Abstract
The past half century has seen the development of the field of post-ejaculatory sexual selection, the sequel to sexual selection for mate-acquisition (pre-ejaculatory) described by Darwin. In richness and diversity of adaptations, post-ejaculatory selection rivals that of pre-ejaculatory sexual selection. Anisogamy—and hence two sexes—likely arose by primeval gamete competition, and sperm competition remains a major force maintaining high sperm numbers. The post-ejaculatory equivalent of male–male competition for matings, sperm competition was an intense ancestral form of sexual selection, typically weakening as mobility and internal fertilization developed in many taxa, when some expenditure became diverted into pre-ejaculatory competition. Sperm competition theory has been relatively successful in explaining variation in relative testes size and sperm numbers per ejaculate and is becoming more successful in explaining variation in sperm phenotype. Sperm competition has generated many other male adaptations such as seminal fluid proteins that variously modify female reproduction towards male interests, and copulatory plugs, prolonged copulations and post-ejaculatory guarding behaviour that reduce female remating probability, many of which result in sexual conflict. This short survey of conceptual developments is intended as a broad overview, mainly as a primer for new researchers.
This article is part of the theme issue ‘Fifty years of sperm competition'.
Keywords: sperm competition, anisogamy, sperm allocation, relative testes size, sexual conflict, sexual cascade
1. Historic introduction
‘The primary cause of intra-masculine selection would thus seem to be that females produce much fewer gametes than males. Consequently there is competition between male gametes for the fertilization of the female gametes. And this competition is vastly more intense than that hitherto considered between zygotes.' (Bateman, 1948 [1, pp 364–365])
Bateman's [1] visionary insight appeared 77 years after Darwin's [2] definitive treatise on sexual selection, which extensively discussed competitive male traits for procuring matings (or ejaculations), but made no significant mention of sexual selection continuing after ejaculation [3]. Darwin's account of mixed paternity in a goose brood nevertheless indicated his awareness of competition for fertilizations [4]. Why he did not discuss the consequences of ejaculate competition has been attributed to his focus on female monogamy [5,6], or alternatively to embarrassment at writing on the topic in the prudish Victorian era, especially under proof-reading censorship by his wife and daughter [3,4,7]. Whatever the reason, Darwin focused on competition for matings in mobile taxa such as arthropods and vertebrates, explicitly excluding many invertebrate groups, such as the coelenterates and echinoderms, because they are often sedentary and monomorphic, or hermaphroditic [8,9].
Sperm competition [10] (more precisely, inter-ejaculate competition [11,12]) is the competition between ejaculates from different males for fertilization of a given set of ova. In sexual selection, it is the post-ejaculatory equivalent of Darwinian male–male competition. Haldane ([13], p. 120–124) may have first clearly recognized, in plants, male gamete competition as a force in evolution—not only between gametes from different males but also between gametes from the same male, equivalent to inter- and intra-ejaculate competition in animals [11,14]—and that competition between plants would lead to increased pollen production. Note that in flowering plants, the pollen grain is functionally analogous, but not homologous to an animal sperm. Two haploid sperm nuclei migrate down the pollen tube; one unites with the ovum nucleus to produce a zygote while the other unites with the two polar nuclei of the central cell to produce the triploid endosperm nucleus. In plants, wind pollination has parallels with invertebrate broadcast spawning by sperm casting (where males shed sperm into the sea and the female retains and broods the eggs), while insect pollination has features in common with internal fertilization in animals.
My 1970 sperm competition review [10] was written when ecology and ethology were dominated by group/species selection interpretations, which may be why interest rose only slowly. While acknowledging that the female ‘cannot be regarded as an inert environment in and around which this form of adaptation evolves' [10, p 559], it was male-centred, stressing simultaneous selection on males both to outcompete previously stored sperm and to prevent this happening, later termed respectively ‘offence' and ‘defence' [15]. Some of my early research considered female interests more fully [16], later elaborated in analyses of sexual conflict [17,18].
Robert Smith's first symposium [19] on the topic was a key driver into the 1980s. That male gamete selection by females may be important in sexual selection appears to have its main origins between 1979 and 1983 ([15,19–23]. It was comprehensively defined as a research field by William Eberhard's major work [24] in the 1990s. The two post-ejaculatory equivalents (sperm competition and cryptic female choice) of Darwinian pre-ejaculatory sexual selection (male–male competition and female choice) were now in place.
A theory base grew rapidly in the 1990s [12], focusing mainly on sperm economics and relative testes expenditure [25], accompanied by an explosion in empirical studies. Theoretical predictions have been quite successful in explaining sperm allocation under different information conditions, and testes size variation in relation to sperm competition levels across taxa. Conceptual developments in all aspects of post-ejaculatory sexual selection have rebalanced how we now view many sexual traits (e.g. [3,4,26]), the functional biology of sperm [27] and the evolutionary dynamics of sexual selection [9]. This very brief synopsis aims to serve as an introductory primer to this now vast field (see glossary for terms as used here); several monographs exist [4,19,28–32]. Throughout the text, references primarily refer to theoretical contributions and review articles. Additional references to theoretical papers and a small sample of related empirical investigations are provided in the electronic supplementary material.
2. Gamete (proto-sperm) competition and evolution of two sexes
Males and females are defined by anisogamy, which most likely arose from isogamous ancestors through gamete competition. Theoretical models [33–35] assume ancestral broadcast spawning parents with fixed reproductive resources, so a size–number tradeoff applies for gamete production. When zygote size is sufficiently important, selection favours one mating type (proto-males) producing numerous small gametes, and the other (proto-females), few large gametes, which survive well as zygotes. Drive to reduce proto-sperm size occurs because releasing more sperm increases a proto-male's share of proto-ova fertilized (primordial sperm competition). If zygote size is less important, the evolutionarily stable strategy (ESS) remains at isogamy. Contrastingly, if gamete competition is almost absent, a similar model generates anisogamy through strong gamete limitation (where many ova remain unfused): producing many small gametes increases fusion probability [36,37]. Gamete limitation may increase ovum mass by increasing its target size for collision [38].
These two models (collectively termed ‘gamete dynamics models') have similar assumptions, differing only in two continuous variables found in broadcast spawners: gamete competition and gamete limitation. They are very robust against changes in assumptions. Though both can generate anisogamy, gamete competition is the stronger selective force unless the average number of competing proto-males is less than two. A major force generating anisogamy from ancestral isogamy may have been the evolution of body complexity; this increases the importance of zygote size, an essential condition for the transition from isogamy to anisogamy under both gamete competition and gamete limitation [33,34,37].
Anisogamy is claimed to establish an ancestral divergence in male and female behaviour (‘sex roles'; §9) [1,33,39]; secondary changes can diversify this ancestral generality. Primeval sperm competition between proto-males, through anisogamy and its consequences, underlies the whole of sexual selection.
