Abstract
Background
Digital technology is an opportunity for public health interventions to reach a large part of the population.
Objective
This systematic literature review aimed to assess the effectiveness of mobile health–based interventions in reducing the risk of cardiovascular disease and type 2 diabetes mellitus.
Methods
We conducted the systematic search in 7 electronic databases using a predefined search strategy. We included articles published between inception of the databases and March 2019 if they reported on the effectiveness of an intervention for prevention of cardiovascular disease or type 2 diabetes via mobile technology. One researcher performed the search, study selection, data extraction, and methodological quality assessment. The steps were validated by the other members of the research team
Results
The search yielded 941 articles for cardiovascular disease, of which 3 met the inclusion criteria, and 732 for type 2 diabetes, of which 6 met the inclusion criteria. The methodological quality of the studies was low, with the main issue being nonblinding of participants. Of the selected studies, 4 used SMS text messaging, 1 used WhatsApp, and the remaining ones used specific smartphone apps. Weight loss and reduction in BMI were the most reported successful outcomes (reported in 4 studies).
Conclusions
Evidence on the effectiveness of mobile health-based interventions in reducing the risk for cardiovascular disease and type 2 diabetes is low due to the quality of the studies and the small effects that were measured. This highlights the need for further high-quality research to investigate the potential of mobile health interventions.
Trial Registration
International Prospective Register of Systematic Reviews (PROSPERO) CRD42019135405; https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=135405
Keywords: systematic review; mobile health; telemedicine; primary prevention; cardiovascular diseases; diabetes mellitus, type 2
Introduction
Description of the Condition
Worldwide, chronic diseases are the main cause of death and years lived with disability [1,2]. Cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) are globally among the top 5 chronic conditions in terms of incidence and prevalence [2]. The behavioral risk factors for these conditions, such as smoking, harmful use of alcohol, poor diet, and physical inactivity, are highly correlated with the disease progression [3]. For example, Gellert et al [4] observed in their meta-analysis a dose-response relationship between the number of cigarettes smoked and premature death. They also found an inverse correlation between time since cessation and all-cause mortality. Wood et al [5] reported that all-cause mortality was positively associated with the level of alcohol intake, based on data from over half a million current drinkers. Chudasama et al [6] found a negative dose-response relationship between physical activity levels and all-cause mortality in their analysis of almost half a million people. Regarding low whole-grain intake, which is the highest risk factor related to poor diet, in their meta-analysis, Zhang et al [7] showed an inverse dose-response relationship between whole-grain intake and all-cause mortality. Hence, targeting these with preventive measures could significantly reduce people’s chronic disease risk [8], and behavior change interventions are well suited for preventing CVD and T2DM [2,3].
Description of the Intervention
To stop noncommunicable diseases from rising further, the World Health Organization (WHO) developed the Global Action Plan 2013-2020 [8]. In this report, the WHO emphasized the importance of early screening and the implementation of preventive programs. Further, the WHO recommended the use of information and communication technologies, such as the internet and mobile phone technologies, to deliver health education and promotion programs. In 2019, the WHO released a guideline with recommendations on digital interventions for health system strengthening [9]. This report outlined how the implementation of technology could overcome current challenges in health care systems and help to achieve the goal of universal health coverage. Health apps have promising potential. Wilson [10] pointed out that digital health interventions have the advantage of being easily accessible and cost-effective. According to the Pew Research Center [11], many people use their smartphones daily. Riley et al [12] reported that new advancements allow apps to be tailored to personal needs and preferences, as well as the integration of dynamic feedback systems. Despite the promising potential of health apps, there is still ambiguity about their effectiveness, as outlined by the WHO guideline [9].
Objective
The aim of this systematic literature review was to assess the current evidence regarding the effectiveness of mobile health–based interventions in reducing the risk for CVD and T2DM. The focus was on multiple behavioral risk–factor interventions, rather than single risk–factor interventions, because of the lack of evidence on their combined effectiveness compared with substantial evidence on single risk–factor interventions [13,14].
Methods
Review Standards
We conducted this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [15] and registered it with International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42019135405).
Search Strategy
We searched the following medical and bioengineering databases to retrieve all relevant articles regarding preventive mobile health intervention for CVD and T2DM: EMBASE (via Ovid), Scopus, ScienceDirect, CINAHL (via EBSCOhost), MEDLINE (via Ovid), ProQuest science and technology databases, and Ei Compendex and Inspec (both via Engineering Village 2). The search strategy (Multimedia Appendix 1) included terms relating to the 2 conditions under study and the intervention; we combined the terms using Boolean operators [16] and adapted the terms to the database-specific requirements. The search included articles published from the inception of the databases until March 25, 2019. We limited the search to English- and German-language publications because these languages were proficiently spoken by the review team. We excluded review articles, conference abstracts, comments, editorials, letters to the editor, and theses. Additionally, we identified studies using “snowballing” techniques by reviewing the reference lists of articles included in the initial search and searching for other publications by authors included in the initial search [17].
Study Selection
Inclusion Criteria
The study selection followed predefined inclusion criteria according to the PICOS system (Table 1). After removing duplicate publications, we reviewed all retrieved articles for eligibility, first by examining the titles and abstracts, and then the full articles if we considered the articles to be relevant in the first step. We included in the review full articles that met the inclusion and exclusion criteria. The steps described above were performed by 1 researcher (VHB). For the title and abstract screening, a 10% random sample of all retrieved articles was validated by a second researcher (shared between the remaining researchers). If discrepancies occurred, a third researcher resolved the issue. A second researcher (SL) independently assessed which of the full articles fulfilled the inclusion and exclusion criteria. The results were compared, and discrepancies were resolved by involving a third researcher (MB).
Table 1.
Inclusion criteria according to the PICOS system.
| Criteria | Description of inclusion criteria |
| Participants | Adults who are free of CVDa or T2DMb. |
| Intervention | Health promotion interventions that use mobile health technology (ie, mobile app or SMS text messaging) aiming to change more than 1 risk factor for 1 of the 2 chronic conditions under study. |
| Comparator | No intervention (ie, standard care), or waitlist control, or intervention delivered in person. |
| Outcome | Onset of disease (CVD or T2DM) or relative risk reduction, which can be in the form of surrogate parameters. |
| Study design | Randomized controlled trial, case-control study, or interrupted time series. |
aCVD: cardiovascular disease.
bT2DM: type 2 diabetes mellitus.
