Abstract
Purpose
A growing body of preclinical and observational research suggests that statins have potential as a therapeutic strategy in patients with cancer. This systematic review of randomised controlled trials (RCTs) in patients with solid tumours aimed to determine the efficacy of statin therapy on mortality outcomes, their safety profile and the risk of bias of included studies.
Methods
Full-text articles comparing statin therapy versus control in solid tumours and reporting mortality outcomes were identified from Medline and Embase from conception to February 2020. A systematic review with qualitative (primarily) and quantitative synthesis was conducted. This systematic review was prospectively registered (Prospero registration CRD42018116364).
Results
Eleven trials of 2165 patients were included. Primary tumour sites investigated included lung, colorectal, gastro-oesophageal, pancreatic and liver. Most trials recruited patients with advanced malignancy and used sub-maximal statin doses for relatively short durations. Aside from one trial which demonstrated benefit with allocation to pravastatin 40 mg in hepatocellular carcinoma, the remaining ten trials did not demonstrate efficacy with statins. The pooled hazard ratio for all-cause mortality with allocation to pravastatin in patients with hepatocellular carcinoma in two trials was 0.69 (95% confidence interval CI 0.30–1.61). Study estimates were imprecise. There were no clinically important differences in statin-related adverse events between groups. Overall, included trials were deemed low risk of bias.
Conclusion
The trial evidence is not sufficiently robust to confirm or refute the efficacy and safety of statins in patients with solid malignant tumours. Study and patient characteristics may explain this uncertainty. The potential role of high-dose statins in adjuvant settings deserves further research.
Electronic supplementary material
The online version of this article (10.1007/s00228-020-02967-0) contains supplementary material, which is available to authorized users.
Keywords: Statin, HMG-CoA, Clinical trials, Cancer, Adverse effects
Background
Hydroxy-3-methylglutaryl-CoA (HMG-CoA) inhibitors, better known as statins, are a class of lipid-lowering agents that are highly effective and used widely in clinical practice for the primary and secondary prevention of cardiovascular disease [1]. Statins inhibit the rate-limiting step of the mevalonate pathway, a ubiquitous metabolic cascade which plays an essential role in the synthesis of downstream sterol (e.g. cholesterol) and non-sterol isoprenoids [2]. There is growing evidence that a number of these biologically active intermediates exert functions which have direct relevance to cancer biology, with roles in proliferative signalling, cell-cycle regulation, angiogenesis, and metastases [3]. Interest in the potential of statins to prevent and treat cancer has grown over the last three decades.
In vitro studies have demonstrated that statins inhibit proliferation, induce apoptosis and limit invasiveness in numerous malignancies, and have demonstrated the functional relevance of mevalonate pathway intermediates in these observations [4–6]. Mutant TP53, the most frequently mutated gene in cancer [7, 8] and consistently associated with poor prognosis [9], has been shown to upregulate transcription of mevalonate pathway products to sustain malignant proliferation [10], a pathway potently inhibited by statins. Furthermore, statins have been shown to selectively destabilise mutant TP53 protein [11]. Preclinical in vivo studies have demonstrated statins effectively inhibit growth of established tumours with no noticeable effect on normal tissues [11, 12]. These preclinical observations underscore the potential for statins as a viable therapeutic strategy in human malignancy.
The most recent systematic review of observational research included 95 cohorts with over 1.1 million cancer patients and demonstrated post-diagnostic statin use was associated with a significant reduction in all-cause mortality (HR 0.70, 95% CI 0.66–0.74 pooled from 55 studies), with broadly similar effect sizes for progression-free survival, cancer-specific mortality and disease-free survival [13]. However, to varying degrees, studies were potentially susceptible to selection bias, immortal-time bias and confounding. Nevertheless, compared with studies with a higher risk of bias (≤ 8 points on a 6-item scale [14]), effect sizes of those with a lower risk of bias (> 8 points) were attenuated, however remained statistically significant. While preclinical and epidemiological evidence is encouraging, causality remains to be established. To determine whether statins are an effective therapeutic option for specific cancers, evidence from well-designed, sufficiently powered, randomised controlled trials (RCTs) are required.
A series of trials have assessed the efficacy and safety of statins in patients with solid tumours; however, there remains considerable uncertainty, and the justification for further trials has been questioned [15]. The conduct of future trials should be reliably informed by critical appraisal of existing randomised studies in patients with cancer. Therefore, we undertook a systematic review of statins in patients with any malignancy to assess the current state of evidence from RCTs. Specifically, in patients with solid tumours, we aimed to determine (i) the efficacy of statin therapy on mortality outcomes, (ii) the safety profile of statins, and (iii) the risk of bias in RCTs of statin therapy.
Methods
This systematic review was registered (CRD42018116364) on the PROSPERO database and conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [16].
Search strategy
We sought relevant published articles by searching MEDLINE (1948 onwards) and Embase (1980 onwards) (Supplementary Table 1) using the OVID interface and manual searches of reference lists of any systematic reviews identified by the previous step. We used the following search terms to search each database: hydroxymethylglutaryl-CoA reductase inhibitors, statin, cancer, carcinoma, neoplasms, malignancy and randomised controlled trial. The literature search was limited to the English language and human subjects. Searches were completed in Feb 2020.
Eligibility criteria
Only RCTs satisfying the following eligibility criteria were included in the systematic review: (i) statin therapy was the intervention, either given alone or in combination with a co-intervention across trial arms; (ii) at least one trial group received placebo, no statin or standard care alone; (iii) participants were diagnosed with a malignant solid tumour prior to enrolment; and (iv) overall survival (OS), progression-free survival (PFS) or response rate (RR) were reported outcomes. No restrictions were placed on the statin administered, posology, frequency or duration of administration. No restrictions were placed on length of follow-up. Two reviewers (JPT and LA) independently screened abstracts and selected full-text articles for inclusion based on the above criteria. Discrepancies were resolved through discussion among two or more reviewers.
