Abstract
Aggressive and cannibalistic female spiders can impose strong selection on male mating and fertilization strategies. Furthermore, the distinctive reproductive morphology of spiders is predicted to influence the outcome of sperm competition. Polyandry is common in spiders, leading to defensive male strategies that include guarding, plugging and self-sacrifice. Paternity patterns are highly variable and unlikely to be determined solely by mating order, but rather by relative copulation duration, deployment of plugs and cryptic female choice. The ability to strategically allocate sperm is limited, either by the need to refill pedipalps periodically or owing to permanent sperm depletion after mating. Further insights now rely on unravelling several proximate mechanisms such as the process of sperm activation and the role of seminal fluids.
This article is part of the theme issue ‘Fifty years of sperm competition’.
Keywords: sperm competition, spiders, cryptic female choice, monogyny, sexual cannibalism
1. Introduction
Since its conception marked by Geoff Parker's seminal paper on sperm competition [1], the fiftieth anniversary of which we honour with this review, sperm competition research has identified morphological, behavioural and ejaculate traits that convey selective advantages to males. It has, however, become increasingly clear that both sexes shape fertilization outcomes, with selection acting on female morphology or behaviour that biases fertilization towards preferred or compatible males. While cryptic female choice is often addressed separately or in opposition to sperm competition, recent research considers the reproductive interests of both sexes for a more comprehensive view of sperm competition.
Here, we argue that in spiders (48 692 described species; World Spider Catalogue [2]) the female role is central to the outcome of sperm competition. This may be why spiders have been key in the development of cryptic female choice in the first place [3]. While researchers have been charmed by some of their specialized behaviours, such as the ability to build silk webs, their distinctive reproductive biology poses a particularly costly challenge for males. Females can be aggressive, predatory, cannibalistic and often substantially larger, imposing strong selection on male mating and fertilization modes. Spider reproductive morphology, most importantly their paired genitalia, are advantageous for the study of post-mating selection as they afford the possibility to experimentally manipulate which male inseminates which spermatheca [4]. The presence of independent sperm storage sites, however, challenges general theoretical predictions because sperm from different males may not overlap spatially.
In this review, we provide an overview of post-copulatory sexual selection mechanisms in spiders with a focus on sperm competition. We first describe the reproductive biology of spiders, then we discuss female mating rates and male adaptations to competitive fertilization, before describing fertilization outcomes in spiders. We provide an overview of how copulation duration relates to sperm transfer and discuss strategic sperm allocation when sperm is limited.
2. Spider fertilization: an overview
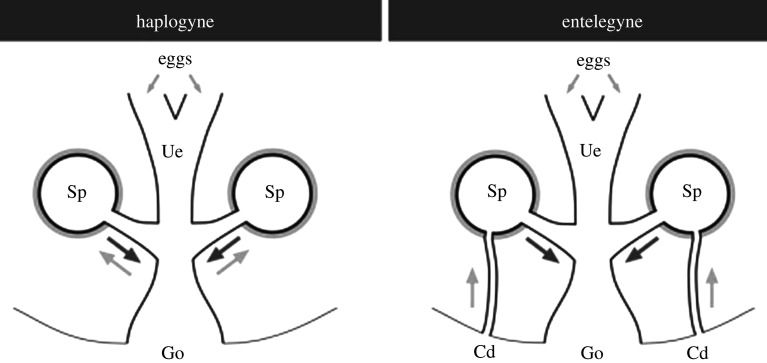
Male spiders possess paired secondary sperm transfer organs (pedipalps) that are not connected to the testes. Consequently, prior to mating, males have to charge their pedipalps with sperm. During sperm induction, males commonly build a small sperm web, release sperm from their genital opening onto it and dip their pedipalps into the droplet to uptake sperm. Sperm are then transferred to the female sperm storage organs (the spermathecae) through pedipalp insertions into the female copulatory openings. The female genital system can be categorized crudely into two types (figure 1). The entelegyne spermathecal type consists of two bilaterally symmetrical copulatory openings, each leading to a distinct sperm storage organ through an insemination duct. From each spermatheca, a fertilization duct leads to the oviduct where fertilization occurs. Fertilized eggs are laid through an oviposition opening that is separate from the copulatory opening. Spiders with a haplogyne spermathecal type possess a single opening that functions as a copulatory and oviposition opening and leads directly to the oviduct (uterus externus). Pouches, paired or multiple sperm storage organs, are connected to the oviduct and the eggs released by the ovary are fertilized in the oviduct by sperm from one of the spermathecae. In all spider species, sperm are encapsulated in the testes and arrive at the spermathecae in this inactive form. In the spermathecae, sperm are activated before oviposition, turning into motile sperm [6,7].
Figure 1.
Schematic representation of the entelegyne and haplogyne female genital systems from Uhl et al. [5]. Arrows indicate sperm entering the spermathecae (grey) for storage and exiting for fertilizing the eggs (black); Cd, copulatory duct; Go, genital opening; Sp, spermathecae; Ue, uterus externus. Reprint with permission from Springer Nature.
These features articulate important differences between spiders and other arthropods, and warrant special consideration within the current sperm competition paradigm. For instance, paired sperm storage organs linked to separate copulatory ducts in entelegyne spermathecal types implies that sperm of different males may not necessarily mix in storage unless the same copulatory opening is used by multiple males. Fertilization outcomes may therefore differ dramatically based on whether sperm from the storage site is activated and released differentially, with females potentially playing an active role in the decapsulation and activation of sperm (see references in [6]). Another important feature is the indirect mode of insemination that separates the amount of sperm available at copulation from the production site. Thus, the insemination ability of a male depends not only on the amount of sperm produced but also on the amount of sperm stored in its pedipalps. Moreover, the frequency and timing of sperm induction into the pedipalps, which varies across species, may have implications for male strategic sperm allocation and other reproductive decisions as described below.
