Abstract
Introduction
When spinal fracture occurred in ankylosing spinal disorder (ASD) patients, it is important to evaluate not only the long lever arm but also bone density and bone quality for the determination of treatment strategies. This case-controlled study examined bone mineral density (BMD), bone metabolism markers, and pentosidine levels in patients with ASD.
Methods
Subjects with bridging of minimum four contiguous vertebral bodies were classified into ASD group and the rest into non-ASD group. The former was further divided into two subgroups based on the presence/absence of sacroiliac joint ankylosis (SJA). We compared BMD, bone metabolism markers, and pentosidine levels in these groups.
Results
The BMD T and Z scores of the femur proximal extremity were lower in the ASD with SJA group than those in the ASD without SJA group. When groups were matched for age, weight, and eGFR, compared with the non-ASD group, the ASD with SJA group had lower BMD of the lumbar spine and femur proximal extremity and the ASD without SJA group had significantly higher BMDs of the lumbar spine and femur proximal extremity. After matching, the ASD without SJA group showed a significantly higher pentosidine level than the non-ASD group.
Conclusions
Patients with SJA have low femur proximal extremity BMD, whereas those with ASD without SJA have a higher BMD of the femur proximal extremity with high pentosidine level. Investigating the presence or absence of SJA is important for the determination of treatment strategies in fractured ASD patients.
Keywords: sacroiliac joint ankylosis, pentosidine, ankylosing spinal disorder, bone mineral density
Introduction
Spinal injuries associated with ankylosing spinal disorders (ASDs) are increasing owing to increasing aging population. In ASD, vertebral linking through interbody bone bridges creates a long, lever arm that increases fracture risk from minor trauma1). ASDs include ankylosing spondylitis (AS) and diffuse idiopathic skeletal hyperostosis (DISH). AS and DISH can be distinguished depending on the presence/absence of sacral joint fusion and histocompatibility leukocyte antigen-B27 (HLA-B27)2). In studies involving young patients with AS, bone mineral density (BMD) values of the lumbar spine and femur proximal extremity are generally low with high osteocalcin levels; low BMD increases the risk of fracture3-9). On the contrary, BMD in patients with DISH has been reported to be either higher than or similar to that in age-matched healthy population10-13).
Based on the above reports, diagnosing AS or DISH, when fractured ASD patients are encountered, affects treatment strategies in terms of BMD or bone quality. But it is difficult to differentiate AS and DISH in the form of vertebral ossification in elderly ASD. Also, fractures with ASD require early surgery, and the results of HLA-B27 cannot be obtained before treatment in many cases. On the other hand, the presence or absence of sacroiliac joint ankylosis (SJA), which is criteria to distinguish AS from DISH, can be confirmed easily by computed tomography (CT) scan before surgery. If there is a difference in BMD or bone quality between the population with or without SJA, even if ASD patients cannot be accurately diagnosed for AS and DISH, we think that it may be useful for planning treatment strategies. Therefore, we examined BMD, bone metabolism markers, and pentosidine levels in patients with ASD.
Materials and Methods
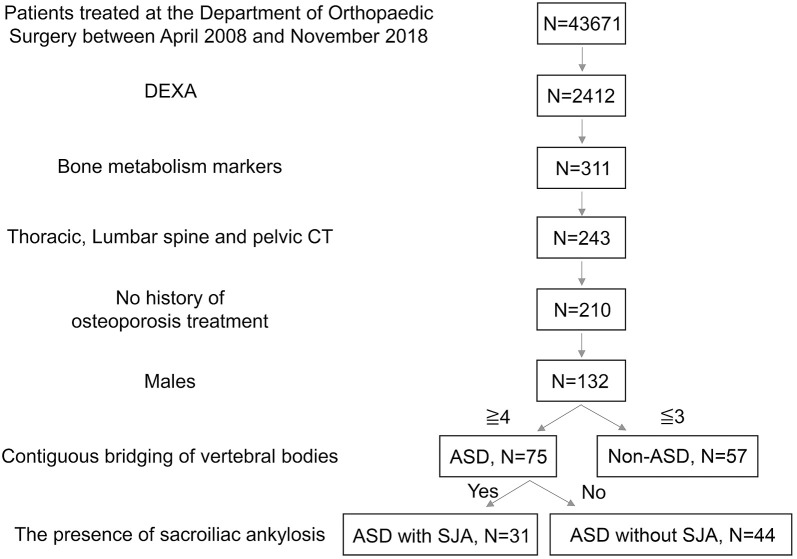
From April 2008 to December 2018, 43,671 patients visited our department, and 2,412 were tested for BMD by dual-energy X-ray absorptiometry (DEXA) (GE Medical Systems LUNAR). Bone metabolism markers were used in 311 patients, and CT scans (Discovery CT 750HD) of the thoracic to lumbar vertebrae and pelvis were acquired for 243 patients. By examining medical records, interviews during examinations, and phone interviews, we identified 210 patients without a history of osteoporosis treatment. From these 210 subjects, only males (n = 132) were included in this study (mean age: 77.6 years, range: 50-98 years) (Fig. 1).
