Abstract
Compensating for wind drift can improve goalward flight efficiency in animal taxa, especially among those that rely on thermal soaring to travel large distances. Little is known, however, about how animals acquire this ability. The great frigatebird (Fregata minor) exemplifies the challenges of wind drift compensation because it lives a highly pelagic lifestyle, travelling very long distances over the open ocean but without the ability to land on water. Using GPS tracks from fledgling frigatebirds, we followed young frigatebirds from the moment of fledging to investigate whether wind drift compensation was learnt and, if so, what sensory inputs underpinned it. We found that the effect of wind drift reduced significantly with both experience and access to visual landmark cues. Further, we found that the effect of experience on wind drift compensation was more pronounced when birds were out of sight of land. Our results suggest that improvement in wind drift compensation is not solely the product of either physical maturation or general improvements in flight control. Instead, we believe it is likely that they reflect how frigatebirds learn to process sensory information so as to reduce wind drift and maintain a constant course during goalward movement.
Keywords: navigation, ontogeny, development, wind drift compensation, seabirds, frigatebird
1. Introduction
For motile animals, the ability to navigate efficiently through space is essential, and for animals moving over long distances early in life, it is necessary that this ability is either innate or develops rapidly. In birds, initial orientation among long-distance migrants is thought to be inherited genetically [1], possibly as a vector [2–4]. However, for any instructions to be meaningful in a stochastic and changeable environment, an animal must account for instantaneous variation in the prevailing conditions, such as wind strength and direction. Any animal moving through a fluid medium is liable to drift, and thus any flying animal moving towards a target is liable to drift with the prevailing air movement. Not all animal movement is goal-oriented, and thus drifting partially or completely with a fluid medium is not necessarily maladaptive [5]; indeed, moving with the overall movement of a fluid could increase the overall energetic efficiency of long-distance movement [6–10]. However, if an animal is to move efficiently through space towards a pre-determined goal, accounting and correcting for wind drift is likely to be beneficial over both large and small spatial scales [11–14].
‘Wind drift compensation’ encompasses multiple behaviours that limit the wind's propensity to displace a navigator from their most efficient goalward route. This is typically thought to include the adjustment of the heading so that the track taken is oriented towards the goal [15], though in practice multiple modifications could be made that contribute to a reduction in wind drift (e.g. increasing the rate at which direction to the goal is updated). Wind drift compensation, by whatever mechanism, is likely to be especially important in seabirds, given their use of wind to efficiently travel vast distances over often visually sparse terrain [16–18]. At least partial wind drift compensation has been noted in several seabird taxa among mature individuals, with the extent to which birds drift postulated to be contingent on the sensory cues available [19,20]. Among seabirds, great frigatebirds (Fregata minor) may be particularly susceptible to wind drift, owing to their reliance on thermalling flight [21] and their inability to land on the water due to non-waterproofed feathering [22]. Whether frigatebirds have an ability to compensate for wind drift, and more generally how such an ability might develop in avian taxa, are, however, unclear. While experience has been shown to affect navigational ability (and migration phenology) across animal taxa [23–25], and age has been specifically implicated as a predictor of drift compensation in birds [24], it is unclear whether improvement in drift compensation with age reflects learning or simply reflects physical maturation (e.g. muscle and feather growth).
Here, we use GPS tracks derived from free-flying fledgling frigatebirds to investigate the development of drift compensation in avian taxa. Frigatebirds have a protracted post-fledging period where they still rely on parental care but are capable of independently moving around the natal site [22], in doing so refining both biomechanical flight and foraging behaviour [26,27]. Because birds were tracked consistently from the moment they fledged, we were able to precisely quantify the extent of individual birds' experience prior to specific excursions from their breeding colony. Using this information, alongside data pertaining to the extent to which land was visible to an individual along its homing trajectory, we sought to investigate (i) whether experience correlates with reduced wind drift during homing flight, and (ii) if so, whether this correlation was contingent on the sensory cues available.
