Abstract
Patterns of habitat use are commonly studied in horizontal space, but this does not capture the four-dimensional nature of ocean habitats (space, depth, and time). Deep-diving marine animals encounter varying oceanographic conditions, particularly at the poles, where there is strong seasonal variation in vertical ocean structuring. This dimension of space use is hidden if we only consider horizontal movement. To identify different diving behaviours and usage patterns of vertically distributed habitat, we use hidden Markov models fitted to telemetry data from an air-breathing top predator, the Weddell seal, in the Weddell Sea, Antarctica. We present evidence of overlapping use of high-density, continental shelf water masses by both sexes, as well as important differences in their preferences for oceanographic conditions. Males spend more time in the unique high-salinity shelf water masses found at depth, while females also venture off the continental shelf and visit warmer, shallower water masses. Both sexes exhibit a diurnal pattern in diving behaviour (deep in the day, shallow at night) that persists from austral autumn into winter. The differences in habitat use in this resident, sexually monomorphic Antarctic top predator suggest a different set of needs and constraints operating at the intraspecific level, not driven by body size.
Keywords: diving behaviour, water mass, continental shelf, Weddell seal, sex-specific variation, hidden Markov model
1. Background
Understanding what parts of an ecosystem are important for species is a cornerstone of ecological research. Important habitat is often detected by proxy; if species regularly occur in a habitat, it must fulfil a life-history function. For large marine vertebrates, occurrence is usually measured using location data. However, identifying the drivers of marine population distributions from horizontal location data alone can be problematic for air-breathing deep-diving marine animals (e.g. [1]). This group spend most of their time underwater and are intrinsically difficult to observe. Depth is a fundamental dimension of their movement, and information is lost if dives are not considered. Vertical structuring of ocean habitats enhances productivity and creates predictable concentrations of resources [2,3]. Deep-divers target the increased prey density at steep physical gradients [4–6] and track its seasonality [7,8]. Seasonal variability in physical gradients is especially strong at the poles [9] due to seasonal extremes. For most deep-diving wide-ranging marine vertebrates, we lack a detailed understanding of the prey they consume and the structure and functioning of the ecosystems that support them.
For air-breathing divers like pinnipeds, seabirds, and cetaceans, dives are the result of the separation of two basic resources: air at the surface and prey at depth. Greater depths are more costly from a time-budget perspective, since transit likely excludes foraging [10], and they are physiologically more costly due to the metabolic requirements of hunting and digestion [11–13]. It follows that the habitat at these depth layers must be profitable in terms of prey resources and that dives to regularly visited depth layers involve hunting and prey acquisition [10,14,15].
Understanding the environmental context of diving is key to linking behaviour to habitat and prey. Recent examples of integrative multi-species studies describing the spatial distribution of different marine animal guilds include [16–18]. However, surface variables do not reflect conditions at depth [19], and the methodology for relating diving behaviour to depth-varying environmental variables is underdeveloped in comparison. The collection of behavioural and in situ environmental data by satellite-linked animal-borne devices [20,21] is a way of filling this data-gap and allows us to relate depth-varying behaviour to depth-varying conditions. We posit that incorporating depth into habitat studies of diving animals (e.g. [22,23]) is critical for making good predictions of future distributions and detecting intraspecific variability that is hidden when taking a bird’s-eye view of movement. We present an example of this in the Weddell seal (Leptonychotes weddellii), in its namesake shelf sea.
The southern Weddell Sea is a large embayment in the Southern Ocean’s Atlantic sector. It is unique environmentally, due to physical processes that take place, leading to the formation of high-salinity water masses on the continental shelf [9,24,25]. Weddell seals occur all around the Antarctic coast and their diving behaviour has been studied since the earliest years of animal-borne instrument development [26,27]. Due to accessibility, most of what we know about their foraging ecology comes from East Antarctica. In these areas, seals seldom leave the continental shelf, where a much warmer and slightly fresher water mass (modified Circumpolar Deep Water) plays a central role [28,29]. In contrast, the southern Weddell Sea is only accessible by ship during austral summer. The interaction of Weddell seals with the hydrographically complex and varied Weddell Sea vertical habitat is not currently understood (but see [30–32]) and is likely to be different, owing to the availability of different water masses and deep ocean habitat. Previous work on foraging ecology in the Weddell Sea has found that dives target the surface, shallow epipelagic, and the mesopelagic region [30,33–35], while commuting through intermediate depths. This pioneering work has gone some way towards finding out what occurs at these depths, but knowledge of Weddell Sea shelf ecosystem remains poor.
Sex-specific differences in space use (horizontal and vertical) have been well-documented in air-breathing divers, but the underlying mechanisms remain unclear [36]. Many diving marine mammals are sexually dimorphic, making it difficult to disentangle size from other drivers (e.g. [37–39]). Being sexually monomorphic, Weddell seals present an opportunity to examine movement patterns independently from size. Several hypotheses exist for explaining sexual segregation in marine vertebrates, the main drivers being energy requirements and risk tolerance [36]. Different combinations of these may lead to divergent habitat use, time spent engaged in different behaviours, or risk exposure.
The high degree of serial correlation in animal movement metrics makes it necessary to use analytical approaches that account for it. Hidden Markov models (HMMs) [40,41] are time-series models, which allow us to learn about the underlying process from multivariate observations (multiple data streams). They have been used to model diving data from several marine predators and are powerful for making inferences about complex temporal patterns and responses to environmental features [42–46]. We use HMMs to identify Weddell seal dive types or vertical movement states, and the effect of temporal covariates on the probability of switching between them. Geographical differences in diving activity [47] suggest that the two sexes may use different vertical habitat. We identify the depths where seals spend time hunting and link hunting activity to in situ oceanographic conditions, making this the first study to describe sex-specific preferences for vertical habitat in this circumpolar resident top predator. Although this is a single-species study, the approach can be used equally effectively for multi-species data.
2. Methods
(a). Data collection
Movement and oceanographic data (salinity, temperature, and depth) were collected using telemetry instruments attached to the fur of adult Weddell seals in the southern Weddell Sea, Antarctica, after their annual moult. We used conductivity-temperature-depth satellite relay data loggers (CTD-SRDLs) [48,49], designed and manufactured by the Sea Mammal Research Unit Instrumentation Group (SMRU-IG), St Andrews, Scotland, UK. CTD-SRDLs employ the CLS Argos satellite system to relay data [50]. This produced a dataset from 19 Weddell seals (10 females, 9 males) instrumented in February 2011 (figure 1), during an oceanographic research program [9,52]. This is the largest single-year Weddell seal telemetry dataset from the Weddell Sea.
Figure 1.