3. Sperm allocation and relative testes size
Eric Charnov derived ESS allocations to male and female ‘function' in hermaphrodites, explicitly including sperm competition level in 1980 [40]. Models relating ESS sperm allocation to sperm competition level for separate-sex species appeared in 1982 [41]. They assumed a fixed reproductive budget and a tradeoff between a male's expenditure on sperm and on finding/acquiring matings. Since then, many ‘sperm competition game' variants on this basic model predict sperm allocation under different assumptions about a male's information at the time of ejaculation [25]. An interesting recent approach investigates when information transmission between males about sperm competition level is likely to evolve [42]. Most models assume that sperm competition follows the ‘raffle principle' [43]—sperm are analogous to tickets in a fertilization lottery. Sperm size and sperm limitation are often not included. Conditions for a ‘fair raffle' occur when sperm from all ejaculates can compete equally and mix randomly before fertilization, while in ‘loaded raffles', sperm from a given male are more likely to be successful (e.g. last male advantage). In nature, a fair raffle may sometimes be approached, especially in vertebrates where the female tract is vast relative to sperm size. But in many invertebrates, one ejaculate may completely fill female sperm stores; typically previously stored sperm are then displaced, either directly or indirectly. In some insects, sperm mixing appears to occur during volumetric displacement of stored sperm, forming a special type of loaded raffle [44], though the precise mechanism of sperm competition is poorly known in most cases.
The initial models [41] divided sperm competition level into two ranges: (i) where N ejaculates compete (e.g. groups of external fertilizers) and (ii) where sometimes there is no sperm competition and sometimes just one other ejaculate competes (e.g. many internal fertilizers), respectively categorized later as ‘intensity' and ‘risk' models [12]. This division, convenient in view of the vast range of sperm competition levels in nature, persists largely for analytical tractability. More biologically realistic models assume that sperm competition levels follow continuous probability distributions; this, however, reduces their mathematical tractability (e.g. [45]). Caution is required to ensure that model assumptions are met when interpreting experimental results [45,46]. Perhaps ironically, the large sperm allocation theory literature predominantly assumes raffle-based sperm competition, while the most detailed quantitative evidence relates to a quite different mechanism—indirect sperm displacement in yellow dung flies, Scathophaga (= Scatophaga) stercoraria.
Sperm allocation models generate many testable predictions [25]. Within species, sperm allocation should typically increase with assessments of (i) female quality [47] (but see [48]) and (ii) risk (assuming that females determine mating frequency [49], and unless special conditions apply [50,51]), but (iii) generally decline with intensities above N = 2. Meta-analyses confirm that many species obey (i) and (ii), but not that sperm allocation decreases with intensities above N = 2. However, some species clearly show the decrease predicted in (iii); those that do not may experience conditions unsuitable for the prediction to be fulfilled. For instance, it is probably more difficult for sequentially mating internal fertilizers to assess N than group-spawning external fertilizers such as fish. Within populations, males with lower mate-finding costs should allocate fewer sperm, and sperm allocation should vary with male mating tactic [52], but not mating order if this is randomly determined [53]. Variation in reproductive resources need not produce selection for differing ejaculate investment strategies [54] (but see [55]).
Testes expenditure, measured as relative testes size (RTS), generally reflects sperm demand rate. Models predict RTS to reflect sperm competition level, mating rate and possibly sperm limitation, particularly in some marine broadcast spawners. Evidence across many taxa shows that RTS increases as predicted with sperm competition level [56]. Since sperm competition level increases with mating rate per set of eggs [57], claims that RTS reflects mating rate rather than sperm competition must be regarded with caution [58].
Internal fertilization generally reduces sperm competition compared to broadcast spawning. RTS ranges from extremely high (over 40% in some broadcast spawners) to very low (less than 1% in some taxa with internal fertilization) [59] and is often used as a proxy for sperm competition level, and though caution has been urged since sperm output can be increased in various ways (e.g. increased density of seminiferous tissue) other than by testis mass per se [60], it remains a useful approximation when sperm competition level cannot be measured directly. For internal fertilizers, ejaculate allocation is predicted to correlate positively with RTS across sperm competition risk levels, and negatively over intensity levels [59].
4. Sperm phenotype
John Sivinski [15] was possibly the first to ask how sperm competition has shaped sperm phenotype. This topic has since attracted considerable attention, with increasing evidence that sperm morphology [56,61] and functional post-ejaculatory sperm modifications such as sperm capacitation [62] are affected by sperm competition.
The most notable features of most ejaculates are that sperm are very small and very numerous [35,41]. The classic explanation is that having copious sperm increases fertilization efficiency. While sperm limitation is not infrequent in external fertilizers, ejaculates of many mammals can be diluted extensively without significant reduction in fertilization probability. Cohen [63] proposed that vast sperm numbers are necessary to overcome chiasmata errors; this fails to explain their small size, or how ova escape meiotic errors. More sperm means more tickets in the fertilization lottery; sperm competition and a fixed reproductive budget have proven a good explanation in many taxa for tiny sperm size in high numbers [41], but this is only part of the story [61]. Sperm size and morphology vary enormously [15,64]—why?
First, consider sperm size. Even extremely low sperm competition levels should prevent sperm increasing in size to contribute resources to the zygote [41]. What about competitiveness? Sperm speed is typically expected to increase competitiveness [56,65] and much evidence supports this [56]. Particularly in external fertilizers, number times velocity should be the best indicator of competitiveness, though the evidence for this is equivocal [61]. Studies show positive, negative and most commonly, no relationship between sperm size and sperm competition level [61]. Early theory suggested that if size increases competitiveness (e.g. through increased speed or survivorship), under the simplest assumptions, optimal size remains independent of sperm competition level, but if relative competiveness changes with sperm density around the ova as sperm competition increases, sperm size can relate to sperm competition level [11,66]. Further analysis [67] showed that sperm numbers always increase with sperm competition risk, but size may increase or decrease. Increasing risk increases ejaculate expenditure (sperm size times number). In raffle systems such as vertebrates, where female tracts are large relative to sperm size, this is mainly owing to increased sperm numbers. However, in displacement systems such as in insects, increased ejaculate expenditure can be more attributable to sperm size than number. Some evidence for this divergence is found in passerine birds compared with drosophilid flies [68].
Giant sperm occur in certain taxa. Some Drosophila species have remarkably long (but few) sperm, accompanied by coevolutionary increases in female sperm storage organ length [64]. This may have evolved by Fisherian runaway selection involving genetic correlations between sperm length, female preference for long sperm and female mating frequency, and longer sperm increasing indirect benefits to females [69].
In external fertilizers, ova are fertilized continuously after release. If sperm size increases speed (competitiveness) but decreases longevity (reserves are used more quickly), size increases with sperm competition intensity from an optimum maximizing fertilizations at zero competition, to an ESS maximizing speed times number at maximum competition. However, if size increases longevity, sperm size decreases with sperm competition intensity [70]. A premium on speed (size) as sperm competition increases was predicted for external fertilizers, but unsupported by comparative studies on fish. Models for internal fertilizers predict decreasing sperm viability with increased sperm competition: viability benefits decline as female remating rates increase, reducing future fertilization prospects [71].
Other forms of variation include sperm and pollen polymorphisms present in various taxa, where a given parent generates more than one sperm or pollen type [15,64,72]. Their function remains largely uncertain, though suggestions that sperm morphs have roles in sperm competition go back 40 years [15,73]. Non-fertilizing sperm found in the lepidopteran Pieris napi delay female remating by filling the spermathecae (and inducing female unreceptivity) more cheaply than fertilizing sperm. The suggestion that mammalian sperm variants (often seen as abnormalities) may have a ‘kamikazi' function in impeding rival sperm competitiveness [28] has so far failed to find support, though plausible models for non-fertilizing ‘soldier' morphs exist [74]. In plants, different parents may produce different pollen morphs. This can be geographic, or linked to the phenomenon of heterostyly, where two or three flower morphs (with characteristic stigma, anther and pollen forms) coexist in a population. In the latter, the pollen morphs are probably related to achieving disassortative pollination between flower morphs [75].