2. Methods
(a). GPS tracking
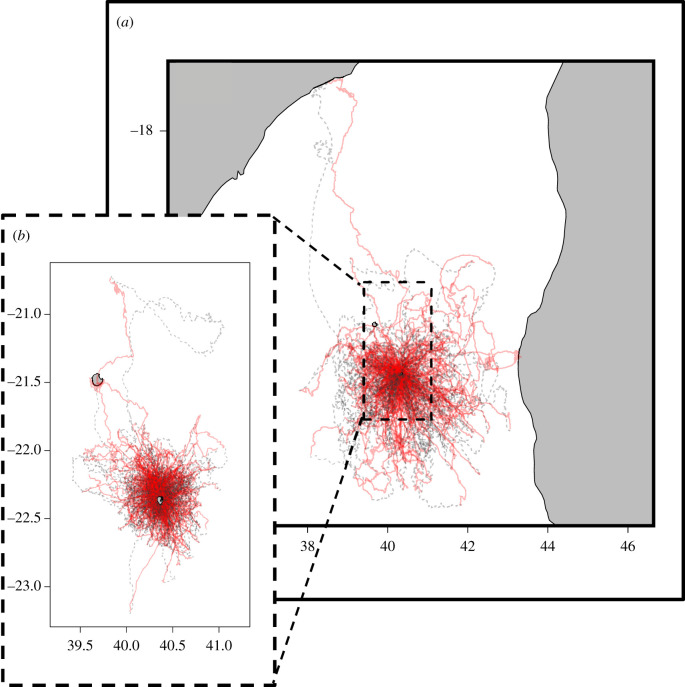
Tracking was carried out on Europa Island in the Mozambique Channel (−22.36° N, 40.37° E) on 13 adult female and 10 juvenile frigatebirds (figure 1). Adult males were not used in this analysis as their role in chick provisioning is limited and, hence, they show little homing motivation. Devices measured 130 × 30 × 12 mm and weighed 30 g (PS-RF, e-obs GmbH, Munich, Germany), representing between 1.88 and 3.55% of the frigatebirds’ mass. Devices were deployed dorsally using Tesa tape and were set to record location every 2 or 5 min. Three-dimensional accelerometer data were also gathered but are not presented here [27].
Figure 1.
The complete GPS tracks used in this analysis. (a) Interpolated GPS tracks from adult female frigate birds and (b) interpolated GPS tracks from fledglings are shown, with the dotted black line denoting the juvenile distribution nested within the adult distribution. Homing sections of tracks are highlighted in red, while outbound sections are highlighted in grey. (Online version in colour.)
(b). Environmental and landmark cue data
Wind data were derived from the NOAA Global Forecast System at a temporal resolution of 3 h and a spatial resolution of 0.5° longitude and 0.5° latitude. Whether or not birds could see any piece of land was ascertained using their altitude, measured using GPS and smoothed using a rolling median over a window of four consecutive fixes, the elevation of local topography, derived from USGS Global Multi-resolution Terrain Elevation Data, and the curvature of Earth. Smoothed altitude was used in analyses as the GPS-derived altitudinal error is substantially higher than that observed in both the longitudinal and latitudinal dimensions [28,29]. Birds were assumed to be able to see land if a line-of-sight could be drawn between their position and the maximum elevation of any piece of land without the Earth's surface intervening. GPS points taken at night were removed from the analysis because it was not known whether reduced visual salience might affect access to landmark cues, and there were insufficient night-time GPS points to statistically test for an effect of daytime/night-time (980 individual fixes, representing only 24 trips from 6 unique individuals). Analysis including GPS fixes taken at night is included in the electronic supplementary material.
(c). Track processing, statistics and analysis
All statistics and processing were conducted in R [30]. Tracks were interpolated using a cubic spline function [31] so that fixes were positioned at precise 5 min intervals. Tracks were also divided into trips out from the colony, with a trip defined as a continuous set of points recorded greater than 500 m from the island's coastline with a maximum distance from the colony of greater than 3 km. Since juveniles were tracked from their very first trips to sea, for a given trip, we attempted to quantify the experience of the bird at that point in its development, measuring experience as the number of trips the focal bird had been on prior to the trip in question. In total, 19 732 interpolated GPS fixes were used in the analysis of fledgling frigatebirds, representing 1001 trips from 10 individual birds (with a mean of 100 and a median of 122 trips per individual), while 35 430 interpolated GPS fixes were used in the analysis of adult frigatebirds, representing 345 trips from 13 individuals (with a mean of 26 and a median of 12 trips per individual).