Types of Participants
Participants could either be healthy or have an increased disease risk. We excluded interventions targeting adults who were already diagnosed with CVD or T2DM (depending on the aim of the intervention, eg, for CVD prevention, people diagnosed with CVD) at baseline. Further, we excluded studies intended for minors (<18 years of age). The conditions under study were CVD and T2DM, for which we applied the following WHO definitions: CVD is a “group of disorders of heart and blood vessels,” including coronary heart disease, cerebrovascular disease, peripheral vascular disease, heart failure, rheumatic heart disease, congenital heart disease, and cardiomyopathies [18]; T2DM “is a chronic disease that occurs...when the body cannot effectively use the insulin it produces” [19].
Types of Intervention
We included primary studies if they evaluated the effectiveness of a mobile phone–based intervention for primary prevention of 1 of the conditions under study. The intervention had to be delivered, at least partially, via mobile health technology (ie, mobile app or SMS text messaging) with the aim of changing more than 1 risk factor for 1 or more of the chronic conditions under study. We defined a mobile app as a software program that can run on mobile devices such as smartphones, and a text message as a written message sent to a mobile phone. The type of interventions that we included needed to be aimed at health promotion using behavior change strategies, including counselling or education regarding disease-related knowledge, healthy diet, physical activity, smoking cessation, motivational messages, and goal setting. We excluded from the review studies that exclusively targeted 1 behavioral risk factor (eg, smoking only, diet only, or step count only).
Types of Comparator
The comparison group could consist of either no intervention (ie, standard care), or a waitlist control, or an intervention delivered in person. Studies were eligible if they included adults who were free of CVD or T2DM at study baseline, depending on the condition targeted in the study.
Types of Outcome
Studies were only eligible for inclusion if their main outcomes were disease incidence (either CVD or T2DM) or a reduction in disease risk, which could be measured using a risk prediction tool (such as the Framingham score for CVD [20]) or surrogate parameters. Examples of surrogate parameters were weight, waist circumference, blood pressure, blood glucose, level of physical activity, dietary intake, or smoking status. Additional outcomes that we included in the review were the feasibility of mobile health interventions, disease knowledge, and quality of life. Respective outcome measures included dropout rates, participants’ acceptability of and adherence to the intervention, and questionnaires assessing disease knowledge and quality of life.
Types of Study Design
We restricted the study design to randomized controlled trials (RCTs), case-control studies, and interrupted time series in order to have a measurement against which the effectiveness of the intervention could be compared.
Data Extraction and Synthesis
Relevant data (study objective, study design, study population, comparator, description of the intervention, duration of the intervention or follow-up, outcomes, main results, and methodology for the assessment of the study’s quality) were extracted by 1 researcher (VHB) using a standardized form in Excel 365 (Microsoft Corporation). This was reviewed by all the other researchers. We synthesized the main results of the included studies in a narrative manner focusing on the intervention delivery and reported outcomes. A meta-analysis was not possible due to the small number of identified studies and the heterogeneity in interventions and outcomes.
Literature Quality Assessment
One researcher (VHB) assessed the risk of bias using the following assessment tools: for RCTs, the Cochrane Collaboration’s tool for assessing risk of bias [21]; and for non-RCTs, the Risk of Bias In Non-randomized Studies - of Intervention assessment tool [22].
Results
Results of the Literature Search and Study Selection
In total, we identified 941 articles using the search strategy for CVD and 732 articles using the search strategy for T2DM. In the validation of the 10% random sample of all retrieved articles, there was a 100% agreement (after initial disagreements were resolved by a third investigator) with the selection conducted by the researcher who screened all articles. Finally, 3 CVD articles [23-25] and 6 T2DM articles [26-31] fulfilled the inclusion and exclusion criteria; we identified no additional articles through the snowballing technique (Figures 1 and 2). We excluded many articles for several of the exclusion criteria.
Figure 1.
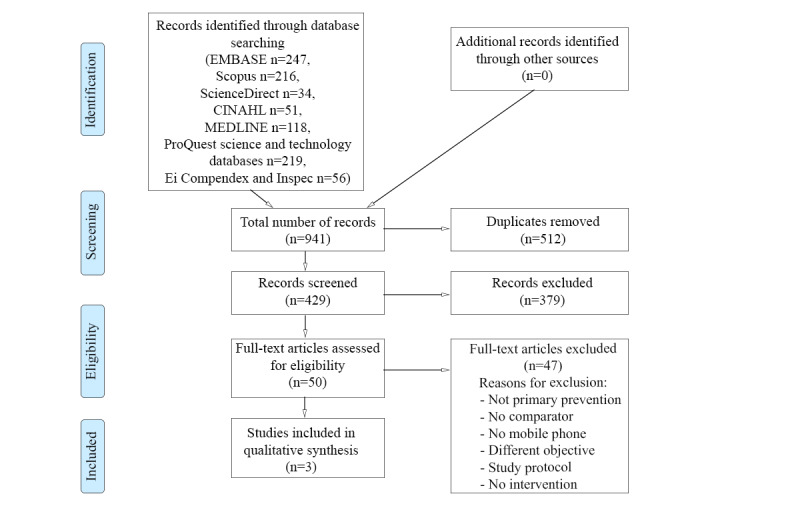
Full article selection process for cardiovascular disease.
Figure 2.
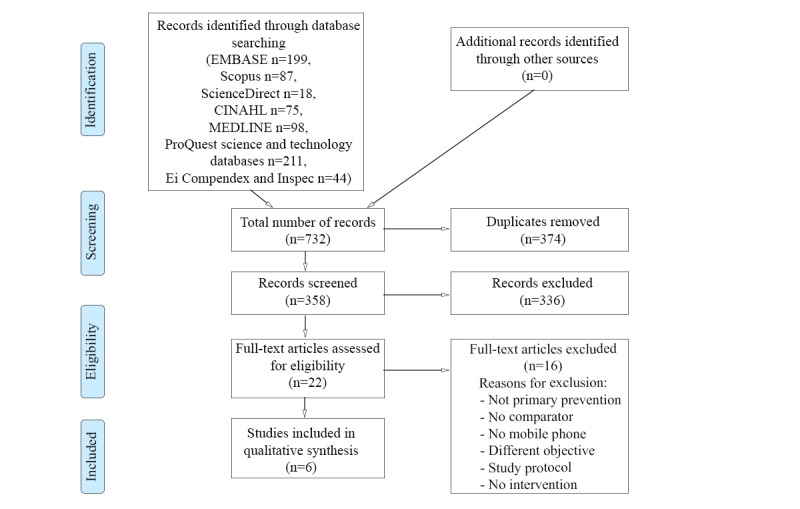
Full article selection process for type 2 diabetes.
