Abstract
In the three decades, since Birkhead and Møller published Sperm competition in birds (1992, Academic Press) more than 1000 papers have been published on this topic, about half of these being empirical studies focused on extrapair paternity. Both technological innovations and theory have moved the field forward by facilitating the study of both the mechanisms underlying sperm competition in both sexes, and the ensuing behavioural and morphological adaptations. The proliferation of studies has been driven partly by the diversity of both behaviours and morphologies in birds that have been influenced by sperm competition, but also by the richness of the theory developed by Geoff Parker over the past 50 years.
This article is part of the theme issue ‘Fifty years of sperm competition’.
Keywords: birds, sperm competition, behaviour, morphology, mating systems, sexual conflict
1. Introduction
We were both undergraduates when Geoff Parker's Sperm competition in insects [1] paper was published in 1970. One of us (T.R.B.) was fortunate to have been taught by Robin Baker, who at that time was a postdoc at the University of Newcastle upon Tyne, UK, and had been a colleague of Parker's when both were graduate students at Bristol. It was in 1971, during a final year entomology course that Baker introduced T.R.B. to Parker's work on sperm competition in dung flies (Scatophaga spp.), as well as to Bob Trivers's (1972) parental investment paper [2]—which had not yet even been published. The combination of individual selection thinking, sex and sexual selection, delivered in Baker's inimitable style, was irresistible, and T.R.B. decided there and then to apply these ideas to birds. To colleagues, it seemed like a non-starter given that everyone knew that birds were monogamous [3]. But luckily, as it turned out, sexual monogamy is rather uncommon in birds.
Our own initial forays into sperm competition were limited to exploring certain behavioural adaptations to sperm competition—a prominent theme in Parker's 1970 paper—including research on the frequency and timing of extrapair copulations (EPCs) and mate guarding in common guillemots Uria aalge [4], Eurasian magpies Pica pica [5] and Lapland longspurs Calcarius lapponicus [6].
During the 1980s, it became clear that sperm competition in birds was a topic with considerable potential, not least because there was a clear set of questions to be addressed. Almost nothing was known, for example, about the copulation behaviour of birds, yet this was clearly a fundamental aspect of sperm competition.
In 1984, T.R.B. sent out a questionnaire about copulation behaviour in birds to ornithologists, only to discover that, at about the same time, Anders Pape Møller (A.P.M.) had done the same. As T.R.B. was to discover, A.P.M. was dynamic, full of ideas and an extremely clear thinker. As T.R.B. and A.P.M. worked on that copulation paper, they decided to write a book covering what was then known about sperm competition in birds. They approached Andy Richford at Academic Press for advice and while they were keen to wait until more studies were available, Richford urged writing a book while the topic was still on the steepest part of the curve—publisher's prescience—and this was excellent advice.
Sperm competition in birds was published in 1992 and comprised a broad overview of our current state of knowledge [7]. As often occurs with book-writing, new questions were identified as the work progressed. The book began with some theory regarding sexual selection and a description of avian mating systems. It then reviewed what was known about anatomical adaptations—such as relative testis size in males and the duration of sperm storage in females—and current ideas about possible mechanisms of sperm competition, based on Parker's insect work. With respect to mechanisms, there was some informative research conducted for an entirely different purpose on domestic poultry. The remainder of the book summarized what little was known about (i) the frequency and timing of both within-pair copulations and EPCs, (ii) the different types of paternity guards employed by males (proximity, frequent pair copulations and territoriality), and (iii) the costs and benefits of promiscuity in each sex. The book also outlined the various methods for detecting extrapair paternity (EPP), especially using the recently developed [8] and exciting method of DNA fingerprinting—an innovation that could hardly have been more apposite for the study of sperm competition. The final chapter emphasized the apparent ubiquity of sperm competition in birds, a taxon once thought to be a model of monogamy, and suggested the many implications of sperm competition for future studies of avian mating systems. Throughout, Sperm competition in birds drew on the ideas generated from the burgeoning study of behavioural ecology in general, and from research on sperm competition in insects in particular.
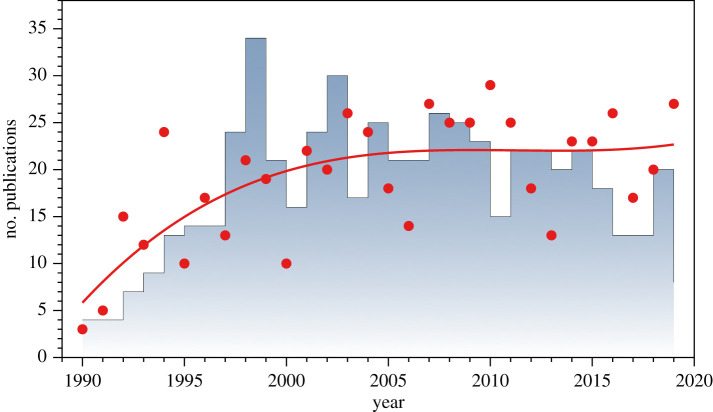
We (T.R.B., R.M.) met at the 1988 International Society for Behavioural Ecology meeting in Vancouver and have collaborated ever since on studies of sperm competition and egg traits in birds, as well as on the history of ornithology. As will be obvious from the other papers in this collection, the study of sperm competition has flourished in the past three decades, in part owing to some new technologies and analytical procedures, but also because this topic has been such a rich source of ideas and has revealed an amazing diversity of patterns within and between taxa. Since 1990, for example, more than 1000 papers have been published on various aspects of sperm competition in birds (figure 1), including more than 500 studies on extrapair paternity in more than 300 species [9]. Our goal here is to highlight a few field and laboratory studies, meta-analyses and reviews, as we cannot review everything in this short paper.
