Abstract
Fifty years of research on sperm competition has led to a very good understanding of the interspecific variation in sperm production traits. The reasons why this variation is often very large within populations have been less investigated. We suggest that the interaction between fluctuating environmental conditions and polyandry is a key phenomenon explaining such variation. We focus here on imminent predation risk (IPR). IPR impacts significantly several aspects of prey behaviour and reproduction, and it is expected to influence the operation of sexual selection before and after mating. We estimated the effect of IPR on the male opportunity for pre- and postcopulatory sexual selection in guppies (Poecilia reticulata), a livebearing fish where females prefer colourful males and mate multiply. We used a repeated-measures design, in which males were allowed to mate with different females either under IPR or in a predator-free condition. We found that IPR increased the total opportunity for sexual selection and reduced the relative contribution of postcopulatory sexual selection to male reproductive success. IPR is inherently variable and our results suggest that interspecific reproductive interference by predators may contribute towards maintaining the variation in sperm production within populations.
This article is part of the theme issue ‘Fifty years of sperm competition'.
Keywords: sperm competition, genetic variance, interspecific reproductive interference, total opportunity for sexual selection
1. Introduction
Polyandry, whereby females mate with multiple males during a single reproductive period, is nearly ubiquitous in the animal kingdom [1] and can extend male–male competition after mating in the form of sperm competition, where sperm from rival males compete to fertilize ova [2]. The number of ejaculated sperm is one of the most important determinants of sperm competition success [3,4]. Differences in the level of sperm competition explain the large variation in sperm production both among species [5,6] and among populations of the same species [7–9].
With the exception of species with alternative male mating tactics [10–12], within-population variation in sperm production should be less pronounced than variation among species or populations, because males from the same population experience similar levels of sperm competition. By contrast, phenotypic and genetic variation in sperm production is often substantial, usually larger than that of other traits under postcopulatory sexual selection [13–15]. This is surprising considering that sperm production has non-trivial costs [16–18], and there should be little variation around the optimal sperm investment expected for the level of sperm competition experienced by the males of the same population. While interspecific variation in sperm production is well understood theoretically [19] and supported empirically [6,20], the causes of this large within-population variation in sperm production are less clear.
Recently, it has been shown that the relative importance of pre- and postcopulatory sexual selection (and their interaction) can fluctuate significantly under variable environmental conditions [21]. For example, population density [22–25] or short-term food limitation [26,27] can significantly alter sexual selection dynamics, including sperm competition. Within-population variability in the strength of sperm competition may also result from interaction with heterospecifics, which may interfere with the operation of sexual selection at many levels [28].
A heterospecific interaction that is ecologically relevant and inherently variable is the imminent risk of predation. Non-consumptive effects of predation significantly impact prey demography [29] and can be even larger than lethal effects [30]. Behavioural and physiological responses to imminent predation risk (hereafter IPR) are particularly relevant for reproduction [31,32] and are expected to strongly influence the operation of sexual selection. The relevance of predation risk for precopulatory sexual selection is obvious: mate searching increases predation risk [33], and conspicuous intra- and intersexual signals are likely to attract predators [34,35]. Indeed, IPR can affect female sexual responsiveness [36] and choosiness [37,38]. Postcopulatory sexual traits are generally inconspicuous to predators [39], and probably, for this reason, the effect of IPR on postcopulatory sexual selection has received relatively less attention. However, sperm competition is mediated by female polyandry [40], which is likely to vary under IPR ([41], but see [42]), possibly weakening the strength of sperm competition. IPR can also affect the operation of sexual selection indirectly, for example, by influencing prey social structure [43] and, consequently, males' access to females [32]. Other environmental factors like food availability, population density or sex ratio are variable and can influence the strength of sperm competition [22,25,26]. IPR, however, is inherently variable over short time and space scales [44–46] and may therefore be a key factor in the maintenance of genetic variability in sperm production. If IPR changes rapidly and unpredictably, affecting, for example, female mating rate, female choosiness and hence males' mating opportunity, the strength of selection on sperm production may continuously vary within populations. To test this hypothesis, it is necessary to combine measures of pre- and postcopulatory selection across contrasting IPR gradients using a total sexual selection approach [21]. This is because in promiscuous species the strength of selection on sperm production depends on female mating rate, but it is also influenced by concomitant changes in precopulatory selection [40] and in the covariation between pre- and postcopulatory selection in response to IPR [21].
The guppy (Poecilia reticulata), the first species used in a sperm competition experiment 100 years ago [47], is an ideal model for testing whether IPR affects the strength of postcopulatory sexual selection. This livebearing fish is characterized by strong precopulatory selection, largely influenced by female preference for male courtship rate and coloration [48], and by a high level of polyandry [49]. Pre- and postcopulatory sexual selection dynamics have been quantified in both static [50] and variable environments [27]. On its native island of Trinidad, variation in predation intensity is a key driver of consistent interpopulation evolutionary differences in life-history strategies, including reproductive investment and behaviour [51,52]. Adaptations to predation include plastic behavioural responses to predation risk cues, which are well documented in both the field and the laboratory [53], even in domestic guppies [54]. These allow for ecologically realistic manipulations of IPR under controlled conditions [55,56], and for separating the effects on sexual selection processes that are due to selective mortality from those associated with prey's antipredator responses. Finally, guppies show a very large phenotypic and genetic variance in sperm production, as estimated from the number of sperm stripped from rested males [14,57]. In the present study, we tested whether IPR affected the operation of sexual selection using a repeated-measures design with the same males tested both in the presences and absence of predation cues with different females. We predicted an increase in female social cohesion, a typical antipredator response in this species [43], a reduction in mean mating rate and a relative increase in the importance of pre- versus postcopulatory sexual selection in response to imminent predation risk.
