Abstract
Although initially lagging behind discoveries being made in other taxa, mammalian sperm competition is now a productive and advancing field of research. Sperm competition in mammals is not merely a ‘sprint-race’ between the gametes of rival males, but rather a race over hurdles; those hurdles being the anatomical and physiological barriers provided by the female reproductive tract, as well as the egg and its vestments. With this in mind, in this review, I discuss progress in the field while focusing on the female perspective. I highlight ways by which sperm competition can have positive effects on female reproductive success and discuss how competitive outcomes are not only owing to dynamics between the ejaculates of rival males, but also attributable to mechanisms by which female mammals bias paternity toward favourable sires. Drawing on examples across different species—from mice to humans—I provide an overview of the accumulated evidence which firmly establishes that sperm competition is a key selective force in the evolution of male traits and detail how females can respond to increased sperm competitiveness with increased egg resistance to fertilization. I also discuss evidence for facultative responses to the sperm competition environment observed within mammal species. Overall, this review identifies shortcomings in our understanding of the specific mechanisms by which female mammals ‘select’ sperm. More generally, this review demonstrates how, moving forward, mammals will continue to be effective animal models for studying both evolutionary and facultative responses to sperm competition.
This article is part of the theme issue ‘Fifty years of sperm competition’.
Keywords: multiple paternity, cryptic female choice, reproductive success, experimental evolution, phenotypic plasticity, social environment
1. Introduction
In mammals, sperm competition occurs when sexually receptive (oestrus) females mate multiply and sperm from rival males compete within the female reproductive tract (FRT) to fertilize the egg(s) [1]. For a long time, the expectation was that mammalian sperm competition should be a rare phenomenon because females do not possess dedicated organs or structures for storing sperm so they are relatively short-lived inside the FRT (i.e. 2–5 days; the exceptions being bats and some marsupials) and a single fertile mating should be sufficient to fertilize the egg(s) [2–4]. Further, relative to male reproductive fitness, which is maximized when paternity success exceeds the cost associated with soliciting multiple mates, maternal fitness in mammals is typically constrained (e.g. by the number of offspring that can be gestated/lactated). Despite this, many studies have now demonstrated that female mammals actively seek out matings from multiple males [5–7] and, with the development of molecular technologies, that sperm competition is a common event (e.g. multiple paternity rates: California ground squirrels, 89% [8]; common shrew, 89%, [9]; dormice, 95% [10]; house mice, up to 80%, [11]).
Mating with multiple males can significantly increase energy expended on reproduction and expose females to a greater risk of predation or disease [12]. Consequently, a number of different theories have been hypothesized to account for the evolution of polyandry and ways in which sperm competition can improve female fitness [13–15]. I therefore begin this review with exploring reasons why female mammals mate multiply and summarize current evidence of the benefits that they can receive from ensuring that sperm are locked in the competition prior to fertilization. In the following section, I review interspecific comparisons that have demonstrated evolutionary responses to postmating sexual selection, which, for a long time, represented the major research ‘tool’ in mammalian sperm competition [1]. In the 1990s, as the evolutionary potential of sperm competition began to emerge from discoveries made in studies of non-mammalian taxa, within species mammal research continued to focus mostly on sperm competition in the context of male copulatory behaviour (e.g. Donald Dewsbury's important early contributions; see [16,17]). This changed as behavioural ecologists began to use small mammal species amendable to laboratory life in an effective way to test sperm competition evolutionary theory and as molecular and in vitro fertilization (IVF) technologies were refined and optimized. To demonstrate this, I provide examples of my own research on house mice, and, in the same section, demonstrate how mammals can exhibit phenotypic plasticity in a number of traits to make adaptive responses to the sperm competition environment. In the final section, I provide a synopsis of what is currently known of sperm competition in our own species. Although historically the field of mammalian sperm competition has been largely male-orientated, throughout this review, I emphasize the female perspective and discuss female traits that separate mammals from other taxonomic groups. I hope to inspire future research that will focus on uncovering the mechanisms that female mammals use to ‘select’ the sperm of desirable sires and further understand how doing so improves their evolutionary fitness.
2. The prerogative of female mammals to mate multiply and benefit from sperm competition
There are different reproductive stages where polyandry can be beneficial to female mammals by influencing offspring survival or performance [18–20]. For example, in some species, the production of offspring sired by multiple or extra-pair males leads to paternity confusion, which effectively functions as a strategy to reduce the likelihood that offspring will fall victim to male infanticide (observed in both rodent and primates; [21,22]). Sperm competition has also been shown to generate an increase in offspring survival even in the absence of male infanticide risk, suggesting that genetic mechanisms which lead to high-quality offspring may be at play [23,24]. In support of this idea, a comparative analysis across 70 mammal species revealed that monogamous species experience an elevated rate of zygote loss compared to polyandrous species [25]. As is the case for all comparative studies, a number of different explanations could account for this evolutionary pattern. Still, this finding is convincingly consistent with the genetic incompatibility avoidance hypothesis [25], which proposes that polyandry can offset reproductive failure resulting from unfavourable combinations of parental genotypes (non-additive genetic benefits) [20]. In this sense, the overlapping of ejaculates within the FRT allows the opportunity for paternity to be skewed toward compatible male genotypes, either via sperm competition or by facilitating mechanisms of cryptic female choice. As an example of the former, sperm competition has been demonstrated to ameliorate the detrimental effects of an autosomal selfish genetic element, the mouse t haplotype (i.e. a gene driver found on chromosome 17, the recessive condition of which is lethal [t/t]) [26]. Although t haplotype carrying males (t/+) produce the same number of sperm as wild-type males (+/+), their sperm show reduced swimming ability, and fewer arrive at the site of fertilization, compared to the sperm of wild-type males (see references in [26]). In this system, sperm competition dynamics, specifically poor sperm performance by t haplotype carriers, allows females to avoid the immediate fitness costs associated with the t haplotype (e.g. reduced litter size).
