Abstract
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive and lethal lung disease characterised by a fibrotic histological pattern found in usual interstitial pneumonia. Its causes, pathogenesis, clinical phenotype and molecular mechanisms are poorly defined. Large-scale, multicentre studies are warranted to better understand IPF as a disease in China, its associated risk factors, clinical characteristics, diagnosis, disease progression and treatment.
Methods and analysis
The Idiopathic Pulmonary Fibrosis Registry China Study (PORTRAY) is a prospective, multicentre registry study of patients with IPF in China. Eight hundred patients will be enrolled over a 36-month period and followed for at least 3 years to generate a comprehensive database on baseline characteristics and various follow-up parameters including patient-reported outcomes. Biological specimens will also be collected from patients to develop a library of blood, bronchoalveolar lavage fluid and lung biopsy samples, to support future research. As of 15 December 2019, 204 patients from 19 large medical centres with relatively high IPF diagnosis and treatment rates had been enrolled. Patient characteristics will be presented using descriptive statistics. The Kaplan-Meier method will be used for survival analyses. Repeated measures will be used to compare longitudinal changes in lung function, imaging and laboratory tests. Results following analysis have been projected to be available by July 2025.
Ethics and dissemination
The study protocol was reviewed and approved by the Institutional Review Board from all the study sites currently recruiting patients. Study results will be published in peer-reviewed journals.
Trial registration number
Keywords: interstitial lung disease, thoracic medicine, epidemiology
Strengths and limitations of this study.
This is the first large-scale, multicentre registry study in China designed to collect demographic and clinical data of Chinese patients with newly diagnosed idiopathic pulmonary fibrosis (IPF).
The multidisciplinary Central Expert Committee will ensure the accuracy of the IPF diagnosis across study sites.
Regular follow-up surveys will enable detailed observation of the natural history of IPF.
Collection of biosamples will allow for future precision medicine research among Chinese patients with IPF.
Patients in this study come from about 30 hospitals that are not randomly selected. Data from this study may not be representative of all patients with IPF in China.
Introduction
Idiopathic pulmonary fibrosis as a disease
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, life-threatening lung disease characterised by irreversible scarring of the lungs, with a fibrotic histological pattern common to usual interstitial pneumonia (UIP), a subclass of idiopathic interstitial pneumonia.1 Despite being the most common subtype of interstitial lung disease (ILD), the causes of IPF are still not well-defined. IPF occurs primarily in the elderly, and potential risk factors include smoking, environmental exposure, microbial infection and genetic predisposition.1
The prognosis of IPF can vary drastically between patients—most patients will experience a slow but predictable decline in lung function, some will experience acute exacerbations of IPF (AE-IPF) and a small proportion will worsen rapidly over a short period of time.2 Lung function will deteriorate in all patients with IPF, irrespective of severity. A recent study of patient with IPF data from the Beijing Institute of Respiratory Medicine Interstitial Lung Disease Group, Beijing Chao-Yang Hospital (affiliated to Capital Medical University), in China revealed a 1, 2 and 5-year survival rate of 61%, 52% and 39%, respectively,3 which is similar to reports from other countries.4
Epidemiology of IPF
Although IPF is a rare disease, recent studies have suggested a global increase in the incidence and prevalence of IPF.5–7 In the UK, the incidence of IPF was estimated to be 4.6/100 000 person-years between 1991 and 2003, increasing to 7.44/100 000 person-years between 2000 and 20085 8 and to 8.65/100 000 person-years between 2000 and 2012.5 9 Epidemiological data from a large US healthcare claims database showed that from 1996 to 2000, using narrow and broad diagnostic criteria, the incidence of IPF in the USA increased from 6.8/100 000 to 16.3/100 000 person-years, and the prevalence increased from 14.0/100 000 to 42.7/100 000 people.10 In Canada, using similar diagnostic criteria, the incidence of IPF between the years 2007 and 2011 increased from 9/100 000 to 18.7/100 000 person-years, and the prevalence from 20/100 000 to 41.8/100 000 persons, respectively.4 In Asia, specifically South Korea, China Taiwan and Japan, based on a narrow or broad criteria, the incidence of IPF has been reported to be much lower, ranging from 1.2 to 4.16/100 000 person-years.11 There is still no population-based epidemiological data of IPF in Mainland of China. Recently, a single-centre study at the Beijing Chao-Yang Hospital (affiliated to Capital Medical University) in China found that, of the total 3568 hospital admissions for further workup of ILD from 2000 to 2012, the number of cases with ILD per year increased from 43 in 2000 to 732 in 2012, accordingly, the proportion of inpatients diagnosed with ILD increased approximately fourfold from 2.8% to 10.5% in the department of pulmonary medicine and sevenfold from 0.2% to 1.4% in the whole hospital.12 Notably, IPF was most common, representing 26.5% of 2615 patients with newly diagnosed ILD.12
There is currently little data demonstrating whether epidemiological differences are driven by geographic, ethnic and cultural factors.