3. Female multiple mating (polyandry)
Polyandry, which sets the stage for sperm competition, is taxonomically widespread [8], particularly in arthropods, for which monandry, mating with just one male, is the exception [9] and polyandry is probably the ancestral state [10]. Females that mate multiply are expected to collect direct and/or indirect benefits that exceed the costs of copulations beyond those needed to fertilize all eggs [9,11,12]. Spiders are no exception. Polyandrous females may gain indirect genetic benefits for their offspring (e.g. higher growth rates and offspring size [13]) via genetic bet-hedging (Linyphia litigiosa; [14,15]), inbreeding avoidance (Oedothorax apicatus; [16]) or by cryptically favouring sperm of unrelated partners (Argiope lobata; [17]). Alternatively, they may derive direct fecundity benefits such as increased egg-laying (Pholcus phalangioides; [13]), by either reducing the costs of rejections and/or foraging interference from males (Li. litigiosa; [18]). Resource and genetic benefits can also operate in concert. In the nuptial feeding spider Pisaura mirabilis, for instance, food donations from multiple mating partners lead to faster oviposition and multiple male ejaculates lead to higher hatching success [19]. Finally, polyandry may not necessarily be adaptive and females may mate multiply owing to sexual conflict, with males manipulating female re-mating behaviour (e.g. Stegodyphus lineatus [20,21]).
Accurate estimates of female mating rates not only ease interpretations on the adaptive value of polyandry, but address the evolutionary consequences of sperm competition, with implications for male mating strategies and patterns of sperm use. Unfortunately, the paucity of data available for spiders does not allow any general patterns to be drawn. In fact, only a handful of studies have investigated female mating frequencies in natural populations using laborious field observations of marked animals [22–25] or paternity assessment from cocoons using allozyme [26–30] or microsatellite [31] markers. The latter might provide an underestimate of the degree of polyandry, given that females can employ post-copulatory choice to use sperm from particular males, reducing the number of sires.
Evidence for polyandry comes instead from experimental laboratory studies using mostly double matings. Double mating trials in quick succession might not represent natural mating frequencies or capture realistic re-mating intervals, both of which might strongly affect female receptivity and sperm dynamics. Indeed, experimental studies often reveal that mated females are particularly reluctant to re-mate (examples across families reviewed in [32]). They are aggressive [33] and unattractive to males [34–36]. While a decrease in female receptivity following mating could reflect increased choosiness in mated females, or male manipulations such as mechanical or physiological effects caused by sperm and/or seminal fluids (e.g. Schizocosa malitiosa [37]), decreased receptivity may not necessarily rule out female polyandry. Females may resume sexual receptivity at later stages, even after egg-laying [38]. Australian redback spiders (Latrodectus hasselti), for example, cease advertising by modifying the chemical composition of their webs immediately after mating, but resume pheromone production months later, after breeding [39]. If this is common across species, re-mating rates are likely to be underestimated. Monandry is seldomly female-driven [40,41], but results from large travelling costs for mate-searching males and female-biased sex ratios [23,40,42,43], or is largely under male control, as discussed below.
4. Paternity protection and fertilization outcome
Spiders offer a spectacular array of male adaptations that protect paternity and exclude rivals [44,45], suggesting a high risk of sperm competition. Behavioural adaptations include mate guarding of the female against rivals either before or after copulation [33,46–51]. Cohabiting males respond agonistically to other males that enter the females' web, with aggression levels depending on male future reproductive prospects [52] or on the degree of paternity certainty [53,54]. Web manipulation (reducing the female's web, wrapping it up in their own silk and sometimes discarding it) is also common. This behaviour may reduce female attractiveness through reduced pheromone dissemination, or release male pheromones that deter other males and decrease female receptivity [55–57]. A rather extreme paternity protection strategy evolved independently in Larinia jeskovi and Cyclosa argentoalba. Here, males remove a female genital structure required for genital coupling [58–60], rendering re-mating impossible even though females remain receptive and attract males.
The males of many species defend their paternity by plugging the female genitalia [5]. Mating plugs may consist of amorphous masses visible on the female genital openings. These may not necessarily be male-derived however; both mating partners may jointly produce the plug [61]. Plugs can be extremely durable and last until oviposition [62], or can be removed partly or entirely by subsequent males, suggesting that female control and male quality affect plug efficacy [63,64]. Although the production of amorphous plugs can be costly [61], it does not prevent males from mating with several females [65]. However, mating plugs are formed from broken male genitalia left inside the female genital opening, which may come at the expense of the male's future reproduction. Depending on the species, either the entire pedipalp or the tip of the intromittent organ are detached [66], rendering the pedipalp dysfunctional after a single use [5]. One-shot genitalia that limit males to a maximum of two copulations are generally associated with a monogynous mating system, a male-biased sex ratio and extreme sexual dimorphism that have evolved several times independently in spiders [67–69]. Under conditions when more males than females mate, monogynous (or rarely bigynous) males that succeed in monopolizing paternity with a single female will gain above-average paternity [70]. Indeed, genital mating plugs can reduce re-mating probability of females considerably [5,71–73]. In some species sterile males survive and guard the female, in others they die during copulating with or without female intervention [66,74,75].
One of the most frequent adaptations to enhance fertilization success is transferring high numbers of sperm (e.g. [76,77]). If ejaculates differ in size, fertilization may become a function of the relative number of sperm transferred by each male, similar to a fair raffle [78]. Accordingly, P2 (the proportion of offspring sired by the second male) often relates to the relative copulation duration of two or more males (e.g. Latrodectus; [79], Argiope bruennichi; [80]; Pardosa agrestis [81]).
Ejaculate characteristics may interact with other important processes, such as storage modalities (e.g. stratification, displacement, sperm loss) to determine paternity outcomes. Mating order is often associated with skewed paternity in other invertebrates [76], with fertilizations biased towards the first or last male to mate. An early attempt to explain patterns of sperm precedence in spiders relied exclusively on female reproductive anatomy, suggesting the two separate ducts (one for insemination and one for fertilization) of entelegynes favours first-male sperm priority, in a first-in first-out fashion, and the single duct of haplogynes favours last-male sperm priority (last-in-first-out) [82]. Despite abundant empirical tests measuring P2 in double matings (electronic supplementary material, table S1), this hypothesis has been largely confuted. Spider spermathecal morphology is far more diverse [83] and the considerable variation in P2 reported in spiders (values range from 0% to 100%) argues against rigid first- or last-male priority patterns.
