Abstract
Background
Toothbrushing is a health-related lifestyle habit and has been reported to contribute not only to oral health but also to some parameters of general health; however, little research has been conducted to understand the association of the frequency and timing of toothbrushing with the development of comprehensive metabolic abnormalities, with consideration of oral health condition. In this study, using longitudinal data, we examined this association in Japanese adults, adjusting for periodontal condition.
Methods
A 5-year longitudinal study was performed with 4,537 participants between 35 and 64 years old who underwent an annual dental examination in both 2003 and 2008. Data about toothbrushing habits and metabolic abnormalities, such as obesity, hyperglycemia, diabetes, hypertension, hypertriglyceridemia, and low levels of high-density lipoprotein-cholesterol, were analyzed using Poisson regression analysis.
Results
The percentage of participants with a toothbrushing frequency ≤1 time/day was 29.4%, and that for those not brushing their teeth at night was 21.4%. The incidences of obesity and hyperglycemia after 5 years were 5.5% and 28.4%, respectively. A toothbrushing frequency ≤1 time/day was associated with development of obesity (prevalence rate ratio [PRR] 1.77; 95% confidence interval [CI], 1.12–2.80), after adjusting for periodontal condition and potential risk factors. A significant association between not brushing teeth at night and hyperglycemia (PRR 1.30; 95% CI, 1.02–1.66) was observed in participants with toothbrushing frequency of 1 time/day. No association was found between toothbrushing habits and other metabolic abnormalities.
Conclusions
This study suggests that toothbrushing habits are associated with the development of obesity and hyperglycemia.
Key words: toothbrushing, obesity, diabetes
INTRODUCTION
In modern affluent societies, unhealthy lifestyle factors are responsible for a large proportion of morbidities and mortalities. For example, smoking, unhealthy dietary habits, lack of regular physical activity, high alcohol consumption, and not maintaining an appropriate body weight have been shown to have an unfavorable influence on cardiovascular disease,1,2 as well as diabetes mellitus3 and hypertension.4 Additionally, metabolic syndrome, a precursor of cardiovascular disease and diabetes mellitus,5 is affected by lifestyle factors, including smoking, diet, physical activity, and alcohol consumption habits.6
These lifestyle factors have been shown to be connected to oral health behaviors, such as toothbrushing, as it has been reported that lower toothbrushing frequency correlates with smoking, unhealthier dietary habits, lower physical activity, and higher consumption of alcohol.7,8 Recent studies have shown that low toothbrushing frequency is associated with obesity,9 metabolic syndrome,10 diabetes mellitus,11,12 and cardiovascular disease.13 Furthermore, it has been found that not brushing teeth at night is related to mortality.14 These previous studies suggest both the clinical and public health importance of toothbrushing, as toothbrushing leads not only to the prevention of oral disease, but also to a reduced risk of health deterioration. Toothbrushing may be an easily applicable preventive method for overall health deterioration. Additionally, toothbrushing may have the hidden potential to become another factor of improving health deterioration when general lifestyle modification has not been effective.
However, these studies10–14 did not simultaneously address oral health condition. Metabolic abnormalities, including obesity, hyperglycemia, dyslipidemia, and high blood pressure, are associated with poor oral health condition, especially periodontal disease.15 The association between toothbrushing and metabolic abnormalities may be influenced not only by lifestyle but also by periodontal condition.16 We previously reported that low toothbrushing frequency was associated with the onset of metabolic syndrome, defined by ≥3 components of metabolic syndrome, after adjusting for lifestyle factors and periodontal condition.17 The previous study suggests the possibility that toothbrushing is an independent related factor for the development of metabolic syndrome. However, it remained unclear as to which specific metabolic abnormalities were associated with toothbrushing habits and whether this association differed based on the timing of toothbrushing.
In the present study, we examined whether toothbrushing habits (toothbrushing frequency and toothbrushing at night) was longitudinally associated with the development of metabolic abnormalities, including diabetes and hypertension, with consideration of oral health condition in Japanese adults. We hypothesized that toothbrushing was an independent risk factor for developing metabolic abnormalities.
METHODS
Study population
This longitudinal study used the medical and dental records of Japanese adults who had undergone workplace health check-ups in 2003 and 2008 at the health examination center of a manufacturing company in Yokohama, Japan. Details of the study design have been described previously.18
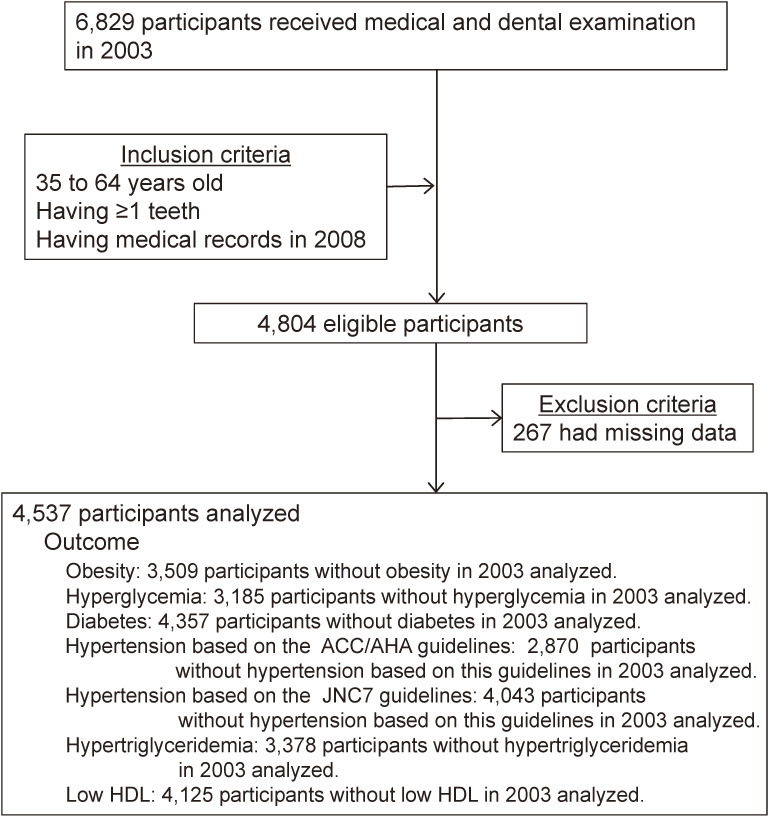
At baseline, 6,829 individuals received both medical and dental examinations. Participants were included if they fulfilled the following criteria at baseline: (1) were 35 to 64 years old, (2) had 1 or more teeth, and (3) had medical records from 2008. Of the 4,804 participants fulfilling the inclusion criteria, 267 participants with missing data were excluded. Finally, 4,537 participants were analyzed in this study (3,643 men and 894 women) (Figure 1).
Figure 1. Flow diagram of the study participants. Of the 4,537 overall participants, we selected 3,509 participants when examining the incidence of obesity during a 5-year period, 3,185 participants for hyperglycemia, 4,357 participants for diabetes, 2,870 participants for hypertension based on ACC/AHA guidelines, 4,043 participants for hypertension based on the JNC7 guidelines, 3,378 participants for hypertriglyceridemia, and 4,125 participants for low HDL. ACC/AHA, American College of Cardiology/American Heart Association; HDL, high-density lipoprotein; JNC7, Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.
