Abstract
Schooling is a collective behaviour that enhances the ability of a fish to sense and respond to its environment. Although schooling is essential to the biology of a diversity of fishes, it is generally unclear how this behaviour is coordinated by different sensory modalities. We used experimental manipulation and kinematic measurements to test the role of vision and flow sensing in the rummy-nose tetra (Hemigrammus rhodostomus), which swims with intermittent phases of bursts and coasts. Groups of five fish required a minimum level of illuminance (greater than 1.5 lx) to achieve the necessary close nearest-neighbour distance and high polarization for schooling. Compromising the lateral line system with an antibiotic treatment caused tetras to swim with greater nearest-neighbour distance and lower polarization. Therefore, vision is both necessary and sufficient for schooling in H. rhodostomus, and both sensory modalities aid in attraction. These results can serve as a basis for understanding the individual roles of sensory modalities in schooling for some fish species.
Keywords: locomotion, flow sensing, collective behaviour
1. Introduction
Schooling is essential to the biology of a diversity of fish species. A school provides a fish with the potential to enhance their ability to identify prey, detect predators and swim efficiently [1–3] by responding to the motion of neighbouring fish [4–6]. Despite this importance, it remains largely unclear how different sensory modalities facilitate communication and decision-making among the individuals in a school. Therefore, the aim of the present study was to test the roles of vision and the lateral-line system in schooling by the rummy-nose tetra (Hemigrammus rhodostomus; Ahl, 1924).
Vision and flow sensing are the two sensory modalities thought to facilitate schooling. Pitcher and Partridge tested the roles of these sensory systems in a pelagic marine species, the pollock (Pollachius virens) [7,8]. They found that blinded pollock were capable of schooling, but only with a functional trunk lateral line, which supports the notion that both modalities are sufficient for the behaviour. Flow sensing may also be sufficient for shoaling in blind cavefish [9,10], but a number of fish species are incapable of schooling in the dark [11–14]. The inability to school in darkness indicates that flow sensing is insufficient and that vision is necessary and likely sufficient. Therefore, the extent that schooling depends on vision or flow sensing varies among fish species.
Vision and flow sensing may play distinct roles in the social behaviour of fishes. Shoaling is a general category of behaviours that cause fish to congregate because they are attracted to one another. In close proximity, fish are commonly repelled by other individuals, which serves to minimize collisions [15]. Schooling is a form of shoaling where the fish move with a similar heading [16], measured as the group polarization. The polarization of a group may be achieved indirectly as a consequence of fish moving towards other fish [4], or results from actively seeking alignment with neighbours [17,18]. Pollock swim more closely together when the lateral line system is compromised, which supports the hypothesis that flow sensing mediates repulsion and vision is the modality used for attraction [8]. This modality-partitioning is compatible with the spatial limitations of the lateral line system [19] and the visual system’s capacity for attraction towards fish at a distance [20]. However, disrupting the lateral line in other species causes the distance between individuals to increase [21,22], which suggests that flow sensing can aid in attraction, unlike what has been reported in some other species [16,23].
The present study evaluated the sensory basis of schooling in a tetra species that swims intermittently with alternating phases of bursts and coasts [24,25]. These distinct locomotor phases may be analysed discretely and have the potential to yield contrasting collective behaviour from species that move continuously [26]. We measured the swimming kinematics of groups of H. rhodostomus individuals both over long durations and with high-speed video in separate experiments. The role of vision was considered by manipulating the illuminance of visible light. The effects of flow sensing were tested by exposure of the lateral line to an aminoglycoside antibiotic, which causes cell death and otherwise attenuates mechanotransduction by lateral-line hair cells [27,28].
2. Material and methods
We video-recorded the swimming of groups of 5 rummy-nose tetra (H. rhodostomus, 2.48 ± 0.14 cm standard length). Our experimental setup used transmitted illumination with IR lights to visualize the fish with high contrast under variable levels of reflected visible light. The fish were enclosed in an arena (), with a shallow water depth (13 cm). Experiments were either performed over a long duration (30 min) at a time-lapse recording rate (, 2.5 ms exposure) or for a brief duration (6–10 s) with high-speed video (, 2 ms exposure) on a total of 105 fish. The long-duration recordings included the entire arena (at ) and thereby offered comprehensive measurements of position, including interactions with the enclosure wall. The high-speed recordings were intended to examine the details of intermittent swimming during schooling through the centre of the tank (approx. 46 cm wide at 2048 × 2048 pixels) and our analysis therefore included the tracking of individuals over time (electronic supplementary material, figure S1a,b).
