Abstract
We review the use of transmission electron microscopy (TEM) and associated techniques for the analysis of beam-sensitive materials and complex, multiphase systems in-situ or close to their native state. We focus on materials prone to damage by radiolysis and explain that this process cannot be eliminated or switched off, requiring TEM analysis to be done within a dose budget to achieve an optimum dose-limited resolution. We highlight the importance of determining the damage sensitivity of a particular system in terms of characteristic changes that occur on irradiation under both an electron fluence and flux by presenting results from a series of molecular crystals. We discuss the choice of electron beam accelerating voltage and detectors for optimizing resolution and outline the different strategies employed for low-dose microscopy in relation to the damage processes in operation. In particular, we discuss the use of scanning TEM (STEM) techniques for maximizing information content from high-resolution imaging and spectroscopy of minerals and molecular crystals. We suggest how this understanding can then be carried forward for in-situ analysis of samples interacting with liquids and gases, provided any electron beam-induced alteration of a specimen is controlled or used to drive a chosen reaction. Finally, we demonstrate that cryo-TEM of nanoparticle samples snap-frozen in vitreous ice can play a significant role in benchmarking dynamic processes at higher resolution.
This article is part of a discussion meeting issue ‘Dynamic in situ microscopy relating structure and function’.
Keywords: TEM, radiolysis, STEM, beam-sensitive materials
1. Introduction
Complex materials often include a component of soft matter that is sensitive to structural or chemical alteration when imaged by electron microscopy. These electron beam-sensitive components might be organic crystals, polymers, interfaces and hybrid organic–inorganic materials, even some inorganic materials such as hydrates and hydroxides, as well as multiphase solid/liquid and solid/gas systems [1,2]. Arguably materials that contain such components constitute the majority of systems of current interest across a wide range of scientific and engineering disciplines including chemistry and chemical engineering, engineering materials, food science, biology and increasingly physics and electronic engineering.
For the last ninety years transmission electron microscopy (TEM) and subsequently scanning electron microscopy (SEM) have been the premier tools for observing matter at the micrometre, nanometre and even atomic scale. This is mainly because of the image resolution obtainable with fast/high energy electrons but also arises, in part, because the interaction between electrons and matter is so strong. However, as a result of the latter property, these techniques have suffered from having to analyse samples in a vacuum leading to specimen dehydration and, in the case of TEM, has necessitated the use of very thin samples that often require considerable sample preparation. Furthermore, much of the progress of both TEM and SEM has often relied on a highly simplified and imprecise accounting of the interaction of the electrons with the material under examination. In order to progress research into new material systems and to observe them in-situ in both their native state and under dynamic conditions, it is imperative we attempt to address this limitation.
For the purposes of this communication, we concentrate almost exclusively on TEM of thin specimens using both near parallel beam, conventional TEM (CTEM) and scanning TEM (STEM) with a focused electron probe. Furthermore, we define native-state analysis of such complex, beam-sensitive materials and systems, as the condition that they remain largely unmodified by the specimen preparation used to bring them into the microscope and that the interaction with the electron beam is controlled such that there is minimal measurable alteration of structure and chemistry of the specimen during analysis. In-situ analysis is the dynamic observation of the reactions in or on a specimen contained within the microscope when stimulated by mechanical, thermal, electrical, optical or chemical means. In-situ analysis, therefore, poses a number of challenges: first the inherent beam sensitivity of both the starting components and the product following in-situ phase transformation and/or reaction must be quantified and secondly, for chemical reactions, the inherent beam sensitivity of the reactant liquids or gases, with which the material is interacting in-situ, must be understood or accounted for.
Initially, we discuss the origins of electron beam sensitivity of materials and ways to mitigate these effects. Electron beam-induced damage has been investigated intermittently over the years including key studies in [3–7]. In fact electron irradiation has even been used as a surrogate for fast neutron damage in the study of materials for application in nuclear fission and fusion (early work is summarized in [8] and more recent works in [9–11]).
The electron dose experienced by a sample is the energy absorbed by the specimen during electron irradiation and is a function of both the incident energy of the accelerated electrons, electron fluence (the number of electrons per unit area) and fluence rate or flux (the number of electrons per unit area per second) in the TEM, as well as parameters associated with the specimen itself such as the thickness and chemical composition. The absorbed dose is strictly measured in Grays (note a Gray (Gy) is 1 Joule of energy deposited per kg of matter), and for 100 keV electrons exposed to a typical organic material an electron fluence of 1 electron/Å2 or ca 1.6 × 10−3 C cm−2 is then equivalent to a total absorbed dose of a few MGy [12]. The effects of ionizing radiation on materials has been a major topic of study for many years, particularly in relation to cancer radiotherapy, atmospheric science, remediation of waste-water, food preservation/treatment, sterilization of pharmaceuticals, and synthesis and nuclear energy production. Yet the issue is often side-stepped in electron microscopy investigations. A distinct difference with electron microscopy is that electron dose rates (and hence total electron doses) are many orders of magnitude higher than those generated by other common radiation sources. This is especially so for the focused electron probes of STEM where understanding of the implications of very high dose rates for dose efficiency is still developing [13,14] and will be briefly discussed later in this work. Nevertheless, the effects of electron beam radiation are almost universally present in electron microscopy experiments, complicating their separate study. It is therefore unsurprising that the electron beam sensitivity of many materials is a major issue limiting their investigation.
We discuss a number of useful concepts for TEM imaging and/or chemical analysis within a TEM, including a dose budget for experiments and a dose-limited resolution [15]. We also highlight the current state of the art in dose control during imaging and spectroscopy including the use of scanning TEM (STEM) as opposed to conventional TEM (CTEM), compressed sensing methods and the cryo-preservation of samples. Finally, we also discuss dynamic studies of such complex materials and systems including the use of in-situ liquid and gas cell TEM holders, which have their own additional limitations due to dose, as well as the use of time-resolved cryo-methods to provide snapshots of dynamic systems.
2. Damage of beam-sensitive materials by electrons
In general, inorganic materials such as metals and metal oxides are more electron beam stable while biological materials, inorganic–organic hybrid materials and organic materials (including polymers and small molecules in both crystalline and non-crystalline forms) are more easily damaged. The mechanisms by which a material is damaged by the electron beam can be categorized by the different types of electron scattering experienced: either, (i) elastic scattering arising from an electron–nucleus interaction which can lead to atomic displacement and electron beam-induced sputtering, or (ii) inelastic scattering arising from an electron–electron interaction which can lead to both electron excitation and heating in the sample, or (iii) a combination of both. Inelastic scattering also causes the production of low energy secondary electrons that can generate highly reactive free radicals and ions that can cause bond breakage, an overall process known as radiolysis. There are a number of comprehensive reviews of these mechanisms and, in the first instance, the reader is referred to an example that considers typical TEM accelerating voltages between 10 and 300 kV [16]. In this work, we focus on how understanding of these principles can be applied for improved experimental design and execution.
The elastic scattering damage mechanism (known as knock-on) is dominant in conducting materials, often containing light elements, with strong primary bonds. Knock-on damage dominates for materials such as graphite and graphene, and is characterized by a threshold incident electron energy required to displace atoms within the specimen [5]. Clearly, this can be mitigated by lowering the energy of the incident electrons to be at or below this threshold and, in combination with spherical aberration correctors that retain image resolution at lower kV, has stimulated the field of low energy TEM [17,18]. Conversely, the predominant mechanism for damage of non-conducting materials containing light elements and weaker secondary bonds, such as molecular crystals, is radiolysis. Radiolytic bond breakage is a secondary mechanism that does not exhibit a distinct incident beam energy threshold, but does lead to a loss of order in crystals which can be observed through the fading of diffraction spots to amorphous rings in electron diffraction patterns [19,20]. A loss of molecular structure can be observed in electron energy loss spectroscopy (EELS) and, if mass loss occurs, there will be changes in chemical composition detectable by EELS and/or energy dispersive X-ray spectroscopy (EDX) [7]. Mass loss of lighter atoms due to bond breakage and sputtering may also be seen as a change in mass-thickness contrast in images, whereby areas from which atoms have been removed appear more intense in the bright field (BF) image [7,20].
The propensity for damage to the sample to be caused by the electron beam can be quantified by measuring the characteristic/critical electron fluence (CF) for an image or spectral feature to significantly change, in units of e−/Å2 [7]. Note the terms electron dose and electron fluence tend to be used interchangeably in the field of electron microscopy, although, as discussed, strictly dose is the energy absorbed by the sample per unit mass (in units of Grays) (e.g. [21]). The critical electron fluence (CF) may be calculated by measuring the change in intensity of an electron scattering feature as a function of irradiation time under a given electron flux, such as an electron diffraction spot in the case of a crystalline material, and determining the total accumulated electron fluence at which the relative intensity of the feature decays to e−1 (ca 37%) of its initial value during exposure. Electron diffraction monitors crystallinity and lattice periodicity, alternatively a CF related to changes in bright field, dark field or phase contrast TEM images may be determined or, for analytical TEM, one related to changes in chemical composition or local chemistry measured by EDX or EEL spectroscopies; the latter may also be used to monitor changes in chemical bonding by tracking spectroscopic fine structure arising from the local electronic structure. These alternative approaches will yield differing sensitivities to the electron beam (i.e. differing values of the critical electron fluence), because the type and extent of observed damage varies from loss of atomic order, loss of material to changes in chemistry, and each of these will depend on the predominant damage mechanism responsible for these particular changes [22].
Fundamentally, the value of the critical fluence depends on the material and a variety of experimental parameters (including fluence rate or flux, discussed below); in general, for irradiation voltages of 80–300 kV, typical for TEM, biological materials have a CF in the range of 1–15 e−/Å2, organic crystals 0.2–120 e−/Å2, zeolites and other hydrated or hydroxylated minerals 100–600 e−/Å2, and transition metal oxides greater than 107 e−/Å2 [3,23–27].
3. Critical fluences of molecular crystals
As an example, we focus on molecular crystalline materials and choose a model organic material theophylline, a metabolite of caffeine that can exist in one hydrated and seven anhydrous polymorphic forms [28]. Anhydrous form II crystals of theophylline can be readily synthesized by recrystallization from a solution in nitromethane and possess a thin platelet morphology. This thin platelet morphology causes particles to adopt a predictable 〈100〉 orientation, and samples tend to be electron transparent—so making them a good candidate for study.
