Abstract
The proinflammatory chemokine interleukin-32 is related to various diseases, including cancer. However, it has never been associated with bladder cancer (BC). To detect whether there is a relationship between the IL-32 gene polymorphisms (rs12934561 C/T and rs28372698 T/A) and BC, the study enrolled 170 non-muscle-invasive bladder cancer (NMIBC) patients, 151 muscle-invasive bladder cancer (MIBC) patients, and 437 healthy controls. The polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method was used for the IL-32 single-nucleotide polymorphism (SNP) genotyping. Statistical analysis was performed using SNPstats online analysis software and SPSS software. Our data revealed that the CC homozygous genotype of rs12934561 in BC patients was significantly higher than that in controls (P = 0.03, OR = 1.47, 95%CI = 1.04‐2.08), and the percentage of TC genotype carriers was relatively less than that of controls (P = 0.001, OR = 0.61, 95%CI = 0.45‐0.82). Furthermore, the TT homozygous genotype of rs28372698 was associated with a significantly lower overall survival rate in MIBC patients (P = 0.028, OR = 2.77, 95%CI = 1.11‐6.90). The IL-32 gene polymorphism rs12934561 might be associated with increased BC risk, and the rs28372698 might participate in the prognosis of BC patients. Therefore, they could be potential forecasting factors for the prognosis of MIBC patients.
1. Introduction
Bladder cancer (BC) is the tenth most common cancer according to the International Agency for Research on Cancer (IARC), with 549,393 new cases worldwide in 2018 (1). Seventy-five percent of the total burden occurs in men, and 60% of the incidence rate and 50% of the mortality rate occur in the less developed regions of the world. In 2018, 82,270 new cases and 38,208 deaths were recorded in China, which revealed an estimated increase of 30,000 cases and 20,000 deaths compared with the data of 2012 (1). According to these reports, only about 20% of BC patients have muscle-invasive bladder cancer (MIBC), which is responsible for most of the cancer-specific deaths. The remaining 80% of the patients present with non-muscle-invasive bladder cancer (NMIBC) (1–3).
Common BC risk factors include tobacco smoking and exposure to industrial paints, petroleum products, and other chemical carcinogens (2–4). However, in recent years, increasing evidence has demonstrated a genetic predisposition towards it (5). Furthermore, the first-degree relatives of BC patients have a twofold higher risk of developing BC, showing that genetic factors play a crucial role in the initiation and progression of this disease.
Interleukin-32, a proinflammatory cytokine, was first detected as the product of natural killer cell transcript 4 (NK4) in 1992 (6) and was officially renamed as IL-32 by Kim et al. in 2005 (7). Its encoding gene IL-32 is located on the human chromosome 16p13.3, is approximately 1,200 bp full-length, and consists of eight exons (6). IL-32 is mainly produced by activated T cells, NK cells, epithelial cells, and blood monocytes (7), and it has nine splice variants IL-32α, IL-32β, IL-32γ, IL-32δ, IL-32ε, IL-32θ, IL-32ζ, IL-32η, and IL-32small (IL-32sm) (8, 9). IL-32 has been implicated in many inflammatory diseases and cancers, including rheumatoid arthritis (10), chronic obstructive pulmonary disease (COPD) (11), lymphoma (12), head and neck squamous cell carcinoma (HNSCC) (13), thyroid cancer (TC) (14), hepatocellular carcinoma (HCC) (15), lung cancer (LC) (16–18), esophageal cancer (19), gastric cancer (GC) (20, 21), pancreatic cancer (22), colorectal cancer (CRC) (23, 24), renal cell carcinoma (RCC) (25), breast cancer (26), and endometrial cancer (EC) (27).
Recently, several reports have clearly indicated that two single nucleotide polymorphisms (SNPs) in the IL-32 gene sequence (rs12934561 and rs28372698) were associated with cancer susceptibility (LC, GC, TC, EC, and CRC) (14, 16, 24, 27–29). However, no relationship has been established between IL-32 and BC. Therefore, we selected these two SNPs (rs12934561 and rs28372698) of IL-32 to determine their differences in BC patients and healthy controls in the Chinese Han population.