Data extraction and quality assessment
Two reviewers (JPT and LA) independently extracted data from each selected article for study characteristics (location, setting, number of randomised patients, recruitment period, primary cancer site, intervention, duration of statin therapy, concomitant therapy and reported outcome measures); patient characteristics at enrolment (number of patients allocated to active and control groups, age, gender, cancer stage and Eastern Cooperative Oncology Group [ECOG] performance status); study outcomes (reported median overall and progression-free survival in allocated groups with corresponding hazard ratios (and confidence intervals) and reported response rates (%) in each group); and toxicity profile. For continuous participant characteristics and outcomes, we extracted means (with corresponding standard deviations) and medians (with corresponding ranges) as appropriate in each arm. To assist the comparison of statin type and posology used between studies, the defined daily dose (DDD) for each trial was calculated [17]. The DDD is a standardised measure of drug exposure relative to the assumed average maintenance dose per day for a drug used, for its main indication in adults was as defined by the World Health Organization. For example, a single dose of simvastatin 30 mg or atorvastatin 20 mg is equivalent to 1 DDD. Two reviewers (JPT and LA) used the Cochrane risk of bias tool to assess internal validity of each eligible study across seven items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias [18]. Given the outcomes of interest were objective (e.g. all-cause mortality), open-label study designs, where applicable, were deemed to pose minimal risk of bias for the domains of “blinding of participants and personnel”, and “blinding of outcome assessment”. Discrepancies were resolved through consensus discussion between reviewers. We contacted authors for additional information where required.
Study outcomes
The primary outcome was overall survival (OS), defined as the time from randomisation to death from any cause [19]. Secondary outcomes were (i) progression-free survival (PFS), defined as time from randomisation to first observed cancer progression or death; (ii) response rate (RR), defined as the proportion of patients with tumour size reduction of a predefined amount and for a minimum time period [19]; and (iii) toxicity (proportions of grade 3–5 and separately statin-related adverse events in each group).
Statistical analysis
From the outset, we decided it would be inappropriate to conduct a quantitative meta-analysis comprising trials with different primary cancers as any resultant summary effect size estimate for mortality outcomes would be difficult to interpret. This is because each distinct cancer has disparate biology, behaviour, prognosis, treatments and responsiveness to therapy. Furthermore, while the mevalonate pathway is ubiquitous to all eukaryotes and will be functional in malignancy, there is insufficient evidence at present to suggest a universally consistent role in effecting cancer prognosis. As a result, we primarily undertook a qualitative assessment of included trials to critically review the study characteristics, participant characteristics, mortality and safety outcomes of eligible studies. We performed a quantitative meta-analysis, where possible, of any trials in patients with the same primary cancer.
Summary study characteristics were calculated and weighted by sample size for gender, cancer stage and ECOG performance status. Where p values were not provided in original study reports for comparisons between intervention and control arm for overall response rate, we calculated these with extracted categorical data using the chi-squared test or Fisher’s exact test, as appropriate. Meta-analysis of trials involving patients with the same primary cancer was performed to quantify the association between statin use and overall survival. Effect estimates were pooled by the inverse of their variance and are presented as pooled hazard ratios (HRs) with corresponding 95% CIs. Due to differences in recruited study populations, concomitant therapies and intervention protocols, we utilised a random-effects meta-analysis using the method of DerSimonian and Laird [20]. Heterogeneity was estimated using the Cochrane’s Q and I2 statistics. A two-tailed p value of less than 0.05 was defined as statistically significant for all analyses apart from Cochrane’s Q test for heterogeneity where a p value of 0.10 was selected as the threshold of significance. Results of this meta-analysis were illustrated by means of a forest plot. Analyses were performed with STATA version 15.1 (StataCorp LP, College Station, TX, USA).
Results
Search and selection of studies
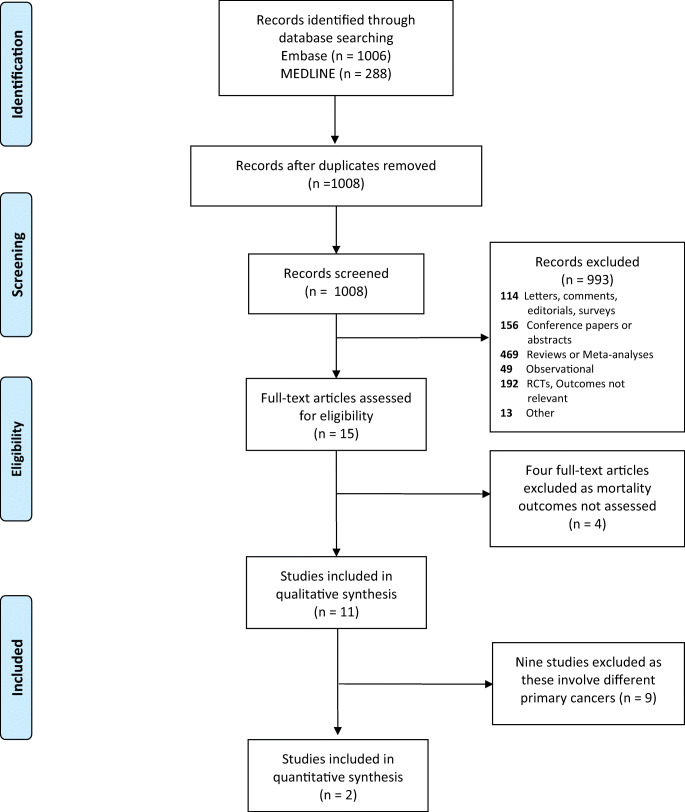
Among 1008 articles identified from the literature search, 15 full-text articles were assessed for eligibility, of which eleven were ultimately eligible for inclusion (Fig. 1) [21–31]. The four excluded articles met all inclusion criteria except for the outcomes of interest, instead focusing on surrogate outcomes [32–35].
Fig. 1.