All participants provided written informed consent for the use of their anonymous data for research. The study design was approved by the Ethics Committee of the Kyushu University Faculty of Dental Sciences, Fukuoka, Japan.
Assessment of toothbrushing and oral health condition
Toothbrushing habits were recorded based on self-reported questionnaires. The frequency of toothbrushing and the time of day when toothbrushing was performed were derived from the following question: ‘How often do you brush your teeth?’. The response options were: ‘once in the morning’; ‘once in the afternoon’; ‘once at night’; ‘two times, in the morning and at night’; ‘two times, in the morning and the afternoon’; ‘two times, in the afternoon and at night’; ‘three times’; and ‘four and more times’. Then, the ‘once in the morning’, ‘once in the afternoon’, and ‘two times in the morning and afternoon’ were combined to create a dichotomous variable to identify the no toothbrushing at night group. Toothbrushing time was also recorded.
Number of teeth, periodontal condition, and oral hygiene status were recorded as indicators of oral health condition at the baseline examination. A periodontal examination was performed on all teeth except the third molars at two sites (mesiobuccal and mid-buccal), following the National Health and Nutrition Examination Survey III method; probing pocket depth (PPD) and clinical attachment level (CAL) were assessed. The percentage of teeth that bled upon probing (%BOP) was also examined. The periodontal examination was described previously in more detail.18 Oral hygiene status was recorded using the Oral Hygiene Index-Simplified.19
Assessment of metabolic abnormalities
The assessment of metabolic abnormalities included the following measurements: fasting glucose, triglyceride, and high-density lipoprotein (HDL) cholesterol concentrations; systolic and diastolic blood pressure; and body mass index (BMI), which was calculated after body weight and height were measured. Metabolic abnormalities were assessed according to the Joint Interim Statement using the criteria for Asians20 as follows: obesity was defined as BMI ≥25.0 kg/m2 (appropriate cut-off for obesity in Asian individuals21); hyperglycemia was defined as fasting glucose ≥100 mg/dL or current antidiabetic treatment; hypertriglyceridemia was defined as triglycerides ≥150 mg/dL or the use of any lipid-lowering medications; and low HDL cholesterol was defined as <40 mg/dL in males and <50 mg/dL in females. Diabetes was determined by fasting glucose ≥126 mg/dL or current antidiabetic treatment.22 The oral glucose tolerance test was not conducted, so the diagnostic criteria of diabetes used in this study did not correspond with the current diagnostic criteria proposed by the American Diabetes Association. Hypertension was defined using criteria from the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines and the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7). In the 2017 ACC/AHA guidelines, hypertension was defined as blood pressure ≥130/80 mm Hg or current antihypertensive treatment,23 and hypertension in the JNC7 was defined as blood pressure ≥140/90 mm Hg or current antihypertensive treatment.24
Assessment of other variables
Information about eating habits, smoking, alcohol consumption, physical activity, sleeping hours, and profession was collected using a self-reported questionnaire. Eating habits were assessed using six questions, and three answer categories were collapsed into dichotomous variables as follows: (1) eating a daily snack between meals: yes versus no; (2) preferred taste of food: salty versus normal or lightly salted; (3) skipping breakfast: yes versus no; (4) eating meat and oily food: more versus normal or lower; (5) eating sweet food: more versus normal or lower; and (6) eating dinner: eating home-cooked meals versus eating outside the home or eating take-out food (ie, seldom eat home-cooked meals). Smoking status was categorized as current smoking or nonsmoker. Alcohol consumption was measured via frequency of alcohol consumption and volume of alcoholic beverages consumed; alcohol consumption was categorized into high consumption (≥30 g ethanol per day for men and ≥20 g ethanol per day for women) or no consumption. Physical activity was assessed via frequency of exercise and was grouped as regular (more than 1 day per week) or none. Sleeping hours were divided into ≤5 hours, 6 hours, and ≥7 hours. Occupational status was classified into office workers, skilled workers, and other workers.
Statistical analysis
Bivariate tests, including the Mann-Whitney U test, the Kruskal-Wallis test, and the chi-squared test, were performed to describe oral condition and lifestyle factors according to toothbrushing habits. As logistic regression might have overestimated the risk of association in a high incidence of outcomes,25 a Poisson regression model with robust standard errors was used.26 The association between toothbrushing habits, including toothbrushing frequency and brushing teeth at night, at baseline (independent variable) and developing each metabolic abnormality (dependent variable) was assessed using Poisson regression analysis. The prevalence rate ratio (PRR) with 95% confidence intervals (CIs) based on robust standard errors was calculated. The Poisson regression models included age, sex, mean CAL, number of teeth, BMI, eating habits (eating snacks between meals, preferring salty dishes, skipping breakfast, eating meat and oily food, eating sweet foods, and seldom eating home-cooked meals), smoking, alcohol consumption, physical activity, sleeping hours, and job as covariates. The model with each metabolic abnormality, except obesity, as the dependent variable additionally included the baseline value of each metabolic abnormality as a covariate (eg, the model for the development of hypertension additionally included systolic blood pressure at baseline). To determine whether brushing teeth at night was associated with metabolic abnormalities regardless of toothbrushing frequency, data stratified by toothbrushing frequency were analyzed. SPSS software (version 25.0 for Windows; IBM SPSS Japan, Tokyo, Japan) was used for data analysis. A two-sided value of P < 0.05 was considered statistically significant in all analyses.
RESULTS
Characteristics of the participants
Age, obesity, and mean values of CAL were different between the analyzed participants and the participants who did not receive a medical examination after the 5-year follow-up period (Table 1). The toothbrushing habits and metabolic conditions were similar between the participants. The prevalence of each metabolic abnormality at baseline was 22.7% for obesity, 29.7% for hyperglycemia, 3.8% for diabetes, 36.7% for hypertension based on the 2017 ACC/AHA guidelines, 10.9% for hypertension based on the JNC7, 25.5% for hypertriglyceridemia, and 9.1% for low HDL cholesterol (Table 1).