2. Methods
(a). Experiment design
Guppies used in this experiment are descendants of a stock collected from Lower Tacarigua river in Trinidad, a high-predation site inhabited by several predator species, and maintained as a self-sustained population at the Botanical Garden of the University of Padova [27]. We used a repeated-measures design, with 20 sets of six adult males. Each set of males was allowed to mate sequentially with two different sets of six virgin females (amounting to a total of 40 sets of females), either in the presence of cues of imminent predation risk (IPR), or in their absence (control, hereafter C; electronic supplementary material, figure S1). Before each mating trial, the sets of males and virgin females were housed separately. Mating trials were conducted over 2 consecutive days (3 h per day) and were separated by 6 days between treatments, with treatment order balanced. We used a repeated-measures design because male mating, insemination and competitive fertilization success are significantly repeatable across females [58,59]. We simulated IPR using visual (predator models consisting of a fish bait resembling a pike cichlid Crenicichla alta, 10.8–12.5 cm long) and olfactory stimuli (adult guppy skin extract mimicking a predation event), following an established procedure [55]. In the IPR group, a predator attack was simulated in the afternoon before each mating trial (both model and skin extract) and before the start of mating trials (skin extract only). In the control treatment, males and females underwent the same housing and mating trial procedures, but in the absence of any predation cues (see electronic supplementary material). After the mating trial, females were isolated into individual tanks until parturition, or for a maximum of 50 days.
(b). Behavioural observations
During each mating trial, three 30 min long behavioural observations were carried out at 30 min intervals (total observation time over the 2 days = 3 h). One observer recorded the time when all six females dispersed (i.e. each female was at a distance more than two body lengths from the other females), and when at least two females in the group were within less than two body lengths distance. Female position was scored every 15 s for the duration of the behavioural observations, amounting to a total of 720 datapoints per female set, and the proportion of times females were not all dispersed was considered an index of social cohesion [60]. Because of logistic problems (second day's observations were missing), one C replicate was excluded from shoaling data analyses. During the same observation sessions, for each male, individually recognizable from colour patterns [27,50], we recorded the number of courtship displays (sigmoid displays, SD), coercive mating attempts (gonopodial thrusts, GT) and the number of successful copulations (hereafter male copulation success, MCS), i.e. copulations followed by postcopulatory jerks performed by the male [61].
(c). Paternity assignment
Caudal fin clips were collected from males after the second mating treatment and from females after parturition. Offspring were sacrificed when 24–48 h old with an overdose of MS222. All samples were stored in absolute ethanol at −20°C until analyses. We assigned paternity from two hypervariable microsatellite loci using Cervus 3.0.7 (http://www.fieldgenetics.com, [62]), with a 95% confidence (further details in the electronic supplementary material). One male died between treatments and was excluded from all analyses. A total of 195 females (81.25%) produced broods, 193 of which consisted of at least 3 young and were retained for paternity analyses. We obtained unambiguous paternity for all but one IPR brood, amounting to 92% of all offspring (IPR: 725/774, 93%; C: 695/758, 89%). Our final paternity dataset included 1420 offspring from 192 females (IPR, N = 99; C, N = 94) and 119 males.
(d). Statistical analyses
For each male in each treatment, we determined male mating success (MMS) as the number of females with which a given male produced offspring divided by the number of females that produced a brood in each female set (genetic MMS, sensu [63]); male reproductive success (MRS), as the number of offspring sired by a given male divided by the number of offspring produced by the set of females; postcopulatory success (PCS), as the mean paternity share of offspring produced by the females successfully inseminated by a male, corrected by the number of males that fertilized the eggs of a given female [50]; and fecundity of mates (FEC), as the average fecundity of females with which a given male produced offspring divided by the average number of offspring produced within the female set [27].
We used linear mixed models (LMM) to compare the proportion of time shoaling between treatments, with treatment and day as fixed factors, and male replicate as a random factor. We used LMM or generalized LMM (according to dependent variable distribution, see Results and electronic supplementary material), with treatment as a fixed factor and replicate and male identity as random factors, to compare male sexual behaviour (MCS, SDs and proportion of SDs out of total sexual behaviours) between IPR and C. Similarly, we investigated whether IPR affected mean female reproductive success (FRS) and polyandry (sires per brood, SPB). For the calculation of FRS, we considered only the offspring with assigned paternity. For the calculation of SPB, we excluded seven females with FRS less than 3, because of the low probability of detecting multiple sires in small broods. Final sample size for SPB was 185 females (C = 90; IPR = 95). Standardized variances in MCS, MMS, MRS and PCS (var./mean2) were used to estimate the opportunity for precopulatory sexual selection (IMCS and IMMS), total sexual selection (IMRS) and postcopulatory sexual selection (IPCS), respectively [64]. We compared the observed difference in these statistics between treatments with a random distribution of the differences obtained by shuffling each male's reproductive success across treatments, using a Monte Carlo simulation (10 000 iterations). The significance of the observed difference between standardized variances was calculated as the proportion of iterations in which the random difference was larger than the observed one. The same approach was used for comparing standardized variances in FRS and SPB. Bateman gradients were calculated as the slope of ordinary least-squares regressions of MRS on MMS with their 95% confidence interval (CI), both excluding and including from each treatment males with MRS and MMS = 0 [63]. Finally, we estimated the relative contribution of MMS, PCS, FEC and their covariances on the variance in male reproductive success in IPR and C following Webster et al. [65]. The difference in the (co)variance components between IPR and C were compared with a random distribution of the differences obtained as above. We used a bootstrap procedure to calculate standard error of standardized variances and covariances. If not otherwise stated, mean ± s.e. are given (see electronic supplementary material for further details).