Figure 1.
The number of publications about some aspect of sperm competition in birds since 1990. Blue-grey bars are publications about extrapair copulations and extrapair paternity; red dots are for all other publications and the red line is a cubic polynomial fit to the data. Source: searches on Web of Science (webofknowledge.com) and LENS (lens.org) with keywords: birds AND sperm competition AND copulation AND extrapair, as well as a few additional studies known to us but not included in the results of those searches. (Online version in colour.)
2. Methodological advances
In the 350 years since Leeuwenhoek used his home-made microscope to discover spermatozoa, the study of these ‘little animalcules’ has been continuously fuelled by new technologies, as is true for most of the sciences [10]. By contrast, however, the study of sperm competition has been driven largely by ideas, generated initially—and often for the past 50 years—by Geoff Parker. Researchers have been quick to adopt new technologies to test Parker's ideas, particularly since 1990 when several new methods bloomed, moving the study of sperm competition along in ways that we had never envisioned when our own research on this topic began. Up to 1990, most of the studies of sperm competition in birds were observational, whereas today almost all research involves one or more technologies. Here, we highlight only a few of the instruments that have yielded the most, and in some ways the most interesting, results.
Early in the 1990s, three previously developed technologies became essential tools for the study of sperm competition in birds—radio-tracking, DNA profiling and computer-assisted sperm analysis (CASA). Refinements in electronics and computing, lower costs and increased accuracy made each of these tools attractive for the study of both mechanisms and adaptations, whether behavioural, anatomical or physiological.
The radio-tracking of individual animals in the wild began in the early 1960s [11], but transmitters were initially too expensive, too short-range and too large to be carried by small birds where much of the interesting action is with respect to sperm competition [9,12,13]. Even by 1990, transmitters were too large to be carried by species weighing less than 100 g. But an explosion of new technologies—geolocators, GPS trackers, satellite and ground receivers—extended the species that could be tracked down to the smallest birds, and with pin-point accuracy at continental and annual scales [11]. Now even short-term extra-territorial forays by both sexes, as well as contacts between females and potential extrapair mates, can be comprehensively documented [14], though it is still not easy to use tracking devices to determine if and when copulations occur.
DNA profiling to identify parents in birds began in 1987 [15,16] and quickly became essential for field studies of avian mating systems. The impetus here, of course, was not just the technology but the uncovering of diverse patterns of EPP in species once thought to be sexually monogamous [9,12,13]. Initial applications involved minisatellite analysis which was expensive, time-consuming and somewhat inaccurate. Since then, progressive development in both DNA and analytical technologies—microsatellites, single nucleotide polymorphisms, genomics and software tools—has reduced the costs, sped up the analyses and improved the accuracy of parentage and kinship assignment.
Because birds are internal fertilizers, the behaviour of their spermatozoa has not (yet) been studied in vivo, limiting our understanding of the underlying anatomical and physiological mechanisms influencing sperm competition. Such mechanisms are more easily studied in external fertilizers [17,18]. The development of commercially available CASA systems in 1985 made the study of sperm behaviour and ejaculate traits feasible on a large scale, and these systems were quickly adopted by those studying humans and domestic animals. Though expensive and sometimes tricky to use, CASA systems by Hamilton Thorne, Inc. (Beverly, MA, USA) and Microptic S.L. (Barcelona, Spain), as well as a free plug-in for ImageJ software [19], have become essential tools for the study of sperm in the past two decades. Despite this, we still do not know very much about how avian sperm behave inside the female where they move along the walls of the oviduct in the presence of various secretions by the female, often assisted by cilia in parts of the oviduct.
A somewhat different method for evaluating sperm quality was developed by poultry scientists in the 1990s, whereby a fresh ejaculate is placed onto a column filled with a solution of Accudenz® (from Accurate Chemicals & Scientific Corporation, Westbury, NY, USA). The proportion of spermatozoa able to penetrate this medium is used as a measure of sperm performance [20]. This method has been used in sperm competition studies of the domestic fowl Gallus gallus domesticus [21] and zebra finch Taeniopygia guttata [22].
Four additional developments hold some promise for understanding sperm competition within the female. First, the study of sperm on the ovum's perivitelline layer (PVL [22]) permits the quantification of sperm actually reaching the site of fertilization. Using this technique, it is now possible to compare the rate that a male's sperm travel from the vagina where ejaculates are deposited to the sperm storage tubules (SSTs), and from there to the infundibulum where ova are fertilized (see [23] for review). Second, the labelling of sperm from different males with a fluorescent protein (green fluorescent protein [24]) has the potential to determine which males contributed sperm that actually reach the PVL when a female is inseminated by multiple males. Third, the study of female oviducts has provided insights into the female's role in male and sperm selection. In some birds, ejaculates are delivered to the female via the male's intromittent organ. Using three-dimensional models of the female's oviduct, the action of that intromittent organ during copulation, and the various restrictions imposed by the female, can now be visualized [25].