In several taxa sperm remain associated after release, then may or may not disperse prior to fertilization; aggregation may relate to sperm competition. Wood mouse Apodemus sylvaticus sperm aggregate secondarily (i.e. after ejaculation) using their apical hooks, hundreds of sperm forming ‘trains' that swim faster than single sperm [76]. Most sperm altruistically lose fertilizing capacity (through premature acrosome reaction) on dispersal from trains. There can be an optimal number of sperm per train. In A. sylvaticus, secondary aggregation is probably beneficial since release of the sperm apical hook (necessary for aggregation) within the male could lead to blockage of his excurrent ducts, with resultant infertility.
That sperm and ejaculate traits may be shaped by male–female coevolution is an emerging field [77].
5. Pre- versus post-ejaculatory tradeoffs
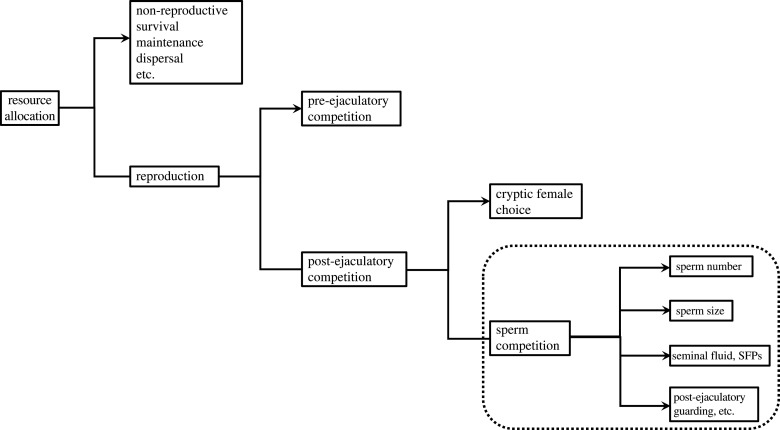
Males have a suite of concatenating expenditures to which they must allocate resources—sperm competition adaptations constitute one of a series of competing allocation branches, and a change in any one expenditure is likely to affect all others (figure 1). However, it is difficult to model such complexity.
Figure 1.
Schema showing investment flow into male non-reproductive, pre- and post-ejaculatory expenditures, emphasizing sperm competition expenditures (within box with dotted outline); see text. Call these male expenditures (arrowheads) , etc. If gain through each investment i follows monotonic diminishing returns, the ideal optimal overall strategy equalizes all marginal gains, i.e. , etc. Hence change in any one expenditure affects expenditure in all others, though the magnitudes of changes depend on the different forms of the curves. Note that is the average expenditure in trait i, which may vary with local information (e.g. sperm number). SFPs, seminal fluid proteins.
Sperm allocation models typically assume a tradeoff between sperm expenditure and expenditure on acquiring matings [25,41], i.e. pre- versus post-ejaculatory traits. While varying sperm competition conditions extensively, most models assume pre-ejaculatory competition by scramble, such as mate-searching. Recent interest has focused on evidence for this tradeoff [78], and how different forms of pre-ejaculatory competition affect sperm allocation [79].
Some studies support this tradeoff and show negative correlations between traits, while others show positive or no correlation between traits and thus no support for a tradeoff between those traits [55,78,80]. Expenditures occur in traits other than reproduction (e.g. growth, dispersal, somatic maintenance), so that pre- and post-ejaculatory expenditures may or may not approximate to direct tradeoff ([78]; figure 1). Even with a direct tradeoff, reproductive resource variation can create positive covariation: individuals (or species) with more resource can expend more on both pre- and post-ejaculatory traits [55]. Negative correlations implying a tradeoff are likely when resources are similar across species and are also possible when species vary in the form of pre-ejaculatory male–male competition [55]. Taxon-specific relative expenditure on weaponry (pre-ejaculatory) versus testes (post-ejaculatory) can switch from positive to negative as the proportion of species showing female monopolization increases within taxa [78,81].
When pre- and post-ejaculatory male traits do trade off, qualitative predictions about sperm and testes allocation are not radically altered whatever the form of pre-ejaculatory male–male competition between scramble and dyadic contest [79]. Recent models of sperm allocation involve male–female interactions, and continue to become more nuanced, involving complex coevolution of female mating frequency or fecundity stimulation and male sperm allocation strategies [82–85]. New models also investigate the case where paternal care, pre- and post-ejaculatory expenditures trade off within a fixed total energy budget [86].
6. Physical defences against future sperm competition
Many insect defensive adaptations were identified in 1970 [10,31]. Mate guarding may or may not involve contact with the female, and can be pre-ejaculatory, to monopolize a female until she becomes receptive, or post-ejaculatory, to prevent or delay further mating with other males. Guarding has mate-searching time costs: whether guarding or searching is favoured depends on timing of fertilization and sperm competition risk if the female is unguarded, and may lead to guarder/non-guarder polymorphisms [87–90]. Post-ejaculatory guarding can be more advantageous than increased sperm allocation [91]. In general, strategic allocation between pre- and post-ejaculatory guarding is complex [92], as is the relationship between non-contact mate-guarding and risk of extra-pair paternity, which depends on male phenotype and female choice [93].
Post-ejaculatory guarding, prolonged copulation [94] and copulatory plugs are alternative defences against female remating. Plugs have evolved independently in many taxa, and are generally male accessory gland products that prevent remating until dislodged by a rival male, or degraded by the female. They may also provide nutrient to the female [95]. In certain spiders and dipterans, plugging occurs by male genitalia being left in the female tract. An evolutionary balance may be expected between plug efficacy and plug failure, since perfect plugs remove selection for plug removal [10,18]. Plug effectiveness should be high under male-biased sex ratios, low number of mating attempts per female, and when males are sperm-limited [96].
7. Seminal fluid proteins (SFPs)
In addition to plug production in some species, SFPs may induce many reproductive female responses that increase male success in sperm competition. These include reduced remating probability, increased ovulation and changes related to sperm transport, use and storage, including action against rival sperm [31,56,95,97–100]. Males may adjust their SFP allocation strategically (following predictions for sperm allocation) in relation to the risk or intensity of sperm competition, or female mating status. SFP manipulation of female reproduction by a prior male is open to exploitation by a subsequent male, hence a possible generator of last male sperm precedence [101].
Models predict that ejaculate composition (sperm versus SFPs) and expenditure depend on how SFPs affect male fitness, and on sperm usage by females [102]. Models of SFPs that act as nuptial gifts to females predict that as sperm competition level increases, the gift fraction decreases and sperm allocation increases [103]. Recent proteomics approaches show that sperm competition can have a pronounced impact on the molecular composition of the male gamete, and that SFP gene expression levels and their rates of evolution are related to sperm competition level.