Treatment of IPF
In light of new evidence over the last decade, IPF therapy has shifted from anti-inflammatory treatment using glucocorticoid and immunosuppressive agents, to antifibrotic treatment with pirfenidone or nintedanib.1 13 14 However, detailed understanding of its pathological and molecular mechanisms is still lacking, both of which are critical to the identification of new treatment targets. Similarly, due to the lack of understanding of the disease mechanism, no sensitive or specific biomarkers have been identified to date to support IPF diagnosis, evaluate disease progression or predict prognosis. Furthermore, most of the available data are from Western population and there is a shortage of data in Chinese.5 6 15
Therefore, large-scale multicentre studies are warranted in China to further understand IPF as a disease, particularly its genetic and clinical characteristics, to improve current diagnostic and treatment practices.
Methods and analysis
Overview
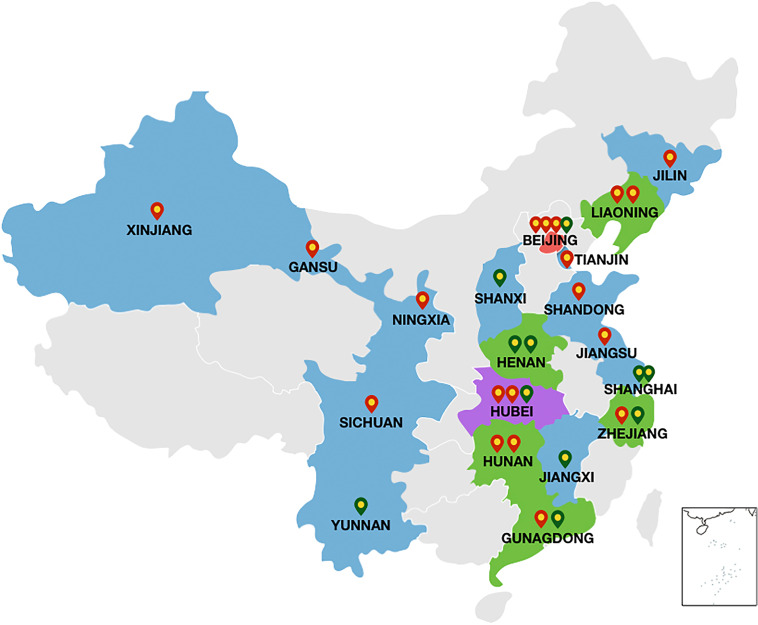
Idiopathic Pulmonary Fibrosis Registry China Study (PORTRAY) is a prospective, multicentre, registry study on patients with IPF from some large institutional hospitals across China. The study aims to enrol at least 800 patients with newly diagnosed IPF. Since the start of the 3-year recruitment period in July 2018, 30 large medical centres from 19 provinces with good experience of IPF diagnosis and treatment have expressed interest in participating in this study, the list of 30 participating centres and key investigators can be found in online supplemental appendix 1. Of these, 25 have completed research-related ethical approvals, the list of ethics committees can be found in online supplemental appendix 2, and 19 have started patient recruitment (figure 1). As of 15 December 2019, 204 patients with IPF had been enrolled. Initial data collection from these centres have proven that the trial design, including the role of the Central Expert Committee and the electronic data collection (EDC) system, is feasible. PORTRAY Research Group assisted with clinical data acquisition. A complete list of the PORTRAY Research Group investigators can be found in online supplemental appendix 3. The original planned recruitment period was 2 years; however, due to the delays in ethics approval at some centres, the recruitment period was changed from 2 to 3 years. The study is expected to be completed within 6 years and results have been projected to be available by July 2025 following analysis.