Figure 1.
Inclusion criteria for the ankylosing spinal disorder (ASD) and non-ASD groups among male patients and the definitions of ASD with and without sacroiliac joint ankylosis (SJA).
Abbreviations: DEXA: dual-energy X-ray absorptiometry, CT: computed tomography
These subjects were categorized into two groups: ASD (N = 75) and non-ASD (N = 57). ASD groups comprised patients with the bridging of at least four contiguous vertebral bodies and relative preservation of intervertebral disc height according to the Resnick's diagnostic criteria14). We identified all patients with SJA assessed by CT. Of these patients, those with ankylosis but without narrowing of the sacroiliac joint were classified into the ASD with SJA (ASD with SJA N = 31) group and those without ankylosis but with osteoarthritic change (narrowing of sacroiliac joint) were classified into the ASD without SJA group (N = 44) (Fig. 2).
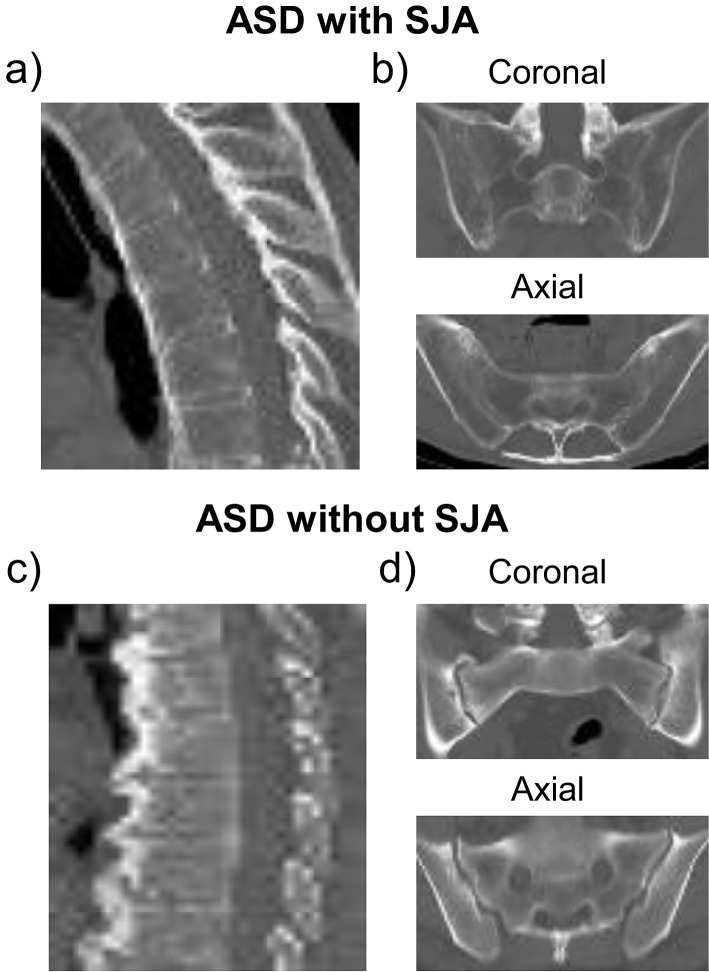
Figure 2.
Example images of ossification patterns of spine and the sacroiliac joint in ankylosing spinal disorder (ASD) with and without ankylosis (SJA).
a) ASD with SJA has smooth and film-shaped ossification pattern of spine.
b) Sacroiliac joint was ankylosed by both coronal and axial CT views in ASD with SJA.
c) ASD without SJA has the ossification pattern with notable protrusions of spine.
d) Sacroiliac joint was not ankylosed by both coronal and axial CT views in ASD without SJA.
