Abstract
Background:
Atrial fibrosis is a hallmark of arrhythmogenic structural remodeling in patients with persistent atrial fibrillation (AF) and is negatively correlated with procedure outcome in patients undergoing ablation. However, noninvasive methods to determine the extent of atrial fibrosis are limited. Here, we used microRNA (miRNA) expression analysis to detect markers of left atrial low-voltage areas (LVAs) in patients with persistent AF undergoing catheter ablation.
Methods:
We performed 3-dimensional voltage mapping in 102 patients (average age 62.1±13.1 years, CHA2DS2-VASc score of 2.3±1.6, LA size 41.5±5.7 mm) undergoing ablation for persistent AF and determined the extent of left atrial low-voltage. LVAs were defined if bipolar electrogram amplitudes were <0.5 mV during sinus rhythm. Before ablation, we obtained a blood sample, isolated miRNAs, and profiled them on a miRCURY LNA Universal RT microRNA PCR Human panel.
Results:
Sixty-nine miRNAs were identified in all samples, with an average of 123 miRNAs detectable per sample. We found that the serum concentration of miR-21, a miRNA that has been previously linked to cardiac fibrosis development, was strongly associated with the extent of LVAs determined by voltage mapping. We could confirm that LVAs were negatively correlated with ablation success in a 1-year follow-up. In addition, miR-21 serum levels were associated with AF-free survival after catheter ablation.
Conclusions:
Circulating miR-21 correlates with left atrial LVAs and is associated with procedure outcome in patients with persistent AF undergoing ablation.
Keywords: atrial fibrillation, biomarkers, fibrosis, microRNAs, pulmonary veins
WHAT IS KNOWN?
Atrial fibrosis is a negative predictor for procedure outcome in patients undergoing ablation for persistent atrial fibrillation. However, noninvasive methods to determine the extent of left atrial fibrosis are limited.
MicroRNA-21 is a major regulator of cardiac fibrosis. As a biomarker, it is detectable in blood.
WHAT THE STUDY ADDS?
MicroRNA-21 serum concentration correlates with the extent of left atrial low-voltage areas measured by voltage mapping.
MicroRNA-21 serum concentration was associated with procedure outcome in patients undergoing ablation for atrial fibrillation.
Within the last decade, the burden of atrial fibrillation (AF) has almost doubled in Europe, affecting between 1.9% and 2.9% of the general population.1,2 Undetected and untreated, AF bears a significant risk for the development of tachycardiomyopathy and stroke.3,4
It is well-established that prolonged episodes of AF can induce various pathways that stabilize and fuel the disease.5 According to a widely accepted model, paroxysmal AF is thought to be the result of rapid triggering by ectopic foci or a single reentry mechanism in the area around the pulmonary veins, whereas the more persistent or permanent forms are based on multiple functional or structural reentry mechanisms, partly caused by structural remodeling of the atria with development of atrial fibrosis.6,7 Left atrial remodeling, in particular, atrial fibrosis, plays a key role in the pathogenesis of AF.8–11 Fibrosis is associated with impaired cellular coupling, heterogeneity of intra-atrial conduction, and dispersion of refractory periods, which provides the substrate for reentry capable of sustaining AF.12,13
Circumferential pulmonary vein isolation is the cornerstone for AF catheter ablation, with long-term success rates of up to 80% in preselected patients.14 However, ablation success is strongly correlated with the extent of preexisting atrial fibrosis,10,15,16 leading to AF recurrence in ≈70% of patients with extensive fibrotic areas in the atrial wall.10
The extent of left atrial fibrosis can be estimated by mapping low-voltage areas (LVAs) in the atrium using a multipolar mapping catheter.16 However, for counseling patients and to plan the ablation procedure, noninvasive markers reflecting atrial fibrosis that can be used in a preclinical setting would be of great clinical benefit.
MicroRNAs (miRNAs) are short, single-stranded noncoding RNAs. They are evolutionary conserved and are involved in the regulation of gene-expression as well as in controlling and fine-tuning diverse cellular pathways.17 They operate by binding to a protein-coding target mRNA leading to its degradation or reduced expression.18,19 It is well-established that miRNAs play an important role in the pathophysiology of fibrosis development, both at the ventricular and on the atrial level.20,21
miRNAs may be suitable as biomarkers of disease, because of their tissue- and pathology-specific expression pattern. They are stable in plasma because they are either incorporated in microparticles (exosomes, microvesicles, and apoptotic bodies) or bound to proteins or high-density lipoproteins and are thereby protected from RNase activity. Moreover, miRNAs are detectable in plasma or serum with high sensitivity and specificity.20
In this study, we found that circulating miR-21 correlates with left atrial LVAs and is associated with ablation success in patients with persistent AF undergoing catheter ablation.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because, at the time of study start, this permission was not implemented in the patient-informed consent.