We tested the roles of the visual and lateral line systems on schooling through experimental manipulation. We varied the white light generated by two lamps, each with a single LED bulb (6 W), controlled with a variable dimmer. This allowed for video recordings of swimming under eight levels of illuminance (0, 0.44, 0.62, 0.66, 0.70, 1.25, 1.80 and 8.15 lx). The lateral-line system was manipulated through exposure to a solution of neomycin sulfate (Fisher BioReagents, Fair Lawn, NJ, USA; see electronic supplemental material for protocol). This treatment was verified in a group of animals that were not used for schooling experiments with a fluorescent vital stain for hair cells (DASPEI, 2-(4-(dumethylamino)styryl)-N-ethylpyridinium iodide, electronic supplementary material, figure S1c). Fish in the control group were handled to the same extent as the treated fish and no anaesthesia was required for the treatment. The effects of the lateral line manipulation were considered for experiments conducted at two light levels (1.80 lx and 8.15 lx) that were found to be sufficient for schooling.
The kinematics of swimming were acquired and analysed with custom software. This software was developed in Matlab (R2018b, MathWorks, Natick, MA, USA; see electronic supplemental material for details). The automated acquisition of kinematics worked by finding the centre of area for each fish, as well as the anterior margin of the rostrum, which allowed for measurements of the heading (θ; electronic supplementary material, figure S1b). For short-duration experiments, we focused on the determinants of the direction and magnitude of turning during bursts by measuring how they varied with the numbers of neighbours to the left and right sides of a focal fish. We similarly measured how the change in speed during a burst varied with the numbers of fish ahead of, and behind, the focal fish. For the long-duration recordings, we calculated the mean speed () and nearest-neighbour distance (), which we found as the mean across time of the mean among individuals. Measurements of the polarization provided a non-dimensional metric of the common alignment of the fish in a school, calculated as follows [6,29]:
| 2.1 |
where i is an index for a particular fish and n is the total number of fish in a school (n = 5). The mean polarization () was calculated as the mean value of a school over time.
3. Results
Tetras schooled with intermittent bursts in speed that were frequently accompanied by changes in heading (figure 1a–c). During these bursts, individuals changed their position within a school and thereby generated large temporal variation of the nearest-neighbour distance and polarization (figure 1d–e). We found that the fish tended to swim faster when they were behind their neighbours (electronic supplementary material, figure S2 and table S2) and that the lateral position of neighbours had a significant effect on the kinematics of turning. In particular, the probability of turning either left or right depended on the number of neighbours on either side of a focal fish (figure 2a). When a fish was balanced by two fish on either side, they would turn towards the right with a probability indistinguishable from 50% (pR = 0.52, 95% CI: 0.43–0.60, n = 15 over 141 experiments). When there were no fish on the right, fish tended to turn towards that side of the body only about one-third of the time (pR = 0.30, 95% CI: 0.23–0.37) and when all four fish were on the right, turns were pointed in that direction around two-thirds of the time (pR = 0.64, 95% CI: 0.56–0.72). These results suggest that turning decisions either considered the numbers of neighbours on opposite sides of the body or resulted from the focal fish moving away from the side of the body with no visible neighbours.
Figure 1.
