Abstract
Purpose of Review
Periprosthetic infection is a relatively rare but potentially devastating complication after shoulder arthroplasty. The purpose of this article is to review the incidence, diagnosis, prevention, and management of periprosthetic infections after reverse shoulder arthroplasty, with a focus on literature published within the last 5 years.
Recent Findings
The 2018 International Consensus Meeting on Musculoskeletal Infection provides us with a framework for the diagnosis and management of periprosthetic infections after shoulder arthroplasty. Reverse shoulder arthroplasty has a higher reported rate of infection compared with anatomic total shoulder arthroplasty. Our current diagnostic tests do not appear to be as sensitive when compared with the hip and knee literature. Similar success has been reported with single and two-stage revision protocols, although prospective comparative data are lacking. The significance of unexpected positive cultures during revision arthroplasty remains unclear.
Summary
We report current diagnostic and therapeutic options for periprosthetic infection after reverse shoulder arthroplasty. Much of the current literature does not distinguish between anatomic and reverse shoulder arthroplasty. Further high-level studies are warranted to refine these definitions and guide management.
Keywords: Reverse shoulder arthroplasty, Prosthetic joint infection, Periprosthetic infection, Shoulder infection
Introduction
Reverse shoulder arthroplasty (RSA) is becoming an increasingly common procedure with expanding indications [1]. The incidence of periprosthetic infection after primary RSA most commonly ranges from 3 to 4% in the literature, although rates as low as 0.5% and as high as 6.7% have been reported [2–9]. According to a 2018 study of 4063 complications after shoulder arthroplasty reported to the FDA, infection after RSA comprised 13.8% of all RSA complications, with instability/dislocation being the most common complication reported at 32% [10].
Although there are well-established guidelines for management of infections after hip and knee arthroplasty, the diagnosis and management of periprosthetic infections of the shoulder remain less well defined. This review will focus on the definition, risk factors, diagnosis, prevention, and management of periprosthetic infections of the shoulder, with an emphasis on reverse shoulder arthroplasty literature published within the last 5 years.
Definition
In 2011, the Musculoskeletal Infection Society (MSIS) established a set of criteria for the diagnosis of periprosthetic infections of the hip and knee [11]. These criteria were updated in 2013 and 2018 and have largely been accepted across the orthopedic community [12, 13]. However, because periprosthetic infections of the shoulder are frequently caused by less virulent organisms, in particular Cutibacterium acnes, there is concern that the criteria used for hip and knee infections may not directly translate to infections of the shoulder. Furthermore, C. acnes colonizes the skin of the shoulder and is frequently identified in cultures even at the time of primary shoulder surgery, which further complicates definition of shoulder prosthetic joint infection (PJI) [14, 15••]. Therefore, the International Consensus Meeting (ICM) on Musculoskeletal Infection in 2018 established new guidelines specifically geared towards the diagnosis and management of periprosthetic infections of the shoulder [16••].
The 2018 ICM divided periprosthetic shoulder infection into four categories: definite infection, probable infection, possible infection, and unlikely infection. A definite infection is defined by the presence of one or more of the following criteria: (1) presence of a sinus tract from the skin surface to the prosthesis, (2) gross intra-articular pus, or (3) two positive tissue cultures with phenotypically identical virulent organisms, such as Staphylococcus aureus. This is in contrast to low-virulence organisms, which includes C. acnes and coagulase-negative Staphylococcus species. In addition to these definite criteria, the ICM also established a set of minor criteria for the definition of shoulder PJI (Table 1). A probable infection is defined as the presence of 6 or more minor criteria with an identified organism. A possible infection is defined as the one of the following: (1) presence of 6 or more minor criteria without an identified organism, (2) fewer than 6 minor criteria with a single positive culture with a virulent organism, or (3) fewer than 6 minor criteria with 2 positive cultures with a low-virulence organism. An unlikely infection is defined as the presence of fewer than 6 minor criteria with negative cultures or only a single positive culture with a low-virulence organism. These definitions are summarized in Table 2.
Table 1.
Minor criteria for shoulder PJI (Garrigues et al. 2019 [16••])
| Criteria | Weight |
|---|---|
| Unexpected wound drainage | 4 |
| Single positive tissue culture with a virulent organism | 3 |
| Single positive tissue culture with a low-virulent organism | 1 |
| Second positive tissue culture (identical low-virulence organism) | 3 |
| Humeral loosening | 3 |
| Positive frozen section (5 neutrophils in ≥ 5 high-power fields) | 3 |
| Positive pre-operative aspirate culture | 3 |
| Synovial neutrophil percentage > 80% | 2 |
| Synovial white blood cell count > 3000 cells/μL beyond 6 weeks from surgery | 2 |
| ESR > 30 mm/h | 2 |
| CRP > 10 mg/L | 2 |
| Elevated synovial alpha-defensin | 2 |
| Cloudy synovial fluid | 2 |
Table 2.
Definition of shoulder PJI (Garrigues et al. 2019 [16••])
| Category | Definition |
|---|---|
| Definite infection | Presence of a sinus tract from the skin surface to the prosthesis ORGross intra-articular pus ORTwo positive tissue cultures with identical virulent organisms |
| Probable infection | Presence of ≥6 minor criteria with an identified organism |
| Possible infection | Presence of ≥6 minor criteria without an identified organism OR< 6 minor criteria with one culture with a virulent organism OR< 6 minor criteria with 2 positive cultures with a low-virulence organism |
| Unlikely infection | < 6 minor criteria with negative cultures OR< 6 minor criteria with 1 positive culture with a low-virulence organism |
In summary, the 2018 ICM guidelines provide a definition specifically for periprosthetic infections of the shoulder [16••]. A standardized definition serves as a useful tool to aid in clinical decision making as well as allows for more consistent reporting in the literature. As these definitions are based on a consensus derived frequently from low-quality evidence and expert opinion, further studies are warranted to establish the validity of the criteria in diagnosing shoulder PJI. Advances in diagnosis should also lead to better ability to define and diagnose definite infections with low virulence organisms such as C. acnes.
Risk Factors
Patient Factors
Multiple studies have reported male gender as a significant risk factor for infection after shoulder arthroplasty, with odds ratios ranging from 1.5 to 3.5 times compared with their female counterparts [4, 7, 17–19]. The exact cause is likely multifactorial, but it has been shown that men have a higher burden of C. acnes at various sites around the shoulder which likely contributes to the risk of infection [20]. Younger patients have also been shown to be at higher risk of shoulder PJI [4, 7, 9, 17, 18].
Overall, the majority of studies currently have shown no correlation between the presence of diabetes and shoulder PJI [4, 7, 21, 22]. One retrospective database study of 18,729 TSA and RSA patients did find an increased risk of wound complications (OR 1.2) and deep infection (OR 1.47) in patients with a hemoglobin A1c > 8 mg/dL [23].
In terms of BMI, the current studies have shown mixed results. A large retrospective review by Richards et al. and meta-analysis by Kunutsor et al. reported no association between BMI and shoulder PJI [4, 7]. Wagner et al. reported a slightly higher rate of superficial infection, but no increased incidence of deep infection [24]. On the other hand, in a systematic review of upper limb arthroplasty, Theodoulou et al. reported an increased risk with increasing BMI, with an odds ratio of 2.37 in BMI > 30 kg/m2 and 5.04 in BMI > 40 kg/m2 [25]. Werner et al. reported similar results in their retrospective review, showing an incremental increase of shoulder PJI with increasing BMI, with an odds ratio of 3.4 in patients with BMI > 50 kg/m2 [26].
Smoking has also been shown to be a risk factor for shoulder PJI. Hatta et al. showed an increased risk of infection in both current (OR 7.3) and former (OR 4.6) smokers [27].
Other medical conditions that have been associated with an increased risk of periprosthetic infection after shoulder arthroplasty include patients with hepatitis C, HIV, Parkinson’s disease, and those on hemodialysis [28–31]. Patients with a history of solid organ transplant on immunosuppression have not been shown to be at increased risk for shoulder PJI [32].