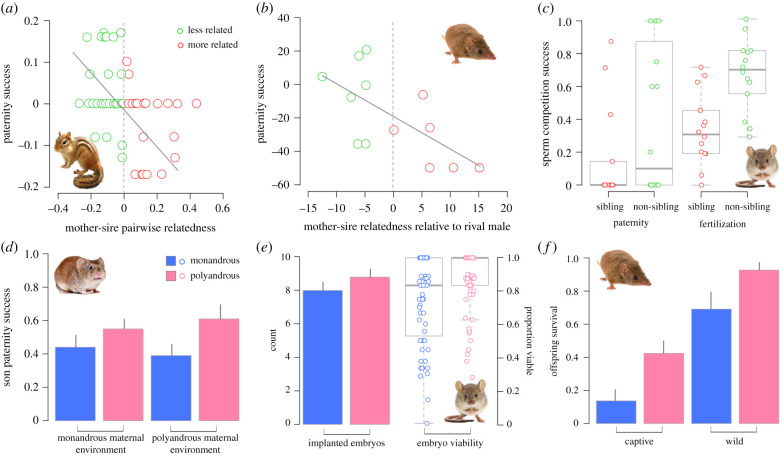
More generally, inbreeding can be viewed as a form of genetic incompatibility because it has the potential to negatively impact maternal fitness by increasing offspring homozygosity levels and elevating the risk of the expression of deleterious mutations [27]. Some degree of inbreeding is usually tolerated and expressed as compromised offspring quality or reduced offspring reproductive performance. In extreme cases, inbreeding will lead to complete reproductive failure (e.g. embryonic death). Studies of mammals have investigated how females might avoid inbreeding and offset the risk of fertilizations by related-male sperm by enabling the ejaculates of different males to co-occur in the FRT. While some of these provide no support [28,29], the consensus seems to be that under competitive conditions fertilization is skewed towards the sperm of more distantly related males [30–33] (figure 1a–c). The mechanisms leading to paternity skews that favour unrelated males is not always clear, but may be linked to interactions between and within ejaculates within the FRT [38], or interactions between the gametes during fertilization [33].
Figure 1.
Studies of small mammals have demonstrated that females can gain both non-additive (a–c) and additive (d–f) genetic benefits from mating with multiple males and enabling their sperm to compete for fertilizations. For example, in providing evidence for the inbreeding avoidance hypothesis, field studies on polytocous species, such as the eastern chipmunk (Tamias striatus) [30] and brown antechinus (Antechinus agilis) [31], have demonstrated reduced paternity success towards males that are more closely related to their partners relative to males that are more distantly related (a,b). In the context of inbreeding, however, paternity outcomes are often confounded by early-stage offspring mortality and, more generally, paternity scores can mask covert mechanisms that operate during fertilization, for example, interactions between the gametes. Laboratory experiments using molecular and in vitro fertilization (IVF) technologies on house mice (Mus musculus domesticus) have addressed these issues and provided compelling evidence that the overlapping of ejaculates can facilitate inbreeding avoidance: both paternity success [32] and IVF rate [33] were found to be skewed towards non-sibling males when they were engaged in sperm competition with a sibling of the mating female (c). These findings support the idea that polyandry can mitigate the negative fitness costs associated with inbreeding via the action of functional mechanisms (e.g. selective fertilization). Sperm competition can have positive effects on female reproductive success via additive genetic benefits (d,e). A study of bank voles (Myodes glareolus) tested whether the sons of polyandrous females experience high competitive ability via paternally inherited traits (e.g. elevated fertilization efficiency), while controlling for potential maternal effects associated with mating regime [34] (d). This study reported that sons of polyandrous females achieved greater paternity success relative to sons produced by monandrously mated females; an outcome that is most parsimoniously explained by sperm competition selecting for genetically superior males who have greater competitive ability [34] (similar results observed in house mice, see [35]). Research on house mice (e) revealed that a selection history of polyandry can enhance female reproductive output by influencing embryo implantation rates and offspring viability [36]. Moreover, an investigation on the brown antechinus showed that offspring which are the product of a polyandrous mating have increased survival compared to the offspring produced via monandry [37] (f). Further, this study eloquently demonstrated how sperm competition can enhance female lifetime fitness [37]. Figure panels have been redrawn from those presented in the corresponding study. (Online version in colour.)
Polyandrous females will benefit when high-quality males are both successful in sperm competition and produce offspring with ‘good genes’ [19] or produce sons that inherit their superior sperm competitiveness (additive genetic benefits) [18]. These so-called ‘good’ and ‘sexy’ sperm hypotheses initially proved challenging to test and decipher in mammals. However, over the past 20 years, experimental manipulations using small mammal species have been instrumental in moving the field forward and there is now accumulated evidence that polyandry may boost female evolutionary potential by influencing offspring quality [34–37,39] (figure 1d–f). Owing to an inherent male-dominated cultural bias that predisposed researchers to only consider male-driven mechanisms, it was once assumed that the outcome of mammalian sperm competition was determined exclusively by males, for example, because the fittest male produced the fastest sperm which were able to reach the eggs first [1,40]. However, we now understand that sperm passage in mammals is not simply an act of ‘passive transportation’ but rather it is mediated by complex interactions between the ejaculate(s) and the FRT epithelium [1,41]. Indeed, female mammals exert considerable control over the small population of sperm that eventually reach the oviduct (the site of fertilization) and fertilize the egg(s) [42]. It is through such mechanisms that females may ‘select’ sperm that will improve the genetic quality of their offspring. Indeed, we now appreciate that in most taxa, including mammals, postmating female-driven mechanisms can result in non-random sperm use and biased paternity share [43–45]. In this sense, sperm competition both begins (via polyandry) and ends (via mechanisms of cryptic female choice) with the female.
3. Evolutionary responses to sperm competition among mammals
When female mammals mate multiply and sperm competition occurs, male fertilization success will depend on both the timing of sperm capacitation relative to the time of ovulation and the male's ability to achieve fertilizations at the expense of his rivals. Generally, the complex mammalian FRT generates extraordinary selective pressure on sperm and other constituents within the seminal fluid. After ejaculation, sperm are required to navigate through the challenging environment of the FRT while undergoing maturation (capacitation), changes in motility pattern (hyperactivation) and an acrosomal reaction, which are essential for the ability to bind to and penetrate the egg. The timing of these processes is critical for successful fertilization (e.g. premature capacitation relative to ovulation may result in a drastic loss of ejaculate fertilizing efficiency) [46]. The rate at which a mammal sperm population matures has been linked to ovulation mode (induced versus spontaneous) [46]. For example, in humans, where ovulation occurs spontaneously, selection has favoured a low steady-state sperm capacitation rate, while in the rabbit, where ovulation is induced by mating, there is a distinct peak in sperm capacitation levels shortly after insemination [47]. Ovulation mode has been implicated to lead to variation in the opportunity for and intensity of sperm competition among species [1]. For example, comparative studies of carnivores have suggested that both the risk and intensity of sperm competition may differ according to ovulation mode; the former being greater for induced ovulators and the latter greater for spontaneous ovulators (see [48,49]).