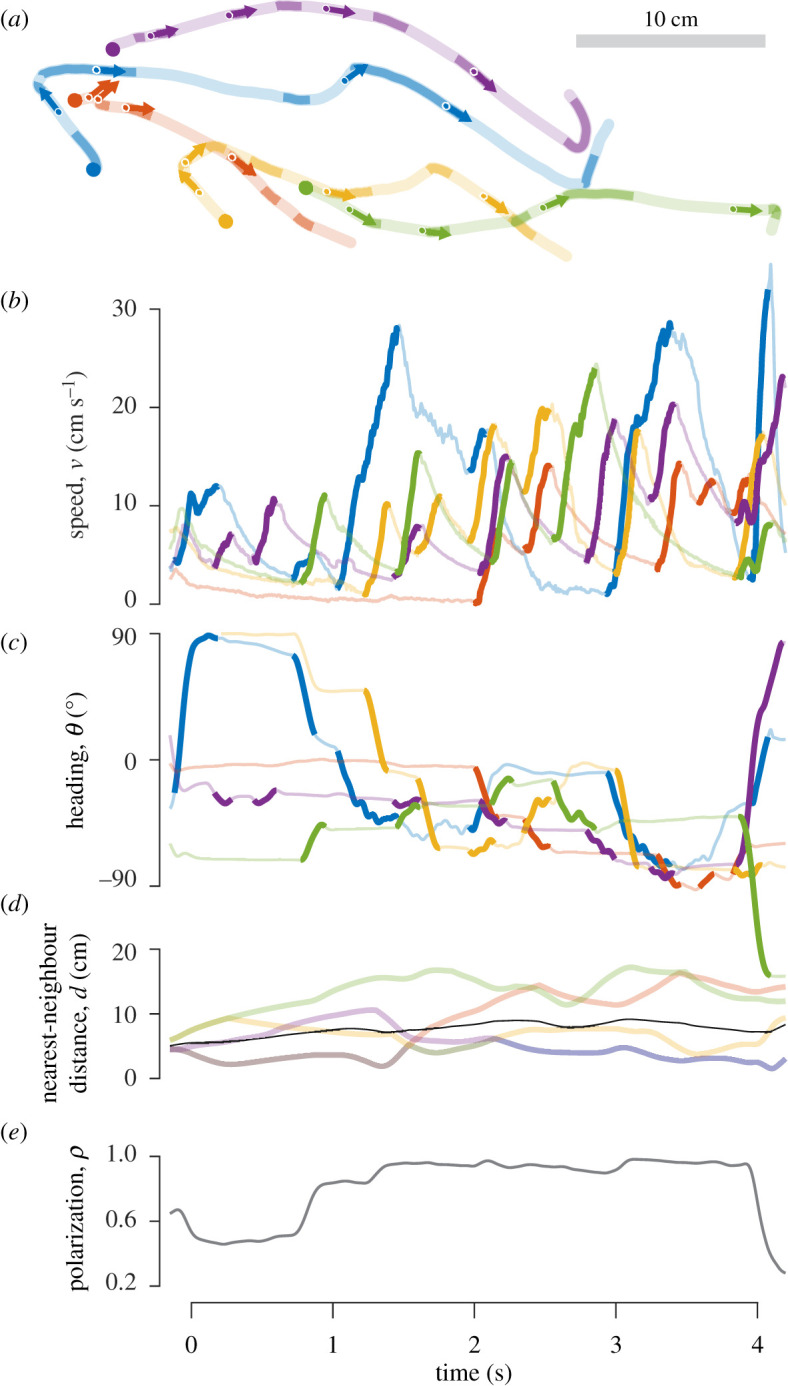
High-speed kinematics of a school of fish swimming intermittently. (a) The trajectory of each fish is displayed with a unique colour, with periods of acceleration indicated by dark curves and decelerations shown in light colours, starting at each filled circle. The arrows indicate the heading of a fish at regular (1 s) intervals, starting at 0.5 s. (b–e) Time series of measurements for the trajectories shown in a. (b) Periods of bursts (dark curves) and coasts (light curves) were found with an automated approach from these measurements of speed. (c) Variation in heading reflects the intermittent changes in direction that were correlated with the bursting phase. (d) The nearest-neighbour distance is indicated with curves that show the shared values between neighbours and the mean among all members (black curve). (e) Polarization was calculated from the heading values in c (equation (2.1)) and indicates the common alignment of fish in the group. (Online version in colour.)
Figure 2.