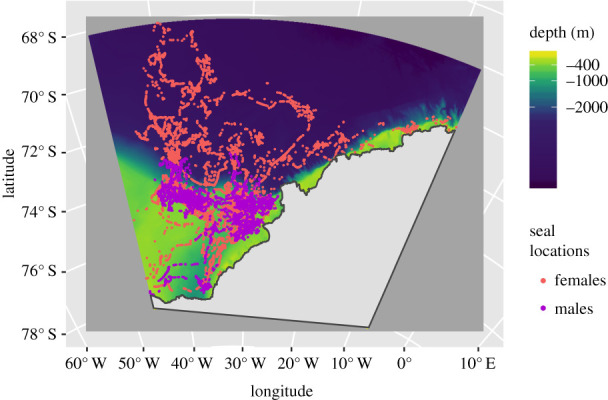
Satellite tracks from 19 instrumented Weddell seals carrying CTD-SRDL tags deployed in February 2011 (10 females and 9 males). The tracks are colour-coded by sex. The background colour represents bathymetry (depth in metres at a 0.5 km resolution). The colour bar is scaled so that light areas represent the continental shelf and dark areas represent the deep ocean. There is a deep trough on the continental shelf at 40° W, the Filchner Depression. These are predicted locations from a correlated random walk model fitted to the original data, accounting for the estimated CLS Argos location error, using the foieGras package in R [51]. (Online version colour.)
A detailed description of the deployment technique and individual deployment data are presented in [47,53]. Dive data were collected using algorithms developed for high-latitude, deep-diving seals [48,49,54] and CTD data were collected, calibrated, and processed according to MEOP (marine mammals exploring the oceans pole to pole) Consortium standards [20]. A summary of the data is presented in electronic supplementary material, S1.
(b). Data processing
We consider a near-complete sequence of behaviour by combining the haulout, surface, and dive information returned by the tag (definitions: electronic supplementary material, S1). We do not consider short surface intervals between these events. We found that the amount of data reduces substantially after week 24 (13–19 June 2011) so we only consider data up to 19 June.
We calculated hunting depth and time spent hunting during dives using methods developed in [15]. They used high-resolution time-depth data and triaxial accelerometer data from Weddell seals to detect prey capture attempts (PrCAs) and found a strong correlation between vertical sinuosity, swimming speed, and the number of prey capture attempts. We use their estimated vertical speed threshold (0.5 m s−1) to extract segments of dives with low vertical speeds (hunting segments) and calculate the total duration of time spent in ‘hunting’ mode per dive [55]. We use the depth of the longest hunting segment, where most PrCAs occur, as the hunting depth in our analyses (electronic supplementary material, S2).
Only some dive profiles have a simultaneous CTD profile. To overcome this, we interpolated variables linearly in time, between time points where CTD data were available, and assigned them to the intervening dive times (electronic supplementary material, S3). We extracted bathymetry at the location of each seal dive after correcting the tracks by fitting a state-space model using the R package foieGras (electronic supplementary material, S3).
(c). Statistical analysis
We fit a multivariate HMM to the haulout, surface, and dive data to classify diving behaviour and describe the relationship between dive types and two temporal covariates; time of day and season. The aim is to describe and classify diving behaviour, which includes foraging. The non-diving states in the model are known a priori from the data, so we only allow additional states to describe different diving behaviours. It was obvious from exploratory data analysis that female and male seals display different movement modes, which are differently affected by covariates, and therefore warrant separate models.
We use behavioural and environmental variables from each behavioural record (dive, surface, or haulout) to derive five data streams (state-dependent variables) which are modelled with the multivariate HMM: (1) behaviour duration (s), (2) hunting depth (m), (3) proportion of dive duration spent hunting (hunting time/duration: 0–1), (4) proportion of bathymetry reached at hunting depth (hunting depth/bathymetry: 0–1) (see electronic supplementary material, S3.3), and (5) salinity (psu) at hunting depth. These are the variables of interest that we wish to learn about and use to describe movement modes. Only behaviour duration is meaningful for non-dive behaviours, so this state-dependent variable alone is used to characterize haulout and surface events. All state-dependent variables are used to characterize dive states. We include the time series of salinity in the state process rather than as a covariate on the state transition probabilities (details: electronic supplementary material, S4).
Assuming contemporaneous conditional independence given the states, we model behaviour duration and hunting depth using a gamma distribution for non-zero positive values, proportion of dive time spent hunting as a beta distribution for values between zero and one, proportion of bathymetry reached as a single probability between zero and one, and salinity as a normal distribution. Salinity values are strictly positive but far from zero, so parameters need not be bounded.
Based on our data and the literature [26,32,56], we choose two covariates that act on the state transition probabilities: local time of day as a circular variable (cosine and sine), week of the year, and their interaction. The model formulation (the state-dependent variables, the temporal covariates, and the way the covariates were included in the model) was chosen carefully to make biological sense. The full model was used for both females and males. See electronic supplementary material, S4 for details on the model formulation and choice of covariates.
We estimate the parameters of the state-dependent distributions and the covariate effects using numerical maximization of the likelihood, implemented in R [57], using the nlm function. The computation of the covariate-dependent transition probability matrices and the forward algorithm were coded in C++ (electronic supplementary material, S4). We used twenty sets of starting values for each model to ensure the algorithm found a global maximum (electronic supplementary material, S4). We use the Viterbi algorithm to calculate the most likely state sequence for each individual [41] (electronic supplementary material, S4). We checked the model fit by (1) computing the pseudo-residuals for each of the state-dependent variables, and by (2) simulating data from the model and checking how well it matched the observed data (electronic supplementary material, S5). There was no evidence of systematic lack of fit for either model.
3. Results
(a). State-dependent distributions
The data from females and males were best described using different numbers of dive states, resulting in a five-state model for males and a six-state model for females (electronic supplementary material, S4). In each case, the two non-diving states were considered known and dive states were estimated from the data (females: 4 dive states, males: 3).
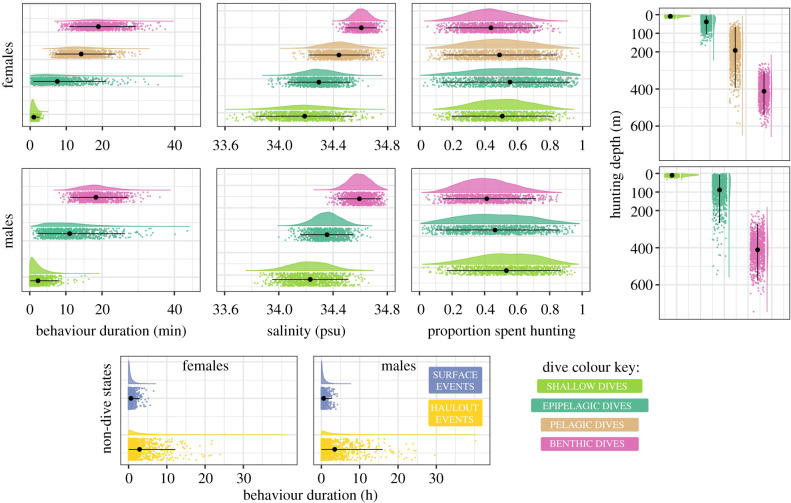
The estimated state-dependent distributions displayed in figure 2 show that female and male Weddell seals have three analogous vertical movement states. These are most easily thought of in terms of their hunting depth and proportion of bathymetry reached: (1) very short shallow dives, (2) slightly deeper dives in the epipelagic layer of the water column, and (3) deep dives with high probability of reaching the bottom (approx. 90%), where they access high salinity water masses found only on the continental shelf (table 1 and figure 2). Females also carry out a fourth dive type, in the pelagic layer of the water column. These dives are shorter than benthic dives and take place over the deep ocean, where the seals access lower salinity water masses (table 1 and figure 2). In males, pelagic dives appear to be absent, although their epipelagic dives are a bit deeper than in females. There was no compelling evidence of differences in proportion of time spent hunting between dive types or sexes.