We first examined the following items: the ratio of ASD with SJA included in the modified New York criteria, treatment history of patients diagnosed with AS, the ratio of DISH in the ASD without SJA group, history of rheumatism in the ASD without SJA group, and clinical diagnosis of individuals in the non-ASD group. Next, the T and Z scores were compared between the ASD and non-ASD groups or ASD with SJA and ASD without SJA groups. BMD and pentosidine levels have been affected by various factors. Therefore, for matching in this study, we identified the factors correlating with bone density or pentosidine levels. Finally, after matching with the correlation factors, we compared BMD, pentosidine level, and bone metabolic markers between the groups.
This study was approved by the Institutional Review Board of our hospital, and informed consent was obtained from the research subjects by opt-out in the hospital and homepage. All procedures were conducted in accordance with the Declaration of Helsinki.
Statistical analysis
All statistical analyses were performed using SPSS Statistics (Ver 22).
The age, height, weight, HbA1c level, and eGFR were compared among the ASD with SJA, ASD without SJA, and non-ASD groups by ANOVA using Dunnett's multiple comparison test. The ratio of the type of diabetes mellitus was compared using the chi-square test.
The BMD T and Z scores of the lumbar spine (L1-L4) and femur proximal extremity (total of the neck, head, Ward's triangle, and greater trochanter) were compared between the ASD and non-ASD or ASD with SJA and ASD without SJA groups using the Mann-Whitney U test. We then examined correlations among age, height, weight, HbA1c level, estimated glomerular filtration rate (eGFR), the BMD of the femur proximal extremity, and pentosidine level using a function of Pearson's correlation coefficient.
We also compared parameters among groups after propensity score matching with age, weight, and eGFR as independent variables. Before and after matching, BMDs of the lumbar spine and femur proximal extremity; eGFR; and HbA1c, Ca, P, 1α,25-dihydroxyvitamin D3, procollagen type 1 N-terminal propeptide (P1NP), tartrate-resistant acid phosphatase 5b (TRAP5b), N-terminal telopeptide of type I collagen (NTx), and pentosidine levels were compared between paired groups matched according to the indicated parameters (e.g., ASD with SJA vs. ASD without SJA group, ASD with SJA vs. non-ASD group, and ASD without SJA vs. non-ASD group) using the Kruskal-Wallis test. The items, which exhibited a significant difference, as assessed by the Kruskal-Wallis test, were next confirmed by Mann-Whitney U test. A P < 0.05* or P < 0.01** was considered statistically significant.
Results
Background of patients in the ASD with SJA, ASD without SJA, and non-ASD groups
Of the 31 patients classified into the ASD with SJA group, five patients have experienced low back pain and stiffness, which improved with activity for >3 months, 21 patients have a limited range of motion of the lumbar spine during forward and lateral bending. Twenty-one patients were diagnosed with AS. No patient with history of AS treatment was included. Based on the Resnick classification, ASD without SJA corresponds to DISH in all cases. There was no patient with history of rheumatism in the ASD without SJA group. The breakdown of clinical diagnosis of non-ASD patients is as follows: 30 cases of lumbar spinal canal stenosis; nine cases of cervical spondylotic myelopathy; six cases of vertebral fracture; four cases of lumbar disk herniation; four cases of lumbar disk herniation; two cases of cervical disc herniation; and one case of cervical spondylosis and lumbar spondylosis.
The contents of age, height, HbA1c, and the ratio the type of diabetes mellitus did not exhibit significant differences among the ASD with SJA, ASD without SJA, and non-ASD groups (Table 1). The population of the ASD with SJA group is older than that of the ASD without SJA and non-ASD groups (P < 0.05). The weight of individuals in the ASD with SJA group is lower than that of individuals in the ASD without SJA group (P < 0.01). The eGFR in the ASD without SJA group is lower than that in the non-ASD group (P < 0.05).
Table 1.