The structural CF of anhydrous form II crystals of theophylline has been investigated for a variety of microscope and sample conditions and is detailed in [29,30]. Figure 1a shows an example of a diffraction pattern of theophylline during prolonged irradiation over ca 10 min at a beam energy of 200 keV and an electron flux of 0.098 e−/(Å2s). Higher-order Bragg reflections (corresponding to smaller lattice spacings) fade first as bond breakage and molecular rotation disrupts short range order, however general long-range molecular packing remains to higher electron fluences [3]. Figure 1b shows the decrease in intensity of the {004} and {011} diffraction spots as a function of accumulated fluence from which the structural critical fluence can be determined as ca 23 e−/Å2 (for the case of the {011} spot).
Figure 1.
(a) Diffraction series of theophylline form II along the 〈100〉 zone axis using an electron flux of 0.098 e−/(Å2s) and at 200 kV. (b) Plot of the logarithmic normalized spot intensity versus total electron fluence for the {004} and {011} diffraction spots. The CF is measured at 1/e of the initial spot intensity which in this case is approximately 11 e−/Å2 and 23 e−/Å2 for the {004} and {011} spots, respectively. (c) Comparison of experimentally determined critical fluence values relative to standard conditions (SC: 200 kV, room temperature and continuous carbon support film) for theophylline form II arranged in order from lowest to highest (least stable to most stable). Values with units of kV refer to electron beam accelerating voltages, while values with units of K refer to sample temperatures. GF stands for graphene film substrate, respectively. (Online version in colour.)
The decay of the diffraction intensity is due to damage to the chemical (i.e. the molecular unit) and/or crystal structure and, in most cases, it appears that both occur simultaneously. However, it has been demonstrated in phthalocyanines that the crystal structure is lost before the chemical structure while studies on tetracene show that the chemical structure is destroyed first [31]. The form of the decay of the diffraction intensity as a function of increasing fluence is most often observed to be exponential, however sometimes variations are observed such as an initial plateau (as seen for the case of the {011} diffraction spot in figure 1b) or even a rise in intensity (followed eventually by an exponential decay) as electron fluence increases. The latter may be attributed to a rapid loss of mass upon irradiation, reorientation of the crystal or conformational changes occurring in the sample, producing a structure that is more stable to the electron beam [32–35]. Reports of this type of initial plateau or rise in recorded diffracted intensities in X-ray crystallography have been attributed to initial changes in orientation or unit cell parameters throughout the sample volume [36]. Similar observations have also been reported in cryo-EM where an initial rise in contrast at high spatial frequencies over a series of frames is attributed to initially rapid particle motion followed by a reduction in motion with increasing exposure time [37,38]. The existence of an initial plateau may also indicate that damage products need to reach a critical concentration before they can diffuse away and initiate further damage [39]; the occurrence of a thermally activated reverse reaction (or healing process) may prevent a proportion of the damage, allowing for the structure to stay intact until the cumulative damage is too great and the structure finally breaks down.
Using averaged measurements from a number of form II theophylline crystals (which averaged out any effect of sample thickness) at room temperature with samples supported on continuous carbon film substrate TEM grids, the critical fluence was determined to be ca 26 e−/Å2 at 200 keV. Due to the inverse dependence of the inelastic cross-section on incident electron beam energy, the CF increased to ca 36 e−/Å2 at 300 keV and decreased to 11 e−/Å2 at 80 keV, as highlighted in figure 1c [29]. At least for continuous carbon substrates, specimen cooling was found to have relatively little impact on CF, while specimen heating showed a slight adverse effect in that it decreased CF, figure 1c. More generally, it would appear that proportionately, relative to room temperature, specimen cooling to liquid nitrogen temperatures has a larger effect the more beam-sensitive the specimen [40]; the rationale here is that cooling may help limit the diffusion of secondary products generated during radiolysis [40]. Overall, the largest value of CF for theophylline form II at 200 keV (ca 42 e−/Å2) was obtained using a graphene support substrate and cooling to liquid nitrogen temperatures (also shown in figure 1c).
This methodology was extended to the analysis of 20 poorly soluble crystalline organic materials (figure 2) that are generic active pharmaceutical ingredients (APIs) and were selected on the basis of a variation in their molecular chemical groups [40]. A range of structural CF values were obtained which did not appear to correlate with traditional or simple measures of sample stability, such as the melting point. As a result, a total of 19 different molecular descriptors that may have influenced CF were used as input parameters for a Principal Component Analysis (PCA) which identified those which were statistically significant. The molecular descriptors which gave a positive correlation to CF were related to the presence of aromatic and conjugated carbons suggesting that delocalization of electrons allows the energy deposited from the electron beam to be shared and dissipated more effectively, so decreasing the formation of damaging radicals [41–44]. The molecular descriptors that exhibited a negative correlation to CF were related to the number of rotatable bonds (which relates to the number of different structural configurations the molecules can undertake) and, more surprisingly, factors that related to the numbers of hydrogen bond donors and acceptors. The latter can be rationalized by the fact that when hydrogen bonding is present, the adjacent covalent bond is weakened making it more susceptible to radiolytic damage. An alternative explanation might be that, during radiolysis, the chemical groups involved in the hydrogen bond may readily form radicals (e.g. OH·) that can propagate and cause further damage. Potentially, the presence of hydrogen bonding may also encourage the incorporation of ambient water during handling/processing.
Figure 2.
Predicted critical fluence (CF) of 20 different pharmaceutical compounds, to calculate the predicted CF a multiple-linear regression equation was created using different chemical parameters that related to the chemical structures such as number of hydrogen bond donors/acceptors and number of conjugated carbons/non-conjugated carbons. The points shown in red are two of the compounds that were poorly predicted and the data point for tolnaftate is not shown due to the experimental CF being twice as large as the next highest compound. Adapted from [40]. (Online version in colour.)
Using the parameters identified as being significant, a multiple-linear regression (MLR) model was generated to allow prediction of critical fluence for structural degradation of a molecular crystal based on the structure of the constituent molecule. The experimentally observed critical fluence versus the predicted value based on the model is shown in figure 2; further details are discussed in [31].
4. Implications of damage for the measurement of beam-sensitive materials
The critical fluence gives a measure of the fluence budget (FBudget) (a surrogate for the dose budget) available to a user during an experiment. This is the accumulated fluence required to locate (FLocate), align (FAlign) and focus (FFocus) the specimen region, prior to recording either an image, diffraction pattern, EDX or EELS spectrum or spectrum image from the specimen area of interest (which requires an additional accumulated fluence of FSignal). Thus, the total fluence budget is given by
| 4.1 |
Beam blanking is often used between these separate experimental steps and the flux reduced so as to avoid unintended irradiation of the specimen. The concept of a dose budget has analogies with dose fractionation in cryo-TEM tomography [45].
Increasing the critical fluence of a beam-sensitive material by appropriate choice of the sample itself, specimen preparation and the electron microscope operating conditions is obviously desirable, however this does not necessarily lead to an improvement in information obtained. Egerton [15] has introduced the concept of dose-limited resolution (DLR) for a polycrystalline or amorphous material:
| 4.2 |
where SNR is the signal-to-noise ratio which must equal or exceed some chosen background value, typically greater than 3–5 times the standard deviation to satisfy the Rose criterion [46]; DQE is the detector quantum efficiency which refers to the efficiency of a detector in converting incident electrons into an imaging signal (this may be modified by a modulation transfer function (MTF) which is a function of the spacing or spatial frequency that is being measured); F is the collection efficiency of incident electrons to detected electrons, which, for CTEM, depends on the sample thickness (t) and the elastic mean free path of electrons (λe) and is equal to F = exp(−t/λe); e is the elementary charge of an electron; C is the contrast which depends on C0 (the initial contrast before electron beam exposure), the accumulated fluence and the critical fluence of the material (CF) and may be given by C = C0exp(−Fluence/CF), assuming an exponential decay of contrast with increasing fluence.
Many of the factors in the DLR are inter-related in a complex fashion. For example, for a given specimen thickness, increasing the electron beam energy in order to lower the inelastic cross-section and hence increase the critical fluence, will conversely lead to a reduction in contrast and hence signal. There is a benefit to increasing sample thickness insofar as the larger number of scattering events in a thicker specimen results in more signal whereas the damage per unit volume remains the same, so the signal/damage ratio improves [15]. The choice of beam energy, however, also depends on the collection efficiency, and higher electron beam energies (e.g. 300 keV) can offer improvements in DLR for thick samples for many imaging modes [15]. Alteration of the electron beam energy will however change the DQE of the detector that may be optimized for a particular electron energy range and future work should also consider this. The optimum resolution for thin (∼10 nm) biological specimens has recently been experimentally determined by Peet et al. [47]. They showed that the elastic cross-section is approximately two times greater at 100 keV than at 300 keV, whereas the radiation damage increased by only 1.57 times; hence 100 keV is recommended for measurements on thin samples, once a direct electron detector with optimal pixelation and performance at 100 keV becomes available.
5. Low-dose TEM approaches for high-resolution imaging
From the above discussion of DLR, besides the optimum choice of experimental variables (incident beam energy, detector etc.) and sample variables (specimen thickness, coating, cooling etc.), the primary task in low-dose microscopy is to control the electron fluence incident on the sample to provide just enough fluence to collect sufficient scattered electrons to achieve contrast before significant structural alteration occurs.
The electron flux in CTEM can be controlled via adjustment of the extraction voltage of the electron field emission source, as well as by the condenser lens system and associated apertures so as to control the brightness and area illuminated. In contrast, for STEM the total electron fluence per image can be controlled via choice of: the magnification (and hence the specimen pixel size), the probe current (typically pA) using the illumination aperture and associated condenser optics or source extraction voltage, and alteration of the dwell time per pixel (typically microseconds). The electron flux and measured probe current in CTEM and STEM can be measured from the exposure time or fluorescent screen current reading which can be directly calibrated using a Faraday cup. In some S/TEM systems, continuous control of the probe or beam current is not possible due to the electron optical design. However, a monochromated electron source will permit continuous control of probe/beam current by various means depending on monochromator design, meaning that any chosen probe/beam current can be used without significant impact on the electron optical alignment lower down the column including, beam/probe size, or focus [48].