PRISMA flow diagram
Study characteristics
The characteristics of selected studies are shown in Table 1. Four were phase III studies [22, 24, 26, 27] and the remainder were phase II/pilot/feasibility trials. Of the eleven RCTs, six originated from East Asia [23, 26–29, 31]. Four studies were performed in Europe [21, 22, 24, 30] and one in Egypt [25]. All studies were conducted in hospital-based settings. In total, 2165 participants were recruited across all trials. Four studies were conducted at a single site and included between 30 and 106 participants [25, 29–31]. The largest trial included 846 patients (LUNGSTAR) across 91 UK centres [24]. The gastro-intestinal tract and accessory digestive organs were the primary site examined in seven trials, including cancers of the gastro-oesophageal junction/stomach [27, 30], oesophagus/gastro-oesophageal junction [21], pancreas [26], liver [22, 31] and colorectum [26]. The remaining studies separately recruited patients with small cell lung cancer [24], non-small cell lung cancer [23, 29] and brain metastases (with various primary tumour sites) [25]. Eight of the trials explicitly excluded prior/current statin users [21, 22, 24–26, 28–30], and no trials reported the proportion of prior/current users among the randomised population. The intervention arm in seven studies was simvastatin [21, 23, 25–29] and in four studies was pravastatin [22, 24, 30, 31]. The highest DDD used in one study was 2.67 [25] and in the remaining ten studies was 1.33. Open-label statins were administered in six studies [22, 23, 25, 29–31], and identical matched placebo was used in those remaining. Reported median duration of statin therapy administration was 3–8.6 months [22, 24, 26, 27, 29]. One trial of pravastatin in hepatocellular carcinoma administered statins for a mean of 16.5 months [31]. Concomitant chemo/radiotherapy was administered in all but one trial [21].
Table 1.
Characteristics of selected randomised controlled trials
| Trial | Location | Centre(s) | Number of patients | Recruitment period | Cancer | Intervention (DDD) | Duration of statin therapy (months) | Previous cancer therapy | Concomitant therapy | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Alexandre et al. [21] | UK | 3 | 32 | Oct 2014–July 2016 | Oesophageal/GOJ adenocarcinoma | Simvastatin 40 mg (1.33) or Placebo | 9.6 | Yesb | Nil | Retention, Absorption, Adherence, OS, PFS, QoL |
| Jouve et al. [22] | France | 61 | 323 | March 2010–Nov 2013 | Hepatocellular Carcinoma | Pravastatin 40 mg (1.33), open label | 4.1Md | Yes | Chemotherapy (S) | OS, PFS, TTP, TTF, QoL |
| Lee et al. [23] | South Korea | 2 | 68 | Nov 2012–Sept 2015 | NA Non-Small Cell Lung Cancer | Simvastatin 40 mg (1.33), open label | NS | Yesc | Afatinib | OS, PFS, RR |
| Seckl et al. [24] | UK | 91 | 846 | Feb 2007–Jan 2012 | Small Cell Lung Cancer | Pravasatatin 40 mg (1.33) or placebo | 8.6Md | Nil | Chemotherapy (ET + C or CB) | OS, PFS, RR |
| El-Hamamsy et al. [25] | Egypt | 1 | 50 | April 2014–Oct 2015 | Brain metastases (various primariesa) | Simvastatin 80 mg (2.67), open label | 0.5 | NS | Whole brain radiotherapy | OS, PFS, Rad R |
| Lim et al. [26] | South Korea | 5 | 269 | April 2010–July 2013 | Colorectal Cancer | Simvastatin 40 mg (1.33) or Placebo | 3–4.5Md | Yesc | Chemotherapy (XELIRI/FOLFIRI) | OS, PFS, RR, TTP |
| Kim et al. [27] | South Korea | 9 | 244 | Feb 2009–Nov 2014 | Gastric/GOJ adenocarcinoma | Simvastatin 40 mg (1.33) or placebo | 4.43Md | Yesd | Chemotherapy (C + X) | OS, PFS, RR |
| Hong et al. [28] | South Korea | 4 | 114 | Dec 2008–April 2012 | Pancreatic cancer | Simvastatin 40 mg (1.33) or Placebo | NS | Nil | Chemotherapy (GC) | OS, RR, TTP, DCR |
| Han et al. [29] | South Korea | 1 | 106 | May 2006–Sept 2008 | Non-Small Cell Lung Cancer | Simvastatin 40 mg (1.33), open label | 8.6Md | Yes | Chemotherapy (GF) | OS, PFS, RR |
| Konings et al. [30] | Netherlands | 1 | 30 | Feb 2005–May 2009 | Gastric adenocarcinoma | Pravastatin 40 mg (1.33), open label | 3.5Mx | Nil | Chemotherapy (E, C + CB) | OS, PFS, RR |
| Kawata et al. [31] | Japan | 1 | 83 | Feb 1990–Jan 1993 | Hepatocellular Carcinoma | Pravastatin 40 mg (1.33), open label | 16.5Me | Nil | TACE +5FU | OS |
5FU 5-Flurouracil, C cisplatin, CB carboplatin, DCR disease control rate, DDD defined daily dose, DX dexamethasone, E epirubicin, ET etoposide, FOLFIRI 5-fluorouracil and irinotecan, GC gemcitabine, GOJ gastro-oesophageal junction, GF gefitanib, OS overall survival, Md median, Me mean, Mx maximum, NA non-adenocarcinomatous, NS not stated, PFS progression-free survival, QoL quality of life, RR response rate, Rad R radiological response, S sorafenib, TACE transcatheter arterial chemoembolisation, TTF time to treatment failure, TTP time to progression, THL thalidomide, X capecitabine, XELIRI regimen capecitabine plus irinotecan
aPrimary cancers were mostly breast and lung cancers (in 88% of patients)
b94% patients received prior chemotherapy
cAll patients received prior chemotherapy
d36.6% in statin group and 45.1% in control group received prior chemotherapy
Patient characteristics
The mean age of recruited participants between trials was between 53 and 68 years (Supplementary Table 2) and was generally well balanced between groups. Of all recruited participants, 64.5% were male. Gender was generally well balanced between groups; however, there were numerical differences (≥ 10%) for three small trials [23, 25, 30]. All but one trial [21] included patients with metastatic disease at enrolment. Eight trials reported exact proportions with metastatic disease [21, 22, 24, 26–29, 31], comprising 2035 recruited participants, of which 65% had metastases (Table 2). Disease staging appeared well balanced between groups. Of nine trials which reported ECOG performance status [21, 22, 24, 26–30], including 2032 patients, 87% were status 0–1, and 13% were status 2–3. ECOG performance status appeared well balanced between groups.