Results of the Data Extraction
There were 3 CVD [23-25] and 6 T2DM studies [26-31]. Table 2 provides details about the CVD studies and Table 3 provides details about the T2DM studies. For each study, the table includes the first author, year of publication, study design and duration, objectives, study population, interventions and comparators, outcomes, and the main results.
Table 2.
Data extraction from cardiovascular disease (CVD) studies.
| First author, date, reference | Study design and duration; objectives | Study population | Intervention and comparator | Outcomes | Main results |
| Gore, 2019 [23] | Non-RCTa for 12 months; effectiveness of an SMS text message intervention to reduce CVD risk | Adults from the United States at high risk of CVD without preexisting coronary artery disease, cerebrovascular disease, and diabetes; intervention n=204, usual care n=408 | Create action plan with community health workers and return 6-12 months after initial screening for retesting; intervention: text messages once/day on advice on healthy eating, PAb, weight loss, contacting community health worker; control: usual care | Engagement, program retention, changes in risk factors (smoking, fat and fiber intake, PA, weight, BMI, BPc, low-density lipoprotein), Framingham risk score | Only statistically significant decrease in fat intake (intervention −26.3% vs control −10.6%; P=.001) |
| Muntaner-Mas, 2017 [24] | Non-RCT for 10 weeks; effectiveness of a WhatsApp-based PA intervention to reduce CVD risk factors | Spanish adults aged 53-73 years without medical conditions or other physical problems requiring special medical attention and who were able to perform rigorous PA; mobile group n=7, training group n=16, control n=9 | Intervention: twice/week functional fitness for training and mobile group; for training group face-to-face sessions, for mobile group training videos for download via WhatsApp, chat function plus motivational messages from study coordinator; control: no intervention | BP, WCd, waist to height ratio, weight, BMI, fat mass index, fat-free mass index, heart rate after exercise, balance, handgrip strength, aerobic capacity | No statistically significant differences between mobile group and control; statistically significant differences between training group and control group (systolic BP P=.038; diastolic BP P=.005; mean arterial BP P=.006; heart rate after exercise P=.002) |
| Rubinstein, 2016 [25] | RCT for 12 months; effectiveness of preventive mobile health intervention in adults with prehypertension | Adults aged 30-60 years with prehypertension from poor urban settings in Argentina, Guatemala, and Peru, free of hypertension, diabetes, and CVD; intervention n=316, usual care n=321 | Intervention: monthly motivational counselling calls (healthy diet and PA) followed by weekly text messages related to behavior goals and readiness to change; control: usual care | Changes in BP, weight, BMI, WC, PA, diet | Mean differences, baseline-adjusted (95% CI): weight −0.66 kg (−1.24 to −0.07), BMI −0.30 kg/m2 (−0.54 to −0.06), daily intake of high-sugar and -fat servings −0.75 (−1.30 to −0.20); change in BP not significant |
aRCT: randomized controlled trial.
bPA: physical activity.
cBP: blood pressure.
dWC: waist circumference.
Table 3.
Data extraction from type 2 diabetes mellitus (T2DM) studies.
| First author, date, reference | Study design and duration; objectives | Study population | Intervention and comparator | Outcomes | Main results |
| Arens, 2018 [26] | Non-RCTa for 12 months; effectiveness of app-based weight reduction program for people with metabolic syndrome | German adults aged 30-65 years treated for metabolic syndrome in 23 medical practices; intervention n=148, usual care n=85 | Health goals regarding weight and PAb; app for feedback; physicians with access to app data could give feedback, initiate messages, or modify goals; ≤9 free classes on diet and PA; control: usual care | 5% weight reduction; change in BMI | 5% weight reduction (adjusted for time in study) (95% CI): 44.8% (34.1 to 57.1) in intervention vs 11.5% (4.6 to 27.0) in control; Cox proportional hazard model for time to 5% weight reduction hazard ratio 6.2 (2.4 to 16.2; P<.001), baseline adjusted between groups change in weight (kg) P=.06 and BMI (kg/m2) P=.10 |
| Bender, 2018 [27] | RCT for 3 months plus 3 months follow-up (no control for follow-up); effectiveness of mobile phone-based weight loss intervention to reduce T2DM risk | Filipino-American overweight or obese adults from United States at increased risk for T2DM, able to walk 20 min; intervention n=33, control n=34 | 5 in-person sessions, daily step count via wearable device, daily food intake and weekly weight logged in app, weekly information on weight loss, PA, and diet via private Facebook page; control: waitlist | Recruitment (goal n=50), retention, 5% weight loss, changes in weight, BMI, WCc, FBGd, HbA1ce | Weight loss ≥5%: intervention 36% vs control 6%; between-group cross-level interaction (95% CI): weight −1.1%/month (−1.7 to −0.53) and −0.85 kg/month (−1.4 to −0.35), BMI −0.93 kg/m2 (−1.5 to −0.40), WC −4.9 cm (−7.5 to −2.6), FBG −1.4 mg/dL (−5.9 to 3.6), HbA1c −0.10% (−0.21 to 0.002) |
| Block, 2015 [28] | RCT for 6 months plus 6 months follow-up (no control for follow-up); effectiveness of digital health intervention for T2DM risk reduction in prediabetics | Prediabetics aged 30-69 years from United States with BMI ≥27 kg/m2, without diabetes medication; intervention n=163, control n=176 | Tailored behavioral support for PA, diet, weight loss, stress, sleep; weekly emails with goals linked to website (tracking tools, coaching, social support, competition, health advice), app and automated phone calls; control: waitlist | Decreased HbA1c, FBG, weight, BMI, WC, triglyceride to HDLf ratio, metabolic syndrome, Framingham diabetes risk score | Mean (95% CI) HbA1c −0.26% (−0.27 to −0.24) in intervention vs control −0.18% (−0.19 to −0.16), FBG −0.41 mmol/L (−0.44 to, −0.12) in intervention vs −0.21 mmol/L (−0.15 to −0.10) in control, all outcomes significantly greater in intervention than control (P<.001) |