Strict first-male precedence can occur when sperm becomes a physical impediment for additional sperm to enter the storage organ or by plugging the female genital opening [5]. However, this pattern may vary with the effectiveness of the mating plug [84,85]. Pronounced last-male precedence can occur owing to sperm removal as in haplogyne pholcids (Ph. phalangioides and Holocnemus pluchei) in which males use pedipalp movements to reach the sperm storage site with shovel-like structures that seem to remove rival sperm [86,87].
Finally, female decisions during mating are pivotal for determining fertilization outcomes and interact inextricably with the processes described above. Females may for instance delay sexual cannibalism at mating, allowing preferred partners to copulate for longer and therefore transfer more sperm [78]. They can selectively store sperm from preferred males [17,88–90], or dump sperm from their spermathecae [91] to bias paternity outcomes.
5. Copulation duration and sperm transfer
Copulation duration among spiders can vary considerably between species, and in some cases within individuals. For example, certain cave-dwelling linyphiids copulate for 18 h while some orb-web spiders (e.g. Argiope spp.) only copulate for a few seconds [92]. Moreover, a male's copulation duration can change substantially between his first and second mating [93].
Intuitively, copulation duration should reflect sperm transfer resulting in a linear relationship between the amount of time in copulation and the amount of sperm stored. However, even if males have ceased sperm transfer, prolonged copulation duration may have a positive impact on fertilization success if it prevents females from ejecting sperm, mating with other males [94], if males remove the sperm from previous males [87] or perform copulatory courtship [86].
The relationship between copulation duration, sperm released by the male, sperm storage by the female and consequently fertilization success is complex. A male that copulates for longer may fertilize more eggs [78,89] because he transferred more sperm or because the female stored more of his sperm. Unravelling this complexity requires information on how much sperm the male released and how much of this sperm was stored by the female. Because the male pedipalps are paired, and most entelegyne species insert one pedipalp at a time, the amount of sperm released from a given pedipalp can be estimated by counting the amount of sperm in the unused pedipalp and the amount of sperm left in the used pedipalp [95].
To date, only a few studies have related copulation duration to sperm release and/or sperm storage in spiders (electronic supplementary material, table S2). While some studies report a linear increase of sperm release/storage with copulation duration, the majority reports no relationship (electronic supplementary material, table S2). It stands to reason that in species with very short copulations the main function of copulation is sperm transfer, in which case, a linear relationship is predicted. Species where copulation duration is much longer however, other functions, such as sperm removal, plugging and copulatory courtship are more likely to generate nonlinear relationships between copulation duration and sperm release/storage. More in-depth studies are needed to comprehensively test these predictions.
6. Strategic sperm allocation, when sperm limitation is at play
Theory predicts that males can maximize their fitness returns by strategically allocating sperm to females depending on the risk of sperm competition, their reproductive prospects (male age, female availability) or partner quality (female fecundity, age) [77,96]. Male spiders can distinguish female reproductive status and mating history [34,97], with males commonly investing more towards unmated females, performing enhanced copulatory behaviour [98,99], releasing more sperm [100] or discriminating against already inseminated genital openings [101] (but see [102]). Given its correlation with fecundity, female size is also an indicator of individual quality. Male Ph. phalangioides, for instance perform more pedipalp movements, which relate to sperm transfer, when mating with larger females [103]. When responding to sperm competition risk (presence of rivals), males are reported either to not adjust (Trichonephila senegalensis) [104] or to reduce (Pi. mirabilis) [105] sperm allocation.
Whether male spiders strategically tailor their sperm investment in response to the above-mentioned factors may depend largely on permanent or temporary sperm limitations, and whether males face physical danger during mating. In nephilids, araneids and theridiids, there is a phylogenetic signal for independently evolved severe sperm limitation [106,107]. In these spider families, the testes shut down sperm production after the male matures as an adult [93,107,108]. Consequently, the males of these species have no opportunity to refill their palps. Permanent sperm depletion appears to have coevolved with mono- or bigyny, genital mutilation and plugging of the female genitalia with male body parts [73]. As a result, males maximize their fertilizations by transferring as much of their sperm during a single copulation with their ‘one-shot genitalia’. Transferring as much sperm as possible during a single mating may represent a terminal investment strategy, especially when facing cannibalistic females. However, strategic sperm allocation is still predicted when trading off current versus future mating opportunities. For example, in T. senegalensis, a species with sperm depletion but no genital mutilation and cannibalism, males can mate up to four times by partitioning sperm among females [108].
In most spider species, however, testes actively produce sperm throughout the male's lifetime. While males in some species can successfully sire broods from two consecutive matings without reloading their palps [109,110], others deplete their entire sperm load after a single mating [94]. The degree of sperm depletion from the pedipalps (examples in the electronic supplementary material, table S2), and the timing and frequency of sperm induction is likely to determine strategic sperm allocation. In some species, sperm induction occurs during the mating sequence itself. In linyphiids, for example, initial copulation without transfer (pseudocopulation) is followed by a sequence of transfers and inductions [54,111]. In other spiders, induction occurs shortly after copulation [112] or at some point between matings with different females [65,113]. The associated costs of recharging the pedipalps may also vary. Cost may include the ability for males to build sperm webs [112,114,115], the risks of losing the mating partner or risk of predation associated with interrupting the mating sequence to reload palps.
7. Outlook
The last decades of sperm competition research in spiders has progressed our understanding of the evolutionary implications of male adaptations to competitive fertilization success tremendously, but the proximate mechanisms involved remain poorly understood. The most pressing outstanding questions include: how intense is sperm competition in natural populations? How does sperm storage (together or separately) affect sperm precedence patterns? What is the site and timing of sperm activation and how does it affect fertilization outcomes? What is the degree of sperm depletion in male pedipalps and what role does seminal fluid play? Integrating these functional processes with the fitness consequences of male traits will fill important gaps in our understanding of spider reproduction and evolution.