Figure 2.
State density distributions for dive states (shallow, epipelagic, pelagic, and benthic dives) in female (top row) and male (middle row) Weddell seals. The state density distributions for non-diving states (haulout, surface) are shown for females (left) and males (right) in the bottom row. The density distributions are colour-coded by state, with a cloud of points below them. The cloud represents a realization of the estimated state-dependent distribution, based on a sample of 1000 points. The black point and line going through each coloured cloud of points represent the mean estimated by the model and the interval covering 95% of the probability mass. In females, all four dive states are present, while in males there are three dive states (no pelagic dives). (Online version colour.)
Table 1.
Parameter estimates (mean, 95% confidence interval) for state-dependent distributions for female and male Weddell seals, from the respective HMMs—six states for females, five states for males. The proportion state occupancy is calculated as the proportion of observations labelled as each state. Note that there are four dive states for females to spread their activity over compared to three in males. This naturally leads to smaller proportions in the six-state scenario. However, if all states occur equally often, the proportions would all be smaller, which we do not see.
| sex | haulout events | surface events | shallow dives | epipelagic dives | pelagic dives | benthic dives | |
|---|---|---|---|---|---|---|---|
| duration | F | 2.8 h (2.7–3.0) | 37.9 min (36.4–39.5) | 1.0 min (59–64 s) | 7.4 min (7.2–7.7) | 14.1 min (13.9–14.2) | 19.0 min (18.7–19.2) |
| M | 3.5 h (3.3–3.7) | 34.3 min (32.6–35.9) | 2.3 min (2.2–2.4) | 11.0 min (10.7–11.3) | — | 18.2 min (18.1–18.4) | |
| depth (m) | F | — | — | 8.8 (8.6–8.9) | 38.5 (37.2–39.8) | 193.8 (189.0–198.6) | 413.1 (409.9–416.3) |
| M | — | — | 10.2 (10.1–10.4) | 90.0 (86.8–93.2) | — | 411.8 (408.5–415.1) | |
| proportion of dive | F | — | — | 0.51 (0.50–0.51) | 0.55 (0.54–0.56) | 0.49 (0.48–0.50) | 0.43 (0.42–0.44) |
| spent hunting | M | — | — | 0.53 (0.52–0.53) | 0.46 (0.46–0.47) | — | 0.42 (0.41–0.42) |
| proportion of | F | — | — | 0.00 | 0.00 | 0.00 | 0.89 (0.72–1.00) |
| bathymetry reached | M | — | — | 0.00 | 0.00 | — | 0.91 (0.77–1.00) |
| salinity at | F | — | — | 34.18 (34.178–34.192) | 34.29 (34.283–34.292) | 34.44 (34.433–34.444) | 34.61 (34.603–34.608) |
| depth (psu) | M | — | — | 34.23 (34.226–34.234) | 34.36 (34.351–34.360) | — | 34.59 (34.591–34.598) |
| state occupancy | F | 0.13 (0.10–0.17) | 0.21 (0.18–0.28) | 0.17 (0.12–0.23) | 0.24 (0.14–0.36) | 0.15 (0.07–0.30) | 0.09 (0.01–0.26) |
| M | 0.13 (0.10–0.17) | 0.20 (0.12–0.26) | 0.31 (0.09-0.42) | 0.19 (0.12–0.31) | — | 0.17 (0.11–0.36) |
The proportion of Viterbi decoded states differed between females and males (table 1). Both sexes are equally likely to haul out, and spend time at the surface, but males are almost twice as likely to carry out shallow dives (F: 17%, M: 31%) and benthic dives (F: 9%, M: 17%), while females are more likely to carry out epipelagic dives (F: 24%, M: 19%). Pelagic dives clearly make up a regularly used movement mode for females with 15% of records being estimated to belong to this state.
(b). Covariate effects
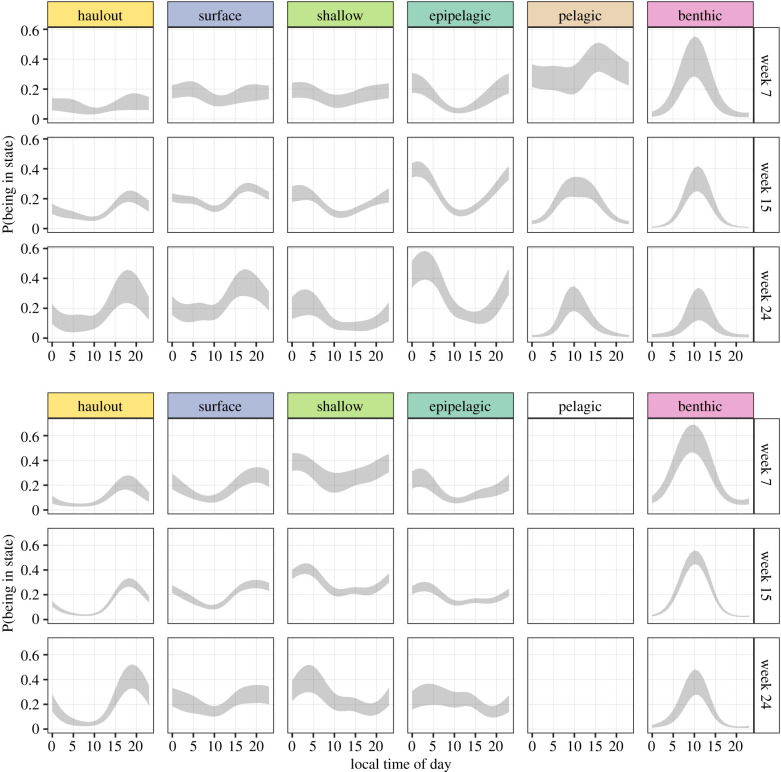
The distribution of state occupancy (i.e. the equilibrium probabilities as functions of covariates) is shown in figure 3. These plots represent the probability (y-axis) of finding a seal in a given state (columns), during a week of the year (rows) throughout the 24-h cycle (x-axis). There is little diurnal variation in female or male shallow dives until winter, when they become more common at night. Female epipelagic dives happen predominantly at night in all weeks, and throughout the day in males. In both sexes, deep dives (pelagic and benthic) happen during the day, centred on local noon, (figure 3). This pattern is present throughout the seasons represented in our dataset, even when there is little light at local noon. We provide the state transition probabilities for the conditions shown in figure 3, in electronic supplementary material, S5.