Treatment Factors
In terms of treatment, there are multiple risk factors that have been associated with shoulder PJI. In a large retrospective study, Florschutz et al. showed a significantly increased risk of shoulder PJI in patients with a history of prior non-arthroplasty shoulder surgery, with a relative risk of 4.8 [33]. A history of recent steroid injection has also been shown to be a risk factor for shoulder PJI. Werner et al. showed that patients who received an ipsilateral shoulder steroid injection within 3 months prior to their arthroplasty had a 2 times increased risk of infection compared with those without a history of injection. They found no increased risk of infection in patients that received an injection 3–12 months prior to arthroplasty [34].
Compared with anatomic total shoulder arthroplasty (TSA), RSA has been shown to carry a higher risk of PJI, with odds ratios ranging from 2 to 6 times in the most recent literature [2, 4, 7, 8]. The cause of this increased risk with RSA is likely multifactorial. Patients who undergo RSA are typically older, have more medical comorbidities, and have higher rates of prior surgery [2, 33]. In addition, due to the frequent absence of a competent rotator cuff, there is an increased theoretical dead space with RSA, which may contribute to the risk of hematoma formation and subsequent infection. The indication for surgery has also been shown to be a risk factor for periprosthetic shoulder infection. Multiple studies have shown arthroplasty for treatment of a proximal humerus fracture has a higher risk of PJI, with 3–4 times greater risk compared with other surgical indications [4, 35]. Revision shoulder arthroplasty has also been shown to be a risk factor for PJI [9, 19].
In terms of post-operative management, Everhart et al. showed a dose-dependent increase in periprosthetic shoulder infection with perioperative blood transfusion, with a relative risk of 1.86 times per unit of packed red blood cells [19]. Grier et al. showed similar results, with an odds ratio for infection of 2 in patients who received a perioperative blood transfusion [36]. Post-operative therapeutic anticoagulation has also been shown to increase the rates of wound complications and PJI in a large retrospective study of 17,272 shoulder arthroplasty patients [37].
Diagnosis
Microbiology
Multiple studies have reported on the pathogens isolated in periprosthetic shoulder infection. By far the most commonly isolated bacterium is Cutibacterium acnes (formerly known as Propionibacterium acnes). C. acnes is an anaerobic gram-positive rod that is a part of the normal skin flora, found primarily in the sebaceous glands of hair follicles [14]. It is a slow-growing bacterium, and cultures must be held for at least 14 days in an anaerobic medium in order to reliably detect the organism [14, 15••]. Although a common misconception, the axilla does not harbor the highest concentrations of C. acnes when it comes to shoulder surgery. Instead, the chest and back regions actually have the highest burden of C. acnes due to a relatively higher density of sebaceous glands in the area [38, 39]. C. acnes is not as virulent as other bacterium that can cause prosthetic joint infection, such as Staph aureus, and the presentation tends to be more indolent in nature. It also tends to form biofilms around prosthetic implants, making its detection and eradication more difficult [14, 15••]. Multiple studies have also shown the presence of C. acnes in the deep tissues of primary shoulder arthroplasty patients without a history of prior surgery, complicating efforts to determine when C. acnes exists purely as a commensurate bacterium and when it is a pathogenic cause of infection [40–42].
The proportion of cases of shoulder PJI caused by C. acnes varies in the literature, ranging from 28 to 79% in the most recent reports [4, 19, 43, 44•, 45–47]. In a 2016 systematic review by Nelson et al., the pooled data showed C. acnes was implicated in 38.9% of all shoulder PJI, followed by Staph aureus at 14.8% and Staph epidermidis at 14.5% [43].
Clinical Presentation
The clinical presentation of periprosthetic infection of the shoulder is variable. Patients may present with symptoms of acute infection, such as redness, swelling, or drainage (Fig. 1), which can be accompanied by systemic symptoms of fevers, chills, and even sepsis. This is more likely when the infection is caused by a more virulent organism, such as Staph aureus. However, a more common presentation is one of a more indolent nature, with pain, stiffness, or limitations in function possibly being the only symptoms [14, 15••]. A draining sinus tract is pathognomonic for PJI but is rarely present.
Fig. 1.
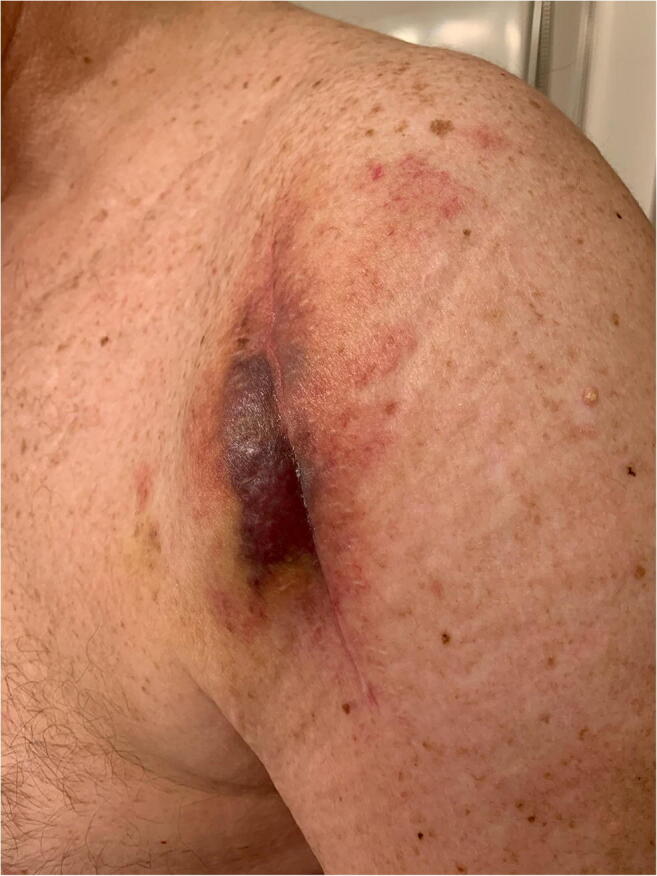
Left shoulder surgical site with erythema and skin changes, concerning for infection
Imaging
Plain radiographs are the initial imaging modality of choice when evaluating any painful shoulder arthroplasty. Although non-specific for infection, radiographs show the overall alignment of the prosthesis and can show signs of loosening or osteolysis [15••]. In a retrospective review of 193 revision shoulder arthroplasties, Pottinger et al. showed that the finding of humeral osteolysis on plain X-ray was associated with a 10-fold increased likelihood of growing C. acnes at the time of revision (Fig. 2) [48].
Fig. 2.
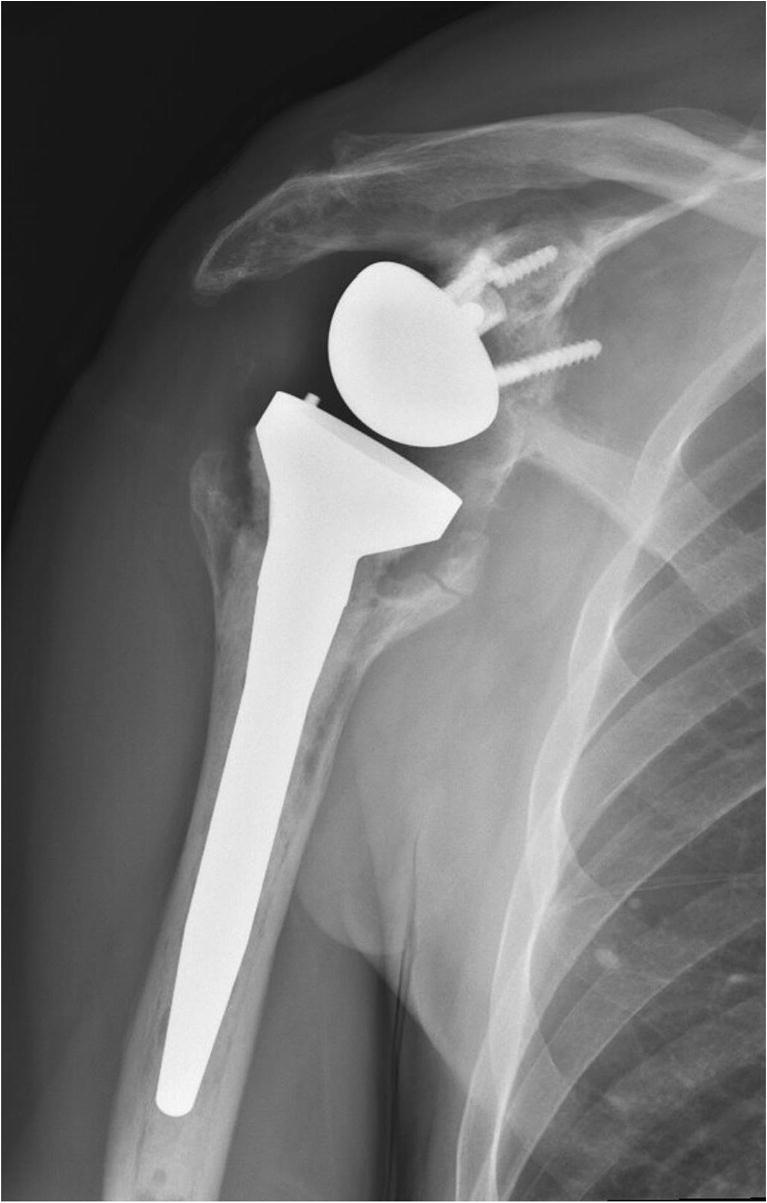
Significant humeral osteolysis and broken glenoid screws status-post reverse shoulder arthroplasty, concerning for infection
In terms of advanced imaging, computed tomography (CT) scans may be used to evaluate implant positioning, component loosening, and remaining bone stock prior to revision surgery; however, their ability to aid in the specific diagnosis of PJI is limited. If component loosening from PJI is evident from plain radiographs, we typically hold off on CT scan until after component explantation and spacer placement. This allows for a better estimation of bone stock for second stage revision due to the reduction in metal artifact (Fig. 3e).
