Abstract
Introduction
Mirogabalin should be equivalent to pregabalin, but with fewer incidences of adverse drug reactions (ADRs). To verify these benefits in actual clinical trials, our study investigated the frequency of ADRs and mirogabalin's analgesic effects during treatment of peripheral neuropathic pain.
Methods
This study included 74 patients with lower limb pain. We surveyed patient reports of ADRs during the follow-up period as the primary endpoint and examined the visual analog scale (VAS) reported for lower limb pain as the secondary endpoint (before administration, and two and four weeks after administration).
Results
The occurrence of ADR was 27.0%, like the frequency of ADRs in the clinical trials for other disorders. However, the discontinuation rate of administration was 10.8%, which was significantly lower than the frequency of ADR occurrences. When the analgesic effect was assessed, a significant decrease in the temporal change of VAS for lower limb pain was observed before administration, and two and four weeks after administration.
Conclusions
In this study, the occurrence of ADRs reported by the patients was like the frequency of ADRs reported in the clinical trials for other disorders. When assessing the analgesic effect, the temporal change of VAS for lower limb pain was found to decrease significantly before administration, and two and four weeks after administration.
Keywords: Mirogabalin, neuropathic pain, adverse drug reactions
Introduction
According to the summarized data from 11 studies on 4951 patients with chronic pain, the prevalence of neuropathic pain caused by chronic low back pain is as high as 36.6%1). In other words, orthopedists often encounter neuropathic pain in daily clinical practice.
In recent years, pregabalin has been used to treat neuropathic pain because it has achieved a certain level of cost-effectiveness2). While many aspects of pregabalin's analgesic mechanism remain unclear, it might supporess calcium influx through calcium channels on the cell surface, eventually releasing neurotransmitters, such as glutamate, through α2δ subunit binding as a subsidiary role for voltage-gated calcium channels in the central nervous system3-5). Furthermore, pregabalin's analgesic effect may also be involved with the pathway of descending pain modulatory system6,7).
However, pregabalin is also known for its frequent adverse drug reactions (ADRs). According to the package inserts of pregabalin sold in Japan, dizziness (20% or higher) or somnolence (20% or higher) may occur frequently. Dizziness and somnolence significantly reduce drug compliance, and in some cases, may force a patient to discontinue pregabalin prematurely8).
Mirogabalin, which was launched in Japan last year, has been receiving attention as a drug with the same efficacy as pregabalin. Mirogabalin demonstrates strong and selective binding affinity for the α2δ-1 and α2δ-2 subunits, and it persistently binds with the α2δ-1 subunit, which plays an important role in neuropathic pain9). However, mirogabalin reportedly exhibited faster dissociation from α2δ-2 subunits than pregabalin. This might contribute to less frequent ADRs, because pregabalin's higher binding affinity with α2δ-2 subunits is suspected to be the underlying cause of ADRs9). Therefore, mirogabalin is expected to be a drug equivalent to pregabalin, but with fewer ADRs.
To verify these benefits in actual clinical trials, our study investigated the frequency of ADRs and the analgesic effects of mirogabalin during treatment of peripheral neuropathic pain.
Materials and Methods
This was a multicenter, retrospective, observational study conducted with the approval of the Ethics Committee at our institution. The opt-out method was adopted to obtain informed consent from the subjects.
This study used data obtained from the records of patients with lower limb pain, who were diagnosed with peripheral neuropathic pain and treated with mirogabalin during the study period from March 2019 to February 2020. These patients visited seven healthcare facilities located in Japan.
This study included 74 patients with lower limb pain. The diagnoses of peripheral neuropathic pain in all cases were confirmed according to clinical practice guidelines for neuropathic pain10). The Japanese Orthopedic Association specialists judge neuropathic pain according to two objective findings: (1) Sensory deficits observed in a region corresponding to the anatomical innervation of the impaired nerve, and (2) Magnetic resonance imaging explaining neuropathic pain, which was also considered in this study10). The study included patients who were treated as follows: 1) The initial dose was 10 mg/day, following the directions in the attached package insert, 2) No other painkillers were added after the initial dose (except the continuous dose before mirogabalin), 3) The dose was gradually increased to 20 mg in two weeks with the ascending dose regimen, 4) Patient-reported ADRs were checked two weeks/four weeks after administration, and 5) Pain assessment using the visual analog scale (VAS) was conducted before administration, and two and four weeks after administration.
For the endpoint, we surveyed the occurrence of ADRs during the follow-up period as primary endpoint and examined VAS for lower limb pain as secondary endpoint. In other words, we assessed the temporal changes of VAS and the improvement rate of VAS for lower limb pain before administration, and two and four weeks after administration ((VAS before administration-VAS 4 weeks after administration) / (VAS before administration) × 100 (%)).