Patients, AF Ablation Procedure, and Follow-Up
One hundred two patients >18 years who underwent ablation for persistent AF at the University Hospital of Tuebingen were eligible for this study. Patients were excluded if they have had a prior left atrial ablation procedure. All patients gave their informed consent. The study was approved by the local ethics committee.
The ablation strategy was at the discretion of the operator. All patients received pulmonary vein isolation, either with a cryoballoon (Arctic Front Advance, Medtronic, 42.2% of patients) or with a force-controlled irrigated radiofrequency ablation catheter (Thermocool Smarttouch, Biosense Webster or TactiCath quartz, Abbott, USA, 57.8% of patients). Additional ablation strategies targeting the substrate were allowed if indicated by the operator. An additional left atrial roof line was done in 22.5%, a mitral line in 21.5%, and a right atrial isthmus line in 19.6% of patients. Ablation of complex fractionated electrograms was performed in 8.8% of cases. All baseline characteristics and the procedure details are listed in Table 1.
Table 1.
Demographic and Clinical Characteristics of Patients Included in the Study

After ablation, antiarrhythmic drugs were discontinued. In all patients, serial 7-day ECGs were performed 3, 6, and 12 months after the procedure. In addition, patients were instructed to seek ECG documentation when symptoms suggestive of AF occurred. Recurrences were defined as documented AF or atrial tachycardia (both symptomatic and asymptomatic, documented in a 7-day Holter ECG with a duration >30 s or in a 12-lead ECG after a blanking period of 3 months).
Acquisition of Electroanatomical Voltage Maps
Before ablation, a 20 mL blood sample was obtained and processed for RNA extraction (see below). If the patient was not in sinus rhythm (SR), an electrical cardioversion was performed. A decapolar spiral mapping catheter (Lasso NAV Eco catheter, Biosense Webster, Diamond Bar, CA or Reflexion spiral, Abbott) was placed into the left atrium. A left atrial voltage map was created using the spiral catheter and a 3-dimensional mapping system (Carto-3, Biosense Webster, or NavX velocity) with the acquisition of 200 to 1000 data points per atrium. LVAs were defined if the local electrogram was <0.5 mV during SR. The Carto-3 built-in software was used to calculate the percentage of LVAs from the left atrial surface. Maps shown in Figure 1 are color-coded from gray (<0.5 mV, substantial LVAs) to purple (>1.5 mV, normal voltage). Patients were divided into 3 groups according to the extents of LVAs: group A (<10% LVAs), group B (10% to 30% LVAs), and group C (>30% LVAs).
Figure 1.

Left atrial low-voltage areas (LVAs) are associated with outcome after atrial fibrillation (AF) ablation. A, Example of left atrial voltage maps. Patients were divided into 3 groups according to the extent of LVAs. B, Kaplan–Meier curves estimating freedom from AF after pulmonary vein isolation (PVI) only, which shows a strong correlation between LVAs and procedure outcome. C, Kaplan–Meier curves for patients that received PVI and—in case of significant LVAs—additional substrate modification.
RNA Purification and miRNA Profiling
Total RNA was purified from whole blood using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Profiling of 372 miRNAs in the serum samples of 2 patient groups was performed by the miRCURY LNA Universal RT microRNA PCR Human panel I (Exiqon, Vedbaek, Denmark). All miRNAs were polyadenylated and reversed transcribed into cDNA using the miRCURY LNA Universal RT microRNA PCR, Polyadenylation, and cDNA synthesis kit (Exiqon). Individual miRNA was amplified and detected in a Roche LightCycler 480. The second derivative method was used for the determination of Cp values.
miRNA Normalizer Analysis
The raw Cps of the 68 miRNAs (except miRNA-21) which were detectable in all the samples from the profiling study were used to find normalizers. The NormFinder software22 was used to assess the stability of miRNAs and the 10 most stable miRNAs were used in a stepwise exclusion of the least stable genes calculation in geNorm.23 Pairwise variation calculation for the determination of the optimal number of genes as normalizers in geNorm had shown that 2 miRNAs were sufficient for the following real-time PCR profiling analysis, as inclusion of a third gene has no significant effect and overall all the pairwise variations were below the default cutoff value 0.15. miRNAs hsa-miR-23a-3p and hsa-miR-23b-3p were selected, and their geometric mean was used as the normalizer in the larger scale real-time PCR profiling.