2. Material
2.1. Participants' Clinical Characteristics
A case-control study which enrolled 321 unrelated BC individuals (mean ± SD: 63.82 ± 12.17 years (NMIBC group: 62.14 ± 12.87 years; MIBC group: 65.70 ± 11.06 years)) and 437 healthy controls (mean ± SD: 63.86 ± 6.94 years) was approved by the hospital ethics committee, and informed consent was provided by all the participants. The subjects were from the West China Hospital of Sichuan University between 2007 and 2012. All participants with personal or family history of BC or other severe diseases such as other types of cancers, or those who had undergone radiotherapy or chemotherapy, were excluded from the study. Patients' clinical and follow-up data were collected every 6 months for 5 years by telephone calls. All tumor tissues resected from BC patients were confirmed by histopathological analysis, and the clinical characteristics are summarized in Table 1. All of the participants were genetically unrelated individuals of the Han population living in the Sichuan province of China.
Table 1.
Characteristics of the study population.
| Characteristics | NMIBC group | MIBC group | Controls |
|---|---|---|---|
| Sample size | 170 | 151 | 437 |
| Sex | |||
| Male | 131 (77.1%) | 122 (80.8%) | 336 (76.9%) |
| Female | 39 (22.9%) | 29 (19.2%) | 101 (23.1%) |
| Age at first diagnosis (mean ± SD) | 62.14 ± 12.87 | 65.70 ± 11.06 | 63.86 ± 6.94 |
| Smoking status | |||
| Smokers | 85 (50.0%) | 82 (54.3%) | 199 (45.5%) |
| Nonsmokers | 85 (50.0%) | 69 (45.7%) | 238 (54.5%) |
| Clinical stage | |||
| Ta | 10 (5.9%) | — | — |
| T1 | 160 (94.1%) | — | — |
| T2 | — | 89 (58.9%) | — |
| T3a | — | 34 (22.5%) | — |
| T3b | — | 17 (11.3%) | — |
| T4 | — | 11 (7.3%) | — |
| Tumor grade | |||
| Low grade | 114 (67.1%) | 23 (15.2%) | — |
| High grade | 56 (32.9%) | 128 (84.8%) | — |
2.2. Genotyping
As shown in Table 2, polymerase chain reaction (PCR) primers of the two SNPs were designed using Primer 3 web version 4.1.0. (http://primer3.ut.ee/) (30). The genetic DNA of each individual was extracted from a 200 μL EDTA-anticoagulated peripheral blood sample using a DNA isolation kit from BioTeke (Peking, China). Genotyping was performed using PCR-restriction fragment length polymorphism (PCR-RFLP). The DNA fragments that contained the polymorphisms were amplified in a volume of 10 μL, including 100 ng extracted genomic DNA, 2.7 picomole primers of each SNP, and 5 μL 2x power Taq PCR Master Mix (BioTeke, Peking, China). The PCR annealing temperature was 60°C for 30 s. After PCR termination, the products were digested by a restriction enzyme, as shown in Table 2, and the digested fragments were separated on a 6% polyacrylamide gel and stained with 1.5 g/L of argent nitrate. Finally, DNA sequencing analysis was used to confirm the genotypes, and approximately 10% of the randomly selected samples were 100% in agreement with the results after performing the repeated assays.
Table 2.
Primer sequences for genotyping two SNPs in the IL-32 gene.
| SNP ID | Primer sequence | Restriction enzyme | Allele (bp) |
|---|---|---|---|
| rs12934561 | F: 5′-GGCCTCACTCCTCACACAGT-3′ | Hpy188III | C (20 + 155) |
| R: 5′-CCCACAGGTGTTGGTTTCC-3′ | T (175) | ||
| rs28372698 | F: 5′-GTCAGAAGGACCTGGTCAGC-3′ | Hpy188III | A (115) |
| R: 5′-GTTGGAGGGGTGGCTAGTC-3′ | T (21 + 94) |
2.3. Statistical Analysis
The SNPstats online analysis software was used to evaluate the genotypic association, including the codominant, dominant, recessive, and overdominant genetic models (31), and the Hardy-Weinberg equilibrium was calculated using the chi-squared test. The effects of different genotypes and alleles were evaluated by odds ratio (OR) and respective 95% confidence intervals (95% CI). Kaplan-Meier univariate analysis plots and Cox regression multivariate survival analysis model were used to estimate the relationships of IL-32 genotypes with patient outcomes. The level of significance was set at P < 0.05.