Previously, high-resolution CTEM images of electron beam-sensitive materials were generally recorded on photographic film, whilst more recently direct electron detectors have been used to obtain HRTEM images at low dose [26,49–52]. One example is by Zhang et al. [52] where atomic resolution images of metal-organic frameworks (MOFs) were obtained by HRTEM using a total electron fluence of 5 e−/Å2 at 300 keV. The use of direct electron detectors increases the signal-to-noise ratio (SNR) and contrast in an image due to the high DQE compared to conventional scintillator-based detectors and this is key to enabling high-resolution CTEM at very low electron fluence. The capability for these detectors to operate in an electron counting mode, when operated at suitably low counts per pixel for charge localization, also enables the elimination of spurious dark current which dominates CCD detectors at low beam currents incident on the scintillator. The counting capability of direct electron detectors also results in improvements in the detector MTF and, in turn, improvements in the DQE. Broadly, the available direct electron detectors may be classified as monolithic active pixel sensors (MAPS) or hybrid pixel array detectors (PAD) with characteristic differences in pixel size, number of pixels, and performance at different accelerating voltages. These have been reviewed in [53] and elsewhere. Furthermore, the rapid acquisition speed of some direct detectors allows any mechanical instability in a sample during the initial stages of damage to be identified and even corrected by post acquisition, cross-correlation of images.
S'ari et al. [54] investigated a model system of crocidolite (a form of asbestos which is moderately beam-sensitive) in order to determine the electron flux/fluence limits for low-dose, high-resolution CTEM imaging for a particular scintillator-based, CMOS electron camera by testing a variety of electron flux and total electron fluence regimes. The results are shown in figure 3a–i and revealed that an electron flux of 10 e−/(Å2s) and total fluence of 10 e−/Å2 provided sufficient contrast and SNR to resolve 0.30 nm lattice spacings at 300 keV. These parameters were then used to image an organic crystalline material, furosemide, which has a critical fluence of a little above 10 e−/Å2 at 300 keV (figure 3j–o).
Figure 3.
HRTEM of a crocidolite needle (low magnification image in (c) shows the magnified area and SAED pattern), used as a model system to determine which electron flux and electron fluence regime would be best suited for clearly resolving lattice information at low dose. Images (a), (d) and (g) were captured using an electron flux of 1 e−/(Å2s) and show the same area of the crocidolite crystal but are exposed for a total electron fluence of 1, 10 and 100 e−/Å2, respectively. Images (b), (e) and (h) were captured using an electron flux of 10 e−/(Å2s) at a total electron fluence of 1, 10 and 100 e−/Å2, respectively. Finally, (f) and (i) were captured using an electron flux of 100 e−/(Å2s) at a total fluence of 10 and 100 e−/Å2, respectively. Inset in images (a–i) is the corresponding FFT with visible lattice points highlighted. The bottom panel shows HRTEM of two different areas (j) and (m) from the same furosemide crystal acquired using an electron flux of 10 e−/(Å2s) and total fluence of 10 e−/Å2. The corresponding FFTs are shown in (k) and (n) and filtered FFTs showing the lattice fringes more clearly are shown in (i) and (o). Adapted from [54]. (Online version in colour.)
6. Low dose STEM versus CTEM
In terms of maximizing the fluence budget (Equation (1)) for actual signal measurement, a common option used in both low-dose CTEM and STEM is to locate and align the specimen under study in a neighbouring (sacrificial) region of the sample and then move to a fresh, undamaged region of interest (ROI) for signal measurement. In terms of practical experimental considerations, in high-resolution CTEM lattice imaging, which illuminates a specimen area in parallel, there is an additional requirement to refocus the image when moving from the sacrificial location/alignment area to a fresh unexposed area, which correspondingly uses up a proportion of the available fluence budget. However, in STEM one can significantly reduce FFocus by focusing the Ronchigram (obtained using a static, focused STEM probe) in a small area directly adjacent to the ROI, so sacrificing only a small region of specimen and retaining a greater proportion of the fluence budget for actual signal measurement.
Unlike CTEM, STEM is a serial technique that collects signal pixel by pixel—making it more ideally suited to provide localized analytical information such as diffraction or chemical composition than CTEM. However, this serial nature means that STEM is relatively inefficient in terms of signal collection. The conditions for bright field STEM that still approximates to bright field phase contrast CTEM can be optimized in terms of signal collection by choosing the bright field detector collection semi-angle to be half of the probe convergence semi-angle (for the case of a non-aberration corrected STEM probe, and two thirds of the probe convergence semi-angle for an aberration corrected STEM Probe) [55]. Despite this inefficiency, the DQE of STEM Photomultiplier Detectors, which, in some cases, can approach 1, is generally higher relative to CTEM cameras (typical DQEs of 0.2 and 0.96 for scintillator-CMOS and direct detection CCD cameras respectively) and, for STEM, in principle there is an absence of a modulation transfer function (MTF) as data are recorded independently pixel by pixel (although this may fail at faster scan speeds [56]). Sader et al. quantitatively compared bright CTEM and bright field STEM for the case of a moderately beam-sensitive hydrous phyllosilicate clay mineral, vermiculite and concluded that whilst absolute contrast levels were higher for STEM, SNRs were lower [57].
Collection efficiency and SNR can be improved through recent developments in STEM, such as (integrated) differential phase contrast (DPC) using a segmented detector as well as focused (or defocused) probe ptychography with a pixelated detector, where the SNR is able to exceed CTEM phase contrast images [58,59]. Both DPC and ptychography have shown promise for the study of beam-sensitive materials and imaging of light element containing materials at atomic resolution is possible due to the efficiency of electron collection at low probe currents, although to date the dwell times per pixel required for suitable frame readouts on MAPS and PAD detectors have remained in the 1–3 ms range [60–62]. These dwell times are significantly longer than those possible with the scanning hardware and currently restrict the beam currents for low-dose applications using pixelated direct electron detectors. A further area at the interface between crystallography and imaging is the use of scanning electron diffraction techniques, where a less than 5 nm diffraction-limited STEM probe combined with direct electron detectors can yield imaging of beam-sensitive materials at estimated fluences of a few e−/Å2 at 200 keV [62].
There may also be further benefits possible to using STEM over CTEM in terms of the ultimate DLR achievable. Potentially the use of a fast scanned focused STEM probe reduces charging of a sample and hence lowers the mechanical stress and specimen movement observed (either due to reduced charging itself or from reduced mechanical stress induced as a result of localized damage) [19]. Furthermore, electron beam heating of the sample can be minimal in STEM, for example, steady state modelling of heat generation by a beam incident on an amorphous carbon film balanced against heat loss due to radial conduction and radiation by the film indicates that a 5 nA electron probe of 1 nm diameter only produces a temperature rise of 1.4 K [7]. Note however that beyond a certain probe current threshold (depending mainly on the thermal conductivity of the specimen) thermal heating can increase diffusion and hence result in greater damage to organic specimens [63]. Furthermore, although electron beam heating of the sample is generally assumed to be minimal, it is possible that the focused, high current density probe in STEM could cause less heating than a parallel beam (although this is thought to be dependent upon the specific probe diameter and current). Note that it has been shown that beyond a certain dose rate, thermal heating can increase diffusion and hence result in greater damage [63]. Furthermore, the intermittent illumination of a scanned, focused STEM probe may allow damage recovery or healing processes to operate. Recent studies by Vandenbusche et al. [64] using pulsed, laser-driven electron beams in CTEM imaging of an alkane crystal have indicated that controlled delivery of dose, both in terms of numbers of electrons per pulse and the time between pulses, can lead to a reduction in irreversible structural damage.
The significant differences in electron flux between STEM and CTEM could also lead to changes in damage for a given irradiation fluence of a particular material. A schematic is provided (figure 4a) which postulates how specimen damage rate might correlate to electron dose rate (or electron flux) [13]. If damage has a direct dose rate dependence (such that radiation sensitivity increases with increasing dose rate to give an above linear response in figure 4a), then a high dose rate may cause an appreciable temperature rise or significant electrostatic charging. The former will increase radiolytic efficiency or even result in thermal damage [66], while the latter can cause specimen movement or rupture, ion migration and even ion emission or hole drilling [67,68]. If however radiation damage is time dependent or diffusion-limited (as can be the case for radiolytic damage of insulating materials such as polymers and organic crystals [13,14]), the relationship between dose rate and damage rate will be sub-linear and the curve can plateau at higher dose rates. In the case of low-dose CTEM, imaging is usually carried out using an electron flux of less than 10 e−/(Å2s). Comparatively for STEM, imaging and analysis is carried out with a much higher localized probe flux on the order of 108 e−/(Å2s) for a 1.4 Å probe size and 60 pA probe current, even when taking into account beam broadening. Note here that we are referring to the probe flux and not the average flux per pixel, where the latter is commonly reported and can be orders of magnitude smaller than the localized probe flux. If the rate of damage of a specimen is indeed diffusion limited, then it may be possible to use a higher electron flux (as in STEM) to obtain a better SNR for a given level of damage. This analysis assumes that the flux in low-dose CTEM corresponds to the linear portion of the schematic in figure 4a and the localized STEM probe flux relates to the region at which the dose rate versus damage rate curve plateaus and hence the damage per unit fluence (i.e. the gradient of the curve) is lower.
Figure 4.
(a) A schematic illustrating the differences in the relationship between damage and dose rates for different samples. It is postulated that diffusion-limited processes correspond to a power law relationship with exponent less than 1 (blue line). For such samples, high flux STEM would be preferential in reducing the damage rate. In comparison samples that exhibit a direct dose v damage relationship (green line) tend to exhibit more damage at higher dose rates and CTEM is predicted to be more advantageous. The schematic in (b) illustrates the concept of beam broadening and delocalized damage in STEM. The predicted minimum delocalized damage is typically a few nm, suggesting a pixel size more than a few nanometres would be preferential to avoid over-sampling. Adapted from [13,65].
Here, we also note that damage is in fact delocalized around the STEM probe (figure 4b), typically over a radius of 3–5 nm for the low energy electronic excitations responsible for radiolysis, which may suggest that an appropriate choice of probe size, specimen pixel size (the distance between scanned points) and also scanning patterns may reduce the effects of damage [69]. That said, we note that low energy EELS spectroscopic signals are also being generated from this larger delocalized volume which may present opportunities for chemical spectroscopy whilst reducing the information obtained in STEM imaging.