8. Sperm competition and sexual conflict
Male–male competition over paternity generates many different forms of sexual conflict [18,31,104–106]. Male defensive adaptations such as mate guarding, prolonged copulation and plugs may delay female foraging and/or oviposition, and reduce mate choice, which can have negative consequences for females. They may sometimes also confer female benefits, such as harassment reduction or predation protection. While most females receive sperm and release eggs through the same genital opening, ditrysian Lepidoptera have separate copulatory and oviposition apertures, permitting plugs that do not prevent oviposition; the plug may nevertheless involve sexual conflict [107]. SFPs generate sexual conflict by manipulating female optima towards male optima [31,100,106], hence increasing male fitness at the female's expense, such as increasing her immediate egg production (likely his offspring) but reducing her lifespan (impacting potential future offspring sired by other males).
Sperm competition may also restrict sperm selection by females [31], such as when two males compete by escalating sperm numbers, and the female has a preference for one male's ejaculate. The ESS sperm allocations in the favoured and disfavoured male roles, and the female's ESS sperm selection depend on whether a given male type is favoured by all females, or male roles occur randomly [108].
How sexual conflicts resolve depends on the assumptions and may generate equilibria or continuous dynamics [17,109,110]. Equilibria may lie anywhere between ‘male win' and ‘female win' limits, the balance often relating to interaction, for the sexes, between how costly it is to win and the value of winning [109].
9. Sperm competition dynamics in the sexual cascade
Sequential transitions in sexual strategy through evolutionary time give rise, under appropriate conditions, to selective forces that generate the next transition—the ‘sexual cascade' [9,111]. Sperm competition levels diversified dramatically during the cascade, generating high sperm expenditure in ancestral sedentary metazoans, to later, much lower sperm expenditure in taxa that acquired advanced mobility and internal fertilization [9].
After syngamy evolved in unicells, anisogamy appears to have arisen from isogamy several times, often associated with complexity and multicellularity (§2). Next, the rise of two sexes generated selection for unity sex ratios [112]. Ancestral complex metazoans were probably sedentary broadcast-spawning marine invertebrates, with sexual selection operating after gamete release, by sperm competition and sperm selection, favouring high gamete expenditure, with similarly high gonad masses in both sexes [58].
The next key step in the cascade is the evolution of mobility. Darwin [2, p 274] noted:
‘…in the case of animals having little power of locomotion, the males must trust the fertilizing element [sperm] to the risk of at least a short transit through the waters of the sea. It would, therefore, be a great advantage to such animals, as their organization became perfected, if the males when ready to emit the fertilizing element, were to acquire the habit of approaching the female as closely as possible…'
Darwin had fertilization efficiency in mind; however, female-targeting behaviour by males also confers a major sperm competition advantage, and transitions from broadcast spawning to primitive forms of copulation are apparent in some weakly mobile marine invertebrates [9]. As mobility advanced further, often accompanied by internal fertilization (catalyzed by sperm competition [10]), this set the scene for the rise of Darwinian pre-ejaculatory sexual selection [2] and reduced testes expenditure. Pronounced anisogamy results in large proportions of sperm remaining unfertilized, favouring—under reduced sperm competition arising by copulation—male-biased diversion of gametic expenditure into acquiring mates [9] (for formal validation, see [113]), vindicating Bateman's pioneering statement (§1).
This ancestral diversion of post- into pre-ejaculatory expenditure in mobile animals, one of the final cascade steps [9], generates what have been termed classic ‘sex roles', in which males typically show more competitive adaptations towards gaining matings than females, often resulting in sexual conflict. Deviations from this ancestral mainstream flow certainly occur, but are secondary. Parental care—a secondary adaptation arising many times independently—is female-biased, a trend also likely rooted in anisogamy [114,115]. Though sexual selection can certainly operate in both sexes, it is generally likely to be stronger in males [113–115]. Nevertheless, sex role reversals can occur in species, especially with parental care, and so circumstances present should be fully ascertained before claiming that results are compatible with ancestral sex roles.
While the causal link between anisogamy and ancestral sex roles forecast by Bateman has extensive empirical [116] and theoretical evidence [113], it has nevertheless been vigorously challenged (reviewed in [117]), and gender-biased assumptions about sex roles current in society are also claimed to threaten scientific objectivity [118]. In human societies, assumptions about sex roles have been used to sustain injustice and inequity, which is entirely deplorable. But evidence for ancestral sex roles in the mainstream sexual cascade of metazoan evolution, particularly in species lacking parental care (i.e. most species), is strong. Scientific evidence and logic cannot be bent to suit a political aim, however desirable.
10. Future prospects
-
1.
Although pollen competition and its consequences have received sporadic focus since Mary Willson's pioneering paper [20], sexual selection and sexual conflict have received much less attention in plants than animals, and though much more could be done [119], recent advances look promising, as are studies on analogous processes in basidiomycete fungi.
-
2.
Haldane in 1932 [13] noted that while haploid expression occurs in pollen, early evidence did not support it in sperm. Sivinski [15] urged for study of the causes and consequences of this difference. Views of haploid expression in sperm have been subsequently modified [120]; the consequences for sperm competition are complex [121] but pose interesting future prospects.
-
3.
Much remains to be discovered about causes of sperm variation [61,64,66] and sperm polymorphisms. Despite theoretical and empirical efforts, how sperm competition affects sperm speed, and how increased sperm size affects sperm velocity, remains controversial [56,65,66,122,123] and in need of general principles.
-
4.
Incorporation of paternal care into sperm competition games has recently generated predictions that offer good subjects for testing [86].
-
5.
Female effects need further investigation. For instance, new work indicates how chemical substances released by eggs and female reproductive fluid affect sperm and selection on sperm traits [124].
-
6.
Pre- versus post-ejaculatory tradeoffs, and potential tradeoffs within ejaculates (sperm size, number, SFPs, etc.) would repay further study.
-
7.
In most species, the exact mechanism by which sperm compete in the female tract is poorly understood and would inform future modelling, which often relies on the raffle principle.
-
8.
The role of selfish genetic elements (replicating mobile elements, segregation distorters, maternally inherited endosymbionts) in sperm competition and sexual conflict is emerging as a field in sexual selection [125] and offers exciting new prospects.