3. Results
3.1. Susceptibility between the IL-32 Genotypes and BC
The genotype distributions of these two SNPs follow the Hardy-Weinberg equilibrium (P > 0.05) in our groups. The effects of IL-32 genotypes and allele frequencies on BC patients are presented in Table 3. As shown, for rs12934561, the homozygous genotype (CC) in the recessive genetic model was significantly higher in BC patients than that in controls (24.6% vs. 18.1%, P = 0.03, OR = 1.47, 95%CI = 1.04‐2.08), indicating an increased risk for BC susceptibility. Compared with the TT/CC genotypes, the TC genotype was associated with a lower risk for BC in the overdominant model (P = 0.001, OR = 0.61, 95%CI = 0.45‐0.82). No significant differences were observed between BC susceptibility and the rs28372698 genotype or allele distribution.
Table 3.
Distribution of SNPs in IL-32 among patients and controls and their association with bladder cancer risk.
| rs28372698 | rs12934561 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | Genotype | Patients N (%) | Controls N (%) | OR (95% CI) | P | Genotype | Patients N (%) | Controls N (%) | OR (95% CI) | P |
| Codominant | AA | 144 (44.9%) | 215 (49.2%) | 1.00 (reference) | TT | 127 (39.6%) | 151 (34.5%) | 1.00 (reference) | ||
| AT | 147 (45.8%) | 193 (44.2%) | 1.15 (0.85-1.54) | 0.24 | TC | 115 (35.8%) | 207 (47.4%) | 0.65 (0.47-0.90) | 0.004 | |
| TT | 30 (9.3%) | 29 (6.6%) | 1.59 (0.91-2.78) | CC | 79 (24.6%) | 79 (18.1%) | 1.18 (0.79-1.75) | |||
| Dominant | AA | 144 (44.9%) | 215 (49.2%) | 1.00 (reference) | TT | 127 (39.6%) | 151 (34.5%) | 1.00 (reference) | ||
| AT/TT | 177 (55.1%) | 222 (50.8%) | 1.20 (0.90-1.61) | 0.21 | TC/CC | 194 (60.4%) | 286 (65.5%) | 0.79 (0.59-1.08) | 0.14 | |
| Recessive | AA/AT | 291 (90.7%) | 408 (93.4%) | 1.00 (reference) | TT/TC | 242 (75.4%) | 358 (81.9%) | 1.00 (reference) | ||
| TT | 30 (9.3%) | 29 (6.6%) | 1.47 (0.86-2.50) | 0.15 | CC | 79 (24.6%) | 79 (18.1%) | 1.47 (1.04-2.08) | 0.03 | |
| Overdominant | AA/TT | 174 (54.2%) | 244 (55.8%) | 1.00 (reference) | TT/CC | 206 (64.2%) | 230 (52.6%) | 1.00 (reference) | ||
| AT | 147 (45.8%) | 193 (44.2%) | 1.08 (0.80-1.43) | 0.64 | TC | 115 (35.8%) | 207 (47.4%) | 0.61 (0.45-0.82) | 0.001 | |
| Allele | ||||||||||
| A | 435 (67.8) | 623 (71.3) | 1.18 (0.95-1.47) | 0.14 | T | 369 (57.5) | 509 (58.2) | 1.03 (0.84-1.27) | 0.77 | |
| T | 207 (32.2) | 251 (28.7) | C | 273 (42.5) | 365 (41.8) | |||||
N corresponds to the number of individuals. Boldfaced values indicate a significant difference at the 5% level.
3.2. Clinical Characteristics
To gain further insights into the relationship between these two SNPs of IL-32 and BC, patients with different genotypes were stratified by mean age (≤64 and >64 years old), sex (male and female), smoking status (smokers and nonsmokers), tumor grade (low-grade and high-grade), and tumor stage (Ta-T1 and T2-T4) (Supplementary Table 1). However, no significant relationship was detected for any subgroup of the SNPs after adjusting for common risk factors (P > 0.05).