Table 2.
Cancer stage and performance status
| Trial | Cancer type | Stage of Cancer | ECOG 0 or 1 in statin group | ECOG 0 or 1 in control group | ECOG 2 or 3 in statin group | ECOG 2 or 3 in control group | |
|---|---|---|---|---|---|---|---|
| Statin group | Control group | ||||||
| Alexandre et al. [21] | Oesophageal/GOJ adenocarcinoma | Up to stage 3 | 93.8% | 100% | 6.2% | 0% | |
| Jouve et al. [22] | Hepatocellular Carcinoma |
Child-Pugh A Extra-hepatic Metastatic disease: 29.0% |
Child-Pugh A Extra-hepatic metastatic disease: 30.4% |
95.7% | 95% | 4.3% | 5% |
| Lee et al. [23] | Non-adenocarcinomatous Non-small cell lung cancer | Stage 3B/4b | 75% | 75% | 25% | 25% | |
| Seckl et al. [24] | Small cell lung cancer | Limited diseasec: 43.4% | Limited diseasec: 42.7%, | 75.6% | 75.2% | 24.4% | 24.8% |
| Extensive diseasec: 56.6% | Extensive diseasec: 49.5% | ||||||
| El-Hamamsy et al. [25] | Brain metastases (various primariesa) | Stage 4 | NSe | NS | NS | NS | |
| Lim et al. [26] | Colorectal Cancer | Stage 4b | 98.5% | 98.5% | 1.5%g | 1.5%g | |
| Kim et al. [27] | Gastric/GOJ adenocarcinoma | Stage 4b | 99.2% | 96.7% | 0.8% | 3.3% | |
| Hong et al. [28] | Pancreatic cancer | Locally advanced disease: 12% | Locally advanced disease: 12.5% | 100% | 100% | 0% | 0% |
| Metastatic disease: 88% | Metastatic disease: 87.5% | ||||||
| Han et al. [29] | Non-small cell lung cancer | Stage 3bb: 6%, stage 4: 94% | Stage 3bb: 11%, stage 4: 89% | 94% | 89% | 6% | 11% |
| Konings et al. [30] | Gastric adenocarcinoma | ≥ 43% metastatic disease | 86.7% | 100% | 13.3%g | 0% | |
| Kawata et al. [31] | Hepatocellular carcinoma | Stage 2–3d: 73%, stage 4: 27% | Stage 2–3d: 69%, stage 4: 31% | NSf | NS | NS | NS |
ECOG Eastern Cooperative Oncology Group, GOJ gastro-oesophageal, NS not stated
aPrimary cancers were mostly breast and lung cancers (in 88% of patients)
bAmerican Joint Committee on Cancer TNM staging
cVeterans Administration Lung Study Group Staging
dPrimary Liver Cancer Study Group of Japan staging
e36% in the statin group and 28% in the control group had a Karnofsky performance scale score of > = 70%
f83% in the statin group and 86% in the control group had a Karnofsky performance scale score of > 70%
gNo ECOG > 2 patients
f83% in the statin group and 86% in the control group had a Karnofsky performance scale score of > 70%
Mortality outcomes
Two trials investigated the effect of pravastatin 40 mg in patients with advanced hepatocellular carcinoma [22, 31]. Allocation to pravastatin therapy was associated with significantly improved overall survival in one of these studies only [31]: median survival was 18 months in the pravastatin group and 9 months in the control group (HR 0.42, 95% CI 0.20–0.83). Meta-analysis of overall survival with pravastatin in both these trials revealed a HR of 0.69 (95% CI: 0.30–1.61) which was not statistically significant (p = 0.392) (Supplementary Fig. 1). The Cochrane Q test (p = 0.024) and I2 statistic (80.5%) demonstrated a statistically significant degree of heterogeneity (p < 0.10). None of the other included trials demonstrated significant improvements in overall survival with statins, including for small-cell lung cancer, non-small cell lung cancer, oesophageal/GOJ/gastric cancers, colorectal cancer and pancreatic cancer. No improvements in progression-free survival were observed with allocation to statins individually in nine studies (n = 2050) in which this outcome was reported [22–30]. There were no significant differences in overall response rate for the eight studies (n = 1727) reporting this outcome [23–30].
Safety profile
Five trials reported grades 3–5 adverse events. None of these trials demonstrated significant differences in grades 3–5 adverse events between statin and control group (n = 1497) outcome [21, 24, 26, 27, 29] (Supplementary Table 3). Statin-related adverse events (myalgia/myopathy or abnormal alanine aminotransferase/aspartate aminotransferase or elevated creatine phosphokinase) were similar in proportion between groups in all nine studies reporting these outcomes [21–28, 30]. Most trials had small sample sizes and may have been inadequately powered to detect clinically relevant differences in adverse events if they existed (Table 3).
Table 3.