Supplementary Material
Acknowledgements
I am deeply indebted to many colleagues over many years, beginning with Bob and Jill Smith for the Tucson 1980 sperm competition symposium, Tim Birkhead and Harry Moore for long-term running of Biology of Spermatozoa conferences, and—particularly—to my wonderful 1990s sperm competition group, all of whom, and many more, have done so much to develop this fascinating subject. I am extremely grateful to three very constructive reviewers.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
Glossary
| ancestral sex roles | Behavioural differences between males and females arising soon after the evolution of mobility, originally defined by [2] and [1] in terms of higher male competitiveness for matings as a result of sexual selection, and now more often described as a higher optimal mating rate for males than females. |
| anisogamy | Occurrence within a species of gametes of two different sizes, resulting in two sexes, males (producing smaller gametes) and females (producing larger gametes). |
| isogamy | Occurrence within a species of gametes of one size; typically fusion occurs between gametes of different mating types. |
| post-ejaculatory traits | Traits evolved by post-ejaculatory (sometimes termed post-copulatory) sexual selection that increase the probability of fertilization. |
| pre-ejaculatory traits | Traits evolved by pre-ejaculatory (sometimes termed pre-copulatory) sexual selection that increase the probability of successful ejaculation (or copulation). |
| raffle principle | Sperm competition mechanism in which sperm from different ejaculates compete for fertilizations numerically, as in a lottery. |
| fair raffle | A given male's fertilization probability is proportional to his number of sperm ejaculated as a proportion of the total sperm from all competing ejaculates. |
| loaded raffle | A given male's fertilization probability is disproportional to his number of sperm ejaculated as a proportion of the total sperm from all competing ejaculates, usually modelled by a loading factor, r, applied to each of his sperm's competitive success. |
| relative testes size | Typically expressed as total testes mass as a proportion of of total body mass; equivalent to male gonadosomatic index as used in marine and fish biology. |
| sexual cascade | The sequence of evolutionary transitions in sexual strategy through time: under appropriate conditions each transition gives rise to the selective forces that generate the next, though some taxa remain ‘frozen’ at a given stage without further change. |
| sperm competition games | Models that seek ESS sperm allocations under various sperm competition conditions. |
| risk models | Sperm competition games where sperm competition is relatively infrequent; e.g. females mate twice with probability q and once with probability (1 – q). |
| intensity models | Sperm competition games where sperm competition is frequent; e.g. N > 1 ejaculates compete simultaneously. |
| sperm (inter-ejaculate) competition | Competition between the ejaculates of two or more males over the fertilization of a given set of ova. This differs from intra-ejaculate competition, i.e. competition between sperm within the same ejaculate over the fertilization of a given set of ova.. |
| sperm displacement | Sperm competition mechanism in which sperm from the last male to mate displaces, either directly or indirectly, sperm from previous males stored in the female's sperm stores. Indirect sperm displacement typically involves action by the female. |
| sperm limitation | Cases where sperm density is insufficient to fertilize all available ova. High sperm limitation refers to cases where few of the available ova are fertilized. |
| syngamy | Fusion of two compatible haploid gametes to form a diploid zygote. |
References
- 1.Bateman AJ. 1948. Intra-sexual selection in Drosophila. Heredity 2, 349–368. ( 10.1038/hdy.1948.21) [DOI] [PubMed] [Google Scholar]
- 2.Darwin CR. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 3.Birkhead TR. 2010. How stupid not to have thought of that: post-copulatory sexual selection. J. Zool. 281, 78–93. ( 10.1111/j.1469-7998.2010.00701.x) [DOI] [Google Scholar]
- 4.Birkhead TR. 2000. Promiscuity. London, UK: Faber and Faber. [Google Scholar]
- 5.Smith RL. 1984. Preface. In Sperm competition and the evolution of animal mating systems (ed. Smith R. L.), pp. xvii–xxix London, UK: Academic Press. [Google Scholar]
- 6.Smith RL. 1998. Foreword. In Sperm competition and sexual selection (eds Birkhead T. R., Moller A. P.), pp. xv–xxiii London, UK: Academic Press. [Google Scholar]
- 7.Birkhead TR. 1997. Darwin on sex. Biologist 44, 397–399. [Google Scholar]
- 8.Evans JP, Sherman CDH. 2013. Sexual selection and the evolution of egg–sperm interactions in broadcast-spawning invertebrates. Biol. Bull. 224, 166–183. ( 10.1086/BBLv224n3p166) [DOI] [PubMed] [Google Scholar]
- 9.Parker GA. 2014. The sexual cascade and the rise of pre-ejaculatory (Darwinian) sexual selection, sex roles, and sexual conflict. Cold Spring Harb. Perspect. Biol. 6, a017509 ( 10.1101/cshperspect.a017509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker GA. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567. ( 10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 11.Parker GA. 1993. Sperm competition games: sperm size and sperm number under adult control. Proc. R. Soc. Lond. B 253, 245–254. ( 10.1098/rspb.1993.0110) [DOI] [PubMed] [Google Scholar]
- 12.Parker GA. 1998. Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection (eds Birkhead T. R., Møller A. P.), pp. 3–90. London, UK: Academic Press. [Google Scholar]
- 13.Haldane JS. 1932. The causes of evolution. London, UK: Longmans, Green and Co. [Google Scholar]
- 14.Parker GA, Begon ME. 1993. Sperm competition games: sperm size and number under gametic control. Proc. R. Soc. B 253, 255–262. ( 10.1098/rspb.1993.0111) [DOI] [PubMed] [Google Scholar]
- 15.Sivinski JL. 1980. Sexual selection and insect sperm. Fla Entomol. 63, 99–111. ( 10.2307/3494659) [DOI] [Google Scholar]
- 16.Parker GA. 1970. The reproductive behaviour and the nature of sexual selection in Scatophaga stercoraria L. (Diptera: Scatophagidae) V. The females's behaviour at the oviposition site. Behaviour 37, 140–168. ( 10.1163/156853970X00277) [DOI] [Google Scholar]
- 17.Parker GA. 1979. Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects (eds Blum M. S., Blum N. A.), pp. 123–166. New York, NY: Academic Press. [Google Scholar]
- 18.Parker GA. 1984. Sperm competition and the evolution of animal mating strategies. In Sperm competition and the evolution of animal mating systems (ed. Smith R. L.), pp. 1–60. Orlando, FL: Academic Press. [Google Scholar]
- 19.Smith RL. 1984. Sperm competition and the evolution of animal mating systems. New York, NY: Academic Press. [Google Scholar]
