in the HPR group being significantly higher than that in the OPR group (unadjusted HR, 1.55; 95% CI, 1.19–2.02 [P=0.001]) (Figure 4A and Table 2). In contrast, the PRU value at 12 to 48 hours after index PCI was not associated with the incidence of major bleeding at 1 year (Figure 4B).
Figure 3. Distribution of P2Y12 reaction unit (PRU) value at 12 to 48 hours after index percutaneous coronary intervention.
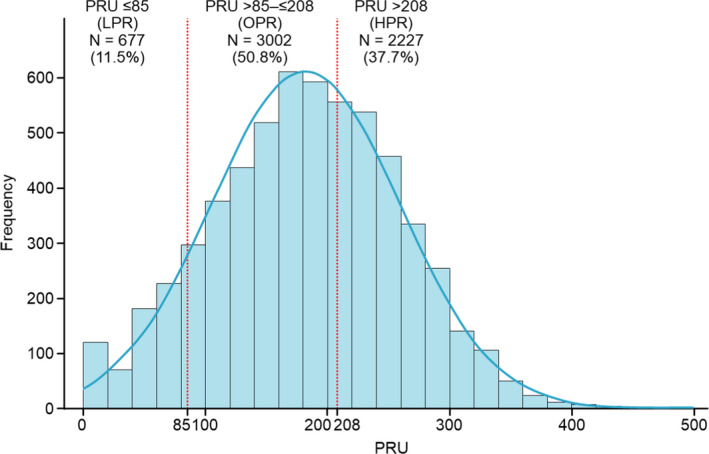
HPR indicates high P2Y12 reaction unit; LPR, low P2Y12 reaction unit; and OPR, optimal P2Y12 reaction unit.
Figure 4. Time‐to‐event curves from baseline to 1 year according to platelet reactivity.
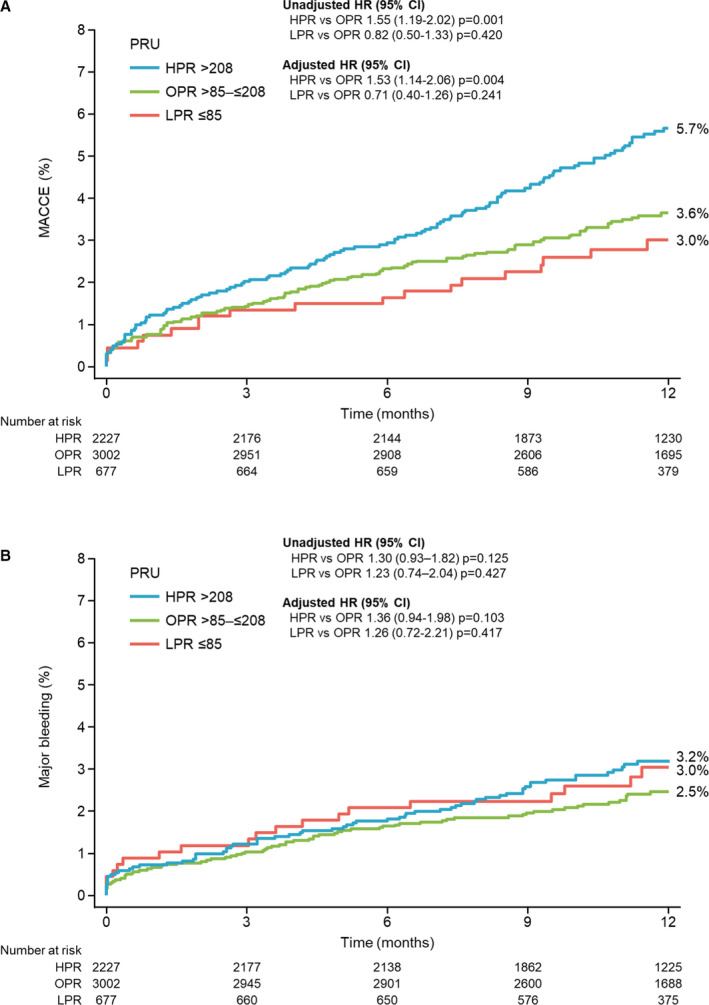
A, Major adverse cardiac and cerebrovascular events (MACCE) (all‐cause death, nonfatal MI, nonfatal stroke, and stent thrombosis); (B) major bleeding. HPR indicates high P2Y12 reaction unit; HR, hazard ratio; LPR, low P2Y12 reaction unit; MI, myocardial infarction; OPR, optimal P2Y12 reaction unit; and PRU, P2Y12 reaction unit.
Table 2.