Statistics
Linear regression analyses were performed to relate normalized miR-21 relative serum expression and left atrial LVAs to individual demographic and clinical covariates. Time to AF recurrence was related to the individual demographic and clinical covariates using separate univariable Cox proportional hazard regression models. Cox regression analyses were also performed to relate time to recurrence of arrhythmia to miR-21 and to LVAs after adjusting for the following covariates: age, sex, hypertension, congestive heart failure, mitral valve disease, and diabetes mellitus status. The unadjusted risks were estimated using the Kaplan–Meier estimator with 95% confidence intervals.
Results
LVAs Correlate With Ablation Success in Patients With Persistent AF
Left atrial voltage mapping in patients with persistent AF showed a wide spectrum of LVAs ranging from 0% to >90% of the left atrial wall, with a mean of 16.31±21.89%. Out of 102 patients, 47 (46.1%) showed no or only minor LVAs (group A, LVAs <10%), 26 patients (25.5%) had moderate LVAs (group B, LVAs 10% to 30%), and 29 patients (28.4%) had severe LVAs (group C, LVAs >30%; Figure 1A). The extent of LVA was associated with age, mitral valve disease, diastolic blood pressure, left ventricular hypertrophy, CHA2DS2-VASc score, and chronic kidney failure.
Consistent with other studies,9,10,24 the extent of LVAs strongly correlated with AF-free survival after ablation. Patients (87.8%) with minor LVAs (group A) were in stable SR 12 months after ablation, whereas only 50.0% of group B and no patient (0%) of group C was in SR, if pulmonary vein isolation without any additional left atrial substrate modifications had been performed (P<0.01; Figure 1B). By adding additional substrate modification to the left atrium, success rates could be increased to 54.5% in group C, resulting in an overall success rate of 63.1% in group B and 34.6% in group C (P<0.01; Figure 1C). A 1% increase in LVA resulted in a hazard ratio for AF recurrence of 1.054 (1.032–1.078; P<0.01).
The hazard ratios of clinical factors related with arrhythmia recurrence are listed in Table 2. Among these factors, only age, mitral valve disease, and CHA2DS2-VASc score was significantly correlated with AF recurrence.
Table 2.
Individual Demographic and Clinical Factors, Expression of miR-21, and Extent of LVAs Based on Separate Binary Logistic Regression Analyses

miRNA-21 Correlates With Left Atrial LVAs
We performed miRNA profiling with blood samples obtained directly before AF ablation. For an initial screen, blood samples were pooled from patients with extreme LVAs (>80%) and from patients without any LVAs (<5%). The extracted miRNA was profiled on a miRCURY LNA Universal RT microRNA PCR Human panel I (Exiqon, Vedbaek, Denmark). Among 372 miRNAs, 69 were identified in all samples, with an average of 123 miRNAs detectable per sample. Several of those miRNAs were significantly dysregulated between the 2 groups (Figure 2A).
Figure 2.