| Fischer, 2016 [29] | RCT for 12 months; effectiveness of text message–supported T2DM prevention program | Obese and overweight adults from United States without prediabetes, English or Spanish speaking; intervention n=82, control n=81 | 6 text messages per week: skills, problem solving, motivation, stress reduction, recipes, web links to additional resources, PA promotion; weekly self-reported weight; eligible for individual motivational phone health coaching; control: usual care | Change in weight; percentage of participants with ≥3% or 5% weight loss, changes in HbA1c and systolic BPg, costs per participant | Weight (95% CI) in intervention −1.2 kg (−2.5 to 0.1) vs control −0.3 kg (−1.2 to 0.7), P=.05; 3% weight loss absolute difference between groups 17.0%, P=.02; no significant difference for 5% weight loss; HbA1c in intervention −0.09% (−0.2 to 0.0) vs control 0.19% (−0.1 to 0.5), systolic BP in intervention 0.35 mmHg (−2.8 to 3.5) vs control 6.4 mmHg (3.2 to 9.5) |
| Fukuoka, 2015 [30] | RCT for 5 months; effectiveness of mobile app-based intervention for T2DM prevention | Overweight adults aged ≥35 years from United States at high risk of diabetes; intervention n=30; control n=31 | 2-week run-in period before randomizing; all daily step count via pedometer; intervention: mobile version of Diabetes Prevention Program, 6 in-person sessions, app: diaries for self-monitoring of weight, PA, and caloric intake, daily reminders and messages; control: pedometer only | % change in weight and BMI; hip circumference, BP, lipid profile, glucose levels, step count, PA, caloric and fat intake | Weight (95% CI) −6.8% (−12.2 to −1.4) in intervention vs 0.3% (−2.7 to 3.3) in control; BMI −6.6% (−12.3 to −0.9) in intervention vs 0.3% (−2.7 to 3.3) in control; both P<.001; also significant differences in hip circumference, BP, step count, and PA for intervention vs control; no effect on lipid profile, glucose levels, caloric or fat intake |
| Ramachandran, 2013 [31] | RCT for 2 years; effectiveness of SMS text messaging to reduce incidence of T2DM in men with impaired glucose tolerance | Indian men aged 35-55 years with impaired glucose tolerance; intervention n=271, control n=266 | All at baseline: healthy lifestyle education and written information on diet and PA, lifestyle changes prescribed; intervention: frequent reinforcing text messages, content tailored to baseline behavior; control: usual care | Incidence of T2DM; BMI, WC, BP, lipid profile, energy intake, PA | Cumulative T2DM incidence: intervention 18%, control 27%; differences in mean change (95% CI): BMI −0.05 kg/m2 (−0.46 to 0.37); WC 0.04 cm (−0.56 to 0.64); systolic BP 0.04 mmHg (−0.96 to 1.03); diastolic BP −0.07 mmHg (−0.64 to 0.49); total cholesterol 0.01 mmol/L (−0.08 to 0.10); HDL 0.033 mmol/L (0.011 to 0.054); triglycerides −0.08 mmol/L (−0.17 to −0.06); energy intake –43.7 kcal (−65.5 to −22.0); PA score −1.0 (−2.0 to 0.0) |
aRCT: randomized controlled trial.
bPA: physical activity.
cWC: waist circumference.
dFBG: fasting blood glucose.
eHbA1c: glycated hemoglobin.
fHDL: high-density lipoprotein.
gBP: blood pressure.
Results of the Synthesis
Summary
We synthesized the results of the data extraction according to the PICOS system. Table 4 summarizes CVD and T2DM data individually and in total. For each parameter, we provide a count, as well as the list of relevant references.
Table 4.
Synthesis of findings.
| Finding | Cardiovascular disease | Type 2 diabetes | Total (n) | |||
| No. of studies | Reference | No. of studies | Reference | |||
| Target population | ||||||
|
|
General population | 1 | [24] | —a | — | 1 |
|
|
At risk of the disease | 2 | [23,25] | 6 | [26-31] | 8 |
| Location | ||||||
|
|
Spain | 1 | [24] | — | — | 1 |
|
|
United States | 1 | [23] | 4 | [27-30] | 5 |
|
|
Germany | — | — | 1 | [26] | 1 |
|
|
Latin America | 1 | [25] | — | — | 1 |
|
|
India | — | — | 1 | [31] | 1 |
| Intervention delivery | ||||||
|
|
SMS text messaging | 2 | [23,25] | 2 | [29,31] | 4 |
|
|
1 | [24] | — | — | 1 | |
|
|
Mobile app | — | — | 4 | [26-28,30] | 4 |
| Comparator | ||||||
|
|
Usual care | 3 | [23-25] | 3 | [26,29,31] | 6 |
|
|
Waitlist | — | — | 2 | [27,28] | 2 |
|
|
Pedometer only | — | — | 1 | [30] | 1 |
|
|
Face-to-face training | 1 | [24] | — | — | 1 |
| Outcomesb | ||||||
|
|
Weight loss | 1 | [25] | 3 | [27,28,30] | 4 |
|
|
Reduced BMI | 1 | [25] | 3 | [27,28,30] | 4 |
|
|
Reduced waist circumference | — | — | 2 | [27,28] | 2 |
|
|
Lower fasting blood glucose/glycated hemoglobin | N/Ac | — | 1 | [28] | 1 |
|
|
Improved diet | 2 | [23,25] | 1 | [31] | 3 |
|
|
Improved physical activity | — | — | 2 | [30,31] | 2 |
|
|
Improved blood pressure | — | — | 1 | [30] | 1 |
| Study design | ||||||
|
|
Randomized controlled trial | 1 | [25] | 5 | [27-31] | 6 |
|
|
Nonrandomized controlled trial | 2 | [23,24] | 1 | [26] | 3 |
a—: data not available.
bStatistically significant compared with control group.
cN/A: not applicable.
Participants
The CVD studies were conducted in Spain, the United States, and Latin America. For the T2DM studies, 1 was conducted in Germany, 1 in India, and 4 in the United States. All studies had small to medium samples, ranging from 32 to 637 participants. For CVD, 2 of the 3 studies targeted populations at higher risk of developing CVD [23,25], whereas the study by Muntaner-Mas et al [24] included healthy people. For T2DM, all studies focused on populations at increased risk of the disease.
Interventions
The duration of the interventions varied from 10 weeks to 2 years. In 4 studies the participants received text messages [23,25,29,31], in 1 study the intervention was delivered via WhatsApp [24], and in the remaining 4 studies a specifically developed mobile phone app was involved [26-28,30]. Only 1 intervention was delivered fully automated [28]; all other interventions included human involvement [23-27,29-31].