Thus, controlling or limiting diffusion may limit the movement of the secondary products of inelastic electron specimen interactions that are so damaging. As discussed previously, specimen cooling is perhaps the most well established approach that has been shown to improve sample stability under the electron beam, enabling higher contrast and so improved resolution to be obtained. This effect is particularly strong in vitreous ice formed by plunge freeze specimen preparation that is discussed in more detail later. Alternatively, as shown in figure 5, Hooley et al. [22] compared the radiolytic damage of calcium carbonate nanoparticles (a moderately sensitive, direct-damage inorganic material) by both CTEM and STEM and concluded that hydrocarbon contamination induced by the focused STEM probe was important in mitigating damage, as has been noted by others [70–72]. The build-up of hydrocarbon contamination may protect the specimen in two ways: by improving the conduction of electrons, thus reducing the extent of radiolysis or electrostatic charging by suppressing charge build-up in a similar manner to intentionally deposited carbon coatings [68]; or by preventing the migration of molecular fragments and facilitating their recombination, meaning that there may be some structural damage without significant chemical change [73]. Hooley et al. used the benefits of contamination to enable low-dose bright STEM lattice imaging of calcite nanoparticles at 0.05% of the observed damage threshold for pore formation in these particles (figure 6).
Figure 5.
(a) A comparison between high-resolution 300 kV CTEM (1–3) and STEM (4–9) imaging of calcite nanoparticles. STEM BF imaging was able to detect a lattice at higher fluences than was possible in CTEM. However, this was found to be due to the contamination deposited during STEM imaging, as when the contamination was removed (7–9), STEM imaging caused more severe specimen degradation than CTEM, converting the specimen to calcium oxide at a lower fluence. (b) Comparison of O : Ca atomic ratios extracted from time-resolved CTEM EDX spectra from both a contaminated and (cleaned) uncontaminated calcite nanoparticle specimen. Adapted from [22]. (Online version in colour.)
Figure 6.
300 keV bright field STEM image of a calcite nanoparticle showing contamination and also 1.6 Å calcite (122) crystal lattice measured with a 1 pA probe current and a fluence per pixel of 165 e−/Å2 which is 0.05% of the measured 300 kV damage threshold. Note 25% electron collection efficiency due to non-reciprocal conditions used.
7. Low-dose STEM approaches
As mentioned above, low-dose STEM involves use of the following: (i) fast scanning of the focused probe by decreasing the pixel dwell time (currently the lower limits are around 0.5 µs pixel−1); (ii) lowering the probe current and; (iii) increasing the specimen pixel size (by using a lower magnification). Buban et al. [74] used a combination of all three strategies to produce low fluence, noisy images of a strontium titanate lattice that showed periodicity in the Fourier transformed image. Fourier filtering a low-dose image for these periodicities can in principle return lattice information (although this is not ideal as it leads to a synthetic processed image), alternatively it is possible to average a stack of undamaged, noisy images to increase the overall SNR.
Non-sequential/randomized pixel scanning has also been reported to reduce damage via lowering dose accumulation effects [75]. An extension of this approach which is employed to obtain low-dose STEM images is the use of compressive sensing methods [74,76] that employ the concept that data can be represented in a sparse form and by recording a sub-sampled image (using appropriate blanking of the beam), the missing data can then be recovered using mathematical algorithms [77,78], so reducing the fluence that the sample is exposed to. There is some debate as to how this approach compares with simply averaging low-dose noisy data; however, this approach may offer a benefit in cases where alteration of the optics (e.g. lowering the probe current) is itself problematic, as the compressed sensing methods can be implemented simply by using add-on hardware and/or software.
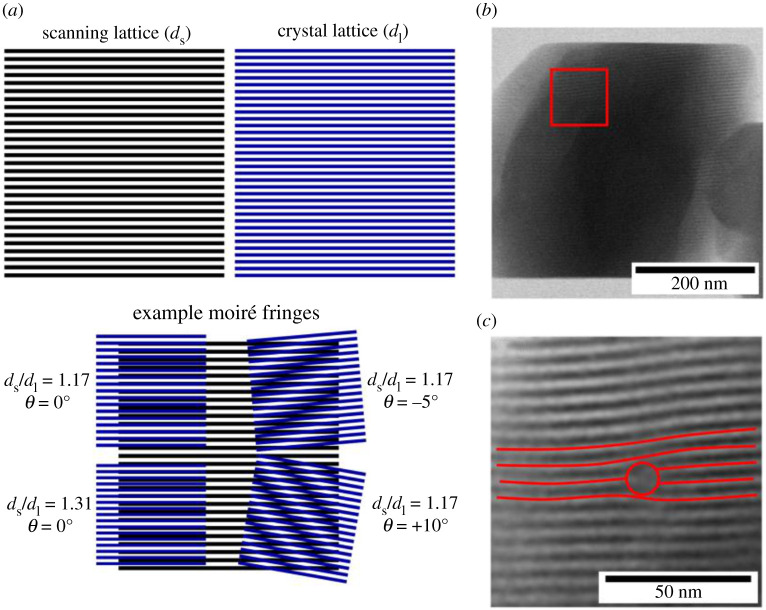
A further technique that has been used to obtain high-resolution, atomic lattice information at low electron fluence is the formation of scanning moiré fringes (SMFs) in STEM [79–81]. Scanning moiré fringes arise from the interference between the atomic plane spacings in a crystal lattice and spacings in a similarly sized reference lattice, produced by the scanning of the electron beam. The spacing of the moiré pattern that is generated depends on the magnitude of the spacings in the sample and reference lattice and their relative misorientation [82]. Effectively, the moiré pattern produces a magnified image of the crystal lattice, including any imperfections, and allows lower magnification acquisitions and therefore larger areas to be imaged at a lower electron fluence than would normally be required. S'ari et al. [54,80] have applied this to molecular crystalline materials and highlighted the ability to image, albeit indirectly, crystalline defects and surface strain, as shown in figure 7.
Figure 7.
(a) Schematic showing example moiré fringes generated from a scanning lattice (ds) and crystal lattice (dl) of a similar size being overlaid at different ds/dl ratios and rotations. (b) Example of a scanning moiré fringe image of furosemide using ds of 1.32 nm and dl of 1.50 nm (c) Magnified area from the area highlighted in (b) showing a defect within the furosemide crystal. Adapted from [80]. (Online version in colour.)
8. Analytical spectroscopy in the TEM
Analytical spectroscopy of beam-sensitive materials using EDX and EELS to determine chemical composition and bonding is significantly more challenging than imaging or diffraction. For CTEM, in general, spatially localized information is achieved by focusing the beam using the condenser lens system that, in turn, leads to a higher fluence on the specimen and the associated risk of damage. Such damage may change both the chemical composition (via preferential mass loss from the illuminated volume) and the chemical bonding and, as previously discussed, may in itself be used to monitor the progression of damage. Energy filtered CTEM is possible by selecting particular ranges of inelastically scattered, energy loss electrons, although, for thin samples, the inherent signals are generally much lower than for elastically scattered signals which provide the information in images and diffraction patterns [83]. Hence, overall, STEM methods are more suited for analytical spectroscopic mapping (known generically as spectrum imaging).
EDX spectroscopy in the TEM is inherently low efficiency since X-rays are emitted isotropically from the sample over a solid angle of 4π steradians. However, the advent of larger solid angle EDX silicon drift detectors (SDDs) and placing several detectors around a specimen have improved signal collection efficiencies (typically up to 1 steradian) and, if specimen damage can be mitigated, it is possible to chemically map beam-sensitive specimens at the scale of a few nanometres (figure 9).
Figure 9.
A comparison between cryo-CTEM and cryo-STEM indicated far less bubbling was observed in STEM. CTEM images were captured using a definitive electron fluence and showed that at 100 e−/Å2 (a) little bubbling in the vitreous ice was observed, but after exposure to 400 e−/Å2 (b) significant bubbling in the ice occurred. In comparison HAADF STEM images taken using 300 e−/Å2 (c) and after exposing to a further 1000 e−/Å2 (d) indicated much less bubbling of the ice. The inset in (d) indicates more clearly the bubbling of the vitreous ice shown by the white arrow. Cryo-analytical STEM has been used to characterize a calcium phosphorus rich corona around BaTiO3 nanoparticles suspended in cell culture media (e–f). STEM/EDX maps are shown in (g) for Ti Kα (blue), Ba Lα (red), P Kα (green), Ca Kα (yellow), Mg Kα (turquoise) and Na Kα (pink) and indicate Ca and P spatially resolve to the position of the coating. The EDX data was acquired using a total electron fluence of 1900 e−/Å2. Adapted from [65].
EELS, while in principle a more efficient technique in terms of signal collection, generally suffers from the problem of a small analytical signal on a relatively large background which increases drastically with specimen thickness. Preliminary studies on model organic crystals of theophylline using low fluence diffraction mode in CTEM have indicated the potential for monitoring radiation damage by both low loss and higher energy core loss EELS spectroscopy on beam-sensitive materials [84]. Li & Egerton [43] have shown that the radiation induced changes to the conjugated organic compound coronene are identified by diffraction at lower doses than changes in molecular structure are identified by EELS. Inorganic oxides, however, can show electron beam induced changes in EELS fine structure at doses where changes in structure are not identified by STEM imaging [85]. Freeman et al. [86] have demonstrated the ability to accurately measure an electron beam induced change in Fe(II)/Fe(III) ratio in green rust: a redox-active, mixed-valence layered double hydroxide using low-dose EELS. Using both anaerobic sample transfer procedures and also cryo-TEM methods, electron fluences above 40 ± 5 e−/Å2 were shown to induce in-situ oxidation of Fe(II) to Fe(III) in green rust platelets without a change in structure being identified by electron diffraction. The advent of direct detection EELS systems (based on the same principle as direct detection cameras for imaging) should enable low fluence EELS measurements to become more commonplace in the future [87]. A further interesting development in recent years has been the improvement in electron monochromator design and hence the improvements in associated EELS energy resolution (down to ca 10 meV) that has allowed the recording of vibrational losses induced by the electron beam. Such ultra-low energy excitations may be significantly delocalized (to the order of nanometres), particularly in non-sample-penetrating geometries, and Rez et al. [88] have demonstrated the ability to record a vibrational EEL signature (analogous to an infra-red spectrum) from an organic crystal of guanine with the beam aloof from the crystal at a distance of a few nanometres. As this aloof distance is greater than that required to cause electronic excitation and hence radiolysis, this makes the vibrational EELS measurement effectively damage free.