As in other taxa, comparative studies of mammal species have provided strong evidence that selection via sperm competition influences the evolution of male reproductive traits (electronic supplementary material, table S1). Evolutionary theory predicts that sperm competition will favour an increase in sperm production [50]. In agreement, studies of different mammalian taxa have shown that relative testes size among species corresponds with evolutionary increases in the strength of selection from sperm competition, quantified by variation in the mating system or female remating behaviour (via multiple paternity rate) (figure 2a). For example, primate, ungulate, bat and rodent species that have been subject to a high level of sperm competition have larger relative testes size compared to those species where the strength of selection is reduced or absent [51–54]. These findings support the assertion that sperm production is costly and that males should optimize their investment in sperm production according to the level of sperm competition (as well as the mating rate) [55,56]. Species comparisons on mammals have demonstrated that sperm competition may select for increased sperm production via avenues other than increased relative testes size, such as increased proportions of sperm-producing tissue within the testes [57,58] and faster sperm production rates (e.g. [57,59]). Sperm competition may also lead to evolutionary changes in components of male reproductive anatomy beyond the testes, for example, accessory gland size [54] (figure 2a). Moreover, among mammals, the sperm competition level has been associated with increased sperm number, size, motility (i.e. higher proportions of motile sperm, faster sperm velocity) and capacitation rate (e.g. [60–63]) (figure 2a).
Figure 2.
Evidence for evolutionary responses to sperm competition both among (a) and within (b) mammal species, as well as facultative response within species (c). The summary figures correspond to detailed information in the electronic supplementary material tables S1 and S2. Traits that have been shown to respond to sperm competition are provided in the ring. The shading within the ring represents the weight of evidence for those traits (i.e. a non-quantitative estimate based on the number of studies that provide support). In some cases, traits have been grouped and named accordingly; e.g. (a) ‘sperm production’ includes studies that have quantified testes composition, sperm production rate and sperm number (see the electronic supplementary material, table S1). Mammal group/species providing evidence are displayed on the outer edge of the ring. (Online version in colour.)
These observed evolutionary responses among males to postmating sexual selection suggest that sperm competition promotes the evolution of a larger population of sperm that are competent to fertilize the egg—but what are the implications for the evolution of female traits? Elevated sperm fertilizing potential under sperm competition has the potential to lead to an increased risk of lethal polyspermic fertilization (i.e. simultaneous entry of the egg by more than one sperm), which may generate gametic sexual conflict and the evolution of female (egg) defences that make it more difficult for fertilization to occur [63]. Sperm will be then selected to overcome these defences, leading to a perpetual arms race in which sperm evolve to become ever more competitive and female gametes more defensive (reviewed in [64]). Under this scenario of antagonistic coevolution, it is predicted that heterospecific fertilization success will be associated with sperm competition level [64]. Evidence supporting this prediction comes from a study of mammals where fertilization success between two closely related Mus species that have been subject to either a high or low level of sperm competition (as defined by relative testes size) differed depending on the direction of the cross (e.g. high sperm competitiveness × low egg defensiveness = high fertilization rate, low sperm competitiveness × high egg defensiveness = low fertilization rate) [65]. Thus, female gametes of high sperm competition species appear to be more resistant to fertilization by heterospecific sperm compared to low sperm competition species, and the male gametes of high sperm competition species have a greater capacity to overcome egg defences [65]. This suggests that sexual conflict between the gametes may contribute to the evolution of postmating prezygotic barriers to reproduction and allow females to have greater control over fertilization with the production of eggs that are more resistant to fertilization and therefore more discriminatory over sperm entry [64].
The intimate association between the mammal ejaculate and FRT provides ample opportunity for female mechanisms to choose the sperm of competing males. Despite this, the research area of mammal cryptic female choice is largely understudied [43]. This is especially true in the context of reproductive delays (reviewed [66]). It has been identified that more than 100 mammalian species exhibit some form of reproductive delay, the best examples being among bat species that demonstrate delays at multiple stages [66]. Delayed fertilization, which occurs following sperm storage in the lining of the FRT, may offer an opportunity for ‘mate choice’ when an opportunity prior to mating is not provided; this has been suggested to be an effective adaptive strategy for female temperate bat species that are mated by multiple males during torpor [66]. An overproduction of eggs is a common mammalian trait and delays between fertilization and implantation can last for a few weeks to a year among bats, creating an option for differential embryo implantation and resorption to facilitate paternity biases towards preferred/compatible male genotype(s) [66] (note there is potential for this to also occur within much shorter fertilization-implantation time frames; e.g. [67]). Finally, in some bat species (and possibly other mammal groups), development may be slowed or stopped after implantation, allowing females to ‘assess and compare’ embryo paternity and retain only those pup(s) sired by desirable males. All in all, although the specific mechanisms are not yet well understood, it is evident that processes such as sperm or embryo selection are likely to operate as effective cryptic choice mechanisms in female mammals (reviewed [43]). By increasing the opportunity for or the intensity of sperm competition, species with reproductive delays might allow such mechanisms to operate more freely and consequently provide intriguing avenues for future discovery (see [66]).
4. Evolutionary and facultative responses to sperm competition within mammals
(a). Of mice: a powerful and effective model system
As the sperm competition field exploded in the 1990s with exciting empirical discoveries being made with the use of insects, mammologists were presented with the challenge of adopting an appropriate model species that could be manipulated to test sperm competition theory in an effective way. Renowned for rapidly ‘adapting’ to laboratory life and reproducing reliably under such conditions [68], as well as fulfilling the criteria that females actively engage in polyandrous matings, the house mouse (Mus musculus domesticus) was a suitable candidate [6]. Studies of wild mouse populations had provided evidence of natural variation in both the risk (polyandry rate: 10–80%) and intensity (paternity: often 2 or 3 sires litter−1) of sperm competition [11,69] and a dense microsatellite map meant that the genetic paternity studies could be conducted with relative ease and at a reasonable cost [70]. These key factors saw the house mouse emerge as an amenable species for exploring evolutionary and facultative responses to sperm competition.
By the early 2000s, experimental evolution had proven to be a powerful tool for investigating the evolutionary implications of sperm competition. Studies of this kind in insects had provided strong empirical evidence of how polyandry influences female evolutionary fitness, male evolutionary responses to sperm competition and the evolutionary costs of male adaptations to females. At this time, a multigenerational approach had not yet been applied to assess the potential evolutionary significance of sperm competition in a vertebrate. Leigh Simmons and I set out to achieve this using house mice. We reinstated postmating sexual selection in a wild-derived laboratory colony of house mice that had experienced a long history of monogamy. We maintained replicate monogamous (single male × single female mating) and polyandrous (three males × single female mating) lines across multiple generations and, once the lines had diverged, performed a series of experiments to address a number of outstanding questions in the field [71].