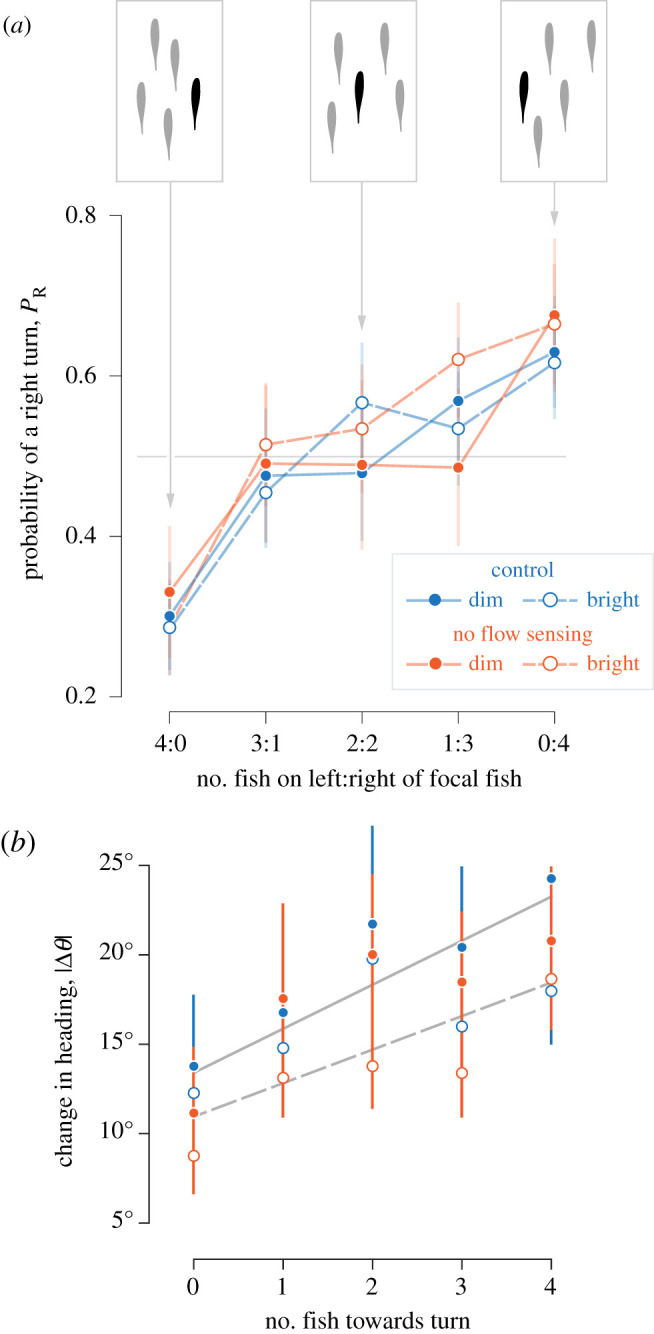
Changes in heading relative to the position of neighbouring fish. Measurements are shown for four neomycin-treated (i.e. ‘no flow sensing’, red, n = 8) and control (blue, n = 8) groups under dim (1.80 lx, filled circles) and bright (8.15 lx, open circles) light for 92–236 turns, depending on the group. (a) Probability of turning towards the right, as a function of the number of fish to the left:right of the focal fish (±95% confidence intervals, assuming binomial distribution). (b) The change in heading during turns is shown with respect to the number of neighbouring fish in the direction of the turn (mean ± 95% confidence intervals, assuming normal distribution) with trend lines for our generalized linear mixed-effects model for experiments under bright (dashed line, r2 = 0.76) and dim (solid line, r2 = 0.82) light (see electronic supplementary material, table S1 for statistics). (Online version in colour.)
The distribution of neighbours around a focal fish affected the magnitude of change in heading during a turn. A generalized linear mixed-effects model found that the number of fish on the side of the body in the direction of a turn showed a highly significant effect on the change in heading during a turn (figure 2b), with an estimated regression coefficient of 1.94° (n = 15, 141 experiments; electronic supplementary material, table S1). This suggests, for example, if there were 4 fish on the left side of a focal fish, then the average left turn would be 7.76° (4 × 1.94°) more than if there were no fish on the left side.
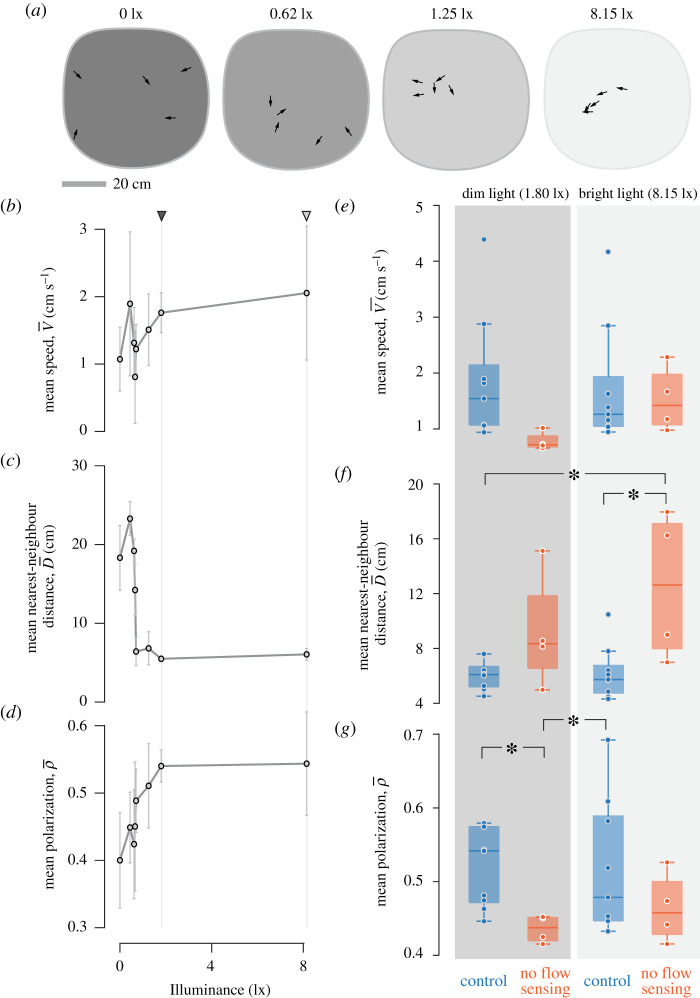
Performing experiments under different levels of illuminance allowed us to address the effects of vision on schooling. Long-duration experiments (electronic supplementary material, figure S3) showed that kinematics varied with the availability of ambient light (figure 3a–d). In darkness and under low illuminance, fish moved with low polarization and a large mean nearest-neighbour distance that spanned many body lengths (greater than 15 cm). Under brighter light (greater than 1.5 lx), the mean distance (±1 s.d.) between nearest neighbours dropped considerably (e.g. at 1.8 lx, n = 5) and fish moved with a more polarized orientation (e.g. ρ = 0.54 ± 0.02 at 1.8 lx, n = 5). The results of our high-speed kinematics showed that fish under brighter light turned to a slightly greater degree during bursts than under dim light (electronic supplementary material, table S1). However, illuminance did not affect turning probability, by comparison of 95% confidence intervals (figure 2a), or the relationship between changes in speed and the position of neighbours (electronic supplementary material, figure S2 and table S2).
Figure 3.