9. Liquid cell TEM/STEM
Recent developments have led to the emergence of liquid cells for (S)TEM, which allows for in-situ characterization of materials in contact with liquids (such as nanoparticles or thin cross-sections produced by focused ion beam (FIB)-SEM methods; figure 8). Dynamic processes such as nanoparticle growth [89], crystallization [90,91] and some biological processes [92] have been captured by this technology. Recently, the so-called four-dimensional liquid cell TEM has been shown to enable single particle reconstruction of the three-dimensional morphology of the iron storage protein ferritin in water [93] and even the structure of water itself has been probed by (vibrational) EELS of water encapsulated between two sheets of boron nitride [94].
Figure 8.
Schematic of typical liquid phase TEM holder (a) and cell (b). Silicon nitride windows are typically 200 µm × 50 µm × 50 nm. An example of using liquid phase TEM to follow the hydration of calcium sulfate in aqueous solution is shown in (c) and (d); bassanite rods in (c) transform to larger gypsum crystals (d). (Online version in colour.)
All liquid cell designs involve a microfluidic device and incorporate a membrane that prevents evaporation of the liquid sample in the microscope vacuum. This membrane is most often fabricated from thin (typically 10–50 nm) silicon nitride that can be functionalized with surfactants or plasma-treated to provide either a positively or negatively charged surface, or (depending on the liquid and nanoparticle type) either a hydrophobic or hydrophilic surface. Functionalization may assist nanoparticle attachment to the membrane that aids the imaging process. However, issues associated with the use of a membrane include: alterations to the particle dispersion arising as a result of the membrane attachment process itself, charging of the membrane under the electron beam, and secondary electron production in the dense, solid membrane which could result in localized or enhanced radiolytic damage to either the sample or the liquid close to the membrane. Initial shortcomings in the design of liquid cell holders meant that it was difficult or even impossible to undertake elemental analysis, but these have now been overcome and Lewis et al. [95] have successfully demonstrated imaging and EDX analysis of a multi-component nanoparticle suspension in a liquid cell.
Besides issues with the membrane, the main artefact in liquid cell TEM analysis arises from beam-induced effects that occur in the liquid suspension. For aqueous samples, the radiolysis of water leads to the production of hydrated electrons (eh) and OH·, H· and H2· radicals. This is a rapid process occurring within 10 ps of energy transfer between the incident electron and a water molecule. Additional reactions of these radiolysis products cause further damage products to form; H2O2, H3O+ and H2O· [12]. These damage products can then diffuse in solution and undergo reactions with each other and any surrounding molecules or particles in the liquid [96]. These effects can lead to beam-induced charging of both the membrane and the sample, gas bubble formation, pH changes (in the absence of competing processes, the increased level of H3O+ may cause a decrease in the solution pH), increases in the ionic strength of the solution and even changes in chemistry of the material under investigation. Furthermore, mass transport of species produced by radiolysis outside the irradiated volume can also occur. These damage mechanisms can be advantageous, in principle, for investigating and driving dynamic processes [97]. However, they are highly detrimental when aiming to accurately characterize a starting solution and any suspended products. A continuous slow flow rate of liquid can partially mitigate these radiolytic effects, but they can all severely affect the dispersion state and chemistry of a nanoparticulate suspension, potentially causing heterogeneous nucleation of particles on the membrane, particle agglomeration (including self-organization of particles on the membrane), aggregation, etching and growth of nanoparticles or even dissolution.
All of the events described above can have severe implications for studying dynamic processes (e.g. crystallization or dissolution) within a dispersion or solution using liquid cell TEM, as any changes in pH, particle charge or concentration including supersaturation can severely modify behaviour. Abellan et al. [97] have discussed the factors influencing quantitative imaging in liquid cells, and Schneider et al. [12] have produced a predictive model to calculate the radiolysis effects in water under a given set of microscope beam conditions. Typically, dose rates have to be kept at or below MGy per second to avoid unwanted alteration [98], although consideration should be given to the effects of different imaging modes, i.e. global irradiation in CTEM versus local irradiation in STEM—generally observation by STEM appears to be advantageous relative to CTEM, possibly because intermittent scanning allows recombination of damage products in aqueous media. Electron beam-induced alteration of aqueous-based suspending media will occur even at relatively low electron fluence (less than 100 e−/Å2) [97]. It has been established that under STEM conditions operating at an averaged flux per pixel/frame close to 140 e−/(Å2s), nanoparticle clusters can break apart and move out of the field of view in seconds due to alterations of their surface charge induced by radiolysis products generated in the suspending solution [99].
A potential option here is to measure the chemical kinetics (of, for example, a nucleation, growth or dissolution process) as a function of electron fluence and then extrapolate to ultra-low fluences. Alternatively, chemical scavengers can be used to mop-up reactive radical species created during radiolysis [97] or non-polar liquids can be employed which are less susceptible to radiolytic damage than polar liquids.
Apart from changes to local chemistry in the liquid cell (S)TEM arising from radiolysis, a further issue to consider is that associated with the volume confinement of the liquid which may potentially influence fluid mixing and alter chemical reaction pathways relative to macroscale environments. Indeed, the induction time, polymorph selection, shape evolution and composition for the crystallization of calcium carbonate [100] and calcium sulphate [101] are known to differ significantly when in confinement [102,103], possibly as a result of changes in ion flux from solution due to differences in diffusion and ion incorporation rates [104].
Similar microfluidic devices can be used to provide in-situ gaseous environments in the TEM allowing the study of gaseous-solid interactions up to gas pressures of typically a few bar [105], however here the damage issues are somewhat reduced relative to liquids due to the much decreased gas density. Alternatively, differential pumping around the sample can be used to allow direct gas injection into the column (so-called true environmental TEM), however here pressures are limited to the order of mbar.
10. Cryogenic-CTEM/STEM
An alternative to liquid cell TEM is the preparation of frozen, hydrated suspensions of a material which are transferred and imaged in the TEM at temperatures of less than −165°C (referred to as cryogenic (cryo-)TEM). A sample is blotted onto a TEM grid and plunged rapidly into a cryogen, commonly liquid ethane, such that the suspension is captured in a thin layer of electron transparent, vitrified ice. Rapid vitrification of the liquid ensures the sample is captured in its native state, i.e. without re-dispersion or crystallization of the suspending solution or vacuum-induced drying of any hydrated surfaces. Time-resolved spraying (at a time resolution of tens of milliseconds) of dispersions onto TEM grids is also possible [106]. Cryo-TEM (almost exclusively CTEM) is most commonly associated with the determination of three-dimensional biomolecular structures at near-atomic resolution [107], either by single particle analysis (essentially viewing many different projections of identical objects) or by tomographic methods (i.e. tilting a single object over a range of projections), both of which have undergone significant advances in recent years. However, cryo-TEM has also been used to investigate liquid crystal structure [108] and the ordering of aggregates of gold nanoparticles within organic solvents [109].
There are significant benefits of cryo-TEM over liquid cell TEM. Firstly, the reduced temperature of vitreous ice relative to liquid water will lower the rates of some, if not all of the reactions involved in the electron beam-induced damage mechanisms [110,111], therefore reducing the rate of radiation damage of both the suspending media and the sample. In addition, radiolysis of water is a diffusion-limited process [96], and the diffusion rates in an amorphous solid held below the glass transition temperature (−137°C for water) are orders of magnitude slower than in the liquid [112]. Reactive species (e.g. eh, OH·, H·, H2· H2O2, H3O+ and H2O·) produced during radiolysis in vitreous ice at -165°C will diffuse more slowly than in liquid water, reducing secondary damage to the vitreous ice [113]. Furthermore, provided the suspending ice remains structurally intact, the native dispersion of the material should remain unaltered; note there is always physical movement of a specimen in vitreous ice during the beginning of irradiation and further charge accumulation in the ice can cause unwanted specimen movement and loss of contrast at atomic lattice resolution, however, recent work shows this can be minimized to only a few per cent of information transfer if a conductive support is used [114,115]. The agglomeration of nanoparticles dispersed in cell culture media has been reported using this technique [116] and flattening of nanoparticle agglomerates by the blotting process, pre-plunge freezing can even be accounted for [117]. Cryo-TEM also removes any compositional drying artefacts as well as preventing physical movement that occurs during conventional drop cast TEM sample preparation [118]. A variation on this technique involves plunge-freezing a particulate dispersion followed by vacuum sublimation of the vitrified ice so as to study snapshots of dynamic behaviour [116] such as agglomeration and also nucleation and growth processes during crystallization. The latter approach has been used to highlight nucleation processes which follow non-classical pathways [119].
Ilett et al. [65] undertook a direct comparison of cryo-TEM and cryo-STEM methods. Bubbling of the vitreous ice caused by the electron beam was shown to occur at far higher electron fluences in STEM (less than 2000 e−/Å2) compared to CTEM (less than 100 e−/Å2) resulting in unwanted movement of entrapped nanoparticles. Bubble visibility is dependent on both breaking of bonds in the water, the diffusion rate of hydrogen within the solid layer and the nucleation of a bubble and so is not itself a primary metric of radiation damage in the ice, however STEM did produce less movement of entrapped nanoparticles than CTEM (figure 9a). They also demonstrated the possibility for STEM/EDX elemental mapping of samples in vitreous ice. This opens up the exciting possibility for studying the structure, chemistry and potentially dynamics of solid/liquid interfaces at high spatial resolution, figure 9b.
11. Conclusion
The native state analysis of complex, beam-sensitive materials by transmission electron microscopy firstly requires an understanding and characterization of the dominant electron beam-induced damage mechanism in the sample under study. In those materials for which radiolysis dominates, it is clear that beam-induced damage to a specimen cannot be eliminated or switched off and so analysis has to be done within a dose budget to achieve an optimum dose-limited resolution. Here thought should be given to the sample thickness, the mode of operation of the microscope (STEM versus CTEM), the detectors, as well as the microscope operating parameters such as accelerating voltage. This understanding can then be carried forward for in-situ analysis of samples interacting with liquids and gases provided the electron beam-induced alterations of these latter phases are controlled or used to drive a chosen reaction. Finally, we stress that cryo-TEM still has a significant place for benchmarking dynamic processes at higher resolution.