Figure 3.
The probability (y-axis) of finding a seal in a given state (columns), during a week of the year (rows) throughout the 24-h cycle (x-axis). The grey ribbon represents the area enclosed by the lower and upper confidence bounds of the 95% interval of the estimated effect, which were calculated using the delta method. Time of day corresponds to local time calculated from the location of each data point. The female plots are shown in the top panel, and the males’ in the bottom panel. Week 7 corresponds to late summer, week 15 to autumn, and week 24 to midwinter. (Online version colour.)
A seasonal effect is evident in female diving behaviour, particularly pelagic and benthic diving. Early in the year (week 7) pelagic dives are common throughout the day, while benthic dives occur mainly in the day. By midwinter (week 24) deep dives are less common, replaced by night-time epipelagic diving. Male diving behaviour appears less seasonal. Daytime benthic dives occur throughout the observation period. Near midwinter they become less dominant and are complemented by shallow dives.
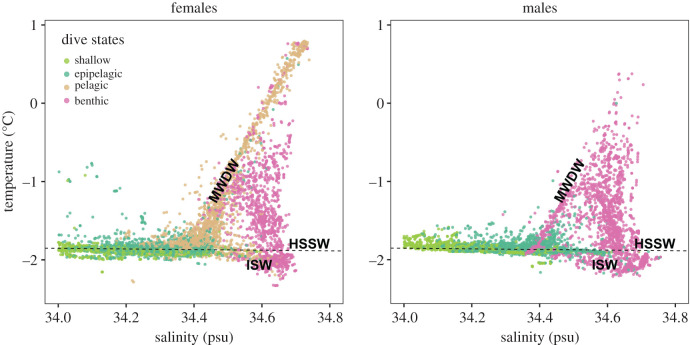
The difference in oceanographic regimes used by female and male seals is clear in the temperature-salinity diagrams associated with their dive profiles. Although temperature was not included in the model we can still visualize the Viterbi-decoded states in temperature-salinity (TS) space (figure 4). The distribution of states in TS space is unsurprising for the shallower states (shallow, epipelagic dives) because there is a limited range of available conditions in these surface layers. However, the deeper states show a trend: females use Modified Warm Deep Water and Warm Deep Water extensively for pelagic dives. These water masses are found along the diagonal line seen in the female TS diagram. By contrast, males in this dataset almost never visited areas with at-depth temperatures warmer than 0°C. The TS conditions associated with benthic dives are relatively consistent between females and males. In both plots, Ice Shelf Water and High Salinity Shelf Water, as well as its interface with precursors to Modified Warm Deep Water, are visited regularly (water mass definitions: fig. 3 in [24]).
Figure 4.
Temperature-salinity diagrams for dives from female and male seals coloured by Viterbi-decoded state. The points in each plot correspond to the conditions encountered by seals at the hunting depth of each dive. The water mass labels correspond to High Salinity Shelf Water (HSSW), Ice Shelf Water (ISW), and Modified Warm Deep Water (MWDW). These labels are transcribed from fig. 3 of Nicholls et al. [24]. (Online version colour.)
4. Discussion
We show that oceanographic data collected in situ by diving marine predators can be used to characterize their preferences for vertically distributed habitat and reveal intraspecific variability. We do this by modelling the relationship between diving and seasonal environmental conditions using HMMs. We find two movement strategies that in our dataset correspond to sex-specific differences. Females and males have some overlap in diving behaviour, but also important differences. Males mainly carry out benthic deep dives, consistent with their spatial location on the continental shelf, into winter (figure 1). By contrast, female deep dives are both benthic and pelagic, consistent with their extensive off-shelf autumn and winter distribution. Both sexes use high-salinity shelf water masses throughout, from February to June. Regular visits to this unique and inaccessible vertical habitat—the coldest possible liquid water habitat at −2°C—is worth the effort of carrying out deep dives, which are energetically costly [13]. Female diving behaviour follows a mixed seasonal pattern. This is in contrast with the comparatively aseasonal diving behaviour of males, suggesting that male diving behaviour does not track seasonally varying limitations or opportunities.
Dive types represent different vertical habitat types. We found that male seals avoid the pelagic zone almost entirely. Dives down to the continental shelf benthos place seals in some of the coldest, densest water in the world. Harcourt et al. [58] found that males that dived deeply during the breeding season lost weight at a slower rate. This is evidence that deeper prey resources are likely to be particularly profitable, hinting at the productivity of on-shelf Antarctic habitats [59]. Possible reasons for leaving this profitable area could be intraspecific competition, density-dependent prey depletion, or pelagic resources being even more profitable. Females can move north to exploit them at shallower, less energetically costly depths, unhindered by the need to establish territories for the spring. This pattern is similar to southern elephant seals from Îles Kerguelen, where the males commonly stay in the sea ice and forage on the Antarctic continental shelf while females move north as winter progresses and forage in the marginal ice zone [60]. The life-history constraints that likely bring about this pattern for sexually dimorphic elephant seals are reversed for monomorphic Weddell seals—males are limited by the need to establish territories, keeping them in the south, not females. In both cases, the marginal ice zone is clearly seasonally attractive for top predators. In light of the need for reliable sea ice habitat for breeding, and male territoriality, it seems likely that habitat segregation is based, at least partly, on females’ lack of place-based constraints. Without knowing how profitable each of the two habitats is, we cannot say whether one sex excludes another or not. However, the increased predation risk from killer whales associated with lower sea ice concentrations, may point towards a high-risk high-rewards strategy in females [36].
The seasonality in female movement behaviour manifests latitudinally and vertically. In late summer, females are most likely to be found diving pelagically throughout the day, and benthically at midday, in contrast to what has been found in East Antarctica ([56] but also [28]). By midwinter, both deep dive types happen in the day. The hydrographic conditions associated with pelagic dives are compellingly clear: Modified Warm Deep Water, mixing with early Winter Water, and the continuum up the temperature gradient towards Warm Deep Water (fig. 4 and fig. 3 in Nicholls et al. [24] for reference). These conditions, like the marginal ice zone, have consistently been shown to be profitable to female phocid seals [4,23,28,61]. Sea ice has a high concentration of detritus and living organisms, which contribute to year-round biological productivity and community development, particularly under older ice floes [62,63]. This leads to a cascade of productivity, perhaps including the prey base exploited during the two shallow dive types we found. The existence of multi-year sea ice in the Weddell Sea may be the critical factor that allows female seals to forage successfully (and haul out) away from the coast, over deep water. The post-moult period finds female Weddell seals in need of replenishing their energy reserves after a period of reduced foraging due to lactation and the annual moult. A shallower pelagic diving strategy could afford the added energetic benefit of minimizing dive costs.