Comparison of Backgrounds among the ASD with SJA, ASD without SJA, and Non-ASD Groups.
| ASD with SJA | ASD without SJA | non-ASD | |
|---|---|---|---|
| Number | 31 | 44 | 57 |
| Age | 82.9±9.2* | 76.8±8.9 | 75.4±7.9 |
| Height | 162.4±7.4 | 164.5±5.3 | 164.4±6.4 |
| Weight | 57.9±9.5** | 67.5±10.9 | 63.1±10.6 |
| HbA1c | 5.9±0.78 | 6.1±0.75 | 5.9±0.62 |
| Ratio of DM type (%) | 12.9 | 18.6 | 15.8 |
| eGFR | 59.4±27.2 | 53.3±15.9* | 63.6±13.7 |
The population in the ASD with SJA group is older than that in ASD without SJA and non-ASD groups (P<0.05*). The weight of patients in the ASD with SJA group is lower than that of those in the ASD without SJA group (P<0.01**). The eGFR of patients in the ASD with SJA group is lower than that of those in the non-ASD group (P<0.05*).
Data are expressed as mean±standard deviation. P<0.05* and P<0.01** indicate statistical significance vs. non-ASD or ASD without SJA groups.
Differences in BMD T and Z scores of the lumbar spine and femur proximal extremity between ASD and non-ASD or ASD with SJA and ASD without SJA
The BMD T and Z scores were higher for the lumbar spine of the ASD group (n = 75) than those for that of the non-ASD group (n = 57). No significant difference was observed for femur proximal extremity between the groups. The BMD T and Z scores of lumbar and the femur proximal extremity were lower in the ASD with SJA group (n = 31) than those in the ASD without SJA group (n = 44) (Table 2).
Table 2.
Comparison between Bone Mineral Density (BMD) T and Z Scores for Lumbar Vertebrae and Femur Proximal Extremity between the ASD and Non-ASD Groups or the ASD with SJA and ASD without SJA Groups.
| N | Lumbar BMD | BMD of the femur proximal extremity | |
|---|---|---|---|
| T score | |||
| ASD | 75 | 1.58±2.57** | −0.27±1.73 |
| Non-ASD | 57 | 0.15±1.89 | −0.19±1.09 |
| ASD with SJA | 31 | 0.17±2.8** | −1.77±1.1** |
| ASD without SJA | 44 | 2.4±2 | 0.79±1.2 |
| Z score | |||
| ASD | 75 | 3.15±2.4** | 1.13±1.62 |
| Non-ASD | 57 | 1.86±1.88 | 1.09±1.06 |
| ASD with SJA | 31 | 2.17±2.75** | −0.19±1.17** |
| ASD without SJA | 44 | 3.71±2 | 2.08±1.17 |
Data expressed as mean and standard deviation. P<0.01** indicates statistical significance vs. non-ASD or ASD without SJA groups.
Abbreviations: ASD: ankylosing spinal disorders, BMD: bone mineral density, SJA: sacroiliac joint ankylosis
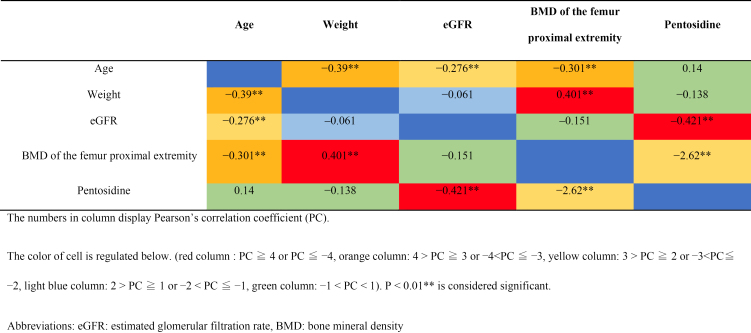
Correlations among age, weight, eGFR, BMD of femur proximal extremity, and pentosidine value
Age correlated with weight, eGFR, the BMD of the femur proximal extremity, weight correlated with the BMD of the femur proximal extremity, and eGFR correlated with pentosidine level (Table 3).
Table 3.
Correlations among Age, Weight, eGFR, BMD of Femur Proximal Extremity, and Pentosidine Value, Displayed by Heat Map Diagram.
Differences in parameters among the ASD with SJA, ASD without SJA, and non-ASD groups before and after matching
Before matching, the age; levels of TRAP5b, NTx, and P1NP; BMDs of the femur proximal extremity; pentosidine level; and weight exhibited significant differences in the ASD with SJA group (n = 31) compared with those in the ASD without SJA group (n = 44). Age; levels of TRAP5b, NTx, and P1NP; BMDs of the lumbar spine, femur proximal extremity, pentosidine level, and weight exhibited significant differences in the ASD with SJA group (n = 31) compared with those in the non-ASD group (n = 57). The BMD of the femur proximal extremity and pentosidine level were significantly higher in the ASD without SJA group (n = 44) than those in the non-ASD group (n = 57) (Table 4).