Supplementary Material
Supplementary Material
Data accessibility
This article has no additional data.
Authors' contributions
C.T. reviewed outline, literature search, analysis and writing; J.S. reviewed outline, literature search, analysis, and writing; G.U. reviewed outline, literature search, analysis, and writing; M.E.H. reviewed outline, literature search, analysis and writing.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Parker GA. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567. ( 10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 2.World Spider Catalogue. 2020. World spider catalogue, version 21.0. Natural History Museum, Bern, online at http://wsc.nmbe.ch (accessed on 02.08.2020) ( 10.24436/2) [DOI]
- 3.Eberhard W. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Eberhard WG. 2004. Why study spider sex: special traits of spiders facilitate studies of sperm competition and cryptic female choice. J. Arachnol. 32, 545–556. ( 10.1636/0161-8202(2004)032[0545:WSSSST]2.0.CO;2) [DOI] [Google Scholar]
- 5.Uhl G, Nessler SH, Schneider JM. 2010. Securing paternity in spiders? A review on occurrence and effects of mating plugs and male genital mutilation. Genetica 138, 75 ( 10.1007/s10709-009-9388-5) [DOI] [PubMed] [Google Scholar]
- 6.Herberstein ME, Schneider JM, Uhl G, Michalik P. 2011. Sperm dynamics in spiders. Behav. Ecol. 22, 692–695. ( 10.1093/beheco/arr053) [DOI] [Google Scholar]
- 7.Vöcking O, Uhl G, Michalik P. 2013. Sperm dynamics in spiders (Avraneae): ultrastructural analysis of the sperm activation process in the garden spider Argiope bruennichi (Scopoli, 1772). PLoS ONE 8, e72660 ( 10.1371/journal.pone.0072660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor M, Price T, Wedell N. 2014. Polyandry in nature: a global analysis. Trends Ecol. Evol. 27, 376–383. ( 10.1016/j.tree.2014.04.005) [DOI] [PubMed] [Google Scholar]
- 9.Arnqvist G, Nilsson T. 2000. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 60, 145–164. ( 10.1006/anbe.2000.1446) [DOI] [PubMed] [Google Scholar]
- 10.Kokko H, Mappes J. 2013. Multiple mating by females is a natural outcome of a null model of mate encounters. Entomol. Exp. Appl. 146, 26–37. ( 10.1111/j.1570-7458.2012.01296.x) [DOI] [Google Scholar]
- 11.Jennions MD, Petrie M. 2000. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. Camb. Philos. Soc. 75, 21–64. ( 10.1017/S0006323199005423) [DOI] [PubMed] [Google Scholar]
- 12.Simmons LW. 2005. The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu. Rev. Ecol. Evol. Syst. 36, 125–146. ( 10.1146/annurev.ecolsys.36.102403.112501) [DOI] [Google Scholar]
- 13.Uhl G, Schmitt S, Schäfer MA. 2005. Fitness benefits of multiple mating versus female mate choice in the cellar spider (Pholcus phalangioides). Behav. Ecol. Sociobiol. 59, 69 ( 10.1007/s00265-005-0010-2) [DOI] [Google Scholar]
- 14.Watson PJ. 1991. Multiple paternity as genetic bet-hedging in female sierra dome spiders, Linyphia litigiosa (Linyphiidae). Anim. Behav. 41, 343–360. ( 10.1016/S0003-3472(05)80486-5) [DOI] [Google Scholar]
- 15.Watson P. 1998. Multi-male mating and female choice increase offspring growth in the spider Neriene litigiosa (Linyphiidae). Anim. Behav. 55, 387–403. ( 10.1006/anbe.1997.0593) [DOI] [PubMed] [Google Scholar]
- 16.Bilde T, Maklakov AA, Schilling N. 2007. Inbreeding avoidance in spiders: evidence for rescue effect in fecundity of female spiders with outbreeding opportunity. J. Evol. Biol. 20, 1237–1242. ( 10.1111/j.1420-9101.2006.01280.x) [DOI] [PubMed] [Google Scholar]
- 17.Welke K, Schneider JM. 2009. Inbreeding avoidance through cryptic female choice in the cannibalistic orb-web spider Argiope lobata. Behav. Ecol. 20, 1056–1062. ( 10.1093/beheco/arp097) [DOI] [Google Scholar]
- 18.Watson PJ. 1993. Foraging advantage of polyandry for female sierra dome spiders (Linyphia litigiosa: Linyphiidae) and assessment of alternative direct benefit hypoteses. Am. Nat. 141, 440–465. ( 10.1086/285483) [DOI] [PubMed] [Google Scholar]
- 19.Tuni C, Albo MJ, Bilde T. 2013. Polyandrous females acquire indirect benefits in a nuptial feeding species. J. Evol. Biol. 26, 1307–1316. ( 10.1111/jeb.12137) [DOI] [PubMed] [Google Scholar]
- 20.Maklakov AA, Lubin Y. 2004. Sexual conflict over mating in a spider: increased fecundity does not compensate for the costs of polyandry. Evolution 58, 1135–1140. ( 10.1111/j.0014-3820.2004.tb00447.x) [DOI] [PubMed] [Google Scholar]
- 21.Schneider JM, Lubin Y. 1996. Infanticidal male eresid spiders. Nature 381, 655–656. ( 10.1038/381655a0) [DOI] [Google Scholar]
- 22.Zimmer SM, Welke KW, Schneider JM. 2012. Determinants of natural mating success in the cannibalistic orb-web spider Argiope bruennichi. PLoS ONE 7, e31389 ( 10.1371/journal.pone.0031389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuni C, Berger-tal R. 2012. High mortality and female-biased operational sex ratio result in low encounter rates and moderate polyandry in a spider. Biol. J. Linn. Soc. 107, 910–919. ( 10.1111/j.1095-8312.2012.01990.x) [DOI] [Google Scholar]
