Abstract
BACKGROUND AND PURPOSE:
The association of arterial tortuosity and connective tissue diseases is widely reported in the literature, but only a few studies were based on a quantitative evaluation of this arterial phenotype, and none of the latter examined the intracranial vasculature. The aim of this study was to evaluate the degree of intracranial arterial tortuosity in patients with Marfan syndrome and those with Loeys-Dietz syndrome, and to assess its usefulness in the differential diagnosis.
MATERIALS AND METHODS:
We performed a retrospective analysis of 68 patients with genetically confirmed Marfan syndrome (n = 36) or Loeys-Dietz syndrome (n = 32), who underwent at least 1 MRA of the brain at our institution. Fifty-two controls were randomly selected among patients who presented with headache and without any known comorbidity. Tortuosity indexes of 4 intracranial arterial segments were measured on a 3D volume-rendered angiogram by using the following formula: .
RESULTS:
Both Marfan syndrome and Loeys-Dietz syndrome showed a significantly higher tortuosity index compared with controls in all examined vessels. The tortuosity index of the vertebrobasilar system showed an excellent interrater reliability (intraclass correlation coefficient, 0.99) and was the strongest independent predictor of Loeys-Dietz syndrome in patients with connective tissue disease (P = .002), with a 97% specificity for this pathology when its value was > 60.
CONCLUSIONS:
The tortuosity index of intracranial arteries is an easily calculated and highly reproducible measure, which shows a high specificity for Marfan syndrome and Loeys-Dietz syndrome and may be useful in differentiating these 2 entities.
The term arterial tortuosity defines the presence of multiple abnormal turns of one or more arteries, which likely develop as a result of aberrant vessel elongation.1 Previous studies showed an association between arterial tortuosity and aging as well as female sex, hypertension, and other cardiovascular risk factors.2-4 A renewed interest in this vascular biomarker emerged in recent years, especially in relation to its possible role in the diagnostic evaluation and prognostic assessment of genetic arteriopathies.5-7
Although arterial tortuosity has been recognized as a hallmark of Loeys-Dietz syndrome (LDS) and arterial tortuosity syndrome,8-10 recent studies suggest that this arterial phenotype is also present in other connective tissue diseases (CTDs), such as Marfan syndrome, and familial thoracic aneurysm and aortic dissection.5,11
LDS is characterized by a disease course that is more severe compared with Marfan syndrome and presents with aortic dissection at a younger age and smaller aortic diameter, with vascular disease extending beyond the aortic root.9,12 An early and accurate differential diagnosis between Marfan syndrome and LDS, therefore, is essential to ensure proper patient care. Nonetheless, a risk of misdiagnosis is present, especially in patients without specific traits of Marfan syndrome (eg, ectopia lentis) or LDS (eg, bifid uvula, cleft palate, and hypertelorism) because several other features of these syndromes demonstrate a considerable overlap.12,13 In these scenarios, the presence of prominent intracranial arterial tortuosity may raise the suspicion of LDS and lead to early genetic testing and closer monitoring of these patients.
Several studies reported the presence of tortuous intracranial arteries in patients with LDS,14-18 but all of them were based on subjective evaluation of tortuosity, which limits the usefulness of this finding due to the lack of standardization in terms of definition and measurement. Recently, more advanced quantitative methods, for example, the tortuosity index (TI), have been proposed to measure arterial tortuosity. TI is calculated as the ratio between the actual length of an arterial segment and the shortest distance measured between its end points, which has been shown to be a reproducible measure of arterial tortuosity in different vascular territories.1,5 Recent studies used TI to measure carotid artery and vertebral artery tortuosity in the cervical region in patients with CTDs,5,6 but no quantitative data are available with regard to the intracranial vasculature. The aim of this study was to evaluate the degree of intracranial arterial tortuosity in patients with Marfan syndrome and those with LDS, and to assess its usefulness in the differential diagnosis of these 2 pathologies.
MATERIALS AND METHODS
We retrospectively reviewed the electronic medical records and imaging studies of all patients with genetically confirmed Marfan syndrome and LDS who were referred until December 2019 to the hub center for heritable CTDs of the University of Bologna (Italy). We included in this study all patients (N= 68) who underwent at least 1 MRA of the brain at our institution.
In accordance with current guidelines,12 at our institution, all patients diagnosed with LDS undergo full vascular imaging, including the intracranial arterial system, every 2 years to screen for the presence of aneurysms and other vascular pathology. In recent years, due to the reported association of Marfan syndrome with extra-aortic vascular pathology,17 our patients diagnosed with Marfan syndrome undergo a screening MRA of the brain, which is not repeated if no pathologic findings are discovered. All MRAs were acquired after the diagnosis of Marfan syndrome or of LDS was already established. When more than one brain imaging study was available, only the most recent MRA was selected for analysis. All included MRAs were acquired between January 2015 and December 2019.
Fifty-two controls were randomly selected among patients who presented with headache and without any intracranial vascular pathology, and who underwent an MRA of the brain during the same period. We reviewed the electronic medical records of the controls and excluded all the patients with systemic arterial disease or cardiovascular risk factors (such as hypertension, diabetes, renal pathology, atrial fibrillation, aortic aneurysm or dissection, and fibromuscular dysplasia). In addition, patients with genetic syndromes or a history of cancer were excluded from the control group. This study was carried out in accordance with ethics standards as set out in the Declaration of Helsinki and was approved by the institutional review board as a retrospective study.