The lack of seasonality in male diving behaviour raises questions about the prey base they are exploiting. The end of summer sees the beginning of a reduction in the inflow of warm water onto the continental shelf in the southern Weddell Sea [52]. Warm water enters the shelf as Modified Warm Deep Water in the region of the Filchner Depression, especially the eastern and western edges of the Filchner Sill (figs 6 and 7 in [24], and also [52]). These physical changes must have some ecosystem effects but do not induce a discernible change in diving behaviour. However, a reduction in the probability of benthic dives and increase in epipelagic dives hint towards a shift in reliance towards surface layers in winter, despite the reduction in light availability. Changes not detectable by our approach include benthic prey type or prey behaviour. The TS diagram for males shows that some benthic dives occur in Modified Warm Deep Water (diagonal line starting at 34.4 psu in figure 4) but the clear majority of them occur in the denser, saltier water characteristic of sub-ice-shelf circulation and melting [64].
The shelf ecosystem that exists under these extreme temperature conditions is not well studied, but is likely to be very stable due to the narrow range of temperature and salinity conditions that can exist there [64]. It is known to support a large amount of biomass through Weddell seals and their likely prey, Antarctic toothfish (Dissostichus mawsoni) and other notothen fish (Antarctic silverfish Pleurogramma antarcticum, scaly rockcod Trematomus loennbergii). Stability and high biomass are attributes of mature ecosystems where large body size, narrow niche specialization and long, complex life cycles are observed [65]. These attributes fit with what we know about the Weddell Sea continental shelf from a top predator perspective. Riotte-Lambert and Matthiopoulos [66] show that learning and memory are emergent properties of animals in a system with moderate-to-high environmental predictability. At slightly lower predictability, more social information use is favoured. Although the degree of social information exchange is not known for Weddell seals, they have a substantial underwater vocal repertoire known to function socially, at least in the breeding season. This would place the predictability of their environment on the mid-to-upper end of the scale (fig. 1 in [66]) at a timescale comparable with the lifetime of a Weddell seal. As non-migratory top predators, Weddell seals are likely to be instrumental in the stability of the Antarctic shelf ecosystem, forming a feedback loop between movement ecology and environmental predictability [66].
Historically, bottom trawls found high notothen biomass at 600 m on the southern slopes of the Filchner Depression (especially D. mawsoni, P. antarcticum, and T. loennbergii) [59]. This is in contrast with the narrow eastern shelf where overall fish biomass was lower (fig. 3 in Ekau [59]), and which seals in our dataset tend to avoid. Over the Filchner Depression Antarctic toothfish were only caught in summer at 420–670 m depth and dominated in terms of biomass, along with scaly rockcod and large specimens of Antarctic silverfish. The distribution and temporal availability of these high-biomass, large-bodied fish is highly consistent with the location and dive depth of Weddell seals on the shelf. In other parts of their range, Weddell seals mainly consume Antarctic silverfish, with seasonally varying contributions of larger prey such as Antarctic toothfish, which may increase with seal body size, or age [67]. We do not have age information for the individuals tagged in this study, but it is possible that there is further intraspecific niche separation beyond sexual segregation [68].
A diurnal dive pattern was observed early on in Weddell seals (Ross Sea [26], East Antarctica [28], Weddell Sea [32]), with seals diving deeply in the day and shallowly at night. The shallow depths visited during night-time are now known to overlap with the diel vertical migration of Antarctic silverfish [28,69]. The depths and dive types described in [26,70] correspond closely to our females dive states. In agreement with previous studies, we find that epipelagic (less than 100 m) dives are more likely at night [26,28,32]. The daytime pelagic (less than 250 m) female dives deviate from what we expected for pelagic diving behaviour based on other species (e.g. southern elephant seals [4,7]). It seems likely that at these depths (approx. 200 m) in the Weddell Gyre seals are foraging on some age class of Antarctic silverfish and other notothen species in the day, and moving shallower at night (approx. 50 m).
The biological inferences we have drawn hinge on our analytical approach. The major advantage of HMMs is that we can combine many aspects of diving behaviour and make inferences about possible behavioural classes in multiple dimensions, e.g. depth, duration, hunting behaviour, distance from features, physiological state etc. A drawback of HMMs based on telemetry data collected without direct observations, is that we do not know how well our state interpretations correspond to reality. If we repeated the study, increasing the sample size and measuring physiological parameters (life stage, energetic, and reproductive status), and learning more about the ecosystem and prey field, would provide further valuable information on diving behaviour. The need for information on prey field, prey type, and prey profitability is highlighted by the lack of a clear trend in the proportion of time spent hunting in different dive types.
5. Conclusions
In this study, we show that Weddell Sea Weddell seals segregate in horizontal, vertical, and hydrographic space from late summer into midwinter. The residency of males on the continental shelf, and the lack of pelagic diving, suggest that benthic and shallow under-ice habitats provide adequate prey resources to support them. Whether by competition avoidance, or in search of ‘better’ (i.e. more reliable, abundant, energetically profitable) foraging opportunities, most of the females in our dataset left the shelf and moved north over the abyssal plain, supported by pelagic and epipelagic resources, before returning east and south towards shelf areas. These movements are associated with access to different hydrographic conditions, which are exploited via the diving patterns we have documented here. This is the earliest evidence both of (1) sex-specific diving patterns in this monomorphic top predator, and of (2) their common reliance on the high-salinity shelf water masses unique to the southern Weddell Sea. Information on intrinsic and external factors, as well as year-round diving behaviour, are needed to begin to understand the mechanisms that give rise to this pattern.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Thank you to Marius Årthun and the officers and crew of RRS Ernest Shackleton for their support during cruise ES054. Special thanks to Keith Nicholls for valuable input on Weddell Sea oceanography, and Mike Fedak for productive discussions on pinniped foraging strategies. The data collection was funded by NERCgrant nos NE/G014833/1 and NE/G014086/1. T.P. was supported by a Royal Society Newton International Fellowship (NF170682). K.H. was supported by a Marie-Skłodowska Curie Research Fellowship. The oceanographic data were processed and made freely available by the International MEOP Consortium and the national programs that contribute to it. (http://www.meop.net).
Ethics
Animal capture and handling protocols were reviewed and approved by the University of St Andrews Teaching and Research Ethics Committee (UTREC) and the Animal Welfare and Ethics Committee (AWEC) as part of the ethical review process. Permission to work in the Antarctic was granted under permit no. S7-4/2010 of the Antarctic Act 1994. Capture and deployment of satellite transmitters was carried out by experienced personnel with UK Animal (Scientific Procedures) Act 1986 Personal Licenses.
Data accessibility
The dataset analysed for this article is available in a Zenodo repository at http://doi.org/10.5281/zenodo.3820359 and R code for running the models can be found on GitHub at https://github.com/theoniphotopoulou/emews. An animated gif showing the tracking data through time is also available in a Zenodo repository at https://doi.org/10.5281/zenodo.3985898.