3. Results
(a). Effect of imminent predation risk on female shoaling and male sexual behaviour
Both female shoaling and male mating behaviour differed significantly between the two treatments according to predictions: IPR females spent a larger proportion of time shoaling (48.4%) compared to their control counterparts (37.6%; treatment: F1,36 = 4.387, p = 0.043; day: F1,37 = 2.542, p = 0.19; random factor, male replicate: z = 3.280, p = 0.001, arcsine transformation). IPR males reduced their courtship rate (SD) both in absolute terms (C = 27.78 ± 1.68, IPR = 23.47 ± 1.59, F1,123 = 8.825, p = 0.004; male.ID, z = 2.464, p = 0.014, Poisson error distribution with log link), and in relation to the total mating attempts (proportion of SD over SD + GT: C = 0.78 ± 0.015, IPR = 0.74 ± 0.015, F1,94 = 8.772, p = 0.004, male.ID: z = 2.092, p = 0.036; binomial error distribution with logit link). Mean MCS was significantly lower in the IPR treatment (C = 1.370 ± 0.102, IPR = 0.891 ± 0.093, F1,129 = 16.82, p < 0.0001, male.ID: z = 2.555, p = 0.011, Poisson error distribution with log link), but the standardized variance in MCS (IMCS) was significantly larger (IPR, 1.234 ± 0.191; C, 0.640 ± 0.098, p = 0.0011), as compared to controls.
(b). Effect of imminent predation risk on female reproductive success and polyandry
FRS did not differ between treatments either when considering brood size mean (C = 7.394 ± 0.310, n = 94; IPR = 7.398 ± 0.303, n = 98; treatment: F1,190 = 0.001, p = 0.99), or its standardized variance (IFRS: C = 0.164 ± 0.028; IPR = 0.163 ± 0.024, difference = 0.01, p = 0.48). SPB varied between 1 and 4, except for one female with six SPB. Considering only broods larger than 2 (N = 185), frequency of SPB differed significantly between groups (Fisher exact test, p = 0.044). In particular, C females produced a higher proportion of broods with 3 or more sires (25.6%) as compared to IPR females (11.6%; electronic supplementary material, figure S2).
(c). Effect of imminent predation risk on sexual selection in males
Across treatments, males showed a significant, although not large, repeatability in MMS (R = 0.198 ± 0.088, F1,118 = 1.494, p = 0.015) and MRS (R = 0.172 ± 0.089, F1,118 = 1.415, p = 0.03, arcsine transformation). The opportunities for precopulatory and total sexual selection were significantly larger in the IPR treatment (IMMS: C = 0.401 ± 0.060; IPR = 0.586 ± 0.087, difference = 0.185, p = 0.035; IMRS: C = 0.619 ± 0.087; IPR = 1.055 ± 0.175, difference = 0.436, p = 0.016). By contrast, the standardized variance in postcopulatory success did not differ between treatments (IPCS: C = 0.123 ± 0.0157; IPR = 0.129 ± 0.0159, difference = 0.006, p = 0.399, figure 1).
Figure 1.
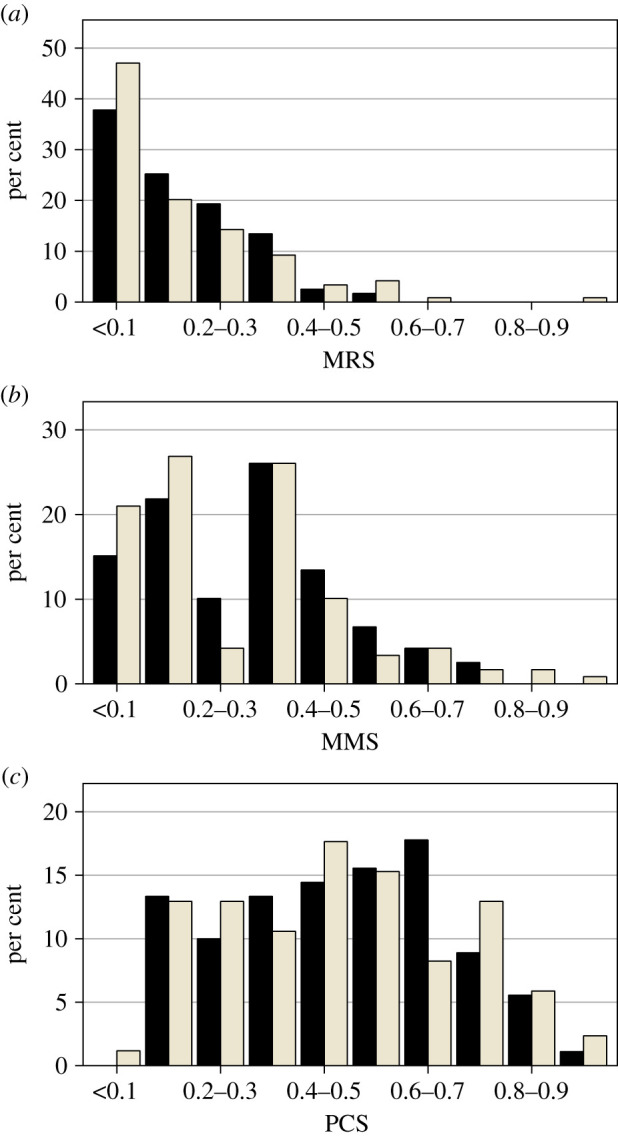
Distribution of (a) male reproductive success, (b) male mating success (number of females a male produced offspring with) and (c) mean fertilization success (standardized for the number of sperm competitors). Bar colours represent mating trials under control conditions (C, black) and under imminent predation risk (IPR, grey).