MRA Acquisition and Image Analysis
MRAs of the brain were performed with a 1.5T superconducting system (Ingenia; Philips Healthcare), with a 20-channel phased array head coil. A commercially available 3D TOF-MRA sequence was used, with the following parameters: field of view, 200 × 189 mm; matrix, 444 × 235; section thickness, 1.2 mm; number of slices, 168; echo time, 6.9 ms; repetition time, 27 ms; flip angle, 20°; and number of acquisitions, 1.
Image analysis was performed by using IntelliSpace 8.0 software (Philips Healthcare) by an examiner who was blinded to clinical data and extracranial imaging findings. A 3D volume-rendered angiogram was created from the source TOF-MRA images for each patient. The actual length of the artery was measured by using the vessel centerline, which was automatically traced by the software algorithm; manual correction was only needed in the presence of extremely tortuous vessels when the ends of a vascular loop came into contact with each other. Both centerline length and straight-line length were automatically measured by the software, whereas the end points of each vessel were manually selected. TI was calculated by using a previously described formula: .5 A second examiner (SD)calculated the TI of 20 randomly selected MRAs of the brain, and interrater reliability was assessed by using the intraclass correlation coefficient.
Arterial Segment Selection
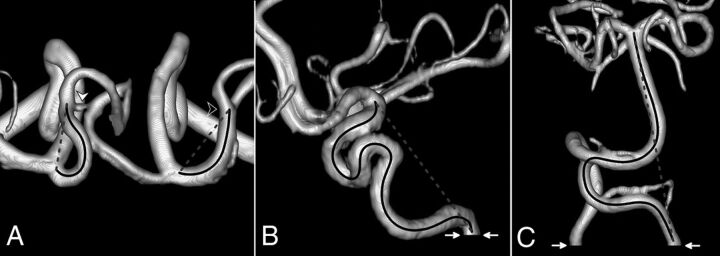
To obtain a comprehensive estimate of intracranial arterial tortuosity, we selected vessels from both the anterior and posterior circulation, which could be defined based on easily identifiable anatomic landmarks, to maximize reproducibility and ease of segmentation (Fig 1). In the anterior circulation, the following arterial segments were analyzed: intracranial internal carotid artery (IICA) (from its entrance into the carotid canal of the temporal bone to its bifurcation into anterior and middle cerebral arteries), the A1 segment of the anterior cerebral artery (from the origin of the anterior cerebral artery to the level of the anterior communicating artery), and the M1 segment of the MCA (from the origin of the MCA to the M1–M2 bifurcation). In the posterior circulation, we segmented the vertebrobasilar system (VBS) starting from the level of the foramen magnum to the tip of the basilar artery. The level of the inferior opening of the carotid canal and the level of the foramen magnum were identified on MPR images. The TI of each arterial segment was calculated on both sides. For the VBS, the TI was calculated starting from both vertebral arteries. Both the average TI and maximum TI values of each segment were used for analyses. To improve clarity, we only reported data on the former because both values yielded similar results at univariate analysis, but receiver operating characteristic curve analysis showed higher sensitivity and specificity for the average TI.
FIG 1.
The TI measurement on 3D volume-rendered TOF-MRA. The solid black line indicates the centerline length, whereas the dashed gray line shows straight-line length. A, Superior view of A1 and M1 segments in a patient with LDS (all anterior cerebral artery segments distal to A2 have been removed; white arrowhead, anterior communicating artery; black arrowhead, right M1–M2 bifurcation). B, Lateral view of the right IICA in a patient with LDS (white arrows, level of the inferior opening of the carotid canal). C, Anterior view of VBS in a patient with Marfan syndrome (white arrows, level of the foramen magnum).
Statistical Analysis
Categoric variables were compared by using the Fisher exact test, whereas the Wilcoxon rank sum test was computed for continuous variables. The Spearman rank correlation coefficient was used to evaluate the association between TI and age. Normalcy was assessed by means of the Shapiro-Wilk test. Receiver operating characteristic curve analysis was performed for each vessel TI and the binary end points of Marfan syndrome versus LDS and CTD versus control. At multivariate analysis, ORs and 95% CIs were estimated with a logistic regression model, including age, A1 TI, and VBS TI. Continuous variables were described as median and interquartile range (IQR), whereas categoric variables were summarized by absolute and relative (%) frequency. All tests were 2-sided, and P < .05 was considered statistically significant. Statistical analysis was performed by using R version 3.6.1 (2019; The R Foundation for Statistical Computing).