Risk of PRU for Ischemic Events (PRU=208) and Bleeding Events (PRU=85) Through 1‐Year Follow‐Up
| Event Rates at 1 y | LPR vs OPR | HPR vs OPR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LPR (n=677) | OPR (n=3002) | HPR (n=2227) | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
| MACCEa | 2.8% (19) | 3.4% (103) | 5.3% (117) | 0.82 (0.50–1.33) | 0.420 | 0.71 (0.40–1.26) | 0.241 | 1.55 (1.19–2.02) | 0.001 | 1.53 (1.14–2.06) | 0.004 |
| All‐cause deatha | 1.8% (12) | 2.0% (60) | 3.1% (70) | 0.89 (0.48–1.65) | 0.710 | 0.71 (0.34–1.51) | 0.377 | 1.58 (1.12–2.24) | 0.009 | 1.44 (0.98–2.12) | 0.064 |
| Nonfatal MIa | 0.3% (2) | 0.8% (24) | 1.4% (32) | 0.37 (0.09–1.57) | 0.177 | 0.39 (0.09–1.64) | 0.198 | 1.81 (1.07–3.08) | 0.028 | 1.66 (0.95–2.89) | 0.075 |
| Nonfatal strokea | 0.6% (4) | 0.8% (23) | 0.9% (20) | 0.77 (0.27–2.24) | 0.637 | 0.72 (0.21–2.46) | 0.596 | 1.18 (0.65–2.15) | 0.583 | 1.46 (0.74–2.89) | 0.276 |
| STb | 0.1% (1) | 0.1% (4) | 0.4% (10) | 1.11 (0.12–9.93) | 0.926 | 0.93 (0.10–8.35) | 0.948 | 3.37 (1.06–10.75) | 0.040 | 4.06 (1.27–13.00) | 0.018 |
| Major bleedingc | 2.8% (19) | 2.3% (69) | 3.0% (66) | 1.23 (0.74–2.04) | 0.427 | 1.26 (0.72–2.21) | 0.417 | 1.30 (0.93–1.82) | 0.125 | 1.36 (0.94–1.98) | 0.103 |
| All bleedingc | 7.4% (50) | 6.3% (188) | 7.1% (158) | 1.19 (0.87–1.63) | 0.270 | 1.27 (0.90–1.80) | 0.167 | 1.15 (0.93–1.42) | 0.207 | 1.22 (0.97–1.54) | 0.094 |
HPR indicates high P2Y12 reaction unit; HR, hazard ratio; LPR, low P2Y12 reaction unit; MACCE, major adverse cardiac and cerebrovascular events; OPR, optimal P2Y12 reaction unit; PRU, P2Y12 reaction unit; and ST, stent thrombosis.
Variables entered in this model include sex, age, body weight, smoking, acute coronary syndrome (ACS)/non‐ACS, and composite of prior myocardial infarction (MI), prior percutaneous coronary intervention, and prior coronary artery bypass graft surgery.
Variables entered in this model include age and ACS/non‐ACS.
Variables entered in this model include sex, age, body weight, smoking, ACS/non‐ACS, and composite of history of cerebral hemorrhage and gastrointestinal hemorrhage.
Relationship Between PRU and Each Component of MACCE and All Bleeding
HPR was associated with a significantly higher incidence of each component of MACCE except for nonfatal stroke, and LPR was not associated with all bleeding events (Figures S2A through S2E). However, results from Kaplan–Meier curve of all bleeding showed numerically higher bleeding events immediately after index PCI for the LPR group.
Adjusted HPR and LPR Hazard Risk for Events
The risk of MACCE and ST was significantly higher in the group with HPR than in the OPR group even after adjusted analysis (adjusted HR for MACCE, 1.53; 95% CI, 1.14–2.06 [P=0.004]; adjusted HR for ST, 4.06; 95% CI, 1.27–13.00 [P=0.018]) (Table 2). In contrast, the risks of major bleeding and all bleeding events were not different between LPR and OPR in either unadjusted or adjusted analyses (adjusted HR for major bleeding, 1.26; 95% CI, 0.72–2.21 [P=0.417]; adjusted HR for all bleeding, 1.27; 95% CI, 0.90–1.80 [P=0.167]) (Table 2).
Subgroup Analysis of ACS and Non‐ACS Groups
In the subgroup analysis, the respective 1‐year cumulative incidences of MACCE and major bleeding were 5.5% (95% CI, 4.6–6.6) and 3.0% (95% CI, 2.3–3.9) in the ACS group, and 3.9% (95% CI, 3.4–4.6) and 2.7% (95% CI, 2.3–3.3) in the non‐ACS group (Figure S3A and S3B). The PRU value at 12 to 48 hours after index PCI was strongly associated with the incidence of MACCE at 1 year in both the ACS (Figure 5A) and non‐ACS groups (Figure 5B); the incidence of MACCE in the HPR group was significantly higher than that in the OPR group for patients in both the ACS and non‐ACS groups (unadjusted HR, 1.61; 95% CI, 1.06–2.46 [P=0.027] in ACS; unadjusted HR, 1.55; 95% CI, 1.11–2.18 [P=0.011] in non‐ACS) (Table 3). In contrast, the PRU value at 12 to 48 hours after index PCI was not associated with the incidence of major bleeding at 1 year (Figure 5B, Table 3). Even after adjustment, HPR was an independent risk factor for MACCE in both ACS (adjusted HR, 1.72; 95% CI, 1.05–2.82 [P=0.031]) and non‐ACS (adjusted HR, 1.45; 95% CI, 1.00–2.09 [P=0.048]) groups (Table 3).