Results
The patient backgrounds in the present study are shown in Table 1. The subjects were 74 patients (35 males and 39 females, average age: 62.1 ± 13.9 years) whose causative disorders were lumbar spinal canal stenosis (34 cases), lumbar spondylolisthesis (13 cases), and lumbar disc hernia (27 cases). The duration of lower limb pain was 14.6 ± 14.9 weeks, and the drugs used for premedication were loxoprofen (29 cases, average dose 142.8 ± 48.7 mg/day), pregabalin (19 cases, average dose 39.5 ± 18.9 mg/day), duloxetine (2 cases, average dose 40.0 ± 23.1 mg/day), and no premedication (24 cases). The concomitant drugs (continuous-use drugs before the test) were loxoprofen (10 cases, average dose 144.0 ± 49.2 mg/day), duloxetine (2 cases, average dose 40.0 ± 23.1 mg/day), and no continuously used drug (62 cases).
Table 1.
The Patient Background in the Present Study.
| n=74 | |
|---|---|
| Age (y/o) | 62.1±13.9 |
| Sex (cases) | Male 35 : Female 39 |
| The causative disorders (cases) | Lumbar spinal canal stenosis 34 |
| Lumbar spondylolisthesis 13 | |
| Lumbar disc hernia 27 | |
| The duration of lower limb pain (weeks) | 14.6±14.9 |
| Drugs used for premedication (cases) | Loxoprofen 29 |
| Pregabalin 19 | |
| Duloxetine 2 | |
| No premedication 24 | |
| The concomitant drugs (cases) | Loxoprofen 10 |
| ※Continuous-use drugs | Duloxetine 2 |
| before the test | No continuously used drug 62 |
The occurrence of ADRs as primary endpoint are shown in Table 2. We found ADRs in 20 out of 74 cases (27.0%); 6 cases for drowsiness (8.1%), 6 cases for edema (8.1%), 4 cases for wamble (5.4%), and four cases for dizziness (5.4%). However, in 8 out of 74 cases (10.8%), mirogabalin was discontinued because of severe ADRs, which interfered with daily life. In addition, ADRs (n = 20) occurred immediately after initial administration (16 cases, 80.0%) and at the time of dose increase (4 cases, 20.0%) (Fig. 1). We examined 66 cases (except the eight cases where mirogabalin was discontinued) for pain assessment (VAS) as secondary endpoint. Since the temporal change of VAS for lower limb pain was 7.1 ± 2.3 (before administration), 5.1 ± 2.8 (2 weeks after administration), and 3.9 ± 2.8 (four weeks after administration), it was significantly lowered at two and four weeks after administration compared to before administration (p < 0.01) (Fig. 2). Furthermore, the improvement rates of VAS for two and four weeks after administration were 27.2 ± 31.8% and 41.3 ± 39.2%, respectively.
Table 2.
Occurrence of ADRs.
| Adverse drug reactions | 20/74 cases (27.0%) |
|---|---|
| Drowsiness | 6 cases (8.1%) |
| Edema | 6 cases (8.1%) |
| Wamble | 4 cases (5.4%) |
| Dizziness | 4 cases (5.4%) |
However, in 8 out of 74 cases (10.8%), mirogabalin was discontinued because of the ADRs.
Figure 1.
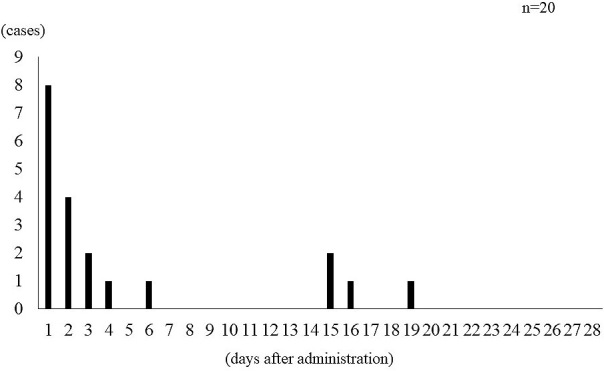
Occurrence of adverse drug reactions over study period.
Figure 2.

Temporal change of VAS for lower limb pain.
Discussion
In this study, the occurrence of patient-reported ADRs was 27.0%, similar to the frequency of ADRs in clinical trials for other disorders (diabetic peripheral neuropathic pain, 31.3%; postherpetic neuralgia, 43.6%)11,12). In addition, we found a similar composition (somnolence, dizziness, weight gain, etc.) in diabetic peripheral neuropathic pain and postherpetic neuralgia11,12). Therefore, mirogabalin may also produce specific ADRs at a fixed frequency.
In contrast, it is extremely interesting that in 10.8% of the cases, mirogabalin was discontinued because of severe ADRs, which interfered with daily life in the present study. In other words, compared to the frequency of ADRs, it was suggested that severe ADRs as a cause of discontinuation would be unlikely to occur or continue because of an extremely low discontinuation rate.