RESULTS
Subjects
Sixty-eight patients with CTD and 52 controls were included in this study. In the CTD group, 36 patients (52.9%) had Marfan syndrome and 32 (47.1%) had LDS. All the patients with Marfan syndrome had a mutation in the FBN-1 gene, whereas, among patients with LDS, 15 (46.9%) had a mutation in SMAD3, 11 (34.4%) in TGFBR2, and 6 (18.7%) in TGFBR1. Univariate analysis did not show a statistically significant difference in age or sex between cases and controls. The median age at MRA was 38.5 years (IQR, 23–48 years) in patients with CTD and 35 years (IQR, 28.5–54 years) among the controls (P = .43); 10 patients and 5 controls were < 18 years old. There were 33 females (48.5%) in the CTD group and 28 females (53.8%) among the controls (P = .59). We did not find any significant difference in the examined demographic and clinical variables between the patients with Marfan syndrome and those with LDS, except for a higher prevalence of peripheral artery dissection in LDS (Table 1).
Table 1:
Demographic and clinical characteristics of patients with CTD
| Characteristic | CTD (N = 68) | Marfan Syndrome (n = 36) | LDS (n = 32) | P |
|---|---|---|---|---|
| Age at MRA, median (IQR), y | 38.5 (23–48) | 32.5 (25–43.5) | 42 (22–50) | .21 |
| Female, n (%) | 33 (48.5) | 15 (41.7) | 18 (56.3) | .33 |
| Aortic root dilation, n (%) | 59 (86.8) | 33 (91.7) | 26 (81.3) | .29 |
| Aortic dissection, n (%) | 9 (13.2) | 6 (16.7) | 3 (9.4) | .48 |
| Arterial dissection, n (%) | 4 (5.9) | 0 (0.0) | 4 (12.5) | .04 |
| Aortic surgery, n (%) | 41 (60.3) | 25 (69.4) | 16 (50.0) | .14 |
| Age at surgery, median (IQR), y | 28 (20–37) | 26 (21–35) | 33 (18–44) | .47 |
Among the patients with LDS, 7 were found to harbor a total of 8 intracranial aneurysms; in 1 patient, a dural arteriovenous fistula was reported. We did not find any intracranial vascular pathology in the patients with Marfan syndrome. The average aneurysm diameter was 3.6 mm (range, 2–5 mm), and the most frequent location was the carotid siphon (50%). No patients presented with subarachnoid hemorrhage. After evaluation by a multidisciplinary team, including neuroradiologists, neurosurgeons, and cardiologists, 2 patients underwent successful treatment of the aneurysm, 1 via endovascular coiling, the other by surgical clipping, without any complications. We did not find any significant difference in tortuosity between the patients with LDS and with and without intracranial aneurysm (P = .71 for A1, P = .10 for M1, P = .37 for IICA, and P = .96 for VBS). Among the patients with CTD, 7 (10.3%) reported a history of smoking, 8 (11.8%) presented with dyslipidemia, 1 (1.5%) had a body mass index >30 kg/m2, and none had diabetes mellitus. No significant difference in TI was found between smokers and nonsmokers (P = .36 for A1, P = .39 for M1, P = .24 for IICA, and P = .69 for VBS) nor between patients with and patients without dyslipidemia (P = .52 for A1, P = .86 for M1, P = .73 for IICA, and P = .89 for VBS).
Interrater Reliability
The TI of the 4 examined arterial segments were measured by 2 observers in 20 randomly chosen subjects (8 patients with Marfan syndrome, 6 patients with LDS, and 6 controls) to assess for interrater reliability. Intraclass correlation coefficient for TI comparison between the 2 observers was 0.97 (P < .001) for A1, 0.76 (P = .03) for M1, 0.99 (P < .001) for IICA, and 0.99 (P < .001) for VBS.
Analysis of Intracranial Arterial Tortuosity
IICA TI and VBS TI showed a positively skewed distribution both in patients with CTD and the controls, whereas the distribution of A1 TI and M1 TI was positively skewed in LDS and normally distributed in those with Marfan syndrome and the controls. The highest TI variability was observed for M1, which may be explained by the highly variable position of the M1–M2 bifurcation relative to the origin of the MCA. The median difference between the TI of the right and left side (expressed as the percentage of the absolute value of the difference between the 2 sides compared with the less tortuous side) was 45.5% for A1 (IQR, 14.3%–64.8%), 40.7% for M1 (IQR, 21.4%–84.6%), 10.2% for IICA (IQR, 5.0%–20.1%), and 19.2% for VBS (IQR, 7.6%–33.3%). The variation in tortuosity between the 2 sides was not significantly different when comparing the patients with CTD and the controls (P = .85 for A1, P = .67 for M1, P = .46 for IICA, and P = .48 for VBS) as well as patients with Marfan syndrome and those with LDS (P = .91 for A1, P = .94 for M1, P = .97 for IICA, and P = .33 for VBS).
No significant differences were found between the TI of vessels from the right and left sides (P = .90 for M1, P = .67 for IICA, and P = .37 for VBS), except for a trend toward significance for the A1 segment (P = .05), which showed a higher TI on the right side (median TI, 11 [IQR, 7–17]) compared with the left side (median TI , 8 [IQR, 7–15]). No significant results were found when stratifying the patients by CTD status. The TI of the examined arterial segments did not differ significantly between males and females, even when stratifying the subjects by CTD status. The median TI in males and females was 11 (IQR, 7.5–14) and 11 (IQR, 7–18) for A1 (P = .42); 7 (IQR, 4–14.5) and 10 (IQR, 5–18) for M1 (P = .50); 117 (IQR, 93–136.5) and 117 (IQR, 97–140) for IICA (P = .68); and 22 (IQR, 12.5–33) and 29 (IQR, 13–49) for VBS (P = .11), respectively. No correlation was found between age and the TI: the correlation coefficient was −0.03 for A1 (P = .73), 0.05 for M1 (P = .62), 0.06 for IICA (P = .50), and 0.06 for VBS (P = .54). Similar results were obtained when assessing each group of patients separately.
