Abstract
The role of non-gametic components of the ejaculate (seminal fluid) in fertility and sperm competitiveness is now well established. Surprisingly, however, we know far less about female reproductive fluid (FRF) in the context of sexual selection, and insights into male–FRF interactions in the context of sperm competition have only recently emerged. Despite this limited knowledge, evidence from taxonomically diverse species has revealed insights into the effects of FRF on sperm traits that have previously been implicated in studies of sperm competition. Specifically, through the differential effects of FRF on a range of sperm traits, including chemoattraction and alterations in sperm velocity, FRF has been shown to exert positive phenotypic effects on the sperm of males that are preferred as mating partners, or those from the most compatible or genetically diverse males. Despite these tantalizing insights into the putative sexually selected functions of FRF, we largely lack a mechanistic understanding of these processes. Taken together, the evidence presented here highlights the likely ubiquity of FRF-regulated biases in fertilization success across a diverse range of taxa, thus potentially elevating the importance of FRF to other non-gametic components that have so far been studied largely in males.
This article is part of the theme issue ‘Fifty years of sperm competition'.
Keywords: female reproductive fluid, sperm competition, cryptic female choice, ovarian fluid, follicular fluid, oviductal fluid
1. Introduction
Sperm competition was originally conceived as an extension of male–male (intrasexual) competition, but in this case through a contest that plays out among ejaculates from rival males after mating has occurred [1]. More recently, the burgeoning field of cryptic female choice [2,3], defined as female-mediated mechanisms that bias fertilization toward the sperm of specific males, emphasizes the critical role that females play in moderating the outcome of sperm competition to suit their reproductive interests. As highlighted in this special issue, the definitions of sperm competition and cryptic female choice have now been broadened considerably to include externally fertilizing species and those with less well-studied mating systems ([4], see also [5]).
The formal definitions for sperm competition and cryptic female choice emphasize how selection acts on, and targets, sperm cells, respectively. Yet an increasing body of evidence highlights the important role that non-gametic ejaculate components (seminal fluids) play during postmating sexual selection. Seminal fluids play a role in moderating the success of sperm when they compete to fertilize eggs, or in influencing or manipulating females' mating behaviour and the way they subsequently use sperm from different males [6]. For example, in Drosophila melanogaster, seminal fluid proteins have effects on female postmating responses, including changes in egg production, sexual receptivity and activation of the immune system, all of which serve to bias sperm utilization patterns [7].
While research on seminal fluid continues apace [see 8,9], we know far less about the potential sexually selected roles of female reproductive fluid. For the purposes of the present review, we define female reproductive fluid (hereafter FRF) as the medium, arising from females, through which sperm must pass on their way to fertilize eggs. In external fertilizers, the females release FRF along with the eggs; in internal fertilizers, the FRF remains inside the female reproductive tract. Components of the FRF may include (but are not limited to) ovarian, follicular, oviductal or coelomic fluid, egg chemoattractants, peripheral surfaces to eggs (e.g. egg jelly or cumulus cells), etc.
The aim of the present review is to broadly synthesize the recent literature on FRF and sexual selection, which together highlights the putative role that this fluid plays in postmating sexual selection (see figure 1 for a graphical overview of the role of FRF in postmating sexual selection). First, we briefly discuss the role that FRF plays before gametes are released, including its effect on males and eggs, and after fertilization, during the early stages of embryo development. Second, we examine the specific ways in which FRF influences sperm traits. Third, we explore the emerging evidence that FRF can play a role in moderating the selection of compatible/preferred sperm when ejaculates compete for fertilization (i.e. sexual selection). Fourth, we consider the putative mechanistic processes that underlie such sexually selected functions of FRF. Finally, we conclude by highlighting key gaps in our knowledge and provide a potential roadmap for future research in this area.
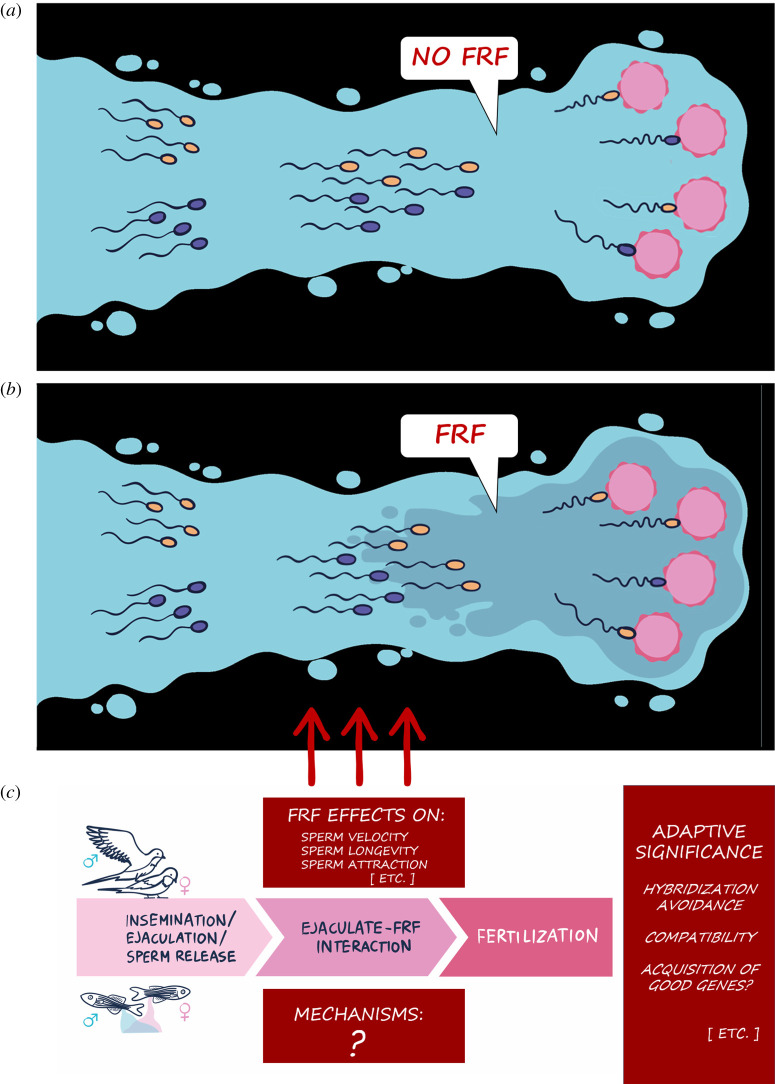
Figure 1.