- 20.Willson MF. 1979. Sexual selection in plants. Am. Nat. 113, 777–790. ( 10.1086/283437) [DOI] [Google Scholar]
- 21.Lloyd JE. 1979. Mating behavior and natural selection. Fla Entomol. 62, 17–34. ( 10.2307/3494039) [DOI] [Google Scholar]
- 22.Walker WF. 1980. Sperm utilization strategies in non-social insects. Am. Nat. 115, 780–799. ( 10.1086/283600) [DOI] [Google Scholar]
- 23.Thornhill R. 1983. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. Am. Nat. 122, 765–788. ( 10.1086/284170) [DOI] [Google Scholar]
- 24.Eberhard WG. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 25.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934. ( 10.1111/j.1469-185X.2010.00140.x) [DOI] [PubMed] [Google Scholar]
- 26.Pitnick S, Hosken DJ. 2010. Postcopulatory sexual selection. In Evolutionary behavioral ecology (eds Westneat D. F., Fox C. W.), pp. 379–399. Oxford, UK: Oxford University Press. [Google Scholar]
- 27.Birkhead TR, Hosken DJ, Pitnick S. 2009. Sperm biology: an evolutionary perspective. London, UK: Academic Press. [Google Scholar]
- 28.Baker RR, Bellis MA. 1995. Human sperm competition: copulation, masturbation and infidelity. London, UK: Chapman and Hall. [Google Scholar]
- 29.Birkhead TR, Møller AP. 1992. Sperm competition in birds: evolutionary causes and consequences. London, UK: Academic Press. [Google Scholar]
- 30.Birkhead TR, Møller AP. 1998. Sperm compeititon and sexual selection. London, UK: Academic Press. [Google Scholar]
- 31.Simmons LW. 2001. Sperm competition and its evolutionary consequences in the insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 32.Shackelford TK, Pound N. 2006. Sperm competition in humans: classic and contemporary readings. New York, NY: Springer. [Google Scholar]
- 33.Parker GA, Baker RR, Smith VGF. 1972. The origin and evolution of gamete dimorphism and the male–female phenomenon. J. Theor. Biol. 36, 529–553. ( 10.1016/0022-5193(72)90007-0) [DOI] [PubMed] [Google Scholar]
- 34.Parker GA. 2011. The origin and maintenance of two sexes (anisogamy), and their gamete sizes by gamete competition. In The evolution of anisogamy: a fundamental phenomenon underlying sexual selection (eds Togashi T., Cox P. A.), pp. 17–74. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 35.Lessells CM, Snook RR, Hosken DJ. 2009. 2 - The evolutionary origin and maintenance of sperm: selection for a small, motile gamete mating type. In Sperm biology (eds Birkhead TR, Hosken DJ, Pitnick S), pp. 43–67. London, UK: Academic Press. [Google Scholar]
- 36.Iyer P, Roughgarden J. 2008. Gametic conflict versus contact in the evolution of anisogamy. Theor. Popul. Biol. 73, 461–472. ( 10.1016/j.tpb.2008.02.002) [DOI] [PubMed] [Google Scholar]
- 37.Lehtonen J, Kokko H. 2011. Two roads to two sexes: unifying gamete competition and gamete limitation in a single model of anisogamy evolution. Behav. Ecol. Sociobiol. 65, 445–459. ( 10.1007/s00265-010-1116-8) [DOI] [Google Scholar]
- 38.Levitan DR. 1996. Effects of gamete traits on fertilization in the sea and the evolution of sexual dimorphism. Nature 382, 153–155. ( 10.1038/382153a0) [DOI] [Google Scholar]
- 39.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man, 1871–1971 (ed. Campbell B.), pp. 136–179. Chicago, IL: Aldine. [Google Scholar]
- 40.Charnov EL. 1980. Sex allocation and local mate competition in barnacles. Mar. Biol. Lett. 1, 269–272. [Google Scholar]
- 41.Parker GA. 1982. Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J. Theor. Biol. 96, 281–294. ( 10.1016/0022-5193(82)90225-9) [DOI] [PubMed] [Google Scholar]
- 42.Engqvist L, Taborsky M. 2017. The evolution of strategic male mating effort in an information transfer framework. J. Evol. Biol. 30, 1143–1152. ( 10.1111/jeb.13083) [DOI] [PubMed] [Google Scholar]
- 43.Parker GA, Simmons LW, Kirk H. 1990. Analysing sperm competition data: simple models for predicting mechanisms. Behav. Ecol. Sociobiol. 27, 55–65. ( 10.1007/BF00183314) [DOI] [Google Scholar]
- 44.Simmons LW, Parker GA, Stockley P. 1999. Sperm displacement in the yellow dung fly, Scatophaga stercoraria: an investigation of male and female processes. Am. Nat. 153, 302–314. ( 10.1086/303171) [DOI] [PubMed] [Google Scholar]
- 45.Turnell BR, Shaw KL, Reeve HK. 2018. Modeling strategic sperm allocation: tailoring the predictions to the species. Evolution 72, 414–425. ( 10.1111/evo.13423) [DOI] [PubMed] [Google Scholar]
- 46.Engqvist L, Reinhold K. 2005. Pitfalls in experiments testing predictions from sperm competition theory. J. Evol. Biol. 18, 116–123. ( 10.1111/j.1420-9101.2004.00792.x) [DOI] [PubMed] [Google Scholar]
- 47.Reinhold K, Kurtz J, Engqvist L. 2002. Cryptic male choice: sperm allocation strategies when female quality varies. J. Evol. Biol. 15, 201–209. ( 10.1046/j.1420-9101.2002.00390.x) [DOI] [Google Scholar]
- 48.Galvani A, Johnstone R. 1998. Sperm allocation in an uncertain world. Behav. Ecol. Sociobiol. 44, 161–168. ( 10.1007/s002650050528) [DOI] [Google Scholar]
- 49.Fromhage L, McNamara JM, Houston AI. 2008. Sperm allocation strategies and female resistance: a unifying perspective. Am. Nat. 172, 25–33. ( 10.1086/587806) [DOI] [PubMed] [Google Scholar]
- 50.Ball MA, Parker GA. 2007. Sperm competition games: the risk model can generate higher sperm allocation to virgin females. J. Evol. Biol. 20, 767–779. ( 10.1111/j.1420-9101.2006.01247.x) [DOI] [PubMed] [Google Scholar]
- 51.Engqvist L, Reinhold K. 2006. Theoretical influence of female mating status and remating propensity on male sperm allocation patterns. J. Evol. Biol. 19, 1448–1458. ( 10.1111/j.1420-9101.2006.01134.x) [DOI] [PubMed] [Google Scholar]
- 52.Parker GA. 1990. Sperm competition games: sneaks and extra-pair copulations. Proc. R. Soc. Lond. B 242, 127–133. ( 10.1098/rspb.1990.0115) [DOI] [Google Scholar]
- 53.Parker GA. 1990. Sperm competition games: raffles and roles. Proc. R. Soc. Lond. B 242, 120–126. ( 10.1098/rspb.1990.0114) [DOI] [Google Scholar]
- 54.Tazzyman SJ, Pizzari T, Seymour RM, Pomiankowski A. 2009. The evolution of continuous variation in ejaculate expenditure strategy. Am. Nat. 174, E71–E82. ( 10.1086/603612) [DOI] [PubMed] [Google Scholar]
- 55.Supriya K, Price TD, Rowe M. 2018. Resource variation generates positive correlations between pre- and postcopulatory sexual traits. Behav. Ecol. 30, 341–347. ( 10.1093/beheco/ary170) [DOI] [Google Scholar]
- 56.Simmons LW, Fitzpatrick JL. 2012. Sperm wars and the evolution of male fertility. Reproduction 144, 519–534. ( 10.1530/REP-12-0285) [DOI] [PubMed] [Google Scholar]