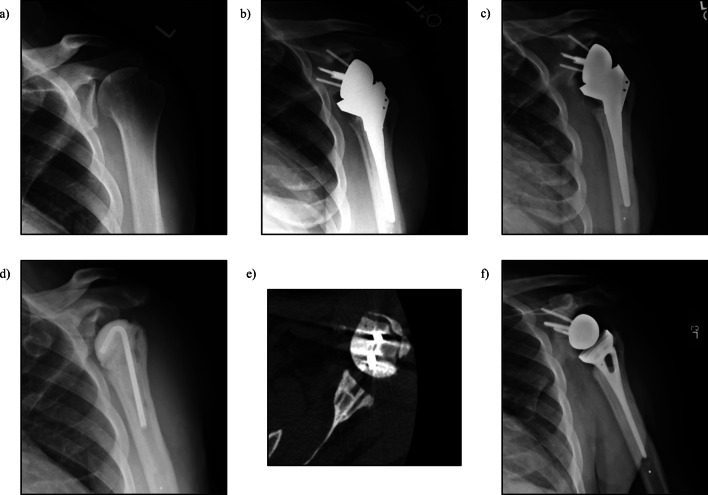
Fig. 3.
a 62-year-old male with Hamada Grade 3 left shoulder rotator cuff arthropathy. b Status-post reverse shoulder arthroplasty. c 7-year post-op with proximal humeral osteolysis. d Status-post explant and antibiotic spacer placement. e CT-scan showing glenoid bone stock remaining after explant and antibiotic spacer placement. f Status-post re-implantation of reverse shoulder arthroplasty after infection was cleared
Magnetic resonance imaging (MRI) is of limited value secondary to the significant metal artifact related to the prosthesis. Other imaging modalities, such as triple-phase bone scan, positron emission tomography (PET)/CT scan, or tagged white blood cell (WBC) combined with single-photon emission tomography (SPECT) scan, have limited sensitivity and/or specificity and are not currently recommended in the routine workup of shoulder PJI [15••, 49, 50].
Serum Labs
In addition to radiographs, a basic set of labs including serum white blood cell count (WBC) and inflammatory markers (ESR, CRP) are a routine part of the initial workup when there is concern for periprosthetic infection. Multiple studies have looked at the sensitivity and specificity of several serum inflammatory markers and overall the results are poor [43, 45, 46]. Shields et al. conducted a systematic review of the current diagnostic options, reporting the pooled sensitivity of serum WBC (7%), CRP (36%), ESR (39%), and IL-6 (13%) [51]. Currently the 2018 ICM recommends obtaining serum WBC, ESR, and CRP as a part of the initial workup [15••]. Overall, while elevated inflammatory markers may increase the suspicion for infection, normal values by no means rule it out. Furthermore, we have found that 25–30% of patients undergoing primary shoulder arthroplasty for non-infectious etiology have elevated inflammatory markers prior to surgery, so even elevated values may frequently be nonspecific.
Synovial Fluid Analysis
Arthrocentesis and synovial fluid analysis is the standard of care in the workup of prosthetic joint infection in the hip and knee literature; however, its role in shoulder arthroplasty is less clear [15••]. There are multiple concerns with regard to joint aspiration in the evaluation of shoulder PJI. First, multiple authors have reported a high incidence of “dry taps” when ordering or attempting imaging-guided arthrocentesis of a shoulder arthroplasty, up to 44% in the available literature, even in the setting of infection confirmed at the time of revision arthroplasty [15••, 52, 53]. Second, when fluid is obtained, there is concern that the sensitivity and specificity of our currently available tests are not high enough to reliably exclude an infection [54].
Regarding synovial fluid WBC, there are currently no high-level studies that provide a threshold to diagnose shoulder PJI. Currently, the 2018 ICM included synovial WBC > 3000 cells/μL and synovial neutrophil percentage > 80% as minor criteria in the diagnosis of shoulder PJI; however, these values are largely based on the hip and knee literature [13].
Synovial fluid alpha-defensin (Synovasure ®) is another lab that has been widely studied. There are currently two available alpha-defensin tests: the lab-based enzyme-linked immunosorbent assay (ELISA) test and the stand-alone alpha-defensin lateral flow test (ALDF). In a retrospective study of 105 patients with painful shoulder arthroplasty, Unter et al. demonstrated a sensitivity of 75% and specificity of 96% in the lab-based ELISA alpha-defensin test in diagnosing shoulder PJI [45]. Frangiamore et al. reported similar results with a sensitivity of 63% and specificity of 95% [55]. Weigelt et al. studied the stand-alone ALDF test, reporting a sensitivity of 60% and specificity of 83% [56]. Overall, while synovial fluid alpha-defensin does seem to have a role in the diagnosis of shoulder PJI, the sensitivity is much lower than the reported sensitivity of 97% in the hip and knee literature [57], and this must be taken into consideration when working up a possibly infected shoulder arthroplasty.
Multiple other synovial fluid biomarkers have been studied, including leukocyte esterase, Il-2, IL-6, and TNF-α. While some have shown promise, specifically when used in combination, additional research is needed to establish their validity, and they are currently not a part of the routine workup of periprosthetic infection of the shoulder [15••, 58, 59].
Pre-revision Tissue Culture
Due to the relatively low sensitivity of our currently available synovial fluid biomarkers, some surgeons have advocated for obtaining a tissue sample prior to undergoing revision surgery. Dilisio et al. studied the efficacy of pre-revision arthroscopic tissue biopsy for diagnosing PJI in 19 patients with suspicion for chronic PJI. They found that all patients with positive open biopsy tissue culture at the time of revision surgery also had positive arthroscopic cultures prior to revision with no false-positives, yielding a 100% sensitivity and specificity. This was in contrast to pre-operative aspiration, which only had a 16.7% sensitivity in their series [60]. Similarly, Lapner et al. studied pre-operative capsular needle biopsy in 17 patients undergoing revision arthroplasty for suspicion of infection. They found an 80% sensitivity and 100% specificity of the capsular needle biopsy using open surgical biopsy as their standard [61]. In all, tissue sampling prior to revision surgery does likely have a role as an adjunct in the workup of PJI in the painful shoulder arthroplasty for select cases where other testing is unrevealing and components are not clearly radiographically loose.
Intra-operative Evaluation
Intra-operative open biopsy with culture remains the gold standard for the diagnosis of shoulder PJI. The 2018 ICM recommends obtaining 5 deep tissue specimens from various surgical sites such as the capsule, humeral canal, and periprosthetic membranes. All cultures should be held for at least 14 days and prepared in aerobic and anaerobic media. Currently, holding antibiotics prior to obtaining intra-operative cultures is not recommended [15••].