A schematic representation of the general mechanism by which female reproductive fluid (FRF) can affect sperm competition among ejaculates from two males (indicated by yellow and blue sperm, respectively). (a) Depicts a situation in which sperm competition occurs with no effect of FRF, or where FRF has been experimentally removed. Sperm are transferred/released (left side) and will fertilize eggs (right side) based on a fair raffle or their intrinsic quality. (b) Depicts the same situation but with FRF present. As an example, FRF differentially influences sperm velocity from the two males, increasing the velocity of the yellow sperm, which then go on to fertilize more eggs than sperm from the other male. (c) Summarizes the successive steps from gamete transfer/release to fertilization and highlights what we have yet to learn about this process. (Online version in colour.)
2. Effects of female reproductive fluid before gametes are released
Before considering the effects of the FRF on sperm traits, it is important to acknowledge that FRF can also affect the fertilization process independently of any effects that directly influence ejaculates. That is, FRF can have effects prior to ejaculation. In this section, we briefly consider these processes, with an emphasis on those effects that can be linked to sexual selection mechanisms and ultimately affect sperm competition.
(a). Effects on the eggs
In internal fertilizers, FRF can play a prominent role in providing the appropriate environment for fertilization and the development of oocytes (see [10] for a review on mammals). FRF can also protect eggs from oxidative stress, which might otherwise compromise embryo viability [11]. Proteomic analyses in insects and mammals showed that FRF is involved in mitigating the deleterious effects of oxidative stress [12,13], which may indicate that this is a highly conserved function of the FRF in internal fertilizers. In external fertilizers, FRF has the additional function of protecting eggs from the adverse environmental conditions into which they are released, thereby prolonging egg viability [14]. Recent work indicates that FRF is also involved in protecting eggs and embryos from pathogens. For example, proteomic analyses of FRF in species such as fishes, insects and humans confirmed the presence of proteins related to the immune system and serving a proteolytic function within the FRF [15–18]. In summary, research on the effects of FRF on female gametes has focused on naturally selected functions and predominantly those that increase egg viability. Intriguingly, some of these effects of FRF on eggs may also indirectly influence postmating sexual selection. For example, recent evidence from zebrafish (Danio rerio) suggests that FRF is also able to extend the fertilization window (time available for fertilization since egg activation), which may increase the opportunity for sperm competition and cryptic female choice (L Pinzoni, MB Rasotto, F Poli, C Gasparini 2020, unpublished data).
(b). Effects on males and ejaculates before spawning/ejaculation
At least two lines of evidence indicate that FRF can have a significant effect on postmating sexual selection through mechanisms that occur before ejaculates interact with FRF. First, by attracting males, FRF increases the opportunity for premating sexual selection, but also for female multiple matings, and hence for postmating sexual selection, as in the rainbow trout (Oncorhynchus mykiss), where males increase their upstream movement in the presence of FRF [19]. Second, by inducing an adjustment in male ejaculate investment, FRF may alter the outcome of postmating sexual selection, as in in the brown trout (Salmo trutta), where males exposed to FRF show increased fertilization success under both competitive and non-competitive circumstances [20] (and see [21] for a similar effect in goldfish, Carassius auratus). If the adjustment of ejaculate quality requires some time, females may indirectly favour males with whom they have recently associated (e.g. dominant males). FRF therefore has the potential to influence the dynamics of postmating sexual selection also through its effects prior to ejaculation.
3. Effects of female reproductive fluid on sperm traits
There is increasing evidence across a diverse range of internally and externally fertilizing taxa that FRF has multiple effects (compared to a neutral/control medium) on sperm traits typically associated with sperm competition success [22,23].
Irrespective of reproductive mode, FRF is involved at almost every stage of the sperm's journey towards the egg, from activation to fertilization. In externally fertilizing fishes, for example, the proportion of sperm that becomes motile after release is generally higher in the presence, compared to the absence, of FRF [24]. In internal fertilizers, such as insects, FRF increases the proportion of viable sperm [25,26], although this effect is not universal [27,28]. By contrast, the positive effect of FRF on sperm longevity is so far proving to be ubiquitous across taxa, including both internally and externally fertilizing fishes [29,30], birds [31], and mammals [32,33]. In mammals, FRF induces both sperm capacitation and the acrosome reaction, which are necessary precursors to fertilization [34,35].
FRF-mediated sperm chemotaxis describes the capacity of sperm to respond to chemical attractants in order to locate and swim towards the egg. Much of the evidence for FRF-mediated sperm chemoattraction comes from marine invertebrates [36], but sperm chemoattraction mediated by the FRF has also been demonstrated in a range of internally and externally fertilizing taxa, including fishes [24,37], mammals [38–40] and indeed humans [41,42], among many other metazoans [43]. Interestingly, despite the fact that sperm chemotaxis was one of the first components of the FRF–ejaculate interaction to be documented, with research on the topic dating back to the end of the nineteenth century (see electronic supplementary material), it has rarely been considered in the context of sperm competition until very recently (see §4).
Finally, sperm swimming behaviour, collectively termed ‘sperm motility', is arguably the most common trait used to assess fertility and is commonly used as a proxy of sperm competitiveness in sexual selection studies [22,44]. Many studies on fishes have shown that sperm swimming speed is usually higher in the presence of FRF compared to a control solution [24,45]. The effects of FRF on parameters such as sperm velocity and sperm trajectory (e.g. linearity) have been reported also in marine invertebrates [36], amphibians [46], mammals [47] and birds ([31], but see [48]).
Overall, the evidence to date indicates that FRF can significantly alter (generally improve) sperm behaviour, including enhanced sperm capacitation, viability and longevity, chemoattraction, and sperm swimming velocity and trajectory.