Figure 1.
The distribution of Idiopathic Pulmonary Fibrosis Registry China Study centres. Red markers represent the currently active recruitment.
bmjopen-2020-036809supp002.pdf (43.7KB, pdf)
bmjopen-2020-036809supp003.pdf (43.5KB, pdf)
Sample size
The primary outcomes are the demographics and clinical characteristics of patients with newly diagnosed IPF in China. Common symptoms of IPF include dyspnoea (91%), coughing (88%), crackling (94%) and digital clubbing (43%).3 Power calculations made based on these expected symptom frequencies at a 95% CI indicate that a sample size of at least 800 patients will be enough to obtain acceptable precision for the estimates of the above symptom frequency.
Inclusion criteria for centres
Included centres must (1) be a medical college teaching hospital or a upper first-class hospital certified by the National Health Commission of the People’s Republic of China with relatively high IPF diagnosis rates and the infrastructure to support ILD diagnosis and treatment; (2) have a multidisciplinary team, including respiratory disease clinical specialists, and imaging and pathology specialists to support comprehensive diagnosis and care for patients with IPF; and (3) have the basic facility to properly store and process blood and bronchoalveolar lavage fluid (BALF) samples.
As a prescreening step, all interested centres will be asked to complete a survey and submit two to three representative high-resolution CT (HRCT) scans for evaluation by the Central Expert Committee of the study. On selection, the sponsoring unit of each included centre must sign and abide by a research agreement, outlining requirements on patient registration, follow-up, completion of case report form(s) and storage of biological samples. Once enrolled, the centres cannot withdraw from the study without acceptable and explicit reasoning.
Inclusion criteria for patients
Patients diagnosed with IPF, aged less than 3 months and 40 years or older were included in the study. Patients were excluded if they were already enrolled in any randomised controlled trial or scheduled to receive a lung transplant within 6 months of study initiation or if the Central Expert Committee reclassified the diagnosis as non-IPF on review. Informed consent was required from all patients during enrolment.
Study outcomes
The primary outcomes are the demographics and clinical characteristics of patients with newly diagnosed IPF in China. Secondary outcomes include descriptions on the natural course of the disease, mortality rate and associated causes of death, and causes and rates of AE-IPF among patients with IPF in China. Other outcomes include (1) IPF risk factors, comorbidities and complications (eg, emphysema, pulmonary hypertension, diabetes, coronary heart disease, gastro-oesophageal reflux, anxiety and depression); (2) the treatment landscape for IPF in China, including preventive and treatment regimens (eg, influenza or pneumonia vaccinations, medications, traditional Chinese medicine, oxygen therapy, mechanical ventilation and rehabilitation); (3) quality of life as per St. George’s Respiratory Questionnaire (SGRQ); (4) IPF treatment-related costs (eg, hospitalisation fees, outpatient expenses and emergency medical expenses); and (5) developing an IPF-biological database, including blood, BALF and lung biopsy samples, from which further research can be drawn to identify specific biomarkers for IPF diagnosis to monitor IPF progression and/or to predict disease prognosis at the individual patient level.
Altogether, the results of this study will serve as the basis for establishing a standardised diagnosis and treatment system in China, which, in turn, will improve clinical outcomes and inform further research.
Roles and responsibilities
Key investigators and physicians at each enrolled centre
Key investigators from each enrolled centre should ensure that patients’ baseline data are entered in the EDC within 1 week of signing the consent form. These include the chest HRCT scan (submitted electronically or mailed in a compact disc) and images from lung biopsy specimens, if available.