Finally, and most recently, research on the proteins in male seminal fluid, using mass spectrometry to identify and quantify the constituent proteins, has given some insights into their role in fertilization and sperm competition. In the domestic fowl, for example, both depletion and enrichment of different male seminal proteins during successive copulations with different females has been detected [26], suggesting that ejaculate allocation is more nuanced than a simple adjustment in sperm numbers. Seminal proteins in sparrows (Passer domesticus and Passer hispaniolensis) [27] and zebra finches [28], also show variation in the seminal proteins that influence both sperm mobility and storage in the female, both processes that influence the outcome of sperm competition.
3. Evolutionary consequences
(a). Mechanisms
The original focus of research in behavioural ecology was on adaptation, with an almost explicit rejection of interest in mechanisms. But, over time, it became clear that knowledge of mechanisms was essential for addressing questions about adaptation. Thus, predicting the timing and temporal pattern of mate guarding depends upon knowing when females are fertile—how long do females store viable sperm? Some relevant information on sperm storage, and hence the female's ‘fertile period’, was available from studies of poultry [29], but it was not until the 1990s that field ornithologists became interested in sperm behaviour, in the duration and mechanisms of sperm storage, and in the fate of sperm from different males within the female tract.
Parker [30] showed that last male sperm precedence (LMSP) occurred in dung flies, with the second of two males to inseminate a female securing about 80% of the fertilizations. Given that male dung flies routinely copulate with already-inseminated females, some LMSP was to be expected. Poultry biologists interested in patterns of female sperm use used genetic plumage markers to assign paternity after two sequential inseminations and also detected a pronounced LMSP effect in domestic fowl [31].
The question was then to identify the mechanism of LMSP. There were three possibilities: (i) stratification of sperm within the female's SSTs, (ii) incoming sperm displaced or destroyed already-stored sperm or (iii) passive sperm loss (i.e. by the time of the final insemination, previously stored sperm would have largely been lost from the SSTs). Modelling these three scenarios suggested that displacement was the most-likely mechanism of LMSP in birds [32]. The first test of this hypothesis [33] revealed an LMSP effect, but not as extreme as that recorded by Compton et al. [31] who found that an interval of just 4 h between inseminations in the domestic fowl resulted in 80% LMSP. This result implied a rate of sperm loss from the SSTs much higher than other poultry data had suggested. It turned out that Compton's study was biased because the first insemination was made soon after oviposition, when the uptake of sperm by the SSTs was disproportionately low. In other words, the successive inseminations were not equal as the first was disadvantaged, resulting in a strong LMSP effect. Subsequent experiments showed that passive sperm loss accounted for LMSP in both domestic fowl and zebra finches [32,33].
In general, however, LMSP has been overestimated as a biologically meaningful mechanism in wild birds. In domesticated birds, LMSP does occur when two sequential inseminations—comprising equal numbers of sperm of comparable fertilizing efficiency—are made under controlled conditions. Among wild birds, the number, size and quality of inseminations are likely to be much more variable and the outcome of sperm competition, therefore, less predictable.
Parker's [30] original sperm competition models were based on sperm numbers. However, animal breeders were aware that the sperm of some males was disproportionately effective—a phenomenon known as differential fertilizing capacity (DFC). Using live-dead fluorescent labels, it was possible to test whether the proportion of live sperm in an ejaculate could account for a DFC effect. As a result, a pronounced difference in the proportion of live sperm was uncovered among insect species experiencing different levels of sperm competition [34]. A few years later, using an assay developed by Froman & Feltmann [20] that linked sperm velocity with fertilizing success in domestic fowl, it was clear that, after controlling for sperm numbers, sperm quality has a marked effect on the outcome of sperm competition experiments [21].
Comparative studies of birds showed similar results to those found in insects [35] and fishes [36], wherein ejaculates of species with relatively large testes (thus higher levels of sperm competition) contained fewer dead or deformed sperm, but also sperm that were longer, more consistent in size and morphology within an ejaculate and with a higher swimming velocity [37]. In the zebra finch, a species with relatively low levels of EPP, sperm length varies markedly from male to male (40–80 µm [38]). Bennison et al. [22] used this variation to create lineages of long- and short-sperm males. In controlled sperm competition experiments, they showed that males with longer sperm secured a higher proportion of fertilizations. This was owing to the higher swimming velocity of longer sperm, resulting in a greater proportion of their sperm getting into storage and then to the site of fertilization. Moreover, sperm length and velocity had a genetic basis with most of the variation in sperm morphology the result of a ‘supergene’ on the Z chromosome [39,40].
The possibility that females were not simply the passive recipients of sperm, and thus might control how sperm from different males in their oviduct are used, was first suggested by Tyler [41] then developed by Eberhard [42]. Such cryptic female choice (CFC) can be difficult to demonstrate unless all male effects are controlled [43]. CFC in birds is most likely in situations where females have little behavioural control over who inseminates them, as, for example, occurs in forced pair or EPCs. Female feral domestic fowl prefer to be inseminated by the dominant cockerel, but are sometimes forcibly inseminated by a subordinate male, in which case they exert their ‘choice’ by ejecting his ejaculate [44]. Artificially inseminated domestic fowl are also able to discriminate between the sperm of different males, presumably physiologically and possibly through an immune response between the semen and the oviduct [45].