Authors' contributions
T.P. and L.B. collected the data and conceived the study. T.P., J.P., and K.H. developed the methods. T.P. carried out the analysis and wrote the manuscript. K.H. carried out the analysis in electronic supplementary material, S2. J.P. provided high-level statistical support. All authors contributed critically to the manuscript and gave approval for publication.
Competing interests
The authors declare no competing interests.
Funding
TP was supported by a Royal Society Newton International Fellowship (NF170682).
References
- 1.Dragon AC, Bar-Hen A, Monestiez P, Guinet C. 2012. Horizontal and vertical movements as predictors of foraging success in a marine predator. Mar. Ecol. Prog. Ser. 447, 243–257. ( 10.3354/meps09498) [DOI] [Google Scholar]
- 2.Woodson CB, Litvin SY. 2015. Ocean fronts drive marine fishery production and biogeochemical cycling. Proc. Natl Acad. Sci. USA 112, 1710–1715. ( 10.1073/pnas.1417143112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoit-Bird KJ, Moline MA, Southall BL. 2017. Prey in oceanic sound scattering layers organize to get a little help from their friends. Limnol. Oceanogr. 62, 2788–2798. ( 10.1002/lno10606) [DOI] [Google Scholar]
- 4.Biuw M. et al. 2007. Variations in behavior and condition of a Southern Ocean top predator in relation to in situ oceanographic conditions. Proc. Natl Acad. Sci. USA 104, 13 705–13 710. ( 10.1073/pnas.0701121104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naito Y, Costa DP, Adachi T, Robinson PW, Peterson SH, Mitani Y, Takahashi A. 2017. Oxygen minimum zone: an important oceanographic habitat for deep-diving northern elephant seals, Mirounga angustirostris. Ecol. Evol. 7, 6259–6270. ( 10.1002/ece3.3202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrahms B, Scales KL, Hazen EL, Bograd SJ, Schick RS, Robinson PW, Costa DP. 2018. Mesoscale activity facilitates energy gain in a top predator. Proc. R. Soc. B 285, 20181101 ( 10.1098/rspb.2018.1101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biuw M, Nøst O, Stien A, Zhou Q, Lydersen C, Kovacs K. 2010. Effects of hydrographic variability on the spatial, seasonal and diel diving patterns of southern elephant seals in the eastern Weddell Sea. PLoS ONE 5, 1–14. ( 10.1371/journal.pone.0013816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Toole MD, Lea MA, Guinet C, Schick R, Hindell MA. 2015. Foraging strategy switch of a top marine predator according to seasonal resource differences. Front. Mar. Sci. 2, 21 ( 10.3389/fmars.2015.00021) [DOI] [Google Scholar]
- 9.Nicholls KW, Boehme L, Biuw M, Fedak MA. 2008. Wintertime ocean conditions over the southern Weddell Sea continental shelf, Antarctica. Geophys. Res. Lett. 35, L21605 ( 10.1029/2008gl035742) [DOI] [Google Scholar]
- 10.Mitani Y, Watanabe Y, Sato K, Cameron MF, Naito Y. 2004. 3D diving behavior of Weddell seals with respect to prey accessibility and abundance. Mar. Ecol. Prog. Ser. 281, 275–281. ( 10.3354/meps281275) [DOI] [Google Scholar]
- 11.Williams TM, Davis RW, Fuiman LA, Francis J, Le BJ, Horning M, Calambokidis J, Croll DA. 2000. Sink or swim: strategies for cost-efficient diving by marine mammals. Science 288, 133–136. ( 10.1126/science.288.5463.133) [DOI] [PubMed] [Google Scholar]
- 12.Sparling CE, Fedak MA, Thompson D. 2007. Eat now, pay later? Evidence of deferred food-processing costs in diving seals. Biol. Lett. 3, 95–99. ( 10.1098/rsbl.2006.0566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuiman LA, Madden KM, Williams TM, Davis RW. 2007. Structure of foraging dives by Weddell seals at an offshore isolated hole in the Antarctic fast-ice environment. Deep Sea Res. Part II 54, 270–289. ( 10.1016/j.dsr2.2006.11.011) [DOI] [Google Scholar]
- 14.Heerah K, Hindell M, Guinet C, Charrassin JB. 2014. A new method to quantify within dive foraging behaviour in marine predators. PLoS ONE 9, e99329 ( 10.1371/journal.pone.0099329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heerah K, Cox SL, Blevin P, Charrassin JB. 2019. Validation of dive foraging indices using archived and transmitted acceleration data: the case of the weddell seal. Front. Ecol. Evol. 7, 1–15. ( 10.3389/fevo.2019.00030) [DOI] [Google Scholar]
- 16.Scales KL, Miller PI, Ingram SN, Hazen EL, Bograd SJ, Phillips RA. 2016. Identifying predictable foraging habitats for a wide-ranging marine predator using ensemble ecological niche models. Divers. Distributions 22, 212–224. ( 10.1111/ddi.12389) [DOI] [Google Scholar]
- 17.Sequeira AMM. et al. 2018. Convergence of marine megafauna movement patterns in coastal and open oceans. Proc. Natl Acad. Sci. USA 115, 3072–3077. ( 10.1073/pnas.1716137115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reisinger RR. et al. 2018. Habitat modelling of tracking data from multiple marine predators identifies important areas in the Southern Indian Ocean. Divers. Distributions 24, 535–550. ( 10.1111/ddi.12702) [DOI] [Google Scholar]
- 19.Saijo D, Mitani Y, Abe T, Sasaki H, Goetsch C, Costa DP, Miyashita K. 2017. Linking mesopelagic prey abundance and distribution to the foraging behavior of a deep-diving predator, the northern elephant seal. Deep Sea Res. Part II 140, 163–170. ( 10.1016/j.dsr2.2016.11.007) [DOI] [Google Scholar]
- 20.Treasure AM. et al. 2017. Marine mammals exploring the oceans pole to pole: a review of the MEOP consortium. Oceanography 30, 132–138. ( 10.5670/oceanog.2017.234) [DOI] [Google Scholar]
- 21.Harcourt R. et al. 2019. Animal-borne telemetry: an integral component of the ocean observing toolkit. Front. Mar. Sci. 6, 326 ( 10.3389/fmars.2019.00326) [DOI] [Google Scholar]
- 22.Bestley S, Jonsen ID, Hindell MA, Harcourt RG, Gales NJ. 2015. Taking animal tracking to new depths: synthesizing horizontal–vertical movement relationships for four marine predators. Ecology 96, 417–427. ( 10.1890/14-0469.1) [DOI] [PubMed] [Google Scholar]
- 23.Hindell MA. et al. 2016. Circumpolar habitat use in the southern elephant seal: implications for foraging success and population trajectories. Ecosphere 7, e01213 ( 10.1002/ecs2.1213) [DOI] [Google Scholar]
- 24.Nicholls KW, Østerhus S, Makinson K, Gammelsrød T, Fahrbach E. 2009. Ice-ocean processes over the continental shelf of the southern Weddell Sea, Antarctica: a review. Rev. Geophys. 47, RG3003 ( 10.1029/2007RG000250) [DOI] [Google Scholar]