Data accessibility
This article has no additional data.
Authors' contributions
The manuscript was written by R.B. with full contributions from all other authors.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Chen Q, Dwyer C, Sheng G, Zhu C, Li X, Zheng C, Zhu Y. 2020. Imaging beam-sensitive materials by electron microscopy. Adv. Mater. 2020, 1907619 ( 10.1002/adma.201907619) [DOI] [PubMed] [Google Scholar]
- 2.Goode AE, Porter AE, Kłosowski MM, Ryan MP, Heutz S, McComb DW. 2017. Analytical transmission electron microscopy at organic interfaces. Curr. Opin. Solid State Mater. Sci. 21, 55–67. ( 10.1016/j.cossms.2016.02.005) [DOI] [Google Scholar]
- 3.Glaeser RM. 1971. Limitations to significant information in biological electron microscopy as a result of radiation damage. J. Ultrastruct. Res. 36, 466–482. ( 10.1016/S0022-5320(71)80118-1) [DOI] [PubMed] [Google Scholar]
- 4.Glaeser RM, Taylor KA. 1978. Radiation damage relative to transmission electron microscopy of biological specimens at low temperature: a review. J. Microsc. 112, 127–138. ( 10.1111/j.1365-2818.1978.tb01160.x) [DOI] [PubMed] [Google Scholar]
- 5.Hobbs LW. 1979. Radiation effescts in analysis of inorganic specimens by TEM (eds Hren JJ, Goldstein JL, Joy DC). New York: NY: Plenum Press. [Google Scholar]
- 6.Reimer L. 1984. Specimen damage by electron irradiation. Berlin, Germany: Springer-Verlag. [Google Scholar]
- 7.Egerton RF, Li P, Malac M. 2004. Radiation damage in the TEM and SEM. Micron 35, 399–409. ( 10.1016/j.micron.2004.02.003) [DOI] [PubMed] [Google Scholar]
- 8.Jenkins ML. 1994. Characterisation of radiation-damage microstructures by TEM. J. Nucl. Mater. 216, 124–156. ( 10.1016/0022-3115(94)90010-8) [DOI] [Google Scholar]
- 9.Farbos B, et al. 2014. Nanoscale structure and texture of highly anisotropic pyrocarbons revisited with transmission electron microscopy, image processing, neutron diffraction and atomistic modeling. Carbon 80, 472–489. ( 10.1016/j.carbon.2014.08.087) [DOI] [Google Scholar]
- 10.Karthik C, Kane J, Butt DP, Windes WE, Ubic R. 2011. In situ transmission electron microscopy of electron-beam induced damage process in nuclear grade graphite. J. Nucl. Mater. 412, 321–326. ( 10.1016/j.jnucmat.2011.03.024) [DOI] [Google Scholar]
- 11.Mironov BE, et al. 2015. Electron irradiation of nuclear graphite studied by transmission electron microscopy and electron energy loss spectroscopy. Carbon 83, 106–117. ( 10.1016/j.carbon.2014.11.019) [DOI] [Google Scholar]
- 12.Schneider NM, Norton MM, Mendel BJ, Grogan JM, Ross FM, Bau HH. 2014. Electron–water interactions and implications for liquid cell electron microscopy. J. Phys. Chem. C. 118, 22 373–22 382. ( 10.1021/jp507400n) [DOI] [Google Scholar]
- 13.Egerton RF. 2019. Radiation damage to organic and inorganic specimens in the TEM. Micron. 119, 72–87. ( 10.1016/j.micron.2019.01.005) [DOI] [PubMed] [Google Scholar]
- 14.Russo CJ, Egerton RF. 2019. Damage in electron cryomicroscopy: lessons from biology for materials science. MRS Bull. 44, 935–941. ( 10.1557/mrs.2019.284) [DOI] [Google Scholar]
- 15.Egerton RF. 2014. Choice of operating voltage for a transmission electron microscope. Ultramicroscopy 145, 85–93. ( 10.1016/j.ultramic.2013.10.019) [DOI] [PubMed] [Google Scholar]
- 16.Egerton RF. 2012. Mechanisms of radiation damage in beam-sensitive specimens, for TEM accelerating voltages between 10 and 300 kV. Microsc. Res. Tech. 75, 1550–1556. ( 10.1002/jemt.22099) [DOI] [PubMed] [Google Scholar]
- 17.Krivanek OL, et al. 2010. Gentle STEM: ADF imaging and EELS at low primary energies. Ultramicroscopy 110, 935–945. ( 10.1016/j.ultramic.2010.02.007) [DOI] [Google Scholar]
- 18.Liu Z, Suenaga K, Harris PJF, Iijima S. 2009. Open and closed edges of graphene layers. Phys. Rev. Lett. 102, 015501 ( 10.1103/PhysRevLett.102.015501) [DOI] [PubMed] [Google Scholar]
- 19.Henderson R, Glaeser RM. 1985. Quantitative analysis of image contrast in electron micrographs of beam-sensitive crystals. Ultramicroscopy 16, 139–150. ( 10.1016/0304-3991(85)90069-5) [DOI] [Google Scholar]
- 20.Jones W, Thomas JM. 1979. Applications of electron microscopy to organic solid-state chemistry. Prog. Solid State Chem. 12, 101–124. ( 10.1016/0079-6786(79)90003-7) [DOI] [Google Scholar]
- 21.Garvie LAJ, Zega TJ, Rez P, Buseck PR. 2004. Nanometer-scale measurements of Fe3+/ΣFe by electron energy-loss spectroscopy: a cautionary note. Am. Mineral. 89, 1610–1616. ( 10.2138/am-2004-11-1204) [DOI] [Google Scholar]
- 22.Hooley R, Brown A, Brydson R. 2019. Factors affecting electron beam damage in calcite nanoparticles. Micron 120, 25–34. ( 10.1016/j.micron.2019.01.011) [DOI] [PubMed] [Google Scholar]
- 23.Jones W. 1976. Interaction of high-energy electrons with organic crystals in the electron microscope: difficulties associated with the study of defects. In Surface and defect properties of solids: volume 5 (eds Roberts MW, Thomas JM), pp. 65–80. The Royal Society of Chemistry. [Google Scholar]
- 24.Kumar S, Adams WW. 1990. Electron beam damage in high temperature polymers. Polymer 31, 15–19. ( 10.1016/0032-3861(90)90341-U) [DOI] [Google Scholar]
- 25.Pan M, Crozier PA. 1993. Quantitative imaging and diffraction of zeolites using a slow-scan CCD camera. Ultramicroscopy 52, 487–498. ( 10.1016/0304-3991(93)90065-6) [DOI] [Google Scholar]
- 26.Revol JF, Manley RSJ. 1986. Lattice imaging in polyethylene single crystals. J. Mater. Sci. Lett. 5, 249–251. ( 10.1007/BF01748066) [DOI] [Google Scholar]
- 27.Pan Y, Brown A, Brydson R. 2006. Electron beam damage studies on 6-line ferrihydrite. J. Phys: Conf. Ser. 26, 46–49. ( 10.1088/1742-6596/26/1/011) [DOI] [PubMed] [Google Scholar]
- 28.Eddleston MD, Bithell EG, Jones W. 2010. Transmission electron microscopy of pharmaceutical materials. J. Pharm. Sci. 99, 4072–4083. ( 10.1002/jps.22220) [DOI] [PubMed] [Google Scholar]
- 29.Cattle J, S'Ari M, Hondow N, Abellán P, Brown AP, Brydson RMD. 2015. Transmission electron microscopy of a model crystalline organic, theophylline. J. Phys: Conf. Ser. 644, 012030 ( 10.1088/1742-6596/644/1/012030) [DOI] [Google Scholar]
- 30.Cattle JE. 2019. Transmission electron microscopy of organic crystalline material and zeolites. PhD thesis, University of Leeds. [Google Scholar]
- 31.Stenn K, Bahr GF. 1970. Specimen damage caused by the beam of the transmission electron microscope, a correlative reconsideration. J. Ultrastruct. Res. 31, 526–550. ( 10.1016/s0022-5320(70)90167-x) [DOI] [PubMed] [Google Scholar]
- 32.Clark WRK, Chapman JN, MacLeod AM, Ferrier RP. 1980. Radiation damage mechanisms in copper phthalocyanine and its chlorinated derivatives. Ultramicroscopy 5, 195–208. ( 10.1016/0304-3991(80)90024-8) [DOI] [Google Scholar]
- 33.Grubb DT. 1974. Radiation damage and electron microscopy of organic polymers. J. Mater. Sci. 9, 1715–1736. ( 10.1007/BF00540772) [DOI] [Google Scholar]
- 34.Howitt DG, Thomas G. 1977. Electron damage in organic crystals. Radiation Effects 34, 209–215. ( 10.1080/00337577708233149) [DOI] [Google Scholar]
- 35.Russo CJ, Passmore LA. 2014. Electron microscopy: ultrastable gold substrates for electron cryomicroscopy. Science 346, 1377–1380. ( 10.1126/science.1259530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warkentin M, Atakisi H, Hopkins J, Walko D, Thorne R. 2017. Lifetimes and spatio-temporal response of protein crystals in intense X-ray microbeams. IUCrJ 4, 785–794. ( 10.1107/S2052252517013495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheres SHW. 2014. Beam-induced motion correction for sub-megadalton cryo-EM particles. eLife 3, e03665 ( 10.7554/eLife.03665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brilot AF, et al. 2012. Beam-induced motion of vitrified specimen on holey carbon film. J. Struct. Biol. 177, 630–637. ( 10.1016/j.jsb.2012.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegel G. 1972. The influence of very low temperature on the radiation damage of organic crystals irradiated by 100keV electrons. Z. Naturforsch. A Physik, Physikalische Chemie, Kosmophysik. 27, 325–332. [Google Scholar]
- 40.S'ari M, Blade H, Brydson R, Cosgrove SD, Hondow N, Hughes LP, Brown A. 2018. Toward developing a predictive approach to assess electron beam instability during transmission electron microscopy of drug molecules. Mol. Pharm. 15, 5114–5123. ( 10.1021/acs.molpharmaceut.8b00693) [DOI] [PubMed] [Google Scholar]
- 41.Fryer JR. 1987. The effect of dose rate on imaging aromatic organic crystals. Ultramicroscopy 23, 321–327. ( 10.1016/0304-3991(87)90242-7) [DOI] [Google Scholar]