The effects of light and flow sensing on schooling kinematics for long-duration recordings. (a) Arrows denote the position and orientation of fish from video stills of experiments performed at different levels of illuminance. (b–d) Measurements of schooling kinematics among multiple schools under varying light intensity. Triangles above the panels and vertical lines designate the light intensities at which we performed additional experiments at dim (dark grey) and bright (light grey) light. Circles indicate the mean value (±1 s.d.) for the (b) mean speed, (c) mean nearest-neighbour distance, and (d) polarization among the schools (3 < n < 5). (e–g) The effects of lateral line manipulation on schooling kinematics at two light intensities. The (e) mean speed, (f) nearest-neighbour distance and (g) polarization among experiments performed under dim (dark grey area, 1.80 lx) and bright (light grey area, 8.15 lx) light. Fish were either treated such that their lateral line system was compromised (red), or served as a control (blue). The box plots indicate the values for individual groups (circles, 4 < n < 8), the median value (centre line), with the box designating the first and third quartiles and the range indicated by the error flags. Values for the mean speed (b,e) and mean nearest-neighbour distance (c,f) were calculated as the mean over time of the mean value among individuals for a school. Significant differences between groups (p < 0.05) indicated by the asterisks, were found by two-way ANOVA with post hoc tests. (Online version in colour.)
Manipulating the lateral-line system altered schooling kinematics. A two-way ANOVA found that the manipulation significantly affected both the minimum distance (p < 0.001) and polarization for our long-duration recordings (p = 0.02, figure 3e–g). Fish schooled at a distance from their nearest neighbour that was 79% greater when they did not have the assistance of the lateral line (, n = 8), compared with the control (, n = 18), when we combined measurements from the two levels of illuminance. Polarization of the control groups was 15% greater on average (, n = 18) than the treated fish (, n = 8). Therefore, schools composed of fish with an ability to sense flow swam more closely together and with greater similarity in heading than groups with a compromised lateral line. However, our lateral-line manipulation showed no significant effects on the relationships between speed and the duration of burst and coast phases (electronic supplementary material, figure S4 and table S3), nor on the relationships in kinematics between focal and neighbouring fish (electronic supplementary material, figure S5 and table S4).
4. Discussion
Our experiments addressed the role of vision in schooling. The inability of the tetras to school in the dark (figure 3a–d) and their capacity to school with a compromised lateral line (figure 3e–g) suggest that vision is both necessary and sufficient for schooling. These results contrast the pollock’s ability to school in the dark [7], but the necessity of vision is not unusual. A variety of other species are similarly incapable of schooling in darkness, including a freshwater cyprinid (Danionella translucida) [11] and a number of pelagic marine species (Scomber scombrus, Pseudocaranx dentex, Thunnus orientalis and Scomber scombrus L.) [12–14]. Schooling therefore is principally facilitated by the visual system in many fish species. The ability to school in the dark may thus be the exception, and not the norm, among fishes.