4. Effects on sperm competitiveness and fertilization outcome
Here, we briefly summarize the direct evidence for FRF-mediated sexual selection from studies reporting fertilization biases when males from the same population compete to fertilize the same batch of eggs, along with evidence that FRF differentially affects sperm traits known to be associated with competitive fertilization success. We further illustrate the role of FRF in postmating sexual selection in species in which males exhibit alternative reproductive tactics, and finally, we briefly illustrate the potential role that FRF plays in reproductive isolation. All these studies are reported in electronic supplementary material tables 1 and 2.
(a). Differential fertilization success
Much of the evidence for FRF-mediated sexual selection suggests that these mechanisms serve to bias fertilization toward the sperm of the most compatible partner. A study of internally fertilizing guppies (Poecilia reticulata) revealed that FRF functions as an inbreeding avoidance mechanism, increasing the velocity of sperm from unrelated males, ultimately affecting competitive fertilization success towards the unrelated males [49]. In the externally fertilizing mussel (Mytilus galloprovincialis), FRF differentially attracts sperm from specific males [50], and these preferences predict both sperm swimming behaviour and offspring survival [51] and ultimately determine competitive fertilization success [52]. In the chinook salmon (Oncorhynchus tshawytscha), sperm swimming velocity measured in the FRF has been shown to better predict competitive paternity success than sperm velocity measured in the absence of FRF [53]. Further evidence for FRF-moderated fertilization biases comes from the ocellated wrasse (Symphodus ocellatus), where FRF was shown to decrease the numbers advantage that would otherwise be enjoyed by sneaking males (producing more sperm), thereby increasing the relative importance of sperm velocity and favouring preferred male phenotypes during sperm competition [54].
(b). Differential effects of female reproductive fluid on sperm performance
Other studies indirectly suggest that FRF affects sperm competition, by reporting differential (male-by-female) effects of FRF on sperm performance. These studies have been performed predominantly on external fertilizers (notably fishes), including arctic charr (Salvelinus alpinus) [55], chinook salmon [56,57] and the zebrafish [30], but there is also evidence in internal fertilizers, including guppies (see above [49]) and humans, where FRF has recently been shown to differentially attract sperm from different males [42]. Although many of these studies implicate FRF as a critical moderator of sperm competition, not all of them have revealed consistent effects. For example, studies of the quacking frog (Crinia georgiana) [46], lake trout (Salvelinus namaycush) [58], arctic charr [59] and capelin (Mallotus villosus) [60] reported no evidence for male-by-female interaction effects moderated by the FRF. Similarly, in the ant (Acromyrmex echinatior), there is no evidence of FRF-mediated inbreeding avoidance [26].
(c). Female reproductive fluid and alternative reproductive tactics
Recent studies have explored the possibility that FRF affects sperm competition in species where males employ alternative reproductive tactics, ultimately favouring males with ‘preferred' mating tactics. Studies investigating FRF-by-tactic interactions have so far focussed on fishes, where alternative male reproductive tactics are more common. In the chinook salmon (O. tshawytscha), for example, FRF increases the velocity of sperm from the dominant males but not those from sneakers, resulting in a paternity bias that ultimately favours dominant males [61]. Similarly, in the masu salmon (Oncorhynchus masou), FRF increases the velocity and motility of sperm from dominant males more than sperm from sneaky males [62]. By contrast, in the ocellated wrasse, FRF does not differentially affect the performance of sperm from alternative male phenotypes, but decreases the sperm numerical advantage that would otherwise favour sneakers in sperm competition (see above [54]).
(d). Hybridization avoidance and reproductive isolation
FRF has been shown to discriminate between conspecific and heterospecific sperm, favouring fertilization from males of the same species and thus serving as an anti-hybridization strategy (see also electronic supplementary material for early studies on sperm chemotaxis). Species-specific effects of FRF on sperm traits have been reported in echinoderms [63], molluscs [64,65], fishes [66,67] and birds [68]. Interestingly, the species-specific discrimination effect of FRF seems to be positively associated with the risk of hybridization, as it was reported in the pied and collared flycatchers (gen. Ficedula), which frequently hybridize [68], but this effect was not reported among bird species with low risk of hybridization [31]. Similarly, FRF differentially affects the velocity of sperm from sympatric and allopatric males of the same species, suggesting that FRF-mediated effects on sperm may be involved in reproductive isolation among populations that ultimately leads to speciation (in guppies and Atlantic cod [69,70]).
5. Female reproductive fluid–ejaculate interactions: mechanisms
The search for underlying mechanisms of the FRF's effect on postmating sexual selection has so far focused on the composition of these fluids. The chemical characteristics of FRF, such as osmolality, pH and ionic concentration (Na+, K+, Mg2+ and Ca2+) are likely to be important in both external [37] and internal fertilizers [6]. However, biochemical components of FRF, such as nutrients (glucose, pyruvate, lactate and fructose), free amino acids, hormones (e.g. prostaglandins, steroids, progesterone and growth factors), and proteins also play a pivotal role [37]. In fruit flies, for example, female reproductive proteomes of sibling species express unique secreted proteins, suggesting their involvement in postmating, prezygotic reproductive isolation [71]. Interestingly, while some FRF proteins, like albumin and immunoglobulins, are also found in the serum, others are FRF-specific, like many glycoproteins [10,13,72], suggesting a specific function in FRF–ejaculate interactions. Macromolecules, like proteins, carbohydrates, lipids or female exosomes, bind to sperm and affect their performance [6,73], while sugars can prolong sperm longevity [74,75], and progesterone is a well-known chemoattractant for mammalian sperm [76]. Mechanisms underlying FRF-mediated paternity biases may also include physiological or structural modifications to sperm [6], although for the most part such functions have not been investigated. One known example of this comes from the mussel M. galloprovincialis, where FRF has been shown to induce modifications on the sperm surface glycans [77] and hence affect the acrosome reaction to favour specific males at fertilization [78]. Chemical and biochemical components of FRF may interact in influencing sperm performance, as FRF Ca2+ concentration and FRF proteins may interact to modulate the activity of Ca2+ sperm-specific channels such as CatSper and pdk2, ultimately affecting sperm hyper-activation [79].