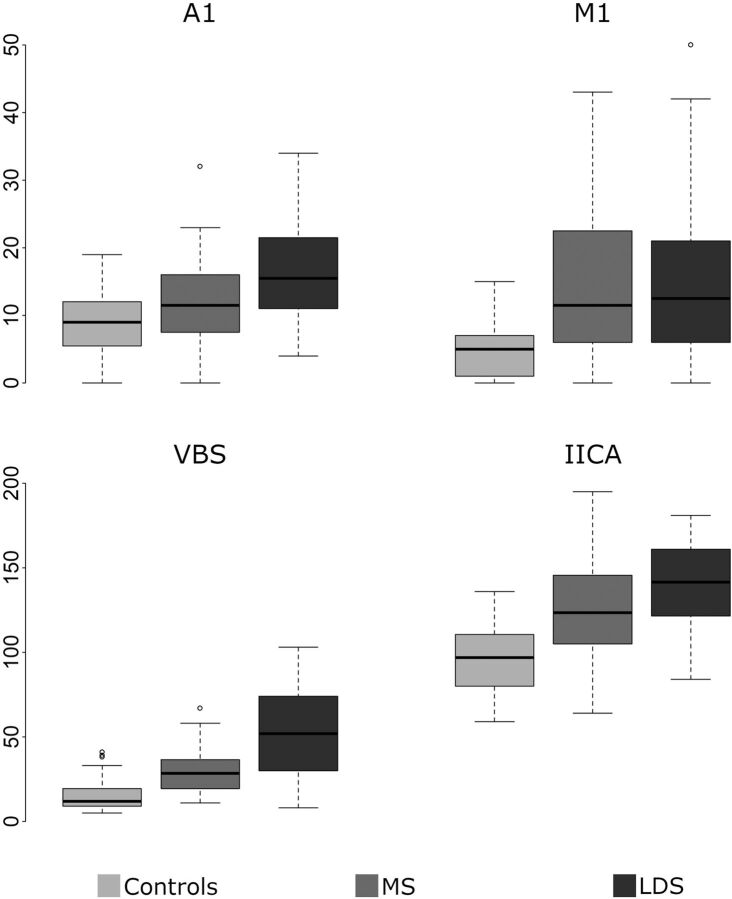
Association between the TI and CTD
Among the patients with LDS, no significant association was found between intracranial arterial tortuosity and gene mutation; therefore, we considered these patients as a single group for further analysis. Both Marfan syndrome and LDS were characterized by a significantly higher intracranial TI compared with controls. In addition, the A1 TI and VBS TI were significantly higher in patients with LDS compared with patients with Marfan syndrome (Table 2 and Fig 2). When considering only the 59 patients with both CTD and aortic root dilation (according to Hager nomograms19), patients with LDS demonstrated a significantly higher TI compared with patients with Marfan syndrome for A1 (16 [IQR, 11–22] versus 11 [IQR, 8–16]; P = .02), IICA (145 [IQR, 125.5–164] versus 125 [IQR, 110–143]; P = .01), and VBS (59 [IQR, 48–80] versus 29 [IQR, 20–36]; P < .001). Examples of different degrees of tortuosity of the IICA and VBS are reported in Fig 3. A multivariate logistic regression model, including the TI of A1 and VBS as well as age confirmed the VBS TI to be the strongest independent predictor of LDS in patients with CTD (OR 1.68 [95% CI, 1.25–2.44]) (Table 3).
Table 2:
The intracranial TI in patients with CTD and the controls
| Intracranial TI, median (IQR) |
P |
|||||
|---|---|---|---|---|---|---|
| Controls (n = 52) | Marfan Syndrome (n = 36) | LDS (n = 32) | Marfan Syndrome vs Controls | LDS vs Controls | LDS vs Marfan Syndrome | |
| A1 | 9 (6–12) | 11.5 (8–16) | 15.5 (11–21) | .02 | <.001 | .02 |
| M1 | 5 (1–7) | 11.5 (6–22) | 12.5 (6–21) | <.001 | <.001 | .92 |
| IICA | 97 (80.5–110) | 123.5 (105–144) | 141.5 (123–160.5) | <.001 | <.001 | .06 |
| VBS | 12 (9–19) | 28.5 (20–36) | 52 (32–73.5) | <.001 | <.001 | <.001 |
FIG 2.
Boxplots of the TI of the 4 examined arterial segments for controls and patients with Marfan syndrome (MS) and LDS.