3.3. The Effects of IL-32 SNP Genotypes on Patient Outcome
During the follow-up, all of the involved BC patients were tracked every six months. At the end of our study, 50 patients (15.6%, NMIBC: 13 cases, MIBC: 37 cases) died of BC and 95 patients (29.6%, NMIBC: 48 cases, MIBC: 47 cases) relapsed. Following the stratification of patients by tumor stage (MIBC and NMIBC), we conducted Kaplan-Meier survival analyses and multivariate Cox survival analyses; the associations between SNPs of IL-32 and BC patient outcomes are summarized in Table 4.
Table 4.
Association between SNPs in IL-32 and patient outcome.
| SNP/genotype | NMIBC | MIBC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alive/dead, N | HR (95% CI)a | P | Recurrence/nonrecurrence | HR (95% CI)a | P | Alive/dead, N | HR (95% CI)a | P | Recurrence/nonrecurrence | HR (95% CI)a | P | |
| rs28372698 | ||||||||||||
| AA | 68/7 | 52/23 | 57/12 | 50/19 | ||||||||
| AT | 72/6 | 59/19 | 50/19 | 46/23 | ||||||||
| TT | 17/0 | 11/6 | 7/6 | 8/5 | ||||||||
| Dominant | 0.66 (0.22-1.98) | 0.46 | 0.72 (0.40-1.28) | 0.26 | 1.83 (0.92-3.65) | 0.09 | 1.54 (0.85-2.78) | 0.16 | ||||
| Recessive | NA | 0.98 | 1.57 (0.66-3.76) | 0.31 | 2.77 (1.11-6.90) | 0.028 | 2.06 (0.79-5.36) | 0.14 | ||||
| Overdominant | 0.86 (0.29-2.59) | 0.79 | 0.60 (0.33-1.09) | 0.09 | 1.22 (0.64-2.32) | 0.56 | 1.24 (0.70-2.21) | 0.46 | ||||
| rs12934561 | ||||||||||||
| TT | 64/5 | 46/23 | 45/13 | 40/18 | ||||||||
| TC | 61/3 | 47/17 | 38/13 | 39/12 | ||||||||
| CC | 32/5 | 29/8 | 31/11 | 25/17 | ||||||||
| Dominant | 0.92 (0.29-2.95) | 0.89 | 0.70 (0.39-1.26) | 0.23 | 1.24 (0.61-2.53) | 0.55 | 0.91 (0.49-1.68) | 0.76 | ||||
| Recessive | 1.92 (0.61-6.08) | 0.27 | 0.57 (0.27-1.24) | 0.16 | 0.95 (0.46-1.93) | 0.88 | 1.46 (0.80-2.68) | 0.22 | ||||
| Overdominant | 0.47 (0.13-1.76) | 0.27 | 1.03 (0.56-1.89) | 0.92 | 1.32 (0.66-2.67) | 0.43 | 0.60 (0.31-1.18) | 0.14 | ||||
N corresponds to the number of individuals. aAdjusted by age, sex, and smoking status. Boldfaced values indicate a significant difference at the 5% level.
Kaplan-Meier plots indicated a significantly worse prognosis of MIBC patients carrying the TT homozygous genotype of IL-32 rs28372698 compared to that of AA or AT genotypes (log-rank test: P = 0.015, Figure 1; P = 0.025, Figure 2). Furthermore, as shown in Table 4, the multivariate survival analyses reiterated that the TT genotype carriers (P = 0.028, OR (95%CI) = 2.77 (1.11-6.90)) had a worse overall survival rate in MIBC patients after adjustment for age, sex, and smoking status. However, no significant relationship was detected between the overall survival rate and another SNP (rs12934561) or between these two SNPs and the recurrence-free survival rate.
Figure 1.