We chose to analyse only the homing sections of trajectories as we had an a priori expectation of where birds were aiming for. Because we had no expectations about the form of homing behaviour in frigatebirds, we conservatively defined homing as any points that occurred after the maximum distance to the colony was recorded on the trajectory. Due to the mechanisms by which frigatebirds generate lift (principally thermalling) and search for prey items (area-restricted search behaviour), we expected individuals of all ages to engage in tortuous, non-navigational behaviours [21]. Track tortuosity was measured using the rolling standard deviation of track bearing over a window of 5 consecutive fixes, and non-navigational behaviours were parsed out using a mixture model to separate GPS fixes into two groups based on tortuosity [32,33]. Only points with tortuosity lower than the mixture model-defined cut-off (of 52°) were retained for use in navigational analyses. We repeated all analyses with multiple tortuosity cut-off points so as to ensure the significance of any findings were robust and unbiased by the threshold at which points were removed based on tortuosity (see electronic supplementary material).
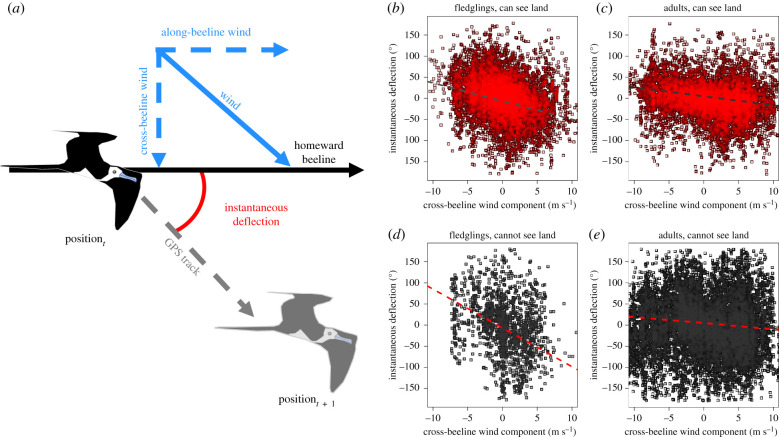
For each point along a homing track, a beeline direction to the colony was assigned along the Great Circle route, from which instantaneous deflection was calculated [34]. Orientation behaviour was modelled using this instantaneous deflection from the beeline (measured in degrees on a −180° to 180° scale) as a response variable. From the calculated beeline direction home, the cross-beeline and along-beeline wind components were calculated per interpolated GPS position. Using these wind components, we modelled the effect of wind drift in a three-way interaction between the cross-beeline wind component, fledgling experience and whether or not the bird could see land as a binary factor (figure 2). The along-beeline wind component was also included in all models. This was because we expected that an increased headwind component might reduce groundspeed, thus halting a bird's forward progress and increasing instantaneous deflection per unit crosswind. By including the along-beeline component, we, therefore, sought to standardize model output coefficients with respect to the along-beeline wind component so as the results presented were not the result of a confound between any variables of interest and the along-beeline wind component.
Figure 2.
The effect of access to landmark cues on wind drift in fledgling and adult frigatebirds. (a) Visual representation of modelled quantities and (b–e) deflection plotted against the cross-beeline wind component in (b) fledgling frigatebirds that could see land, (c) adult frigatebirds that could see land, (d) fledgling frigatebirds that could not see land and (e) adult frigatebirds that could not see land. Regression lines show the mean effect size as estimated using linear mixed-effects models. Negative values for crosswind component/deflection angle indicate that the crosswind/deflection is anti-clockwise with respect to the bird's movement. (Online version in colour.)
The effect of wind drift was modelled using linear mixed-effects models with trip ID, nested within bird ID, used as random effects [34,35]. p-values were calculated using likelihood ratio tests between the hypothesized (alternative) model and a null model that did not contain the variable or interaction of interest.
3. Results
We found that, for fledgling frigatebirds, there was a significant effect of the cross-beeline wind component on deflection from the beeline to home (LR test; p < 0.001; figure 2). Linear mixed-effect model outputs suggest that, between consecutive GPS fixes, inexperienced frigatebirds with no line-of-sight to land drifted by 10.5° (±2.21° (s.e.); table 1) per metre per second of crosswind. We found, however, that there was a significant interaction between the cross-beeline wind component and whether or not land was in principle visible (LR test; p < 0.001), and that drift per metre per second of wind reduced by 5.72° (±2.23°) in sight of land. This suggests that juvenile frigatebirds drifted around twice as much per unit wind speed when they could not see land when compared with when they could (figure 2).
Table 1.