- 57.Parker GA, Ball MA. 2005. Sperm competition, mating rate and the evolution of testis and ejaculate sizes: a population model. Biol. Lett. 1, 235–238. ( 10.1098/rsbl.2004.0273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parker GA, Ramm SA, Lehtonen J, Henshaw JM. 2018. The evolution of gonad expenditure and gonadosomatic index (GSI) in male and female broadcast-spawning invertebrates. Biol. Rev. Camb. Philos. Soc. 93, 693–753. ( 10.1111/brv.12363) [DOI] [PubMed] [Google Scholar]
- 59.Parker GA. 2016. The evolution of expenditure on testes. J. Zool. 298, 3–19. ( 10.1111/jzo.12297) [DOI] [Google Scholar]
- 60.Ramm SA, Schärer L. 2014. The evolutionary ecology of testicular function: size isn't everything. Biol. Rev. Camb. Philos. Soc. 89, 874–888. ( 10.1111/brv.12084) [DOI] [PubMed] [Google Scholar]
- 61.Snook RR. 2005. Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 20, 46–53. ( 10.1016/j.tree.2004.10.011) [DOI] [PubMed] [Google Scholar]
- 62.Pitnick S, Wolfner MF, Dorus S. 2020. Post-ejaculatory modifications to sperm (PEMS). Biol. Rev. 95, 365–392. ( 10.1111/brv.12569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohen J. 1969. Why so many sperms? An essay on the arithmetic of reproduction. Sci. Prog. 57, 23–41. [PubMed] [Google Scholar]
- 64.Pitnick S, Hosken DJ, Birkhead TR. 2009. 3 Sperm morphological diversity. In Sperm biology (eds Birkhead T. R., Hosken D. J., Pitnick S.), pp. 69–149. London, UK: Academic Press. [Google Scholar]
- 65.Fitzpatrick JL, Lüpold S. 2014. Sexual selection and the evolution of sperm quality. Mol. Hum. Reprod. 20, 1180–1189. ( 10.1093/molehr/gau067) [DOI] [PubMed] [Google Scholar]
- 66.Pizzari T, Parker GA. 2009. 6 - Sperm competition and sperm phenotype. In Sperm biology (eds Birkhead T. R., Hosken D. J., Pitnick S.), pp. 207–245. London, UK: Academic Press. [Google Scholar]
- 67.Parker GA, Immler S, Pitnick S, Birkhead TR. 2010. Sperm competition games: sperm size (mass) and number under raffle and displacement, and the evolution of P2. J. Theor. Biol. 264, 1003–1023. ( 10.1016/j.jtbi.2010.03.003) [DOI] [PubMed] [Google Scholar]
- 68.Immler S, Pitnick S, Parker GA, Durrant KL, Lupold S, Calhim S, Birkhead TR. 2011. Resolving variation in the reproductive tradeoff between sperm size and number. Proc. Natl Acad. Sci. USA 108, 5325–5330. ( 10.1073/pnas.1009059108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lüpold S, Manier MK, Puniamoorthy N, Schoff C, Starmer WT, Luepold SHB, Belote JM, Pitnick S. 2016. How sexual selection can drive the evolution of costly sperm ornamentation. Nature 533, 535–538. ( 10.1038/nature18005) [DOI] [PubMed] [Google Scholar]
- 70.Ball MA, Parker GA. 1996. Sperm competition games: external fertilization and ‘adaptive’ infertility. J. Theor. Biol. 180, 141–150. ( 10.1006/jtbi.1996.0090) [DOI] [PubMed] [Google Scholar]
- 71.Engqvist L. 2012. Evolutionary modeling predicts a decrease in postcopulatory sperm viability as a response to increasing levels of sperm competition. Am. Nat. 179, 667–677. ( 10.1086/665000) [DOI] [PubMed] [Google Scholar]
- 72.Till-Bottraud I, Joly D, Lachaise D, Snook RR. 2005. Pollen and sperm heteromorphism: convergence across kingdoms? J. Evol. Biol. 18, 1–18. ( 10.1111/j.1420-9101.2004.00789.x) [DOI] [PubMed] [Google Scholar]
- 73.Silberglied RE, Shepherd JG, Dickinson JL. 1984. Eunuchs: the role of apyrene sperm in Lepidoptera? Am. Nat. 123, 255–265. ( 10.1086/284200) [DOI] [Google Scholar]
- 74.Kura T, Nakashima Y. 2000. Conditions for the evolution of soldier sperm classes. Evolution 54, 72–80. ( 10.1111/j.0014-3820.2000.tb00009.x) [DOI] [PubMed] [Google Scholar]
- 75.Costa J, Castro S, Loureiro J, Barrett SCH. 2017. Experimental insights on the function of ancillary pollen and stigma polymorphisms in plants with heteromorphic incompatibility. Evolution 71, 121–134. ( 10.1111/evo.13082) [DOI] [PubMed] [Google Scholar]
- 76.Moore H, Dvoráková K, Jenkins N, Breed W. 2002. Exceptional sperm cooperation in the wood mouse. Nature 418, 174–177. ( 10.1038/nature00832) [DOI] [PubMed] [Google Scholar]
- 77.Pitnick S, Wolfner MF, Suarez SS. 2009. 7 - Ejaculate–female and sperm–female interactions. In Sperm biology (eds Birkhead TR, Hosken DJ, Pitnick S), pp. 247–304. London, UK: Academic Press. [Google Scholar]
- 78.Simmons LW, Lüpold S, Fitzpatrick JL. 2017. Evolutionary trade-off between secondary sexual traits and ejaculates. Trends Ecol. Evol. 32, 964–976. ( 10.1016/j.tree.2017.09.011) [DOI] [PubMed] [Google Scholar]
- 79.Parker GA, Lessells CM, Simmons LW. 2013. Sperm competition games: a general model for precopulatory male–male competition. Evolution 67, 95–109. ( 10.1111/j.1558-5646.2012.01741.x) [DOI] [PubMed] [Google Scholar]
- 80.Mautz BS, Møller AP, Jennions MD. 2013. Do male secondary sexual characters signal ejaculate quality? A meta-analysis. Biol. Rev. 88, 669–682. ( 10.1111/brv.12022) [DOI] [PubMed] [Google Scholar]
- 81.Lüpold S, Tomkins JL, Simmons LW, Fitzpatrick JL. 2014. Female monopolization mediates the relationship between pre- and postcopulatory sexual traits. Nat. Commun. 5, 3184 ( 10.1038/ncomms4184) [DOI] [PubMed] [Google Scholar]
- 82.Alonzo SH, Pizzari T. 2010. Male fecundity stimulation: conflict and cooperation within and between the sexes: model analyses and coevolutionary dynamics. Am. Nat. 175, 174–185. ( 10.1086/649596) [DOI] [PubMed] [Google Scholar]
- 83.Alonzo SH, Pizzari T. 2013. Selection on female remating interval is influenced by male sperm competition strategies and ejaculate characteristics. Phil. Trans. R. Soc. B 368, 20120044 ( 10.1098/rstb.2012.0044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abe J, Kamimura Y. 2015. Sperm economy between female mating frequency and male ejaculate allocation. Am. Nat. 185, 406–416. ( 10.1086/679586) [DOI] [PubMed] [Google Scholar]
- 85.Bocedi G, Reid JM. 2016. Coevolutionary feedbacks between female mating interval and male allocation to competing sperm traits can drive evolution of costly polyandry. Am. Nat. 187, 334–350. ( 10.1086/684746) [DOI] [PubMed] [Google Scholar]
- 86.Requena GS, Alonzo SH. 2017. Sperm competition games when males invest in paternal care. Proc. R. Soc. B 284, 20171266 ( 10.1098/rspb.2017.1266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parker GA. 1974. Courtship persistence and female-guarding as male time investment strategies. Behaviour 48, 157–184. () [DOI] [Google Scholar]
- 88.Yamamura N. 1986. An evolutionarily stable strategy (ESS) model of postcopulatory guarding in insects. Theor. Popul. Biol. 29, 438–455. ( 10.1016/0040-5809(86)90018-3) [DOI] [Google Scholar]
- 89.Yamamura N, Tsuji N. 1989. Postcopulatory guarding strategy in a finite mating period. Theor. Popul. Biol. 35, 36–50. ( 10.1016/0040-5809(89)90009-9) [DOI] [Google Scholar]
- 90.Fryer T, Cannings C, Vickers GT. 1999. Sperm Competition. II—Post-copulatory guarding. J. Theor. Biol. 197, 343–360. ( 10.1006/jtbi.1998.0879) [DOI] [PubMed] [Google Scholar]