We tested the function of visual cues by measuring schooling kinematics over a range of light intensities. The nearest-neighbour distance, speed and polarization were at similar values of illuminance above a threshold (greater than 1.5 lx). Under dimmer light, the inability to school was manifested by a greater nearest-neighbour distance and low polarization (figure 3c,d). Our results do not distinguish between whether visually mediated polarization occurs as a consequence of attraction [4] or results from fish actively matching the heading of their neighbours [18,20], as either mechanism could be enhanced by a stronger visual stimulus. Similar results have been observed in some marine species [12,13,30], which illustrates a general role for the visual system in attracting fish together when provided with sufficient illumination and water clarity.
Schooling was altered by our manipulation of the lateral line system. The lateral line is generally thought to offer information about other fishes through flow cues that emerge in relatively close spatial interactions [19]. We found that the tetras moved at a distance that was 79% greater from their nearest neighbour with a compromised lateral line than the control group (figure 3f), similar to what was previously reported [21]. This result is consistent with the idea that flow serves as an attractive sensory cue when in close proximity. By contrast, ablating the trunk lateral line in pollock [8] and the golden shiner [23] reduced the spacing between neighbours, which suggests that flow serves as a cue for repulsion. However, the ability of pollock to school in the dark [7] also indicates that flow may serve an attractive function in the same species that uses flow for repulsion. Both the lateral line and visual systems may therefore facilitate attraction at relatively large distances and repulsion in close proximity.
Differences in sensing between species may be affected by the degree by which they move intermittently [26,31]. We found that bursts of swimming frequently caused tetras to advance in their position in the school, which they would forfeit during the coasting phase, as the other fish accelerated (figure 1a). These persistent changes in relative position contrast the kinematics of some steady-swimming species that maintain their station in a school [4] and probably presents a fish with highly dynamic stimuli. The visual field of an intermittent swimmer is exposed to changing visual angles for its neighbours and flow stimuli should reflect the unsteady motion of conspecifics. Our results suggest that decisions about turning (figure 2b) and changes in speed (electronic supplementary material, figure S2) during swimming bursts depend on the spatial distribution of neighbours, which is consistent with previous observations of the rummy-nose tetra [21,24,25,32] and other intermittent swimmers [5,26,31,33]. This spatial accounting apparently depends on visual cues and is probably beyond the receptive field of the lateral line [19]. Steady swimming that depends on group members in close proximity may more easily use flow as a means of information for schooling.
5. Summary
The present study evaluated the roles of vision and flow sensing in the schooling behaviour of a species that swims intermittently. By manipulating the intensity of light, we found that vision serves to attract and align the members of a school (figure 3c,d). We found that vision is sufficient to school when we compromised the lateral line system, but that flow sensing draws fish together and increases polarization (figure 3f,g). Therefore, flow sensing in the rummy-nose tetra enhances schooling that is facilitated by the visual system.
Supplementary Material
Acknowledgements
This project received valuable input at all stages from members of the McHenry Lab (A. Peterson, A. Carrillo and T. Po) as well as D. Paley and E. Kanso.
Ethics
All rearing and experimental protocols were conducted with the approval of the Institutional Animal Care and Use Committee at the University of California, Irvine (IACUC Protocol no. AUP-17-012).
Data accessibility
Data and analysis code is available from the Dryad Digital Repositoy: https://doi.org/10.7280/D1N96K [34].
Authors' contributions
This study was designed in collaboration among all authors. Experiments were performed by A.M. and P.C. and data analysis was designed by M.J.M., with coding contributions by A.P.S. and A.M. The manuscript was written by A.M. and M.J.M., with feedback from A.P.S.
Competing interests
We declare we have no competing interests.
Funding
This project was supported by grants from the National Science Foundation (IOS-1354842) and the Office of Naval Research (N00014-19-1-2035).