Figure 5. Time‐to‐event curves of major adverse cardiac and cerebrovascular events (MACCE) from baseline to 1 year according to platelet reactivity.
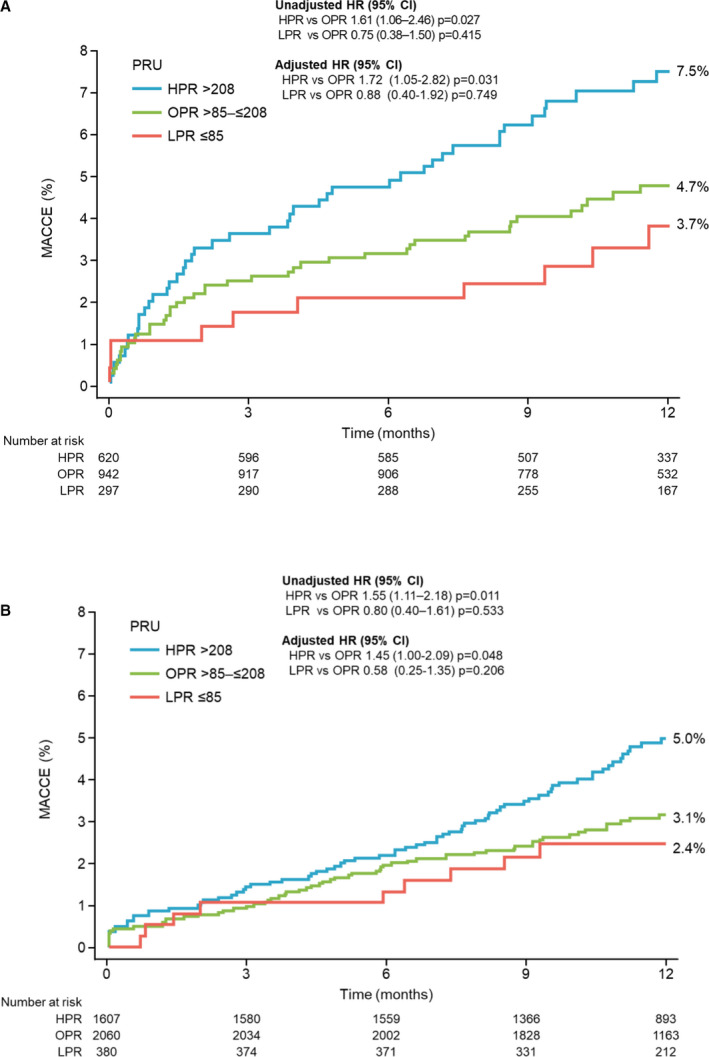
A, Patients with acute coronary syndrome (ACS); (B) patients without ACS. HPR indicates high P2Y12 reaction unit; LPR, low P2Y12 reaction unit; OPR, optimal P2Y12 reaction unit; and PRU, P2Y12 reaction unit.
Table 3.
Risk of PRU for MACCE (PRU=208) and Major Bleeding Events (PRU=85) Through 1‐Year Follow‐Up According to ACS or Non‐ACS
| Event Rates at 1 y | LPR vs OPR | HPR vs OPR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LPR | OPR | HPR | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value | |
| MACCEa | |||||||||||
| ACS | 3.4% (10/297) | 4.5% (42/942) | 7.1% (44/620) | 0.75 (0.38–1.50) | 0.415 | 0.88 (0.40–1.92) | 0.749 | 1.61 (1.06–2.46) | 0.027 | 1.72 (1.05–2.82) | 0.031 |
| Non‐ACS | 2.4% (9/380) | 3.0% (61/2060) | 4.5% (73/1607) | 0.80 (0.40–1.61) | 0.533 | 0.58 (0.25–1.35) | 0.206 | 1.55 (1.11–2.18) | 0.011 | 1.45 (1.00–2.09) | 0.048 |
| Major bleedingb | |||||||||||
| ACS | 2.4% (7/297) | 2.8% (26/942) | 3.2% (20/620) | 0.85 (0.37–1.95) | 0.698 | 0.90 (0.33–2.43) | 0.836 | 1.17 (0.65–2.10) | 0.594 | 1.55 (0.81–2.96) | 0.188 |
| Non‐ACS | 3.2% (12/380) | 2.1% (43/2060) | 2.9% (46/1607) | 1.54 (0.81–2.91) | 0.190 | 1.61 (0.82–3.17) | 0.166 | 1.39 (0.92–2.11) | 0.120 | 1.31 (0.83–2.07) | 0.251 |
ACS indicates acute coronary syndrome; HPR, high P2Y12 reaction unit; HR, hazard ratio; LPR, low P2Y12 reaction unit; MACCE, major adverse cardiac and cerebrovascular events; OPR, optimal P2Y12 reaction unit; and PRU, P2Y12 reaction unit.