Major study outcomes
| Median OS (months) | Median PFS (months) | Overall response rate (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial | Statin group | Control group | HR (95% CI), p value | Statin group | Control group | HR (95% CI), p value | Statin | Control | p value |
| Alexandre et al. [21] | NS | NS | HR 1.56 (0.14-17.28), p = 0.716 | NS | NS | HR 0.78 (0.11-5.61), p = 0.807 | NS | NS | NS |
| Jouve et al. [22] | 10.7 (7.7–14.3) | 10.5 (8.2–12.4) | 1.00 (0.79–1.28), p = 0.975 | 5.0 (3.4–6.0) | 4.4 (3.3–5.6) | 1.00 (0.80–1.25), p = 0.986 | NS | NS | NS |
| Lee et al. [23] | 10.0 (6.4–13.8) | 7.0 (6.1–7.9) | 1.03 (0.58–1.80), p = 0.466 | 1.0 (0.5–1.4) | 3.6 (3.0–4.1) | 1.38 (0.84–2.29), p = 0.898 | 5.70% | 9.40% | 0.43 |
| Seckl et al. [24] | 10.7 | 10.6 | 1.01 (0.88–1.16), p = 0.90 | 7.7 | 7.3 | 0.98 (0.85–1.13), p = 0.81 | 69% | 69.10% | 0.963c |
| El-Hamamsy et al. [25] | 3.4 (0.69–6.01) | 3 (2.46–3.54) | NS, p = 0.880 | 1.6 (0.68–2.52) | 1.47 (0.91–2.02) | NS, p = 0.392 | 78.6%b | 60%b | 0.427 |
| Lim et al. [26] | 15.3 (12.1–18.5) | 19.2 (16.8–21.6) | NS, p = 0.826a | 5.9 (4.5–7.3) | 7 (5.4–8.6) | 1.03 (0.77–1.37), p = 0.858 | 11.90% | 11.80% | 1 |
| Kim et al. [27] | 11.6 (9.2–13.9) | 11.5 (9.9–13.1) | NS, p = 0.818 | 5.2 (4.3–6.1) | 4.6 (3.5–5.7) | 0.93 (0.68–1.26), p = 0.664 | 27.50% | 29.00% | 0.936 |
| Hong et al. [28] | 6.6 (4.4–8.2) | 8.7 (4.8–12.6) | NS, p = 0.98 | 2.4 (0.7–4.1) | 3.6 (3.1–4.1) | NS, 0.903 | 6.90% | 14.30% | 0.23 |
| Han et al. [29] | 13.6 (7.1–20.1) | 12 (7.8–16.2) | 0.88 (0.57–1.35), p = 0.491 | 3.3 (1.4–5.2) | 1.9 (1.0–2.8) | 0.891 (0.60–1.32), p = 0.549 | 38.50% | 31.50% | 0.666 |
| Konings et al. [30] | 8 (3.02–12.98) | 6 (4.93–7.08) | NS | 6 (3.39–8.61) | 5 (3.83–6.17) | NS | 33.30% | 46.70% | 0.473 |
| Kawata et al. [31] | 18 | 9 | 0.42 (0.20–0.83), p = 0.006 | NS | NS | NS | NS | NS | NS |
NS not stated, OS overall survival, PFS progression-free survival
Figures in parenthesis indicate 95% confidence intervals
ap value calculated using log-rank test
bRadiological response
cp value calculated using chi-squared test
Figures in parenthesis indicate 95% confidence intervals
Risk of bias
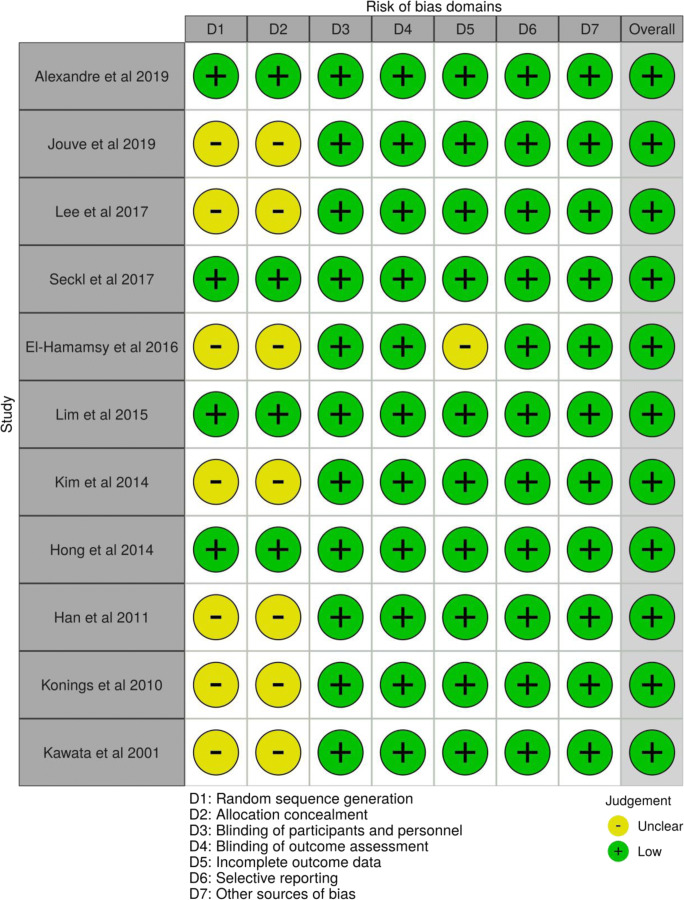
Figure 2 shows the assessment of risk of bias in the included trials as per the Cochrane risk of bias tool, illustrated using the robvis application [36]. Four trials reported random sequence generation and allocation concealment adequately [21, 24, 26, 28], while this was insufficiently reported in the remaining seven. While six trials were open-label studies, any deviations from intended intervention were unlikely to impact on the outcome and therefore were deemed at low risk of performance bias [22, 23, 25, 29–31]. Risk of detection bias for all trials overall was determined to be low given that knowledge of statin allocation (where applicable to open-label studies) would seem unlikely to bias reported outcomes not involving subjective judgement, such as mortality outcomes or measures of treatment response. All trials were deemed to be at low risk of selective reporting.
Fig. 2.
Risk of bias of selected studies using the Cochrane risk of bias tool
Discussion
In summary, this systematic review included eleven trials of statin therapy in 2165 patients with solid tumours in total, including small cell lung cancer (n = 846), non-small cell lung cancer (n = 106 and n = 68), colorectal cancer (n = 269), gastric adenocarcinoma (n = 244 and n = 30), oesophageal adenocarcinoma (n = 32), pancreatic cancer (n = 114), hepatocellular cancer (n = 83 and n = 323) and patients with brain metastases (from mainly breast and lung primaries) (n = 50). Most patients recruited had advanced malignancy and received concomitant palliative chemotherapy. Most patients received 40 mg of simvastatin or pravastatin (1.33 DDD), and typically for short durations (on average fewer than 9 months). Most trials did not demonstrate significant improvements in overall survival (aside from one trial of pravastatin 40 mg in hepatocellular carcinoma [31]), and no trials reported improvements in progression-free survival or overall response rate. Meta-analysis of the two trials involving pravastatin 40 mg in advanced hepatocellular cancer [22, 31] revealed no significant improvements in overall survival (Supplementary Fig. 1). There was no indication in any trial of an increased rate of adverse events in those allocated to statins. Overall, included trials were deemed to be at low risk of bias using the Cochrane risk of bias tool [18].