Comparators
Of the studies, 6 used usual care as the control group. In 1 trial, the control group received pedometers only [30]; 1 study had a second comparator group, additional to usual care, which received face-to-face training sessions [24]; and 2 studies used waitlist controls [27,28], meaning that the control group received the intervention after the intervention group had completed it.
Outcomes
The mobile phone interventions led to statistically significant weight loss compared with the control group in 4 studies [25,27,28,30], ranging from a difference of −0.66 kg (P=.04) over 12 months [25] to −6.2 kg for the intervention compared with 0.3 kg for the control group (P<.001) over 5 months [30]. The same studies reported a statistically significant decrease in BMI compared with the control group [25,27,28,30], ranging from a difference of −0.3 kg/m2 (P=.02) over 12 months [25] to −2.2 kg/m2 for the intervention compared with 0.1 kg/m2 for the control group (P<.001) over 5 months [30]. A smaller waist circumference due to the intervention was measured in 2 T2DM studies [27,28], from −4.56 cm for the intervention compared with −2.22 cm (P<.001) for the control group over 6 months [28] to a cross-level interaction of −4.9 cm (95% CI −7.5 to −2.6) over 3 months [27]. One T2DM study reported statistically significantly lower fasting blood glucose (−0.41 mmol/L in the intervention compared with −0.12 mmol/L in the control group; P<.001) and glycated hemoglobin levels (−0.26% in the intervention compared with −0.18% in the control group; P<.001) over 6 months [28]. Statistically significantly greater changes in the lipid profile were observed in the intervention group than in the control group in 2 of the T2DM trials [28,31], from a difference in mean change of high-density lipoprotein cholesterol of 0.033 mmol/L (95% CI 0.011 to 0.054) and triglycerides of −0.080 mmol/L (95% CI −0.17 to −0.06) over 2 years [31] to a triglyceride to high-density lipoprotein ratio of –0.21 in the intervention compared with 0.21 in the control group (P=.04) over 6 months [28]. Improved diet patterns that were statistically superior to the control group were observed in 3 studies [23,25,31], of which 2 studies aimed at CVD prevention. Improvements in physical activity were reported in 2 T2DM studies [30,31]. Blood pressure was statistically significantly improved in the intervention groups compared with the control group in 1 T2DM study [30].
Study Design
A total of 6 studies were RCTs [25,27-31]; the remaining 3 were non-RCTs [23,24,26].
Results of Literature Quality Assessment
All RCTs used acceptable methods for randomization [25,27-31], but in none of the studies were the participants blinded to the design, which is an inherent problem with this type of intervention. Figure 3 summarizes the risk of bias for the RCTs. Of the 6 studies, 3 ensured blinding of the study personnel [25,27,28] and 3 ensured the blinding of the outcome assessors [25,28,31]. Apart from the study by Fukuoka et al [30], all RCTs published study protocols on the ClinicalTrials.gov database. Overall, due to performance bias, all studies were at high risk of bias.
Figure 3.
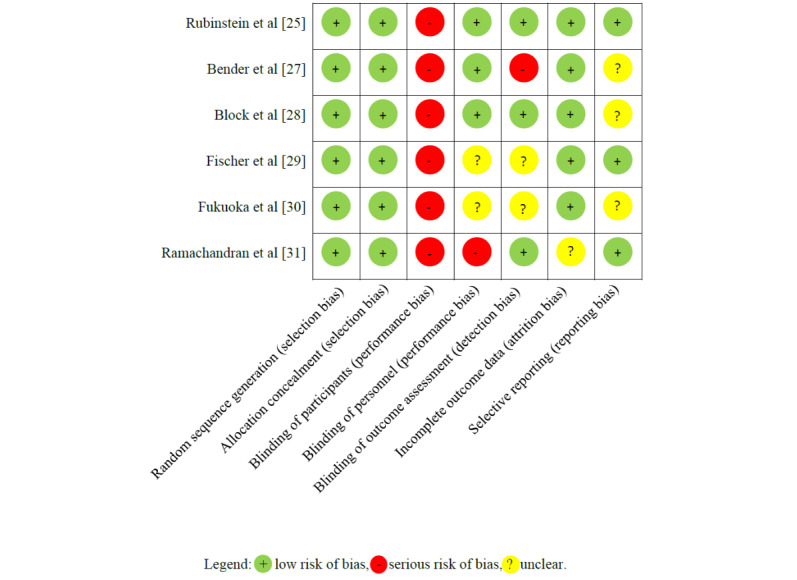
Risk-of-bias summary table for the randomized controlled trials. The upper 1 is a cardiovascular disease study and the remainder are type 2 diabetes studies.
Of the 3 non-RCTs [23,24,26], the study by Muntaner-Mas et al [24] was at moderate risk of bias, the study by Gore et al [23] was at high risk of bias, and the study by Arens et al [26] was at critical risk of bias. Figure 4 summarizes the risk of bias for the non-RCTs. The biggest issue with the study by Arens et al [26] was that missing data were not handled adequately, putting the study at critical risk of bias. We assessed the study by Gore et al [23] to be at high risk of confounding because some of the measurements that were used to control for confounding were based on nonvalidated questionnaires.
Figure 4.
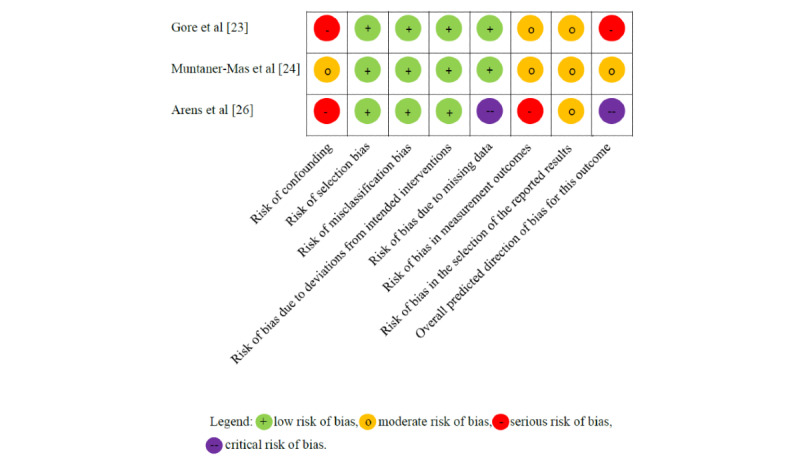
Risk-of-bias summary table for the nonrandomized controlled trials. The upper 2 are cardiovascular disease studies and the lower 1 is a type 2 diabetes study.