Fixed-effect estimates for wind drift compensation. Fixed effects, as estimated using a linear mixed-effects model, as used in our analysis of wind drift compensation in fledgling frigatebirds. Because the overall correlation between the deflection angle and wind speed is negative (i.e. winds anti-clockwise of the beeline displace a bird clockwise and vice versa), a positive effect indicates a reduction in wind drift.
| effect | estimate | s.e. | t-value |
|---|---|---|---|
| (intercept) | −10.84 | 6.50 | −1.67 |
| cross-beeline wind | −10.51 | 2.21 | −4.75 |
| experience | 0.17 | 0.06 | 2.59 |
| can see land = TRUE | 13.53 | 6.01 | 2.25 |
| along-beeline wind | −0.08 | 0.15 | −0.56 |
| cross-beeline wind × experience | 0.06 | 0.02 | 2.73 |
| cross-beeline wind × can see land = TRUE | 5.72 | 2.23 | 2.57 |
| experience × can see land = TRUE | −0.14 | 0.06 | −2.36 |
| cross-beeline wind × can see land = TRUE × experience | −0.04 | 0.02 | −2.04 |
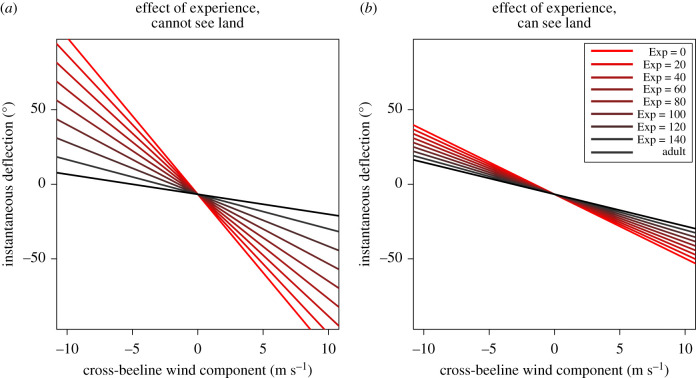
As well as finding evidence that naive fledglings seemed to drift with the wind, we also found that the magnitude of this effect varied significantly with experience (figure 3; LR test; p < 0.001). For each trip away from the island, the angular effect of wind drift was reduced by 0.0586° (±0.0214°; table 1) for birds that did not have a direct line of sight to land. Since during the tracking period, we found that birds undertook a median 122 trips from the colony meaning that, after the median number of trips were completed, birds were estimated to have reduced their drift per metre per second crosswind by 7.14° (±2.61°) when out of sight of land. By contrast, we found that the effect of experience was significantly reduced when birds were in sight of land (LR test; p < 0.001; table 1), with the effect of experience on drift reduced by 0.0439° (±0.0216°) when birds could see land, suggesting that the effect of experience was substantially greater when access to visual landmarks is limited.
Figure 3.
The development of wind drift in fledgling frigatebirds. The LMM-estimated mean effect of wind speed on instantaneous deflection for fledglings of varying experiences (Exp, the number of trips undertaken) and adults. (a) The estimated effect sizes for birds moving out of sight of land and (b) the estimated effect sizes for birds moving in sight of land. As in figure 1, negative values for crosswind component/deflection angle indicate that the crosswind/deflection is anti-clockwise with respect to the bird's movement. (Online version in colour.)
While there was a significant effect of wind drift on adult frigatebirds (LR test; p < 0.001), it was of a markedly lower magnitude compared to that observed in fledglings. The modelled estimate of wind drift was 1.43° (±0.12°) drift per metre per second of wind in adult frigatebirds when out of sight of land (figure 2). Unlike fledgling frigatebirds, we found that this drift component increased slightly but significantly by 0.701° (±0.172°) when birds could see land (LR test; p < 0.001; figure 2).
4. Discussion
Based on complete GPS records of the free-ranging post-fledging movements of great frigatebirds, we found that wind drift was reduced by both access to visual landmark cues and increased experience. Experience interacted with access to visual cues, reducing wind drift to a greater extent when birds were out of sight of land (figure 2). While previous studies have shown age to be a strong predictor of wind drift compensation in other avian taxa [24], the role of experience has so far remained unclear. Here, we postulate that at least part of the improvement in wind drift compensation with age is a learnt ability, gained progressively, probably involving the interpretation of sensory inputs.