(b). Adaptations
Since 1990, about half of the published work on sperm competition in birds every year (figure 1) has focused on the patterns and causes of EPCs and EPP. This topic has generated so much interest because it was clear early on that many socially monogamous birds were in fact sexually polygynous and polyandrous, and the variability in EPP rates was enormous both within and between species. EPP is relatively uncommon in non-passerine birds. But in passerines, the majority of species show some level of EPP ranging from 5 to 75% (average about 19%) of offspring being sired by non-pair males and a mean of about 33% of broods containing extrapair offspring in socially monogamous species with biparental care [9]. Lower levels of EPP (less than 5%) may simply be ‘accidental’.
Explanations for extrapair parentage have been numerous both within and between species, including (i) high breeding densities, (ii) breeding asynchrony, (iii) mate choice, (iv) male coercion [46], (v) high rates of adult mortality, and (vi) the need for paternal care [9,12,13]. Another alternative, suggested by Halliday & Arnold [47], is that female birds engage in extrapair interactions as the result of a genetic correlation with male extrapair behaviour. Cheng & Siegel [48] found no evidence for this in domestic fowl, Japanese quail Coturnix japonica, or fruit flies Drosophila, although Forstmeier et al. [49] did for captive zebra finches (in which levels of EPP in the wild are low [50]). Similar studies of species with more typical levels of EPP are now needed.
The study of EPP has provided some fascinating insights into the behaviour of males and females in the face of such intense sperm competition—mate guarding, sperm ejection and the timing, frequency and duration of copulations. It is now reasonably clear, for example, that mate guarding is common when there is any threat of EPP, whether that guarding is accomplished by establishing and maintaining exclusive territories by aggression and song, staying close to a mate, prolonged and repeated copulations [51], or maintaining contact between mates with calls and duets. While proximity might be the most effective guard, it is often impossible when food resources are widely distributed, or the breeding habitat is dense. In species with high levels of sperm competition resulting in EPP, there can also be downstream effects with males sometimes adjusting their contribution to parental duties in proportion to their perceived levels of cuckoldry [52].
While copulations—and particularly extrapair copulations—can be difficult to observe, there is increasing evidence that EPCs are often timed to maximize the chance of fertilization during both the season and the day (e.g. [53]). The response of pair males to this threat of lost paternity has been to increase the intensity of both mate guarding and copulation rates during these critical periods. At times of highest fertility, females sometimes also change their behaviour to increase the potential for an EPC that will result in an extrapair offspring [14,54].
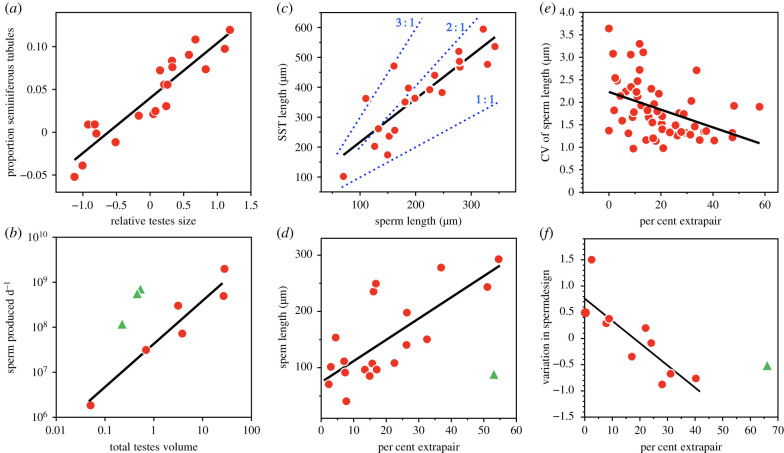
Although not initially predicted from theory, studies of birds were among the first to reveal the effects of sperm competition on the evolution of spermatozoa [55]. Thus, species undergoing high levels of sperm competition have longer [56] and thus faster swimming sperm [37], as well as sperm that are less variable in length and structure [57] (figure 2d–f). In this case, empirical studies encouraged the development of useful theory [63], although the broader study of sperm quality in relation to other ejaculate and oviductal traits is still in its infancy.
Figure 2.
Morphological adaptations to sperm competition in birds. (a) In species undergoing more intense sperm competition (as measured by relative testes size) males devote a higher proportion of their testes to sperm production [58]. (b) In species with larger testes, males produce more sperm per day [59]. Green triangles are fairy wrens (Malurus spp.) where sperm competition is intense. (c) Sperm storage tubules (SSTs) in females can be two to three times as long as male sperm [60]. SST to sperm length ratios shown as dotted blue lines. (d) Sperm length increases with the intensity of sperm competition as measured by the proportion of offspring that are extrapair [56]. The outlier (green triangle) is the American robin Turdus migratorius studied in a high-density population [61]. (e) Sperm length is less variable within males in species with higher levels of sperm competition [62]. (f) In species with higher levels of sperm competition, sperm design is less variable [57]; outlier is a fairy wren. All regression lines exclude the outliers (green triangles); figures drawn from data or redrawn from figures in the sources listed. (Online version in colour.)