- 24.Masumoto T. 1991. Males' visits to females’ webs and female mating receptivity in the spider, Agelena limbata (Araneae: Agelenidae). J. Ethol. 9, 1–7. ( 10.1007/BF02350291) [DOI] [Google Scholar]
- 25.Segev O, Ziv M, Lubin Y. 2003. The male mating system in a desert widow spider. J. Arachnol. 31, 379–393. ( 10.1636/S01-101) [DOI] [Google Scholar]
- 26.Ramirez MG, Wight EC, Chirikian VA, Escobedo ES, Quezada LK, Schamberger A, Kagihara JA, Hoey CL. 2009. Evidence for multiple paternity in broods of the green lynx spider Peucetia viridans (Araneae: Oxyopidae). J. Arachnol. 37, 375–378. ( 10.1636/Hi09-04SC.1) [DOI] [Google Scholar]
- 27.Yoward PJ, Oxford GS. 2014. Evolutionary implications of sperm competition in the silver stretch spider, Tetragnatha montana Simon (Araneae: Tetragnathidae). Arachnology 16, 175–182. ( 10.13156/arac.2012.16.5.175) [DOI] [Google Scholar]
- 28.Martyniuk J, Jaenike J. 1982. Multiple mating and sperm usage patterns in natural populations of Prolinyphia marginata (Araneae: Linyphiidae). Ann. Entomol. Soc. Am. 75, 516–518. ( 10.1093/aesa/75.5.516) [DOI] [Google Scholar]
- 29.Oxford GS. 1993. Patterns of sperm usage in large house spiders (Tegenaria spp.): genetics of esterase markers. Heredity (Edinb). 70, 413–419. ( 10.1038/hdy.1993.58) [DOI] [Google Scholar]
- 30.Watson PJ. 1991. Multiple paternity and first mate sperm precedence in the sierra dome spider, Linyphia litigiosa Keyserling (Linyphiidae). Anim. Behav. 41, 135–148. ( 10.1016/S0003-3472(05)80509-3) [DOI] [Google Scholar]
- 31.Tuni C, Goodacre S, Bechsgaard J, Bilde T. 2012. Moderate multiple parentage and low genetic variation reduces the potential for genetic incompatibility avoidance despite high risk of inbreeding. PLoS ONE 7, e29636 ( 10.1371/journal.pone.0029636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elgar MA. 1998. Sperm competition and sexual selection in spiders. In Sperm competition and sexual selection (eds TR Birkhead, AP Møller), pp. 307–339. London, UK: Academic Press London. [Google Scholar]
- 33.Elgar MA, Bathgate R. 1996. Female receptivity and male mate-guarding in the jewel spider Gasteracantha minax thorell (Araneidae). J. Insect Behav. 9, 729–738. ( 10.1007/BF02213553) [DOI] [Google Scholar]
- 34.Rypstra AL, Wieg C, Walker SE, Persons MH. 2003. Mutual mate assessment in wolf spiders: differences in the cues used by males and females. Ethology 109, 315–325. ( 10.1046/j.1439-0310.2003.00874.x) [DOI] [Google Scholar]
- 35.Baruffaldi L, Costa FG. 2010. Changes in male sexual responses from silk cues of females at different reproductive states in the wolf spider Schizocosa malitiosa. J. Ethol. 28, 75–85. ( 10.1007/s10164-009-0158-8) [DOI] [Google Scholar]
- 36.Stoltz JA, McNeil JN, Andrade MCB. 2007. Males assess chemical signals to discriminate just-mated females from virgins in redback spiders. Anim. Behav. 74, 1669–1674. ( 10.1016/j.anbehav.2007.03.011) [DOI] [Google Scholar]
- 37.Aisenberg A, Costa FG. 2005. Females mated without sperm transfer maintain high sexual receptivity in the wolf spider Schizocosa malitiosa. Ethology 111, 545–558. ( 10.1111/j.1439-0310.2005.01077.x) [DOI] [Google Scholar]
- 38.Uhl G. 1993. Mating behaviour and female sperm storage in Pholcus phalangioides (Fuesslin)(Araneae). Mem. Queensl. Museum. Brisbane. 33, 667–674. [Google Scholar]
- 39.Perampaladas K, Stoltz JA, Andrade MCB. 2008. Mated redback spider females re-advertise receptivity months after mating. Ethology 114, 589–598. ( 10.1111/j.1439-0310.2008.01513.x) [DOI] [Google Scholar]
- 40.Norton S, Uetz GW. 2005. Mating frequency in Schizocosa ocreata (Hentz) wolf spiders: evidence for a mating system with female monandry and male polygyny. J. Arachnol. 33, 16–24. ( 10.1636/S02-72) [DOI] [Google Scholar]
- 41.Jiao X, Guo L, Chen Z, Wu J, Chen J, Liu F, Li D. 2011. Experimental evidence for female-driven monandry in the wolf spider, Pardosa astrigera. Behav. Ecol. Sociobiol. 65, 2117–2123. ( 10.1007/s00265-011-1220-4) [DOI] [Google Scholar]
- 42.Singer F, Riechert SE. 1995. Mating system and mating success of the desert spider Agelenopsis aperta. Behav. Ecol. Sociobiol. 36, 313–322. ( 10.1007/BF00167792) [DOI] [Google Scholar]
- 43.Berger-Tal R, Lubin Y. 2011. High male mate search costs and a female-biased sex ratio shape the male mating strategy in a desert spider. Anim. Behav. 82, 853–859. ( 10.1016/j.anbehav.2011.07.021) [DOI] [Google Scholar]
- 44.Schneider JM, Andrade MCB. 2011. Mating behaviour and sexual selection. In Spider behaviour: flexibility and versatility (ed. ME Herberstein), pp. 215–274. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 45.Huber BA. 2005. Sexual selection research on spiders: progress and biases. Biol. Rev. Camb. Philos. Soc. 80, 363–385. ( 10.1017/S1464793104006700) [DOI] [PubMed] [Google Scholar]