Table 1. Descriptive statistics comparing analyzed participants with participants who dropped out of the health examination in 2008.
| Analyzed | Dropped out | P-value | |
| (n = 4,537) | (n = 1,659) | ||
| Age, years, mean (SD) | 45.0 (8.4) | 46.9 (9.2) | <0.001 |
| Sex | <0.001 | ||
| Men | 3,643 (80.3) | 1,203 (72.5) | |
| Women | 894 (19.7) | 456 (27.5) | |
| Obesity | 0.015 | ||
| BMI <25.0 kg/m2 | 3,509 (77.3) | 1,234 (74.4) | |
| BMI ≥25.0 kg/m2 | 1,028 (22.7) | 425 (25.6) | |
| Fasting glucosea | 0.518 | ||
| Normal | 3,185 (70.2) | 1,150 (69.3) | |
| Elevated | 1,346 (29.7) | 506 (30.5) | |
| Diabetesa | 0.102 | ||
| No | 4,357 (96.2) | 1,577 (95.1) | |
| Yes | 174 (3.8) | 79 (4.8) | |
| Hypertension based on the 2017 ACC/AHA guidelines |
0.911 | ||
| No | 2,870 (63.3) | 1,052 (63.4) | |
| Yes | 1,667 (36.7) | 607 (36.6) | |
| Hypertension based on the JNC7 guidelines |
0.070 | ||
| No | 4,043 (89.1) | 1,451 (87.5) | |
| Yes | 494 (10.9) | 208 (12.5) | |
| Triglycerides | 0.743 | ||
| Normal | 3,378 (74.5) | 1,242 (74.9) | |
| Elevated | 1,159 (25.5) | 417 (25.1) | |
| HDL cholesterol | 0.593 | ||
| Normal | 4,125 (90.9) | 1,501 (90.5) | |
| Reduced | 412 (9.1) | 158 (9.5) | |
| Toothbrushing frequency | 0.600 | ||
| ≥3 times | 844 (18.6) | 304 (18.3) | |
| 2 times | 2,361 (52.0) | 846 (51.0) | |
| ≤1 time | 1,332 (29.4) | 509 (30.7) | |
| Toothbrushing at night | 0.983 | ||
| Yes | 3,565 (78.6) | 1,304 (78.6) | |
| No | 972 (21.4) | 355 (21.4) | |
| CAL, mean (SD) | 2.52 (0.72) | 2.57 (0.77) | 0.018 |
ACC/AHA, American College of Cardiology/American Heart Association; BMI, body mass index; CAL, clinical attachment level; HDL, high-density lipoprotein; JNC7, Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; SD, standard deviation.
Values reported as n (%), unless otherwise noted.
Chi-square test was performed for categorical variables, and Mann-Whitney U test was performed for continuous variables.
aExcluding individuals with a missing value (n = 6 in analyzed and n = 3 in dropped out).
Toothbrushing habits, oral condition, and lifestyle factors
The percentage of participants with a toothbrushing frequency of ≤1 time/day and who did not brushing their teeth at night was 29.4% and 21.4%, respectively. The association between toothbrushing frequency and brushing teeth at night is shown in Table 2. Of the 1,332 participants with a toothbrushing frequency of 1 time/day, 885 brushed their teeth in the morning, 10 in the afternoon, and 437 at night. Participants with a toothbrushing frequency of ≤1 time/day had higher mean values of CAL, more plaque and calculus, higher %BOP, a greater prevalence of current smoking, higher alcohol consumption, lower physical activity, greater preference for salty foods, greater prevalence of skipping breakfast, higher consumption of meat and oily food, lower consumption of home-cooked meals and shorter sleeping hours than those with a toothbrushing frequency of ≥3 times/day (Table 3). The association of not brushing teeth at night with oral condition and lifestyle factors was similar to that of toothbrushing frequency.
Table 2. Association between toothbrushing frequency and toothbrushing at night.
| Toothbrushing frequency | ||||
| ≤1 time | 2 times | ≥3 times | P-value | |
| (n = 1,332) | (n = 2,361) | (n = 844) | ||
| Toothbrush at night | <0.001 | |||
| Yes | 437 (32.8) | 2,284 (96.7) | 844 (100) | |
| No | 895 (67.2) | 77 (3.3) | 0 (0) | |
n (%), Chi-square test.
Table 3. Oral condition and lifestyle factors according to toothbrushing habits at baseline.
| Toothbrushing frequency | Brushing teeth at night | ||||||
| ≤1 time | 2 times | ≥3 times | P-value | No | Yes | P-value | |
| (n = 1,332) | (n = 2,361) | (n = 844) | (n = 972) | (n = 3,565) | |||
| Age, years, mean (SD) | 44.9 (8.3) | 44.9 (8.3) | 45.8 (8.5) | 0.020 | 44.5 (8.2) | 45.2 (8.4) | 0.022 |
| Men, % | 94.5 | 80.6 | 57.1 | <0.001 | 76.1 | 95.7 | <0.001 |
| CAL, mean (SD) | 2.55 (0.72) | 2.52 (0.73) | 2.46 (0.67) | 0.015 | 2.57 (0.73) | 2.50 (0.71) | 0.005 |
| Dental plaque (debris index simplified), mean (SD) | 0.81 (0.44) | 0.69 (0.39) | 0.58 (0.38) | <0.001 | 0.80 (0.45) | 0.68 (0.40) | <0.001 |
| Calculus (calculus index simplified), mean (SD) | 0.57 (0.51) | 0.45 (0.44) | 0.36 (0.39) | <0.001 | 0.58 (0.52) | 0.44 (0.43) | <0.001 |
| %BOP, mean (SD) | 24.1 (23.0) | 19.4 (21.0) | 17.1 (19.6) | <0.001 | 23.7 (22.2) | 19.5 (21.2) | <0.001 |
| Number of teeth, mean (SD) | 28.0 (2.5) | 27.8 (2.6) | 27.7 (2.4) | 0.049 | 28.0 (2.5) | 27.8 (2.6) | 0.009 |
| Occupational status, % | <0.001 | <0.001 | |||||
| Office workers | 29.8 | 27.6 | 40.5 | 30.1 | 30.8 | ||
| Skilled workers | 40.5 | 35.2 | 21.1 | 41.4 | 32.2 | ||
| Others | 29.7 | 37.1 | 38.4 | 28.5 | 37.0 | ||
| Current smoking, % | 38.4 | 24.9 | 11.8 | <0.001 | 40.2 | 22.7 | <0.001 |
| High alcohol consumption, % | 30.0 | 25.2 | 17.3 | <0.001 | 34.1 | 22.7 | <0.001 |
| Lack of regular physical activity, % | 70.0 | 64.3 | 53.9 | <0.001 | 70.2 | 62.3 | <0.001 |
| Eats snacks between meals, % | 27.1 | 31.5 | 42.1 | <0.001 | 24.7 | 34.2 | <0.001 |
| Prefers salty dishes, % | 17.5 | 13.0 | 9.5 | <0.001 | 20.4 | 11.9 | <0.001 |
| Skips breakfast, % | 26.6 | 12.9 | 7.7 | <0.001 | 28.0 | 12.7 | <0.001 |
| Eats more meat and oily food, % | 21.1 | 15.8 | 10.9 | <0.001 | 23.8 | 14.4 | <0.001 |
| Eats more sweet food, % | 11.8 | 13.0 | 13.5 | <0.001 | 10.7 | 13.3 | 0.033 |
| Seldom eats home-cooked meals for dinner, % | 27.0 | 19.6 | 13.4 | <0.001 | 28.0 | 18.6 | <0.001 |
| Sleeping hours, % | 0.028 | 0.004 | |||||
| ≥7 hours | 32.7 | 33.9 | 36.7 | 32.0 | 34.6 | ||
| 6 hours | 44.7 | 46.3 | 46.1 | 44.1 | 46.3 | ||
| ≤5 hours | 22.6 | 19.8 | 17.2 | 23.9 | 19.1 | ||
CAL, clinical attachment level; %BOP, percentage of sites that bled upon probing; SD, standard deviation.
Chi-square test was used for categorical variables, and Mann-Whitney U test or Kruskal-Wallis test was used for continuous variables.