Table 4.
Comparisons among Groups Matched for Age, Weight, and eGFR.
| Before matching | After matching | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Lumbar BMD |
BMD of femur proximal extremity |
Pentosidine | N | Lumbar BMD |
BMD femur of proximal extremity |
Pentosidine | |
| ASD with SJA vs. | 31 | 1.2±0.36** | 0.72±0.15** | 0.081±0.036 | 20 | 1.25±0.29* | 0.77±0.13** | 0.087±0.4 |
| ASD without SJA | 44 | 1.5±0.32 | 1.1±0.18 | 0.081±0.043 | 20 | 1.49±0.28 | 1.00±0.19 | 0.076±0.22 |
| - | - | |||||||
| ASD with SJA vs. | 31 | 1.2±0.36 | 0.72±0.15** | 0.081±0.036* | 23 | 1.27±0.38 | 0.76±0.15** | 0.083±0.38 |
| non-ASD | 57 | 1.23±0.28 | 0.91±0.14 | 0.062±0.025 | 23 | 1.21±0.18 | 0.91±0.15 | 0.066±0.25 |
| ASD without SJA vs. | 44 | 1.5±0.32** | 1.1±0.18** | 0.081±0.043* | 30 | 1.48±0.35** | 1.04±0.18** | 0.084±0.046* |
| non-ASD | 57 | 1.23±0.28 | 0.91±0.14 | 0.059±0.026 | 30 | 1.24±0.33 | 0.91±0.14 | 0.067±0.03 |
Data are expressed as mean±standard deviation. P<0.05* and P<0.01** are considered significant.
Abbreviations: ASD: ankylosing spinal disorders, BMD: bone mineral density, SJA: sacroiliac joint ankylosis, eGFR: estimated glomerular filtration rate
Then, we made comparisons between the groups matched for age, weight, and eGFR. The BMD of the lumbar spine and femur proximal extremity were significantly lower in the ASD with SJA group (n = 20 cases) than those in the ASD without SJA group (n = 20). The BMD of the femur proximal extremity was significantly lower in the ASD with SJA group (n = 23) than that in the non-ASD group (n = 23). However, the BMD of the lumbar spine, BMD of the femur proximal extremity, and pentosidine level were significantly higher in the ASD without SJA group (n = 30) than those in the non-ASD group (n = 30) (Table 3). P1NP, TRAP5b, and NTx levels, which exhibited a significant difference before matching, no longer exhibited significant differences in all combinations after matching.
Discussion
We speculated that low BMD is associated with osteoporosis and that the long lever arm created by AS contributes to a high vertebral fracture risk in patients with ASD. Therefore, we compared BMD among male patients with bridging over multiple vertebral bodies in the presence/absence of SJA (ASD with/without SJA groups) and non-ASD group. As expected, we found that ASD with or without SJA was involved in BMD and bone quality.
Patients with AS reportedly have low BMDs of the lumbar spine, whereas patients with DISH have high BMDs of the lumbar spine9,10,15-18). The T and Z scores of the lumbar spine were higher in the ASD group than those in the non-ASD group. Conversely, there is a contradiction that the T and Z scores of the femur proximal extremity did not exhibit significant differences in this report. Past reports and the present report could not elucidate how bone bridge contributes to BMD. Westerveld et al. performed DEXA on cadavers in directions that did not include the bone bridge and found no difference in the BMD of the lumbar spine and that of the controls, suggesting that the bone bridge results in the overestimation of BMD. Diederichs et al. reported that the BMD of the lumbar spine was higher in patients with ASD than in those without DISH as measured using DEXA, whereas there was no significant difference in QCT11,12). Since it is clinically difficult to perform spinal DEXA or QCT without including the bone bridge, the measurement of BMD should be made at the femur proximal extremity (Table 5). A possible reason for the absence of a significant difference for the T and Z scores of the femur proximal extremity between the ASD and non-ASD groups is that the ASD group included different pathologies. Therefore, we further divided the ASD group according to the presence/absence of SJA. The BMD T and Z scores of the femur proximal extremity were significantly lower in the ASD with SJA group than those in the ASD without SJA group.
Table 5.