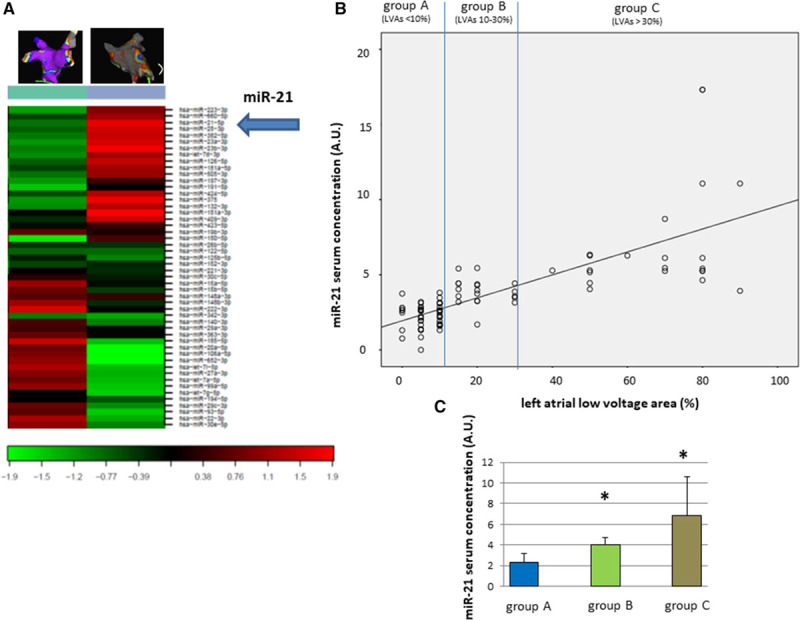
miR-21 correlates with left atrial low-voltage areas (LVAs) in patients with persistent atrial fibrillation. A, MicroRNA profiling of patients with extreme and no left atrial LVAs revealed dysregulation of several miRNAs. B, One of the dysregulated microRNAs, miR-21, correlates with LVAs in a linear pattern. C, Relative miR-21 serum expression according to LVA group (group 1: LVAs <10%; group 2: LVAs 10% to 30%; group 3: LVAs >30%, *P<0.05).
Because miR-21 has been suggested to be involved in cardiac remodeling,21,25,26 we analyzed miR-21 expression in all 102 blood samples and found a strong linear correlation between miR-21 serum concentration and extent of LVAs (Spearman ρ test: P<0.01, R=0.735; LVA (%)=miR-21*12.5–1.94 AU; Figure 2B). The average miR-21 expression in group A was 2.33±0.8 AU (lowest Ct value, 0.76 AU; highest Ct value, 3.80 AU), whereas it was 4.0±0.75 AU (lowest Ct value, 3.15 AU; highest Ct value, 5.45 AU) in group B, and 6.85±3.73 AU (lowest Ct value, 3.92 AU; highest Ct value, 17.32 AU) in group C (P<0.01 between group A and B and between B and C; Figure 2C).
miR-21 Is Associated With Event-Free Survival After AF Ablation
We assigned patients in 3 groups according to their miR-21 serum concentration: group I with very low relative miR-21 expression (<2.5 AU), group II with intermediate relative miR-21 expression (2.5–4.5 AU), and group III with high miR-21 serum concentration (>4.5 AU). Figure 3A shows the Kaplan–Meier curve on event-free survival after ablation of persistent AF. Patients (86.5%) in group I were in stable SR during the observation period, whereas only 76% of patients in group II and 23% of patients in group III had SR 12 months after ablation.
Figure 3.

miR-21 correlates with ablation success in patients with persistent atrial fibrillation (AF). A, Kaplan–Meier curves estimating AF-free survival dependent on miR-21 relative serum expression. B, Receiver operating characteristics (ROC) curve analysis shows an area under the curve (AUC) of 0.822. C, Extreme miR-21 serum concentrations are strongly correlated with ablation success in patients with persistent AF.
Although group sizes are small and the study is underpowered to show significant findings, miR-21 serum concentration seems to be in particular relevant for extreme values. Patients with a very low miR-21 concentration (<2 AU) had an excellent outcome after AF ablation with 94.1% in stable SR after 12 months, whereas patients with very high miR-21 concentrations (>5 AU) had a probability of AF recurrence of 87.5% (Figure 3C). An increase of miR-21 serum concentration by 1 AU resulted in a hazard ratio for AF recurrence of 1.736 (1.301–2.316, P<0.01).
Receiver operating characteristics curve analysis gave an area under the curve of 0.864 for a miR-21 serum concentration <3 AU to be a predictor for a positive ablation result in patients with persistent AF.
Follow-Up of miR-21 Expression
We assessed miR-21 serum concentration in follow-up blood samples that were obtained 6 months after AF catheter ablation. We found that miR-21 expression was strongly decreased in patients that were in stable SR after 6 months (relative reduction of miR-21 by 70.41±14.9%). miR-21 expression was also decreased in patients that had AF recurrence (42.41±12.9%), but the relative reduction was significantly smaller than in patients with a successful ablation (Figure 4; P<0.05).
Figure 4.

Follow-up miR-21 serum concentration 6 mo after ablation. Although patients in stable sinus rhythm (SR) had a strong reduction in miR-21 expression (42.41±12.9% from baseline), patients with atrial fibrillation (AF) recurrence only showed a mild decrease in miR-21 (70.41±14.9% from baseline, P<0.05).