Toothbrushing habits and development of metabolic abnormalities
After excluding participants with each metabolic abnormality at baseline, the incidence of the development of each metabolic abnormality was calculated. We analyzed 3,509 participants when examining incidences of obesity, 3,185 for hyperglycemia, 3,378 for hypertriglyceridemia, 4,357 for diabetes, 2,870 for hypertension based on the 2017 ACC/AHA guidelines, 4,043 for hypertension based on the JNC7 guidelines, 3,378 for hypertriglyceridemia, and 4,125 for low HDL cholesterol (Table 4). The incidence of the development of each metabolic abnormality was 5.5% for obesity, 28.4% for hyperglycemia, 2.3% for diabetes, 16.8% for hypertension based on the 2017 ACC/AHA guidelines, 7.9% for hypertension based on the JNC7, 12.8% for hypertriglyceridemia, and 2.7% for low HDL cholesterol (Table 4).
Table 4. Incidence of each metabolic abnormality.
| Without each metabolic abnormality at baseline |
Incidence of each metabolic abnormality |
|
| Obesity | 3,509 | 5.5% |
| Hyperglycemia | 3,185 | 28.4% |
| Diabetes | 4,357 | 2.3% |
| Hypertension based on the 2017 ACC/AHA guidelines |
2,870 | 16.8% |
| Hypertension based on the JNC7 guidelines |
4,043 | 7.9% |
| Hypertriglyceridemia | 3,378 | 12.8% |
| Low HDL | 4,125 | 2.7% |
ACC/AHA, American College of Cardiology/American Heart Association; JNC7, Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.
Poisson regression models showed that a toothbrushing frequency of ≤1 time/day was positively associated with the development of obesity (PRR 1.77; 95% CI, 1.12–2.80) after adjusting for age, sex, number of teeth, mean CAL, BMI, eating snacks between meals, preferring salty dishes, skipping breakfast, eating meat and oily food, eating sweet food, seldom eating home-cooked meals, smoking, consuming alcohol, physical activity, sleeping hours, job, and the baseline value of each metabolic abnormality (Table 5). Not brushing teeth at night was significantly associated with the development of obesity (PRR 1.49; 95% CI, 1.13–1.96) and hyperglycemia (PRR 1.14; 95% CI, 1.00–1.29) (Table 6). When we examined the association between brushing teeth at night and each metabolic abnormality stratified by toothbrushing frequency, participants who had a toothbrushing frequency of 1 time/day and were not brushing at night were more likely to develop hyperglycemia (PRR 1.30; 95% CI, 1.02–1.66) than those brushing at night (Table 7). A significant association between brushing at night and obesity was not found (Table 7). Regarding periodontal condition, mean CAL, PPD, calculus, and %BOP were higher in participants who had a toothbrushing frequency of 1 time/day and were not brushing at night than in those who brushed at night (eTable 1). In participants with toothbrushing frequency of 1 time/day, not brushing at night was not significantly associated with hyperglycemia in the model with %BOP (eTable 2).
Table 5. Association between toothbrushing frequency and the development of each metabolic abnormality.
| Development of each metabolic abnormality | Crude PRR (95% CI) | Adjusted PRRa (95% CI) | |||
| No | Yes | Model 1 | Model 2 | ||
| Obesity | |||||
| Toothbrushing frequency, n (%) | |||||
| ≥3 times | 692 (97.2) | 20 (2.8) | 1 | 1 | 1 |
| 2 times | 1,757 (94.8) | 96 (5.2) | 1.84 (1.15–2.96) | 1.22 (0.78–1.89) | 1.20 (0.77–1.88) |
| ≤1 time | 866 (91.7) | 78 (8.3) | 2.94 (1.82–4.76) | 1.80 (1.14–2.84) | 1.77 (1.12–2.80) |
| CAL, mean (SD) | 2.49 (0.72) | 2.58 (0.74) | 1.16 (0.99–1.35) | 1.13 (0.97–1.31) | |
| Hyperglycemia | |||||
| Toothbrushing frequency, n (%) | |||||
| ≥3 times | 495 (77.8) | 141 (22.2) | 1 | 1 | 1 |
| 2 times | 1,224 (72.0) | 475 (28.0) | 1.26 (1.07–1.49) | 1.03 (0.88–1.21) | 1.04 (0.89–1.21) |
| ≤1 time | 561 (66.0) | 289 (34.0) | 1.53 (1.29–1.82) | 1.14 (0.96–1.38) | 1.14 (0.96–1.36) |
| CAL, mean (SD) | 2.47 (0.67) | 2.51 (0.72) | 1.05 (0.97–1.14) | 0.98 (0.91–1.06) | |
| Diabetes | |||||
| Toothbrushing frequency, n (%) | |||||
| ≥3 times | 808 (98.7) | 11 (1.3) | 1 | 1 | 1 |
| 2 times | 2,234 (98.2) | 42 (1.8) | 1.37 (0.71–2.66) | 1.02 (0.56–1.85) | 1.02 (0.56–1.87) |
| ≤1 time | 1,215 (96.3) | 47 (3.7) | 2.77 (1.45–5.31) | 1.62 (0.91–2.88) | 1.62 (0.91–2.88) |
| CAL, mean (SD) | 2.51 (0.70) | 2.71 (1.12) | 1.35 (1.11–1.65) | 1.07 (0.78–1.47) | |
| Hypertension based on the 2017 ACC/AHA guidelines | |||||
| Toothbrushing frequency, n (%) | |||||
| ≥3 times | 502 (87.8) | 70 (12.2) | 1 | 1 | 1 |
| 2 times | 1,271 (83.5) | 251 (16.5) | 1.35 (1.05–1.72) | 1.14 (0.90–1.45) | 1.14 (0.90–1.45) |
| ≤1 time | 616 (79.4) | 160 (20.6) | 1.69 (1.30–2.18) | 1.27 (0.97–1.66) | 1.27 (0.97–1.66) |
| CAL, mean (SD) | 2.47 (0.70) | 2.55 (0.75) | 1.13 (1.02–1.25) | 1.03 (0.93–1.15) | |
| Hypertension based on the JNC7 guidelines | |||||
| Toothbrushing frequency, n (%) | |||||
| ≥3 times | 710 (92.6) | 57 (7.4) | 1 | 1 | 1 |
| 2 times | 1,955 (92.3) | 163 (7.7) | 1.04 (0.78–1.38) | 0.92 (0.70–1.23) | 0.93 (0.70–1.23) |
| ≤1 time | 1,057 (91.3) | 101 (8.7) | 1.17 (0.86–1.60) | 0.88 (0.64–1.22) | 0.89 (0.64–1.22) |
| CAL, mean (SD) | 2.50 (0.71) | 2.62 (0.80) | 1.21 (1.07–1.36) | 1.09 (0.96–1.25) | |
| Hypertriglyceridemia | |||||
| Toothbrushing frequency, n (%) | |||||
| ≥3 times | 644 (91.5) | 60 (8.5) | 1 | 1 | 1 |
| 2 times | 1,563 (87.2) | 230 (12.8) | 1.51 (1.15–1.97) | 1.12 (0.87–1.45) | 1.12 (0.87–1.45) |
| ≤1 time | 738 (83.8) | 143 (16.2) | 1.90 (1.43–2.53) | 1.24 (0.94–1.63) | 1.23 (0.94–1.63) |
| CAL, mean (SD) | 2.50 (0.73) | 2.51 (0.68) | 1.01 (0.90–1.14) | 1.04 (0.93–1.17) | |
| Low HDL | |||||
| Toothbrushing frequency, n (%) | |||||
| ≥3 times | 786 (97.6) | 19 (2.4) | 1 | 1 | 1 |
| 2 times | 2,102 (97.4) | 56 (2.6) | 1.07 (0.64–1.80) | 1.06 (0.65–1.73) | 1.08 (0.66–1.76) |
| ≤1 time | 1,143 (96.9) | 37 (3.1) | 1.30 (0.75–2.24) | 1.21 (0.67–2.20) | 1.23 (0.68–2.23) |
| CAL, mean (SD) | 2.51 (0.72) | 2.44 (0.62) | 0.88 (0.68–1.13) | 0.86 (0.67–1.11) | |
ACC/AHA, American College of Cardiology/American Heart Association; CI, confidence interval; CAL, clinical attachment level; HDL, high-density lipoprotein; JNC7, Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; PRR, prevalence rate ratio.