Our investigations confirmed within a single mammal species that sperm competition selects for (i) an elevation in the sperm number attributable to increased proportions of sperm-producing tissue within the testes (and not an increase in overall testes mass) [71,72], (ii) greater proportions of motile sperm and faster swimming sperm [71,73,74], (iii) increased in vivo and in vitro sperm competitiveness [75,76], (iv) increased mating duration [77], (v) an elevation in seminal vesicle protein (SVS1, SVS2) expression (i.e. those that contribute to mating plug formation and sperm longevity) [78], and (vi) a wider baculum [79] (i.e. a genital shape which has been linked to male reproductive success; see [80,81]) (figure 2b). Our experimental evolution design accounted for differential inbreeding effects between treatment lines and further experimentation confirmed that male responses to sperm competition were owing to selection acting on standing genetic variation rather than the purging of a mutation load that had accumulated in the monogamously bred source population [71,74]. However, the outcome of a collaborative investigation with Andri Manser and Anna Lindholm revealed that competitive ability among males from the polyandrous lines was partly attributed to gene drive [82]. Here, we found that drive-carrying males were substantially compromised in their sperm competitive ability and as a consequence t haplotype frequencies declined significantly in the polyandrous lines while remaining at stable, high levels in the monogamous lines [82]. Thus, the body of work from our house mouse lines provided direct empirical evidence that sperm competition has important repercussions for male fertility at the individual level (figure 2b), as well as the spread of a drive gene at the population level [82].
In collaboration with Eduardo Roldan and Montse Gomendio, we performed reciprocal IVF assays between the sperm and eggs of individuals from our evolved lines to provide intraspecific evidence of increased egg defensiveness under elevated sperm competition (i.e. polyandrous line sperm × monogamous line eggs = high fertilization rate, monogamous line sperm × polyandrous line egg = low fertilization rate) [75]. It was possible that in addition to gametic sexual conflict, directional selection via cryptic female choice also contributed to divergence in egg defensiveness among our experimental populations [75], for example, in the case that egg defences mediate sperm entry to bias fertilization towards a specific sperm type that provides a fitness advantage [35,36]. In agreement, IVF studies of mice have shown that females can benefit from egg discernment of sperm types and avoid fertilizations by related males (figure 1c; [33]) or males with compromised health [83]. Further support comes from observed facultative responses in egg defensiveness in response to the sperm competition environment; eggs produced by females that have developed from weaning until sexual maturity under a high sperm competition (HSC) risk (high-male density) were shown to be more resistant to fertilization compared with females that had developed under a low sperm competition risk (low-male density) (figure 2c; [84]). An adjustment in egg fertilizability in response to prevailing social conditions is an adaptive female strategy that would counter the observed male strategy, which is an increase in sperm production, and therefore competitiveness, under an HSC risk ([85,86]; observed in a number of rodent species, figure 2c). When sperm densities are anticipated to be high based on cues perceived within the social environment, increased egg defensiveness will minimize the risk of polyspermy and safeguard against reproductive failure, but may also be beneficial by allowing females to discriminate between males, and select potential sires, at the gametic level [84].
The house mouse has also proven useful for studying facultative male responses to the sperm competition environment beyond plasticity in sperm production (electronic supplementary material, table S1; figure 2c). Experimental work by Steve Ramm and Paula Stockley has shown that males exposed to varied risk(s) or intensity of sperm competition during sexual development can lead to differences in the size of the seminal vesicle, as well as the expression of seminal proteins that are involved in mating plug formation [87]. Further, an investigation from our own group has demonstrated plasticity in baculum shape, changes that are likely to have adaptive implications [81,88] (figure 2c). The ease at which the house mouse social environment can be experimentally manipulated primes it to be an ideal model species for future work on responses to sperm competition risk.
(b). Of men and women: sperm competition in humans
How best to characterize the human mating system, and the extent to which human females are polyandrous, is a subject of intense debate (reviewed [89,90]). High offspring dependence, which generates overlapping interests between the sexes, is argued to favour long-term monogamous relationships [89]. Long-term pair bonds, typically recognized as marriages, are characteristic of most human societies. Still, sperm competition is often prevalent in varying degrees. For example, young adults of the Macushi of Guyana are encouraged to engage freely in pre-marital sex to facilitate mutual mate choice in the formation of lasting, long-term partnerships (i.e. marriage) [91]. In other societies, post-marital female extra-pair copulations (EPCs) are socially permissible. This is the case for many lowland South American groups who believe that the contribution of multiple men is required for the formation of a fetus (partible paternity) [92] and the Inuit, where wives and husbands engage in partner-swapping if agreed upon by all parties [93]. In most human societies, however, EPCs are clandestine transgressions that, when revealed, may lead to personal shame, social judgement and even criminal punishment [89]. This is probably the reason for studies of western human populations reporting fairly low rates of EPCs (e.g. approximately 14% of women <30 years of age have engaged in at least one EPC; [94]).
From a postmating sexual selection perspective, female EPC behaviour is only relevant when it generates sperm competition and has the potential to lead to extra-pair paternity (EPP; i.e. within the fertile period and contraceptives are not used). Rigorous, high-quality evidence of EPP rates in humans has accumulated over the last 5–10 years. Among the Himba people of northern Nambia, where EPP is associated with elevated female reproductive success, the rate is reported to be as high as 48% [95,96]. More consistently, however, genetic data has revealed very low rates of EPP (e.g. approximately 1 to 2%; [96] and references therein). The most conclusive evidence of sperm competition in humans comes from doubly sired dizygotic twins, although again this is believed to occur at very low rates (e.g. <0.01% twin births in the USA; [97]).
If sperm competition has persisted as a present, but not overly powerful, selective force in human evolution then we might expect relative testes size to be positioned somewhere between strictly monogamous (e.g. gorillas) and highly polyandrous (e.g. chimps) primates, which is precisely what we see [98]. But what do we know about the strength of selection via sperm competition acting on male traits within modern human populations today? Relevant to this question, a controversial study led to the proposition that within-population variation in human testes size was reflective of alternative male reproductive strategies of sperm competition (via EPCs; large testes) and monogamy (small testes) [99]. My own research confirmed that human males with larger testes ejaculate greater numbers of sperm, suggesting that perhaps larger testes do bestow a selective advantage in sperm competition [94]. Despite this, however, we found no evidence to link testes size to permissive sexual behaviour; combined testis volume was comparable among men who engaged in EPCs and those that did not [94]. The collective data suggest that the risk of sperm competition in modern human populations is relatively low compared to that for other nonhuman taxa. This could well be because humans have evolved a defensive mate-guarding strategy, the success of which may have relaxed selection on physiological and morphological adaptations to tackle sperm competition offensively [90]. There is evidence to suggest that male humans may elicit facultative responses when presented with an immediate risk of sperm competition, for example, with changes in sperm quality (figure 1c; [100]). Further, as a potential mechanism of cryptic female choice, exciting new research has demonstrated that sperm chemoattractants produced by eggs may facilitate gamete-mediated ‘mate choice’ in humans [101]. The study of adaptations to human sperm competition has traditionally bordered the fields of behavioural ecology and evolutionary psychology [102]. Now it seems that this fascinating area of research is reaching into the realm of reproductive biology in relation to the role that the FRT, specifically oviductal fluid and sperm chemoattractants, plays in determining postmating ‘mate choice’ outcomes.