FRF can also affect competitive fertilization success by interacting with seminal fluid in addition to, or instead of, the sperm. Empirical evidence comes from bees and ants, where FRF inhibits seminal fluid serine proteases found in the seminal fluid, resulting in an increase in the viability of sperm stored by the female [17,80]. Specific FRF protein components putatively involved in the interactions with seminal fluid have been identified by proteomic analysis [81,82], or by comparing their sequence evolution rate [83,84]. For example, mammalian oviductal glycoproteins show signs of positive Darwinian selection [85], suggesting that they may play a role in FRF–seminal fluid interaction [86].
The scant knowledge of FRF-mediated mechanisms means that we can only speculate about the patterns of among-individual variation in their composition. Three distinct, non-mutually exclusive scenarios may be envisaged. First, FRF composition may show little among-female variation and instead may universally favour a preferred male phenotype [54], thus facilitating directional (i.e. consistent) postmating sexual selection. Second, FRF may vary among females in the relative quantity of one or more of its components [16,70], which in turn might differentially affect male sperm competition success. In this way, selection would be non-directional, in the sense that FRF would function to select the sperm from specific males, and these preferences would not be universal across females. Such a mechanism could act in conjunction with among-male variation in sperm/ejaculate characteristics (e.g. analogous to gamete reproductive proteins in eggs and their specific sperm ligands; see [36]). For example, variability in FRF concentration of specific ions, odorants or amino acids could interact with ‘taste' receptors expressed in sperm, influencing sperm traits and ultimately fertilization success [87,88]. Third, FRF can be involved in the recognition of ejaculates from genetically related males, generating patterns of disassortative postmating sexual selection [49,53]. Under this scenario, a matched variability in the protein products of hypervariable genes, such as MHC, odorant and taste receptors, should be observed in the sperm/ejaculate and FRF. The observed pattern of postmating sexual selection mediated by the FRF may therefore help us to identify the mechanisms underlying FRF–ejaculate interactions.
6. Conclusion and future directions
Our review highlights the putative sexually selected functions of female reproductive fluid, which have only recently emerged in the literature. In the same way that research on cryptic female choice lagged a long way behind sperm competition, research on FRF has lagged a long way behind that of seminal fluid and we are only now starting to appreciate the potential for FRF to serve a role in sexual selection.
Much of the explicit evidence for sexually selected roles of FRF comes from a small handful of organisms, notably fishes [49,53,54] and mussels [52]. Thus, one of the key messages of this review is that we require greater taxonomic breadth in studies that search for sexually selected functions of FRF. Similarly, the considerable evidence from diverse taxa that FRF can promote changes in sperm traits compared to a neutral medium (§3) has only rarely been extended to evaluate the possible significance of such effects for sexual selection (§4). Some limitations can be attributed to logistics, with FRF being available only seasonally, or its amount being limited. However, in some cases, these limitations can be overcome, for example, by freezing FRF (e.g. salmon [89]), and/or using small quantities (few µl) of FRF for the assays (e.g. zebrafish [30] or ants [26]). The bias toward external fertilizers (see electronic supplementary material tables 1 and 2) is attributable to the fact that FRF is easier to collect and manipulate in external fertilizers than in internal fertilizers. However, FRF collection techniques have been developed in many internal fertilizing animals (e.g. [26,42,49,90]), making it possible to manipulate and test the effect of FRF on sperm traits in a sperm competition context. Furthermore, in vitro fertilization can be performed in many internal fertilizers, making it possible to extend the research on the effect of FRF on competitive fertilization to these taxa. To this end, rodents are promising candidates for further research, as in vitro fertilization techniques are well developed and routinely used for sexual selection studies in this group (e.g. in the mouse [91,92]). The combination of proteomic studies on FRF composition, associated with genetic manipulation, can provide another promising tool to investigate the role of FRF in postmating sexual selection in model species with internal fertilization, such as Drosophila [6].
Finally, our review highlights a number of possible mechanisms by which FRF may function to selectively bias fertilization towards specific males. Many such mechanisms, which have been discussed recently in terms of their putative roles in postmating sexual selection [6,93], involve FRF, but only a handful of studies have implicated it as an agent of sexual selection. Moreover, evidence that extends these studies to an understanding of the molecular and physiological processes that underlie FRF-modulated processes of sexual selection is extremely rare (e.g. [17]). Thus, we see enormous scope for future studies that employ mechanistic (e.g. molecular, proteomic and physiological) approaches to understand the fine-scale processes that enable FRF to selectively bias fertilizations. We anticipate that such mechanisms are likely to be widespread, given that many of these processes have been evolutionarily conserved during the ‘cascade' (see [94]) that led to the evolutionary diversification of animals and the rise of pre- and postmating sexual selection.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Leigh Simmons and Nina Weddell for the invitation to contribute to this exciting special issue and Geoff Parker for his inspirational work that has opened the doors for the field. We thank three anonymous referees who helped to improve the manuscript. Also, a special thanks to Jacopo Sacquegno for his help with the figure.
Data accessibility
This article has no additional data.
Authors' contributions
All authors have contributed to the review and wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by the University of Padova STARS@UNIPD grant (STARS-CoG-2019, ‘SPSELECT’) to C.G.