- 25.Purkey SG, Smethie WM Jr, Gebbie G, Gordon AL, Sonnerup RE, Warner MJ, Bullister JL. 2018. A synoptic view of the ventilation and circulation of Antarctic bottom water from chlorofluorocarbons and natural tracers. Annu. Rev. Mar. Sci. 10, 503–527. ( 10.1146/annurev-marine-121916-063414) [DOI] [PubMed] [Google Scholar]
- 26.Kooyman G. 1975. A comparison between day and night diving in the Weddell seal. J. Mammal. 56, 563–574. ( 10.2307/1379469) [DOI] [Google Scholar]
- 27.Kooyman G, Ponganis P. 1998. The physiological basis of diving to depth: birds and mammals. Annu. Rev. Physiol. 60, 19–32. ( 10.1146/annurev.physiol.60.1.19) [DOI] [PubMed] [Google Scholar]
- 28.Heerah K, Andrews-Goff V, Williams G, Sultan E, Hindell M, Patterson T, Charrassin JB. 2013. Ecology of Weddell seals during winter: influence of environmental parameters on their foraging behaviour. Deep Sea Res. Part II 88-89, 23–33. ( 10.1016/j.dsr2.2012.08.025) [DOI] [Google Scholar]
- 29.Shero MR, Goetz KT, Costa DP, Burns JM. 2018. Temporal changes in Weddell seal dive behavior over winter: are females increasing foraging effort to support gestation? Ecol. Evol. 8, 11857–11874. ( 10.1002/ece3.4643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plötz J, Bornemann H, Knust R, Schröder A, Bester M. 2001. Foraging behaviour of Weddell seals, and its ecological implications. Polar Biol. 24, 901–909. ( 10.1007/s003000100297) [DOI] [Google Scholar]
- 31.McIntyre T, Stansfield L, Bornemann H, Plötz J, Bester M. 2013. Hydrographic influences on the summer dive behaviour of Weddell seals (Leptonychotes weddellii) in Atka Bay, Antarctica. Polar Biol. 36, 1693–1700. ( 10.1007/s00300-013-1384-7) [DOI] [Google Scholar]
- 32.Nachtsheim DA, Ryan S, Schröder M, Jensen L, Oosthuizen WC, Bester MN, Hagen W, Bornemann H. 2019. Foraging behaviour of Weddell seals (Leptonychotes weddellii) in connection to oceanographic conditions in the southern Weddell Sea. Prog. Oceanogr. 173, 165–179. ( 10.1016/j.pocean.2019.02.013) [DOI] [Google Scholar]
- 33.Watanabe Y, Bornemann H, Liebsch N, Plötz J, Sato K, Naito Y, Miyazaki N. 2006. Seal-mounted cameras detect invertebrate fauna on underside of Antarctic ice shelf. Mar. Ecol. Prog. Ser. 309, 297–300. ( 10.3354/meps309297) [DOI] [Google Scholar]
- 34.Liebsch N, Wilson RP, Bornemann H, Adelung D, Plöz J. 2007. Mouthing off about fish capture: jaw movement in pinnipeds reveals the real secrets of ingestion. Deep Sea Res. Part II 54, 256–269. ( 10.1016/j.dsr2.2006.11.014) [DOI] [Google Scholar]
- 35.Naito Y, Bornemann H, Takahashi A, McIntyre T, Plötz J. 2010. Fine-scale feeding behavior of Weddell seals revealed by a mandible accelerometer. Polar Sci. 4, 309–316. ( 10.1016/j.polar.2010.05.009) [DOI] [Google Scholar]
- 36.Wearmouth VJ, Sims DW. 2008. Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications. Adv. Mar. Biol. 54, 107–170. ( 10.1016/S0065-2881(08)00002-3) [DOI] [PubMed] [Google Scholar]
- 37.Beck CA, Bowen WD, McMillan JI, Iverson SJ. 2003. Sex differences in the diving behaviour of a size-dimorphic capital breeder: the grey seal. Anim. Behav. 66, 777–789. ( 10.1006/anbe.2003.2284) [DOI] [Google Scholar]
- 38.Lewis R, O’Connell TC, Lewis M, Campagna C, Hoelzel AR. 2006. Sex-specific foraging strategies and resource partitioning in the southern elephant seal (Mirounga leonina). Proc. R. Soc. B 273, 2901–2907. ( 10.1098/rspb.2006.3642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labrousse S. et al. 2017. Variability in sea ice cover and climate elicit sex specific responses in an Antarctic predator. Sci. Rep. 7, 43236 ( 10.1038/srep43236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langrock R, King R, Matthiopoulos J, Thomas L, Fortin D, Morales JM. 2012. Flexible and practical modeling of animal telemetry data: hidden Markov models and extensions. Ecology 93, 2336–2342. ( 10.1890/11-2241.1) [DOI] [PubMed] [Google Scholar]
- 41.Zucchini W, MacDonald IL, Langrock R. 2016. Hidden Markov models for time series: an introduction using R. Second Edition. Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- 42.Langrock R, Marques Ta, Baird RW, Thomas L. 2014. Modeling the diving behavior of whales: a latent-variable approach with feedback and semi-Markovian components. J. Agric. Biol. Environ. Stat. 19, 82–100. ( 10.1007/s13253-013-0158-6) [DOI] [Google Scholar]
- 43.DeRuiter SL, Langrock R, Skirbutas T, Goldbogen JA, Calambokidis J, Friedlaender AS, Southall BL. 2017. A multivariate mixed hidden Markov model for blue whale behaviour and responses to sound exposure. Ann. Appl. Stat. 11, 362–392. ( 10.1214/16-AOAS1008) [DOI] [Google Scholar]
- 44.Michelot T, Langrock R, Bestley S, Jonsen ID, Photopoulou T, Patterson TA. 2017. Estimation and simulation of foraging trips in land-based marine predators. Ecology 98, 1932–1944. ( 10.1002/ecy.1880) [DOI] [PubMed] [Google Scholar]
- 45.van Beest FM. et al. 2019. Classifying grey seal behaviour in relation to environmental variability and commercial fishing activity - a multivariate hidden Markov model. Sci. Rep. 9, 5642 ( 10.1038/s41598-019-42109-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ngô MC, Heide-Jørgensen MP, Ditlevsen S. 2019. Understanding narwhal diving behaviour using Hidden Markov Models with dependent state distributions and long range dependence. PLoS Comput. Biol. 15(3), e1006425 ( 10.1371/journal.pcbi.1006425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langley I, Fedak M, Nicholls K, Boehme L. 2018. Sex-related differences in the postmolt distribution of Weddell seals (Leptonychotes weddellii) in the southern Weddell Sea. Mar. Mammal Sci. 34, 403–419. ( 10.1111/mms.12461) [DOI] [Google Scholar]
- 48.Boehme L, Lovell P, Biuw M, Roquet F, Nicholson J, Thorpe SE, Meredith MP, Fedak M. 2009. Technical note: animal-borne CTD-satellite relay data loggers for real-time oceanographic data collection. Ocean Sci. 5, 685–695. ( 10.5194/os-5-685-2009) [DOI] [Google Scholar]
- 49.Photopoulou T, Fedak MA, Matthiopoulos J, McConnell B, Lovell P. 2015. The generalized data management and collection protocol for conductivity-temperature-depth satellite relay data loggers. Anim. Biotelem. 3, 21 ( 10.1186/s40317-015-0053-8) [DOI] [Google Scholar]
- 50.Argos. 2016. Argos User’s Manual:Worldwide tracking and environmental monitoring by satellite.