(d). Bateman gradients and variance partitioning
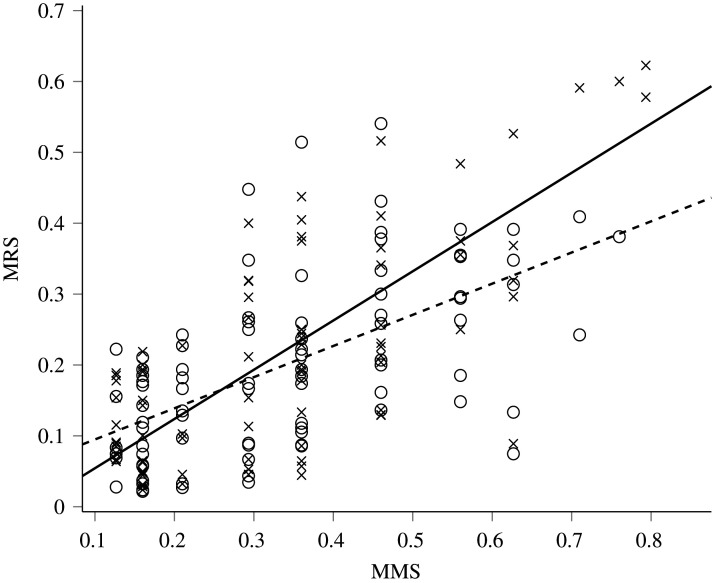
The Bateman gradient was significantly steeper under IPR (β = 0.695, 95% CI = 0.573–0.817) than under control conditions (β = 0.440, 95% CI = 0.320–0.560, figure 2). Similar results were obtained including males with MMS = 0 (electronic supplementary material). The results of the variance partitioning analysis indicate that, concordantly with the lower polyandry under IPR, the variance in MRS was significantly less influenced by PCS (table 1). None of the covariance components was significantly different from 0 (electronic supplementary material, table S1).
Figure 2.
Relationship between male mating success (MMS) and male reproductive success (MRS) of male guppies in the two experimental conditions (control, crosses; imminent predation risk, dots). Lines represent the least square linear regressions for control (continuous line) and predation risk group (dotted line). To magnify scale and reduce overlapping between points, a control male with MMS and MRS = 1 is not visible.
Table 1.
Variance in male reproductive success (MRS) and its partitioning among male mating success (MMS), competitive fertilization success (PCS), female fecundity (FEC) and their respective covariances (Cov), in relation to imminent predation risk (IPR). D represents an error term. None of the covariances were significantly different from 0 (see electronic supplementary material). Significant p values are in italics.
| component | MRS | MMS | PCS | FEC | Cov(MMS, PCS) | Cov(PCS, FEC) | Cov(MMS, FEC) | D |
|---|---|---|---|---|---|---|---|---|
| imminent predation risk (IPR) | ||||||||
| (co)variances | 1.073 | 0.599 | 0.129 | 0.069 | −0.015 | −0.004 | −0.020 | |
| standardized (co) variances (%) | 55.8 ± 6.2 | 12.0 ± 2.4 | 6.5 ± 1.7 | −1.4 ± 2.1 | −0.4 ± 0.9 | −1.9 ± 1.5 | 29.3 | |
| control (C) | ||||||||
| (co)variances | 0.633 | 0.413 | 0.123 | 0.077 | −0.020 | −0.008 | −0.008 | |
| standardized (co) variances (%) | 65.2 ± 7.0 | 19.4 ± 3.2 | 12.2 ± 3.3 | −3.1 ± 2.8 | −1.3 ± 1.5 | −1.3 ± 2.1 | 8.8 | |
| P | 0.19 | 0.039 | 0.042 | 0.31 | 0.28 | 0.41 | ||
4. Discussion
Our manipulation of perceived IPR was effective as we observed the typical behavioural responses to IPR in both females, which significantly increased their social cohesion and decreased their sexual responsiveness, and males, which shifted from courtship displays to coercive copulatory attempts, as reported for this species [53]. As predicted, IPR significantly affected the operation of sexual selection: (i) the number of sires per brood was lower for females exposed to predation risk than for their control counterparts; and (ii) IPR was associated with an increase in the total opportunity for sexual selection and with a decrease in the relative contribution of postcopulatory sexual selection to male reproductive success, as indicated by the larger variance in male copulation and mating success, and by the steeper Bateman gradient in replicates exposed to predation risk compared to their control counterparts. By contrast, (iii) the variance in postcopulatory success did not change between treatments, indirectly suggesting that patterns of male ejaculation [59] and/or cryptic female choice [66] were not influenced by the risk of predation. Guppy antipredator responses measured in the laboratory are similar to those observed in the field [67]. Therefore, our results are most likely representative of the effect of imminent predation risk in natural populations.
Our study provides evidence that the imminent risk of predation, an intrinsically variable environmental factor, can significantly change the fitness return of male postcopulatory investment, by reducing the female mating rate and the relative contribution of postcopulatory success on male reproductive fitness. Results from an artificial selection and experimental evolution study indicate that sperm production is traded-off against mating success in this guppy population [57], suggesting that males cannot simultaneously maximize their investments in sperm production and mate acquisition traits. The reproductive fitness of a male, related to his sperm production investment, is therefore expected to show continuous temporal fluctuation in association with variations in imminent predation risk: males with large sperm production are expected to have higher reproductive fitness when the risk is low, females are more polyandrous and a male's mating success is less strongly associated with his attractiveness. By contrast, the presence of a predator is expected to favour males that invested relatively more in mate acquisition traits, at the expense of sperm production traits. A multivariate selection analysis of pre- and postcopulatory traits under contrasting gradients of predation risk will allow us to test this prediction [27,50].