In addition to tissue culture, other techniques for diagnosing PJI intra-operatively have been studied. Intra-operative frozen section has been widely studied in the diagnosis of PJI in the hip and knee literature, with the presence of greater than 5 neutrophils per high power field in 5 high power fields included as a minor criterion in the diagnosis [12]. Grosso et al. studied the efficacy of frozen section in diagnosing shoulder PJI. They found that using the guidelines of 5 neutrophils per high powered field in 5 high power fields yielded a sensitivity of 50% in the diagnosis of C. acnes infection. Using an optimized receiver operating curve, they found that using a guideline of 10 neutrophils total in 5 high power fields improved the sensitivity to 72% without sacrificing specificity [62]. We recommend obtaining frozen section at the time of revision surgery as an adjunct in the diagnosis of shoulder PJI; however, it requires an experienced pathologist and a negative result does not rule out infection.
Implant sonication is another adjunct test that has been studied in shoulder PJI. This process involves using ultrasonication via the application of sound waves to the explanted hardware in a liquid media, which has been shown to disrupt biofilms that may be present on the implants and allow for culture and quantification [63, 64]. The current literature does not support the use of routine implant sonication due to the low sensitivity as well as the lack of established diagnostic cutoffs for the quantification of bacteria in the obtained samples [15••, 65–67].
Prevention
Topical Treatments
A variety of topical regimens aimed at decreasing the cutaneous bacterial load pre-operatively have been studied. Both 3% hydrogen peroxide and 5% benzoyl peroxide applied topically have been shown to decrease the C. acnes bacterial burden on the skin surface without significant adverse reactions in multiple recent studies [38, 68–70, 71•, 72]. Chlorhexidine, on the other hand, has been shown to decrease overall bacterial load but without significant decrease in C. acnes burden after topical application [73]. Although the 2018 ICM does not recommend for or against topical skin treatments, they may provide a low-cost, low-risk adjunct in the prevention of shoulder PJI [74••].
Antibiotics
Cefazolin is currently the perioperative antibiotic of choice in shoulder arthroplasty in patients without a severe beta-lactam allergy. Two grams of IV cefazolin (3 g if patient weight > 120 kg) should be given 30–60 min prior to incision. Currently, the 2018 ICM states post-operative antibiotics are not required, but if given, should not be continued beyond 24 h post-operatively [74••]. For patients with a severe allergy to beta-lactams, vancomycin is the antibiotic of choice. The use of vancomycin requires a coordinated effort to start an infusion of 15 mg/kg (max 2 g) 2 h prior to skin incision [74••]. Yian et al. recently compared the efficacy of vancomycin and clindamycin versus cefazolin in a retrospective review of 7140 shoulder arthroplasties. They found no difference in the rate of infection between patients who received cefazolin and vancomycin, but a 3.5 times increased risk of infection in those that received clindamycin alone [44]. In addition, Wyles et al. showed a 32% lower risk of hip and knee PJI in those who received cefazolin compared with an alternative antibiotic [75]. Lastly, Rao et al. found no difference in rates of shoulder PJI when peri-operative doxycycline was administered in addition to cefazolin [76]. At our institution, the protocol is peri-operative vancomycin and aztreonam in patients with a severe beta-lactam allergy. For patients with a known history of MRSA infection or colonization, the 2018 ICM recommends vancomycin and cefazolin [74••].
Intra-operative Prophylaxis
There is significant interest in the use of intra-operative adjuncts, such as diluted povidone-iodine solution or vancomycin powder, in the prevention of PJI. To our knowledge, there is no currently available literature showing efficacy in shoulder arthroplasty; however, these agents have been studied in other areas of orthopedics. Iorio et al. recently retrospectively studied the effects of a “vanco-povidone protocol” in which high-risk patients undergoing total hip or total knee arthroplasty received a diluted povidone-iodine lavage following implantation and 2 g of vancomycin powder in the wound during closure. They found a 27.8% reduction in PJI in patients who received their povidone-iodine/vancomycin powder protocol without medical complications or increase in vancomycin-resistant organisms [77]. Vancomycin powder has also been shown to reduce surgical site infection in the spine literature [78]. Hatch et al. conducted a cost analysis of vancomycin powder in the prevention of shoulder PJI, showing the need for only a 0.04% reduction in absolute risk of PJI to be cost effective [79]. The 2018 ICM concluded that there may be a role for povidone-iodine and vancomycin powder in the prevention of shoulder PJI; however, further shoulder-specific studies are warranted [74••].
Management
Surgical Options
Once periprosthetic infection after shoulder arthroplasty has been diagnosed, there are a variety of surgical treatment methods that have been studied, including irrigation and debridement (I&D) with or without modular component exchange, one-stage revision, two-stage revision, and permanent treatment with an antibiotic spacer.
The current indications for I&D with component retention are unknown, and there is a paucity of shoulder-specific literature to guide decision-making. Dennison et al. reported a case series of 10 shoulder arthroplasties with either acute infection (defined as occurring within 6 weeks of the index procedure) or delayed acute hematogenous infection (defined as more than 6 weeks after the index surgery but with less than 3 weeks of symptoms). Three patients underwent arthroscopic I&D and 7 underwent open I&D. No patients had exchange of the polyethylene components. They reported a success rate of 70% with 30% recurrence of infection [80]. The 2018 ICM currently concluded that there is not enough evidence to support or discourage the use of I&D with implant retention for acute or chronic shoulder PJI, but it may play a role in select patients [81••].
There have been multiple retrospective studies looking at the efficacy of one-stage and two-stage revision arthroplasty in the treatment of shoulder PJI [43, 47, 82–88]. A 2020 meta-analysis by Aïm et al. showed pooled reinfection rates of 7% in 1-stage revisions and 21% in 2-stage revisions. They also reported a pooled complication rate of 17% in 1-stage revisions and 33% in 2-stage revisions [82]. The 2018 ICM reported similar results in their pooled analysis of the literature and concluded that the current literature demonstrates that 1-stage revision may be superior to 2-stage revision with lower re-infection and complication rates. However, there is likely selection bias in the currently available data and there are no high-level studies directly comparing the two treatments [81••].
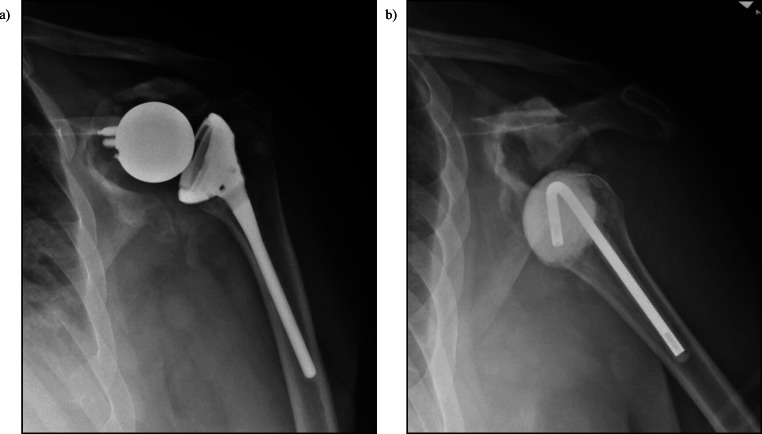
Definitive treatment with an antibiotic spacer is another viable treatment option in select patients. Pellegrini et al. reported a case series of 19 shoulder PJI that were definitively treated with an antibiotic cement spacer. They reported no recurrent infections with good pain relief and improvement in outcome scores but shoulder range of motion remained poor. They concluded that a definitive antibiotic spacer is a good option for low-demand, elderly individuals who do not wish to or are not otherwise able to undergo another operation (Fig. 4) [89]. However, antibiotic spacers are not without complication. McFarland et al. retrospectively studied 60 antibiotic spacers used for the treatment of PJI as a part of a 2-stage revision protocol. They reported 18 complications in 14 patients including glenoid erosion, humeral shaft erosion, fracture or rotation of the spacer, and humerus fractures [90].