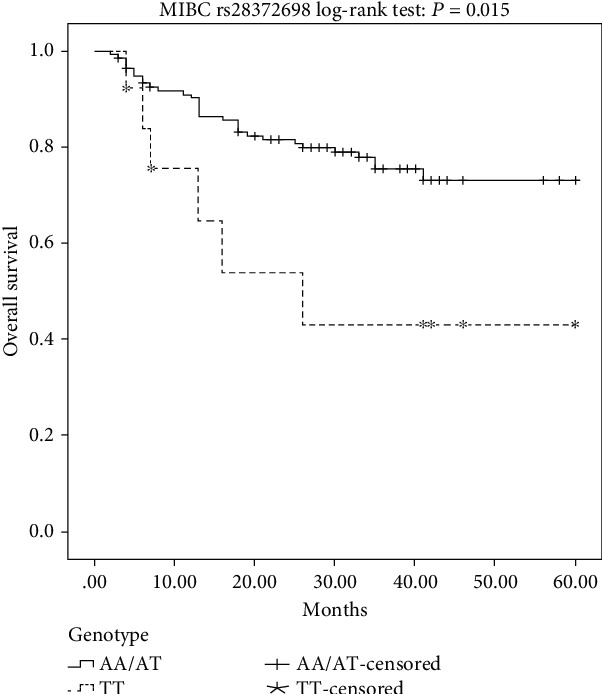
Kaplan-Meier overall survival curves for all of the analyzed MIBC patients categorized by IL-32 rs28372698 in the recessive genetic models.
Figure 2.
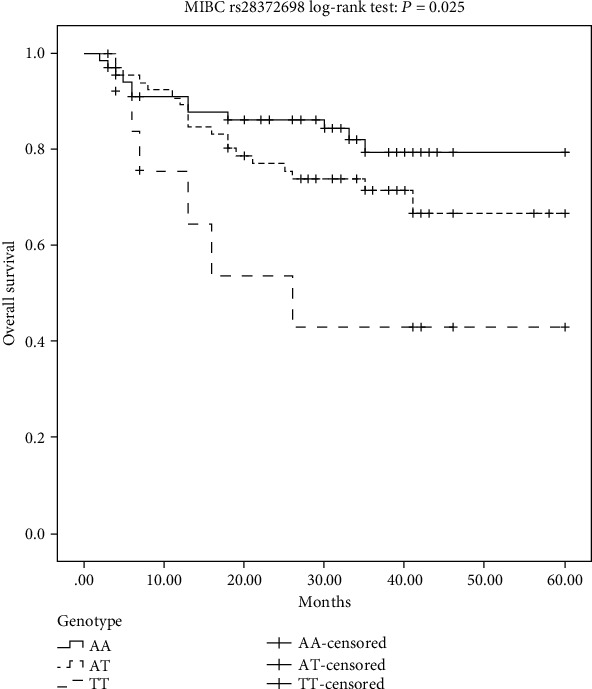
Kaplan-Meier overall survival curves for all of the analyzed MIBC patients categorized by IL-32 rs28372698 in the codominant genetic models.
4. Discussion
Proinflammatory cytokines, such as tumor necrosis factor α (TNF-α), interleukin-8 (IL-8), interleukin-6 (IL-6), and interleukin-1β (IL-1β), could be induced by IL-32, which is often associated with inflammatory and oncogenic diseases (7, 32–34). However, no homologous relationship has been found between the structural basis of IL-32 and the known cytokines, and no extracellular signaling receptor of IL-32 1has been detected until now (7, 35, 36). IL-32 has nine splice variants, and all of the isoforms present differences in secondary structures, which lead to the variant tertiary protein structure and protein function (37).
Substantial reports have shown that IL-32 has different roles in various situations and pathways. Mabilleau and Sabokbar reported that IL-32 is capable of inducing a strong activation of ERK1/2 and Akt signaling and stimulating the release of interleukin-4 (IL-4) and interferon-γ (IFN-γ) in osteoclast formation and activation (38). The transcriptional coactivator p300 (EP300) and death-associated protein kinase-1 (DAPK-1) were found to occupy the inflammatory network nodes of IL-32, which affect both TNF-receptor 1-dependent and TNF-receptor 1-independent pathways (39). Yousif et al. demonstrated that IL-32 is associated with NF-κB and p38 MAPK pathways in esophageal tumors in vivo (19), whereas in vitro, Oh et al. found that it is involved in the NF-κB-STAT3 signaling pathway in colon cancer cells (40). Park et al. suggested that IL-32β could increase the invasion and migration of breast cancer through the EGFR-STAT3 pathway (26).