The effect of imminent predation risk on the operation of sexual selection [21] seems largely driven by its effect on female mating rate in guppies, at least in our experimental conditions. Contrary to previous evidence from other Trinidadian guppy populations that females reduce their proximity preference for most attractive (colourful) males under imminent predation [38], the larger standardized variances in male copulation success and genetic mating success in the replicates exposed to predation cues suggest that female choosiness did not decrease when actual mate choice (i.e. mating) could be expressed. This is also confirmed by the significant repeatability in male mating and reproductive success across treatments, indicating that the same individual males tended to be successful across treatments. As predicted theoretically and demonstrated empirically [68], our results confirm that polyandry is associated with a reduction in the total opportunity for sexual selection, at least in species in which females are highly polyandrous.
Male coercive mating tactics have been suggested to undermine female preference in guppies [69]. This hypothesis is based on the reasonable prediction that when females are distracted by a predator's presence, they may be more exposed to coercive mating attempts by non-preferred males. Indeed, in our experiment males switched from courtship displays to coercive mating attempts under IPR. However, their mating rate and the number of sires per brood were lower in the presence of predation cues than in controls, indicating that (at least in the experimental conditions tested here) imminent risk of predation did not improve significantly the low insemination success of this coercive mating tactic [70]. This may be because females' shoaling response to predation [43] may also reduce the opportunity for a male to approach them without being detected. Indirect evidence that female shoaling may reduce the rate of coercive copulations comes from a closely related species, the eastern mosquitofish (Gambusia holbrooki), in which females increase their social cohesion in response to harassment by males [71].
The imminent risk of predation is certainly a relevant ecological factor with marked effects on prey population dynamics [30], and processes associated with mating are expected to be particularly sensitive to it [28]. Considering that predation is expected to increase the cost of mating [72], a reduction of polyandry under imminent risk may be a general phenomenon. However, its specific effect on postcopulatory sexual selection may vary idiosyncratically among species, depending on its interaction with prey demography (e.g. density and adult sex ratio), temporal and spatial distribution of mates, and sociality [73]. The effect of these interactions on the operation of sexual selection may not be easy to predict. For example, a recent study on the socially monogamous passerine Cyanistes cyaneus [42] found that predation cues significantly increased the frequency of extrapair paternity (and hence, possibly, the relative strength of postcopulatory selection) through disruptions of morning routines, either inhibiting within-pair copulations or increasing opportunities for females to engage in extra-pair copulations.
In conclusion, 50 years of research on sperm competition after Parker's seminal paper [2] has led to a very good understanding of the interspecific and interpopulation variation in sperm production traits [6,19]. The reason why within-population variation in sperm production is often very large is, by contrast, relatively less understood. We demonstrated here that the strength of postcopulatory sexual selection, probably mediated by female sexual responsiveness and social behaviour, fluctuates in response to imminent predation risk, which may be an important factor contributing to explain the large intraspecific variation in sperm production traits.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are indebted to Geoff Parker for his invaluable insights into sperm competition that have so deeply influenced the course of research in sexual selection. We thank Ingo Schlupp and an anonymous reviewer for their helpful comments on an earlier version of the manuscript, and Nina Wedell and Leigh Simmons for inviting us to participate in this Theme Issue. We are also very grateful to Sara Biamonte, Martina Bonaldi and Marta Guerra for their assistance with the experiment.
Ethics
We have complied with all relevant ethical regulations, and our study protocol was approved by the University of Padova Institutional Ethical Committee (permit no. 256 /2018).
Data accessibility
The datasets supporting this article are available as electronic supplementary material.
Authors' contributions
A.P. conceived the study and designed the experiment with A.Gl. and S.C.; A.Gl. performed the experiment and collected the data; A.Gr. and A.Gl. performed the paternity analyses; A.P., A.Gl. and S.C. analysed the data; A.P. wrote the paper with contributions from all authors, who approved the final version of the paper.
Competing interests
The authors declare no competing interests.
Funding
This research was funded by grants to A.P. (University of Padova, PRAT-CPDA120105-2012 and BIRD-175144-2017) and to A.Gr. (MIUR Finanziamento delle attività base di ricerca 2018 and University of Padova PRID-SEED 2019). S.C. was supported by a post-doc fellowship from the University of Padova, and A.Gl. was supported by a CARIPARO PhD fellowship for international students.