Comparison with previous work
This is the second systematic review of RCTs to examine both the clinical efficacy and safety profile of statins in patients with solid tumours. The first included a meta-analysis of eight RCTs included in this systematic review [37]. This review provided a brief description of study characteristics and the overwhelming focus was on quantitative synthesis of the effect of statins on OS, PFS, RR and adverse events. In contrast, our review is primarily a qualitative synthesis of included trials and provides more detail regarding important characteristics relating to included studies (country, blinding, duration of statin therapy, DDD) and participants (demography, cancer staging, performance status) to aid interpretation. Another more recent systematic review focused on a meta-analysis of nine of the included RCTs in our review to examine the effect of allocation to statins on OS and PFS [38]. As previously stated, we deliberately did not conduct a meta-analysis of all RCTs given irreconcilable heterogeneity of included studies and uncertainty surrounding the assumption of a uniform treatment effect, with resultant difficulties in interpretation of summary estimates.
The cholesterol treatment trialists’ collaboration individual patient data (IPD) meta-analysis of 22 RCTs of statin vs. control (primary or secondary prevention of cardiovascular disease, n = 134,537) and 5 RCTs of high-dose vs. low-dose statins (secondary prevention, n = 39,612) demonstrated no evidence of reduced incident cancer overall (RR 1.00, 95% CI 0.96–1.04) or related cancer-specific mortality (RR 0.98, 95% CI 0.92–1.05) for those allocated to the active arm [39]. No significant associations for mortality were demonstrated individually for any of the 23 primary sites examined. However, only cancers diagnosed after randomisation were considered (1.4% developed cancer per year after randomisation), and it is not clear how many of these patients were receiving study drug from the point of cancer diagnosis. It is therefore difficult to make inferences of the effect of allocation to statins on mortality outcomes in patients with cancer from this IPD meta-analysis.
Limitations
It is possible that statins do not exert clinically relevant effects in patients with solid tumours; however, other explanations for the divergence of trial evidence from the promising pre-clinical and epidemiological data deserve consideration. Of included studies, only four were phase III studies, and the remaining seven were not powered to detect significant differences in mortality outcomes. Of the phase III studies, three [22, 26, 27] were powered to detect relatively large effect sizes (HR 0.74, HR 0.65, and HR 0.67 respectively) and were at risk of type II error should the actual effect sizes have been more conservative. The largest trial to date in small cell lung cancer (n = 846) was powered to detect a HR of 0.82 [24]. Treatment response to statins could feasibly differ between palliative and adjuvant settings, depending on their primary mechanism of action in individual tumour types (for example, a primary effect on inhibition of metastases as seen in colorectal cancer may favour response in the adjuvant setting [40]) and the influence of baseline tumour burden. All but one trial included patients with metastatic disease at baseline (65% of participants overall where reported); in such patients with poor prognosis in receipt of statins for short durations, precluding a marked cytotoxic effect of statins (which would seem unlikely), it may not be possible to elicit or demonstrate treatment response. Furthermore, it is difficult to generalize these trial findings to the adjuvant setting. Although an effective statin dose has yet to be defined in the setting of cancer therapy, and may differ from the licenced doses prescribed for the prevention of cardiovascular disease, the dose of statins assessed in these trials may have been insufficient. All trials used statins at sub-maximal doses (ten with a DDD of 1.33 and one with a DDD of 2.67); higher doses (e.g. atorvastatin 80 mg—DDD 4) are clinically licenced in cardiovascular prevention [41] and could be investigated in a trial. Stratification of effect sizes according to statin type, dose (as defined by DDD) and intended duration of therapy may have been informative; however, such comparisons would have included trials with different primary sites in each strata, and the resulting estimates and tests for interaction would have been difficult to interpret. It is unclear whether statin use prior to randomisation is an effect modifier for the association between statin allocation and mortality outcomes, as most studies excluded prior/current statin use; and those studies which did not specifically exclude such users did not report the proportion of existing users in the randomised population.
Recommendations
Given the imprecise estimates for efficacy and the limitations of previous trials discussed above, the current trial evidence base does not preclude the conduct of future statin trials in patients with solid malignancies. Further definitive phase III trials are required to determine the efficacy and safety profile of statins in individual tumour types, provided there exists sufficient scientific justification for their conduct: including the proposed mechanism of action applicable to underlying tumour biology and the relevance of the pharmacokinetic properties of the selected statin. High-dose statin therapy should be considered to maximize the probability of observing clinically relevant effects: given the dose-dependent effects of statins in pre-clinical research [42] and trial data for their current licenced indications [41]. Future trials should be adequately powered to detect more conservative effect sizes than previously examined; indeed, relatively small clinically significant differences in primary outcomes may be justifiable given that statins are easily administered, low-cost medications with a favourable safety profile when used for their licenced indications [43]. Investigators should consider the merits of investigating statins in the adjuvant setting, where there is mounting pre-trial evidence [44]. Future trials should ideally collect blood and fresh frozen tissue to permit translational research studies including biomarkers predictive of treatment response.
Conclusions
Overall, the trial evidence is not sufficiently robust to confirm or refute the efficacy and safety of statins in addition to the current standard of care in patients with solid malignant tumours. Most trials were not adequately powered to detect more conservative differences in efficacy outcomes, and statins were administered for short durations at submaximal doses in patients with predominantly advanced malignancy. Based on this evidence, it may be premature to disregard a potential beneficial role of statins in cancer therapy and there is insufficient evidence to preclude the conduct of future trials. The potential role of high-dose statins in adjuvant settings deserves further research.