References
- 1.Day RL, MacDonald T, Brown C, Laland KN, Reader SM. 2001. Interactions between shoal size and conformity in guppy social foraging. Anim. Behav. 62, 917–925. ( 10.1006/anbe.2001.1820) [DOI] [Google Scholar]
- 2.Ashraf I, Bradshaw H, Ha TT, Halloy J, Godoy-Diana R, Thiria B. 2017. Simple phalanx pattern leads to energy saving in cohesive fish schooling. Proc. Natl Acad. Sci. USA 114, 9599–9604. ( 10.1073/pnas.1706503114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godin J-GJ, Classon LJ, Abrahams MV. 1988. Group vigilance and shoal size in a small Characin fish. Behavior 104, 29–40. ( 10.1163/156853988X00584) [DOI] [Google Scholar]
- 4.Katz Y, Tunstrom K, Ioannou CC, Huepe C, Couzin ID. 2011. Inferring the structure and dynamics of interactions in schooling fish. Proc. Natl Acad. Sci. USA 108, 18 720–18 725. ( 10.1073/pnas.1107583108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arganda S, Pérez-Escudero A, De Polavieja GG. 2012. A common rule for decision making in animal collectives across species. Proc. Natl Acad. Sci. USA 109, 20 508–20 513. ( 10.1073/pnas.1210664109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jolles JW, Boogert NJ, Sridhar VH, Couzin ID, Manica A. 2017. Consistent individual differences drive collective behavior and group functioning of schooling fish. Curr. Biol. 27, 2862–2868; e7. ( 10.1016/J.CUB.2017.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitcher TJ, Partridge BL, Wardle C. 1976. A blind fish can school. Science 194, 963–965. ( 10.1126/science.982056) [DOI] [PubMed] [Google Scholar]
- 8.Partridge BL, Pitcher TJ. 1980. The sensory basis of fish schools: relative roles of lateral line and vision. J. Comp. Phys. A 135, 315–325. ( 10.1007/BF00657647) [DOI] [Google Scholar]
- 9.Timmermann M, Schlupp I, Plath M. 2004. Shoaling behaviour in a surface-dwelling and a cave-dwelling population of a barb Garra barreimiae (Cyprinidae, Teleostei). Acta Ethologica 7, 59–64. ( 10.1007/s10211-004-0099-8) [DOI] [Google Scholar]
- 10.John KR. 1964. Illumination, vision, and schooling of Astyanax mexicanus (Fillipi). J. Fish. Res. Board Can. 21, 1453–1473. ( 10.1139/f64-122) [DOI] [Google Scholar]
- 11.Schulze L. et al. 2018. Transparent Danionella translucida as a genetically tractable vertebrate brain model. Nat. Methods 15, 977–983. ( 10.1038/s41592-018-0144-6) [DOI] [PubMed] [Google Scholar]
- 12.Hunter JR. 1968. Effects of light on schooling and feeding of jack mackerel, Trachurus symmetricus. J. Fish. Res. Board Canada 25, 393–407. ( 10.1139/f68-031) [DOI] [Google Scholar]
- 13.Torisawa S, Takagi T, Fukuda H, Ishibashi Y, Sawada Y, Okada T, Miyashita S, Suzuki K, Yamane T. 2007. Schooling behaviour and retinomotor response of juvenile Pacific bluefin tuna Thunnus orientalis under different light intensities. J. Fish Biol. 71, 411–420. ( 10.1111/j.1095-8649.2007.01498.x) [DOI] [Google Scholar]
- 14.Ali M. 2001. The ocular structure, retinomotor and photo-behavioral responses of juvenile pacific salmon. Can. J. Zool. 37, 965–996. ( 10.1139/z59-092) [DOI] [Google Scholar]
- 15.Breder C. 1954. Equations descriptive of fish schools and other animal aggregations. Ecology 35, 361–370. ( 10.2307/1930099) [DOI] [Google Scholar]
- 16.Pitcher TJ. 1983. Heuristic definitions of fish shoaling behaviour. Anim. Behav. 31, 611–613. ( 10.1016/S0003-3472(83)80087-6) [DOI] [Google Scholar]
- 17.Gautrais J, Ginelli F, Fournier R, Blanco S, Soria M, Chaté H, Theraulaz G. 2012. Deciphering interactions in moving animal groups. PLoS Comp. Biol. 8, e1002678 ( 10.1371/journal.pcbi.1002678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zienkiewicz AK, Ladu F, Barton DA, Porfiri M, Bernardo MD. 2018. Data-driven modelling of social forces and collective behaviour in zebrafish. J. Theor. Biol. 443, 39–51. ( 10.1016/j.jtbi.2018.01.011) [DOI] [PubMed] [Google Scholar]
- 19.McHenry MJ, Liao JC. 2013. The hydrodynamics of flow stimuli. In The lateral line (eds S Coombs, H Bleckmann, RR Fay, AN Popper), vol. 48, p. 26. New York, NY: Springer.