5. Conclusion
Behavioural ecologists have seen the field of mammalian sperm competition grow rapidly over the past 30 years and develop from interspecies comparisons and field observations to empirical investigations with the use of molecular and IVF technologies. We have learnt that sperm competition has profound effects on the evolution of male reproductive traits and can have a positive influence on female reproductive success, both among and within mammal species. Looking forward, an expanded focus on disentangling male and female effects on competitive paternity outcomes would help to identify covert sperm-biasing mechanisms that afford females a fitness advantage. A clearer understanding on the role that various regions of the FRT play in differentially filtering the sperm of different males will help with this endeavour (reviewed [42]). Certainly, as we are now armed with the knowledge that both sperm and eggs elicit changes in the biochemical composition of mammalian oviductal fluid [42], and we have recently learnt that these processes can influence fertilization outcomes [101], there is a great opportunity for explorations into how female reproductive fluid mediates gametic interactions when sperm are locked in competition (as appreciated in other taxa; see [103]). Indeed, mammals have a clear role to play in uncovering new and exciting discoveries as we embark on the next 50 years of studying the fascinating phenomenon that is sperm competition.
Supplementary Material
Acknowledgements
I thank all the people who have made important contributions to the field of mammalian sperm competition. I give a huge thank you to my many collaborators who have worked on my own contributions, especially Leigh Simmons who allowed me to pull him away from insects (although not entirely!) all those years ago. I am grateful to the two anonymous reviewers for their helpful comments on the manuscript.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
I was supported by an Australian Research Council Future Fellowship (FT180100625) while writing this review.
References
- 1.Gomendio M, Harcourt AH, Roldan ERS. 1998. Sperm competition in mammals. In Sperm competition and sexual selection (eds Birkhead TR, Møller AP), pp. 667–781. London, UK: Academic Press. [Google Scholar]
- 2.Holt WV, Fazeli A. 2016. Sperm storage in the female reproductive tract. Ann. Rev. Anim. Biosci. 4, 291–310. ( 10.1146/annurev-animal-021815-111350) [DOI] [PubMed] [Google Scholar]
- 3.Parker GA. 1984. Sperm competition and the evolution of animal mating strategies. In Sperm competition and the evolution of animal mating systems (ed. Smith RL.), pp. 1–60. Orlando, FL: Academic Press. [Google Scholar]
- 4.Orr TJ, Brennan PLR. 2015. Sperm storage: distinguishing selective processes and evaluating criteria. Trends Ecol. Evol. 30, 261–272. ( 10.1016/j.tree.2015.03.006) [DOI] [PubMed] [Google Scholar]
- 5.Huchard E, Canale CI, Le Gros C, Perret M, Henry P-Y, Kappeler PM. 2012. Convenience polyandry or convenience polygyny? Costly sex under female control in a promiscuous primate. Proc. R. Soc. B 279, 1371–1379. ( 10.1098/rspb.2011.1326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rolland C, Macdonald DW, de Fraipont M, Berdoy M. 2003. Free female choice in house mice: leaving best for last. Behaviour 140, 1371–1388. ( 10.1163/156853903771980639) [DOI] [Google Scholar]
- 7.Fisher DO, Double MC, Moore BD. 2006. Number of mates and timing of mating affect offspring growth in the small marsupial Antechinus agilis. Anim. Behav. 71, 289–297. ( 10.1016/j.anbehav.2005.03.041) [DOI] [Google Scholar]
- 8.Boellstroff DE, Owings DH, Penedo MCT, Hersek MJ. 1994. Reproducitve behaviour and multiple paternity of California ground squirrels. Anim. Behav. 47, 1057–1064. ( 10.1006/anbe.1994.1144) [DOI] [Google Scholar]
- 9.Stockley P, Searle JB, Macdonald DW, Jones CS. 1993. Female multiple mating behaviour in the common shrew as a strategy to reduce inbreeding. Proc. R. Soc. B 254, 173–179. ( 10.1098/rspb.1993.0143) [DOI] [PubMed] [Google Scholar]
- 10.Naim D, Telfer S, Sanderson S, Kemp SJ, Watts PC. 2011. Prevalence of multiple mating by female common dormice, Muscardinus avellanarius. Conserv. Genetics 12, 971–979. ( 10.1007/s10592-011-0200-6) [DOI] [Google Scholar]
- 11.Firman RC, Simmons LW. 2008. The frequency of multiple paternity predicts variation in testes size among island populations of house mice. J. Evol. Biol. 21, 1524–1533. ( 10.1111/j.1420-9101.2008.01612.x) [DOI] [PubMed] [Google Scholar]
- 12.Daly M. 1978. The cost of mating. Am. Nat. 112, 771–774. ( 10.1086/283319) [DOI] [Google Scholar]
- 13.Jennions MD, Petrie M. 2000. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 75, 21–64. ( 10.1017/S0006323199005423) [DOI] [PubMed] [Google Scholar]
- 14.Simmons LW. 2005. The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu. Rev. Ecol. Evol. Syst. 36, 125–146. ( 10.1146/annurev.ecolsys.36.102403.112501) [DOI] [Google Scholar]
- 15.Wolff JO, Macdonald DW. 2004. Promiscuous females protect their young. Trends Ecol. Evol. 19, 127–134. ( 10.1016/j.tree.2003.12.009) [DOI] [PubMed] [Google Scholar]
- 16.Dewsbury DA. 1993. Sperm competition and effects of mating order on copulatory behavior in meadow voles (Microtus pennsylvanicus). Bullet. Psychonomic Soc. 31, 437–439. ( 10.3758/BF03334955) [DOI] [Google Scholar]
- 17.Dewsbury DA, Hartung TG. 1980. Copulatory behaviour and differential reproduction of laboratory rats in a two-male, one-female competitive situation. Anim. Behav. 28, 95–102. ( 10.1016/S0003-3472(80)80012-1) [DOI] [Google Scholar]
- 18.Keller L, Reeve HK. 1995. Why do females mate with multiple males? The sexually selected sperm hypothesis. Adv. Stud. Behav. 24, 291–315. ( 10.1016/S0065-3454(08)60397-6) [DOI] [Google Scholar]
- 19.Yasui Y. 1997. A ‘good sperm’ model can explain the evolution of costly multiple mating by females. Am. Nat. 149, 573–584. ( 10.1086/286006) [DOI] [Google Scholar]