References
- 1.Parker GA. 1970. Sperm competition and its evolutionary consequences in the insects. Biol Rev 45, 525–567. ( 10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 2.Thornhill R. 1983. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigricepts. Am. Nat. 122, 765–788. ( 10.1086/284170) [DOI] [Google Scholar]
- 3.Firman RC, Gasparini C, Manier MK, Pizzari T. 2017. Postmating female control: 20 years of cryptic female choice. Trends Ecol. Evol. 32, 368–382. ( 10.1016/j.tree.2017.02.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans JP, Lymbery RA. 2020. Sexual selection after gamete release in broadcast spawning invertebrates. Phil. Trans. R. Soc. B 375, 20200069 ( 10.1098/rstb.2020.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beekman M, Nieuwenhuis B, Ortiz-Barrientos D, Evans JP. 2016. Sexual selection in hermaphrodites, sperm and broadcast spawners, plants and fungi. Phil. Trans. R. Soc. B 371, 20150541 ( 10.1098/rstb.2015.0541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitnick S, Wolfner MF, Dorus S. 2020. Post-ejaculatory modifications to sperm (PEMS). Biol. Rev. 95, 365–392. ( 10.1111/brv.12569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry JC, Sirot L, Wigby S. 2013. The seminal symphony: how to compose an ejaculate. Trends Ecol. Evol. 28, 414–422. ( 10.1016/j.tree.2013.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramm SA. 2020. Seminal fluid and accessory male investment in sperm competition. Phil. Trans. R. Soc. B 375, 20200068 ( 10.1098/rstb.2020.0068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wigby S, Brown NC, Allen SE, Misra S, Sitnik JL, Sepil I, Clark AG, Wolfner MF. 2020. The Drosophila seminal proteome and its role in postcopulatory sexual selection. Phil. Trans. R. Soc. B 375, 20200072 ( 10.1098/rstb.2020.0072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguilar J, Reyley M. 2005. The uterine tubal fluid: secretion, composition and biological effects. Anim. Reprod. 2, 91–105. [Google Scholar]
- 11.Agarwal A, Gupta S, Sharma R. 2005. Oxidative stress and its implications in female infertility - a clinician's perspective. Reprod. Biomed. Online 11, 641–650. ( 10.1016/S1472-6483(10)61174-1) [DOI] [PubMed] [Google Scholar]
- 12.Fu Q, Huang Y, Wang Z, Chen F, Huang D, Lu Y, Liang X, Zhang M. 2016. Proteome profile and quantitative proteomic analysis of buffalo (Bubalus bubalis) follicular fluid during follicle development. Int. J. Mol. Sci. 17, 618 ( 10.3390/ijms17050618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baer B, Eubel H, Taylor NL, O'Toole N, Millar AH. 2009. Insights into female sperm storage from the spermathecal fluid proteome of the honeybee Apis mellifera. Genome Biol. 10, R67 ( 10.1186/gb-2009-10-6-r67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich MA, Dabrowski K, Arslan M, Ware K, Van Tassell J. 2012. Quantifying quality attributes of walleye eggs prior to fertilization—impact of time of ovulation and gametes storage. J. Gt Lakes Res. 38, 445–450. ( 10.1016/j.jglr.2012.06.009) [DOI] [Google Scholar]
- 15.Nynca J, Arnold GJ, Fröhlich T, Ciereszko A. 2015. Shotgun proteomics of rainbow trout ovarian fluid. Repr. Fert. Dev. 27, 504–512. ( 10.1071/RD13224) [DOI] [PubMed] [Google Scholar]
- 16.Johnson SL, Villarroel M, Rosengrave P, Carne A, Kleffmann T, Lokman PM, Gemmell NJ. 2014. Proteomic analysis of chinook salmon (Oncorhynchus tshawytscha) ovarian fluid. PLoS ONE 9, e104155 ( 10.1371/journal.pone.0104155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dosselli R, Grassl J, den Boer SP, Kratz M, Moran JM, Boomsma JJ, Baer B. 2019. Protein-level interactions as mediators of sexual conflict in ants. Mol. Cell Proteom. 18, S34–S45. ( 10.1074/mcp.RA118.000941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zamah AM, Hassis ME, Albertolle ME, Williams KE. 2015. Proteomic analysis of human follicular fluid from fertile women. Clin. Proteom. 12, 5 ( 10.1186/s12014-015-9077-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emanuel ME, Dodson JJ. 1979. Modification of the rheotropic behavior of male rainbow trout (Salmo gairdneri) by ovarian fluid. J. Fish Res. Board Can. 36, 63–68. ( 10.1139/f79-008) [DOI] [Google Scholar]
- 20.Hellström G, Prestegaard T, Dannewitz J, Olsén KH. 2013. Sperm from pheromone primed brown trout (Salmo trutta L.) produce more larvae. Fish Physiol. Biochem. 39, 471–478. ( 10.1007/s10695-012-9712-3) [DOI] [PubMed] [Google Scholar]
- 21.Hoysak DJ, Stacey NE. 2008. Large and persistent effect of a female steroid pheromone on ejaculate size in goldfish Carassius auratus. J. Fish Biol. 73, 1573–1584. ( 10.1111/j.1095-8649.2008.02032.x) [DOI] [Google Scholar]
- 22.Fitzpatrick JL, Lüpold S. 2014. Sexual selection and the evolution of sperm quality. Mol. Hum. Reprod. 20, 1180–1189. ( 10.1093/molehr/gau067) [DOI] [PubMed] [Google Scholar]
- 23.Snook RR. 2005. Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 20, 46–53. ( 10.1016/j.tree.2004.10.011) [DOI] [PubMed] [Google Scholar]
- 24.Zadmajid V, Myers JN, Sørensen SR, Butts IAE. 2019. Ovarian fluid and its impacts on spermatozoa performance in fish: a review. Theriogenology 132, 144–152. ( 10.1016/j.theriogenology.2019.03.021) [DOI] [PubMed] [Google Scholar]
- 25.den Boer SPA, Boomsma JJ, Baer B. 2009. Honey bee males and queens use glandular secretions to enhance sperm viability before and after storage. J. Insect. Physiol. 55, 538–543. ( 10.1016/j.jinsphys.2009.01.012) [DOI] [PubMed] [Google Scholar]