- 20.Collignon B, Séguret A, Halloy J. 2016. A stochastic vision-based model inspired by zebrafish collective behaviour in heterogeneous environments. R. Soc. Open Sci. 3, 150473 ( 10.1098/rsos.150473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faucher K, Parmentier E, Becco C, Vandewalle N, Vandewalle P. 2010. Fish lateral system is required for accurate control of shoaling behaviour. Anim. Behav. 79, 679–687. ( 10.1016/j.anbehav.2009.12.020) [DOI] [Google Scholar]
- 22.Mekdara PJ, Schwalbe MAB, Coughlin LL, Tytell ED. 2018. The effects of lateral line ablation and regeneration in schooling giant danios. J. Exp. Biol. 221, jeb175166 ( 10.1242/jeb.175166) [DOI] [PubMed] [Google Scholar]
- 23.Burgess JW, Shaw E. 1981. Effects of acoustico-lateralis denervation in a facultative schooling fish: a nearest-neighbor matrix analysis. Behav. Neural Biol. 497, 488–497. ( 10.1016/S0163-1047(81)91869-0) [DOI] [PubMed] [Google Scholar]
- 24.Calovi DS, Litchinko A, Lecheval V, Lopez U, Pérez Escudero A, Chaté H, Sire C, Theraulaz G. 2018. Disentangling and modeling interactions in fish with burst-and-coast swimming reveal distinct alignment and attraction behaviors. PLoS Comp. Biol. 14, 1–28. ( 10.1371/journal.pcbi.1005933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecheval V, Jiang L, Tichit P, Sire C, Hemelrijk CK, Theraulaz G. 2018. Social conformity and propagation of information in collective u-turns of fish schools. Proc. R. Soc. B 285, 20180251 ( 10.1098/rspb.2018.0251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harpaz R, Tkačik G, Schneidman E. 2017. Discrete modes of social information processing predict individual behavior of fish in a group. Proc. Natl Acad. Sci. USA 114, 10 149–10 154. ( 10.1073/pnas.1703817114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. 2003. Neomycin-induced hair cell death and rapid regeneration in the laterl line of zebrafish Danio rerio. J. Assoc. Res. Otolaryn. 4, 219–234. ( 10.1002/wdev.160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Trump WJ, Coombs S, Duncan K, McHenry MJ. 2010. Gentamicin is ototoxic to all hair cells in the fish lateral line system. Hear. Res. 261, 42–50. ( 10.1016/j.heares.2010.01.001) [DOI] [PubMed] [Google Scholar]
- 29.Couzin ID, Krause J, James R, Ruxton GD, Franks NR. 2002. Collective memory and spatial sorting in animal groups. J. Theor. Biol. 218, 1–11. ( 10.1006/jtbi.2002.3065) [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki T, Shiozawa S, Kogane T, Masuda R, Maruyama K, Tsukamoto K. 2000. Developmental changes of the light intensity threshold for school formation in the striped jack Pseudocaranx dentex. Mar. Ecol. Prog. Ser. 192, 267–275. ( 10.3354/meps192267) [DOI] [Google Scholar]
- 31.Puckett JG, Pokhrel AR, Giannini JA. 2018. Collective gradient sensing in fish schools. Sci. Rep. 8, 1–11. ( 10.1038/s41598-018-26037-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang L, Giuggioli L, Perna A, Escobedo R, Lecheval V, Sire C, Han Z, Theraulaz G. 2017. Identifying influential neighbors in animal flocking. PLoS Comp. Biol. 13, 1–32. ( 10.1371/journal.pcbi.1005822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinz RC, de Polavieja GG. 2020. Data from:. Proc. Natl Acad. Sci. USA 114, 2295–2300. ( 10.1073/pnas.1616926114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKee A, Soto AP, Chen P, McHenry MJ. 2020. Data from: The sensory basis of schooling by intermittent swimming in the rummy-nose tetra (Hemigrammus rhodostomus). Dryad Digital Repositoy ( 10.7280/D1N96K) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- McKee A, Soto AP, Chen P, McHenry MJ. 2020. Data from: The sensory basis of schooling by intermittent swimming in the rummy-nose tetra (Hemigrammus rhodostomus). Dryad Digital Repositoy ( 10.7280/D1N96K) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data and analysis code is available from the Dryad Digital Repositoy: https://doi.org/10.7280/D1N96K [34].