Poisson regression models with robust standard error; each metabolic abnormality was the dependent variable and toothbrushing frequency was the independent variable.
The crude model included one independent variable and one dependent variable.
aModel 1 adjusted for age, sex, number of teeth, BMI, eating snacks between meals, preferring salty dishes, skipping breakfast, eating meat and oily food, eating sweet food, seldom eating home-cooked meals, smoking, alcohol consumption, physical activity, sleeping hours, job, and baseline value of each metabolic abnormality, and model 2 additionally included mean CAL.
Table 6. Association between brushing teeth at night and the development of each metabolic abnormality.
| Development of each metabolic abnormality |
Crude PRR (95% CI) | Adjusted PRRa (95% CI) | |||
| No | Yes | Model 1 | Model 2 | ||
| Obesity | |||||
| Brushing teeth at night, n (%) | |||||
| Yes | 2,702 (95.2) | 135 (4.8) | 1 | 1 | 1 |
| No | 613 (91.2) | 59 (8.8) | 1.85 (1.37–2.48) | 1.51 (1.14–1.99) | 1.49 (1.13–1.96) |
| CAL, mean (SD) | 2.49 (0.72) | 2.58 (0.74) | 1.16 (0.99–1.35) | 1.13 (0.97–1.32) | |
| Hyperglycemia | |||||
| Brushing teeth at night, n (%) | |||||
| Yes | 1,898 (73.5) | 685 (26.5) | 1 | 1 | 1 |
| No | 382 (63.5) | 220 (36.5) | 1.38 (1.22–1.56) | 1.14 (1.00–1.29) | 1.14 (1.00–1.29) |
| CAL, mean (SD) | 2.47 (0.67) | 2.51 (0.72) | 1.05 (0.97–1.14) | 0.98 (0.91–1.06) | |
| Diabetes | |||||
| Brushing teeth at night, n (%) | |||||
| Yes | 3,369 (98.0) | 68 (2.0) | 1 | 1 | 1 |
| No | 888 (96.5) | 32 (3.5) | 1.76 (1.16–2.66) | 1.08 (0.73–1.60) | 1.07 (0.72–1.60) |
| CAL, mean (SD) | 2.51 (0.70) | 2.71 (1.12) | 1.35 (1.11–1.65) | 1.09 (0.80–1.48) | |
| Hypertension based on the 2017 ACC/AHA guidelines | |||||
| Brushing teeth at night, n (%) | |||||
| Yes | 1,960 (84.4) | 361 (15.6) | 1 | 1 | 1 |
| No | 429 (78.1) | 120 (21.9) | 1.41 (1.17–1.69) | 1.16 (0.97–1.40) | 1.16 (0.96–1.39) |
| CAL, mean (SD) | 2.47 (0.70) | 2.55 (0.75) | 1.13 (1.02–1.25) | 1.03 (0.92–1.14) | |
| Hypertension based on the JNC7 guidelines | |||||
| Brushing teeth at night, n (%) | |||||
| Yes | 2,955 (92.4) | 243 (7.6) | 1 | 1 | 1 |
| No | 767 (90.8) | 78 (9.2) | 1.21 (0.95–1.55) | 1.02 (0.80–1.31) | 1.02 (0.80–1.31) |
| CAL, mean (SD) | 2.50 (0.71) | 2.62 (0.80) | 1.21 (1.07–1.36) | 1.09 (0.96–1.25) | |
| Hypertriglyceridemia | |||||
| Brushing teeth at night, n (%) | |||||
| Yes | 2,424 (88.2) | 324 (11.8) | 1 | 1 | 1 |
| No | 521 (82.7) | 109 (17.3) | 1.47 (1.20–1.79) | 1.08 (0.89–1.31) | 1.08 (0.89–1.31) |
| CAL, mean (SD) | 2.50 (0.73) | 2.51 (0.68) | 1.01 (0.90–1.14) | 1.04 (0.93–1.17) | |
| Low HDL | |||||
| Brushing teeth at night, n (%) | |||||
| Yes | 3,174 (97.3) | 89 (2.7) | 1 | 1 | 1 |
| No | 839 (97.3) | 23 (2.7) | 0.98 (0.62–1.54) | 0.93 (0.58–1.49) | 0.94 (0.59–1.50) |
| CAL, mean (SD) | 2.51 (0.72) | 2.44 (0.62) | 0.88 (0.68–1.13) | 0.87 (0.67–1.12) | |
ACC/AHA, American College of Cardiology/American Heart Association; CI, confidence interval; CAL, clinical attachment level; HDL, high-density lipoprotein; JNC7, Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; PRR, prevalence rate ratio.
Poisson regression models with robust standard error; each metabolic abnormality was the dependent variable and not brushing teeth at night was the independent variable.
The crude model included one independent variable and one dependent variable.
aModel 1 adjusted for age, sex, number of teeth, BMI, eating snacks between meals, preferring salty dishes, skipping breakfast, eating meat and oily food, eating sweet food, seldom eating home-cooked meals, smoking, alcohol consumption, physical activity, sleeping hours, job, and baseline value of each metabolic abnormality, and model 2 additionally included mean CAL.