- 26.Liberti J, Baer B, Boomsma JJ. 2016. Queen reproductive tract secretions enhance sperm motility in ants. Biol. Lett. 12, 20160722 ( 10.1098/rsbl.2016.0722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernasconi G, Hellriegel B, Heyland A, Ward PI. 2002. Sperm survival in the female reproductive tract in the fly Scathophaga stercoraria (L.). J. Insect. Physiol. 48, 197–203. ( 10.1016/S0022-1910(01)00164-0) [DOI] [PubMed] [Google Scholar]
- 28.Holman L, Snook RR. 2008. A sterile sperm caste protects brother fertile sperm from female-mediated death in Drosophila pseudoobscura. Curr. Biol. 18, 292–296. ( 10.1016/j.cub.2008.01.048) [DOI] [PubMed] [Google Scholar]
- 29.Gasparini C, Evans JP. 2013. Ovarian fluid mediates the temporal decline in sperm viability in a fish with sperm storage. PLoS ONE 8, e64431 ( 10.1371/journal.pone.0064431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poli F, Immler S, Gasparini C. 2019. Effects of ovarian fluid on sperm traits and its implications for cryptic female choice in zebrafish. Behav. Ecol. 30, 1298–1305. ( 10.1093/beheco/arz077) [DOI] [Google Scholar]
- 31.Cramer ER, et al. 2016. Sperm performance in conspecific and heterospecific female fluid. Ecol. Evol. 6, 1363–1377. ( 10.1002/ece3.1977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J, Barratt CL, Lippes J, Pacey AA, Lenton EA, Cooke ID. 1994. Human oviductal fluid prolongs sperm survival. Fertil. Steril 61, 360–366. ( 10.1016/S0015-0282(16)56532-7) [DOI] [PubMed] [Google Scholar]
- 33.Sidhu K, Mate K, Molinia F, Glazier A, Rodger J. 1999. Secretory proteins from the female reproductive tract of the brushtail possum Trichosurus vulpecula: binding to sperm and effects on sperm survival in vitro. Reprod. Fertil. Dev. 11, 329–336. ( 10.1071/RD00010) [DOI] [PubMed] [Google Scholar]
- 34.McNutt TL, Killian GJ. 1991. Influence of bovine follicular and oviduct fluids on sperm capacitation in vitro. J. Androl. 12, 244–252. [PubMed] [Google Scholar]
- 35.Bravo Z, Valdivia M. 2018. Follicular fluid stimulates capacitation and acrosome reaction in alpaca sperm (Vicugna pacos). Reprod. Domest. Anim. 53, 629–635. ( 10.1111/rda.13151) [DOI] [PubMed] [Google Scholar]
- 36.Evans JP, Sherman CD. 2013. Sexual selection and the evolution of egg–sperm interactions in broadcast-spawning invertebrates. Biol. Bull. 224, 166–183. ( 10.1086/BBLv224n3p166) [DOI] [PubMed] [Google Scholar]
- 37.Kholodnyy V, Gadêlha H, Cosson J, Boryshpolets S. 2019. How do freshwater fish sperm find the egg? The physicochemical factors guiding the gamete encounters of externally fertilizing freshwater fish. Rev. Aquacult. 12, 1165–1192. ( 10.1111/raq.12378) [DOI] [Google Scholar]
- 38.Eisenbach M, Giojalas LC. 2006. Sperm guidance in mammals—an unpaved road to the egg. Nat. Rev. Mol. 7, 276–285. ( 10.1038/nrm1893) [DOI] [PubMed] [Google Scholar]
- 39.Cosson JJ. 2015. Flagellar mechanics and sperm guidance. Bentham Science Publishers; ( 10.2174/97816810812811150101) [DOI] [Google Scholar]
- 40.Fabro G, Rovasio RA, Civalero S, Frenkel A, Caplan SR, Eisenbach M, Giojalas LC. 2002. Chemotaxis of capacitated rabbit spermatozoa to follicular fluid revealed by a novel directionality-based assay. Biol. Reprod. 67, 1565–1571. ( 10.1095/biolreprod.102.006395) [DOI] [PubMed] [Google Scholar]
- 41.Eisenbach M, Tur-Kaspa I. 1994. Human sperm chemotaxis is not enigmatic anymore. Fertil. Steril 62, 233–235. ( 10.1016/S0015-0282(16)56869-1) [DOI] [PubMed] [Google Scholar]
- 42.Fitzpatrick JL, Willis C, Devigili A, Young A, Carroll M, Hunter HR, Brison DR. 2020. Chemical signals from eggs facilitate cryptic female choice in humans. Proc. R. Soc. B 287, 20200805 ( 10.1098/rspb.2020.0805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller RL. 1985. Sperm chemo-orientation in the metazoa. Biol. Fertil. 2, 275–337. ( 10.1016/B978-0-12-492602-8.50015-2) [DOI] [Google Scholar]
- 44.Simmons LW, Fitzpatrick JL. 2012. Sperm wars and the evolution of male fertility. Reproduction 144, 519–534. ( 10.1530/REP-12-0285) [DOI] [PubMed] [Google Scholar]
- 45.Myers JN, Senior A, Zadmajid V, Sørensen SR, Butts IAE. 2020. Associations between ovarian fluid and sperm swimming trajectories in marine and freshwater teleosts: a meta-analysis. Rev. Fish Sci. Aquac. 28, 322–339. ( 10.1080/23308249.2020.1739623) [DOI] [Google Scholar]
- 46.Simmons LW, Roberts JD, Dziminski MA. 2009. Egg jelly influences sperm motility in the externally fertilizing frog, Crinia georgiana. J. Evol. Biol. 22, 225–229. ( 10.1111/j.1420-9101.2008.01628.x) [DOI] [PubMed] [Google Scholar]
- 47.Oliveira R, Tomasi L, Rovasio R, Giojalas L. 1999. Increased velocity and induction of chemotactic response in mouse spermatozoa by follicular and oviductal fluids. J. Reprod. Fertil. 115, 23–27. ( 10.1530/jrf.0.1150023) [DOI] [PubMed] [Google Scholar]
- 48.Møller AP, Mousseau TA, Rudolfsen G. 2008. Females affect sperm swimming performance: a field experiment with barn swallows Hirundo rustica. Behav. Ecol. 19, 1343–1350. ( 10.1093/beheco/arn068) [DOI] [Google Scholar]