- 20.Zeh JA, Zeh DW. 1997. The evolution of polyandry II: post-copulatory defences against genetic incompatibility. Proc. R. Soc. Lond. B 264, 69–75. ( 10.1098/rspb.1997.0010) [DOI] [Google Scholar]
- 21.Klemme I, Ylonen H. 2010. Polyandry enhances offspring survival in an infanticidal species. Biol. Lett. 6, 24–26. ( 10.1098/rsbl.2009.0500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Connell SM, Cowlishaw GB. 1994. Infanticide avoidance, sperm competition and mate choice: the function of copulation calls in female baboons. Anim. Behav. 48, 687–694. ( 10.1006/anbe.1994.1288) [DOI] [Google Scholar]
- 23.Firman RC, Simmons LW. 2008. Polyandry, sperm competition, and reproductive success in mice. Behav. Ecol. 19, 695–702. ( 10.1093/beheco/arm158) [DOI] [Google Scholar]
- 24.Keil A, Sachser N. 1998. Reproductive benefits from female promiscuous mating in a small mammal. Ethology 104, 897–903. ( 10.1111/j.1439-0310.1998.tb00039.x) [DOI] [Google Scholar]
- 25.Stockley P. 2003. Female multiple mating behaviour, early reproductive failure and litter size variation in mammals. Proc. R. Soc. Lond. B 270, 271–278. ( 10.1098/rspb.2002.2228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutter A, Lindholm AK. 2015. Detrimental effects of an autosomal selfish genetic element on sperm competitiveness in house mice. Proc. R. Soc. B 282, 20150974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pusey A, Wolf M. 1996. Inbreeding avoidance in animals. Trends Ecol. Evol. 11, 201–206. ( 10.1016/0169-5347(96)10028-8) [DOI] [PubMed] [Google Scholar]
- 28.Lane JE, Boutin S, Gunn MR, Slate J, Coltman DW. 2007. Genetic relatedness of mates does not predict patterns of parentage in North American red squirrels. Anim. Behav. 74, 611–619. ( 10.1016/j.anbehav.2006.12.017) [DOI] [Google Scholar]
- 29.Stockley P. 1997. No evidence of sperm selection by female common shrews. Proc. R. Soc. Lond. B 264, 1497–1500. ( 10.1098/rspb.1997.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergeron P, Reale D, Humphries MM, Garant D. 2011. Evidence of multiple paternity and mate selection for inbreeding avoidance in wild eastern chipmunks. J. Evol. Biol. 24, 1685–1694. ( 10.1111/j.1420-9101.2011.02294.x) [DOI] [PubMed] [Google Scholar]
- 31.Kraaijeveld FJL, Ward SJ, Temple-Smith PD, Paetkau D. 2002. Factors influencing paternity success in Antechinus agilis: last-male sperm precedence, timing of mating and genetic compatibility. J. Evol. Biol. 15, 100–107. ( 10.1046/j.1420-9101.2002.00367.x) [DOI] [Google Scholar]
- 32.Firman RC, Simmons LW. 2008. Polyandry facilitates postcopulatory inbreeding avoidance in house mice. Evolution 62, 601–611. ( 10.1111/j.1558-5646.2007.00307.x) [DOI] [PubMed] [Google Scholar]
- 33.Firman RC, Simmons LW. 2015. Gametic interactions promote inbreeding avoidance in house mice. Ecol. Lett. 18, 937–943. ( 10.1111/ele.12471) [DOI] [PubMed] [Google Scholar]
- 34.Klemme I, Bäumer J, Eccard JA, Ylönen H. 2014. Polyandrous females produce sons that are successful at post-copulatory competition. J. Evol. Biol. 27, 457–465. ( 10.1111/jeb.12334) [DOI] [PubMed] [Google Scholar]
- 35.Firman RC. 2011. Polyandrous females benefit by producing sons that achieve high reproductive success in a competitive environment. Proc. R. Soc. B 278, 2823–2831. ( 10.1098/rspb.2010.2791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Firman RC, Simmons LW. 2012. Male house mice evolving with post-copulatory sexual selection sire embryos with increased viability. Ecol. Lett. 15, 42–46. ( 10.1111/j.1461-0248.2011.01706.x) [DOI] [PubMed] [Google Scholar]
- 37.Fisher DO, Double MC, Blomberg SP, Jennions MD, Cockburn A. 2006. Post-mating sexual selection increase lifetime fitness of polyandrous females in the wild. Nature 444, 89–92. ( 10.1038/nature05206) [DOI] [PubMed] [Google Scholar]
- 38.Fisher HS, Hoekstra HE. 2010. Competition drives cooperation among closely related sperm of deer mice. Nature 463, 801–803. ( 10.1038/nature08736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klemme I, Ylonen H, Eccard JA. 2008. Long-term fitness benefits of polyandry in a small mammal, the bank vole Clethrionomys glareolus. Proc. R. Soc. B 275, 1095–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birkhead TR. 2000. Promiscuity. An evolutionary history of sperm competition. Cambridge, MA: Harvard University Press. [Google Scholar]
- 41.Ishikawa Y, Usui T, Yamashita M, Kanemori Y, Baba T. 2016. Surfing and swimming of ejaculated sperm in the mouse oviduct. Biol. Reprod. 94, 89 ( 10.1095/biolreprod.115.135418) [DOI] [PubMed] [Google Scholar]
- 42.Holt WV, Fazeli A. 2016. Sperm selection in the female mammalian reproductive tract. Focus on the oviduct: hypotheses, mechanisms, and new opportunities. Theriogenology 85, 105–112. ( 10.1016/j.theriogenology.2015.07.019) [DOI] [PubMed] [Google Scholar]
- 43.Firman RC, Gasparini C, Manier MK, Pizzari T. 2017. Postmating female control: 20 years of cryptic female choice. Trends Ecol. Evol. 32, 368–382. ( 10.1016/j.tree.2017.02.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeder DM. 2003. The potential for cryptic female choice in primates: behavioural anatomical, and physiological considerations. In Sexual selection and reproductive competition in primates: new perspectives and directions (ed. Jones CB.), pp. 255–303, Norman, OK: American Society of Primatologists. [Google Scholar]
- 45.Eberhard WG. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 46.Pizzari T. 2006. Of mice and sperm. Proc. Natl Acad. Sci. USA 103, 14 983–14 984. ( 10.1073/pnas.0607091103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giojalas LC, Rovasio RA, Fabro G, Gakamsky A, Eisenbach M. 2004. Timing of sperm capacitation appears to be programmed according to egg availability in the female genital tract. Fertil. Steril. 82, 247–249. ( 10.1016/j.fertnstert.2003.11.046) [DOI] [PubMed] [Google Scholar]