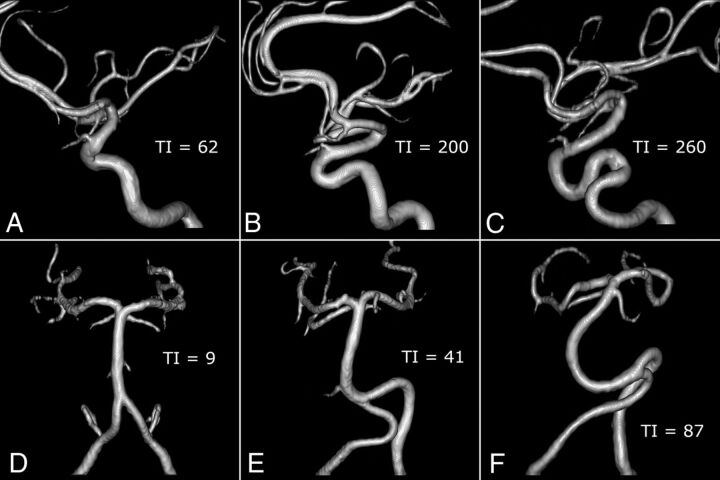
FIG 3.
Examples of different degrees of tortuosity of IICA and VBS. A–C, Lateral view of the right IICA in a control (A), in a patient with Marfan syndrome (B), and in a patient with LDS (C). D–F, Anterior view of VBS in a control (D), in a patient with MS (E), and in a patient with LDS (F).
Table 3:
Multivariate model for LDS versus Marfan syndrome by TI and age
Computed for a 10-unit increase of the variable.
Specificity and Sensitivity Analysis
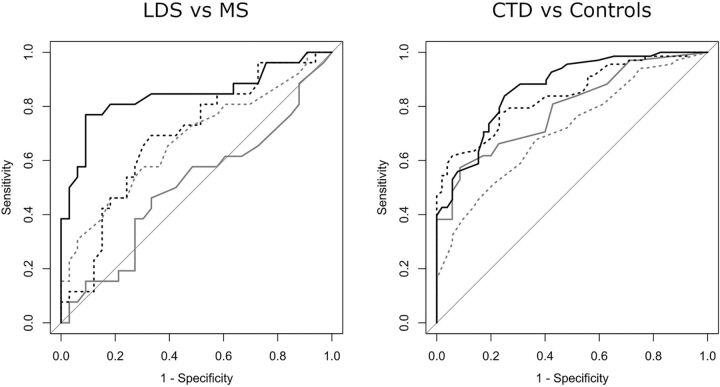
Receiver operating characteristic curve analysis of the intracranial TI for the binary outcome of LDS versus Marfan syndrome showed the VBS TI to be the best classifier, with an area under the curve of 0.84 (Fig 4). The “optimum” cutoff value of the VBS TI for maximizing sensitivity and specificity was 48, which yielded a sensitivity of 77% (95% CI, 56%–91%) and a specificity of 91% (95% CI, 76%–98%), with a positive likelihood ratio of 8.46 (95% CI, 2.82–25.40) and a negative likelihood ratio of 0.25 (95% CI, 0.12–0.52). When maximizing specificity for a value of sensitivity of at least 50%, a VBS TI > 60 returned a specificity of 97% (95% CI, 84%–100%).
FIG 4.
Receiver operating characteristic curves for the intracranial TI, with the outcome of LDS versus Marfan syndrome and CTD versus controls. Dashed gray line indicates the A1 TI; solid gray line, M1 TI; dashed black line, IICA TI; and solid black line, VBS TI.
To evaluate how the VBS TI performed in differentiating patients without discriminating features of either Marfan syndrome or LDS, we grouped patients with Marfan syndrome without ectopia lentis (n = 24 [66.6% of the patients with Marfan syndrome]) and patients with LDS without bifid uvula and/or cleft palate and hypertelorism (n = 22 [68.7% of the patients with LDS]). When applying the previously calculated cutoff of 48 for the VBS TI in this group of patients, it yielded a sensitivity of 82% (95% CI, 57%–96%) and a specificity of 86% (95% CI, 65%–97%) for the diagnosis of LDS. When analyzing the ability of the intracranial TI to discriminate between the patients with CTD and the controls, the IICA TI and VBS TI showed similar overall results, with an area under the curve of 0.84 and 0.87, respectively (Fig 4), but the IICA TI allowed for a higher specificity and the VBS TI allowed for a higher sensitivity. When maximizing specificity for a value of sensitivity of at least 50%, an IICA TI > 132 yielded a specificity of 98% (95% CI, 90%–100%). When maximizing sensitivity for a value of specificity of at least 50%, a VBS TI > 13 yielded a sensitivity of 96% (95% CI, 88%–99%).
DISCUSSION
This study demonstrates that the TI can be reliably used in the assessment of intracranial arterial tortuosity in patients with CTDs and yields a highly reproducible quantitative measure. Importantly, our findings confirmed, on a quantitative basis, the widely reported association of LDS with intracranial arterial tortuosity but also demonstrated a significantly higher tortuosity of the intracranial vasculature in patients with Marfan syndrome compared with the controls. The TI of all examined arterial segments, except for M1, showed excellent interrater reliability, with an intraclass correlation coefficient that ranged from 0.97 to 0.99. The highly variable distance of the M1–M2 bifurcation from the origin of the MCA, as well as the presence of many different branching patterns, could explain the lower interrater reliability for the M1 segment (intraclass correlation coefficient, 0.76).