Past Reports on Bone Mineral Density and Bone Metabolism Markers in Ankylosing Spondylitis and Diffuse Idiopathic Skeletal Hyperostosis.
| N | Sex | Mean age | Diagnostic criteria | Methods | BMD of the proximal extremity or neck of femur | Lumbar BMD | Bone marker | Journal | Year | |
|---|---|---|---|---|---|---|---|---|---|---|
| AS | ||||||||||
| Singh HJ | 100 | Combined | 35.2 | Modified New York criteria | Case-control study | DEXA: low | DEXA: low | 1,25(OH)2D3 low | J Clin Densitom | 2013 |
| Bronson WD | 19 | Male | 50.5 | Modified New York criteria | Case-control study | DEXA: low | DEXA: low | No difference | J Rheumatol | 1998 |
| Sivri A | 22 | Combined | 36.8 | New York criteria | Randomized control | DEXA: low | DEXA: low | Not estimated | Clin Rheumatol | 1996 |
| Mullaji AB | 33 | Combined | 37 | Modified New York criteria | Case-control study | Not estimated | DEXA: low | Not estimated | JBJS | 1994 |
| DISH | ||||||||||
| Horie S | 25 | Male | 62.8 | Resnick | Not described | DEXA: high | DEXA: high | P1NP low | SICOT J | 2018 |
| Sohn S | 65 | Combined | 73.2 | Resnick | Case-control study | DEXA: no difference | DEXA: high | Not estimated | J Clin Densitom | 2016 |
| Diederichs G | 129 | Male | 74.2 | Resnick | Randomized control | Not estimated | DEXA high QCT no difference | Not estimated | Osteoporos Int | 2011 |
| Westerveld LA | 10 | Combined | 80.4 | Resnick and Niwayama | Not described | Not estimated | DEXA: no difference | Not estimated | Rheumatology | 2009 |
Abbreviations
AS: ankylosing spondylitis, BMD: bone mineral density, DISH: diffuse idiopathic skeletal hyperostosis, 1,25(OH)2D3: 1alpha,25-dihydroxyvitamin D3, DEXA: dual-energy X-ray absorptiometry
To the best of our knowledge, no previous study has assessed BMD in patients with based on SJA stratification. AS is usually diagnosed based on the modified New York criteria19), and patients in studies are generally in their 30s and 40s. A general finding of these studies is that BMD is low16-18). The mean age of the population included in this study was 77 years, which is substantially higher than that reported in past studies. Because we did not assess HLA-B27 levels in patients with ASD and classified subjects based on the presence/absence of SJA, the study population is not a perfect match. Although the precise definition of AS in elderly is unclear, ASD with SJA resembles AS because 21 out of 31 patients in the ASD with SJA group were applied with the modified New York diagnostic criteria.
Horie et al. reported a high BMD of the femur neck in patients with DISH among a cohort of patients with ossification of the posterior longitudinal ligament (OPLL). In a case-controlled study, Sohn et al. also reported a significantly higher BMD of the femur neck among patients with DISH matched for age, sex, and BMI than the general population10,15). In addition, ASD without SJA completely matched DISH according to Resnick's definition. The BMD of the femur proximal extremity was higher in the ASD without SJA group than that in the ASD with SJA group when matched for age, weight, and eGFR (Table 5).
The ASD with SJA group did carry a high vertebral fracture risk due to bone fragility caused by decreased BMD. Conversely, BMD was actually higher in the ASD without SJA group, indicating that another factor is responsible for elevated bone fracture risk due to fragility in this patient group.
The ASD without SJA group exhibited higher pentosidine level than the non-ASD group, even when the groups were matched for age, weight, and eGFR. Pentosidine is an advanced glycation end product whose level increases in various connective tissues due to decreased kidney function caused by oxidative and glycation stress or diabetes and is considered an index of bone quality deterioration20). To date, no reports have assessed pentosidine level in patients with ASD; however, Yoshimura et al. reported high pentosidine level in patients with OPLL21). OPLL occasionally develops as a complication of DISH, and pentosidine may be high in patients prone to ossification.
Serum pentosidine level increases in the early stage of renal diseases, rheumatoid arthritis, diabetes, and arteriosclerosis and does not necessarily reflect pentosidine levels in bone. However, in the present study, no patient had history of rheumatism in ASD without SJA, and the prevalence of diabetes and HbA1c level are the same among individuals in all groups. In addition, after matching for age, weight, and eGFR, the ASD without SJA group was found to have a higher pentosidine level than that in the non-ASD group. Yamamoto et al. reported that serum pentosidine level was associated with vertebral fractures in postmenopausal women with type 2 diabetes, suggesting that vertebral fractures are caused by deterioration in bone quality in the ASD without SJA group22).