Table 7. Association between toothbrushing at night and the development of each metabolic abnormality based on toothbrushing frequency.
| Development of each metabolic abnormality |
Crude PRR (95% CI) | Adjusted PRR (95% CI)a | |||
| No | Yes | Model 1 | Model 2 | ||
| Twice a day toothbrushing | |||||
| Obesity | |||||
| Toothbrushing at night | |||||
| Yes | 1,699 (94.9) | 91 (5.1) | 1 | 1 | 1 |
| No | 58 (92.1) | 5 (7.9) | 1.56 (0.66–3.71) | 1.48 (0.66–3.32) | 1.49 (0.66–3.33) |
| CAL, mean (SD) | 2.50 (0.74) | 2.52 (0.71) | 1.05 (0.83–1.32) | 1.03 (0.80–1.32) | |
| Hyperglycemia | |||||
| Toothbrushing at night | |||||
| Yes | 1,190 (71.9) | 465 (28.1) | 1 | 1 | 1 |
| No | 34 (77.3) | 10 (22.7) | 0.81 (0.47–1.40) | 0.68 (0.39–1.19) | 0.68 (0.39–1.19) |
| CAL, mean (SD) | 2.47 (0.68) | 2.53 (0.78) | 0.99 (0.87–1.15) | 1.00 (0.90–1.11) | |
| Diabetes | |||||
| Toothbrushing at night | |||||
| Yes | 2,163 (98.2) | 40 (1.8) | 1 | 1 | 1 |
| No | 71 (97.3) | 2 (2.7) | 1.51 (0.37–6.12) | 0.77 (0.19–3.09) | 0.79 (0.19–3.28) |
| CAL, mean (SD) | 2.51 (0.71) | 2.79 (1.47) | 1.39 (1.08–1.79) | 1.38 (1.04–1.83) | |
| Hypertension in 2017 ACC/AHA guideline | |||||
| Toothbrushing at night | |||||
| Yes | 1,228 (83.6) | 241 (16.4) | 1 | 1 | 1 |
| No | 43 (81.1) | 10 (18.9) | 1.15 (0.65–2.03) | 1.11 (0.66–1.90) | 1.11 (0.65–1.89) |
| CAL, mean (SD) | 2.48 (0.73) | 2.57 (0.74) | 1.14 (1.00–1.29) | 1.07 (0.93–1.24) | |
| Hypertension in JNC7 | |||||
| Toothbrushing at night | |||||
| Yes | 1,890 (92.3) | 158 (7.7) | 1 | 1 | 1 |
| No | 65 (92.9) | 5 (7.1) | 0.93 (0.39–2.18) | 1.09 (0.50–2.38) | 1.10 (0.50–2.41) |
| CAL, mean (SD) | 2.50 (0.74) | 2.58 (0.73) | 1.13 (0.97–1.33) | 1.03 (0.86–1.24) | |
| Hypertriglyceridemia | |||||
| Toothbrushing at night | |||||
| Yes | 1,516 (87.2) | 222 (12.8) | 1 | 1 | 1 |
| No | 47 (85.5) | 8 (14.5) | 1.14 (0.59–2.19) | 0.85 (0.44–1.61) | 0.84 (0.44–1.60) |
| CAL, mean (SD) | 2.51 (0.75) | 2.49 (0.68) | 0.98 (0.84–1.14) | 1.04 (0.89–1.21) | |
| Low HDL | |||||
| Toothbrushing at night | |||||
| Yes | 2,031 (97.4) | 55 (2.6) | 1 | ||
| No | 71 (98.6) | 1 (1.4) | 0.53 (0.07–3.75) | ||
| CAL, mean (SD) | 2.51 (0.73) | 2.57 (0.71) | 0.70 (0.45–1.09) | ||
| Once a day toothbrushing | |||||
| Obesity | |||||
| Toothbrushing at night | |||||
| Yes | 238 (94.4) | 14 (5.6) | 1 | 1 | 1 |
| No | 402 (91.4) | 38 (8.6) | 1.56 (0.86–2.81) | 1.20 (0.66–2.17) | 1.15 (0.63–2.12) |
| CAL, mean (SD) | 2.52 (0.71) | 2.73 (0.74) | 1.39 (1.05–1.84) | 1.12 (0.85–2.12) | |
| Hyperglycemia | |||||
| Toothbrushing at night | |||||
| Yes | 156 (73.9) | 55 (26.1) | 1 | 1 | 1 |
| No | 245 (61.1) | 156 (38.9) | 1.49 (1.15–1.93) | 1.30 (1.02–1.66) | 1.30 (1.02–1.66) |
| CAL, mean (SD) | 2.51 (0.67) | 2.55 (0.66) | 1.05 (0.90–1.23) | 0.99 (0.84–1.16) | |
| Diabetes | |||||
| Toothbrushing at night | |||||
| Yes | 280 (96.6) | 10 (3.4) | 1 | 1 | 1 |
| No | 587 (96.2) | 23 (3.8) | 1.09 (0.53–2.27) | 0.56 (0.24–1.31) | 0.57 (0.24–1.36) |
| CAL, mean (SD) | 2.55 (0.70) | 2.76 (0.85) | 1.42 (0.95–2.12) | 0.94 (0.63–1.39) | |
| Hypertension in 2017 ACC/AHA guideline | |||||
| Toothbrushing at night | |||||
| Yes | 158 (82.3) | 34 (17.7) | 1 | 1 | 1 |
| No | 268 (77.0) | 80 (23.0) | 1.30 (0.91–1.86) | 1.17 (0.81–1.67) | 1.17 (0.81–1.69) |
| CAL, mean (SD) | 2.51 (0.67) | 2.56 (0.79) | 1.09 (0.86–1.39) | 0.98 (0.77–1.24) | |
| Hypertension in JNC7 | |||||
| Toothbrushing at night | |||||
| Yes | 245 (92.5) | 20 (7.5) | 1 | 1 | 1 |
| No | 504 (90.5) | 53 (9.5) | 1.26 (0.77–2.06) | 1.10 (0.67–1.79) | 1.09 (0.66–1.77) |
| CAL, mean (SD) | 2.54 (0.72) | 2.67 (0.79) | 1.24 (0.95–1.60) | 1.11 (0.86–1.44) | |
| Hypertriglyceridemia | |||||
| Toothbrushing at night | |||||
| Yes | 193 (86.9) | 29 (13.1) | 1 | 1 | 1 |
| No | 356 (84.0) | 68 (16.0) | 1.23 (0.82–1.84) | 0.87 (0.57–1.33) | 0.87 (0.57–1.32) |
| CAL, mean (SD) | 2.52 (0.70) | 2.60 (0.74) | 1.15 (0.90–1.46) | 0.96 (0.90–1.02) | |
| Low HDL | |||||
| Toothbrushing at night | |||||
| Yes | 266 (96.7) | 9 (3.3) | 1 | 1 | 1 |
| No | 549 (97.2) | 16 (2.8) | 0.87 (0.39–1.93) | 1.14 (0.48–2.73) | 1.23 (0.52–2.89) |
| CAL, mean (SD) | 2.54 (0.70) | 2.40 (0.57) | 0.73 (0.41–1.28) | 0.71 (0.38–1.34) | |
CI, confidence interval; CAL, clinical attachment level; HDL, high-density lipoprotein; PRR, prevalence rate ratio.
Poisson regression models with robust standard error; each metabolic abnormalities was the dependent variable and no toothbrushing at night was the independent variable.
The crude model included one independent variable and dependent variable.
aModel 1 adjusted for age, sex, number of teeth, BMI, eating snacks between meals, preferring salty dishes, skipping breakfast, eating meat and oily food, eating sweet food, seldom eating home-cooked meals, smoking, alcohol consumption, physical activity, sleeping hours, job, and baseline value of each metabolic abnormality, and model 2 additionally included mean CAL.