Discussion
Consistent with other studies,9,10,15,16,24,27 we could confirm the association between left atrial LVAs and the outcome after catheter ablation in patients with persistent AF. We found that LVAs >30% of the total left atrial wall lead to a high chance of AF recurrence, regardless of the ablation strategy that was used. On the other hand, AF recurrence rate was very low if there was only minimal LVAs detectable in the atrium.
We found a significant correlation between miR-21 serum concentration and extent of LVAs detected in the left atrium. This is in accordance with a study by Adam et al,25 who also found a linear correlation between miR-21 tissue concentration and extent of atrial fibrosis determined by immunostainings of left atrial appendages.
In addition, we found a significant correlation between miR-21 serum concentrations and 1-year outcome after catheter ablation in patients with persistent AF. Circulating miR-21 might, therefore, be useful for the stratification of patients and to plan the procedure (eg, to perform a pulmonary vein isolation only in patients with very low miR-21 serum concentrations or to plan extensive substrate modification in patients with high miR-21 levels), although this needs to be confirmed in further studies.
miRNA-21, which is highly expressed in fibroblasts, has been associated with increased cardiac fibrosis, on the ventricular as well as on the atrial level.21,25,26 Several mechanisms have been proposed for the potential effect of miR-21 on fibrosis, but most attention has been paid to miR-21 repression of SPRY1 (Sprouty 1). Sprouty 1 inhibits the ERK (extracellular signal–regulated kinases) signaling pathway, which promotes fibrosis.21,25,26 Upregulation of miR-21 was associated with downregulation of SPRY1 in patients with valvular AF and in rats with myocardial ischemia.25,26 In vivo inhibition with antagomir-21 in mice or a 15-mer locked nuclear acid-based anti–miR-21 in rats suppressed fibrosis and AF.26
miR-21 can also induce inflammation-associated atrial fibrosis through activation of the STAT3 (signal transducer and activator of transcription 3) pathway. Inhibition of miR-21 with an antagomir-suppressed STAT3 phosphorylation, expression of fibrosis-related genes, and AF vulnerability in rats. Expression of miR-21 itself was also found to be positively regulated by phosphorylated STAT3 and may therefore form a feedback loop. Stimulation of cardiac fibroblasts with IL (interleukin)-6 resulted in STAT3 phosphorylation and an increased miR-21 expression. This pathway may therefore link atrial inflammation to fibrosis formation.28
Another signaling pathway of miR-21 in AF involves the downregulation of Smad7 (mothers against decapentaplegic homolog 7), which is an inhibitor of the TGF (transforming growth factor)-β1 pathway. Rapid atrial pacing in rabbits, a common AF animal model, lead to a loss of Smad7 with induction of collagen I and III, whereas miR-21 expression was significantly increased. In vivo inhibition of miR-21 increased Smad7 and decreased collagen I/III production. Luciferase assays validated Smad7 as a direct target of miR-21.29
It is noteworthy to mention that there is divergent data available about the role of miR-21 in AF. A study by McManus et al30 as well as a study by Dawson et al31 found lower miR-21 concentrations in patients with AF compared with healthy controls. Luo et al32 reviewed the available miRNA biomarker studies in AF and miR-21 was not identified in those. We hypothesize that different blood sample collections, processing, miRNA isolation, and normalization might contribute to these discrepancies. In addition, LVAs or atrial fibrosis was not assessed in these studies, which makes any comparisons difficult.
miR-21 expression is not restricted to the heart, but can also be found in a wide range of human tissues,33 with the atria contributing in a relatively small mass. Although we assume that the increase in serum expression reflects changes in atrial expression, we cannot exclude that the circulating miR-21 comes from another source. Therefore, caution should be exercised when comparing atrial miRNA expression with blood miRNA expression.
In our study, we exclusively included patients with persistent AF because we assumed that patients with paroxysmal AF are unlikely to present with significant LVAs. Although we have not measured miR-21 in patients with paroxysmal AF, it is tempting to speculate that miR-21 serum concentrations would be low. However, this needs to be assessed in further studies.