Variables entered in this model include sex, age, body weight, smoking, and composite of prior myocardial infarction, prior percutaneous coronary intervention, and prior coronary artery bypass graft surgery.
Variables entered in this model include sex, age, body weight, smoking, and composite of history of cerebral hemorrhage and gastrointestinal hemorrhage.
Subgroup Analysis of P2Y12 Inhibitors
The cumulative incidence of MACCE in patients with HPR was significantly higher than that in patients with OPR, irrespective of the prescribed type of drug before adjustment (clopidogrel: unadjusted HR, 1.61 [P=0.042]; prasugrel: unadjusted HR, 1.50 [P=0.028]). However, after adjustment, the trend remained significant only for the prasugrel subgroup (clopidogrel: adjusted HR, 1.50 [P=0.102]; prasugrel: adjusted HR, 1.54 [P=0.033]) (Figure S4A and S4B).
Discussion
Our prospective study was the largest registry study to date to include PRU measurements of East Asian patients who underwent PCI. We found the following: (1) high platelet reactivity was an independent risk factor for the occurrence of MACCE and ST, one of the components of MACCE; and (2) high platelet reactivity was associated with MACCE in both patients with and patients without ACS; the association was stronger in patients with compared with patients without ACS.
East Asian patients are believed to be more susceptible to bleeding events than patients from Europe or the United States but are relatively resistant to thromboembolic events. However, differences in genetic polymorphisms result in higher incidences of reduced response to P2Y12 inhibitors in East Asian patients,21, 22, 23 leading to the East Asian paradox. A recent randomized controlled study comparing standard doses of ticagrelor and clopidogrel in Korean patients with ACS strongly supported and facilitated this theory of regional differences in ischemic and bleeding risks. Consistent with the PHILO (Phase the International Study of Ticagrelor and Clinical Outcomes in Asian ACS Patients) study, which included Japanese patients in majority,19 and in contrast to the PLATO (Study of Platelet Inhibition and Patient Outcomes) trial conducted mainly in Western countries,20 Korean patients with ACS have a statistically higher incidence of bleeding events and numerically higher incidence of ischemic events under standard‐dose ticagrelor treatment.24 The precise reason for the East Asian paradox has not been completely elucidated, but differences in body mass index, pharmacokinetic and pharmacodynamic profiles of P2Y12 inhibitors, intrinsic thrombogenicity, genetic polymorphisms, hemostatic factor, and the like have been proposed. These findings suggest a higher bleeding risk in East Asian patients and endorse the strategy of optimizing antiplatelet drug dosage to minimize ischemic and bleeding event risks. This might be essential for managing thrombotic and bleeding risks after PCI in East Asian patients. Lower doses of antithrombotic drugs have been recommended in Japan based on a pivotal study conducted in Japan.25, 26 The present study provided an opportunity to elucidate current practice in Japanese patients undergoing PCI from the perspective of platelet reactivity.
In the present study, the 1‐year cumulative incidence of MACCE was 4.4%, which was comparable to the expected rate in the planning stage of this study and that reported in previously published registry studies such as the PARIS (Patterns of Non‐adherence to Anti‐platelet Regimens in Stented Patients)27 and ADAPT‐DES (Assessment of Dual Antiplatelet Therapy With Drug Eluting Stents)28 registries; however, the incidence of ST was low (0.3%) compared with the PARIS (1.1%) and ADAPT‐DES (0.8%) registry studies.
HPR was independently associated with MACCE and ST, even after adjustment. Notably, even though the incidence of ST was 0.3%, patients with HPR had an occurrence rate of ST that was 4 times the rate of that in patients with OPR. The low ST rate probably reflects the routine use of imaging‐guided DES deployment (94.4%). These procedural characteristics may have mitigated the risk of ST after DES deployment in the present study. This explanation is supported by the recent subanalysis of ADAPT‐DES in which Maehara et al29 showed that high platelet reactivity and intravascular ultrasound guidance were both independent predictors of ST. Furthermore, early improvement in clinical events after DES implantation with intravascular ultrasound guidance was increased with longer‐term (2‐year) follow‐up. This finding highlights the role of platelet reactivity in ST even in an era of imaging‐guided DES deployment.