DISCUSSION
We found that a low frequency of toothbrushing was associated with the development of obesity after adjusting for covariates and, furthermore, that not brushing teeth at night in participants with toothbrushing frequency of 1 time/day was associated with hyperglycemia. This longitudinal study reinforced the results of a previous cross-sectional study,9 which showed the association between a low frequency of toothbrushing and obesity. Moreover, the current study extends the findings of prior longitudinal studies10,12,17 in regard to toothbrushing frequency and health conditions by specifying the link between the time of day of toothbrushing and metabolic abnormalities.
The association between toothbrushing habits and obesity was found, even though periodontal condition and several lifestyle factors were adjusted for in multivariate models, suggesting that this association is explained by other related factors. Our results are similar to those of a previous study demonstrating a cross-sectional association between low toothbrushing frequency and obesity after adjusting for lifestyle factors and periodontal condition (odds ratio [OR] 1.22 of 1 time/day toothbrushing and OR 1.48 of 0 times/day toothbrushing).9 In this study, PRR for obesity with ≤1 time/day toothbrushing was 1.77, indicating a relatively stronger association than that in the Park et al study.9 This might be related to unobserved confounders, such as diet quality, including total energy intake and vegetable and fruit intake.
One possible pathway leading from toothbrushing to obesity may involve endotoxemia. It has been demonstrated that metabolic endotoxemia induced by lipopolysaccharides results in an increase in total body fat mass in mice.27 The microbiome of subgingival plaque mainly consists of Gram-negative bacteria that contain a lipopolysaccharide on the outer membrane28 and subgingival plaque contributes significantly to endotoxemia. From this perspective, it seems likely that the accumulation of subgingival plaque caused by poor toothbrushing habits contributes to endotoxemia and subsequently leads to obesity. Another possible explanation for the association between toothbrushing and obesity may be related to leptin, which regulates appetite and energy balance primarily through hypothalamic neurons in the central nervous system. Oral proprioceptive signals modulate the hypothalamic histamine neurons, which are concordant with the leptin signaling system.29 One study reported that the serum leptin level within 3 hours after a meal was changed after toothbrushing in non-obese young men.30 This suggests that mechanical stimulation of the gingiva by toothbrushing may play a role in appetite regulation by influencing leptin, which can control obesity.
The effect of brushing teeth at night on hyperglycemia was observed in participants with toothbrushing frequency of 1 time/day. Not brushing teeth at night in individuals with low frequency of toothbrushing can easily cause poor oral hygiene status, because saliva flow is low while sleeping, and bacterial clearance is reduced, resulting in greater bacterial colonization of oral tissues.31 In fact, participants who did not brush their teeth at night had more calculus and higher level of BOP than those who brushed their teeth at night among participants with toothbrushing frequency of 1 time/day (eTable 1). When we added oral hygiene status to the model, the association between not brushing teeth at night and hyperglycemia was attenuated (PRR 1.29; 95% CI, 0.99–1.68 in the model including %BOP; eTable 2), suggesting that gingival inflammation may play a role in mediating this association. However, a significant association between toothbrushing and hyperglycemia remained even in the model adjusted for mean CAL (Table 6 and Table 7). CAL reflects a historical accumulation of periodontal destruction, while BOP is a better real-time indicator of inflammation than CAL.32 The present study indicates that the intensity of existing gingival inflammation is a mediator between toothbrushing habits and hyperglycemia. It is considered that gingival inflammation caused by poor toothbrushing is linked with increased systemic inflammation/endothelial dysfunction and low adiponectin levels, which in turn may lead to an increased risk of hyperglycemia.33 For individuals who had toothbrushing frequency of once a day without brushing teeth at night, increased frequency of toothbrushing may have the hidden potential to reduce a risk of hyperglycemia. In this context, this study showed a lack of a significant association between toothbrushing habits and diabetes, which was probably due to the low incidence of diabetes (2.3%). A longer follow-up study is necessary to confirm this association.
Although we were not able to clearly explain the direct link between toothbrushing and metabolic abnormalities, one potential pathway may be that the removal of bacterial plaque by toothbrushing decreases chronic low-grade systemic inflammation that can contribute to metabolic abnormalities, such as hyperglycemia. An animal experiment showed that subgingival bacteria, such as Porphyromonas gingivalis, induced a change in the bacterial composition of gut microbiota accompanied by increased systemic inflammation,34 implying that oral pathogens may lead to metabolic abnormalities.
Our results were different from those of Kuwabara et al,12 who found that individuals who did not brush their teeth after every meal had a higher risk of developing diabetes during a 5-year follow-up period than those who brushed their teeth after every meal. However, the study by Kuwabara et al12 did not consider lifestyle factors, such as eating habits, smoking, alcohol consumption, physical activity, and sleeping hours, which contribute to diabetes development. Therefore, their results did not exclude the possibility of residual confounding due to unmeasured factors. Our results regarding hyperglycemia are similar to those reported by Kobayashi et al.10 Their finding of no association between toothbrushing frequency and hyperglycemia was the same as in this study, although Kobayashi et al did not evaluate the timing of toothbrushing.10 More longitudinal studies are required to confirm whether the timing of toothbrushing affects hyperglycemia.
Low frequency of toothbrushing and not brushing teeth at night was inversely associated with eating habits, such as eating snacks between meals and eating more sweet foods (Table 2). This finding may be partly because participants are prone to brushing their teeth after eating a snack or a sweet food to prevent dental caries and maintain oral hygiene. Regarding metabolic abnormalities, participants who ate snacks between meals were less likely to have hyperglycemia, hypertriglyceridemia, and hypertension based on the cross-sectional data (data not shown). It has been reported that individuals who eat snacks more frequently eat more fruits, vegetables, milk, and dairy products and less fried food than those with a lower eating frequency.35 The inverse association between eating snacks and metabolic abnormalities may be explained via diet quality.
This study had several limitations. First, diet quality, such as total energy intake and vegetable and fruit intake, was not investigated, although eating habits were included in our study. It has been reported that toothbrushing frequency is positively associated with intake of vegetables and fruits,36 which contributes to the prevention of metabolic abnormalities.37 We also did not investigate inflammatory parameters, such as high-sensitivity C-reactive protein, which is considered to be responsible for the link between gingival and systemic inflammation. These unmeasured potential confounders may have biased our estimates of the association between toothbrushing habits and metabolic abnormalities owing to using the data of health check-ups; these variables that might have been important were not recorded and could not be included in the model. Future studies are needed to assess these factors. Second, we did not obtain glucose tolerance test data, which defines diabetes more accurately, since a glucose tolerance test is more time consuming in the health examination setting and a fasting blood examination was more practical. Third, we used a partial mouth assessment for periodontal condition, which did not include an examination of lingual or palatal sites. Our results potentially underestimate periodontal condition. Finally, the sample investigated was not representative of the general population because this study included individuals who received workplace health checkups, and the prevalences of obesity and periodontal conditions were different between the analyzed participants and those who dropped out of the follow-up examination. This limitation should be taken into consideration when applying the findings to other populations.
In conclusion, this longitudinal study confirmed that low frequency of toothbrushing was associated with the development of obesity, even after adjusting for potential confounders. An association between not brushing teeth at night and hyperglycemia was found in individuals with a toothbrushing frequency of 1 time/day.