- 46.Calbacho-Rosa L, Córdoba-Aguilar A, Peretti AV. 2010. Occurrence and duration of post-copulatory mate guarding in a spider with last sperm precedence. Behaviour 147, 1267–1283. ( 10.1163/000579510X514544) [DOI] [Google Scholar]
- 47.Bel-Venner MC, Venner S. 2006. Mate-guarding strategies and male competitive ability in an orb-weaving spider: results from a field study. Anim. Behav. 71, 1315–1322. ( 10.1016/j.anbehav.2005.08.010) [DOI] [Google Scholar]
- 48.Cohn J, Balding FV, Christenson TE.. 1988. In defense of Nephila clavipes: postmate guarding by the male golden orb-weaving spider. J. Comp. Psychol. 102, 319 ( 10.1037/0735-7036.102.4.319) [DOI] [Google Scholar]
- 49.Elias DO, Sivalinghem S, Mason AC, Andrade MCB, Kasumovic MM. 2014. Mate-guarding courtship behaviour: tactics in a changing world. Anim. Behav. 97, 25–33. ( 10.1016/j.anbehav.2014.08.007) [DOI] [Google Scholar]
- 50.Herberstein ME, Barry KL, Turoczy MA, Wills E, Youssef C, Elgar MA. 2005. Post-copulation mate guarding in the sexually cannibalistic St Andrew's Cross spider (Araneae Araneidae). Ethol. Ecol. Evol. 17, 17–26. ( 10.1080/08927014.2005.9522612) [DOI] [Google Scholar]
- 51.Prenter J, Elwood RW, Montgomery IW. 2003. Mate guarding, competition and variation in size in male orb-web spiders, Metellina segmentata: a field experiment. Anim. Behav. 66, 1053–1058. ( 10.1006/anbe.2003.2266) [DOI] [Google Scholar]
- 52.Fromhage L, Schneider JM. 2005. Virgin doves and mated hawks: contest behaviour in a spider. Anim. Behav. 70, 1099–1104. ( 10.1016/j.anbehav.2005.02.020) [DOI] [Google Scholar]
- 53.Austad SN. 1983. A game theoretical interpretation of male combat in the bowl and doily spider (Frontinella pyramitela). Anim. Behav. 31, 59–73. ( 10.1016/S0003-3472(83)80173-0) [DOI] [Google Scholar]
- 54.Austad SN. 1982. First male sperm priority in the bowl and doily spider, Frontinella pyramitela (Walckenaer). Evolution 36, 777–785. ( 10.1111/j.1558-5646.1982.tb05444.x) [DOI] [PubMed] [Google Scholar]
- 55.Scott C, Kirk D, McCann S, Gries G. 2015. Web reduction by courting male black widows renders pheromone-emitting females' webs less attractive to rival males. Anim. Behav. 107, 71–78. ( 10.1016/j.anbehav.2015.06.009) [DOI] [Google Scholar]
- 56.Watson PJ. 1986. Transmission of a female sex pheromone thwarted by males in the spider Linyphia litigiosa (Linyphiidae). Science 233, 219–221. ( 10.1126/science.3726530) [DOI] [PubMed] [Google Scholar]
- 57.Fischer A, Goh XH, Varney J-LS, Blake AJ, Takács S, Gries G. 2020. Multimodal and multifunctional signaling?–Web reduction courtship behavior in a North American population of the false black widow spider. PLoS ONE 15, e0228988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mouginot P, Prügel J, Thom U, Steinhoff POM, Kupryjanowicz J, Uhl G. 2015. Securing paternity by mutilating female genitalia in spiders. Curr. Biol. 25, 2980–2984. ( 10.1016/j.cub.2015.09.074) [DOI] [PubMed] [Google Scholar]
- 59.Nakata K. 2016. Female genital mutilation and monandry in an orb-web spider. Biol. Lett. 12, 20150912 ( 10.1098/rsbl.2015.0912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mouginot P, Uhl G, Fromhage L. 2017. Evolution of external female genital mutilation: why do males harm their mates? R. Soc. Open Sci. 4, 171195 ( 10.1098/rsos.171195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aisenberg A, Eberhard WG. 2009. Female cooperation in plug formation in a spider: effects of male copulatory courtship. Behav. Ecol. 20, 1236–1241. ( 10.1093/beheco/arp117) [DOI] [Google Scholar]
- 62.Kunz K, Witthuhn M, Uhl G. 2014. Do the size and age of mating plugs alter their efficacy in protecting paternity? Behav. Ecol. Sociobiol. 68, 1321–1328. ( 10.1007/s00265-014-1742-7) [DOI] [Google Scholar]
- 63.Sentenská L, Pekár S, Uhl G. 2018. Deposition, removal and production site of the amorphous mating plug in the spider Philodromus cespitum. Sci. Nat. 105, 50 ( 10.1007/s00114-018-1575-8) [DOI] [PubMed] [Google Scholar]
- 64.Sentenská L, Pekár S, Lipke E, Michalik P, Uhl G. 2015. Female control of mate plugging in a female-cannibalistic spider (Micaria sociabilis). BMC Evol. Biol. 15, 18 ( 10.1186/s12862-014-0278-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uhl G, Kunz K, Vöcking O, Lipke E. 2014. A spider mating plug: origin and constraints of production. Biol. J. Linn. Soc. 113, 345–354. ( 10.1111/bij.12359) [DOI] [Google Scholar]
- 66.Kuntner M, Agnarsson I, Li D. 2015. The eunuch phenomenon: adaptive evolution of genital emasculation in sexually dimorphic spiders. Biol. Rev. 90, 279–296. ( 10.1111/brv.12109) [DOI] [PubMed] [Google Scholar]
- 67.Miller JA. 2007. Repeated evolution of male sacrifice behavior in spiders correlated with genital mutilation. Evol. Int. J. Org. Evol. 61, 1301–1315. ( 10.1111/j.1558-5646.2007.00115.x) [DOI] [PubMed] [Google Scholar]
- 68.Kuntner M, Coddington JA. 2020. Sexual size dimorphism: evolution and perils of extreme phenotypes in spiders. Annu. Rev. Entomol. 65, 57–80. ( 10.1146/annurev-ento-011019-025032) [DOI] [PubMed] [Google Scholar]