One result of selection for larger spermatozoa when sperm competition is intense has been the evolution of larger SSTs in the female. Thus, the length of SSTs sometimes tracks sperm size, presumably evolving in concert to facilitate sperm storage. However, in some species, SSTs are disproportionately longer than the length of spermatozoa (figure 2c) suggesting an arms race between males and females over paternity [60]. In certain waterfowl, where forced EPCs are frequent, such an arms race is also manifested in the design of female vaginas, potentially limiting the ability of unwanted males to achieve successful fertilization [25].
In most studies of sperm competition in birds and mammals, relative testis size (as a proportion of male mass) is used as an index of the intensity of sperm competition. The underlying assumption is that larger testes relative to male size mean more seminiferous tubules (figure 2a), where sperm are produced, and thus a higher rate of sperm production (figure 2b) resulting in more sperm per ejaculate [64]. While there is considerable evidence that relative testis size is a useful index, correlated with higher rates of EPP, it is now clear that the relationship between testis size and ejaculates is more complex than originally thought. For example, although species with larger testes do produce spermatozoa at a higher rate, there can be advantages for a male to have smaller testes that generate spermatozoa at a lower rate, producing larger ejaculates by copulating less frequently [58].
Westneat & Stewart [12] perceptively argued that EPP in birds is most usefully studied in the context of sexual conflict—as opposed to a focus solely on female strategies as had previously dominated the literature. Thus, they said, the behaviour and morphologies of three players all deserve attention if we hope to understand variation in EPP rates within and between species: the pair male and female, and the extrapair male. Few studies have yet to take this perspective [65] but perhaps the most progress has been made in the study of genitalia. In waterfowl, for example, male intromittent organs (IOs) are larger in species with more intense sperm competition [46,66], where females also have more structurally complex vaginas that may impede inseminations from unwanted males [25]. Only about 3% of bird species have male IOs, so there is still much to learn about the conflict between the sexes in species that mate solely by cloacal apposition.
In Japanese quail, for example, males produce a cloacal foam that seems to facilitate insemination and fertilization [67], but it is unknown whether this is a product of sperm competition and whether there is a counteradaptation in females. In the red-billed buffalo weaver Bubalornis niger, sperm competition is intense, and males have a phallus-like cloacal appendage that through protracted copulation stimulates female response and presumably facilitates the uptake and storage of spermatozoa [68], but more work is needed to test these ideas.
4. The future
A number of outstanding questions remain to be addressed. First, our understanding of sperm dynamics within the female's oviduct and SSTs during sperm competition would be greatly enhanced if we had at least two distinctive sperm labels—one for each competing male—that do not interfere with any other aspect of sperm function. Second, the ability to test paternity associated with individual spermatozoa would allow us to establish whether physiological polyspermy in birds results in sperm competition within the germinal disc of the ovum. In birds, several sperm must enter the ovum (polyspermy) to ensure fertilization and embryo development [69], but the reasons for such polyspermy are unknown [70].
Finally, and what is perhaps the most fundamental question of all, why do females engage in EPCs? Despite three decades of work on this question and more than 500 studies of EPP, no general, convincing answer has emerged [9,49]. The last thirty years of progress in the study of sperm competition in birds has revealed such an exceptional degree of complexity that the time has perhaps come to stop looking for broad-scale, general explanations. Instead, a focus on the variation in EPP within and among closely related species seems likely to be most productive [9], with studies of the behavioural, ecological and morphological traits that influence sperm competition and sexual conflict and the resulting levels of EPP. The rich literature developed so far on this topic is likely to inspire research for some time to come.
Acknowledgements
We are particularly grateful to Nicola Hemmings, Tommaso Pizzari and an anonymous reviewer for insightful comments on our manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
Both authors contributed equally to the writing, editing and revision of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
For our research on sperm competition, T.R.B. has been funded by the European Research Council (ERC), Natural Environment Research Council (NERC), Royal Society, Science and Engineering Research Council (SERC), Nuffield Foundation, Association for the Study of Animal Behaviour (ASAB), National Geographic Society, Biotechnology and Biological Sciences Research Council (BBSRC) and University of Sheffield; R.M. by the Natural Sciences and Engineering Research Council of Canada (NSERC), National Geographic Society, and Northern Studies Program of Indian and Northern Affairs Canada, as well as a Canada Council Killam Fellowship and a Queen's University Research Chair.