Several studies on the SNP of IL-32 in cancer have been reported in recent years, and IL-32 has even been linked to the patient outcome in some cancers. Our data revealed that the CC genotype of rs12934561 in IL-32 was associated with an increased risk of BC, which is consistent with findings of previous studies in which the CC genotype was shown to relate closely with an increased susceptibility in lung cancer and endometrial cancer (16, 27). Moreover, Wang et al. showed that lung squamous carcinoma patients with the TT genotype of rs12934561 present a relatively poor survival rate compared with that of other patients (16). However, in our study, we demonstrated that the TC heterozygotes of rs12934561 are associated with a decreased risk of BC, which might be caused by the variant effects and functional pathways of nine isoforms in different organizations.
The rs28372698 T/A genetic variants were located on the 5′-UTR in the promoter region of IL-32. Plantinga et al. investigated a cohort of 139 TC patients and 138 healthy controls who carried the rs28372698 T/A genetic variants, revealing an increased risk of TC in patients with genetic variants of IL-32. Those patients required higher doses of cumulative radioactive iodine (RAI) to achieve successful tumor remission (14). Gonzalez-Hormazabal et al. used a combined attribute network implemented in multifactor dimensionality reduction software to analyze the gene-gene interactions between IL-8-251 A>T and IL-32 rs28372698 T/A, and their results showed that the homozygote for both IL-8-251 T and IL-32 rs28372698 T alleles presents a 2.63-fold risk in the developing gastric cancer (29). Furthermore, in moderate and well-differentiated lung cancer, the T allele of rs28372698 is associated with a poor prognosis (16), which is consistent with our data. In our study, the TT genotype of rs28372698 in IL-32 was associated with a lower overall survival rate of MIBC patients, which indicated that IL-32 might be a potential biomarker for the prognosis of BC.
5. Conclusions
In conclusion, to the best of our knowledge, this is the first study to demonstrate the relationship between IL-32 and BC. The results indicate that SNP rs12934561 may be a potential risk factor for BC processes, and SNP rs28372698 is a significant forecast factor for BC prognosis. Nevertheless, our study has some limitations in terms of sample size and in the absence of the expression level of IL-32 in participants. The types and frequencies of genetic polymorphisms in variant ethnic populations differ, whereas only a cohort of southwest China was genotyped in this study. Thus, further studies in different populations and with larger sample sizes are required to reveal the potential function and mechanism of IL-32 in BC and to confirm these findings.
Data Availability
The data used to support the findings of this study are currently under embargo while the research findings are commercialized. Requests for data, 6 months after publication of this article, will be considered by the corresponding authors.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Authors' Contributions
Jie Yang and Zhongyu Jian contributed equally to this work.
Supplementary Materials
Supplementary Table 1: association between SNPs in IL-32 and patient's characteristics.
References
- 1.Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2015. CA: A Cancer Journal for Clinicians. 65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M., Böhle A., Burger M., et al. EAU Guidelines on Non–Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. European Urology. 71(3):447–461. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Burger M., Catto J. W. F., Dalbagni G., et al. Epidemiology and risk factors of urothelial bladder cancer. European Urology. 63(2):234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Freedman N. D., Silverman D. T., Hollenbeck A. R., Schatzkin A., Abnet C. C. Association between smoking and risk of bladder cancer among men and women. JAMA. 306(7):737–745. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volanis D., Kadiyska T., Galanis A., Delakas D., Logotheti S., Zoumpourlis V. Environmental factors and genetic susceptibility promote urinary bladder cancer. Toxicology Letters. 193(2):131–137. doi: 10.1016/j.toxlet.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Dahl C. A., Schall R. P., He H. L., Cairns J. S. Identification of a novel gene expressed in activated natural killer cells and T cells. Journal of Immunology. 1992;148:597–603. [PubMed] [Google Scholar]