- 69.Schneider J, Fromhage L. 2010. Monogynous mating strategies in spiders. In Animal behaviour: evolution and mechanisms (ed. P Kappeler), pp. 441–464. Berlin, Germany: Springer. [Google Scholar]
- 70.Fromhage L, Elgar MA, Schneider JM. 2005. Faithful without care: the evolution of monogyny. Evolution 59, 1400–1405. ( 10.1111/j.0014-3820.2005.tb01790.x) [DOI] [PubMed] [Google Scholar]
- 71.Nessler SH, Uhl G, Schneider JM. 2007. Genital damage in the orb-web spider Argiope bruennichi (Araneae: Araneidae) increases paternity success. Behav. Ecol. 18, 174–181. ( 10.1093/beheco/arl074) [DOI] [Google Scholar]
- 72.Fromhage L, Schneider JM. 2006. Emasculation to plug up females: the significance of pedipalp damage in Nephila fenestrata. Behav. Ecol. 17, 353–357. ( 10.1093/beheco/arj037) [DOI] [Google Scholar]
- 73.Herberstein ME, Wignall AE, Nessler SH, Harmer AMT, Schneider JM. 2012. How effective and persistent are fragments of male genitalia as mating plugs? Behav. Ecol. 23, 1140–1145. ( 10.1093/beheco/ars088) [DOI] [Google Scholar]
- 74.Kralj-Fišer S, Gregorič M, Zhang S, Li D, Kuntner M. 2011. Eunuchs are better fighters. Anim. Behav. 81, 933–939. ( 10.1016/j.anbehav.2011.02.010) [DOI] [Google Scholar]
- 75.Uhl G, Zimmer SM, Renner D, Schneider JM. 2015. Exploiting a moment of weakness: male spiders escape sexual cannibalism by copulating with moulting females. Sci. Rep. 5, 16928 ( 10.1038/srep16928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Birkhead TR, Hunter FM. 1990. Mechanisms of sperm competition. Trends Ecol. Evol. 5, 48–52. ( 10.1016/0169-5347(90)90047-H) [DOI] [PubMed] [Google Scholar]
- 77.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934. ( 10.1086/656840) [DOI] [PubMed] [Google Scholar]
- 78.Elgar MA, Schneider JM, Herberstein ME. 2000. Female control of paternity in the sexually cannibalistic spider Argiope keyserlingi. Proc. Biol. Sci. 267, 2439–2443. ( 10.1098/rspb.2000.1303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andrade MCB. 1996. Sexual selection for male sacrifice in the Australian redback spider. Science 271, 70–72. ( 10.1126/science.271.5245.70) [DOI] [Google Scholar]
- 80.Schneider JM, Gilberg S, Fromhage L, Uhl G. 2006. Sexual conflict over copulation duration in a cannibalistic spider. Anim. Behav. 71, 781–788. ( 10.1016/j.anbehav.2005.05.012) [DOI] [Google Scholar]
- 81.Kiss B, Rádai Z, Toft S, Samu F. 2019. Sperm competition tactics shape paternity: adaptive role of extremely long copulations in a wolf spider. Anim. Behav. 156, 121–128. ( 10.1016/j.anbehav.2019.08.013) [DOI] [Google Scholar]
- 82.Austad SN. 1984. Evolution of sperm priority patterns in spiders. In Sperm Compet. Evol. Anim. mating Syst. (ed. RL Smith), pp. 223–249. Orlando, FL: Academic Press. [Google Scholar]
- 83.Uhl G. 2000. Female genital morphology and sperm priority patterns in spiders (Araneae). Eur. Arachnol. 2000, 145–156. [Google Scholar]
- 84.Masumoto T. 1993. The effect of the copulatory plug in the funnel-web spider, Agelena limbata (Araneae: Agelenidae). J. Arachnol. 21, 55–59. [Google Scholar]
- 85.Snow LSE, Abdel-Mesih A, Andrade MCB. 2006. Broken copulatory organs are low-cost adaptations to sperm competition in redback spiders. Ethology 112, 379–389. ( 10.1111/j.1439-0310.2006.01163.x) [DOI] [Google Scholar]
- 86.Schäfer MA, Uhl G. 2002. Determinants of paternity success in the spider Pholcus phalangioides (Pholcidae: Araneae): the role of male and female mating behaviour. Behav. Ecol. Sociobiol. 51, 368–377. ( 10.1007/s00265-001-0448-9) [DOI] [Google Scholar]
- 87.Calbacho-Rosa L, Galicia-Mendoza I, Dutto MS, Córdoba-Aguilar A, Peretti AV. 2013. Copulatory behavior in a pholcid spider: males use specialized genitalic movements for sperm removal and copulatory courtship. Naturwissenschaften 100, 407–416. ( 10.1007/s00114-013-1038-1) [DOI] [PubMed] [Google Scholar]
- 88.Albo MJ, Bilde T, Uhl G.. 2013. Sperm storage mediated by cryptic female choice for nuptial gifts. Proc. R. Soc. B 280, 20131735 ( 10.1098/rspb.2013.1735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schneider JM, Lesmono K.. 2009. Courtship raises male fertilization success through post-mating sexual selection in a spider. Proc. R. Soc. B 276, 3105–3111. ( 10.1098/rspb.2009.0694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Albo MJ, Costa FG. 2017. Female wolf spiders exert cryptic control drastically reducing ejaculate size. Ethology 123, 659–666. ( 10.1111/eth.12640) [DOI] [Google Scholar]
- 91.Burger M. 2010. Complex female genitalia indicate sperm dumping in armored goblin spiders (Arachnida, Araneae, Oonopidae). Zoology 113, 19–32. ( 10.1016/j.zool.2009.04.002) [DOI] [PubMed] [Google Scholar]
- 92.Mammola S, Michalik P, Hebets EA, Isaia M. 2017. Record breaking achievements by spiders and the scientists who study them. PeerJ 5, e3972 ( 10.7717/peerj.3972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herberstein ME, Gaskett AC, Schneider JM, Vella NGF, Elgar MA. 2005. Limits to male copulation frequency: sexual cannibalism and sterility in St Andrew's Cross spiders (Araneae, Araneidae). Ethology 111, 1050–1061. ( 10.1111/j.1439-0310.2005.01114.x) [DOI] [Google Scholar]