Discussion
Principal Findings
We identified only a small number (n=9) of articles that fulfilled the preset inclusion and exclusion criteria. We assessed most of the studies to be at high risk of bias. Additionally, 3 studies were underpowered (sample size <100), and 2 studies had short follow-up times (<6 months). Ideally, to show the effectiveness in reducing the risk of CVD or T2DM, the studies should have reported disease incidence rates. The only study that did this was that by Ramachandran et al [31], with their primary outcome being a decrease of T2DM incidence due to the SMS text messaging intervention over 2 years. Block et al [28] reported the percentage of people with metabolic syndrome as defined by the International Diabetes Federation Task Force on Epidemiology and Prevention [32]. Block et al [28] also measured change in the Framingham 8-year diabetes risk score [33], and Gore et al [23] measured change in the Framingham 10-year CVD risk score [20]. All other studies reported single risk factors rather than multivariable absolute risk of disease. None of the identified studies directly targeted tobacco smoking cessation or responsible alcohol intake. Rubinstein et al [25] mentioned in their article that their original protocol included both lifestyle factors, but they were later excluded. According to the authors, alcohol intake was considered a sensitive matter requiring face-to-face interactions, while tobacco smoking was excluded because supposedly, compared with physical activity and diet, it had less effect on the onset of hypertension and was more difficult to target via a mobile health intervention [25]. Overall, there were some positive findings suggesting that mobile health-based interventions can achieve at least small to moderate reductions in CVD and T2DM risk, although these were based on weak evidence.
Strengths and Limitations
The strength of this literature review was that it followed the PRISMA statement. We systematically searched several databases to identify all relevant published articles. Further, we conducted a manual search through the snowballing technique. For the title and abstract screening, a 10% random sample of all retrieved articles was validated by a second researcher, and 2 reviewers independently performed the full article selection. However, only 1 researcher conducted the database search, the data extraction, and the risk-of-bias assessment. Although we a priori restricted the search to English- and German-language articles, we did not exclude any articles because they were not available in these 2 languages. We did not perform a meta-analysis due to the small number of publications that met the inclusion criteria and the differences in their interventions and outcome measures.
Comparison With Prior Work
Previous mobile health research has focused more on self-management of chronic diseases than on prevention. In their systematic review and meta-analysis, Wu et al [34] investigated the effectiveness of mobile phone apps for diabetes self-management (including prediabetes, gestational diabetes, type 1 diabetes, and T2DM). They identified 3 studies that targeted prediabetes, 2 of which we also included in this review. The overall conclusion of Wu et al [34] was that there was evidence for the effectiveness of app interventions in T2DM self-management, but not for prediabetes. Lunde et al [35] conducted a systematic review looking at various types of noncommunicable diseases and lifestyle advice. Most of the identified studies (8 out of 9) targeted T2DM patients for whom the authors measured improvements in lifestyle factors, particularly reduced glycated hemoglobin levels (in 5 of the 8 studies). For CVD patients, Lunde et al [35] found only 2 relevant articles, and these were without statistically significant improvements in any of the outcomes of interest (weight, BMI, waist circumference, physical activity, and quality of life). A systematic review by Coorey et al [36] focused on self-management of CVD via mobile apps, in which they concluded that short-term improvements in behavior and risk factors were possible but there was insufficient evidence for long-term effects. Alessa et al [37] reported from their systematic review of 21 studies that mobile apps could reduce blood pressure, although the evidence originated mainly from studies that had a high risk of bias.
Palmer et al [14] conducted a systematic review of noncommunicable disease prevention through smoking cessation, alcohol reduction, physical activity, and diet using mobile technology. In total, they found 71 articles, but only 2 of the studies were aimed at the combination of physical activity, diet, and smoking cessation, with both studies targeting secondary prevention of CVD. Among the studies they reviewed, 8 RCTs focused on alcohol reduction but did not include any other lifestyle advice, with these studies specifically targeting heavy drinkers [14]. In general, it appears that many interventions are designed to provide advice for 1 or 2 behavioral risk factors that are associated with increased chronic disease risk, whereas there were only a few evaluation studies of comprehensive mobile health interventions addressing the 4 common behavioral risk factors (ie, tobacco smoking, excessive alcohol consumption, physical inactivity, and poor diet) [14]. Noble et al [13] stated in their systematic review that there were clustering patterns between the 4 behavioral risk factors—tobacco smoking, excessive alcohol consumption, physical inactivity, and poor diet—which indicated similar or the same reasons causing these behaviors. Hence, the authors suggested that future interventions should apply a holistic approach instead of targeting single risk factors. Similarly, Geller et al [38] called for future research studies to focus on improved lifestyles, meaning a change in multiple health behaviors rather than 1, even if it might be harder to achieve. Meader et al [39] reported in their systematic review that targeting smoking simultaneously with other behaviors resulted in negative outcomes for diet and physical activity, suggesting that it might be more beneficial to apply a sequential approach. In a Cochrane review published in 2016, Whittaker et al [40] stated that studies have demonstrated that mobile phone-based interventions can be effective in achieving smoking cessation over 6 months, particularly SMS text messaging in high-income countries.
Implications and Future Directions
Most studies that were conducted according to the review’s inclusion criteria were at high risk of bias. This review only considered studies of multirisk factor interventions, which resulted in only 9 studies being included. There is a lack of research evaluating interventions that address the 4 common behavioral risk factors (ie, tobacco smoking, excessive alcohol consumption, physical inactivity, and poor diet) in a single mobile health intervention. Researchers may have preferred to focus on 1 risk factor at a time due to simplicity for participants and clarity of intervention-outcome relationships. Hence, future studies should further explore the use of mobile technology for primary disease prevention, by applying a rigorous study design.
Conclusions
According to the findings of this systematic review, evidence for the effectiveness of mobile health-based interventions in reducing the risk of CVD and T2DM is scarce due to the quality of the included studies and the small effects that were measured. This highlights the need for further high-quality research to investigate the potential of mobile health interventions.
Acknowledgments
This research was supported by a joint stipend from the University of New South Wales and the Commonwealth Scientific and Industrial Research Organisation.
Abbreviations
- CVD
cardiovascular disease
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
- RCT
randomized controlled trial
- T2DM
type 2 diabetes mellitus
- WHO
World Health Organization
Appendix
Search strategy.
Footnotes
Authors' Contributions: VHB participated in research design, data collection, data analysis, and writing of the manuscript. SL participated in data collection, data analysis, and revision of the manuscript. MV, MB, and MH contributed to research design, data collection, data analysis, and revision of the manuscript. All authors provided final approval of the version to be published.