- 51.Jonsen I, Patterson T. 2019. foieGras: fit continuous-time state-space and latent variable models for filtering argos satellite (and other) telemetry data and estimating movement behaviour. CRAN. R package version 0.4.01. Available from: https://cran.r-project.org/package=foieGras.
- 52.Årthun M, Nicholls KW, Makinson K, Fedak MA, Boehme L. 2012. Seasonal inflow of warm water onto the southern Weddell Sea continental shelf, Antarctica. Geophys. Res. Lett. 39, L17601 ( 10.1029/2012GL052856) [DOI] [Google Scholar]
- 53.Boehme L, Baker A, Fedak M, Årthun M, Nicholls K, Robinson P, Costa D, Biuw M, Photopoulou T. 2016. Bimodal winter haul-out patterns of adult Weddell seals (Leptonychotes weddellii) in the Southern Weddell Sea. PLoS ONE 11(5), e0155817 ( 10.1371/journal.pone.0155817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Photopoulou T, Lovell P, Fedak MA, Thomas L, Matthiopoulos J. 2015. Efficient abstracting of dive profiles using a broken-stick model. Methods Ecol. Evol. 6, 278–288. ( 10.1111/2041-210X.12328) [DOI] [Google Scholar]
- 55.Heerah K, Hindell MA, Guinet C, Charrassin JB. 2015. From high-resolution to low-resolution dive datasets: a new index to quantify the foraging effort of marine predators. Anim. Biotelem. 3, 1–12. ( 10.1186/s40317-015-0074-3) [DOI] [Google Scholar]
- 56.Watanabe Y, Mitani Y, Sato K, Cameron MF, Naito Y. 2003. Dive depths of Weddell seals in relation to vertical prey distribution as estimated by image data. Mar. Ecol. Prog. Ser. 252, 283–288. ( 10.3354/meps252283) [DOI] [Google Scholar]
- 57.R Core Team. 2019. R: A Language and Environment for Statistical Computing. Vienna, Austria. Available from: https://www.R-project.org/.
- 58.Harcourt R, Kingston J, Waas JR, Hindell MA. et al. 2008. Foraging while breeding: alternative mating strategies by male Weddell seals? Aquat. Conserv. 17, S68 ( 10.1002/aqc.915) [DOI] [Google Scholar]
- 59.Ekau W. 1990. Demersal fish fauna of the Weddell Sea, Antarctica. Antarct. Sci. 2, 129–137. ( 10.1017/S0954102090000165) [DOI] [Google Scholar]
- 60.Bailleul F, Charrassin JB, Ezraty R, Girard-Ardhuin F, McMahon CR, Field IC, Guinet C. 2007. Southern elephant seals from Kerguelen Islands confronted by Antarctic Sea ice. Changes in movements and in diving behaviour. Deep Sea Res. Part II: Top. Stud. Oceanogr. 54, 343–355. ( 10.1016/j.dsr2.2006.11.005) [DOI] [Google Scholar]
- 61.Labrousse S. et al. 2015. Winter use of sea ice and ocean water mass habitat by southern elephant seals: the length and breadth of the mystery. Prog. Oceanogr. 137, 52–68. ( 10.1016/j.pocean.2015.05.023) [DOI] [Google Scholar]
- 62.Garrison DL, Close AR. 1993. Winter ecology of the sea ice biota in Weddell Sea pack ice. Mar. Ecol. Prog. Ser. 96, 17–31. ( 10.3354/meps096017) [DOI] [Google Scholar]
- 63.Vernet M. et al. 2019. The Weddell Gyre, Southern ocean: present knowledge and future challenges. Rev. Geophys. 57, 623–708. ( 10.1029/2018RG000604) [DOI] [Google Scholar]
- 64.Jenkins A, Dutrieux P, Jacobs S, Steig EJ, Gudmundsson GH, Smith J, Heywood KJ. 2016. Decadal ocean forcing and Antarctic ice sheet response: lessons from The Amundsen Sea. Oceanography 29, 106–117. ( 10.5670/oceanog.2016.103) [DOI] [Google Scholar]
- 65.Christensen V. 1995. Ecosystem maturity–towards quantification. Ecol. Modell. 77, 3–32. ( 10.1016/0304-3800(93)E0073-C) [DOI] [Google Scholar]
- 66.Riotte-Lambert L, Matthiopoulos J. 2020. Environmental predictability as a cause and consequence of animal movement. Trends Ecol. Evol. 35, 163–174. ( 10.1016/j.tree.2019.09.009) [DOI] [PubMed] [Google Scholar]
- 67.Goetz KT, Burns JM, Hückstädt LA, Shero MR, Costa DP. 2017. Temporal variation in isotopic composition and diet of Weddell seals in the western Ross Sea. Deep Sea Res. Part II 140, 36–44. ( 10.1016/j.dsr2.2016.05.017) [DOI] [Google Scholar]
- 68.Burns JM, Trumble SJ, Castellini MA, Testa JW. 1998. The diet of Weddell seals in McMurdo Sound, Antarctica as determined from scat collections and stable isotope analysis. Polar Biol. 19, 272–282. ( 10.1007/s003000050245) [DOI] [Google Scholar]
- 69.Fuiman L, Davis R, Williams T. 2002. Behavior of midwater fishes under the Antarctic ice: observations by a predator. Mar. Biol. 140, 815–822. ( 10.1007/s00227-001-0752-y) [DOI] [Google Scholar]
- 70.Davis RW, Fuiman LA, Williams TM, Horning M, Hagey W. 2003. Classification of Weddell seal dives based on 3 dimensional movements and video-recorded observations. Mar. Ecol. Prog. Ser. 264, 109–122. ( 10.3354/meps264109) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analysed for this article is available in a Zenodo repository at http://doi.org/10.5281/zenodo.3820359 and R code for running the models can be found on GitHub at https://github.com/theoniphotopoulou/emews. An animated gif showing the tracking data through time is also available in a Zenodo repository at https://doi.org/10.5281/zenodo.3985898.