References
- 1.Parker GA. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567. ( 10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 2.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and descent of Man (ed. Campbell B.), pp. 136–179. Chicago, IL: Aldine. [Google Scholar]
- 3.Lack D. 1968. Ecological adaptations for breeding in birds. London, UK: Chapman and Hall. [Google Scholar]
- 4.Birkhead TR. 1978. Behavioural adaptations to high density nesting in the common guillemot Uria aalge. Anim. Behav. 26, 321–331. ( 10.1016/0003-3472(78)90050-7) [DOI] [Google Scholar]
- 5.Birkhead TR. 1982. Timing and duration of mate guarding in magpies, Pica pica. Anim. Behav. 30, 277–283. ( 10.1016/S0003-3472(82)80264-9) [DOI] [Google Scholar]
- 6.Montgomerie RD. 1986. Seasonal patterns of mate guarding in lapland longspurs. In Proceedings of the 19th International Ornithological Congress, pp. 447–453. Ottawa, Canada: University of Ottawa Press. [Google Scholar]
- 7.Birkhead TR, Møller AP. 1992. Sperm competition in birds: evolutionary causes and consequences. London, UK: Academic Press. [Google Scholar]
- 8.Jeffreys AJ, Wilson V, Thein SL. 1985. Hypervariable ‘minisatellite’ regions in human DNA. Nature 314, 67–73. ( 10.1038/314067a0) [DOI] [PubMed] [Google Scholar]
- 9.Brouwer L, Griffith SC. 2019. Extra-pair paternity in birds. Mol. Ecol. 28, 4864–4882. ( 10.1111/mec.15259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price DJS. 1963. Little science, big science New York, NY: Columbia University Press. [Google Scholar]
- 11.López-López P. 2016. Individual-based tracking systems in ornithology: welcome to the era of big data. Ardeola 63, 103–136. ( 10.13157/arla.63.1.2016.rp5) [DOI] [Google Scholar]
- 12.Westneat DF, Stewart IRK. 2003. Extra-pair paternity in birds: causes, correlates, and conflict. Annu. Rev. Ecol. Evol. Syst. 34, 365–396 ( 10.1146/annurev.ecolsys.34.011802.132439) [DOI] [Google Scholar]
- 13.Griffith SC, Owens IPF, Thuman KA. 2002. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212. ( 10.1046/j.1365-294X.2002.01613.x) [DOI] [PubMed] [Google Scholar]
- 14.Double M, Cockburn A. 2000. Pre-dawn infidelity: females control extra-pair mating in superb fairy-wrens. Proc. R. Soc. B 267, 465–470 ( 10.1098/rspb.2000.1023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke T, Bruford MW. 1987. DNA fingerprinting in birds. Nature 327, 149–152. ( 10.1038/327149a0) [DOI] [PubMed] [Google Scholar]
- 16.Wetton JH, Carter RE, Parkin DT, Walters D. 1987. Demographic study of a wild house sparrow population by DNA fingerprinting. Nature 327, 147–149. ( 10.1038/327147a0) [DOI] [PubMed] [Google Scholar]
- 17.Montgomerie R, Fitzpatrick JL. 2009. Testes, sperm and sperm competition. In Reproductive biology and phylogeny of fishes (Agnathans and bony fishes), part B (ed. Jamieson BGM.), pp. 1–53. Enfield, NH: Science Publishers. [Google Scholar]
- 18.Fitzpatrick JL, Simmons LW, Evans JP. 2012. Complex patterns of multivariate selection on the ejaculate of a broadcast spawning marine invertebrate. Evolution 66, 2451–2460. ( 10.1111/j.1558-5646.2012.01627.x) [DOI] [PubMed] [Google Scholar]
- 19.Wilson-Leedy JG, Ingermann RL. 2007. Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology 67, 661–672. ( 10.1016/j.theriogenology.2006.10.003) [DOI] [PubMed] [Google Scholar]
- 20.Froman DP, Feltmann AJ. 1998. Sperm mobility: a quantitative trait of the domestic fowl (Gallus domesticus). Biol. Reprod. 58, 379–384. ( 10.1095/biolreprod58.2.379) [DOI] [PubMed] [Google Scholar]
- 21.Birkhead TR, Martinez JG, Burke T, Froman DP. 1999. Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc. R. Soc. B 266, 1759–1764. ( 10.1098/rspb.1999.0843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennison C, Hemmings N, Slate J, Birkhead TR. 2015. Long sperm fertilize more eggs in a bird. Proc. R. Soc. B 282, 20141897 ( 10.1098/rspb.2014.1897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasanami T, Matsuzaki M, Mizushima S, Hiyamm G. 2013. Sperm storage in the female reproductive tract in birds. J. Reprod. Dev. 59, 334–338 ( 10.1262/jrd.2013-038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manier MK, Belote JM, Berben KS, Novikov D, Stuart WT, Pitnick S. 2010. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science 328, 354–357. ( 10.1126/science.1187096) [DOI] [PubMed] [Google Scholar]