References
- 1.Taylor ML, Price TAR, Wedell N. 2014. Polyandry in nature: a global analysis. Trends Ecol. Evol. 29, 376–383. ( 10.1016/j.tree.2014.04.005) [DOI] [PubMed] [Google Scholar]
- 2.Parker GA. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567. ( 10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 3.Stoltz JA, Neff BD. 2006. Sperm competition in a fish with external fertilization: the contribution of sperm number, speed and length. J. Evol. Biol. 19, 1873–1881. ( 10.1111/j.1420-9101.2006.01165.x) [DOI] [PubMed] [Google Scholar]
- 4.Boschetto C, Gasparini C, Pilastro A. 2011. Sperm number and velocity affect sperm competition success in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 65, 813–821. ( 10.1007/s00265-010-1085-y) [DOI] [Google Scholar]
- 5.Parker GA. 2016. The evolution of expenditure on testes. J. Zool. 298, 3–19. ( 10.1111/jzo.12297) [DOI] [Google Scholar]
- 6.Simmons LW, Fitzpatrick JL. 2012. Sperm wars and the evolution of male fertility. Reproduction 144, 519–534. ( 10.1530/rep-12-0285) [DOI] [PubMed] [Google Scholar]
- 7.Hosken DJ, Ward PI. 2001. Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 4, 10–13. ( 10.1046/j.1461-0248.2001.00198.x) [DOI] [Google Scholar]
- 8.Simmons LW, Garcia-Gonzalez F. 2008. Evolutionary reduction in testes size and competitive fertilization success in response to the experimental removal of sexual selection in dung beetles. Evolution 62, 2580–2591. ( 10.1111/j.1558-5646.2008.00479.x) [DOI] [PubMed] [Google Scholar]
- 9.Firman RC, Simmons LW. 2008. The frequency of multiple paternity predicts variation in testes size among island populations of house mice. J. Evol. Biol. 21, 1524–1533. ( 10.1111/j.1420-9101.2008.01612.x) [DOI] [PubMed] [Google Scholar]
- 10.Scaggiante M, Mazzoldi C, Petersen CW, Rasotto MB. 1999. Sperm competition and mode of fertilization in the grass goby Zosterisessor ophiocephalus (Teleostei: Gobiidae). J. Exp. Zool. 283, 81–90. () [DOI] [Google Scholar]
- 11.Küpper C, et al. 2016. A supergene determines highly divergent male reproductive morphs in the ruff. Nat. Genet. 48, 79–83. ( 10.1038/ng.3443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gage MJG, Stockley P, Parker GA. 1995. Effects of alternative male mating strategies on characteristics of sperm production in the Atlantic salmon (Salmo salar): theoretical and empirical investigations. Phil. Trans. R. Soc. Lond. B 350, 391–399. ( 10.1098/rstb.1995.0173) [DOI] [Google Scholar]
- 13.Simmons LW, Moore AJ. 2009. Evolutionary quantitative genetics of sperm. In Sperm biology (eds Tim RB, David JH, Scott P), pp. 405–434. London, UK: Academic Press. [Google Scholar]
- 14.Gasparini C, Devigili A, Dosselli R, Pilastro A. 2013. Pattern of inbreeding depression, condition dependence, and additive genetic variance in Trinidadian guppy ejaculate traits. Ecol. Evol. 3, 4940–4953. ( 10.1002/ece3.870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasson DA, Munoz PR, Gezan SA, Miller CW. 2016. Resource quality affects weapon and testis size and the ability of these traits to respond to selection in the leaf-footed cactus bug, Narnia femorata. Ecol. Evol. 6, 2098–2108. ( 10.1002/ece3.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsson M, Madson T, Shine R. 1997. Is sperm really so cheap? Costs of reproduction in male adders, Vipera berus. Proc. R. Soc. Lond. B 264, 455–459. (doi:10.1098/rspb.1997.0065 1471-2954) [Google Scholar]
- 17.Hayward A, Gillooly JF. 2011. The cost of sex: quantifying energetic investment in gamete production by males and females. PLoS ONE 6, e16557 ( 10.1371/journal.pone.0016557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomsen R, Soltis J, Matsubara M, Matsubayashi K, Onuma M, Takenaka O. 2006. How costly are ejaculates for Japanese macaques? Primates 47, 272–274. ( 10.1007/s10329-005-0171-7) [DOI] [PubMed] [Google Scholar]
- 19.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934. ( 10.1086/656840) [DOI] [PubMed] [Google Scholar]
- 20.Rowley AG, Daly-Engel TS, Fitzpatrick JL. 2018. Testes size increases with sperm competition risk and intensity in bony fish and sharks. Behav. Ecol. 30, 364–371. ( 10.1093/beheco/ary174) [DOI] [Google Scholar]
- 21.Evans JP, Garcia-Gonzalez F. 2016. The total opportunity for sexual selection and the integration of pre- and post-mating episodes of sexual selection in a complex world. J. Evol. Biol. 29, 2338–2361. ( 10.1111/jeb.12960) [DOI] [PubMed] [Google Scholar]
- 22.Morimoto J, Pizzari T, Wigby S. 2016. Developmental environment effects on sexual selection in male and female Drosophila melanogaster. PLoS ONE 11, e0154468 ( 10.1371/journal.pone.0154468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue D-X, Zhang T, Liu J-X. 2016. Influences of population density on polyandry and patterns of sperm usage in the marine gastropod Rapana venosa. Sci. Rep. 6, 1–10. ( 10.1038/srep23461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullough EL, Buzatto BA, Simmons LW. 2018. Population density mediates the interaction between pre- and postmating sexual selection. Evolution 72, 893–905. ( 10.1111/evo.13455) [DOI] [PubMed] [Google Scholar]