Initial IPF diagnosis should be made at the patient’s enrolled centre by a multidisciplinary team consisting of pulmonologists, imaging specialists/radiologists, pathologists and other specialists when applicable. The diagnosis should be made by first confirming the diagnosis of ILD, then excluding other diagnoses/causes (eg, environmental causes, drug toxicity and connective tissue disease), followed by confirmation with HRCT and/or pathological diagnosis of UIP or probable UIP, in line with the recommendations of the 2011 American Thoracic Society (ATS)/European Respiratory Society (ERS)/Japanese Respiratory Society (JRS)/Latin American Thoracic Association (ALAT) guidelines.1
The pulmonary physician (a member of the clinical team) should review all patient information and develop the final clinical diagnosis within 2 weeks of receiving the pathologist’s/imaging specialist’s diagnosis. They should provide treatment based on the patient’s diagnosis with the patient’s consent and are advised to follow the recommendations from 2015 ATS/ERS/JRS/ALAT Clinical Practice Guideline for IPF where possible.14 Basic treatment and treatment for comorbidities should be provided based on the attending physician’s judgement as per routine clinical practice.
Central expert committee
The Central Expert Committee consists of three respiratory specialists (Dr Huaping Dai, Dr Yanhong Ren and Dr Qiao Ye), three radiologists (Dr Qihang Chen, Dr Xie Sheng and Dr Liu Min) and three pathologists (Dr Ruie Feng, Dr Fang Fang and Dr Ling Zhao) from different hospitals in Beijing. Together, they will be responsible for confirmation of IPF diagnoses. An imaging specialist/pathologist from the Central Expert Committee must complete a blinded review of uploaded HRCT scans and histological images from lung biopsy specimens within 1 month and submit an independent entry of the patient’s diagnosis in the EDC. The final diagnosis should be made based on two independent clinician reviews.
If there are conflicting diagnoses, the Central Expert Committee must review the patient’s case again and come to a consensus. The final diagnosis should be reported back to the key investigator from the patient’s enrolled centre. If the final diagnosis is not IPF, the patient will be removed from the study and its database.
Clinical research organisation
To ensure quality control, a clinical research organisation (CRO) has been engaged for data management and coordination of this study. A quality assurance inspector from the CRO team will conduct a full-scale audit of all study medical records, including researcher-related documents, documentation of informed consent and regular checks on the data recorded.
Data collection
Data will be collected on an EDC with built-in logic checks. Each enrolled centre should designate one to two knowledgeable clinical research coordinators to collect and record patient data in the EDC system to ensure completeness and accuracy of entered data. The dedicated clinical research coordinators should uniformly receive training and assessments, and data entered should be continuously tracked. Timely feedback on recorded data will be provided regularly by the CRO for quality control purposes.
All research materials will be in Chinese, and the source files will be saved at each research centre.
Follow-up
The follow-up schedule has been designed to occur once every 3 months for the first two follow-ups after diagnosis, then once every 6 months thereafter until completion of the study (table 1). All baseline and follow-up parameters are shown in table 1, including demographics, potential IPF risk factors, existing comorbidities, clinical characteristics, diagnostic tests used, treatment and progression, patient-reported outcomes, blood and biopsy specimen collection status, and follow-up plan and results.
Table 1.
Parameters for data collection and follow-up plan
| Evaluation | Visit 0 | Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | Visit 6 | Visit 7 |
| Baseline | FU 1 | FU 2 | FU 3 | FU 4 | FU 5 | FU 6 | FU 7 | |
| Month | 0 | 3 (±2 weeks) |
6 (±1 month) |
12 (±1 month) |
18 (±1 month) |
24 (±1 month) |
30 (±1 month) |
36 (±1 month) |
| Informed consent | X | |||||||
| Inclusion criteria met | X | |||||||
| Demographics* | X | |||||||
| Existing risk factors† | X | |||||||
| IPF medical history‡ | X | |||||||
| Comorbidities§ | X | X | X | X | X | X | X | X |
| IPF clinical symptoms¶ | X | X | X | X | X | X | X | X |
| mMRC score | ||||||||
| GerdQ score | ||||||||
| Lung function evaluation** | X | X | X | X | X | X | X | X |
| GAP score | ||||||||
| 6-minute walking test | X | X | X | X | X | X | X | X |
| Arterial blood gas test | X | X | X | X | X | X | X | X |
| HRCT examination | X | X | X | X | X | |||
| Fibrosis score†† | ||||||||
| Routine blood, liver function and kidney function tests | X | X | X | X | X | X | X | X |
| Connective tissue disease workup | X | X | X | X | X | |||
| Tumor-related examination | X | X | X | X | ||||
| ECG, echocardiography | X | X | X | X | ||||
| BAL±TBLB/BLC‡‡ | X± | |||||||
| VATS/OLB§§ | X± | |||||||
| Duration, symptoms and management of AE-IPF¶¶ | X | X | X | X | X | X | X | X |
| IPF treatment prescribed*** | X | X | X | X | X | X | X | X |
| Non-pharmacological interventions††† | X | X | X | X | X | X | X | X |
| Significant changes to patient’s conditions/survival status‡‡‡ | X | X | X | X | X | X | X | |
| Significant clinical events§§§ | X | X | X | X | X | X | X | |
| IPF-related costs¶¶¶ | X | X | X | X | X | X | X | X |
| Patient-reported outcomes surveys**** | X | X | X | X | X | X | X | X |
| Collection of blood sample(s)†††† | X | X | X | X | X± | X± | ||
| Collection of BALF sample(s)‡‡‡‡ | X± | |||||||
| Lung biopsy specimen(s)§§§§ | X± |
*Demographics include, but are not limited to, age, gender, ethnicity, health insurance and so on. Patients have the right to decline providing answers for questions related to personal privacy.