Besides the TI, other quantitative methods for measuring arterial tortuosity include the sum of angles metric, defined as the sum of the deviations from the straight line (expressed in degrees) at each point of angulation normalized by the length of the vessel, and the inflection count metric, which is calculated by multiplying the length of a vessel by the number of inflection points along its path and then dividing it by the distance between its end points.1 Although these methods are more sensitive to local tortuosity and are effective for measuring vessels with high-frequency–low-amplitude coils, as in the coronary circulation, they require either to segment the vessel at each point of angulation or to identify inflection points, which makes their measurement more complex compared with the TI. In addition, these methods are mainly validated for 2D analysis.1,20
Diedrich et al21 used tortuosity score curves to measure intracranial arterial tortuosity in a heterogeneous patient population, which also included 5 patients with LDS. Compared with the TI, this method is not dependent on the choice of 2 end points along the vessel centerline and may be used to compare images that contained different lengths of the selected artery. However, to our knowledge, no commercially available software allows for calculation of tortuosity score curves, and this method, albeit giving a more comprehensive estimate of tortuosity, might have limited applicability outside the research setting for the time being.
Although previous studies showed that older age and female sex were associated with arterial tortuosity,2,4 the TI of the intracranial arteries did not differ significantly between males and females in our cohort, and we did not find any correlation between age and the TI (correlation coefficients ranged from −0.03 for A1 to 0.06 for the IICA). Even though we were not able to assess changes in intracranial tortuosity over time due to the small number of patients with serial MRA studies, the lack of a significant association between age and the TI is in agreement with previously reported data on the vertebral artery TI in the cervical region5 and may support the hypothesis of a relative stability of the head and neck artery TIs over time in young patients. Although a lack of association between older age and increased arterial tortuosity is in agreement with previous studies on CTD,5-7 this finding cannot be generalized to older patients due to the relatively young age of the subjects included in this study as well as in other studies that focused on patients with CTD. We were not able to evaluate the association of hypertension with arterial tortuosity because all of our patients were treated with antihypertensive drugs, either angiotensin II receptor blockers or β-blockers. Current guidelines recommend blood pressure control in individuals with Marfan syndrome and those with LDS to reduce hemodynamic stress and the aortic root dilation rate.12,22
Intracranial arterial tortuosity is a frequently reported neuroradiologic manifestation of LDS14-18 but has never been described in Marfan syndrome. A possible explanation could be found in the lower degree of tortuosity in Marfan syndrome compared with LDS, which may not be conspicuous enough to be detected by qualitative assessment. On the contrary, quantitative analysis found a significantly higher intracranial TI in patients with Marfan syndrome compared with the controls. This finding is consistent with the increased tortuosity of the cervical vertebral arteries previously reported in patients with Marfan syndrome5 and seems to suggest the presence of a more widespread arterial tortuosity in this syndrome, which goes beyond the aorta.7,23
The presence of arterial tortuosity in both LDS and Marfan syndrome could be the expression of underlying vessel wall abnormality.11,12,24 In fact, these 2 conditions share a common pathogenic mechanism because the causative mutations of both Marfan syndrome and LDS affect the transforming growth factor beta signaling pathway, which results in a disturbance in biogenesis and maintenance of elastic fiber, which, in turn, could lead to vessel wall weakening and clinical vascular manifestations.11,24,25
Increased arterial tortuosity has been associated with reduced perfusion pressure in different vascular territories, including the posterior circulation,26,27 but no data are available on the association between this arterial phenotype and cerebral blood flow in patients with CTD. Although it is possible that the intracranial arterial tortuosity observed in this patient population might have an impact on cerebral perfusion, the potential clinical implications of this association have not been elucidated.
We did not find any significant difference in the examined clinical variables between patients with Marfan syndrome and patients with LDS, except for a higher prevalence of peripheral artery dissection in the latter (Table 1). This result is in contrast with early reports of LDS, which describe a more severe clinical course in this patient population compared with Marfan syndrome.8,9 Nonetheless, our findings are congruent with more recent studies,5 a possible explanation being the wide phenotypic variability of patients with LDS, even among individuals who belong to the same family12,28 as well as the tendency of early reports to characterize the most severe cases.
Although previous studies showed higher arterial tortuosity of head and neck arteries in patients with intracranial aneurysm,20,29,30 we did not find any significant difference in tortuosity between the patients with LDS and with or without intracranial aneurysm. This finding may suggest a difference in some of the underlying mechanisms of aneurysm formation in patients with CTD compared with the general population, but further studies, including greater numbers of patients with CTD and intracranial aneurysm, are needed to further elucidate these results. Our results show that the intracranial TI could be helpful in the differential diagnosis between Marfan syndrome and LDS. The VBS TI is the strongest independent predictor of LDS (Table 3) and yields the best results in classifying these 2 entities compared with other arterial segments (Fig 2), which demonstrates a 97% specificity for LDS when its value is > 60.