Fig. 4.
a RSA with significant glenoid osteolysis. b 6 months status-post explant and antibiotic spacer placement with poor remaining glenoid bone stock and subluxation. Despite this, patient was pain-free with functional range of motion without signs of infection and did not desire further surgery
Antibiotic Treatment
The 2018 ICM recommends prolonged antibiotic treatment in conjunction with surgical management of shoulder PJI, including I&D with component retention, 1-stage, and 2-stage revision procedures; however, there is no specific guidance on the optimal antibiotic, route of administration, or duration of treatment. There may be a difference in optimal treatment based on the surgical procedure performed, the virulence of the infecting organism, and the chronicity of infection, but there is minimal current literature to guide any specific protocols. They recommend individualized treatment with culture-specific antibiotics in addition to consultation with local infectious disease specialists. There also may be a role for chronic suppressive antibiotic therapy in select patients that have retention of components or have failed previous curative attempts [81••].
Unexpected Positive Cultures
Unexpected positive cultures (UPC) at the time apparent aseptic revision arthroplasty pose a unique challenge without clear guidelines in the literature. Hsu et al. retrospectively studied 55 revision arthroplasties without signs of infection and compared outcomes between those who had > 2 unexpected positive cultures with C. acnes and those with 0 or 1 positive culture. All patients were treated with antibiotics for 3 weeks until cultures were finalized and patients with > 2 positive cultures received 6 months total of antibiotics. They reported a 49% rate of UPCs, with a significantly higher rate in males compared with females, with no difference in pain or functional outcome between those with > 2 UPCs and those without [91].
The 2018 ICM concludes there is insufficient literature to guide the optimal treatment for UPCs, with options including antibiotics, re-operation, and no additional treatment. They do, however, state that post-operative antibiotics beyond 24 h after revision arthroplasty with UPCs with an indolent organism do not appear to reduce the risk of subsequent infection [81••]. Overall, further studies are warranted to determine the significance and optimal management of unexplained positive cultures at the time of revision arthroplasty.
Author’s Preferred Treatment
When evaluating the painful reverse shoulder arthroplasty, we begin with a complete history, assessment of comorbidities that may be associated with infection, physical examination, and radiographs. Records are reviewed including details of prior surgeries, operative reports, and postoperative course for anything raising suspicion of infection. Patients with acute PJI may have more obvious symptoms including pain, erythema, drainage, and fever, and we evaluate such patients with ESR, CRP, CBC, and ultrasound-guided aspiration of the shoulder sent for cell count with differential and cultures, holding the anaerobic medium for 14 days.
Patients with indolent PJI due to C. acnes or other similar organisms do not typically have such acute symptoms and instead present later after surgery with more subtle symptoms as previously discussed. In these cases, we also obtain ESR, CRP, CBC, and aspiration. As has been discussed, these tests are often normal or nearly normal due to the poor sensitivity and specificity in this scenario. In a patient with normal labs, no lucency on radiographs, and a negative or dry aspiration, we typically will rule out other causes of pain such as acromial stress reaction or fracture, and recommend repeat evaluation at an appropriate interval. This allows for detection of progressive lucency/loosening on X-ray, which raises the suspicion for PJI, as this can develop over time with C. acnes. Arthroscopic or open biopsy can be considered as a last resort for well-fixed implants with other testing unrevealing for infection. We use this infrequently for the painful RSA. Additional laboratory and synovial fluid tests are not routinely used in our evaluation, although they are important areas of ongoing research.
For patients with acute PJI suspected or confirmed, we proceed to surgery urgently for an I&D. We will exchange modular components and short stemmed humeral components to remove as much bacterial load and biofilm as possible. Full revision of the glenoid baseplate or long-stemmed humeral components can be considered but must be balanced against morbidity of extraction and difficulty with revision fixation.
For the painful RSA proceeding to surgery for revision, we always maintain suspicion for infection since preoperative testing has limited sensitivity. Pre-incision antibiotics are not held in our practice. During surgery, we carefully assess synovial fluid and membrane formation, component loosening, and send multiple frozen sections. Gross purulence or a sinus tract is certainly pathognomonic for PJI but rarely is present. Components are explanted, and a complete I&D is performed while obtaining five deep tissue cultures from multiple locations including interfaces of the prosthesis and bone with membrane. If intraoperative assessment and frozen section are not concerning for infection, we proceed with single-stage revision of both components. If there is concern for infection, our preference is to place an antibiotic spacer with vancomycin and tobramycin, consult infectious disease for postoperative antibiotics, and plan for a two-stage revision (Fig. 3d). The second stage typically occurs after an antibiotic holiday and repeat laboratory work, at around 3 months from the first stage (Fig. 3f). In some cases the glenoid bone stock as determined by radiographs and CT scan may not support a revision reverse baseplate, and we will consider definitive treatment with retention of the antibiotic spacer (Fig. 4).
Patients who undergo revision of RSA in which there is low concern for infection may have unexpected positive cultures identified, while the cultures incubate for 14 days following surgery. There is controversy regarding significance and management of these as has been previously mentioned, and we discuss these cases with the patient and infectious disease team to generate a plan in each case. Typically if there is one positive culture out of five, we will simply observe the patient. If there are two or more positive cultures, we will not recommend further surgery as they already had a full one-stage revision. But in these cases, typically infectious disease will treat the patient with an antibiotic course intravenously and with discussion of further oral suppression thereafter.
Conclusion
Periprosthetic infection after reverse shoulder arthroplasty is a relatively rare complication with a variety of risk factors. The 2018 International Consensus Meeting on Musculoskeletal Infection provides a general framework for the diagnosis and management of shoulder PJI. There are many questions that remain unanswered, including optimal surgical treatment, antibiotic duration, and route of administration, as well as the significance of unexpected positive cultures. Further high-level, shoulder-specific studies are warranted to elucidate the answers to these important questions.
Compliance with Ethical Standards
Conflict of Interest
Erik Contreras, Travis Frantz, Julie Bishop, and Gregory Cvetanovich declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Reverse Shoulder Arthroplasty
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Erik S. Contreras, Email: Erik.Contreras@osumc.edu
Travis L. Frantz, Email: Travis.Frantz@osumc.edu
Julie Y. Bishop, Email: Julie.Bishop@osumc.edu