The temporal change of VAS for lower limb pain was lowered, with a significant difference between before administration and four weeks after administration in the present study. In addition, the average improvement rates of VAS two and four weeks after administration indicated a favorable result of 27.2 ± 31.8% and 41.3 ± 39.2% which suggested that mirogabalin may have an analgesic effect on peripheral neuropathic pain. According to the clinical report on mirogabalin's analgesic effects, the mirogabalin group (30 mg/day) indicated a statistically significant improvement on the 14th week-pain score compared to the placebo group in the international collaborative clinical trial for patients with diabetic peripheral neuropathic pain11). Furthermore, the mirogabalin group (15 mg/day, 20 mg/day, 30 mg/day) had a statistically significant improvement on the 14th week-pain score compared to the placebo group in the international collaborative clinical trial for patients with postherpetic neuralgia12). Although these reports technically used different subjects, doses, and durations of administration, they still proved the contents in the present study reporting mirogabalin's analgesic effects.
However, this study is limited by the inherent disadvantages of a retrospective study design, the absence of a control group, the very small number of cases, bias due to premedication, and the lack of comparisons with other drugs (especially pregabalin) and dose differences (except 10 mg/day→20 mg/day). Therefore, we plan to conduct a large-scale survey by following a prospective study design, selecting a control group, and including wide dose ranges in future.
Conclusions
In conclusion, when we retrospectively examined mirogabalin's efficacy for peripheral neuropathic pain in the present study, the occurrence of ADR was similar to the frequency of ADRs in the clinical trials for other disorders. When assessing the analgesic effect, the temporal change of VAS for lower limb pain significantly decreased before administration, and two and four weeks after administration.
Disclaimer: Sumihisa Orita is one of the Editors of Spine Surgery and Related Research and on the journal's Editorial Committee. He was not involved in the editorial evaluation or decision to accept this article for publication at all.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Ethical Approval: 2894 (School of Medicine, Chiba University)
Author Contributions: KI, YE, SuO, YS, and SeO designed research, analyzed and/or interpreted the data. KI, TS, TF, TO, YA, MI, YE, SuO, YS and SeO performed experiments. KI wrote the article and MK, TA, TF, JN, HT, MiS, SM, HK, MN, TU, TS, MasasS, MasahS, KE, HT, NM, TH, RT, GK, TM, TM, and SeO gave critical comments on the draft of the manuscript. All authors read and approved the final version of the manuscript.
Informed Consent: Opt-out method
References
- 1.Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med. 2014;15(1):4-15. [DOI] [PubMed] [Google Scholar]
- 2.Igarashi A, Akazawa M, Murata T, et al. Cost-effectiveness analysis of pregabalin for treatment of chronic low back pain in patients with accompanying lower limb pain (neuropathic component) in Japan. Clinicoecon Outcomes Res. 2015;7:505-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci. 2009;29(13):4076-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fink K, Dooley DJ, Meder WP, et al. Inhibition of neuronal Ca (2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42(2):229. [DOI] [PubMed] [Google Scholar]
- 5.Maneuf YP, Hughes J, McKnight AT. Gabapentin inhibits the substance P-facilitated K(+)-evoked release of glutamate from rat caudal trigeminal nucleus slices. Pain. 2001;93(2):191-6. [DOI] [PubMed] [Google Scholar]
- 6.Tanabe M, Takasu K, Takeuchi Y, et al. Pain relief by gabapentin and pregabalin via supraspinal mechanisms after peripheral nerve injury. J Neurosci Res. 2008;86(15):3258-64. [DOI] [PubMed] [Google Scholar]
- 7.Bee LA, Dickenson AH. Descending facilitation from the brainstem determines behavioural and neuronal hypersensitivity following nerve injury and efficacy of pregabalin. Pain. 2008;140(1):209-23. [DOI] [PubMed] [Google Scholar]
- 8.Satoh J, Yagihashi S, Baba M, et al. Efficacy and safety evaluation of pregabalin treatment over 52 weeks in patients with diabetic neuropathic pain extended after a double-blind placebo-controlled trial. J Diabetes Investig. 2011;2(6):457-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domon Y, Arakawa N, Inoue T, et al. Binding characteristics and analgesic effects of mirogabalin, a novel ligand for the α2δ subunit of voltage-gated calcium channels. J Pharmacol Exp Ther. 2018;365(3):573-82. [DOI] [PubMed] [Google Scholar]
- 10.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630-5. [DOI] [PubMed] [Google Scholar]
- 11.Baba M, Matsui N, Kuroha M, et al. Mirogabalin for the treatment of diabetic peripheral neuropathic pain: A randomized, double-blind, placebo-controlled phase III study in Asian patients. J Diabetes Investig. 2019;10(5):1299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato J, Matsui N, Kakehi Y, et al. Mirogabalin for the management of postherpetic neuralgia: a randomized, double-blind, placebo-controlled phase 3 study in Asian patients. Pain. 2019;160(5):1175-85. [DOI] [PMC free article] [PubMed] [Google Scholar]