- 25.Buzatto BA, Roberts JD, Simmons LW. 2015. Sperm competition and the evolution of precopulatory weapons: increasing male density promotes sperm competition and reduces selection on arm strength in a chorusing frog. Evolution 69, 2613–2624. ( 10.1111/evo.12766) [DOI] [PubMed] [Google Scholar]
- 26.Janicke T, David P, Chapuis E. 2015. Environment-dependent sexual selection: Bateman's parameters under varying levels of food availability. Am. Nat. 185, 756–768. ( 10.1086/681128) [DOI] [PubMed] [Google Scholar]
- 27.Cattelan S, Evans JP, Garcia-Gonzalez F, Morbiato E, Pilastro A. 2020. Dietary stress increases the total opportunity for sexual selection and modifies selection on condition-dependent traits. Ecol. Lett. 23, 447–456. ( 10.1111/ele.13443) [DOI] [PubMed] [Google Scholar]
- 28.McDonald GC, Gardner A, Pizzari T. 2019. Sexual selection in complex communities: integrating interspecific reproductive interference in structured populations. Evolution 73, 1025–1036. ( 10.1111/evo.13726) [DOI] [PubMed] [Google Scholar]
- 29.Lima SL. 1998. Nonlethal effects in the ecology of predator-prey interactions. Bioscience 48, 25–34. ( 10.2307/1313225) [DOI] [Google Scholar]
- 30.Matassa CM, Trussell GC. 2011. Landscape of fear influences the relative importance of consumptive and nonconsumptive predator effects. Ecology 92, 2258–2266. ( 10.1890/11-0424.1) [DOI] [PubMed] [Google Scholar]
- 31.Magnhagen C. 1991. Predation risk as a cost of reproduction. Trends Ecol. Evol. 6, 31–38. ( 10.1016/0169-5347(91)90210-O) [DOI] [PubMed] [Google Scholar]
- 32.Sih A. 1994. Predation risk and the evolutionary ecology of reproductive behaviour. J. Fish Biol. 45, 111–130. ( 10.1006/jfbi.1994.1217) [DOI] [Google Scholar]
- 33.Gwynne DT. 1987. Sex-biased predation and the risky mate-locating behaviour of male tick-tock cicadas (Homoptera: Cicadidae). Anim. Behav. 35, 571–576. ( 10.1016/S0003-3472(87)80283-X) [DOI] [Google Scholar]
- 34.Zuk M, Kolluru GR. 1998. Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol. 73, 415–438. ( 10.1086/420412) [DOI] [Google Scholar]
- 35.Pocklington R, Dill LM. 1995. Predation on females or males: who pays for bright male traits? Anim. Behav. 49, 1122–1124. ( 10.1006/anbe.1995.0141) [DOI] [Google Scholar]
- 36.Kelly CD, Godin JGJ. 2001. Predation risk reduces male-male sexual competition in the Trinidadian guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 51, 95–100. ( 10.1007/s002650100410) [DOI] [Google Scholar]
- 37.Edomwande C, Barbosa F. 2020. The influence of predation risk on mate signaling and mate choice in the lesser waxmoth Achroia grisella. Sci. Rep. 10, 524 ( 10.1038/s41598-020-57481-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godin JGJ, Briggs SE. 1996. Female mate choice under predation risk in the guppy. Anim. Behav. 51, 117–130. ( 10.1006/anbe.1996.0010) [DOI] [Google Scholar]
- 39.Sato N, Uchida Y, Takegaki T. 2018. The effect of predation risk on post-copulatory sexual selection in the Japanese pygmy squid. Behav. Ecol. Sociobiol. 72, 129 ( 10.1007/s00265-018-2540-4) [DOI] [Google Scholar]
- 40.Kvarnemo C, Simmons LW. 2013. Polyandry as a mediator of sexual selection before and after mating. Phil. Trans. R. Soc. B 368, 20120042 ( 10.1098/rstb.2012.0042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sih A, Krupa JJ. 1995. Interacting effects of predation risk and male and female density on male/female conflicts and mating dynamics of stream water striders. Behav. Ecol. 6, 316–325. ( 10.1093/beheco/6.3.316) [DOI] [Google Scholar]
- 42.Santema P, Valcu M, Kempenaers B. 2019. Exposure to predator models during the fertile period leads to higher levels of extra-pair paternity in blue tits. J. Anim. Ecol. 89, 647–657. ( 10.1111/1365-2656.13114) [DOI] [PubMed] [Google Scholar]
- 43.Magurran AE. 1990. The adaptive significance of schooling as an antipredator defense in fish. Ann. Zool. Fenn. 27, 51–66. [Google Scholar]
- 44.Cimprich DA, Woodrey MS, Moore FR. 2005. Passerine migrants respond to variation in predation risk during stopover. Anim. Behav. 69, 1173–1179. ( 10.1016/j.anbehav.2004.07.021) [DOI] [Google Scholar]
- 45.Valeix M, Loveridge AJ, Chamaillé-Jammes S, Davidson Z, Murindagomo F, Fritz H, Macdonald DW. 2009. Behavioral adjustments of African herbivores to predation risk by lions: spatiotemporal variations influence habitat use. Ecology 90, 23–30. ( 10.1890/08-0606.1) [DOI] [PubMed] [Google Scholar]
- 46.Sih A. 1992. Prey uncertainty and the balancing of antipredator and feeding needs. Am. Nat. 139, 1052–1069. ( 10.1086/285372) [DOI] [Google Scholar]
- 47.Schmidt J. 1920. Racial investigations IV. The genetic behaviour of a secondary sexual character. Compt. Rend. Trav. Lab. Carlsberg 14, 1–23. [Google Scholar]
- 48.Houde AE. 1997. Sex, color, and mate choice in guppies Princeton, NJ: Princeton University Press. [Google Scholar]
- 49.Hain TJA, Neff BD. 2007. Multiple paternity and kin recognition mechanisms in a guppy population. Mol. Ecol. 16, 3938–3946. ( 10.1111/j.1365-294X.2007.03443.x) [DOI] [PubMed] [Google Scholar]