- 25.Brennan PLR, Prum RO, McCracken KGA, Sorenson MD, Wilson RE, Birkhead TR. 2007. Coevolution of male and female genital morphology in waterfowl. PLoS ONE 2, e0000418 ( 10.1371/journal.pone.0000418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Fernandez A, Borziak K, McDonald GC, Dorus S, Pizzari T. 2019. Female novelty and male status dynamically modulate ejaculate expenditure and seminal fluid proteome over successive matings in red junglefowl. Sci. Rep. 9, 5852 ( 10.1038/s41598-019-41336-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowe M, Whittington E, Borziak K, Ravinet M, Eroukhmanoff F, Sætre G-P, Dorus S. 2019. Molecular diversification of the seminal fluid proteome in a recently diverged passerine species pair. Mol. Biol. Evol. 37, 488–506 ( 10.1093/molbev/msz235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowe M, Skerget S, Rosenow MA, Karr TL. 2019. Identification and characterization of the zebra finch (Taeniopygia guttata) sperm proteome. J. Proteomics 193, 192–204 ( 10.1016/j.jprot.2018.10.009) [DOI] [PubMed] [Google Scholar]
- 29.Lake PE. 1975. Gamete production and the fertile period with particular reference to domesticated birds. Symp. Zool. Soc. Lond. 35, 225–244. [Google Scholar]
- 30.Parker GA. 1984. Sperm competition and the evolution of animal mating systems. In Sperm competition and the evolution of animal mating strategies (ed. Smith RL.), pp. 1–60. Orlando, FL: Academic Press. [Google Scholar]
- 31.Compton MM, Van Krey HP, Siegel PB.. 1978. The filling and emptying of the uterovaginal sperm-host glands in the domestic hen. Poult. Sci. 57, 1696–1700 ( 10.3382/ps.0571696) [DOI] [PubMed] [Google Scholar]
- 32.Lessells CM, Birkhead TR. 1990. Mechanisms of sperm competition in birds: mathematical models. Behav. Ecol. Sociobiol. 27, 325–337. ( 10.1007/BF00164003) [DOI] [Google Scholar]
- 33.Birkhead TR, Wishart GJ, Biggins JD. 1995. Sperm precedence in the domestic fowl. Proc. R. Soc. B 261, 285–292 ( 10.1098/rspb.1995.0149) [DOI] [Google Scholar]
- 34.Hunter FM, Birkhead TR. 2002. Sperm viability and sperm competition in insects. Curr. Biol. 12, 121–123 ( 10.1016/S0960-9822(01)00647-9) [DOI] [PubMed] [Google Scholar]
- 35.Simmons LW, Parker GA, Hoskin DJ. 2020. Evolutionary insight from a humble fly: sperm competition and the yellow dungfly. Phil. Trans. R. Soc. B 375, 20200062 ( 10.1098/rstb.2020.0062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitzpatrick JL. 2020. Sperm competition and fertilization mode in fishes. Phil. Trans. R. Soc. B 375, 20200074 ( 10.1098/rstb.2020.0074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lüpold S, Calhim S, Immler S, Birkhead TR. 2009. Sperm morphology and sperm velocity in passerine birds. Proc. R. Soc. B 276, 1175–1181 ( 10.1098/rspb.2008.1645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birkhead TR, Pellat EJ, Brekke P, Yeates R, Castillo-Juarez H. 2005. Genetic effects on sperm design in the zebra finch. Nature 434, 383–387. ( 10.1038/nature03374) [DOI] [PubMed] [Google Scholar]
- 39.Kim KW, Bennison C, Hemmings N, Brookes L, Hurley LL, Griffith SC, Burke T, Birkhead TR, Slate J. 2017. A sex-linked supergene controls sperm morphology and swimming speed in a songbird. Nat. Ecol. Evol. 1, 1168–1176. ( 10.1038/s41559-017-0235-2) [DOI] [PubMed] [Google Scholar]
- 40.Knief U, et al. 2017. A sex-chromosome inversion causes strong overdominance for sperm traits that affect siring success. Nat. Ecol. Evol. 1, 1177–1184. ( 10.1038/s41559-017-0236-1) [DOI] [PubMed] [Google Scholar]
- 41.Tyler A. 1948. Fertilization and immunity. Physiol. Rev. 28, 180–219. ( 10.1152/physrev.1948.28.2.180) [DOI] [PubMed] [Google Scholar]
- 42.Eberhard W. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 43.Birkhead TR. 1998. Cryptic female choice: criteria for establishing female sperm choice. Evolution 52, 1212–1218. ( 10.1111/j.1558-5646.1998.tb01848.x) [DOI] [PubMed] [Google Scholar]
- 44.Pizzari T, Birkhead TR. 2000. Female feral fowl eject sperm of subdominant males. Nature 405, 787–789. ( 10.1038/35015558) [DOI] [PubMed] [Google Scholar]
- 45.Birkhead TR, Chaline N, Biggins JD, Burke T, Pizzari T. 2004. Nontransitivity of paternity in a bird. Evolution 58, 416–420 ( 10.1111/j.0014-3820.2004.tb01656.x) [DOI] [PubMed] [Google Scholar]
- 46.Briskie JV, Montgomerie R. 1997. Sexual selection and the intromittent organ of birds. J. Avian Biol. 28, 73–86 ( 10.2307/3677097) [DOI] [Google Scholar]
- 47.Halliday T, Arnold SJ. 1987. Multiple mating by females: a perspective from quantitative genetics. Anim. Behav. 35, 939–941. ( 10.1016/S0003-3472(87)80138-0) [DOI] [Google Scholar]
- 48.Cheng KM, Siegel PB. 1990. Quantitative genetics of multiple mating. Anim. Behav. 40, 406–407 ( 10.1016/S0003-3472(05)80939-X) [DOI] [Google Scholar]