- 42.Fryer JR, McConnell CH, Zemlin F, Dorset DL. 1992. Effect of temperature on radiation damage to aromatic organic molecules. Ultramicroscopy 40, 163–169. ( 10.1016/0304-3991(92)90057-Q) [DOI] [Google Scholar]
- 43.Li P, Egerton RF. 2004. Radiation damage in coronene, rubrene and p-terphenyl, measured for incident electrons of kinetic energy between 100 and 200 kev. Ultramicroscopy 101, 161–172. ( 10.1016/j.ultramic.2004.05.010) [DOI] [PubMed] [Google Scholar]
- 44.Isaacson M. 1975. Inelastic scattering and beam damage in biological molecules. In Physical aspects of electron microscopy and microbeam analysis (eds BM Seigel, DR Beaman). New York, NY: Wiley. [Google Scholar]
- 45.McEwen BF, Downing KH, Glaeser RM. 1995. The relevance of dose-fractionation in tomography of radiation-sensitive specimens. Ultramicroscopy 60, 357–373. ( 10.1016/0304-3991(95)00082-8) [DOI] [PubMed] [Google Scholar]
- 46.Rose A. 1973. Vision: human and electronic. New York, NY: Plenum Press. [Google Scholar]
- 47.Peet MJ, Henderson R, Russo CJ. 2019. The energy dependence of contrast and damage in electron cryomicroscopy of biological molecules. Ultramicroscopy 203, 125–131. ( 10.1016/j.ultramic.2019.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roncal-Herrero T, Harrington J, Zeb A, Milne S, Brown A. 2018. Nanoscale compositional segregation and suppression of polar coupling in a relaxor ferroelectric. Acta Mater. 158, 53 ( 10.1016/j.actamat.2018.07.053) [DOI] [Google Scholar]
- 49.Murata Y, Fryer JR, Baird T. 1976. Molecular image of copper phthalocyanine. J. Microsc. 108, 261–275. ( 10.1111/j.1365-2818.1976.tb01098.x) [DOI] [Google Scholar]
- 50.Smith DJ, Fryer JR. 1981. Molecular detail in electron micrographs of quaterrylene C40H20. Nature 291, 481–482. ( 10.1038/291481a0) [DOI] [Google Scholar]
- 51.Zemlin F, Reuber E, Beckmann E, Zeitler E, Dorset DL. 1985. Molecular resolution electron micrographs of monolamellar paraffin crystals. Science 229, 461–462. ( 10.1126/science.4012326) [DOI] [PubMed] [Google Scholar]
- 52.Zhang D, Zhu Y, Liu L, Ying X, Hsiung C-E, Sougrat R, Li K, Han Y. 2018. Atomic-resolution transmission electron microscopy of electron beam-sensitive crystalline materials. Science 359, 675–679. ( 10.1126/science.aao0865) [DOI] [PubMed] [Google Scholar]
- 53.McMullan G, Chen S, Henderson R, Faruqi AR. 2009. Detective quantum efficiency of electron area detectors in electron microscopy. Ultramicroscopy 109, 1126–1143. ( 10.1016/j.ultramic.2009.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.S'Ari M, Koniuch N, Brydson R, Hondow N, Brown A. 2020. High-resolution imaging of organic pharmaceutical crystals by transmission electron microscopy and scanning moiré fringes. J. Microsc. 279, 197–206. ( 10.1111/jmi.12866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rose H. 1974. Phase-contrast in scanning-transmission-electron-microscopy. Optik 39, 416–436. [Google Scholar]
- 56.Jones L, Downing C. 2018; The MTF & DQE of Annular Dark Field STEM: implications for low-dose imaging and compressed sensing. Microsc. Microanal. 24, 478–479. ( 10.1017/S143192761800288X)30334517 [DOI] [Google Scholar]
- 57.Sader K, Brown A, Brydson R, Bleloch A. 2010. Quantitative analysis of image contrast in phase contrast STEM for low dose imaging. Ultramicroscopy 110, 1324–1331. (doi:101016/jultramic201006008) [Google Scholar]
- 58.Yücelen E, Lazić I, Bosch EGT. 2018. Phase contrast scanning transmission electron microscopy imaging of light and heavy atoms at the limit of contrast and resolution. Sci. Rep. 8, 2676 ( 10.1038/s41598-018-20377-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sagawa R, Hashiguchi H, Kondo Y. 2018. 3aA_MI-5Low Dose Ptychographic STEM observation using fast pixelated detector. Microscopy 67(Suppl. 2), i27 ( 10.1093/jmicro/dfy080) [DOI] [Google Scholar]
- 60.Gallagher-Jones M, et al. 2019. Nanoscale mosaicity revealed in peptide microcrystals by scanning electron nanodiffraction. Commun. Biol. 2, 26 ( 10.1038/s42003-018-0263-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang Y, et al. 2018. Electron ptychography of 2D materials to deep sub-ångström resolution. Nature 559, 343–349. ( 10.1038/s41586-018-0298-5) [DOI] [PubMed] [Google Scholar]
- 62.Johnstone D, Firth F, Grey C, Midgley PA, Cliffe M, Collins SM. 2020. Direct imaging of correlated defect nanodomains in a metal-organic framework. ChemRXiv. 142, 13081–13089. ( 10.26434/chemrxiv.12024402.v1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Egerton RF, Rauf I. 1999. Dose-rate dependence of electron-induced mass loss from organic specimens. Ultramicroscopy 80, 247–254. ( 10.1016/S0304-3991(99)00114-X) [DOI] [Google Scholar]
- 64.VandenBussche EJ, Flannigan DJ. 2019. Reducing radiation damage in soft matter with femtosecond-timed single-electron packets. Nano Lett. 19, 6687–6694. ( 10.1021/acs.nanolett.9b03074) [DOI] [PubMed] [Google Scholar]
- 65.Ilett M, Brydson R, Brown A, Hondow N. 2019. Cryo-analytical STEM of frozen, aqueous dispersions of nanoparticles. Micron 120, 35–42. ( 10.1016/j.micron.2019.01.013) [DOI] [PubMed] [Google Scholar]
- 66.Hayashida M, Kawasaki T, Kimura Y, Takai Y. 2006. Estimation of suitable condition for observing copper–phthalocyanine crystalline film by transmission electron microscopy. Nucl. Instrum. Methods Phys. Res., Sect. B. 248, 273–278. ( 10.1016/j.nimb.2006.04.168) [DOI] [Google Scholar]
- 67.Humphreys J. 1990. References. In Reflection groups and coxeter groups. Cambridge studies in advanced mathematics (ed. Humphreys JE.), pp. 185–202. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 68.Cazaux J. 1995. Correlations between ionization radiation damage and charging effects in transmission electron microscopy. Ultramicroscopy 60, 411–425. ( 10.1016/0304-3991(95)00077-1) [DOI] [Google Scholar]
- 69.Egerton RF. 2018. Calculation, consequences and measurement of the point spread function for low-loss inelastic scattering. Microscopy 67(Suppl. 1), i52–i9. ( 10.1093/jmicro/dfx089) [DOI] [PubMed] [Google Scholar]
- 70.Egerton RF. 2013. Control of radiation damage in the TEM. Ultramicroscopy 127, 100–108. ( 10.1016/j.ultramic.2012.07.006) [DOI] [PubMed] [Google Scholar]
- 71.Muller DA, Silcox J. 1995. Radiation damage of Ni3Al by 100keV electrons. Philos. Mag. A 71, 1375–1387. ( 10.1080/01418619508244380) [DOI] [Google Scholar]
- 72.Ward MB, et al. 2013. Investigating the role of microbes in mineral weathering: nanometre-scale characterisation of the cell-mineral interface using FIB and TEM. Micron 47, 10–17. ( 10.1016/j.micron.2012.12.006) [DOI] [PubMed] [Google Scholar]
- 73.Fryer JR. 1984. Radiation damage in organic crystalline films. Ultramicroscopy 14, 227–236. ( 10.1016/0304-3991(84)90091-3) [DOI] [Google Scholar]
- 74.Buban JP, Ramasse Q, Gipson B, Browning ND, Stahlberg H. 2010. High-resolution low-dose scanning transmission electron microscopy. J. Electron Microsc. 59, 103–112. ( 10.1093/jmicro/dfp052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zobelli A, et al. 2019. Spatial and spectral dynamics in STEM hyperspectral imaging using random scan patterns. Ultramicroscopy 2019, 112912 ( 10.1016/j.ultramic.2019.112912) [DOI] [PubMed] [Google Scholar]
- 76.Stevens A, Luzi L, Yang H, Kovarik L, Mehdi BL, Liyu A, Gehm ME, Browning ND. 2018. A sub-sampled approach to extremely low-dose STEM. Appl. Phys. Lett. 112, 043104 ( 10.1063/1.5016192) [DOI] [Google Scholar]
- 77.Candes EJ, Romberg J, Tao T. 2006. Robust uncertainty principles: exact signal reconstruction from highly incomplete frequency information. IEEE Trans. Inform. Theory. 52, 489–509. ( 10.1109/TIT.2005.862083) [DOI] [Google Scholar]
- 78.Donoho DL. 2006. Compressed sensing. IEEE Trans. Inform. Theory. 52, 1289–1306. ( 10.1109/TIT.2006.871582) [DOI] [Google Scholar]
- 79.Naden AB, O'Shea KJ, MacLaren DA. 2018. Evaluation of crystallographic strain, rotation and defects in functional oxides by the moiré effect in scanning transmission electron microscopy. Nanotechnology 29, 165704 ( 10.1088/1361-6528/aaae50) [DOI] [PubMed] [Google Scholar]
- 80.S'Ari M, Cattle J, Hondow N, Brydson R, Brown A. 2019. Low dose scanning transmission electron microscopy of organic crystals by scanning moiré fringes. Micron 120, 1–9. ( 10.1016/j.micron.2019.01.014) [DOI] [PubMed] [Google Scholar]
- 81.Su D, Zhu Y. 2010. Scanning moiré fringe imaging by scanning transmission electron microscopy. Ultramicroscopy 110, 229–233. ( 10.1016/j.ultramic.2009.11.015) [DOI] [PubMed] [Google Scholar]