General increases in flight control, due to physical maturation or improvements in the cognitive control of flight, might facilitate successful wind drift compensation and, indeed, previous studies have reported differences in wing length between adult and fledgling frigatebirds [27]. However, we might expect that, if such maturation processes were to underpin the results presented here, the rate of drift both in and out of sight of land should reduce at similar rates with experience. By contrast, we find that the change in wind drift with experience is much more prominent out of sight of land. Hence, we instead suggest that experience acts on the cognitive processes that transform sensory input into the motor actions required to counter drift. We reason this is the case because experience interacts with access to visual landmark cues, suggesting that the effect of experience is in part contingent on the sensory input available. We suggest that this reflects a process where birds learn, possibly using simple associative learning [36], how sensory input relates to drift, and in turn use this input to reduce drift on subsequent trips. Although these effects are consistent with processes dominated by individual learning, it is also possible that social learning effects, learning involving the observation and mimicry of conspecifics [37], might also contribute since frigatebirds are a colonially nesting species.
Drift compensation is typically thought to involve the quantitative adjustment of heading into the wind so as the resultant track taken over the ground is more goalward-oriented [5]. This process requires that a bird can, first, use sensory input to assess correctly the extent to which it is drifting before, second, adjusting its heading accordingly. Given that we find that birds drift less when in sight of land, this could involve visual cues, possibly as optic flow (the relative movement of objects across the field of view) since this has been implicated in wind drift reduction and course retention both in avian taxa and in animals more generally [38–41]. However, given that multiple cues covary with access to visual landmark cues, this is difficult to parse out using only correlative studies.
Heading alteration is not, however, the only mechanism by which wind drift might be reduced with experience. The rate at which birds are displaced by the wind might be reduced by learnt navigational mechanisms that are not specific to wind drift reduction, such as through a more precise understanding of an individual's position; if, at the start of goal-oriented navigation, a bird calculated the bearing home only once and then maintained that heading, then the angle between the bird's realized trajectory and the target would increase if a bird drifted with the wind. Consequently, even a bird inattentive to instantaneous wind conditions could reduce the rate at which it drifts by increasing the rate at which it attends to its displacement relative to the goal. This could be, for example, because of an increase in the resolution at which it perceives spatial information (e.g. an increase in the resolution of a navigational map). As such, it is unclear from our analyses whether frigatebirds learn to attend to specific drift-related cues or whether the observed reduction in wind drift is a product of a learnt ability to attend to more general navigational cues.
In addition to finding that fledglings drift less when more experienced, we find that adult frigatebirds drift less than fledglings (figure 2). The estimated effect sizes derived from linear mixed-effects models imply that, even after the prolonged pre-migratory parental care period, fledgling frigatebirds still drift more than their adult conspecifics (table 1). Indeed, model output would suggest around 151 trips are required to equal the drift compensation seen in adults when out of sight of land. In turn, this suggests that the development of wind drift compensation continues as the fledglings start to migrate. While this is consistent with experience improving drift compensation, we note that, unlike in fledglings, adults drift slightly (but significantly) more when in sight of land. However, the magnitude of this difference is substantially smaller (by almost an order of magnitude) than the difference observed in fledglings. Given that there is no apparent sensory explanation why drift might be increased with increased salient visual information in experienced individuals, we suggest that such a difference may reflect differences in the motivation to home when approaching the colony rather than an inability to compensate for drift when in sight of land.
Taken together, our results suggest that frigatebird drift compensation is, at least in part, learnt. As a tropical seabird with an extended post-fledging parental care period that is reliant on thermals to gain lift, it is possible that the development of wind drift compensation in frigatebirds reflects their unique ecology. Further study is therefore required in order to investigate the generalizability of these results to other avian taxa. Nonetheless, we believe these results are informative in the context of the development and mechanism of avian navigation, and demonstrate the power of analysing free-ranging GPS tracks when examining the ontogeny of animal behaviour.
Supplementary Material
Acknowledgements
Logistical support in Europa Island was kindly provided by the administration of the ‘Terres Australes et Antarctiques Françaises' and the ‘Forces Armées de la Zone Sud de l'Océan Indien’. We thank J. B. Pons, S. Jacquemet, M. LeCorre, M. Bastien and R. Weimerskirch for assistance in the field. We would like to thank the members of the Oxford navigation group for their input into the analysis and for many useful discussions.