†Existing risk factors for IPF include smoking, environmental exposure, occupational exposure/hazards, drug exposure, family history and so on.
‡IPF medical history includes the date of initial symptoms and whether an HRCT scan was taken, along with the date and associated examination results.
§Comorbidities refer to systemic conditions/diseases other than IPF, for example, diabetes, hyperlipidemia and gastrointestinal ulcers. The time of initial diagnosis and associated treatment for these conditions should be recorded.
¶Clinical features of IPF include chronic exertional dyspnoea, cough, bibasilar inspiratory velcro crackles on auscultation, digital clubbing, accompanying extrapulmonary manifestations and so on. mMRC dyspnoea score21 and GerdQ gastro-oesophageal reflux score22 should be evaluated and recorded.
**GAP score should be evaluated after lung function evaluation.23 24
††Fibrosis score from HRCT chest examination should be recorded.25
‡‡To obtain a definitive diagnosis, patients may require a bronchoscopy, including BAL and/or TBLB or BLC, which would be conducted or not depending on the clinical decision.
§§To obtain a definitive diagnosis, patients may require VATS or OLB, which would be conducted or not depending on the clinical decision.
¶¶AE-IPF is defined as (1) previous or simultaneous diagnosis of IPF; (2) typical acute dyspnoea symptoms or symptoms worsening within 30 days; (3) chest CT imaging pattern of usual interstitial pneumonia with new bilateral ground-glass opacities; and (4) symptoms cannot be completely explained by heart failure or fluid overload.
***Treatment details of IPF include the treatment plan, the type of drug, the dose, the start and end date and the outcome of the treatment, and the time of treatment change or addition of the drug. Treatments that are used in less than 2% of the study population would be grouped and analysed as a whole. Common treatments include, but are not limited to, pirfenidone, nintedanib, glucocorticoids, N-acetylcysteine, azithromycin, cyclophosphamide, azathioprine or other immunosuppressants, antiplatelet drugs, anticoagulants, anti-reflux drugs, anti-pulmonary hypertension drugs, airway drugs and Chinese medicine/proprietary Chinese medicine. Adverse reactions associated with IPF treatments should be specified.
†††Non-pharmacological treatments include long-term oxygen therapy, mechanical ventilation, pulmonary rehabilitation, lung transplantation and so on.
‡‡‡Changes in the patient’s conditions include clinical improvement, disease progression or death. The cause and time of death should be recorded. Patients should be followed for the full 3-year period as per study design or until death, whichever occurs first. Survival status data will be used for mortality analysis.
§§§Clinical events of concern include AE-IPF, hospitalisation, lung transplantation, pulmonary infections, respiratory failure, pulmonary hypertension, pulmonary embolism, lung cancer, acute myocardial infarction, stroke, other arterial embolism, deep vein thrombosis, haemorrhage, gastrointestinal perforation and other diseases that impact the prognosis of patients.
¶¶¶IPF-related costs include direct medical costs (hospitalisation costs, outpatient/emergency visits, including laboratory tests, imaging studies, medications and non-pharmacological treatments).
††††Blood samples, including serum, plasma and buffy coat cells, should be collected within the first year of follow-up; further sample collection after the first year of follow-up is not required.