Conflicts of Interest: None declared.
References
- 1.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018 Nov 10;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018 Dec 10;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018 Nov 10;392(10159):1923–1994. doi: 10.1016/S0140-6736(18)32225-6. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gellert C, Schöttker B, Brenner H. Smoking and all-cause mortality in older people: systematic review and meta-analysis. Arch Intern Med. 2012 Jun 11;172(11):837–44. doi: 10.1001/archinternmed.2012.1397. [DOI] [PubMed] [Google Scholar]
- 5.Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M, Burgess S, Bell S, Astle W, Stevens D, Koulman A, Selmer RM, Verschuren WMM, Sato S, Njølstad I, Woodward M, Salomaa V, Nordestgaard BG, Yeap BB, Fletcher A, Melander O, Kuller LH, Balkau B, Marmot M, Koenig W, Casiglia E, Cooper C, Arndt V, Franco OH, Wennberg P, Gallacher J, de la Cámara AG, Völzke H, Dahm CC, Dale CE, Bergmann MM, Crespo CJ, van der Schouw YT, Kaaks R, Simons LA, Lagiou P, Schoufour JD, Boer JMA, Key TJ, Rodriguez B, Moreno-Iribas C, Davidson KW, Taylor JO, Sacerdote C, Wallace RB, Quiros JR, Tumino R, Blazer DG, Linneberg A, Daimon M, Panico S, Howard B, Skeie G, Strandberg T, Weiderpass E, Nietert PJ, Psaty BM, Kromhout D, Salamanca-Fernandez E, Kiechl S, Krumholz HM, Grioni S, Palli D, Huerta JM, Price J, Sundström J, Arriola L, Arima H, Travis RC, Panagiotakos DB, Karakatsani A, Trichopoulou A, Kühn T, Grobbee DE, Barrett-Connor E, van Schoor N, Boeing H, Overvad K, Kauhanen J, Wareham N, Langenberg C, Forouhi N, Wennberg M, Després J, Cushman M, Cooper JA, Rodriguez CJ, Sakurai M, Shaw JE, Knuiman M, Voortman T, Meisinger C, Tjønneland A, Brenner H, Palmieri L, Dallongeville J, Brunner EJ, Assmann G, Trevisan M, Gillum RF, Ford I, Sattar N, Lazo M, Thompson SG, Ferrari P, Leon DA, Smith GD, Peto R, Jackson R, Banks E, Di Angelantonio E, Danesh J, Emerging Risk Factors Collaboration/EPIC-CVD/UK Biobank Alcohol Study Group Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018 Dec 14;391(10129):1513–1523. doi: 10.1016/S0140-6736(18)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chudasama YV, Khunti KK, Zaccardi F, Rowlands AV, Yates T, Gillies CL, Davies MJ, Dhalwani NN. Physical activity, multimorbidity, and life expectancy: a UK Biobank longitudinal study. BMC Med. 2019 Jun 12;17(1):108. doi: 10.1186/s12916-019-1339-0. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-019-1339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang B, Zhao Q, Guo W, Bao W, Wang X. Association of whole grain intake with all-cause, cardiovascular, and cancer mortality: a systematic review and dose-response meta-analysis from prospective cohort studies. Eur J Clin Nutr. 2018 Dec;72(1):57–65. doi: 10.1038/ejcn.2017.149. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . Global action plan for the prevention and control of noncommunicable diseases 2013-2020. Geneva, Switzerland: World Health Organization; 2013. [2019-12-19]. https://apps.who.int/iris/bitstream/handle/10665/94384/9789241506236_eng.pdfjsessionid=75D5722BE3A73FAE254F414C58825563?sequence=1. [Google Scholar]
- 9.World Health Organization . WHO guideline recommendations on digital interventions for health system strengthening. Geneva, Switzerland: World Health Organization; 2019. [2019-12-19]. https://apps.who.int/iris/bitstream/handle/10665/311941/9789241550505-eng.pdf?ua=1. [PubMed] [Google Scholar]
- 10.Wilson K. Mobile cell phone technology puts the future of health care in our hands. CMAJ. 2018 Apr 03;190(13):E378–E379. doi: 10.1503/cmaj.180269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor K, Silver L. Smartphone ownership is growing rapidly around the world, but not always equally. Washington, DC: Pew Research Center; 2019. Feb 05, [2019-10-12]. https://www.pewresearch.org/global/2019/02/05/smartphone-ownership-is-growing-rapidly-around-the-world-but-not-always-equally/ [Google Scholar]
- 12.Riley WT, Rivera DE, Atienza AA, Nilsen W, Allison SM, Mermelstein R. Health behavior models in the age of mobile interventions: are our theories up to the task? Transl Behav Med. 2011 Mar;1(1):53–71. doi: 10.1007/s13142-011-0021-7. http://europepmc.org/abstract/MED/21796270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble N, Paul C, Turon H, Oldmeadow C. Which modifiable health risk behaviours are related? A systematic review of the clustering of Smoking, Nutrition, Alcohol and Physical activity ('SNAP') health risk factors. Prev Med. 2015 Dec;81:16–41. doi: 10.1016/j.ypmed.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Palmer M, Sutherland J, Barnard S, Wynne A, Rezel E, Doel A, Grigsby-Duffy L, Edwards S, Russell S, Hotopf E, Perel P, Free C. The effectiveness of smoking cessation, physical activity/diet and alcohol reduction interventions delivered by mobile phones for the prevention of non-communicable diseases: a systematic review of randomised controlled trials. PLoS One. 2018;13(1):e0189801. doi: 10.1371/journal.pone.0189801. http://dx.plos.org/10.1371/journal.pone.0189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009 Aug 18;151(4):264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 16.Lefebvre C, Manheimer E, Glanville J. Boolean operators (AND, OR and NOT). In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, UK: Cochrane Collaboration; 2011. [2019-09-26]. https://handbook-5-1.cochrane.org/index.htm#chapter_6/6_4_7_boolean_operators_and_or_and_not.htm. [Google Scholar]
- 17.Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ. 2005 Nov 05;331(7524):1064–5. doi: 10.1136/bmj.38636.593461.68. http://europepmc.org/abstract/MED/16230312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . Cardiovascular disease. Geneva, Switzerland: WHO; 2019. [2019-06-19]. https://www.who.int/cardiovascular_diseases/about_cvd/en/ [Google Scholar]
- 19.World Health Organization . Diabetes. Geneva, Switzerland: WHO; 2018. Oct 30, [2019-12-16]. https://www.who.int/news-room/fact-sheets/detail/diabetes. [Google Scholar]