Data accessibility
The data used in our analyses are stored and freely available on the Dryad Digital Repository: https://doi.org/10.5061/dryad.4tmpg4f7s [42].
Authors' contributions
Analysis was based on data gathered by H.W. and A.P. and processed by A.C. and J.C. The analyses were designed by J.W. and J.C. with input from O.P. and T.G. Analyses were conducted by J.W., J.C. and O.P. An initial draft was written by J.W. with input from J.C., upon which all authors commented to produce the submitted manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was funded as part of the EARLYLIFE program by a European Research Council Advanced Grant under the European Community's Seven Framework Program FP7/2007–2013 (Grant agreement ERC-2012-ADG_20120314 to H.W.) and was also part of the program ‘FREGATE—Iles Eparses' fund by The French National Centre for Scientific Research (CNRS), the French National Research Institute for Sustainable Development (IRD) and the ‘Agence des Aires Marines Protégées’. J.W. was funded by a UKRI BBSRC scholarship (grant no. BB/M011224/1), J.C. was supported by the Templeton World Charity Foundation (grant no. TWCF0316 to D. Biro), O.P. was funded by a Junior Research Fellowship at St John's College, University of Oxford and T.G.'s research was supported by Merton College, University of Oxford, and by the Mary Griffiths award.
References
- 1.Helbig AJ. 1991. Inheritance of migratory direction in a bird species: a cross-breeding experiment with SE- and SW-migrating blackcaps (Sylvia atricapilla). Behav. Ecol. Sociobiol. 28, 9–12. ( 10.1007/BF00172133) [DOI] [Google Scholar]
- 2.Perdeck A. 1958. Two types of orientation in migrating starlings, Sturnus yulgaris L., and chaffinches, Fringilla coelebs L., as revealed by displacement experiments. Ardea 55, 1–3. [Google Scholar]
- 3.Yoda K, Yamamoto T, Suzuki H, Matsumoto S, Muller M, Yamamoto M. 2017. Compass orientation drives naive pelagic seabirds to cross mountain ranges. Curr. Biol. 27, R1152–R1153. ( 10.1016/j.cub.2017.09.009) [DOI] [PubMed] [Google Scholar]
- 4.Mouritsen H. 1998. Modelling migration: the clock-and-compass model can explain the distribution of ringing recoveries. Anim. Behav. 56, 899–907. ( 10.1006/anbe.1998.0826) [DOI] [PubMed] [Google Scholar]
- 5.Chapman JW, Klaassen RHG, Drake VA, Fossette S, Hays GC, Metcalfe JD, Reynolds AM, Reynolds DR, Alerstam T. 2011. Animal orientation strategies for movement in flows. Curr. Biol. 21, R861–R870. ( 10.1016/j.cub.2011.08.014) [DOI] [PubMed] [Google Scholar]
- 6.Alerstam T. 1979. Optimal use of wind by migrating birds—combined drift and overcompensation. J. Theor. Biol. 79, 341–353. ( 10.1016/0022-5193(79)90351-5) [DOI] [PubMed] [Google Scholar]
- 7.Pinti J, Celani A, Thygesen UH, Mariani P. 2020. Optimal navigation and behavioural traits in oceanic migrations. Theor. Ecol. 13, 1–11. [Google Scholar]
- 8.Liechti F. 1995. Modelling optimal heading and airspeed of migrating birds in relation to energy expenditure and wind influence. J. Avian Biol. 26, 330–336. ( 10.2307/3677049) [DOI] [Google Scholar]
- 9.Lambardi P, Lutjeharms JRE, Mencacci R, Hays GC, Luschi P. 2008. Influence of ocean currents on long-distance movement of leatherback sea turtles in the Southwest Indian Ocean. Marine Ecol. Prog. Ser. 353, 289–301. ( 10.3354/meps07118) [DOI] [Google Scholar]
- 10.Hays GC, Fossette S, Katselidis KA, Mariani P, Schofield G. 2010. Ontogenetic development of migration: Lagrangian drift trajectories suggest a new paradigm for sea turtles. J. R. Soc. Interface. 7, 1319–1327. ( 10.1098/rsif.2010.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley JR, Reynolds DR, Smith AD, Edwards AS, Osborne JL, Williams IH, McCartney HA. 1999. Compensation for wind drift by bumble-bees. Nature 400, 126 ( 10.1038/22029) [DOI] [Google Scholar]