- 49.Forstmeier W, Nakagawa S, Griffith SC, Kempenaers B. 2014. Female extra-pair mating: adaptation or genetic constraint? Trends Ecol. Evol. 29, 456–464. ( 10.1016/j.tree.2014.05.005) [DOI] [PubMed] [Google Scholar]
- 50.Griffith SC, Holleley CE, Mariette MMA, Pryke SR, Svedin N. 2010. Low level of extrapair parentage in wild zebra finches. Anim. Behav. 79, 261–264 ( 10.1016/j.anbehav.2009.11.031) [DOI] [Google Scholar]
- 51.Harts AMF, Booksmythe I, Jennions MD. 2016. Mate guarding and frequent copulation in birds: a meta-analysis of their relationship to paternity and male phenotype. Evolution 70, 2789–2808 ( 10.1111/evo.13081) [DOI] [PubMed] [Google Scholar]
- 52.Møller AP, Birkhead TR. 1993. Certainty of paternity covaries with paternal care in birds. Behav. Ecol. Sociobiol. 33, 261–268 ( 10.1007/bf02027123) [DOI] [Google Scholar]
- 53.Kupper C, Kis J, Kosztolanyi A, Szekely T, Cuthill IC, Blomqvist D. 2004. Genetic mating system and timing of extra-pair fertilizations in the Kentish plover. Behav. Ecol. Sociobiol. 57, 32–39 ( 10.1007/s00265-004-0832-3) [DOI] [Google Scholar]
- 54.Amrhein V. 1999. Sexual selection and the evolution of extra-pair copulation: rules of the game from the females' point of view. J. Ornithol. 140, 431–441 ( 10.1007/BF01650987) [DOI] [Google Scholar]
- 55.Briskie JV, Montgomerie R. 1992. Sperm size and sperm competition in birds. Proc. R. Soc. B 247, 89–95 ( 10.1098/rspb.1992.0013) [DOI] [PubMed] [Google Scholar]
- 56.Briskie JV, Montgomerie R, Birkhead TR. 1997. The evolution of sperm size in birds. Evolution 51, 937–945 ( 10.2307/2411167) [DOI] [PubMed] [Google Scholar]
- 57.Calhim S, Immler S, Birkhead TR. 2007. postcopulatory sexual selection is associated with reduced variation in sperm morphology. PLoS ONE 2, e413 ( 10.1371/journal.pone.0000413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lüpold S, Linz GM, Rivers JW, Westneat DF, Birkhead TR. 2009. Sperm competition selects beyond relative testes size in birds. Evolution 63, 391–402 ( 10.1111/j.1558-5646.2008.00571.x) [DOI] [PubMed] [Google Scholar]
- 59.Tuttle EM, Pruett-Jones S, Webster MS. 1996. Cloacal protuberances and extreme sperm production in Australian fairy-wrens. Proc. R. Soc. B 263, 1359–1364 ( 10.1098/rspb.1996.0199) [DOI] [Google Scholar]
- 60.Briskie JV, Montgomerie R. 1993. Patterns of sperm storage in relation to sperm competition in passerine birds. The Condor 95, 442–454. ( 10.2307/1369366) [DOI] [Google Scholar]
- 61.Rowe KMC, Weatherhead PJ. 2007. Social and ecological factors affecting paternity allocation in American robins with overlapping broods. Behav. Ecol. Sociobiol. 61, 1283–1291. ( 10.1007/s00265-007-0359-5) [DOI] [Google Scholar]
- 62.Lifjeld JT, Laskemoen T, Kleven O, Albrecht T, Robertson RJ. 2010. Sperm length variation as a predictor of extrapair paternity in passerine birds. PLoS ONE 5, e13456 ( 10.1371/journal.pone.0013456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parker GA. 1998. Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection (ed Birkhead TR, Møller AP), pp. 3–54. New York, NY: Academic Press. [Google Scholar]
- 64.Calhim S, Birkhead TR. 2007. Testes size in birds: quality versus quantity — assumptions, errors, and estimates. Behav. Ecol. 18, 271–275. ( 10.1093/beheco/arl076) [DOI] [Google Scholar]
- 65.Chaine AS, Montgomerie R, Lyon BE. 2015. Sexual conflict arising from extrapair matings in birds. In The genetics and biology of sexual conflict (eds Rice W, Gavrilets S), pp. 141–153. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Montgomerie R. 2010. Sexual conflict and the intromittent organs of male birds. In The evolution of primary sexual characters in animals (eds Leonard J, Cordoba-Aguilar A), pp. 453–470. Oxford, UK: Oxford University Press. [Google Scholar]
- 67.Adkins-Regan E. 1999. Foam produced by male Coturnix quail: what is its function. The Auk 116, 184–193 ( 10.2307/4089465) [DOI] [Google Scholar]
- 68.Winterbottom M, Burke T, Birkhead TR. 2001. The phalloid organ, orgasm and sperm competition in a polygynandrous bird: the red-billed buffalo weaver (Bubalornis niger). Behav. Ecol. Sociobiol. 50, 474–482 ( 10.1007/s002650100384) [DOI] [Google Scholar]
- 69.Hemmings N, Birkhead TR. 2015. Polyspermy in birds: sperm numbers and embryo survival. Proc. R. Soc. B 282, 20151682 ( 10.1098/rspb.2015.1682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Snook RR, Hosken DJ, Karr TL. 2011. The biology and evolution of polyspermy: insights from cellular and functional studies of sperm and centrosomal behavior in the fertilized egg. Reproduction 142, 779–792 ( 10.1530/REP-11-0255) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.