Electronic supplementary material
(DOCX 13 kb)
(DOCX 16 kb)
(XLSX 11 kb)
(DOCX 970 kb)
Abbreviations
- 5FU
5-Fluorouracil
- CB
Carboplatin
- ALT
Alanine aminotransferase
- AST
Aspartate transaminase
- C
Cisplatin
- DCR
Disease control rate
- DDD
Defined daily dose
- DX
Dexamethasone
- ECOG
Eastern Cooperative Oncology Group
- E
Epirubicin
- ET
Etoposide
- FOLFIRI
5-Fluorouracil and irinotecan
- GC
Gemcitabine
- GOJ
Gastro-oesophageal junction
- GF
Gefitinib
- HMG-CoA
Hydroxy-3-methylglutaryl-CoA
- OS
Overall survival
- Md
Median
- Me
Mean
- Mx
Maximum
- NA
Non-adenocarcinomatous
- NS
Not stated
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PFS
Progression-free survival
- Rad R
Radiological response
- RR
Response rate
- RCT
Randomised controlled trial
- TACE
Transcatheter arterial chemoembolisation
- TTP
Time to progression
- THL
Thalidomide
- X
Capecitabine
- XELIRI regimen
Capecitabine plus irinotecan
Authors’ contributions
JPT: methodology, search strategy, data extraction, and writing original draft. YPL: methodology and editing. LA: conceived the review, methodology, search strategy, data extraction, editing and supervision.
Funding information
JPT is an Academic Clinical Fellow and LA is a Clinical Lecturer, both funded by the National Institute of Health Research (NIHR). The funding source had no input regarding the design, conduct, data collection, data analysis, interpretation, manuscript preparation or publication decision.
Data availability
All data reported in this manuscript are found in the literature as cited in the text.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Disclaimer
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cholesterol Treatment Trialists C, Fulcher J, O’Connell R, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 3.Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. 2016;16:718–731. doi: 10.1038/nrc.2016.76. [DOI] [PubMed] [Google Scholar]
- 4.Al-Haidari AA, Syk I, Thorlacius H. HMG-CoA reductase regulates CCL17-induced colon cancer cell migration via geranylgeranylation and RhoA activation. Biochem Biophys Res Commun. 2014;446:68–72. doi: 10.1016/j.bbrc.2014.02.078. [DOI] [PubMed] [Google Scholar]
- 5.Wang T, Seah S, Loh X, et al. Simvastatin-induced breast cancer cell death and deactivation of PI3K/Akt and MAPK/ERK signalling are reversed by metabolic products of the mevalonate pathway. Oncotarget. 2016;7:2532–2544. doi: 10.18632/oncotarget.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusama T, Mukai M, Iwasaki T, Tatsuta M, Matsumoto Y, Akedo H, Inoue M, Nakamura H. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology. 2002;122:308–317. doi: 10.1053/gast.2002.31093. [DOI] [PubMed] [Google Scholar]
- 7.Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 8.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26:1268–1286. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petitjean A, Achatz M, Borresen-Dale A, et al. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 10.Freed-Pastor WA, Mizuno H, Zhao X, Langerød A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li W, Polotskaia A, Bissell MJ, Osborne TF, Tian B, Lowe SW, Silva JM, Børresen-Dale AL, Levine AJ, Bargonetti J, Prives C. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148:244–258. doi: 10.1016/j.cell.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parrales A, Ranjan A, Iyer SV, Padhye S, Weir SJ, Roy A, Iwakuma T. DNAJA1 controls the fate of misfolded mutant p53 through the mevalonate pathway. Nat Cell Biol. 2016;18:1233–1243. doi: 10.1038/ncb3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi Y, Kashima H, Wu RC, Jung JG, Kuan JC, Gu J, Xuan J, Sokoll L, Visvanathan K, Shih IM, Wang TL. Mevalonate pathway antagonist suppresses formation of serous tubal intraepithelial carcinoma and ovarian carcinoma in mouse models. Clin Cancer Res. 2015;21:4652–4662. doi: 10.1158/1078-0432.CCR-14-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mei Z, Liang M, Li L, Zhang Y, Wang Q, Yang W. Effects of statins on cancer mortality and progression: a systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int J Cancer. 2017;140:1068–1081. doi: 10.1002/ijc.30526. [DOI] [PubMed] [Google Scholar]
- 14.Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- 15.Popat S. Do statins improve survival in small-cell lung cancer? J Clin Oncol. 2017;35:1497–1498. doi: 10.1200/JCO.2016.72.0870. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization WHO International Working Group for Drug Statistics Methodology WHO Collaborating Centre for Drug Statistics Methodology
- 18.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/BMJ.D5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FDA (2007) Guidance for industry clinical trial endpoints for the approval of cancer drugs and biologics. Rockville
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Alexandre L, Clark AB, Walton S et al (2019) Adjuvant statin therapy for oesophageal adenocarcinoma: the STAT-ROC feasibility study. BJS Open. 10.1002/bjs5.50239 [DOI] [PMC free article] [PubMed]
- 22.Jouve JL, Lecomte T, Bouché O, Barbier E, Khemissa Akouz F, Riachi G, Nguyen Khac E, Ollivier-Hourmand I, Debette-Gratien M, Faroux R, Villing AL, Vergniol J, Ramee JF, Bronowicki JP, Seitz JF, Legoux JL, Denis J, Manfredi S, Phelip JM, PRODIGE-11 investigators/collaborators Pravastatin combination with sorafenib does not improve survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71:516–522. doi: 10.1016/j.jhep.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y, Lee KH, Lee GK, Lee SH, Lim KY, Joo J, Go YJ, Lee JS, Han JY. Randomized phase II study of afatinib plus simvastatin versus afatinib alone in previously treated patients with advanced nonadenocarcinomatous non-small cell lung cancer. Cancer Res Treat. 2017;49:1001–1011. doi: 10.4143/crt.2016.