Young patients with AS have low levels of active vitamin D3 and sclerostin (secreted from bone tissues and inhibits Wnt, a signaling factor necessary for bone formation by osteoblasts) and high dickkopf-1 levels23-27). Senolt et al. reported that the critical bone formation factor, DKK-1, is low in the serum of patients with DISH. Alternatively, Daniel et al. found no difference in the serum DKK-1 levels between patients with DISH and healthy controls28,29). In this study, P1NP, TRAP, and NTx, which showed a significant difference in the comparison before matching, did not show a significant difference after matching, and the mechanism for the difference in bone density between each group has not been elucidated yet.
Conclusions
To conclude, for analyzing BMD and pentosidine level, elderly patients with ASD were divided into two groups based on the presence or absence of SJA. Patients with ASD with SJA had low BMD of the femur proximal extremity; therefore, bone fragility due to low BMD and a long lever arm are possible risk factors for vertebral fracture. Patients with ASD without SJA have a high BMD of the femur proximal extremity with high pentosidine level, indicating possible bone quality deterioration. It is useful for the determination of treatment strategies in fractured ASD patients to investigate the presence or absence of SJA.
Limitations
The present study classified patients with ASD on the basis of the presence/absence of SJA. Compared with the sacroiliac joint of young people, age-related changes in the elderly make it difficult to ASD between the ASD without SJA and ASD with SJA groups based on X-ray images. Therefore, we used CT for assessment of all cases to diagnose as accurately as possible. The number of patients in each group was small, which may account for the lack of significant differences in bone metabolism markers.
Conflicts of Interest: We have no financial or personal relationships to disclose with other individuals or organizations that could inappropriately influence or bias this work.
Ethical Approval: This study was approved by the institutional review board.
Informed Consent: Informed consent was obtained from the research patients by posting a paper with an announcement on a bulletin board at the orthopedic outpatient clinic of the hospital.
References
- 1.Westerveld LA, Verlaan JJ, Oner FC. Spinal fractures in patients with ankylosing spinal disorders: a systematic review of the literature on treatment, neurological status and complications. Eur Spine J. 2009;18(2):145-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun J, Bollow M, Remlinger G, et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum. 1998;41(1):58-67. [DOI] [PubMed] [Google Scholar]
- 3.Julkunen H, Heinonen OP, Knekt P, et al. The epidemiology of hyperostosis of the spine together with its symptoms and related mortality in a general population. Scand J Rheumatol. 1975;4(1):23-7. [PubMed] [Google Scholar]
- 4.Klingberg E, Geijer M, Gothlin J, et al. Vertebral fractures in ankylosing spondylitis are associated with lower bone mineral density in both central and peripheral skeleton. J Rheumatol. 2012;39(10):1987-95. [DOI] [PubMed] [Google Scholar]
- 5.Karberg K, Zochling J, Sieper J, et al. Bone loss is detected more frequently in patients with ankylosing spondylitis with syndesmophytes. J Rheumatol. 2005;32(7):1290-8. [PubMed] [Google Scholar]
- 6.Gilgil E, Kacar C, Tuncer T, et al. The association of syndesmophytes with vertebral bone mineral density in patients with ankylosing spondylitis. J Rheumatol. 2005;32(2):292-4. [PubMed] [Google Scholar]
- 7.Przepiera-Bedzak H. [The value of researches, which assess bone mineral density and bone metabolism in patients with ankylosing spondylitis, in detecting osteoporosis]. Ann Acad Med Stetin. 2007;53:39-47. [PubMed] [Google Scholar]
- 8.Ulu MA, Batmaz I, Dilek B, et al. Prevalence of osteoporosis and vertebral fractures and related factors in patients with ankylosing spondylitis. Chin Med J (Engl). 2014;127(15):2740-7. [PubMed] [Google Scholar]