- 50.Devigili A, Evans JP, Di Nisio A, Pilastro A.. 2015. Multivariate selection drives concordant patterns of pre- and postcopulatory sexual selection in a livebearing fish. Nat. Commun. 6, 8291 ( 10.1038/ncomms9291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reznick DN, Travis J. 2019. Experimental studies of evolution and eco-evo dynamics in guppies (Poecilia reticulata). Annu. Rev. Ecol. Evol. Syst. 50, 335–354. ( 10.1146/annurev-ecolsys-110218-024926) [DOI] [Google Scholar]
- 52.Endler JA. 1995. Multiple-trait coevolution and environmental gradients in guppies. Trends Ecol. Evol. 10, 22–29. ( 10.1016/S0169-5347(00)88956-9) [DOI] [PubMed] [Google Scholar]
- 53.Magurran AE. 2005. Evolutionary ecology: the Trinidadian guppy. Oxford, UK: Oxford University Press. [Google Scholar]
- 54.Swaney WT, Cabrera-Alvarez MJ, Reader SM.. 2015. Behavioural responses of feral and domestic guppies (Poecilia reticulata) to predators and their cues. Behav. Process. 118, 42–46. ( 10.1016/j.beproc.2015.05.010) [DOI] [PubMed] [Google Scholar]
- 55.Monteforte S, Cattelan S, Morosinotto C, Pilastro A, Grapputo A. 2020. Maternal predator-exposure affects offspring size at birth but not telomere length in a live-bearing fish. Ecol. Evol. 10, 2030–2039. ( 10.1002/ece3.6035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans JP, Gasparini C, Pilastro A. 2007. Female guppies shorten brood retention in response to predator cues. Behav. Ecol. Sociobiol. 61, 719–727. ( 10.1007/s00265-006-0302-1) [DOI] [Google Scholar]
- 57.Cattelan A, Di Nisio A, Pilastro A.. 2018. Stabilizing selection on sperm number revealed by artificial selection and experimental evolution. Evolution 72, 698–706. ( 10.1111/evo.13425) [DOI] [PubMed] [Google Scholar]
- 58.Evans JP, Rutstein AN. 2008. Postcopulatory sexual selection favours intrinsically good sperm competitors. Behav. Ecol. Sociobiol. 62, 1167–1173. ( 10.1007/s00265-008-0545-0) [DOI] [Google Scholar]
- 59.Magris M, Zanata I, Rizzi S, Cattelan S, Pilastro A. 2020. Trade-offs of strategic sperm adjustments and their consequences under phenotype-environment mismatches in guppies. Anim. Behav. 166, 171–181. ( 10.1016/j.anbehav.2020.06.016) [DOI] [Google Scholar]
- 60.Seghers BH, Magurran AE. 1994. Predator inspection behaviour covaries with schooling tendency amongst wild guppy, Poecilia reticulata, populations in Trinidad. Behaviour 128, 121–134. ( 10.1163/156853994X00073) [DOI] [Google Scholar]
- 61.Liley NR. 1966. Ethological isolating mechanisms in four sympatric species of poeciliid fishes. Behaviour (Suppl.) 13, 1–197. ( 10.1086/190135) [DOI] [Google Scholar]
- 62.Kalinowski ST, Taper ML, Marshall TC. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106. ( 10.1111/j.1365-294X.2007.03089.x) [DOI] [PubMed] [Google Scholar]
- 63.Anthes N, Häderer IK, Michiels NK, Janicke T, Schielzeth H. 2017. Measuring and interpreting sexual selection metrics: evaluation and guidelines. Methods Ecol. Evol. 8, 918–931. ( 10.1111/2041-210x.12707) [DOI] [Google Scholar]
- 64.Jones AG. 2009. On the opportunity for sexual selection, the Bateman gradient and the maximum intensity of sexual selection. Evolution 63, 1673–1684. ( 10.1111/j.1558-5646.2009.00664.x) [DOI] [PubMed] [Google Scholar]
- 65.Webster MS, Pruett-Jones S, Westneat DF, Arnold SJ. 1995. Measuring the effects of pairing success, extra-pair copulations and mate quality on the opportunity for sexual selection. Evolution 49, 1147–1157. ( 10.1111/j.1558-5646.1995.tb04441.x) [DOI] [PubMed] [Google Scholar]
- 66.Pilastro A, Simonato M, Bisazza A, Evans JP. 2004. Cryptic female preference for colorful males in guppies. Evolution 58, 665–669. ( 10.1111/j.0014-3820.2004.tb01690.x) [DOI] [PubMed] [Google Scholar]
- 67.Brown GE, Godin JGJ. 1999. Chemical alarm signals in wild Trinidadian guppies (Poecilia reticulata). Can. J. Zool. 77, 562–570. ( 10.1139/z99-035) [DOI] [Google Scholar]
- 68.Morimoto J, McDonald GC, Smith E, Smith DT, Perry JC, Chapman T, Pizzari T, Wigby S. 2019. Sex peptide receptor-regulated polyandry modulates the balance of pre- and post-copulatory sexual selection in Drosophila. Nat. Commun. 10, 283 ( 10.1038/s41467-018-08113-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Magurran AE, Nowak MA.. 1991. Another battle of the sexes: the consequences of sexual asymmetry in mating costs and predation risk in the guppy, Poecilia reticulata. Proc. R. Soc. Lond. B 246, 31–38. ( 10.1098/rspb.1991.0121) [DOI] [PubMed] [Google Scholar]
- 70.Pilastro A, Bisazza A.. 1999. Insemination efficiency of two alternative male mating tactics in the guppy (Poecilia reticulata). Proc. R. Soc. Lond. B 266, 1887–1891. (doi:10.1098/rspb.1999.0862 1471-2954) [Google Scholar]
- 71.Dadda M, Pilastro A, Bisazza A. 2005. Male sexual harassment and female schooling behaviour in the eastern mosquitofish. Anim. Behav. 70, 463–471. ( 10.1016/j.anbehav.2004.12.010) [DOI] [Google Scholar]
- 72.Siemers BM, Kriner E, Kaipf I, Simon M, Greif S. 2012. Bats eavesdrop on the sound of copulating flies. Curr. Biol. 22, R563–R564. ( 10.1016/j.cub.2012.06.030) [DOI] [PubMed] [Google Scholar]
- 73.Rowe L, Arnqvist G, Sih A, Krupa J. 1994. Sexual conflict and the evolutionary ecology of mating patterns: water striders as a model system. Trends Ecol. Evol. 9, 289–293. ( 10.1016/0169-5347(94)90032-9) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available as electronic supplementary material.