Although it has been proposed that East Asian patients have a high risk of complicated bleeding events, major bleeding events according at BARC 3 and 5 were lower than expected (4.0%), occurring in 2.8% of patients. This observation might be attributable to the high frequency of use of the transradial approach.30 A similar low bleeding rate was observed in the recent SENIOR (Synergy II Everolimus Eluting Stent in Patients Older Than 75 Years Undergoing Coronary Revascularisation Associated With a Short Dual Antiplatelet) trial,31 which used the transradial approach in 80% of cases. The high prevalence of proton pump inhibitor treatment (84.5%) and Helicobacter pylori eradication might contribute to the lower incidence of gastrointestinal bleeding, which is known as a main cause of major bleeding.32
Contrary to the ischemic event findings, bleeding events were not associated with LPR. It has been reported that LPR is a strong independent predictor of bleeding events during antiplatelet therapy.33 Part of the reason for this resides in the findings of the low prevalence of LPR (11.5%), which may not be enough to evaluate the relationship between bleeding events and LPR. Thus, this study might be underpowered to detect any difference in bleeding events. According to a recent report, the incidence of bleeding events after PCI in Japanese patients has decreased34 compared with the known incidence in the period when the present study was planned. However, the numerically higher periprocedural bleeding events in the LPR group strongly suggests the importance of procedure‐related management (Figure S2E). In fact, the periprocedural bleeding events in the LPR group were mainly related to components of the PCI procedure (ie, puncture related and urethral catheterization).
In the present study, 2 types of antiplatelet drugs, clopidogrel and prasugrel, were used. In contrast, clopidogrel was the only antiplatelet drug used in the ADAPT‐DES study.28 It is possible that the relationship between HPR and ischemic events observed in this study could be attributed to the type of drug administered, as the PRU may vary depending on which of the 2 types of drugs were administered (clopidogrel: 212.9±71.1; prasugrel: 163.5±74.5). However, the cumulative incidence of MACCE in patients with HPR was significantly higher than that in patients with OPR, irrespective of the prescribed type of drug before adjustment. However, in the clopidogrel group, the adjusted HR is not statistically significant, but it is reasonable to believe that this was caused by the small sample size in each stratified subgroup. Based on the HRs between the 2 drugs, the clinical significance should be considered as similar regardless of the drug. Taken together, the results of the present study suggest that the association of HPR and high ischemic event rate was also present in East Asian populations. While a PRU of 208 might also be applicable to Japanese patients, additional exploration of the optimal PRU thresholds for the efficacy of antiplatelet drugs is required.
The observation described above was consistent with patients with ACS, in whom HPR was associated with a higher risk of ischemic events, and is likely to be in line with the finding that potent P2Y12 inhibitors improve clinical outcomes in ACS.20, 35 Notably, a similar trend was observed in patients without ACS even after adjustment. To the best of our knowledge, this is the first study to show an association between ischemic events and high platelet reactivity in patients without ACS. Although the clinical usefulness of a point‐of‐care approach using the platelet function test has not been proven beneficial in previously reported randomized controlled trials, which indicates that hypothesis‐generated studies to address this are essential, the present study suggests the importance of avoiding HPR, regardless of ACS status, for preventing ischemic events.
Study Limitations
There are several important limitations in this study. First, given the nature of observational studies, the findings should be interpreted with caution. Selection bias was inevitable because not all patients undergoing PCI at each institution could be enrolled in the study. Reasons for lack of enrollment included enrollment in other randomized controlled trials, difficulty in obtaining informed consent because of a high level of urgency with PCI procedures, and refusal of some patients to give informed consent. Second, all patients were Japanese and the proportion of patients with ACS was relatively low. The generalizability of our findings beyond East Asia is unclear. However, the relationship between HPR and ischemic events seems consistent with the findings of the ADAPT‐DES study. Third, although HPR was an independent risk factor for MACCE and ST, there are known (eg, chronic kidney disease, diabetes mellitus, and anemia) and unknown confounding factors that were not used for adjustment. Fourth, it was mandatory to assess platelet reactivity at one point between 12 to 48 hours after index PCI. The timing of PRU measurements was set based on the ADAPT‐DES study, the only registry that measured the PRU of a sample size equivalent to the present study. The PRU measurement at 12 to 48 hours was presumed to be earlier than the time‐to‐maximum drug efficacy of clopidogrel with its loading dose. Almost all of clopidogrel was already prescribed during the preprocedural period. Furthermore, the clinical implications of HPR were similar, regardless of antiplatelet drugs used. Fifth, the investigators were not blinded from the PRU values and thus were able to access this information. Despite this, there were few cases (<2%) of switching between the 2 P2Y12 inhibitors through to the time of discharge. Finally, PRU was the only measure of platelet reactivity used in this study, although it is the most widely applied and investigated method.34, 36, 37
Conclusions
In real‐world patients undergoing PCI in Japan, HPR was independently associated with MACCE. The same trend was observed in both patients with and patients without ACS.