Gregory L. Cvetanovich, Email: Gregory.Cvetanovich@osumc.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Rugg CM, Coughlan MJ, Lansdown DA. Reverse total shoulder arthroplasty: biomechanics and indications. Curr Rev Musculoskelet Med. 2019;12(4):542–553. doi: 10.1007/s12178-019-09586-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villacis D, Sivasundaram L, Pannell WC, Heckmann N, Omid R, Hatch GF., III Complication rate and implant survival for reverse shoulder arthroplasty versus total shoulder arthroplasty: results during the initial 2 years. J Shoulder Elb Surg. 2016;25(6):927–935. doi: 10.1016/j.jse.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elb Surg. 2011;20(1):146–157. doi: 10.1016/j.jse.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Richards J, Inacio MCS, Beckett M, Navarro RA, Singh A, Dillon MT, Sodl JF, Yian EH. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. 2014;472(9):2809–2815. doi: 10.1007/s11999-014-3696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang JR, Dubiel MT, Cofield RH, Steinmann SP, Elhassan BT, Morrey ME, et al. Primary reverse shoulder arthroplasty using contemporary implants is associated with very low reoperation rates. J Shoulder Elb Surg. 2019;28(6S):S175–S180. doi: 10.1016/j.jse.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Chelli M, Cunsolo LL, Gauci MO, Gonzalez JF, Domos P, Bronsard N, et al. Reverse shoulder arthroplasty in patients aged 65 years or younger: a systematic review of the literature. JSES Open Access. 2019;3(3):162–167. doi: 10.1016/j.jses.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunutsor SK, Barrett MC, Whitehouse MR, Craig RS, Lenguerrand E, Beswick AD, Blom AW. Incidence, temporal trends and potential risk factors for prosthetic joint infection after primary total shoulder and elbow replacement: systematic review and meta-analysis. J Inf Secur. 2020;80(4):426–436. doi: 10.1016/j.jinf.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Moeini S, Rasmussen JV, Salomonsson B, Domeij-Arverud E, Fenstad AM, Hole R, Jensen SL, Brorson S. Reverse shoulder arthroplasty has a higher risk of revision due to infection than anatomical shoulder arthroplasty: 17 730 primary shoulder arthroplasties from the Nordic Arthroplasty Register Association. Bone Joint J. 2019;101-B(6):702–707. doi: 10.1302/0301-620X.101B6.BJJ-2018-1348.R1. [DOI] [PubMed] [Google Scholar]
- 9.Morris BJ, O'Connor DP, Torres D, Elkousy HA, Gartsman GM, Edwards TB. Risk factors for periprosthetic infection after reverse shoulder arthroplasty. J Shoulder Elb Surg. 2015;24(2):161–166. doi: 10.1016/j.jse.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Somerson JS, Hsu JE, Neradilek MB, Matsen FA., III Analysis of 4063 complications of shoulder arthroplasty reported to the US Food and Drug Administration from 2012 to 2016. J Shoulder Elb Surg. 2018;27(11):1978–1986. doi: 10.1016/j.jse.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Parvizi J, Zmistowski B, Berbari EF, Bauer TW. Springer BD; workgroup convened by the musculoskeletal infection society. New definition for periprosthetic joint infection. J Arthroplast. 2011;26(8):1136–1138. doi: 10.1016/j.arth.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Parvizi J, Gehrke T, International Consensus Group on Periprosthetic Joint Infection Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331. doi: 10.1016/j.arth.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Shohat N, Bauer T, Buttaro M, Budhiparama N, Cashman J, Della Valle CJ, Drago L, Gehrke T, Marcelino Gomes LS, Goswami K, Hailer NP, Han SB, Higuera CA, Inaba Y, Jenny JY, Kjaersgaard-Andersen P, Lee M, Llinás A, Malizos K, Mont MA, Jones RM, Parvizi J, Peel T, Rivero-Boschert S, Segreti J, Soriano A, Sousa R, Spangehl M, Tan TL, Tikhilov R, Tuncay I, Winkler H, Witso E, Wouthuyzen-Bakker M, Young S, Zhang X, Zhou Y, Zimmerli W. Hip and knee section, what is the definition of a periprosthetic joint infection (PJI) of the knee and the hip? Can the same criteria be used for both joints?: proceedings of international consensus on orthopedic infections. J Arthroplast. 2019;34(2S):S325–S327. doi: 10.1016/j.arth.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 14.Boisrenoult P. Cutibacterium acnes prosthetic joint infection: diagnosis and treatment. Orthop Traumatol Surg Res. 2018;104(1S):S19–S24. doi: 10.1016/j.otsr.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Garrigues GE, Zmistowski B, Cooper AM, Green A, ICM Shoulder Group Proceedings from the 2018 International Consensus Meeting on Orthopedic Infections: evaluation of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2019;28(6S):S32–S66. doi: 10.1016/j.jse.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Garrigues GE, Zmistowski B, Cooper AM, Green A, ICM Shoulder Group Proceedings from the 2018 International Consensus Meeting on Orthopedic Infections: the definition of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2019;28(6S):S8–S12. doi: 10.1016/j.jse.2019.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Matsen FA 3rd, Whitson A, Neradilek MB, Pottinger PS, Bertelsen A, Hsu JE. Factors predictive of Cutibacterium periprosthetic shoulder infections: a retrospective study of 342 prosthetic revisions. J Shoulder Elbow Surg. 2019. 10.1016/j.jse.2019.08.008. [DOI] [PubMed]
- 18.Werthel JD, Hatta T, Schoch B, Cofield R, Sperling JW, Elhassan BT. Is previous nonarthroplasty surgery a risk factor for periprosthetic infection in primary shoulder arthroplasty? J Shoulder Elb Surg. 2017;26(4):635–640. doi: 10.1016/j.jse.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Everhart JS, Bishop JY, Barlow JD. Medical comorbidities and perioperative allogeneic red blood cell transfusion are risk factors for surgical site infection after shoulder arthroplasty. J Shoulder Elb Surg. 2017;26(11):1922–1930. doi: 10.1016/j.jse.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Patel A, Calfee RP, Plante M, Fischer SA, Green A. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elb Surg. 2009;18(6):897–902. doi: 10.1016/j.jse.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 21.McElvany MD, Chan PH, Prentice HA, Paxton EW, Dillon MT, Navarro RA. Diabetes disease severity was not associated with risk of deep infection or revision after shoulder arthroplasty. Clin Orthop Relat Res. 2019;477(6):1358–1369. doi: 10.1097/CORR.0000000000000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lung BE, Bisogno M, Kanjiya S, Komatsu DE, Wang ED. Early postoperative complications and discharge time in diabetic patients undergoing total shoulder arthroplasty. J Orthop Surg Res. 2019;14(1):9. doi: 10.1186/s13018-018-1051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancienne JM, Brockmeier SF, Werner BC. Association of perioperative glycemic control with deep postoperative infection after shoulder arthroplasty in patients with diabetes. J Am Acad Orthop Surg. 2018;26(11):e238–45. 10.5435/JAAOS-D-16-00784. [DOI] [PubMed]
- 24.Wagner ER, Houdek MT, Schleck C, Harmsen WS, Sanchez-Sotelo J, Cofield R, Sperling JW, Elhassan BT. Increasing body mass index is associated with worse outcomes after shoulder arthroplasty. J Bone Joint Surg Am. 2017;99(11):929–937. doi: 10.2106/JBJS.15.00255. [DOI] [PubMed] [Google Scholar]
- 25.Theodoulou A, Krishnan J, Aromataris E. Risk of complications in patients who are obese following upper limb arthroplasty: a systematic review and meta-analysis. Obes Res Clin Pract. 2020;14(1):9–26. doi: 10.1016/j.orcp.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Werner BC, Burrus MT, Browne JA, Brockmeier SF. Superobesity (body mass index >50 kg/m2) and complications after total shoulder arthroplasty: an incremental effect of increasing body mass index. J Shoulder Elb Surg. 2015;24(12):1868–1875. doi: 10.1016/j.jse.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 27.Hatta T, Werthel JD, Wagner ER, Itoi E, Steinmann SP, Cofield RH, Sperling JW. Effect of smoking on complications following primary shoulder arthroplasty. J Shoulder Elb Surg. 2017;26(1):1–6. doi: 10.1016/j.jse.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Cancienne JM, Dempsey IJ, Holzgrefe RE, Brockmeier SF, Werner BC. Is hepatitis C infection associated with a higher risk of complications after total shoulder arthroplasty? Clin Orthop Relat Res. 2016;474(12):2664–2669. doi: 10.1007/s11999-016-4979-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bala A, Penrose CT, Visgauss JD, Seyler TM, Randell TR, Bolognesi MP, Garrigues GE. Total shoulder arthroplasty in patients with HIV infection: complications, comorbidities, and trends. J Shoulder Elb Surg. 2016;25(12):1971–1979. doi: 10.1016/j.jse.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Burrus MT, Werner BC, Cancienne JM, Gwathmey FW, Brockmeier SF. Shoulder arthroplasty in patients with Parkinson's disease is associated with increased complications. J Shoulder Elb Surg. 2015;24(12):1881–1887. doi: 10.1016/j.jse.2015.05.048. [DOI] [PubMed] [Google Scholar]