- 7.Kim S.-H., Han S.-Y., Azam T., Yoon D.-Y., Dinarello C. A. Interleukin-32: A Cytokine and Inducer of TNFα. Immunity. 22(1):131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Sohn D. H., Nguyen T. T., Kim S., et al. Structural characteristics of seven IL-32 variants. Immune Network. 2019;19(2, article e8) doi: 10.4110/in.2019.19.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sloot Y. J. E., Smit J. W., Joosten L. A. B., Netea-Maier R. T. Insights into the role of IL-32 in cancer. Seminars in Immunology. 38:24–32. doi: 10.1016/j.smim.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Joosten L. A. B., Netea M. G., Kim S.-H., et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proceedings of the National Academy of Sciences. 103(9):3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calabrese F., Baraldo S., Bazzan E., et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2008;178(9):894–901. doi: 10.1164/rccm.200804-646OC. [DOI] [PubMed] [Google Scholar]
- 12.Suga H., Sugaya M., Miyagaki T., et al. The role of IL-32 in cutaneous T-cell lymphoma. Journal of Investigative Dermatology. 134(5):1428–1435. doi: 10.1038/jid.2013.488. [DOI] [PubMed] [Google Scholar]
- 13.Guenin S., Mouallif M., Hubert P., et al. Interleukin-32 expression is associated with a poorer prognosis in head and neck squamous cell carcinoma. Molecular Carcinogenesis. 53(8):667–673. doi: 10.1002/mc.21996. [DOI] [PubMed] [Google Scholar]
- 14.Plantinga T. S., Costantini I., Heinhuis B., et al. A promoter polymorphism in human interleukin-32 modulates its expression and influences the risk and the outcome of epithelial cell-derived thyroid carcinoma. Carcinogenesis. 2013;34(7):1529–1535. doi: 10.1093/carcin/bgt092. [DOI] [PubMed] [Google Scholar]
- 15.Kang Y. H., Park M.-Y., Yoon D.-Y., et al. Dysregulation of overexpressed IL-32α in hepatocellular carcinoma suppresses cell growth and induces apoptosis through inactivation of NF-κB and Bcl-2. Cancer Letters. 318(2):226–233. doi: 10.1016/j.canlet.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Yang Y., Zhu Y., Li L., Chen F., Zhang L. Polymorphisms and expression of IL-32: impact on genetic susceptibility and clinical outcome of lung cancer. Biomarkers. 2017;22(2):165–170. doi: 10.1080/1354750X.2016.1252956. [DOI] [PubMed] [Google Scholar]
- 17.Sorrentino C., Di Carlo E. Expression of IL-32 in human lung cancer is related to the histotype and metastatic phenotype. American Journal of Respiratory and Critical Care Medicine. 180(8):769–779. doi: 10.1164/rccm.200903-0400oc. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Q., Li S., Zhou Y., et al. Interleukin-32 contributes to invasion and metastasis of primary lung adenocarcinoma via NF-kappaB induced matrix metalloproteinases 2 and 9 expression. Cytokine. 65(1):24–32. doi: 10.1016/j.cyto.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Yousif N. G., Al-amran F. G., Hadi N., Lee J., Adrienne J. Expression of IL-32 modulates NF-κB and p38 MAP kinase pathways in human esophageal cancer. Cytokine. 61(1):223–227. doi: 10.1016/j.cyto.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Pavlovic M., Gajovic N., Jurisevic M., et al. Diverse expression of IL-32 in diffuse and intestinal types of gastric cancer. Gastroenterology Research and Practice. 2018;2018:9. doi: 10.1155/2018/6578273.6578273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishigami S., Arigami T., Uchikado Y., et al. IL-32 expression is an independent prognostic marker for gastric cancer. Medical Oncology. 30(2) doi: 10.1007/s12032-013-0472-4. [DOI] [PubMed] [Google Scholar]
- 22.Nishida A., Andoh A., Inatomi O., Fujiyama Y. Interleukin-32 expression in the pancreas. The Journal of Biological Chemistry. 2009;284(26):17868–17876. doi: 10.1074/jbc.M900368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y., Wang Z., Zhou Y., Wang X., Xiang J., Chen Z. Dysregulation of over-expressed IL-32 in colorectal cancer induces metastasis. World Journal of Surgical Oncology. 13(1) doi: 10.1186/s12957-015-0552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shamoun L., Kolodziej B., Andersson R. E., Dimberg J. Protein expression and genetic variation of IL32 and association with colorectal cancer in Swedish patients. Anticancer Research. 2018;38(1):321–328. doi: 10.21873/anticanres.12225. [DOI] [PubMed] [Google Scholar]