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seckl MJ, Ottensmeier CH, Cullen M, Schmid P, Ngai Y, Muthukumar D, Thompson J, Harden S, Middleton G, Fife KM, Crosse B, Taylor P, Nash S, Hackshaw A. Multicenter, phase III, randomized, double-blind, placebo-controlled trial of pravastatin added to first-line standard chemotherapy in small-cell lung cancer (LUNGSTAR) J Clin Oncol. 2017;35:1506–1514. doi: 10.1200/JCO.2016.69.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Hamamsy M, Elwakil H, Saad AS, Shawki MA. A randomized controlled open-label pilot study of simvastatin addition to whole-brain radiation therapy in patients with brain metastases. Oncol Res Featur Preclin Clin Cancer Ther. 2016;24:521–528. doi: 10.3727/096504016X14719078133528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim SH, Kim TW, Hong YS, Han SW, Lee KH, Kang HJ, Hwang IG, Lee JY, Kim HS, Kim ST, Lee J, Park JO, Park SH, Park YS, Lim HY, Jung SH, Kang WK. A randomised, double-blind, placebo-controlled multi-centre phase III trial of XELIRI/FOLFIRI plus simvastatin for patients with metastatic colorectal cancer. Br J Cancer. 2015;113:1421–1426. doi: 10.1038/bjc.2015.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim ST, Kang JH, Lee J, Park SH, Park JO, Park YS, Lim HY, Hwang IG, Lee SC, Park KW, Lee HR, Kang WK. Simvastatin plus capecitabine–cisplatin versus placebo plus capecitabine–cisplatin in patients with previously untreated advanced gastric cancer: a double-blind randomised phase 3 study. Eur J Cancer. 2014;50:2822–2830. doi: 10.1016/j.ejca.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Hong JY, Nam EM, Lee J, Park JO, Lee SC, Song SY, Choi SH, Heo JS, Park SH, Lim HY, Kang WK, Park YS. Randomized double-blinded, placebo-controlled phase II trial of simvastatin and gemcitabine in advanced pancreatic cancer patients. Cancer Chemother Pharmacol. 2014;73:125–130. doi: 10.1007/s00280-013-2328-1. [DOI] [PubMed] [Google Scholar]
- 29.Han J-Y, Lee S-H, Yoo NJ, Hyung LS, Moon YJ, Yun T, Kim HT, Lee JS. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. 2011;17:1553–1560. doi: 10.1158/1078-0432.CCR-10-2525. [DOI] [PubMed] [Google Scholar]
- 30.Konings IRHM, van der Gaast A, van der Wijk LJ, de Jongh FE, Eskens FALM, Sleijfer S. The addition of pravastatin to chemotherapy in advanced gastric carcinoma: a randomised phase II trial. Eur J Cancer. 2010;46:3200–3204. doi: 10.1016/j.ejca.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 31.Kawata S, Yamasaki E, Nagase T, Inui Y, Ito N, Matsuda Y, Inada M, Tamura S, Noda S, Imai Y, Matsuzawa Y. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br J Cancer. 2001;84:886–891. doi: 10.1054/bjoc.2000.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garwood ER, Kumar AS, Baehner FL, Moore DH, Au A, Hylton N, Flowers CI, Garber J, Lesnikoski BA, Hwang ES, Olopade O, Port ER, Campbell M, Esserman LJ. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res Treat. 2010;119:137–144. doi: 10.1007/s10549-009-0507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linden KG, Leachman SA, Zager JS, Jakowatz JG, Viner JL, McLaren CE, Barr RJ, Carpenter PM, Chen WP, Elmets CA, Tangrea JA, Lim SJ, Cochran AJ, Meyskens FL. A randomized, double-blind, placebo-controlled phase II clinical trial of lovastatin for various endpoints of melanoma pathobiology. Cancer Prev Res (Phila) 2014;7:496–504. doi: 10.1158/1940-6207.CAPR-13-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Limburg PJ, Mahoney MR, Ziegler KLA, Sontag SJ, Schoen RE, Benya R, Lawson MJ, Weinberg DS, Stoffel E, Chiorean M, Heigh R, Levine J, Della’Zanna G, Rodriguez L, Richmond E, Gostout C, Mandrekar SJ, Smyrk TC, for the Cancer Prevention Network Randomized phase II trial of sulindac, atorvastatin, and prebiotic dietary fiber for colorectal cancer chemoprevention. Cancer Prev Res (Phila) 2011;4:259–269. doi: 10.1158/1940-6207.CAPR-10-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji Y, Rounds T, Crocker A, Sussman B, Hovey RC, Kingsley F, Muss HB, Garber JE, Wood ME. The effect of atorvastatin on breast cancer biomarkers in high-risk women. Cancer Prev Res (Phila) 2016;9:379–384. doi: 10.1158/1940-6207.CAPR-15-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGuinness LA (2019) Robvis: an R package and web application for visualising risk-of-bias assessments [DOI] [PubMed]
- 37.Jang HC, Kim HS, Kim JH, Lee L. The effect of statin added to systemic anticancer therapy: a meta-analysis of randomized, controlled trials. J Clin Med. 2018;7:E325. doi: 10.3390/jcm7100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farooqi M, Malhotra N, Mukherjee S, et al. Statin therapy in the treatment of active cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2018;13:e0209486. doi: 10.1371/journal.pone.0209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cholesterol Treatment Trialists C. Emberson JR, Kearney PM, et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849. doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juneja M, Kobelt D, Walther W, Voss C, Smith J, Specker E, Neuenschwander M, Gohlke BO, Dahlmann M, Radetzki S, Preissner R, von Kries JP, Schlag PM, Stein U. Statin and rottlerin small-molecule inhibitors restrict colon cancer progression and metastasis via MACC1. PLoS Biol. 2017;15:e2000784. doi: 10.1371/journal.pbio.2000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cholesterol Treatment Trialists C, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogunwobi OO, Beales ILP. Statins inhibit proliferation and induce apoptosis in Barrett’s esophageal adenocarcinoma cells. Am J Gastroenterol. 2008;103:825–837. doi: 10.1111/j.1572-0241.2007.01773.x. [DOI] [PubMed] [Google Scholar]
- 43.Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 44.Ahern TP, Lash TL, Damkier P, Christiansen PM, Cronin-Fenton DP. Statins and breast cancer prognosis: evidence and opportunities. Lancet Oncol. 2014;15:e461–e468. doi: 10.1016/S1470-2045(14)70119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 13 kb)
(DOCX 16 kb)
(XLSX 11 kb)
(DOCX 970 kb)
Data Availability Statement
All data reported in this manuscript are found in the literature as cited in the text.