- 48.Lariviere S, Ferguson SH. 2003. Evolution of induced ovulation in North American carnivores. J. Mammal. 84, 937–947. ( 10.1644/BME-003) [DOI] [Google Scholar]
- 49.Iossa G, Soulsbury CD, Baker PJ, Harris S. 2008. Sperm competition and the evolution of testes size in terrestrial mammalian carnivores. Funct. Ecol. 22, 655–662. ( 10.1111/j.1365-2435.2008.01409.x) [DOI] [Google Scholar]
- 50.Parker GA. 1990. Sperm competition games: raffles and roles. Proc. R. Soc. Lond. B 242, 120–126. ( 10.1098/rspb.1990.0114) [DOI] [Google Scholar]
- 51.Harcourt AH, Harvey PH, Larson SG, Short RV. 1981. Testis weight, body weight and breeding system in the primates. Nature 293, 55–57. ( 10.1038/293055a0) [DOI] [PubMed] [Google Scholar]
- 52.Ginsberg JR, Rubenstein DI. 1990. Sperm competition and variation in zebra mating behavior. Behav. Ecol. Sociobiol. 26, 427–434. ( 10.1007/BF00170901) [DOI] [Google Scholar]
- 53.Hosken DJ. 1997. Sperm competition in bats. Proc. R. Soc. Lond. B 264, 385–392. ( 10.1098/rspb.1997.0055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramm SA, Parker GA, Stockley P. 2005. Sperm competition and the evolution of male reproductive anatomy in rodents. Proc. R. Soc. B 272, 949–955. ( 10.1098/rspb.2004.3048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dewsbury DA. 1982. Ejaculate cost and male choice. Am. Nat. 119, 601–610. ( 10.1086/283938) [DOI] [Google Scholar]
- 56.Short RV. 1979. Sexual selection and its component parts, somatic and genital selection, as illustrated by man and the great apes. Adv. Stud. Behav. 9, 131–158. ( 10.1016/S0065-3454(08)60035-2) [DOI] [Google Scholar]
- 57.delBarco-Trillo J, Tourmente M, Roldan ERS. 2013. Metabolic rate limits the effect of sperm competition on mammalian spermatogenesis. PLoS ONE 8, e76510 ( 10.1371/journal.pone.0076510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montoto LG, Arregui L, Sánchez NM, Gomendio M, Roldan ERS. 2012. Postnatal testicular development in mouse species with different levels of sperm competition. Reproduction 143, 333–346. ( 10.1530/REP-11-0245) [DOI] [PubMed] [Google Scholar]
- 59.Ramm SA, Stockley P. 2010. Sperm competition and sperm length influence the rate of mammalian spermatogenesis. Biol. Lett. 6, 219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montoto LG, Magana C, Tourmente M, Martin-Coello J, Crespo C, Luque-Larena JJ, Gomendio M, Roldan ERS. 2011. Sperm competition, sperm numbers and sperm quality in muroid rodents. PLoS ONE 6, e18173 ( 10.1371/journal.pone.0018173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tourmente M, Gomendio M, Roldan ERS. 2011. Sperm competition and the evolution of sperm design in mammals. BMC Evol. Biol. 11, 12 ( 10.1186/1471-2148-11-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tourmente M, Varea-Sanchez M, Roldan ERS. 2019. Faster and more efficient swimming: energy consumption of murine spermatozoa under sperm competition. Biol. Reprod. 100, 420–428. ( 10.1093/biolre/ioy197) [DOI] [PubMed] [Google Scholar]
- 63.Gomendio M, Martin-Coello J, Crespo C, Magana C, Roldan ERS. 2006. Sperm competition enhances functional capacity of mammalian spermatozoa. Proc. Natl Acad. Sci. USA 103, 15 113–15 117. ( 10.1073/pnas.0605795103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Firman RC. 2018. Post-mating sexual conflict and female control over fertilization during gamete interaction. Ann. N Y Acad. Sci. 1422, 48–64. ( 10.1111/nyas.13635) [DOI] [PubMed] [Google Scholar]
- 65.Martin-Coello J, Benavent-Corai J, Roldan ERS, Gomendio M. 2009. Sperm competition promotes asymmetries in reproductive barriers between closely related species. Evolution 63, 613 ( 10.1111/j.1558-5646.2008.00585.x) [DOI] [PubMed] [Google Scholar]
- 66.Orr TJ, Zuk M. 2014. Reproductive delays in mammals: an explored avenue for post-copulatory sexual selection. Biol. Rev. 89, 889–912. ( 10.1111/brv.12085) [DOI] [PubMed] [Google Scholar]
- 67.Firman RC. 2020. Exposure to high-male density causes maternal stress and female-biased sex ratios in a mammal. Proc. R. Soc. B 287, 20192909 ( 10.1098/rspb.2019.2909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rugh R. 1968. The mouse: its reproduction and development. Minneapolis, MI: Burgess Publishing Company. [Google Scholar]
- 69.Dean MD, Ardlie KG, Nachman MW. 2006. The frequency of multiple paternity suggests that sperm competition is common in house mice (Mus domesticus). Mol. Ecol. 15, 4141–4151. ( 10.1111/j.1365-294X.2006.03068.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dietrich W, Katz H, Lincoln SE, Shin H, Friedman J, Dracopoli NC, Lander ES. 1992. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics 131, 423–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Firman RC, Simmons LW. 2010. Experimental evolution of sperm quality via postcopulatory sexual selection in house mice. Evolution 64, 1245–1256. [DOI] [PubMed] [Google Scholar]
- 72.Firman RC, Garcia-Gonzalez F, Thyer E, Wheeler S, Yamin Z, Yuan M, Simmons LW. 2015. Evolutionary change in testes tissue composition among experimental populations of house mice. Evolution 69, 848–855. ( 10.1111/evo.12603) [DOI] [PubMed] [Google Scholar]
- 73.Firman RC, Simmons LW. 2010. Sperm midpiece length predicts sperm swimming velocity in house mice. Biol. Lett. 6, 513–516. ( 10.1098/rsbl.2009.1027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Firman RC, Cheam LY, Simmons LW. 2011. Sperm competition does not influence sperm hook morphology in selection lines of house mice. J. Evol. Biol. 24, 856–862. ( 10.1111/j.1420-9101.2010.02219.x) [DOI] [PubMed] [Google Scholar]
- 75.Firman RC, Gomendio M, Roldan ERS, Simmons LW. 2014. The coevolution of ova defensiveness with sperm competitiveness in house mice. Am. Nat. 183, 565–572. ( 10.1086/675395) [DOI] [PubMed] [Google Scholar]