- 12.Srygley RB. 2001. Compensation for fluctuations in crosswind drift without stationary landmarks in butterflies migrating over seas. Anim. Behav. 61, 191–203. ( 10.1006/anbe.2000.1551) [DOI] [PubMed] [Google Scholar]
- 13.Krupczynski P, Schuster S. 2008. Fruit-catching fish tune their fast starts to compensate for drift. Curr. Biol. 18, 1961–1965. ( 10.1016/j.cub.2008.10.066) [DOI] [PubMed] [Google Scholar]
- 14.Chapman JW, Reynolds DR, Mouritsen H, Hill JK, Riley JR, Sivell D, Smith AD, Woiwod IP. 2008. Wind selection and drift compensation optimize migratory pathways in a high-flying moth. Curr. Biol. 18, 514–518. ( 10.1016/j.cub.2008.02.080) [DOI] [PubMed] [Google Scholar]
- 15.Richardson WJ. 1990. Wind and orientation of migrating birds—a review. Experientia 46, 416–425. ( 10.1007/BF01952175) [DOI] [PubMed] [Google Scholar]
- 16.Gibb R, Shoji A, Fayet AL, Perrins CM, Guilford T, Freeman R. 2017. Remotely sensed wind speed predicts soaring behaviour in a wide-ranging pelagic seabird. J. R. Soc. Interface 14, 20170262 ( 10.1098/rsif.2017.0262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weimerskirch H, Chastel O, Barbraud C, Tostain O. 2003. Frigatebirds ride high on thermals—this bird's bizarre physique and sparse hunting grounds account for its languid lifestyle. Nature 421, 333–334. ( 10.1038/421333a) [DOI] [PubMed] [Google Scholar]
- 18.Ventura F, Granadeiro JP, Padget O, Catry P. 2020. Gadfly petrels use knowledge of the windscape, not memorized foraging patches, to optimize foraging trips on ocean-wide scales. Proc. R. Soc. B 287, 20191775 ( 10.1098/rspb.2019.1775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goto Y, Yoda K, Sato K. 2017. Asymmetry hidden in birds' tracks reveals wind, heading, and orientation ability over the ocean. Sci. Adv. 3, e1700097 ( 10.1126/sciadv.1700097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarroux A, et al. 2016. Flexible flight response to challenging wind conditions in a commuting Antarctic seabird: do you catch the drift? Anim. Behav. 113, 99–112. ( 10.1016/j.anbehav.2015.12.021) [DOI] [Google Scholar]
- 21.Weimerskirch H, Bishop C, Jeanniard-du-Dot T, Prudor A, Sachs G. 2016. Frigate birds track atmospheric conditions over months-long transoceanic flights. Science 353, 74–78. ( 10.1126/science.aaf4374) [DOI] [PubMed] [Google Scholar]
- 22.Nelson JB. 2005. Pelicans, cormorants and their relatives: Pelecanidae, Sulidae, Phalacrocoracidae, Anhingidae, Fregatidae, Phaethontidae. Oxford, UK: Oxford University Press. [Google Scholar]
- 23.Capaldi EA, et al. 2000. Ontogeny of orientation flight in the honeybee revealed by harmonic radar. Nature 403, 537–540. ( 10.1038/35000564) [DOI] [PubMed] [Google Scholar]
- 24.Thorup K, Alerstam T, Hake M, Kjellen N. 2003. Bird orientation: compensation for wind drift in migrating raptors is age dependent. Proc. R. Soc. Lond. B 270, S8–S11. ( 10.1098/rsbl.2003.0014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campioni L, Dias MP, Granadeiro JP, Catry P. 2020. An ontogenetic perspective on migratory strategy of a long-lived pelagic seabird: timings and destinations change progressively during maturation. J. Anim. Ecol. 89, 29–43. ( 10.1111/1365-2656.13044) [DOI] [PubMed] [Google Scholar]
- 26.Collet J, Prudor A, Corbeau A, Mendez L, Weimerskirch H. 2020. First explorations: ontogeny of central place foraging directions in two tropical seabirds. Behav. Ecol. 31, 815–825. [Google Scholar]