- 49.Gasparini C, Pilastro A. 2011. Cryptic female preference for genetically unrelated males is mediated by ovarian fluid in the guppy. Proc. R. Soc. B 278, 2495–2501. ( 10.1098/rspb.2010.2369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans JP, Garcia-Gonzalez F, Almbro M, Robinson O, Fitzpatrick JL. 2012. Assessing the potential for egg chemoattractants to mediate sexual selection in a broadcast spawning marine invertebrate. Proc. R. Soc. B 279, 2855–2861. ( 10.1098/rspb.2012.0181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliver M, Evans JP. 2014. Chemically moderated gamete preference predict offspring fitness in a broadcast spawning invertebrate. Proc. R. Soc. B 281, 20140148 ( 10.1098/rspb.2014.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lymbery RA, Kennington WJ, Evans JP. 2017. Egg chemoattractants moderate intraspecific sperm competition. Evol. Lett. 1, 317–327. ( 10.1002/evl3.34) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosengrave P, Montgomerie R, Gemmell N. 2016. Cryptic female choice enhances fertilization success and embryo survival in chinook salmon. Proc. R. Soc. B 283, 20160001 ( 10.1098/rspb.2016.0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alonzo SH, Stiver KA, Marsh-Rollo SE. 2016. Ovarian fluid allows directional cryptic female choice despite external fertilization. Nat. Commun. 7, 12452 ( 10.1038/ncomms12452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urbach D, Folstad I, Rudolfsen G. 2005. Effects of ovarian fluid on sperm velocity in Arctic charr (Salvelinus alpinus). Behav. Ecol. Sociobiol. 57, 438–444. ( 10.1007/s00265-004-0876-4) [DOI] [Google Scholar]
- 56.Rosengrave P, Gemmell NJ, Metcalf V, McBride K, Montgomerie R. 2008. A mechanism for cryptic female choice in chinook salmon. Behav. Ecol. 19, 1179–1185. ( 10.1093/beheco/arn089) [DOI] [Google Scholar]
- 57.Evans JP, Rosengrave P, Gasparini C, Gemmell NJ. 2013. Delineating the roles of males and females in sperm competition. Proc. R. Soc. B 280, 20132047 ( 10.1098/rspb.2013.2047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galvano PM, Johnson K, Wilson CC, Pitcher TE, Butts IA. 2013. Ovarian fluid influences sperm performance in lake trout, Salvelinus namaycush. Reprod. Biol. 13, 172–175. ( 10.1016/j.repbio.2013.02.001) [DOI] [PubMed] [Google Scholar]
- 59.Kleppe SA, Nordeide JT, Rudolfsen G, Figenschou L, Larsen B, Reiss K, Folstad I. 2018. No support for cryptic choice by ovarian fluid in an external fertilizer. Ecol. Evol. 8, 11 763–11 774. ( 10.1002/ece3.4628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orbach DN, Rooke AC, Evans JP, Pitcher TE, Purchase CF. 2019. Assessing the potential for post-ejaculatory female choice in a polyandrous beach-spawning fish. J. Evol. Biol. 33, 449–459. ( 10.1111/jeb.13579) [DOI] [PubMed] [Google Scholar]
- 61.Lehnert SJ, Butts IA, Flannery E, Peters K, Heath DD, Pitcher TE. 2017. Effects of ovarian fluid and genetic differences on sperm performance and fertilization success of alternative reproductive tactics in Chinook salmon. J. Evol. Biol. 30, 1236–1245. ( 10.1111/jeb.13088) [DOI] [PubMed] [Google Scholar]
- 62.Makiguchi Y, Torao M, Kojima T, Pitcher TE. 2016. Reproductive investment patterns and comparison of sperm quality in the presence and absence of ovarian fluid in alternative reproductive tactics of masu salmon, Oncorhynchus masou. Theriogenology 86, 2189–2193.e2182. ( 10.1016/j.theriogenology.2016.07.009) [DOI] [PubMed] [Google Scholar]
- 63.Dakin WJ, Fordham MGC. 1924. The chemotaxis of spermatozoa and its questioned occurrence in the animal kingdom. J. Exp. Biol. 1, 183. [Google Scholar]
- 64.Riffell JA, Krug PJ, Zimmer RK. 2004. The ecological and evolutionary consequences of sperm chemoattraction. Proc. Natl Acad. Sci. USA 101, 4501–4506. ( 10.1073/pnas.0304594101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller RL, Mojares JJ, Ram JL. 1994. Species-specific sperm attraction in the zebra mussel, Dreissena polymorpha, and the quagga mussel, Dreissena bugensis. Can. J. Zool. 72, 1764–1770. ( 10.1139/z94-238) [DOI] [Google Scholar]
- 66.Yeates SE, Diamond SE, Einum S, Emerson BC, Holt WV, Gage MJG. 2013. Cryptic choice of conspecific sperm controlled by the impact of ovarian fluid on sperm swimming behavior. Evolution 67, 3523–3536. ( 10.1111/evo.12208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Myers J, Bradford A, Hallas V, Lawson L, Pitcher T, Dunham R, Butts I. 2020. Channel catfish ovarian fluid differentially enhances blue catfish sperm performance. Theriogenology 149, 62–71. ( 10.1016/j.theriogenology.2020.03.022) [DOI] [PubMed] [Google Scholar]
- 68.Cramer ERA, Ålund M, McFarlane SE, Johnsen A, Qvarnström A. 2016. Females discriminate against heterospecific sperm in a natural hybrid zone. Evolution 70, 1844–1855. ( 10.1111/evo.12986) [DOI] [PubMed] [Google Scholar]
- 69.Devigili A, Fitzpatrick JL, Gasparini C, Ramnarine IW, Pilastro A, Evans JP. 2018. Possible glimpses into early speciation: the effect of ovarian fluid on sperm velocity accords with post-copulatory isolation between two guppy populations. J. Evol. Biol. 31, 66–74. ( 10.1111/jeb.13194) [DOI] [PubMed] [Google Scholar]
- 70.Beirão J, Purchase C, Wringe B, Fleming I. 2015. Inter-population ovarian fluid variation differentially modulates sperm motility in Atlantic cod Gadus morhua. J. Fish Biol. 87, 54–68. ( 10.1111/jfb.12685) [DOI] [PubMed] [Google Scholar]
- 71.McCullough EL, McDonough CE, Pitnick S, Dorus S. 2020. Quantitative proteomics reveals rapid divergence in the postmating response of female reproductive tracts among sibling species. Proc. R. Soc. B 287, 20201030 ( 10.1098/rspb.2020.1030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lahnsteiner F, Weismann T, Patzner RA. 1995. Composition of the ovarian fluid in 4 salmonid species: Oncorhynchus mykiss, Salmo trutta f lacustris, Salvelinus alpinus and Hucho hucho. Reprod. Nutr. Dev. 35, 465–474. ( 10.1051/rnd:19950501) [DOI] [PubMed] [Google Scholar]