‡‡‡‡BALF specimens from bronchoscopy should be collected.
§§§§If a surgical lung biopsy is performed, lung tissue specimens should be collected.
AE-IPF, acute exacerbations of IPF; BAL, bronchoalveolar lavage; BALF, bronchoalveolar lavage fluid; BLC, bronchoscopic lung cryobiopsy; FU, follow-up; GAP, gender–age–physiology; GerdQ, Gastro-oesophageal Reflux Disease Questionnaire; HADS, Hospital Anxiety and Depression Scale; HRCT, high-resolution CT; IPF, idiopathic pulmonary fibrosis; mMRC, modified medical research council; OLB, open-lung biopsy; SGRQ, St. George's Respiratory Questionnaire; TBLB, transbronchial lung biopsy; VATS, video-assisted thoracoscopic surgery.
Patient-reported outcomes will be assessed using the modified Medical Research Council score for dyspnoea, the Gastro-oesophageal Reflux Disease Questionnaire (GerdQ) for monitoring reflux, the SGRQ for quality of life, and the Hospital Anxiety and Depression Scale (HADS) for anxiety. The relevant support staff can assist patients in completing these assessments, if required.
All patient data and results shall be preserved in their original form in the EDC database as reference materials without alterations.
Statistical analysis
All registered patients will be included in the final analysis. Data will first be analysed by the centre to check for IPF epidemiological differences between centres. The overall analysis of all patients may be stratified by these differences, if appropriate.
Data from this study will mainly be analysed using SAS software, V.9.4 (SAS Institute). All analyses will be evaluated at a significance level of 5% with a 95% CI. For missing values, no imputation will be performed and listwise deletion will be used in the analysis.
χ2 tests, Fisher’s exact test, Wilcoxon rank-sum test and Kruskal-Wallis test will be used to analyse the categorical data as appropriate. Relevant adjustments to covariates will be made when necessary. The Kaplan-Meier method will be used for survival analyses. The log-rank test, the Wilcoxon rank-sum test and the likelihood ratio test will be used to compare survival curve between groups, and the Cox proportional hazard model will be used to analyse factors affecting survival. Repeated measures will be used to compare longitudinal changes in lung function, imaging and laboratory tests. Multiple linear regression analysis will be used to identify the factors influencing acute exacerbation and the variables will be selected based on backwards stepwise selection, p<0.10.
Ethics and research responsibilities
This study is a non-interventional, observational study that will protect patients' rights and interests in accordance with research protocols, medical regulations and diagnostic protocols in China. The patient’s attending physician is required to provide standard and appropriate clinical care for the patient, including diagnosis, preventive measures and therapeutic procedures.
The sponsor (China-Japan Friendship Hospital) and principal investigator (Dr Huaping Dai) of this study are responsible for the design and management of the research project through oversight of the steering committee. The research conducted in this study will comply with the Helsinki Declaration, national and local regulations on ethical requirements and patient data protection regulations. Informed consent will be obtained from patients or their families if the patient unable to sign.
Patient data will be de-identified during analysis. Data and results generated from the study may be examined/inspected by key investigator and designated participating physicians within the same centre, sponsor representatives, ethics committees and regulatory agencies. Patients’ medical information will be kept confidential and will not be disclosed to third parties in any way without written consent from the sponsor (China-Japan Friendship Hospital) and principal investigator (Dr Dai Huaping), except to meet the requirements of the relevant national administrative departments. Each enrolled centre should maintain clinical research data related to this study for at least 5 years after the completion of the study and data collection. Proposals from enrolled centres involving use of registry data and biological samples will be evaluated for scientific value, feasibility and prioritisation.
Patient and public involvement
Patients and public were not involved in the design of the study, and in the recruitment to and conduct of the study.
Study limitations
Apart from the inherent limitations of registry studies (namely selection bias of included physicians and centres), the other limitations of the study are associated with the patient inclusion criteria. This study only enrolled patients with newly diagnosed IPF (diagnosed less than 3 months ago) who will not require lung transplantation within 6 months. Since patients who fit this criterion would have relatively mild and stable disease, they should generally respond well to treatment; therefore, the results of this study may underestimate the severity of clinical outcomes in Chinese patients with IPF.