No formal diagnostic criteria have been established for the diagnosis of LDS, which is currently based on the presence of a mutation in one of the known LDS genes in a patient with aortic aneurysms or dissection.13 Nevertheless, some of these patients may not undergo genetic testing due to a misdiagnosis of Marfan syndrome. According to the Revised Ghent Nosology,31 Marfan syndrome can be diagnosed without genetic testing in the presence of aortic root dilation (Z > 2) and either ectopia lentis or a systemic score of >7 if no discriminating feature of LDS or other CTDs are present. Although ectopia lentis is not reported in LDS, many of the features included in the systemic score can also be found among these patients, including scoliosis, pes planus, anterior chest deformity, spontaneous pneumothorax, joint hyperextension, mitral valve prolapse, and dural ectasia.12,13 In addition, craniofacial anomalies that are absent in Marfan syndrome, such as bifid uvula, cleft palate, and hypertelorism, are not always found in patients with LDS.9
In our cohort, 24 patients with Marfan syndrome (66.6%) did not show ectopia lentis and 22 patients with LDS (68.7%) had neither bifid uvula and/or cleft palate nor hypertelorism. The VBS TI also demonstrated high sensitivity and specificity in this group of patients. In the absence of ectopia lentis, an increased VBS TI, therefore, should prompt genetic testing, even if characteristic craniofacial features are lacking, to avoid misdiagnosis of patients with LDS. All the examined arterial segments show a significantly higher tortuosity in both Marfan syndrome and LDS compared with the controls. An increased intracranial TI, therefore, may raise the suspicion for CTD in the appropriate clinical setting. IICA and the VBS TI demonstrated similar overall results in classifying patients and controls, but the IICA TI showed a higher specificity for CTD, which reached 98% for values > 132.
Limitations
The present study has some limitations, including its retrospective nature and the small number of enrolled patients. In addition, the study design did not allow estimating CTD prevalence, which thus precluded computation of positive and negative predictive values. Because our control group had similar demographic characteristics to the CTD group and included only healthy subjects, without cardiovascular risk factors, our data on intracranial tortuosity cannot be generalized to older individuals or those with comorbidities, for example, hypertension, because these factors are known to be associated with increased vascular tortuosity. Our sample is limited to Marfan syndrome and LDS, therefore, our results need to be validated in larger prospective studies, enrolling a larger number of subjects and also including patients with other CTDs.
CONCLUSIONS
Our results confirmed a significantly increased tortuosity of the intracranial vasculature in LDS compared with Marfan syndrome and controls but also demonstrated that patients with Marfan syndrome have significantly more tortuous intracranial arteries compared with healthy subjects, which has not been previously reported. The TI of the intracranial arteries is an easily calculated and highly reproducible measure, which shows a high specificity for CTD and may raise the suspicion for the diagnosis of Marfan syndrome or LDS in the appropriate clinical setting as well as helping in differentiating between these 2 entities.
ABBREVIATIONS:
- CTD
connective tissue disease
- IICA
intracranial internal carotid artery
- IQR
interquartile range
- LDS
Loeys-Dietz syndrome
- TI
tortuosity index
- VBS
vertebrobasilar system
References
- 1.Ciurică S, Lopez-Sublet M, Loeys BL, et al. Arterial tortuosity. Hypertension 2019;73:951–60 10.1161/HYPERTENSIONAHA.118.11647 [DOI] [PubMed] [Google Scholar]
- 2.Del Corso L, Moruzzo D, Conte B, et al. Tortuosity, kinking, and coiling of the carotid artery: expression of atherosclerosis or aging? Angiology 1998;49:361–71 10.1177/000331979804900505 [DOI] [PubMed] [Google Scholar]
- 3.Pancera P, Ribul M, Presciuttini B, et al. Prevalence of carotid artery kinking in 590 consecutive subjects evaluated by echocolordoppler. Is there a correlation with arterial hypertension? J Intern Med 2000;248:7–12 10.1046/j.1365-2796.2000.00611.x [DOI] [PubMed] [Google Scholar]
- 4.Cha KS, Kim MH, Kim HJ. Prevalence and clinical predictors of severe tortuosity of right subclavian artery in patients undergoing transradial coronary angiography. Am J Cardiol 2003;92:1220–22 10.1016/j.amjcard.2003.07.038 [DOI] [PubMed] [Google Scholar]
- 5.Morris SA, Orbach DB, Geva T, et al. Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation 2011;124:388–96 10.1161/CIRCULATIONAHA.110.990549 [DOI] [PubMed] [Google Scholar]
- 6.Chu LC, Haroun RR, Beaulieu RJ, et al. Carotid artery tortuosity index is associated with the need for early aortic root replacement in patients with Loeys-Dietz syndrome. J Comput Assist Tomogr 2018;42:747–53 10.1097/RCT.0000000000000764 [DOI] [PubMed] [Google Scholar]