- 73.Olson JH, Xiang X, Ziegert T, Kittelson A, Rawls A, Bieber AL, Chandler DE. 2001. Allurin, a 21-kDa sperm chemoattractant from Xenopus egg jelly, is related to mammalian sperm-binding proteins. Proc. Natl Acad. Sci. USA 98, 11 205–11 210. ( 10.1073/pnas.211316798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Austin CR, Mann CL, Mann TRR. 1964. Spermatophores and spermatozoa of the squid Loligo pealii. Proc. R. Soc. B 161, 143–152. ( 10.1098/rspb.1964.0085) [DOI] [PubMed] [Google Scholar]
- 75.Mann T. 1967. Sperm metabolism. In Fertilization (eds Metz CB, Monroy A), pp. 99–116. New York, NY: Academic Press. [Google Scholar]
- 76.Teves ME, Barbano F, Guidobaldi HA, Sanchez R, Miska W, Giojalas LC. 2006. Progesterone at the picomolar range is a chemoattractant for mammalian spermatozoa. Fertil. Steril 86, 745–749. ( 10.1016/j.fertnstert.2006.02.080) [DOI] [PubMed] [Google Scholar]
- 77.Kekäläinen J, Larma I, Linden M, Evans JP. 2015. Lectin staining and flow cytometry reveals female-induced sperm acrosome reaction and surface carbohydrate reorganization. Sci. Rep. 5, 15321 ( 10.1038/srep15321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kekäläinen J, Evans JP. 2017. Female-induced remote regulation of sperm physiology may provide opportunities for gamete-level mate choice. Evolution 71, 238–248. ( 10.1111/evo.13141) [DOI] [PubMed] [Google Scholar]
- 79.Cooper JC, Phadnis N. 2017. Parallel evolution of sperm hyper-activation Ca2+ channels. Genome Biol. Evol. 9, 1938–1949. ( 10.1093/gbe/evx131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.den Boer SPA, Baer B, Boomsma JJ. 2010. Seminal fluid mediates ejaculate competition in social insects. Science 327, 1506–1509. ( 10.1126/science.1184709) [DOI] [PubMed] [Google Scholar]
- 81.McDonough CE, Whittington E, Pitnick S, Dorus S. 2016. Proteomics of reproductive systems: towards a molecular understanding of postmating, prezygotic reproductive barriers. J. Proteom. 135, 26–37. ( 10.1016/j.jprot.2015.10.015) [DOI] [PubMed] [Google Scholar]
- 82.Findlay GD, Sitnik JL, Wang W, Aquadro CF, Clark NL, Wolfner MF. 2014. Evolutionary rate covariation identifies new members of a protein network required for Drosophila melanogaster female post-mating responses. PLoS Genet. 10, e1004108 ( 10.1371/journal.pgen.1004108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Swanson WJ, Vacquier VD. 2002. Reproductive protein evolution. Annu. Rev. Ecol. Evol. Syst. 33, 161–179. ( 10.1146/annurev.ecolsys.33.010802.150439) [DOI] [Google Scholar]
- 84.Sirot LK, Findlay GD, Sitnik JL, Frasheri D, Avila FW, Wolfner MF. 2014. Molecular characterization and evolution of a gene family encoding both female- and male-specific reproductive proteins in Drosophila. Mol. Biol. Evol. 31, 1554–1567. ( 10.1093/molbev/msu114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swanson WJ, Zhang ZH, Wolfner MF, Aquadro CF. 2001. Positive Darwinian selection drives the evolution of several female reproductive proteins in mammals. Proc. Natl Acad. Sci. USA 98, 2509–2514. ( 10.1073/pnas.051605998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patil K, Yelamanchi S, Kumar M, Hinduja I, Prasad TSK, Gowda H, Mukherjee S. 2019. Quantitative mass spectrometric analysis to unravel glycoproteomic signature of follicular fluid in women with polycystic ovary syndrome. PLoS ONE 14, e0214742 ( 10.1371/journal.pone.0214742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luddi A, Governini L, Wilmskotter D, Gudermann T, Boekhoff I, Piomboni P. 2019. Taste receptors: new players in sperm biology. Int. J. Mol. Sci. 20, 967 ( 10.3390/ijms20040967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ziegler A, Santos PSC, Kellermann T, Uchanska-Ziegler B. 2010. Self/nonself perception, reproduction, and the extended MHC. Self/nonself 1, 176–191. ( 10.4161/self.1.3.12736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Purchase CF, Rooke AC. 2020. Freezing ovarian fluid does not alter how it affects fish sperm swimming performance: creating a cryptic female choice ‘spice rack' for use in split-ejaculate experimentation. J. Fish Biol. 96, 693–699. ( 10.1111/jfb.14263) [DOI] [PubMed] [Google Scholar]
- 90.Cramer ERA, Laskemoen T, Eroukhmanoff F, Haas F, Hermansen JS, Lifjeld JT, Rowe M, Sætre G-P, Johnsen A. 2014. Testing a post-copulatory pre-zygotic reproductive barrier in a passerine species pair. Behav. Ecol. Sociobiol. 68, 1133–1144. ( 10.1007/s00265-014-1724-9) [DOI] [Google Scholar]
- 91.Whittingham D. 1968. Fertilization of mouse eggs in vitro. Nature 220, 592–593. ( 10.1038/220592a0) [DOI] [PubMed] [Google Scholar]
- 92.Firman RC. 2018. Postmating sexual conflict and female control over fertilization during gamete interaction. Ann. N. Y. Acad. Sci. 1422, 48–64. ( 10.1111/nyas.13635) [DOI] [PubMed] [Google Scholar]
- 93.Kekäläinen J, Evans JP. 2018. Gamete-mediated mate choice: towards a more inclusive view of sexual selection. Proc. R. Soc. B 285, 20180836 ( 10.1098/rspb.2018.0836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parker GA. 2014. The sexual cascade and the rise of pre-ejaculatory (Darwinian) sexual selection, sex roles, and sexual conflict. Cold Spring Harb. Perspect. Biol. 6, a017509 ( 10.1101/cshperspect.a017509) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.