Implications
China is a developing country with a large population spread out over a large geographical area. As such, the availability and quality of medical resources vary, affecting diagnosis and standard of care for diseases, including IPF and ILD. With only a few teaching hospitals and ILD centres where the diagnosis and management were comparable to international standard until recent years, China was trying to improve the standard of care for IPF.
As an effort to improve IPF care, China-specific IPF guidelines were first published in 2002 and updated in 2016, providing IPF treatment and management recommendations that are consistent with that of the ATS/ERS/JRS/ALAT guidelines. Through widespread use of China’s IPF guidelines and with growing knowledge on ILD, the diagnosis and treatment practice have improved. The use of chest HRCT is also now widely promoted, and pirfenidone and nintedanib have also been approved for the treatment of IPF by the Chinese Drug Administration in 2014 and 2017, respectively. These improvements provide the basis for conducting multicentre research on IPF in China.
This is the first large-scale, multicentre, registry study in China and also the largest real-world, prospective registry study involving experts from various disciplines to ensure accurate diagnosis and data collection to develop a systematic database describing the phenotypic characteristics of IPF. The biological data collected as part of the database provides the basis for studying genotypic relationships with clinical phenotypes of IPF, the pathogenesis of IPF, and relevant biomarkers for diagnosis, treatment response and prognosis of IPF. Furthermore, as patients’ quality of life and complexity of IPF clinical manifestations are often affected by the presence of comorbidities,16–20 including combined emphysema, pulmonary hypertension, lung cancer, pulmonary embolism and infections, the PORTRAY database may reveal trends that could potentially inform overall prognosis.
Ethics and dissemination
The study will be conducted in accordance with the Helsinki Declaration, national and local regulations on ethical requirements, and patient data protection regulations. The study protocol was reviewed and approved by the Institutional Review Board from all the study sites currently recruiting patients; a complete list of ethics committees that have approved our study can be found in online supplemental appendix 1. Study results will be disseminated through publications in peer-reviewed journals.
bmjopen-2020-036809supp001.pdf (33.8KB, pdf)
Supplementary Material
Footnotes
Collaborators: PORTRAY Research Group.
Contributors: BX contributed to the study design, the construction of case report form, EDC system and database, data collection and the draft of the manuscript. YR, XH, CB, SW, DJ and SL contributed to the improvement of the study methods and case report form. JG contributed to the improvement of the study methods, construction and management of biological specimen library. QC, ML, RF and LZ contributed to the collection and interpretation of high-resolution CT and pathological features. CW contributed to the study conception, the revision of the manuscript. HD contributed to the study conception and design, the construction of case report form, electronic data collection system and database, the revision of the manuscript and final approval of the version to be published. Idiopathic Pulmonary Fibrosis Registry China Study (PORTRAY) Research Group contributed to clinical data acquisition. All authors contributed to the development of the manuscript, review and approval of the final submitted version of the manuscript. All authors have agreed to be accountable for all aspects of the manuscript, ensuring that all questions related to the accuracy and integrity of the manuscript are appropriately addressed.
Funding: This study was supported by the National Key Technologies R&D Programme Precision Medicine Research (No.2016YFC0901101). Data collection for this study was funded by Boehringer Ingelheim.
Map disclaimer: The depiction of boundaries on the map(s) in this article does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. The map(s) are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer-reviewed.