- 31.Cancienne JM, Kew ME, Deasey MJ, Brockmeier SF, Werner BC. Dialysis dependence and modality impact complication rates after shoulder arthroplasty. J Shoulder Elb Surg. 2019;28(3):e71–e77. doi: 10.1016/j.jse.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 32.Hatta T, Statz JM, Itoi E, Cofield RH, Sperling JW, Morrey ME. Shoulder arthroplasty in patients with immunosuppression following solid organ transplantation. J Shoulder Elb Surg. 2020;29(1):44–49. doi: 10.1016/j.jse.2019.05.042. [DOI] [PubMed] [Google Scholar]
- 33.Florschütz AV, Lane PD, Crosby LA. Infection after primary anatomic versus primary reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2015;24(8):1296–1301. doi: 10.1016/j.jse.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 34.Werner BC, Cancienne JM, Burrus MT, Griffin JW, Gwathmey FW, Brockmeier SF. The timing of elective shoulder surgery after shoulder injection affects postoperative infection risk in Medicare patients. J Shoulder Elb Surg. 2016;25(3):390–397. doi: 10.1016/j.jse.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 35.Lung BE, Kanjiya S, Bisogno M, Komatsu DE, Wang ED. Preoperative indications for total shoulder arthroplasty predict adverse postoperative complications. JSES Open Access. 2019;3(2):99–107. doi: 10.1016/j.jses.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grier AJ, Bala A, Penrose CT, Seyler TM, Bolognesi MP, Garrigues GE. Analysis of complication rates following perioperative transfusion in shoulder arthroplasty. J Shoulder Elb Surg. 2017;26(7):1203–1209. doi: 10.1016/j.jse.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 37.Cancienne JM, Awowale JT, Camp CL, Degen RM, Shiu B, Wang D, et al. Therapeutic postoperative anticoagulation is a risk factor for wound complications, infection, and revision after shoulder arthroplasty. J Shoulder Elbow Surg. 2020. 10.1016/j.jse.2019.11.029. [DOI] [PubMed]
- 38.Duvall G, Kaveeshwar S, Sood A, Klein A, Williams K, Kolakowski L, Lai J, Enobun B, Hasan SA, Henn RF, III, Gilotra MN. Benzoyl peroxide use transiently decreases Cutibacterium acnes load on the shoulder. J Shoulder Elb Surg. 2020;29(4):794–798. doi: 10.1016/j.jse.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 39.Hsu JE, Bumgarner RE, Matsen FA., 3rd Propionibacterium in shoulder arthroplasty: what we think we know today. J Bone Joint Surg Am. 2016;98(7):597–606. doi: 10.2106/JBJS.15.00568. [DOI] [PubMed] [Google Scholar]
- 40.Wong JC, Schoch BS, Lee BK, Sholder D, Nicholson T, Namdari S, Getz CL, Lazarus MD, Ramsey ML, Williams GR, Jr, Abboud JA. Culture positivity in primary total shoulder arthroplasty. J Shoulder Elb Surg. 2018;27(8):1422–1428. doi: 10.1016/j.jse.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Torrens C, Marí R, Alier A, Puig L, Santana F, Corvec S. Cutibacterium acnes in primary reverse shoulder arthroplasty: from skin to deep layers. J Shoulder Elb Surg. 2019;28(5):839–846. doi: 10.1016/j.jse.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Achermann Y, Goldstein EJ, Coenye T, Shirtliff ME. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev. 2014;27(3):419–440. doi: 10.1128/CMR.00092-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson GN, Davis DE, Namdari S. Outcomes in the treatment of periprosthetic joint infection after shoulder arthroplasty: a systematic review. J Shoulder Elb Surg. 2016;25(8):1337–1345. doi: 10.1016/j.jse.2015.11.064. [DOI] [PubMed] [Google Scholar]
- 44.Yian EH, Chan PH, Burfeind W, Navarro RA, Singh A, Dillon MT. Perioperative clindamycin use in penicillin allergic patients is associated with a higher risk of infection after shoulder Arthroplasty. J Am Acad Orthop Surg. 2020;28(6):e270–e276. doi: 10.5435/JAAOS-D-19-00168. [DOI] [PubMed] [Google Scholar]
- 45.Unter Ecker N, Koniker A, Gehrke T, Salber J, Zahar A, Hentschke M, Citak M. What is the diagnostic accuracy of alpha-defensin and leukocyte esterase test in periprosthetic shoulder infection? Clin Orthop Relat Res. 2019;477(7):1712–1718. doi: 10.1097/CORR.0000000000000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmadi S, Lawrence TM, Sahota S, Schleck CD, Harmsen WS, Cofield RH, et al. Significance of perioperative tests to diagnose the infection in revision total shoulder arthroplasty. Arch Bone Jt Surg. 2018;6(5):359–364. [PMC free article] [PubMed] [Google Scholar]
- 47.Mercurio M, Castioni D, Iannò B, Gasparini G, Galasso O. Outcomes of revision surgery after periprosthetic shoulder infection: a systematic review. J Shoulder Elb Surg. 2019;28(6):1193–1203. doi: 10.1016/j.jse.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 48.Pottinger P, Butler-Wu S, Neradilek MB, Merritt A, Bertelsen A, Jette JL, Warme WJ, Matsen FA., III Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am. 2012;94(22):2075–2083. doi: 10.2106/JBJS.K.00861. [DOI] [PubMed] [Google Scholar]
- 49.Falstie-Jensen T, Lange J, Daugaard H, Vendelbo MH, Sørensen AK, Zerahn B, et al. 18F FDG-PET/CT has poor diagnostic accuracy in diagnosing shoulder PJI. Eur J Nucl Med Mol Imaging. 2019;46(10):2013–2022. doi: 10.1007/s00259-019-04381-w. [DOI] [PubMed] [Google Scholar]
- 50.Falstie-Jensen T, Daugaard H, Søballe K, Ovesen J, Arveschoug AK, Lange J, Olsen BS, Sørensen AK, Gormsen LC, Zerahn B, Johanssen HVS, Elmengaard B, Thillemann TM, Bolvig L. Labeled white blood cell/bone marrow single-photon emission computed tomography with computed tomography fails in diagnosing chronic periprosthetic shoulder joint infection. J Shoulder Elb Surg. 2019;28(6):1040–1048. doi: 10.1016/j.jse.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 51.Shields MV, Abdullah L, Namdari S. The challenge of Propionibacterium acnes and revision shoulder arthroplasty: a review of current diagnostic options. J Shoulder Elb Surg. 2016;25(6):1034–1040. doi: 10.1016/j.jse.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Ince A, Seemann K, Frommelt L, Katzer A, Loehr JF. One-stage exchange shoulder arthroplasty for peri-prosthetic infection. J Bone Joint Surg Br. 2005;87(6):814–818. doi: 10.1302/0301-620X.87B6.15920. [DOI] [PubMed] [Google Scholar]
- 53.Strahm C, Zdravkovic V, Egidy C, Jost B. Accuracy of synovial leukocyte and polymorphonuclear cell count in patients with shoulder prosthetic joint infection. J Bone Jt Infect. 2018;3(5):245–248. doi: 10.7150/jbji.29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hecker A, Jungwirth-Weinberger A, Bauer MR, Tondelli T, Uçkay I, Wieser K. The accuracy of joint aspiration for the diagnosis of shoulder infections. J Shoulder Elb Surg. 2020;29(3):516–20. 10.1016/j.jse.2019.07.016. [DOI] [PubMed]
- 55.Frangiamore SJ, Saleh A, Grosso MJ, Kovac MF, Higuera CA, Iannotti JP, Ricchetti ET. α-Defensin as a predictor of periprosthetic shoulder infection. J Shoulder Elb Surg. 2015;24(7):1021–1027. doi: 10.1016/j.jse.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 56.Weigelt L, Plate A, Stadler L, Sutter R, Frustaci D, Zbinden R, et al. Alpha-defensin lateral flow test does not appear to be useful in predicting shoulder periprosthetic joint infections. Int Orthop. 2020. 10.1007/s00264-020-04532-x. [DOI] [PubMed]
- 57.Lee YS, Koo KH, Kim HJ, Tian S, Kim TY, Maltenfort MG, Chen AF. Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2017;99(24):2077–2084. doi: 10.2106/JBJS.17.00123. [DOI] [PubMed] [Google Scholar]
- 58.Frangiamore SJ, Saleh A, Grosso MJ, Farias Kovac M, Zhang X, Daly TM, Bauer TW, Derwin KA, Iannotti JP, Ricchetti ET. Neer award 2015: analysis of cytokine profiles in the diagnosis of periprosthetic joint infections of the shoulder. J Shoulder Elb Surg. 2017;26(2):186–196. doi: 10.1016/j.jse.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 59.Frangiamore SJ, Saleh A, Kovac MF, Grosso MJ, Zhang X, Bauer TW, et al. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97(1):63–70. doi: 10.2106/JBJS.N.00104. [DOI] [PubMed] [Google Scholar]