- 9.Singh HJ, Nimarpreet K, Ashima, et al. Study of bone mineral density in patients with ankylosing spondylitis. J Clin Diagn Res. 2013;7(12):2832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sohn S, Chung CK, Han I, et al. Increased bone mineral density in cervical or thoracic Diffuse Idiopathic Skeletal Hyperostosis (DISH): A case-control study. J Clin Densitom. 2018;21(1):68-74. [DOI] [PubMed] [Google Scholar]
- 11.Westerveld LA, Verlaan JJ, Lam MG, et al. The influence of diffuse idiopathic skeletal hyperostosis on bone mineral density measurements of the spine. Rheumatology (Oxford). 2009;48(9):1133-6. [DOI] [PubMed] [Google Scholar]
- 12.Diederichs G, Engelken F, Marshall LM, et al. Diffuse idiopathic skeletal hyperostosis (DISH): relation to vertebral fractures and bone density. Osteoporos Int. 2011;22(6):1789-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuperus JS, Samsour L, Buckens CF, et al. Bone mineral density changes over time in diffuse idiopathic skeletal hyperostosis of the thoracic spine. Bone. 2018;112:90-6. [DOI] [PubMed] [Google Scholar]
- 14.Resnick D, Shaul SR, Robins JM. Diffuse idiopathic skeletal hyperostosis (DISH): Forestier's disease with extraspinal manifestations. Radiology. 1975;115(3):513-24. [DOI] [PubMed] [Google Scholar]
- 15.Horie S, Sawaji Y, Endo K, et al. Factors associated with bone metabolism in patients with cervical ossification of the posterior longitudinal ligament accompanied with diffuse idiopathic skeletal hyperostosis. SICOT J. 2018;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bronson WD, Walker SE, Hillman LS, et al. Bone mineral density and biochemical markers of bone metabolism in ankylosing spondylitis. J Rheumatol. 1998;25(5):929-35. [PubMed] [Google Scholar]
- 17.Sivri A, Kilinc S, Gokce-Kutsal Y, et al. Bone mineral density in ankylosing spondylitis. Clin Rheumatol. 1996;15(1):51-4. [DOI] [PubMed] [Google Scholar]
- 18.Mullaji AB, Upadhyay SS, Ho EK. Bone mineral density in ankylosing spondylitis. DEXA comparison of control subjects with mild and advanced cases. J Bone Joint Surg Br. 1994;76(4):660-5. [PubMed] [Google Scholar]
- 19.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361-8. [DOI] [PubMed] [Google Scholar]
- 20.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21(2):195-214. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura N, Nagata K, Muraki S, et al. Prevalence and progression of radiographic ossification of the posterior longitudinal ligament and associated factors in the Japanese population: a 3-year follow-up of the ROAD study. Osteoporos Int. 2014;25(3):1089-98. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Yamaguchi T, Yamauchi M, et al. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(3):1013-9. [DOI] [PubMed] [Google Scholar]
- 23.Zhao S, Duffield SJ, Moots RJ, et al. Systematic review of association between vitamin D levels and susceptibility and disease activity of ankylosing spondylitis. Rheumatology (Oxford). 2014;53(9):1595-603. [DOI] [PubMed] [Google Scholar]
- 24.Weivoda MM, Oursler MJ. Developments in sclerostin biology: regulation of gene expression, mechanisms of action, and physiological functions. Curr Osteoporos Rep. 2014;12(1):107-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossini M, Viapiana O, Idolazzi L, et al. Higher level of Dickkopf-1 is associated with low bone mineral density and higher prevalence of vertebral fractures in patients with ankylosing spondylitis. Calcif Tissue Int. 2016;98(5):438-45. [DOI] [PubMed] [Google Scholar]
- 26.Winkler DG, Sutherland MS, Ojala E, et al. Sclerostin inhibition of Wnt-3a-induced C3H10T1/2 cell differentiation is indirect and mediated by bone morphogenetic proteins. J Biol Chem. 2005;280(4):2498-502. [DOI] [PubMed] [Google Scholar]
- 27.Pelletier S, Dubourg L, Carlier MC, et al. The relation between renal function and serum sclerostin in adult patients with CKD. Clin J Am Soc Nephrol. 2013;8(5):819-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senolt L, Hulejova H, Krystufkova O, et al. Low circulating Dickkopf-1 and its link with severity of spinal involvement in diffuse idiopathic skeletal hyperostosis. Ann Rheum Dis. 2012;71(1):71-4. [DOI] [PubMed] [Google Scholar]
- 29.Aeberli D, Schett G, Eser P, et al. Serum Dkk-1 levels of DISH patients are not different from healthy controls. Joint Bone Spine. 2011;78(4):422-3. [DOI] [PubMed] [Google Scholar]