- 91.Alonzo SH, Warner RR. 2000. Allocation to mate guarding or increased sperm production in a Mediterranean wrasse. Am. Nat. 156, 266–275. ( 10.1086/303391) [DOI] [PubMed] [Google Scholar]
- 92.Harts AMF, Kokko H. 2013. Understanding promiscuity: when is seeking additional mates better than guarding an already found one? Evolution 67, 2838–2848. ( 10.1111/evo.12163) [DOI] [PubMed] [Google Scholar]
- 93.Kokko H, Morrell LJ. 2005. Mate guarding, male attractiveness, and paternity under social monogamy. Behav. Ecol. 16, 724–731. ( 10.1093/beheco/ari050) [DOI] [Google Scholar]
- 94.Linn CD, Molina Y, Difatta J, Christenson TE. 2007. The adaptive advantage of prolonged mating: a test of alternative hypotheses. Anim. Behav. 74, 481–485. ( 10.1016/j.anbehav.2007.02.004) [DOI] [Google Scholar]
- 95.Poiani A. 2006. Complexity of seminal fluid: a review. Behav. Ecol. Sociobiol. 60, 289–310. ( 10.1007/s00265-006-0178-0) [DOI] [Google Scholar]
- 96.Fromhage L. 2012. Mating unplugged: a model for the evolution of mating plug (dis-)placement. Evolution 66, 31–39. ( 10.1111/j.1558-5646.2011.01406.x) [DOI] [PubMed] [Google Scholar]
- 97.Chapman T. 2001. Seminal fluid-mediated fitness traits in Drosophila. Heredity 87, 511–521. ( 10.1046/j.1365-2540.2001.00961.x) [DOI] [PubMed] [Google Scholar]
- 98.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. 2011. Insect seminal fluid proteins: identification and function. Annu. Rev. Entomol. 56, 21–40. ( 10.1146/annurev-ento-120709-144823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Perry JC, Sirot L, Wigby S. 2013. The seminal symphony: how to compose an ejaculate. Trends Ecol. Evol. 28, 414–422. ( 10.1016/j.tree.2013.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sirot LK, Wong A, Chapman T, Wolfner MF. 2015. Sexual conflict and seminal fluid proteins: a dynamic landscape of sexual interactions. Cold Spring Harb. Perspect. Biol. 7, a017533 ( 10.1101/cshperspect.a017533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hodgson DJ, Hosken DJ. 2006. Sperm competition promotes the exploitation of rival ejaculates. J. Theor. Biol. 243, 230–234. ( 10.1016/j.jtbi.2006.06.024) [DOI] [PubMed] [Google Scholar]
- 102.Cameron E, Day T, Rowe L. 2007. Sperm competition and the evolution of ejaculate composition. Am. Nat. 169, E158–E172. ( 10.1086/516718) [DOI] [PubMed] [Google Scholar]
- 103.Kura T, Yoda K. 2001. Can voluntary nutritional gifts in seminal flow evolve? J. Ethol. 19, 9–15. ( 10.1007/s101640170011) [DOI] [Google Scholar]
- 104.Rice WR, Holland B. 1997. The enemies within: intergenomic conflict, interlocus contest evolution (ICE), and the intraspecific Red Queen. Behav. Ecol. Sociobiol. 41, 1–10. ( 10.1007/s002650050357) [DOI] [Google Scholar]
- 105.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press. [Google Scholar]
- 106.Edward DA, Stockley P, Hosken DJ. 2014. Sexual conflict and sperm competition. Cold Spring Harb. Perspect. Biol. 7, a017707 ( 10.1101/cshperspect.a017707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carvalho APS, Orr AG, Kawahara AY. 2017. A review of the occurrence and diversity of the sphragis in butterflies (Lepidoptera, Papilionoidea). Zookeys 694, 41–70. ( 10.3897/zookeys.694.13097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ball MA, Parker GA. 2003. Sperm competition games: sperm selection by females. J. Theor. Biol. 224, 27–42. ( 10.1016/S0022-5193(03)00118-8) [DOI] [PubMed] [Google Scholar]
- 109.Parker GA. 2006. Sexual conflict over mating and fertilization: an overview. Phil. Trans. R. Soc. B 361, 235–259. ( 10.1098/rstb.2005.1785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gavrilets S. 2000. Rapid evolution of reproductive barriers driven by sexual conflict. Nature 403, 886–889. ( 10.1038/35002564) [DOI] [PubMed] [Google Scholar]
- 111.Parker GA, Pizzari T. 2015. Sexual selection: the logical imperative. In Current perspectives on sexual selection (ed. Hoquet T.), pp. 119–163. Berlin, Germany: Springer. [Google Scholar]
- 112.Edwards AWF. 2000. Carl Düsing (1884) on The regulation of the sex-ratio. Theor. Popul. Biol. 58, 255–257. ( 10.1006/tpbi.2000.1482) [DOI] [PubMed] [Google Scholar]
- 113.Lehtonen J, Parker GA, Scharer L. 2016. Why anisogamy drives ancestral sex roles. Evolution 70, 1129–1135. ( 10.1111/evo.12926) [DOI] [PubMed] [Google Scholar]
- 114.Queller DC. 1997. Why do females care more than males? Phil. Trans. R. Soc. Lond. B 264, 1555–1557. ( 10.1098/rspb.1997.0216) [DOI] [Google Scholar]
- 115.Fromhage L, Jennions MD. 2016. Coevolution of parental investment and sexually selected traits drives sex-role divergence. Nat. Commun. 7, 12517 ( 10.1038/ncomms12517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Janicke T, Häderer IK, Lajeunesse MJ, Anthes N. 2016. Darwinian sex roles confirmed across the animal kingdom. Sci. Adv. 2, e1500983 ( 10.1126/sciadv.1500983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parker GA, Birkhead TR. 2013. Polyandry: the history of a revolution. Phil. Trans. R. Soc. B 368, 20120335 ( 10.1098/rstb.2012.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ahnesjö I, Brealey JC, Günter KP, Martinossi-Allibert I, Morinay J, Siljestam M, Stångberg J, Vasconcelos P. 2020. Considering gender-biased assumptions in evolutionary biology. Evol. Biol. 47, 1–5. ( 10.1007/s11692-020-09492-z) [DOI] [Google Scholar]
- 119.Lankinen Å, Karlsson Green K. 2015. Using theories of sexual selection and sexual conflict to improve our understanding of plant ecology and evolution. AoB PLANTS 7, plv008 ( 10.1093/aobpla/plv008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Immler S. 2019. Haploid selection in ‘Diploid’ organisms. Ann. Rev. Ecol. Evol. Syst. 50, 219–236. ( 10.1146/annurev-ecolsys-110218-024709) [DOI] [Google Scholar]
- 121.Immler S. 2008. Sperm competition and sperm cooperation: the potential role of diploid and haploid expression. Reproduction 135, 275–283. ( 10.1530/rep-07-0482) [DOI] [PubMed] [Google Scholar]
- 122.Humphries S, Evans JP, Simmons LW. 2008. Sperm competition: linking form to function. BMC Evol. Biol. 8, 319 ( 10.1186/1471-2148-8-319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Simpson JL, Humphries S, Evans JP, Simmons LW, Fitzpatrick JL. 2014. Relationships between sperm length and speed differ among three internally and three externally fertilizing species. Evolution 68, 92–104. ( 10.1111/evo.12199) [DOI] [PubMed] [Google Scholar]
- 124.Hadlow JH, Evans JP, Lymbery RA. 2020. Egg-induced changes to sperm phenotypes shape patterns of multivariate selection on ejaculates. J. Evol. Biol. 33, 797–807. ( 10.1111/jeb.13611) [DOI] [PubMed] [Google Scholar]
- 125.Wedell N. 2020. Selfish genes and sexual selection: the impact of genomic parasites on host reproduction. J. Zool. 311, 1–12. ( 10.1111/jzo.12780) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.