Sources of Funding
This work was supported by Daiichi Sankyo Co., Ltd.
Disclosures
Nakamura has received research funding from Daiichi Sankyo Co., Ltd. and Sanofi K.K., and personal fees from Daiichi Sankyo Co., Ltd., Sanofi K.K., and Terumo Corporation. Iijima has received personal fees from Daiichi Sankyo Co., Ltd. Kadota received personal fees from Daiichi Sankyo Co., Ltd., during the conduct of the study, and personal fees from Sanofi, outside the submitted work. Kanda has received personal fees from Daiichi Sankyo Co., Ltd. Anzai has received personal fees from Daiichi Sankyo Co., Ltd. Yasaka has received personal fees from Daiichi Sankyo Co., Ltd., outside the submitted work. Yamaguchi has received personal fees from Daiichi Sankyo Co., Ltd., during the conduct of the study; and grants from Abbott Vascular, Boston Scientific, Medtronic, and TERUMO, as well as personal fees from Daiichi Sankyo Co., Ltd., outside the submitted work. Ishii has received personal fees from Daiichi Sankyo Co., Ltd. Takahashi has received personal fees from Daiichi Sankyo Co., Ltd. Shibata has received personal fees from Daiichi Sankyo Co., Ltd. Takamisawa has received personal fees from Daiichi Sankyo Co., Ltd., outside the submitted work. Harada and Motohashi are employees of Daiichi Sankyo Co., Ltd. Uemura has received research funding from Daiichi Sankyo Co., Ltd., and scholarship grants from Astellas Pharma Inc., Abbot Vascular Japan Co., Ltd., Sanofi K.K., Daiichi Sankyo Co., Ltd., Terumo Corporation, Nippon Boehringer Ingelheim Co., Ltd., Goodman Co., Ltd., Bayer Yakuhin Ltd., Shionogi and Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Boston Scientific Japan K.K., Amgen Astellas BioPharma Co., Ltd., Kaken Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Taisho Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharmaceutical Co., Ltd., Japan Lifeline Co., Ltd., MSD K.K., Nipro Corporation, Actelion Pharmaceuticals Japan Ltd., and Pfizer Japan Inc. Murakami has received personal fees from Daiichi Sankyo Co., Ltd., SRD, and Sanofi K.K. Takeda has no disclosures to report.
Supporting information
Appendix S1 Data S1 Tables S1–S2 Figures S1–S4
Acknowledgments
We appreciate the support and collaboration of the coinvestigators participating in the PENDULUM registry. The authors wish to thank Keyra Martinez Dunn, MD, and David Murdoch, BSc (Hons), of Edanz Medical Writing for providing medical writing assistance, which was funded by Daiichi Sankyo Co., Ltd.
Author contributions: Masato Nakamura conceived of the article. Yoshitaka Murakami verified analytical methods. All authors discussed the results and contributed to the final article.
(J Am Heart Assoc. 2020;9:e015439 DOI: 10.1161/JAHA.119.015439.)
For Sources of Funding and Disclosures see page 12.
References
- 1. Costa F, Van Klaveren D, Feres F, James S, Räber L, Pilgrim T, Hong MK, Kim HS, Colombo A, Steg PG, et al Dual antiplatelet therapy duration based on ischemic and bleeding risks after coronary stenting. J Am Coll Cardiol. 2019;73:741–754. [DOI] [PubMed] [Google Scholar]
- 2. Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, Pilgrim T, Hong MK, Kim HS, Colombo A, et al Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE‐DAPT) score: a pooled analysis of individual‐patient datasets from clinical trials. Lancet. 2017;389:1025–1034. [DOI] [PubMed] [Google Scholar]
- 3. Secemsky EA, Yeh RW, Kereiakes DJ, Cutlip DE, Cohen DJ, Steg PG, Cannon CP, Apruzzese PK, D'Agostino RB Sr, Massaro JM, et al Mortality following cardiovascular and bleeding events occurring beyond 1 year after coronary stenting: a secondary analysis of the dual antiplatelet therapy (DAPT) study. JAMA Cardiol. 2017;2:478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, et al Coronary thrombosis and major bleeding after PCI with drug‐eluting stents: risk scores from PARIS. J Am Coll Cardiol. 2016;67:2224–2234. [DOI] [PubMed] [Google Scholar]
- 5. Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, et al Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J. 2019;40:2632–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, Collet JP, Cuisset T, Franchi F, Gross L, et al Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1521–1537. [DOI] [PubMed] [Google Scholar]
- 7. Goto Y. Guidelines for the management of patients with acute myocardial infarction (ST‐elevation type). [In Japanese]. Nihon Rinsho. 2011;69(suppl 9):573–582. [PubMed] [Google Scholar]