- 7.Welby JP, Kim ST, Carr CM, et al. Carotid artery tortuosity is associated with connective tissue diseases. AJNR Am J Neuroradiol 2019;40:1738–43 10.3174/ajnr.A6218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loeys BL, Chen J, Neptune ER, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005;37:275–81 10.1038/ng1511 [DOI] [PubMed] [Google Scholar]
- 9.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006;355:788–98 10.1056/NEJMoa055695 [DOI] [PubMed] [Google Scholar]
- 10.Beyens A, Albuisson J, Boel A, et al. Arterial tortuosity syndrome: 40 new families and literature review. Genet Med 2018;20:1236–45 10.1038/gim.2017.253 [DOI] [PubMed] [Google Scholar]
- 11.Morris SA. Arterial tortuosity in genetic arteriopathies. Curr Opin Cardiol 2015;30:587–93 10.1097/HCO.0000000000000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacCarrick G, Black JH III, Bowdin S, et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med 2014;16:576–87 10.1038/gim.2014.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meester JAN, Verstraeten A, Schepers D, et al. Differences in manifestations of Marfan syndrome, Ehlers-Danlos syndrome, and Loeys-Dietz syndrome. Ann Cardiothorac Surg 2017;6:582–94 10.21037/acs.2017.11.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson PT, Chen JK, Loeys BL, et al. Loeys-Dietz syndrome: MDCT angiography findings. AJR Am J Roentgenol 2007;189:W29–35 10.2214/AJR.06.1316 [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues VJ, Elsayed S, Loeys BL, et al. Neuroradiologic manifestations of Loeys-Dietz syndrome type 1. AJNR Am J Neuroradiol 2009;30:1614–19 10.3174/ajnr.A1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Laer L, Dietz H, Loeys B. Loeys-Dietz syndrome In: Halper J, eds. Progress in Heritable Soft Connective Tissue Diseases. Dordrecht: Springer-Verlag; 2014:95–105 [Google Scholar]
- 17.Kim ST, Brinjikji W, Lanzino G, et al. Neurovascular manifestations of connective-tissue diseases: a review. Interv Neuroradiol 2016;22:624–37 10.1177/1591019916659262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spinardi L, Mariucci E, Vornetti G, et al. High prevalence of arterial dissection in patients with Loeys–Dietz syndrome and cerebral aneurysm. Vasc Med 2020;25:218–20 10.1177/1358863X19900923 [DOI] [PubMed] [Google Scholar]
- 19.Hager A, Kaemmerer H, Rapp-Bernhardt U, et al. Diameters of the thoracic aorta throughout life as measured with helical computed tomography. J Thorac Cardiovasc Surg 2002;123:1060–66 10.1067/mtc.2002.122310 [DOI] [PubMed] [Google Scholar]
- 20.Kliś KM, Krzyżewski RM, Kwinta BM, et al. Computer-aided analysis of middle cerebral artery tortuosity: association with aneurysm development. J Neurosurg 2019;130:1478–84 10.3171/2017.12.JNS172114 [DOI] [PubMed] [Google Scholar]
- 21.Diedrich KT, Roberts JA, Schmidt RH, et al. Medical record and imaging evaluation to identify arterial tortuosity phenotype in populations at risk for intracranial aneurysms. AMIA Annu Symp Proc 2011;2011:295–304 [PMC free article] [PubMed] [Google Scholar]
- 22.Brooke BS, Habashi JP, Judge DP, et al. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008;358:2787–95 10.1056/NEJMoa0706585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kono AK, Higashi M, Morisaki H, et al. High prevalence of vertebral artery tortuosity of Loeys-Dietz syndrome in comparison with Marfan syndrome. Jpn J Radiol 2010;28:273–77 10.1007/s11604-010-0420-6 [DOI] [PubMed] [Google Scholar]
- 24.Pepe G, Giusti B, Sticchi E, et al. Marfan syndrome: current perspectives. Appl Clin Genet 2016;9:55–65 10.2147/TACG.S96233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franken R, El Morabit A, de Waard V, et al. Increased aortic tortuosity indicates a more severe aortic phenotype in adults with Marfan syndrome. Int J Cardiol 2015;194:7–12 10.1016/j.ijcard.2015.05.072 [DOI] [PubMed] [Google Scholar]
- 26.Vorobtsova N, Chiastra C, Stremler MA, et al. Effects of vessel tortuosity on coronary hemodynamics: an idealized and patient-specific computational study. Ann Biomed Eng 2016;44:2228–39 10.1007/s10439-015-1492-3 [DOI] [PubMed] [Google Scholar]
- 27.Peng YF, Zhang HL, Zhang DP, et al. Perfusion by delayed time to peak in vertebrobasilar dolichoectasia patients with vertigo. Ann Clin Transl Neurol 2018;5:1562–73 10.1002/acn3.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jondeau G, Ropers J, Regalado E, et al. International Registry of Patients Carrying TGFBR1 or TGFBR2 Mutations: results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet 2016;9:548–58 10.1161/CIRCGENETICS.116.001485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labeyrie P-E, Braud F, Gakuba C, et al. Cervical artery tortuosity is associated with intracranial aneurysm. Int J Stroke 2017;12:549–52 10.1177/1747493016687577 [DOI] [PubMed] [Google Scholar]
- 30.Kim BJ, Lee SH, Kwun BD, et al. Intracranial aneurysm is associated with high intracranial artery tortuosity. World Neurosurg 2018;112:e876–80 10.1016/j.wneu.2018.01.196 [DOI] [PubMed] [Google Scholar]
- 31.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet 2010;47:476–85 10.1136/jmg.2009.072785 [DOI] [PubMed] [Google Scholar]