References
- 1.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431–40. 10.1164/rccm.201006-0894CI [DOI] [PubMed] [Google Scholar]
- 3.Cai M, Zhu M, Ban C, et al. Clinical features and outcomes of 210 patients with idiopathic pulmonary fibrosis. Chin Med J 2014;127:1868–73. [PubMed] [Google Scholar]
- 4.Hopkins RB, Burke N, Fell C, et al. Epidemiology and survival of idiopathic pulmonary fibrosis from national data in Canada. Eur Respir J 2016;48:187–95. 10.1183/13993003.01504-2015 [DOI] [PubMed] [Google Scholar]
- 5.Hutchinson J, Fogarty A, Hubbard R, et al. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J 2015;46:795–806. 10.1183/09031936.00185114 [DOI] [PubMed] [Google Scholar]
- 6.Wakwaya Y, Brown KK. Idiopathic pulmonary fibrosis: epidemiology, diagnosis andOutcomes. Am J Med Sci 2019;357:359–69. 10.1016/j.amjms.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 7.Nalysnyk L, Cid-Ruzafa J, Rotella P, et al. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev 2012;21:355–61. 10.1183/09059180.00002512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navaratnam V, Fleming KM, West J, et al. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax 2011;66:462–7. 10.1136/thx.2010.148031 [DOI] [PubMed] [Google Scholar]
- 9.Maher T, Strongman H, Boggon R, et al. P18 Idiopathic pulmonary fibrosis survival has not improved in the 21st century; analysis of CPRD gold primary care data. Thorax 2013;68:A82.2–3. 10.1136/thoraxjnl-2013-204457.168 [DOI] [Google Scholar]
- 10.Raghu G, Weycker D, Edelsberg J, et al. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006;174:810–6. 10.1164/rccm.200602-163OC [DOI] [PubMed] [Google Scholar]
- 11.Olson AL, Gifford AH, Inase N, et al. The epidemiology of idiopathic pulmonary fibrosis and interstitial lung diseases at risk of a progressive-fibrosing phenotype. Eur Respir Rev 2018;27:180077. 10.1183/16000617.0077-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ban C, Yan W, Xie B, et al. Spectrum of interstitial lung disease in China from 2000 to 2012. Eur Respir J 2018;52:1701554. 10.1183/13993003.01554-2017 [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society American thoracic Society. idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American thoracic Society (ats), and the European respiratory society (ERS). Am J Respir Crit Care Med 2000;161:646–64. 10.1164/ajrccm.161.2.ats3-00 [DOI] [PubMed] [Google Scholar]
- 14.Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 2015;192:e3–19. 10.1164/rccm.201506-1063ST [DOI] [PubMed] [Google Scholar]
- 15.Nakamura Y, Suda T. Idiopathic pulmonary fibrosis: diagnosis and clinical manifestations. Clin Med Insights Circ Respir Pulm Med 2015;9:CCRPM.S39897–171. 10.4137/CCRPM.S39897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghu G, Amatto VC, Behr J, et al. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J 2015;46:1113–30. 10.1183/13993003.02316-2014 [DOI] [PubMed] [Google Scholar]
- 17.King CS, Nathan SD. Idiopathic pulmonary fibrosis: effects and optimal management of comorbidities. Lancet Respir Med 2017;5:72–84. 10.1016/S2213-2600(16)30222-3 [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Zhu M, Geng J, et al. Incidence and radiologic-pathological features of lung cancer in idiopathic pulmonary fibrosis. Clin Respir J 2018;12:1700–5. 10.1111/crj.12732 [DOI] [PubMed] [Google Scholar]
- 19.Yan W, Peng L-Y, Ban C-J, et al. Incidence and clinical characteristics of pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chin Med J 2015;128:896–901. 10.4103/0366-6999.154284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye Q, Huang K, Ding Y, et al. Cigarette smoking contributes to idiopathic pulmonary fibrosis associated with emphysema. Chin Med J 2014;127:469–74. [PubMed] [Google Scholar]
- 21.Fletcher CM, Elmes PC, Fairbairn AS, et al. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J 1959;2:257–66. 10.1136/bmj.2.5147.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther 2009;30:1030–8. 10.1111/j.1365-2036.2009.04142.x [DOI] [PubMed] [Google Scholar]
- 23.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684–91. 10.7326/0003-4819-156-10-201205150-00004 [DOI] [PubMed] [Google Scholar]
- 24.Ryerson CJ, Vittinghoff E, Ley B, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest 2014;145:723–8. 10.1378/chest.13-1474 [DOI] [PubMed] [Google Scholar]
- 25.Kazerooni EA, Martinez FJ, Flint A, et al. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol 1997;169:977–83. 10.2214/ajr.169.4.9308447 [DOI] [PubMed] [Google Scholar]
- 26.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 27.Stern AF. The hospital anxiety and depression scale. Occup Med 2014;64:393–4. 10.1093/occmed/kqu024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-036809supp002.pdf (43.7KB, pdf)
bmjopen-2020-036809supp003.pdf (43.5KB, pdf)
bmjopen-2020-036809supp001.pdf (33.8KB, pdf)