- 8. JCS Joint Working Group . Guidelines for elective percutaneous coronary intervention in patients with stable coronary artery disease (JCS 2011) published in 2012–digest version. Circ J. 2013;77:1590–1607. [DOI] [PubMed] [Google Scholar]
- 9. Mehran R, Rao SV, Bhatt DL, Bibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 10. Rao SV, O'Grady K, Pieper KS, Granger CB, Newby LK, Van de Werf F, Mahaffey KW, Califf RM, Harrington RA. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–1206. [DOI] [PubMed] [Google Scholar]
- 11. Nakamura M, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, Takayama M, Kitagawa K, Ikeda Y, Saito S. Optimal cutoff value of P2Y12 reaction units to prevent major adverse cardiovascular events in the acute periprocedural period: post‐hoc analysis of the randomized PRASFIT‐ACS study. Int J Cardiol. 2015;182:541–548. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura M, Muramatsu T, Yokoi H, Okada H, Ochiai M, Suwa S, Hozawa H, Kawai K, Awata M, Mukawa H, et al Outcomes of the largest multi‐center trial stratified by the presence of diabetes mellitus comparing sirolimus‐eluting stents (SES) and paclitaxel‐eluting stents (PES) in patients with coronary artery disease. The Japan drug‐eluting stents evaluation: a randomized trial (J‐DESsERT). Cardiovasc Interv Ther. 2015;30:103–114. [DOI] [PubMed] [Google Scholar]
- 13. Kimura T, Morimoto T, Natsuaki M, Shiomi H, Igarashi K, Kadota K, Tanabe K, Morino Y, Akasaka T, Takatsu Y, et al Comparison of everolimus‐eluting and sirolimus‐eluting coronary stents: 1‐year outcomes from the Randomized Evaluation of Sirolimus‐eluting Versus Everolimus‐eluting stent Trial (RESET). Circulation. 2012;126:1225–1236. [DOI] [PubMed] [Google Scholar]
- 14. Park KW, Lee JM, Kang SH, Ahn HS, Yang HM, Lee HY, Kang HJ, Koo BK, Cho J, Gwon HC, et al Safety and efficacy of second‐generation everolimus‐eluting Xience V stents versus zotarolimus‐eluting resolute stents in real‐world practice: patient‐related and stent‐related outcomes from the multicenter prospective EXCELLENT and RESOLUTE‐Korea registries. J Am Coll Cardiol. 2013;61:536–544. [DOI] [PubMed] [Google Scholar]
- 15. Natsuaki M, Kozuma K, Morimoto T, Kadota K, Muramatsu T, Nakagawa Y, Akasaka T, Igarashi K, Tanabe K, Morino Y, et al Biodegradable polymer biolimus‐eluting stent versus durable polymer everolimus‐eluting stent: a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2013;62:181–190. [DOI] [PubMed] [Google Scholar]
- 16. Steg PG, Bhatt DL, Wilson PW, D'Agostino R Sr, Ohman EM, Röther J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, et al One‐year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. [DOI] [PubMed] [Google Scholar]
- 17. Uchiyama S, Goto S, Matsumoto M, Nagai R, Origasa H, Yamazaki T, Shigematsu H, Shimada K, Yamada N, Bhatt DL, et al Cardiovascular event rates in patients with cerebrovascular disease and atherothrombosis at other vascular locations: results from 1‐year outcomes in the Japanese REACH Registry. J Neurol Sci. 2009;287:45–51. [DOI] [PubMed] [Google Scholar]
- 18. Tada T, Natsuaki M, Morimoto T, Furukawa Y, Nakagawa Y, Byrne RA, Kastrati A, Kadota K, Iwabuchi M, Shizuta S, et al Duration of dual antiplatelet therapy and long‐term clinical outcome after coronary drug‐eluting stent implantation: landmark analyses from the CREDO‐Kyoto PCI/CABG Registry Cohort‐2. Circ Cardiovasc Interv. 2012;5:381–391. [DOI] [PubMed] [Google Scholar]
- 19. Goto S, Huang CH, Park SJ, Emanuelsson H, Kimura T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome—randomized, double‐blind, phase III PHILO study. Circ J. 2015;79:2452–2460. [DOI] [PubMed] [Google Scholar]
- 20. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et al Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 21. Mak KH, Bhatt DL, Shao M, Hankey GJ, Easton JD, Fox KA, Topol EJ. Ethnic variation in adverse cardiovascular outcomes and bleeding complications in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study. Am Heart J. 2009;157:658–665. [DOI] [PubMed] [Google Scholar]
- 22. Kang J, Park KW, Palmerini T, Stone GW, Lee MS, Colombo A, Chieffo A, Feres F, Abizaid A, Bhatt DL, et al Racial differences in ischaemia/bleeding risk trade‐off during anti‐platelet therapy: individual patient level landmark meta‐analysis from seven RCTs. Thromb Haemost. 2019;119:149–162. [DOI] [PubMed] [Google Scholar]