- 20.D'Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008 Feb 12;117(6):743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 21.Cochrane Collaboration . The Cochrane Collaboration's tool for assessing risk of bias: 8.5.1 Overview. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, UK: Cochrane Collaboration; 2011. [2019-10-24]. https://handbook-5-1.cochrane.org/index.htm#chapter_8/8_5_1_overview.htm. [Google Scholar]
- 22.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016 Oct 12;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gore MO, Krantz MJ, Albright K, Beaty B, Coronel-Mockler S, Bull S, Estacio RO. A controlled trial of mobile short message service among participants in a rural cardiovascular disease prevention program. Prev Med Rep. 2019 Mar;13:126–131. doi: 10.1016/j.pmedr.2018.11.021. https://linkinghub.elsevier.com/retrieve/pii/S2211-3355(18)30277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muntaner-Mas A, Vidal-Conti J, Borràs PA, Ortega FB, Palou P. Effects of a Whatsapp-delivered physical activity intervention to enhance health-related physical fitness components and cardiovascular disease risk factors in older adults. J Sports Med Phys Fitness. 2017;57(1-2):90–102. doi: 10.23736/S0022-4707.16.05918-1. [DOI] [PubMed] [Google Scholar]
- 25.Rubinstein A, Miranda JJ, Beratarrechea A, Diez-Canseco F, Kanter R, Gutierrez L, Bernabé-Ortiz A, Irazola V, Fernandez A, Letona P, Martínez H, Ramirez-Zea M. Effectiveness of an mHealth intervention to improve the cardiometabolic profile of people with prehypertension in low-resource urban settings in Latin America: a randomised controlled trial. Lancet Diabetes Endocrinol. 2016 Jan;4(1):52–63. doi: 10.1016/S2213-8587(15)00381-2. [DOI] [PubMed] [Google Scholar]
- 26.Arens JH, Hauth W, Weissmann J. Novel app- and web-supported diabetes prevention program to promote weight reduction, physical activity, and a healthier lifestyle: observation of the clinical application. J Diabetes Sci Technol. 2018 Jul;12(4):831–838. doi: 10.1177/1932296818768621. http://europepmc.org/abstract/MED/29584454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender MS, Cooper BA, Flowers E, Ma R, Arai S. Filipinos Fit and Trim - a feasible and efficacious DPP-based intervention trial. Contemp Clin Trials Commun. 2018 Dec;12:76–84. doi: 10.1016/j.conctc.2018.09.004. https://linkinghub.elsevier.com/retrieve/pii/S2451-8654(18)30071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Block G, Azar KM, Romanelli RJ, Block TJ, Hopkins D, Carpenter HA, Dolginsky MS, Hudes ML, Palaniappan LP, Block CH. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015 Oct 23;17(10):e240. doi: 10.2196/jmir.4897. http://www.jmir.org/2015/10/e240/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer HH, Fischer IP, Pereira RI, Furniss AL, Rozwadowski JM, Moore SL, Durfee MJ, Raghunath SG, Tsai AG, Havranek EP. Text message support for weight loss in patients with prediabetes: a randomized clinical trial. Diabetes Care. 2016 Dec;39(8):1364–70. doi: 10.2337/dc15-2137. [DOI] [PubMed] [Google Scholar]
- 30.Fukuoka Y, Gay CL, Joiner KL, Vittinghoff E. A novel diabetes prevention intervention using a mobile app: a randomized controlled trial with overweight adults at risk. Am J Prev Med. 2015 Aug;49(2):223–37. doi: 10.1016/j.amepre.2015.01.003. http://europepmc.org/abstract/MED/26033349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramachandran A, Snehalatha C, Ram J, Selvam S, Simon M, Nanditha A, Shetty AS, Godsland IF, Chaturvedi N, Majeed A, Oliver N, Toumazou C, Alberti KG, Johnston DG. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2013 Nov;1(3):191–8. doi: 10.1016/S2213-8587(13)70067-6. [DOI] [PubMed] [Google Scholar]
- 32.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J, James WPT, Loria CM, Smith SC, International Diabetes Federation Task Force on Epidemiology and Prevention. National Heart, Lung, and Blood Institute; American Heart Association. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009 Oct 20;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 33.Wilson PWF, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino RB. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007 May 28;167(10):1068–74. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Guo X, Zhang Z. The efficacy of mobile phone apps for lifestyle modification in diabetes: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2019 Jan 15;7(1):e12297. doi: 10.2196/12297. http://mhealth.jmir.org/2019/1/e12297/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lunde P, Nilsson BB, Bergland A, Kværner KJ, Bye A. The effectiveness of smartphone apps for lifestyle improvement in noncommunicable diseases: systematic review and meta-analyses. J Med Internet Res. 2018 May 04;20(5):e162. doi: 10.2196/jmir.9751. http://www.jmir.org/2018/5/e162/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coorey GM, Neubeck L, Mulley J, Redfern J. Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: systematic review with meta-synthesis of quantitative and qualitative data. Eur J Prev Cardiol. 2018 Mar;25(5):505–521. doi: 10.1177/2047487317750913. [DOI] [PubMed] [Google Scholar]
- 37.Alessa T, Abdi S, Hawley MS, de Witte L. Mobile apps to support the self-management of hypertension: systematic review of effectiveness, usability, and user satisfaction. JMIR Mhealth Uhealth. 2018 Jul 23;6(7):e10723. doi: 10.2196/10723. http://mhealth.jmir.org/2018/7/e10723/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geller K, Lippke S, Nigg CR. Future directions of multiple behavior change research. J Behav Med. 2017 Feb;40(1):194–202. doi: 10.1007/s10865-016-9809-8. [DOI] [PubMed] [Google Scholar]
- 39.Meader N, King K, Wright K, Graham HM, Petticrew M, Power C, White M, Sowden AJ. Multiple risk behavior interventions: meta-analyses of RCTs. Am J Prev Med. 2017 Jul;53(1):e19–e30. doi: 10.1016/j.amepre.2017.01.032. https://linkinghub.elsevier.com/retrieve/pii/S0749-3797(17)30091-0. [DOI] [PubMed] [Google Scholar]
- 40.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2016;4:CD006611. doi: 10.1002/14651858.CD006611.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy.