- 94.Linn CD, Molina Y, Difatta J, Christenson TE. 2007. The adaptive advantage of prolonged mating: a test of alternative hypotheses. Anim. Behav. 74, 481–485. ( 10.1016/j.anbehav.2007.02.004) [DOI] [Google Scholar]
- 95.Gabel E, Uhl G. 2013. How to prepare spider sperm for quantification. Arachnology 16, 109–112. ( 10.13156/100.016.0301) [DOI] [Google Scholar]
- 96.Wedell N, Gage MJG, Parker GA. 2002. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320. ( 10.1016/S0169-5347(02)02533-8) [DOI] [Google Scholar]
- 97.Tuni C, Berger-Tal R. 2012. Male preference and female cues: males assess female sexual maturity and mating status in a web-building spider. Behav. Ecol. 23, 582–587. ( 10.1093/beheco/ars001) [DOI] [Google Scholar]
- 98.Elgar M, Gaskett A, Downes B, Herberstein M. 2004. Changes in male mate choice in a sexually cannibalistic orb-web spider (Araneae: Araneidae). Behaviour 141, 1197–1210. ( 10.1163/1568539042729676) [DOI] [Google Scholar]
- 99.Morse DH. 2010. Male mate choice and female response in relation to mating status and time since mating. Behav. Ecol. 21, 250–256. ( 10.1093/beheco/arp183) [DOI] [Google Scholar]
- 100.Bukowski TC, Christenson TE. 1997. Determinants of sperm release and storage in a spiny orbweaving spider. Anim. Behav. 53, 381–395. ( 10.1006/anbe.1996.0329) [DOI] [Google Scholar]
- 101.Jones TM, Elgar MA. 2008. Male insemination decisions and sperm quality influence paternity in the golden orb–weaving spider. Behav. Ecol. 19, 285–291. ( 10.1093/beheco/arm126) [DOI] [Google Scholar]
- 102.Danielson-François AM, Bukowski TC. 2005. Female mating history influences copulation behavior but not sperm release in the orb-weaving spider Tetragnatha versicolor (Araneae, Tetragnathidae). J. Insect Behav. 18, 131–148. ( 10.1007/s10905-005-9352-x) [DOI] [Google Scholar]
- 103.Schäfer MA, Misof B, Uhl G. 2008. Effects of body size of both sexes and female mating history on male mating behaviour and paternity success in a spider. Anim. Behav. 76, 75–86. ( 10.1016/j.anbehav.2008.01.011) [DOI] [Google Scholar]
- 104.Schneider JM, Lucass C, Brandler W, Fromhage L. 2011. Spider males adjust mate choice but not sperm allocation to cues of a rival. Ethology 117, 970–978. ( 10.1111/j.1439-0310.2011.01960.x) [DOI] [Google Scholar]
- 105.Tuni C, Weber S, Bilde T, Uhl G. 2017. Male spiders reduce pre-and postmating sexual investment in response to sperm competition risk. Behav. Ecol. 28, 1030–1036. ( 10.1093/beheco/arx061) [DOI] [Google Scholar]
- 106.Michalik P, Ramírez MJ. 2014. Evolutionary morphology of the male reproductive system, spermatozoa and seminal fluid of spiders (Araneae, Arachnida): current knowledge and future directions. Arthropod Struct. Dev. 43, 291–322. ( 10.1016/j.asd.2014.05.005) [DOI] [PubMed] [Google Scholar]
- 107.Michalik P, Rittschof CC. 2011. A comparative analysis of the morphology and evolution of permanent sperm depletion in spiders. PLoS ONE 6, e16014 ( 10.1371/journal.pone.0016014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schneider JM, Michalik P. 2011. One-shot genitalia are not an evolutionary dead end: regained male polygamy in a sperm limited spider species. BMC Evol. Biol. 11, 197 ( 10.1186/1471-2148-11-197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Costa FG. 1998. Copulatory pattern and fertilization success in male wolf spiders without pre-or post-copulatory sperm induction. J. Arachnol. 26 106–112. [Google Scholar]
- 110.Fernández-Montraveta C, Cuadrado M. 2013. Hogna radiata males do not deplete their sperm in a single mating. J. Arachnol. 41, 102–107. ( 10.1636/Hi12-72.1) [DOI] [Google Scholar]
- 111.Weldingh DL, Toft S, Larsen ON. 2011. Mating duration and sperm precedence in the spider Linyphia triangularis. J. Ethol. 29, 143–152. ( 10.1007/s10164-010-0237-x) [DOI] [Google Scholar]
- 112.Buffet CR, Viera C. 2016. Loading the male pedipalps: sperm induction in a subsocial spider. J. Arachnol. 44, 96–98. ( 10.1636/J15-57.1) [DOI] [Google Scholar]
- 113.Gong D, Zhang S, Jiao X, Hu Z, Sha X, Zhang S, Peng Y. 2019. Mating experience affects male mating success, but not female fecundity in the wolf spider Pardosa pseudoannulata (Araneae: Lycosidae). Behav. Processes 167, 103921 ( 10.1016/j.beproc.2019.103921) [DOI] [PubMed] [Google Scholar]
- 114.Eberhard WG, Huber BA. 1998. Courtship, copulation, and sperm transfer in Leucauge mariana (Araneae, Tetragnathidae) with implications for higher classification. J. Arachnol. 26, 342–368. [Google Scholar]
- 115.Costa FG, Pérez-Miles F. 2002. Reproductive biology of uruguayan theraphosids (Araneae, Mygalomorphae). J. Arachnol. 30, 571–587. ( 10.1636/0161-8202(2002)030[0571:RBOUTA]2.0.CO;2) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.