- 23. Kohsaka S, Miyata H, Ueda I, Masoudi FA, Peterson ED, Maekawa Y, Kawamura A, Fukuda K, Roe MT, Rumsfeld JS, et al An international comparison of patients undergoing percutaneous coronary intervention: a collaborative study of the National Cardiovascular Data Registry (NCDR) and Japan Cardiovascular Database‐Keio interhospital Cardiovascular Studies (JCD‐KiCS). Am Heart J. 2015;170:1077–1085. [DOI] [PubMed] [Google Scholar]
- 24. Park DW, Kwon O, Jang JS, Yun SC, Park H, Kang DY, Ahn JM, Lee PH, Lee SW, Park SW, et al Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial. Circulation. 2019;140:1865–1877. [DOI] [PubMed] [Google Scholar]
- 25. Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, Takayama M, Kitagawa K, Nishikawa M, Miyazaki S, et al Efficacy and safety of adjusted‐dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT‐ACS study. Circ J. 2014;78:1684–1692. [DOI] [PubMed] [Google Scholar]
- 26. Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, Takayama M, Kitagawa K, Nishikawa M, Miyazaki S, Ikeda Y, et al Prasugrel, a third‐generation P2Y12 receptor antagonist, in patients with coronary artery disease undergoing elective percutaneous coronary intervention. Circ J. 2014;78:2926–2934. [DOI] [PubMed] [Google Scholar]
- 27. Mehran R, Baber U, Steg PG, Ariti C, Weisz G, Witzenbichler B, Henry TD, Kini AS, Stuckey T, Cohen DJ, et al Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet. 2013;382:1714–1722. [DOI] [PubMed] [Google Scholar]
- 28. Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri E, et al Platelet reactivity and clinical outcomes after coronary artery implantation of drug‐eluting stents (ADAPT‐DES): a prospective multicentre registry study. Lancet. 2013;382:614–623. [DOI] [PubMed] [Google Scholar]
- 29. Maehara A, Mintz GS, Witzenbichler B, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, Henry TD, Cox DA, Duffy PL, et al Relationship between intravascular ultrasound guidance and clinical outcomes after drug‐eluting stents. Circ Cardiovasc Interv. 2018;11:e006243. [DOI] [PubMed] [Google Scholar]
- 30. Fujii T, Ikari Y, Hashimoto H, Kadota K, Amano T, Uemura S, Takashima H, Nakamura M; J‐PCI Investigators . Post‐interventional adverse event risk by vascular access site among patients with acute coronary syndrome in Japan: observational analysis with a national registry J‐PCI database. Cardiovasc Interv Ther. 2019;34:297–304. [DOI] [PubMed] [Google Scholar]
- 31. Varenne O, Cook S, Sideris G, Kedev S, Cuisset T, Carrié D, Hovasse T, Garot P, El Mahmoud R, Spaulding C, et al Drug‐eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single‐blind trial. Lancet. 2018;391:41–50. [DOI] [PubMed] [Google Scholar]
- 32. Nakamura M, Kozuma K, Kitazono T, Iizuka T, Sekine T, Shiosakai K, Usui I, Kogure S. Prasugrel for Japanese patients with ischemic heart disease in long‐term clinical practice (PRASFIT‐Practice II)—a 3‐month interim analysis of a postmarketing observational study. Circ J. 2019;83:637–646. [DOI] [PubMed] [Google Scholar]
- 33. Aradi D, Gross L, Trenk D, Geisler T, Merkely B, Kiss RG, Komócsi A, Dézsi CA, Ruzsa Z, Ungi I, et al Platelet reactivity and clinical outcomes in acute coronary syndrome patients treated with prasugrel and clopidogrel: a pre‐specified exploratory analysis from the TROPICAL‐ACS trial. Eur Heart J. 2019;40:1942–1951. [DOI] [PubMed] [Google Scholar]
- 34. Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, Ohya M, Suwa S, Takagi K, Nanasato M, et al Effect of 1‐month dual antiplatelet therapy followed by clopidogrel vs 12‐month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT‐2 randomized clinical trial. JAMA. 2019;321:2414–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, et al Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 36. Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, Puri S, Robbins M, Garratt KN, Bertrand OF, et al Standard‐ vs high‐dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–1105. [DOI] [PubMed] [Google Scholar]
- 37. Collet JP, Cayla G, Cuisset T, Elhadad S, Rangé G, Vicaut E, Montalescot G. Randomized comparison of platelet function monitoring to adjust antiplatelet therapy versus standard of care: rationale and design of the assessment with a double randomization of (1) a fixed dose versus a monitoring‐guided dose of aspirin and clopidogrel after DES implantation, and (2) treatment interruption versus continuation, 1 year after stenting (ARCTIC) study. Am Heart J. 2011;161:5–12.e5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Data S1 Tables S1–S2 Figures S1–S4