- 27.Corbeau A, Prudor A, Kato A, Weimerskirch H. 2020. Development of flight and foraging behaviour in a juvenile seabird with extreme soaring capacities. J. Anim. Ecol. 89, 20–28. ( 10.1111/1365-2656.13121) [DOI] [PubMed] [Google Scholar]
- 28.Bouten W, Baaij EW, Shamoun-Baranes J, Camphuysen KCJ. 2013. A flexible GPS tracking system for studying bird behaviour at multiple scales. J. Ornithol. 154, 571–580. ( 10.1007/s10336-012-0908-1) [DOI] [Google Scholar]
- 29.Ross-Smith VH, Thaxter CB, Masden EA, Shamoun-Baranes J, Burton NHK, Wright LJ, Rehfisch MM, Johnston A. 2016. Modelling flight heights of lesser black-backed gulls and great skuas from GPS: a Bayesian approach. J. Appl. Ecol. 53, 1676–1685. ( 10.1111/1365-2664.12760) [DOI] [Google Scholar]
- 30.Team R Core. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 31.Tremblay Y, et al. 2006. Interpolation of animal tracking data in a fluid environment. J. Exp. Biol. 209, 128–140. ( 10.1242/jeb.01970) [DOI] [PubMed] [Google Scholar]
- 32.Benaglia T, Chauveau D, Hunter DR, Young DS. 2009. mixtools: an R package for analyzing finite mixture models. J. Stat. Softw. 32, 1–29. ( 10.18637/jss.v032.i06) [DOI] [Google Scholar]
- 33.Dean B, Kirk H, Fayet A, Shoji A, Freeman R, Leonard K, Perrins CM, Guilford T. 2015. Simultaneous multi-colony tracking of a pelagic seabird reveals cross-colony utilization of a shared foraging area. Mar. Ecol. Prog. Ser. 538, 239–248. ( 10.3354/meps11443) [DOI] [Google Scholar]
- 34.Padget O, Bond SL, Kavelaars MM, van Loon E, Bolton M, Fayet AL, Syposz M, Roberts S, Guilford T. 2018. In situ clock shift reveals that the sun compass contributes to orientation in a pelagic seabird. Curr. Biol. 28, 275–279. ( 10.1016/j.cub.2017.11.062) [DOI] [PubMed] [Google Scholar]
- 35.Bates D, Machler M, Bolker BM, Walker SC. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 36.Guilford T, de Perera TB. 2017. An associative account of avian navigation. J. Avian Biol. 48, 191–195. ( 10.1111/jav.01355) [DOI] [Google Scholar]
- 37.Hoppitt W, Laland KN. 2008. Social processes influencing learning in animals: a review of the evidence. Adv. Study Behav 38, 105–165. ( 10.1016/S0065-3454(08)00003-X) [DOI] [Google Scholar]
- 38.Hedenstrom A, Akesson S. 2017. Adaptive airspeed adjustment and compensation for wind drift in the common swift: differences between day and night. Anim. Behav. 127, 117–123. ( 10.1016/j.anbehav.2017.03.010) [DOI] [Google Scholar]
- 39.Bhagavatula PS, Claudianos C, Ibbotson MR, Srinivasan MV. 2011. Optic flow cues guide flight in birds. Curr. Biol. 21, 1794–1799. ( 10.1016/j.cub.2011.09.009) [DOI] [PubMed] [Google Scholar]
- 40.Esch HE, Zhang SW, Srinivasan MV, Tautz J. 2001. Honeybee dances communicate distances measured by optic flow. Nature 411, 581–583. ( 10.1038/35079072) [DOI] [PubMed] [Google Scholar]
- 41.Ros IG, Biewener AA. 2016. Optic flow stabilizes flight in ruby-throated hummingbirds. J. Exp. Biol. 219, 2443–2448. ( 10.1242/jeb.128488) [DOI] [PubMed] [Google Scholar]
- 42.Wynn J, Collet J, Prudor A, Corbeau A, Padget O, Guilford T, Weimerskirch H. 2020. Data from: Young frigatebirds learn how to compensate for wind-drift Dryad Digital Repository. ( 10.5061/dryad.4tmpg4f7s) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Wynn J, Collet J, Prudor A, Corbeau A, Padget O, Guilford T, Weimerskirch H. 2020. Data from: Young frigatebirds learn how to compensate for wind-drift Dryad Digital Repository. ( 10.5061/dryad.4tmpg4f7s) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data used in our analyses are stored and freely available on the Dryad Digital Repository: https://doi.org/10.5061/dryad.4tmpg4f7s [42].