- 25.Lee H.-J., Liang Z. L., Huang S. M., et al. Overexpression of IL-32 is a novel prognostic factor in patients with localized clear cell renal cell carcinoma. Oncology Letters. doi: 10.3892/ol.2011.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park J. S., Choi S. Y., Lee J. H., et al. Interleukin-32β stimulates migration of MDA-MB-231 and MCF-7cells via the VEGF-STAT3 signaling pathway. Cellular Oncology (Dordrecht) 2013;36(6):493–503. doi: 10.1007/s13402-013-0154-4. [DOI] [PubMed] [Google Scholar]
- 27.Yu X., Zhou B., Zhang Z., et al. Significant association between IL-32 gene polymorphisms and susceptibility to endometrial cancer in Chinese Han women. Tumour Biology. 2015;36(7):5265–5272. doi: 10.1007/s13277-015-3186-8. [DOI] [PubMed] [Google Scholar]
- 28.GONZALEZ-HORMAZABAL P. A. T. R. I. C. I. O., ROMERO S. A. N. D. R. A., MUSLEH M. A. H. E. R., et al. IL-8-251T>A (rs4073) polymorphism is associated with prognosis in gastric cancer patients. Anticancer Research. 38(10):5703–5708. doi: 10.21873/anticanres.12907. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Hormazabal P., Musleh M., Bustamante M., et al. Role of cytokine gene polymorphisms in gastric cancer risk in Chile. Anticancer Research. 34(7):3523–3530. [PubMed] [Google Scholar]
- 30.Untergasser A., Cutcutache I., Koressaar T., et al. Primer3new capabilities and interfaces. Nucleic Acids Research. 40(15):p. e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sole X., Guino E., Valls J., Iniesta R., Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 22(15):1928–1929. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
- 32.Sunaga N., Imai H., Shimizu K., et al. Oncogenic KRAS-induced interleukin-8 overexpression promotes cell growth and migration and contributes to aggressive phenotypes of non-small cell lung cancer. International Journal of Cancer. 130(8):1733–1744. doi: 10.1002/ijc.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan N. J., Sasser A. K., Axel A. E., et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 28(33):2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y., Wang L., Pappan L., Galliher-Beckley A., Shi J. IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Molecular Cancer. 11(1):p. 87. doi: 10.1186/1476-4598-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong J. T., Son D. J., Lee C. K., Yoon D. Y., Lee D. H., Park M. H. Interleukin 32, inflammation and cancer. Pharmacology & therapeutics. 2017;174:127–137. doi: 10.1016/j.pharmthera.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 36.Joosten L. A. B., Heinhuis B., Netea M. G., Dinarello C. A. Novel insights into the biology of interleukin-32. Cellular and Molecular Life Sciences. 70(20):3883–3892. doi: 10.1007/s00018-013-1301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinhuis B., Koenders M. I., van den Berg W. B., Netea M. G., Dinarello C. A., Joosten L. A. B. Interleukin 32 (IL-32) contains a typical α-helix bundle structure that resembles focal adhesion targeting region of focal adhesion kinase-1. Journal of Biological Chemistry. 287(8):5733–5743. doi: 10.1074/jbc.m111.288290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mabilleau G., Sabokbar A. Hartl D., editor. Interleukin-32 promotes osteoclast differentiation but not osteoclast activation. PLoS ONE. 4(1):p. e4173. doi: 10.1371/journal.pone.0004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner-Brannen E., Choi K.-Y. G., Arsenault R., El-Gabalawy H., Napper S., Mookherjee N. Inflammatory cytokines IL-32 and IL-17 have common signaling intermediates despite differential dependence on TNF-receptor 1. The Journal of Immunology. 186(12):7127–7135. doi: 10.4049/jimmunol.1002306. [DOI] [PubMed] [Google Scholar]
- 40.Oh J. H., Cho M.-C., Kim J.-H., et al. IL-32γ inhibits cancer cell growth through inactivation of NF-κB and STAT3 signals. Oncogene. 30(30):3345–3359. doi: 10.1038/onc.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: association between SNPs in IL-32 and patient's characteristics.
Data Availability Statement
The data used to support the findings of this study are currently under embargo while the research findings are commercialized. Requests for data, 6 months after publication of this article, will be considered by the corresponding authors.