- 82.Read DT, Dally JW. 1996. Theory of electron beam moiré. J. Res. Natl Inst. Stand. Technol. 101, 47–67. ( 10.6028/jres.101.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brydson R. 2001. Electron energy loss spectroscopy. Oxford, UK: Bios in association with the Royal Microscopical Society. [Google Scholar]
- 84.Brydson R, Eddleston MD, Jones W, Seabourne CR, Hondow N. 2014. EELS from organic crystalline materials. J. Phys: Conf. Ser. 522, 012060 ( 10.1088/1742-6596/522/1/012060) [DOI] [Google Scholar]
- 85.Nord M, Vullum PE, Hallsteinsen I, Tybell T, Holmestad R. 2016. Assessing electron beam sensitivity for SrTiO3 and La0.7Sr0.3MnO3 using electron energy loss spectroscopy. Ultramicroscopy 169, 98–106. ( 10.1016/j.ultramic.2016.07.004) [DOI] [PubMed] [Google Scholar]
- 86.Freeman HM, Perez JPH, Hondow N, Benning LG, Brown AP. 2019. Beam-induced oxidation of mixed-valent Fe (oxyhydr)oxides (green rust) monitored by STEM-EELS. Micron 122, 46–52. ( 10.1016/j.micron.2019.02.002) [DOI] [PubMed] [Google Scholar]
- 87.Zachman MJ, Tu Z, Choudhury S, Archer LA, Kourkoutis LF. 2018. Cryo-STEM mapping of solid–liquid interfaces and dendrites in lithium-metal batteries. Nature 560, 345–349. ( 10.1038/s41586-018-0397-3) [DOI] [PubMed] [Google Scholar]
- 88.Rez P, et al. 2016. Damage-free vibrational spectroscopy of biological materials in the electron microscope. Nat. Commun. 7, 10945 ( 10.1038/ncomms10945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Evans JE, Jungjohann KL, Browning ND, Arslan I. 2011. Controlled growth of nanoparticles from solution with in situ liquid transmission electron microscopy. Nano Lett. 11, 2809–2813. ( 10.1021/nl201166k) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ievlev AV, Jesse S, Cochell TJ, Unocic RR, Protopopescu VA, Kalinin SV. 2015. Quantitative description of crystal nucleation and growth from in situ liquid scanning transmission electron microscopy. ACS Nano 9, 11 784–11 791. ( 10.1021/acsnano.5b03720) [DOI] [PubMed] [Google Scholar]
- 91.Cookman J, Hamilton V, Price LS, Hall SR, Bangert U. 2020. Visualising early-stage liquid phase organic crystal growth via liquid cell electron microscopy. Nanoscale 12, 4636–4644. ( 10.1039/C9NR08126G) [DOI] [PubMed] [Google Scholar]
- 92.de Jonge N, Peckys DB, Kremers GJ, Piston DW.. 2009. Electron microscopy of whole cells in liquid with nanometer resolution. Proc. Natl Acad. Sci. USA 106, 2159–2164. ( 10.1073/pnas.0809567106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marchello G, De Pace C, Wilkinson N, Ruiz-Perez L, Battaglia G. 2019. 4D liquid-phase electron microscopy of ferritin by Brownian single particle analysis.
- 94.Jokisaari JR, et al. 2018. Vibrational spectroscopy of water with high spatial resolution. Adv. Mater. 30, 1802702 ( 10.1002/adma.201802702) [DOI] [PubMed] [Google Scholar]
- 95.Lewis EA, Haigh SJ, Slater TJA, He Z, Kulzick MA, Burke MG, Zaluzec NJ. 2014. Real-time imaging and local elemental analysis of nanostructures in liquids. Chem. Commun. 50, 10 019–10 022. ( 10.1039/C4CC02743D) [DOI] [PubMed] [Google Scholar]
- 96.Le Caër S. 2011. Water radiolysis: influence of oxide surfaces on H2 production under ionizing radiation. Water 3, 235 ( 10.3390/w3010235) [DOI] [Google Scholar]
- 97.Abellan P, Woehl TJ, Parent LR, Browning ND, Evans JE, Arslan I. 2014. Factors influencing quantitative liquid (scanning) transmission electron microscopy. Chem. Commun. 50, 4873–4880. ( 10.1039/C3CC48479C) [DOI] [PubMed] [Google Scholar]
- 98.Woehl TJ, Jungjohann KL, Evans JE, Arslan I, Ristenpart WD, Browning ND. 2013. Experimental procedures to mitigate electron beam induced artifacts during in situ fluid imaging of nanomaterials. Ultramicroscopy 127, 53–63. ( 10.1016/j.ultramic.2012.07.018) [DOI] [PubMed] [Google Scholar]
- 99.White ER, Mecklenburg M, Shevitski B, Singer SB, Regan BC. 2012. Charged nanoparticle dynamics in water induced by scanning transmission electron microscopy. Langmuir 28, 3695–3698. ( 10.1021/la2048486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zeng M, Kim Y-Y, Anduix-Canto C, Frontera C, Laundy D, Kapur N, Christenson HK, Meldrum FC. 2018. Confinement generates single-crystal aragonite rods at room temperature. Proc. Natl Acad. Sci. USA 115, 7670–7675. ( 10.1073/pnas.1718926115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y-W, Christenson HK, Meldrum FC. 2013. Confinement leads to control over calcium sulfate polymorph. Adv. Funct. Mater. 23, 5615–5623. ( 10.1002/adfm.201300861) [DOI] [Google Scholar]
- 102.Wang Y, Zeng M, Meldrum FC, Christenson HK. 2017. Using confinement to study the crystallization pathway of calcium carbonate. Crystal Growth Des. 17, 6787–6792. ( 10.1021/acs.cgd.7b01359) [DOI] [Google Scholar]
- 103.Stephens CJ, Kim Y-Y, Evans SD, Meldrum FC, Christenson HK. 2011. Early stages of crystallization of calcium carbonate revealed in picoliter droplets. J. Am. Chem. Soc. 133, 5210–5213. ( 10.1021/ja200309m) [DOI] [PubMed] [Google Scholar]
- 104.Kröger R, Verch A. 2018. Liquid cell transmission electron microscopy and the impact of confinement on the precipitation from supersaturated solutions. Minerals 8, 21 ( 10.3390/min8010021) [DOI] [Google Scholar]
- 105.Wu J, Shan H, Chen W, Gu X, Tao P, Song C, Shang W, Deng T. 2016. In situ environmental TEM in imaging gas and liquid phase chemical reactions for materials research. Adv. Mater. 28, 9686–9712. ( 10.1002/adma.201602519) [DOI] [PubMed] [Google Scholar]
- 106.Klebl DP, et al. 2020. Sample deopsition onto cryo-EM grids: from sprays to jets and back. Acta Crystallogr. Sect. D Struct. Biol. 76, 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheng Y, Grigorieff N, Penczek PA, Walz T. 2015. A primer to single-particle cryo-electron microscopy. Cell 161, 438–449. ( 10.1016/j.cell.2015.03.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gao M, et al. 2014. Direct observation of liquid crystals using cryo-TEM: specimen preparation and low-dose imaging. Microsc. Res. Tech. 77, 754–772. ( 10.1002/jemt.22397) [DOI] [PubMed] [Google Scholar]
- 109.Balmes O, Malm J-O, Karlsson G, Bovin J-O. 2004. Cryo-TEM observation of 3-dimensionally ordered aggregates of 5-nm gold particles in organic solvents. J. Nanopart. Res. 6, 569–576. ( 10.1007/s11051-004-2164-7) [DOI] [Google Scholar]
- 110.Elliot AJ, Bartels DM. 2009. The reaction set, rate constants and g-values for the simulation of the radiolysis of light water over the range 20 deg to 350 deg C based on information available in 2008. Canada: Atomic Energy of Canada Ltd; (Report number AECL-153-127160-450-001. Report No.: AECL–153-127160-450-001 Contract No.: AECL–153-127160-450-001). [Google Scholar]
- 111.Sterniczuk M, Bartels DM. 2016. Source of molecular hydrogen in high-temperature water radiolysis. J. Phys. Chem. A. 120, 200–209. ( 10.1021/acs.jpca.5b12281) [DOI] [PubMed] [Google Scholar]
- 112.Speedy RJ, Angell CA. 1976. Isothermal compressibility of supercooled water and evidence for a thermodynamic singularity at −45°C. J. Chem. Phys. 65, 851–858. ( 10.1063/1.433153) [DOI] [Google Scholar]
- 113.Warkentin M, Thorne RE. 2010. Glass transition in thaumatin crystals revealed through temperature-dependent radiation-sensitivity measurements. Acta Crystallogr. Sect. D Biol. Crystallogr. 66, 1092–1100. ( 10.1107/S0907444910035523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Russo CJ, Henderson R. 2018. Microscopic charge fluctuations cause minimal contrast loss in cryoEM. Ultramicroscopy 187, 56–63. ( 10.1016/j.ultramic.2018.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Russo CJ, Henderson R. 2018. Charge accumulation in electron cryomicroscopy. Ultramicroscopy 187, 43–49. ( 10.1016/j.ultramic.2018.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hondow N, et al. 2012. Quantitative characterization of nanoparticle agglomeration within biological media. J. Nanopart. Res. 14, 977 ( 10.1007/s11051-012-0977-3) [DOI] [Google Scholar]
- 117.Wills JW, et al. 2017. Characterizing nanoparticles in biological matrices: tipping points in agglomeration state and cellular delivery in vitro. ACS Nano 11, 11 986–12 000. ( 10.1021/acsnano.7b03708) [DOI] [PubMed] [Google Scholar]
- 118.Roncal-Herrero T, Micklethwaite S, Ilett M, Hitchcock J, Cayre O, Hondow N. 2019. Analytical cryo electron microscopy for characterization of pickering emulsions. Microsc. Microanal. 25, 1706–1707. ( 10.1017/S1431927619009267) [DOI] [Google Scholar]
- 119.Van Driessche AES, Benning LG, Rodriguez-Blanco JD, Ossorio M, Bots P, García-Ruiz JM.. 2012. The role and implications of bassanite as a stable precursor phase to gypsum precipitation. Science 336, 69–72. ( 10.1126/science.1215648) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.