Our study was not powered to compare different ablation strategies. Because there is no clear evidence on the effectiveness of adjuvant substrate modification in addition to pulmonary vein isolation in patients with persistent AF,34 the therapeutic decision was selected by the operator. Pulmonary vein isolation without additional ablation targeting LVAs resulted in a very poor outcome in patients with advanced LVAs, which is in accordance with existing literature.9,15,16 Success rates could be improved by adding linear ablation lines or complex fractionated electrogram ablation in this cohort. However, further studies need to be performed to compare the effectiveness of the different ablation strategies.
In conclusion, we found that circulating miR-21 strongly correlates with left atrial LVAs assessed by electrophysiological mapping and is associated with a favorable ablation outcome in patients with persistent AF. Measuring miR-21 serum concentration might, therefore, be helpful to find good candidate patients for catheter ablation and to plan the procedure.
Acknowledgments
We thank Klaudia Posavec for providing outstanding technical assistance.
Sources of Funding
This study was supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung) within the framework of the e:Med research and funding concept (grant number 01ZX1407B to Dr Gramlich), a research fund of the Centre for Personalized Medicine (Zentrum für Personalisierte Medizin), University of Tuebingen, and a research fund from the “Sonderlinie Medizin” initiative of the Ministry of Science, Research and Arts, Baden-Wuerttemberg, Germany.
Disclosures
None.
Supplementary Material
Footnotes
Drs Zhou and Maleck contributed equally as first authors.
Drs Seizer and Gramlich contributed equally as senior authors.
References
- 1.Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–220. doi: 10.2147/CLEP.S47385. doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schotten U, Greiser M, Benke D, Buerkel K, Ehrenteidt B, Stellbrink C, Vazquez-Jimenez JF, Schoendube F, Hanrath P, Allessie M. Atrial fibrillation-induced atrial contractile dysfunction: a tachycardiomyopathy of a different sort. Cardiovasc Res. 2002;53:192–201. doi: 10.1016/s0008-6363(01)00453-9. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 5.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 6.Markides V, Schilling RJ. Atrial fibrillation: classification, pathophysiology, mechanisms and drug treatment. Heart. 2003;89:939–943. doi: 10.1136/heart.89.8.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 8.Kottkamp H. Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J. 2013;34:2731–2738. doi: 10.1093/eurheartj/eht194. doi: 10.1093/eurheartj/eht194. [DOI] [PubMed] [Google Scholar]
- 9.Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, Schweikert RA, Saliba W, Cummings J, Burkhardt JD, Bhargava M, Belden WA, Abdul-Karim A, Natale A. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol. 2005;45:285–292. doi: 10.1016/j.jacc.2004.10.035. doi: 10.1016/j.jacc.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 10.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 11.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 12.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 13.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 14.Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le Marec H, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Kircher S, Arya A, Altmann D, Rolf S, Bollmann A, Sommer P, Dagres N, Richter S, Breithardt O-A, Dinov B, Husser D, Eitel C, Gaspar T, Piorkowski C, Hindricks G. Individually tailored vs. standardized substrate modification during radiofrequency catheter ablation for atrial fibrillation: a randomized study [published online ahead of print November 21, 2017]. Europace. doi: 10.1093/europace/eux310. [DOI] [PubMed] [Google Scholar]
- 16.Jadidi AS, Lehrmann H, Keyl C, Sorrel J, Markstein V, Minners J, Park C-I, Denis A, Jaïs P, Hocini M, Potocnik C, Allgeier J, Hochholzer W, Herrera-Sidloky C, Kim S, Omri Y El, Neumann F-J, Weber R, Haïssaguerre M, Arentz T. Ablation of persistent atrial fibrillation targeting low-voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol. 2016;9:e002962. doi: 10.1161/CIRCEP.115.002962. [DOI] [PubMed] [Google Scholar]