ACKNOWLEDGEMENTS
Funding sources: This work was supported by JSPS KAKENHI Grant Number 18K09882 from the Ministry of Education, Science, Sports, and Culture of Japan, Tokyo, Japan.
Conflicts of interest: None declared.
APPENDIX A. SUPPLEMENTARY DATA
The following is the supplementary data related to this article:
eTable 1. Oral condition according to toothbrushing frequency and brushing teeth at night
eTable 2. Association between toothbrushing at night and hyperglycemia adjusted for mean PPD, oral hygiene status, or %BOP in participants with toothbrushing frequency of once a day and without hyperglycemia at baseline
REFERENCES
- 1.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. 10.1056/NEJM200007063430103 [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Hawken S, Ounpuu S, et al. ; INTERHEART Study Investigators . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. 10.1056/NEJMoa010492 [DOI] [PubMed] [Google Scholar]
- 4.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302:401–411. 10.1001/jama.2009.1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. 10.1161/CIRCULATIONAHA.105.539528 [DOI] [PubMed] [Google Scholar]
- 6.Zhu S, St-Onge MP, Heshka S, Heymsfield SB. Lifestyle behaviors associated with lower risk of having the metabolic syndrome. Metabolism. 2004;53:1503–1511. 10.1016/j.metabol.2004.04.017 [DOI] [PubMed] [Google Scholar]
- 7.Sakki TK, Knuuttila ML, Vimpari SS, Hartikainen MS. Association of lifestyle with periodontal health. Community Dent Oral Epidemiol. 1995;23:155–158. 10.1111/j.1600-0528.1995.tb00220.x [DOI] [PubMed] [Google Scholar]
- 8.Tada A, Matsukubo T. Relationship between oral health behaviors and general health behaviors in a Japanese adult population. J Public Health Dent. 2003;63:250–254. 10.1111/j.1752-7325.2003.tb03508.x [DOI] [PubMed] [Google Scholar]
- 9.Park JB, Nam GE, Han K, Ko Y, Park YG. Obesity in relation to oral health behaviors: an analysis of the Korea National Health and Nutrition Examination Survey 2008–2010. Exp Ther Med. 2016;12:3093–3100. 10.3892/etm.2016.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi Y, Niu K, Guan L, et al. Oral health behavior and metabolic syndrome and its components in adults. J Dent Res. 2012;91:479–484. 10.1177/0022034512440707 [DOI] [PubMed] [Google Scholar]
- 11.Su L, Liu W, Xie B, et al. Toothbrushing, blood glucose and HbA1c: findings from a random survey in Chinese population. Sci Rep. 2016;6:28824. 10.1038/srep28824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwabara M, Motoki Y, Sato H, et al. Low frequency of toothbrushing practices is an independent risk factor for diabetes mellitus in male and dyslipidemia in female: a large-scale, 5-year cohort study in Japan. J Cardiol. 2017;70:107–112. 10.1016/j.jjcc.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira C, Watt R, Hamer M. Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish Health Survey. BMJ. 2010;340:c2451. 10.1136/bmj.c2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paganini-Hill A, White SC, Atchison KA. Dental health behaviors, dentition, and mortality in the elderly: the leisure world cohort study. J Aging Res. 2011;2011:156061. 10.4061/2011/156061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuta M, Shimazaki Y, Takeshita T, et al. Gender differences in the association between metabolic syndrome and periodontal disease: the Hisayama Study. J Clin Periodontol. 2013;40:743–752. 10.1111/jcpe.12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito T. Toothbrushing may reduce risk of metabolic syndrome. J Evid Based Dent Pract. 2012;12:151–153. 10.1016/j.jebdp.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 17.Tanaka A, Takeuchi K, Furuta M, et al. Relationship of toothbrushing to metabolic syndrome in middle-aged adults. J Clin Periodontol. 2018;45:538–547. 10.1111/jcpe.12876 [DOI] [PubMed] [Google Scholar]
- 18.Furuta M, Liu A, Shinagawa T, et al. Tooth loss and metabolic syndrome in middle-aged Japanese adults. J Clin Periodontol. 2016;43:482–491. 10.1111/jcpe.12523 [DOI] [PubMed] [Google Scholar]
- 19.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. 10.14219/jada.archive.1964.0034 [DOI] [PubMed] [Google Scholar]
- 20.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 21.Ota T, Takamura T, Hirai N, Kobayashi K. Preobesity in World Health Organization classification involves the metabolic syndrome in Japanese. Diabetes Care. 2002;25:1252–1253. 10.2337/diacare.25.7.1252 [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. 10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- 23.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, et al. ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee . The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 26.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 27.Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279–288. 10.4161/gmic.19625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liljestrand JM, Paju S, Buhlin K, et al. Lipopolysaccharide, a possible molecular mediator between periodontitis and coronary artery disease. J Clin Periodontol. 2017;44:784–792. 10.1111/jcpe.12751 [DOI] [PubMed] [Google Scholar]
- 29.Sakata T, Yoshimatsu H, Masaki T, Tsuda K. Anti-obesity actions of mastication driven by histamine neurons in rats. Exp Biol Med (Maywood). 2003;228:1106–1110. 10.1177/153537020322801002 [DOI] [PubMed] [Google Scholar]
- 30.Yildirim A, Atmaca H, Bedir A, Devrim I. Gingival stimulation: an important metabolic regulator? J Obes Oerweig. 2017;3:204 10.15744/2455-7633.3.204 [DOI] [Google Scholar]
- 31.Dawes C. Salivary flow patterns and the health of hard and soft oral tissues. J Am Dent Assoc. 2008;139(Suppl):18S–24S. 10.14219/jada.archive.2008.0351 [DOI] [PubMed] [Google Scholar]
- 32.Greenstein G. The role of bleeding upon probing in the diagnosis of periodontal disease. A literature review. J Periodontol. 1984;55:684–688. 10.1902/jop.1984.55.12.684 [DOI] [PubMed] [Google Scholar]
- 33.Casanova L, Hughes FJ, Preshaw PM. Diabetes and periodontal disease: a two-way relationship. Br Dent J. 2014;217:433–437. 10.1038/sj.bdj.2014.907 [DOI] [PubMed] [Google Scholar]
- 34.Arimatsu K, Yamada H, Miyazawa H, et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep. 2014;4:4828. 10.1038/srep04828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S, Park GH, Yang JH, Chun SH, Yoon HJ, Park MS. Eating frequency is inversely associated with blood pressure and hypertension in Korean adults: analysis of the Third Korean National Health and Nutrition Examination Survey. Eur J Clin Nutr. 2014;68:481–489. 10.1038/ejcn.2014.9 [DOI] [PubMed] [Google Scholar]
- 36.Liu W, Su L, Xie X, Xiang X, Huang J, Ji P. Association between toothbrushing and behavioral risk factors of non-communicable diseases: a population based survey of 4500 adults in China. Sci Rep. 2019;9:8498. 10.1038/s41598-019-44662-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bazzano LA, Serdula MK, Liu S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr Atheroscler Rep. 2003;5:492–499. 10.1007/s11883-003-0040-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.