- 76.Firman RC, Simmons LW. 2011. Experimental evolution of sperm competitiveness in a mammal. BMC Evol. Biol. 11, 19 ( 10.1186/1471-2148-11-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klemme I, Firman RC. 2013. Male house mice that have evolved with sperm competition have increased mating duration and paternity success. Anim. Behav. 85, 751–758. ( 10.1016/j.anbehav.2013.01.016) [DOI] [Google Scholar]
- 78.Simmons LW, Sloan NS, Firman RC. 2020. Sexual selection shapes seminal vesicle secretion gene expression in house mice. Mol. Biol. Evol. 37, 1114–1117. ( 10.1093/molbev/msz295) [DOI] [PubMed] [Google Scholar]
- 79.Simmons LW, Firman RC. 2014. Experimental evidence for the evolution of the mammalian baculum by sexual selection. Evolution 68, 276–283. ( 10.1111/evo.12229) [DOI] [PubMed] [Google Scholar]
- 80.Stockley P, Ramm SA, Sherbourne AL, Thom MDF, Paterson S, Hurst JL. 2013. Baculum morphology predicts reproductive success of male house mice under sexual selection. BMC Biol. 11, 66 ( 10.1186/1741-7007-11-66) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.André GI, Firman RC, Simmons LW. 2020. Baculum shape and paternity success in house mice: evidence for genital coevolution. Phil. Trans. R. Soc. B 375, 20200150 ( 10.1098/rstb.2020.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Manser A, Lindholm AK, Simmons LW, Firman RC. 2017. Sperm competition suppresses gene drive among experimentally evolving populations of house mice. Mol. Ecol. 26, 5784–5792. ( 10.1111/mec.14215) [DOI] [PubMed] [Google Scholar]
- 83.Rulicke T, Chapuisat M, Homberger FR, Macas E, Wedekind C. 1998. MHC-genotype of progeny influenced by parental infection. Proc. R. Soc. Lond. B 265, 711–716. ( 10.1098/rspb.1998.0351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Firman RC, Simmons LW. 2013. Sperm competition risk generates phenotypic plasticity in ovum fertilizability. Proc. R. Soc. B 280, 20132097 ( 10.1098/rspb.2013.2097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ramm SA, Stockley P. 2009. Adaptive plasticity of mammalian sperm production in response to social experience. Proc. R. Soc. B 276, 745–751. ( 10.1098/rspb.2008.1296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Firman RC, Klemme I, Simmons LW. 2013. Strategic adjustments in sperm production within and between two island populations of house mice. Evolution 67, 3061–3070. [DOI] [PubMed] [Google Scholar]
- 87.Ramm SA, Edward DA, Claydon AJ, Hammond DE, Brownridge P, Hurst JL, Beynon RJ, Stockley P. 2015. Sperm competition risk drives plasticity in seminal fluid composition. BMC Biol. 13, 87 ( 10.1186/s12915-015-0197-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.André GI, Firman RC, Simmons LW. 2018. Phenotypic plasticity in genitalia: baculum shape responds to sperm competition risk in house mice. Proc. R. Soc. B 285, 20181086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schacht R, Kramer KL. 2019. Are we monogamous? A review of the evolution of pair-bonding in humans and its contemporary variation cross-culturally. Front. Ecol. Evol. 7, 230 ( 10.3389/fevo.2019.00230) [DOI] [Google Scholar]
- 90.Leivers S, Simmons LW. 2014. Human sperm competition: playing a defensive strategy. Adv. Stud. Behav. 46, 1–44. ( 10.1016/B978-0-12-800286-5.00001-8) [DOI] [Google Scholar]
- 91.Schacht R, Bell A. 2016. Cassava and the Makushi: a shared history of resiliency and transformation. In Food and identity in the Caribbean (ed. Garth H.), pp. 15–29. London, UK: Berg Publishers. [Google Scholar]
- 92.Walker RS, Flinn MV, Hill KR. 2010. Evolutionary history of partible paternity in lowland South America. Proc. Natl Acad. Sci. USA 107, 19 195–19 200. ( 10.1073/pnas.1002598107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burch ES. 1975. Eskimo kinsmen: changing family relationships in northwest Alaska. Minnesota, MI: West Publishing. [Google Scholar]
- 94.Simmons LW, Firman RC, Rhodes G, Peters M. 2004. Human sperm competition: testis size, sperm production and rates of extrapair copulations. Anim. Behav. 68, 297–302. ( 10.1016/j.anbehav.2003.11.013) [DOI] [Google Scholar]
- 95.Scelza BA. 2011. Female choice and extra-pair paternity in a traditional human population. Biol. Lett. 7, 889–891. ( 10.1098/rsbl.2011.0478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scelza BA, Prall SP, Swinford N, Goplan S, Atkinson EG, McElreath R, Sheehama J, Henn BM. 2020. High rate of extrapair paternity in a human population demonstrates diversity in human reproductive strategies. Sci. Adv. 6, eaay6195 ( 10.1126/sciadv.aay6195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.James WH. 1993. The incidence of superfecundation and of double paternity in the general population. Acta Geneicae Medicae et Gemellologiae 42, 257–262. ( 10.1017/S0001566000003263) [DOI] [PubMed] [Google Scholar]
- 98.Harcourt AH, Purvis A, Liles L. 1995. Sperm competition: mating system, not breeding season, affects testes size of primates. Funct. Ecol. 9, 468–476. ( 10.2307/2390011) [DOI] [Google Scholar]
- 99.Baker RR, Bellis MA. 1995. Human sperm competition: copulation, masturbation and infidelity. London, UK: Chapman and Hall. [Google Scholar]
- 100.Kilgallon SJ, Simmons LW. 2005. Image content influences men's semen quality. Biol. Lett. 1, 253–255. ( 10.1098/rsbl.2005.0324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fitzpatrick JL, Willis C, Devigili A, Young AJ, Carroll M, Hunter HR, Brison DR. 2020. Chemical signals from eggs facilitate cryptic female choice in humans. Proc. R. Soc. B 287, 20200805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pham MN, et al. 2018. Do men produce higher quality ejaculates when primed with thoughts of partner infidelity? Evol. Psychol. 16, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gasparini C, Pilastro A, Evans JP. 2020. The role of female reproductive fluids in sperm competition. Phil. Trans. R. Soc. B 375, 20200077 ( 10.1098/rstb.2020.0077) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.