- 17.Krützfeldt J, Poy MN, Stoffel M. Strategies to determine the biological function of microRNAs. Nat Genet. 2006;38(suppl):S14–S19. doi: 10.1038/ng1799. doi: 10.1038/ng1799. [DOI] [PubMed] [Google Scholar]
- 18.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabian MR, Sundermeier TR, Sonenberg N. Understanding how miRNAs post-transcriptionally regulate gene expression. Prog Mol Subcell Biol. 2010;50:1–20. doi: 10.1007/978-3-642-03103-8_1. doi: 10.1007/978-3-642-03103-8_1. [DOI] [PubMed] [Google Scholar]
- 20.van den Berg NWE, Kawasaki M, Berger WR, Neefs J, Meulendijks E, Tijsen AJ, de Groot JR. MicroRNAs in atrial fibrillation: from expression signatures to functional implications. Cardiovasc Drugs Ther. 2017;31:345–365. doi: 10.1007/s10557-017-6736-z. doi: 10.1007/s10557-017-6736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 22.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 23.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang D, Li JB, Zghaib T, Gucuk Ipek E, Balouch M, Spragg DD, Ashikaga H, Tandri H, Sinha SK, Marine JE, Berger RD, Calkins H, Nazarian S. The extent of left atrial low-voltage areas included in pulmonary vein isolation is associated with freedom from recurrent atrial arrhythmia. Can J Cardiol. 2018;34:73–79. doi: 10.1016/j.cjca.2017.10.012. doi: 10.1016/j.cjca.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Adam O, Löhfelm B, Thum T, Gupta SK, Puhl SL, Schäfers HJ, Böhm M, Laufs U. Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res Cardiol. 2012;107:278. doi: 10.1007/s00395-012-0278-0. doi: 10.1007/s00395-012-0278-0. [DOI] [PubMed] [Google Scholar]
- 26.Cardin S, Guasch E, Luo X, Naud P, Le Quang K, Shi Y, Tardif JC, Comtois P, Nattel S. Role for MicroRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ Arrhythm Electrophysiol. 2012;5:1027–1035. doi: 10.1161/CIRCEP.112.973214. doi: 10.1161/CIRCEP.112.973214. [DOI] [PubMed] [Google Scholar]
- 27.Vlachos K, Efremidis M, Letsas KP, Bazoukis G, Martin R, Kalafateli M, Lioni L, Georgopoulos S, Saplaouras A, Efremidis T, Liu T, Valkanas K, Karamichalakis N, Asvestas D, Sideris A. Low-voltage areas detected by high-density electroanatomical mapping predict recurrence after ablation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28:1393–1402. doi: 10.1111/jce.13321. doi: 10.1111/jce.13321. [DOI] [PubMed] [Google Scholar]
- 28.Huang Z, Chen X, Qian C, Dong Q, Ding D, Wu Q, Li J, Wang H, Li W, Xie Q, Cheng X, Zhao N, Du Y, Liao Y. Signal transducer and activator of transcription 3/MicroRNA-21 feedback loop contributes to atrial fibrillation by promoting atrial fibrosis in a rat sterile pericarditis model. Circ Arrhythmia Electrophysiol. 2016;9:e003396. doi: 10.1161/CIRCEP.115.003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He X, Zhang K, Gao X, Li L, Tan H, Chen J, Zhou Y. Rapid atrial pacing induces myocardial fibrosis by down-regulating Smad7 via microRNA-21 in rabbit. Heart Vessels. 2016;31:1696–1708. doi: 10.1007/s00380-016-0808-z. doi: 10.1007/s00380-016-0808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McManus DD, Tanriverdi K, Lin H, Esa N, Kinno M, Mandapati D, Tam S, Okike ON, Ellinor PT, Keaney JF, Jr, Donahue JK, Benjamin EJ, Freedman JE. Plasma microRNAs are associated with atrial fibrillation and change after catheter ablation (the miRhythm study). Heart Rhythm. 2015;12:3–10. doi: 10.1016/j.hrthm.2014.09.050. doi: 10.1016/j.hrthm.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawson K, Wakili R, Ordög B, Clauss S, Chen Y, Iwasaki Y, Voigt N, Qi XY, Sinner MF, Dobrev D, Kääb S, Nattel S. MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation. 2013;127:1466–1475, 1475e1. doi: 10.1161/CIRCULATIONAHA.112.001207. doi: 10.1161/CIRCULATIONAHA.112.001207. [DOI] [PubMed] [Google Scholar]
- 32.Luo X, Yang B, Nattel S. MicroRNAs and atrial fibrillation: mechanisms and translational potential. Nat Rev Cardiol. 2015;12:80–90. doi: 10.1038/nrcardio.2014.178. doi: 10.1038/nrcardio.2014.178. [DOI] [PubMed] [Google Scholar]
- 33.Ludwig N, Leidinger P, Becker K, Backes C, Fehlmann T, Pallasch C, Rheinheimer S, Meder B, Stähler C, Meese E, Keller A. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016;44:3865–3877. doi: 10.1093/nar/gkw116. doi: 10.1093/nar/gkw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P STAR AF II Investigators. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]