- 60.Dilisio MF, Miller LR, Warner JJ, Higgins LD. Arthroscopic tissue culture for the evaluation of periprosthetic shoulder infection. J Bone Joint Surg Am. 2014;96(23):1952–1958. doi: 10.2106/JBJS.M.01512. [DOI] [PubMed] [Google Scholar]
- 61.Lapner PLC, Hynes K, Sheikh A. Capsular needle biopsy as a pre-operative diagnostic test for peri-prosthetic shoulder infection. Shoulder Elbow. 2019;11(3):191–198. doi: 10.1177/1758573217743943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grosso MJ, Frangiamore SJ, Ricchetti ET, Bauer TW, Iannotti JP. Sensitivity of frozen section histology for identifying Propionibacterium acnes infections in revision shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(6):442–447. doi: 10.2106/JBJS.M.00258. [DOI] [PubMed] [Google Scholar]
- 63.Tunney MM, Patrick S, Gorman SP, Nixon JR, Anderson N, Davis RI, Hanna D, Ramage G. Improved detection of infection in hip replacements. A currently underestimated problem. J Bone Joint Surg Br. 1998;80(4):568–572. doi: 10.1302/0301-620x.80b4.8473. [DOI] [PubMed] [Google Scholar]
- 64.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357(7):654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 65.Grosso MJ, Frangiamore SJ, Yakubek G, Bauer TW, Iannotti JP, Ricchetti ET. Performance of implant sonication culture for the diagnosis of periprosthetic shoulder infection. J Shoulder Elb Surg. 2018;27(2):211–216. doi: 10.1016/j.jse.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Akgün D, Maziak N, Plachel F, Siegert P, Minkus M, Thiele K, et al. The role of implant sonication in the diagnosis of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2020. 10.1016/j.jse.2019.10.011. [DOI] [PubMed]
- 67.Torrens C, Santana F, Puig L, Sorli L, Alier A. Results of cement spacer sonication in the second stage of two-stage treatment of shoulder arthroplasty infection. J Orthop Surg Res. 2018;13(1):58. doi: 10.1186/s13018-018-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stull JD, Nicholson TA, Davis DE, Namdari S. Addition of 3% hydrogen peroxide to standard skin preparation reduces Cutibacterium acnes-positive culture rate in shoulder surgery: a prospective randomized controlled trial. J Shoulder Elbow Surg. 2020;29(2):212–216. doi: 10.1016/j.jse.2019.09.038. [DOI] [PubMed] [Google Scholar]
- 69.Hernandez P, Sager B, Fa A, Liang T, Lozano C, Khazzam M. Bactericidal efficacy of hydrogen peroxide on Cutibacterium acnes. Bone Joint Res. 2019;8(1):3–10. doi: 10.1302/2046-3758.81.BJR-2018-0145.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chalmers PN, Beck L, Stertz I, Tashjian RZ. Hydrogen peroxide skin preparation reduces Cutibacterium acnes in shoulder arthroplasty: a prospective, blinded, controlled trial. J Shoulder Elbow Surg. 2019;28(8):1554–1561. doi: 10.1016/j.jse.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 71.Singh AM, Sethi PM, Romeo AA, Anakwenze OA, Abboud JA, Namdari S. Strategies to decolonize the shoulder of Cutibacterium acnes: a review of the literature. J Shoulder Elbow Surg. 2020;29(4):660–666. doi: 10.1016/j.jse.2019.11.037. [DOI] [PubMed] [Google Scholar]
- 72.Kolakowski L, Lai JK, Duvall GT, Jauregui JJ, Dubina AG, Jones DL, Williams KM, Hasan SA, Henn RF, III, Gilotra MN. Neer award 2018: benzoyl peroxide effectively decreases preoperative Cutibacterium acnes shoulder burden: a prospective randomized controlled trial. J Shoulder Elb Surg. 2018;27(9):1539–1544. doi: 10.1016/j.jse.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Matsen FA, Whitson AJ, Hsu JE. While home chlorhexidine washes prior to shoulder surgery lower skin loads of most bacteria, they are not effective against Cutibacterium (Propionibacterium) Int Orthop. 2020;44(3):531–534. doi: 10.1007/s00264-019-04477-w. [DOI] [PubMed] [Google Scholar]
- 74.Garrigues GE, Zmistowski B, Cooper AM, Green A, ICM Shoulder Group Proceedings from the 2018 International Consensus Meeting on Orthopedic Infections: prevention of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2019;28(6S):S13–S31. doi: 10.1016/j.jse.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 75.Wyles CC, Hevesi M, Osmon DR, Park MA, Habermann EB, Lewallen DG, et al. John Charnley Award: increased risk of prosthetic joint infection following primary total knee and hip arthroplasty with the use of alternative antibiotics to cefazolin: the value of allergy testing for antibiotic prophylaxis. Bone Joint J. 2019;101-B(6_Supple_B):9–15. doi: 10.1302/0301-620X.101B6.BJJ-2018-1407.R1. [DOI] [PubMed] [Google Scholar]
- 76.Rao AJ, Chalmers PN, Cvetanovich GL, O'Brien MC, Newgren JM, Cole BJ, et al. Preoperative doxycycline does not reduce Propionibacterium acnes in shoulder Arthroplasty. J Bone Joint Surg Am. 2018;100(11):958–964. doi: 10.2106/JBJS.17.00584. [DOI] [PubMed] [Google Scholar]
- 77.Iorio R, Yu S, Schwarzkopf R, Vigdorchik J, Slover J, Riesgo AM, et al. Vancomycin powder and dilute povidone-iodine lavage for infection prophylaxis in high-risk total joint arthroplasty. J Arthroplasty. 2020. 10.1016/j.arth.2020.02.060. [DOI] [PubMed]
- 78.Hey HW, Thiam DW, Koh ZS, Thambiah JS, Kumar N, Lau LL, et al. Is intraoperative local vancomycin powder the answer to surgical site infections in spine surgery? Spine (Phila Pa 1976) 2017;42(4):267–274. doi: 10.1097/BRS.0000000000001710. [DOI] [PubMed] [Google Scholar]
- 79.Hatch MD, Daniels SD, Glerum KM, Higgins LD. The cost effectiveness of vancomycin for preventing infections after shoulder arthroplasty: a break-even analysis. J Shoulder Elb Surg. 2017;26(3):472–7. 10.1016/j.jse.2016.07.071. [DOI] [PubMed]
- 80.Dennison T, Alentorn-Geli E, Assenmacher AT, Sperling JW, Sánchez-Sotelo J, Cofield RH. Management of acute or late hematogenous infection after shoulder arthroplasty with irrigation, débridement, and component retention. J Shoulder Elb Surg. 2017;26(1):73–78. doi: 10.1016/j.jse.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 81.Garrigues GE, Zmistowski B, Cooper AM, Green A, ICM Shoulder Group Proceedings from the 2018 International Consensus Meeting on Orthopedic Infections: management of periprosthetic shoulder infection. J Shoulder Elbow Surg. 2019;28(6S):S67–S99. doi: 10.1016/j.jse.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 82.Aïm F, Marion B, Kerroumi Y, Meyssonnier V, Marmor S. One- or two-stage exchange for periprosthetic shoulder infection: systematic review and meta-analysis. Orthop Traumatol Surg Res. 2020;106(1):5–15. doi: 10.1016/j.otsr.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 83.Patrick M, Vincent HK, Farmer KW, King JJ, Struk AM, Wright TW. Management of infected shoulder arthroplasty: a comparison of treatment strategies. J Shoulder Elb Surg. 2019;28(9):1658–1665. doi: 10.1016/j.jse.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 84.Tseng WJ, Lansdown DA, Grace T, Zhang AL, Feeley BT, Hung LW, Ma CB. Outcomes of revision arthroplasty for shoulder periprosthetic joint infection: a three-stage revision protocol. J Shoulder Elb Surg. 2019;28(2):268–275. doi: 10.1016/j.jse.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 85.Grubhofer F, Imam MA, Wieser K, Achermann Y, Meyer DC, Gerber C. Staged revision with antibiotic spacers for shoulder prosthetic joint infections yields high infection control. Clin Orthop Relat Res. 2018;476(1):146–152. doi: 10.1007/s11999.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stone GP, Clark RE, O'Brien KC, Vaccaro L, Simon P, Lorenzetti AJ, Stephens BC, Frankle MA. Surgical management of periprosthetic shoulder infections. J Shoulder Elb Surg. 2017;26(7):1222–1229. doi: 10.1016/j.jse.2016.11.054. [DOI] [PubMed] [Google Scholar]
- 87.Assenmacher AT, Alentorn-Geli E, Dennison T, Baghdadi YMK, Cofield RH, Sánchez-Sotelo J, Sperling JW. Two-stage reimplantation for the treatment of deep infection after shoulder arthroplasty. J Shoulder Elb Surg. 2017;26(11):1978–1983. doi: 10.1016/j.jse.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Buchalter DB, Mahure SA, Mollon B, Yu S, Kwon YW, Zuckerman JD. Two-stage revision for infected shoulder arthroplasty. J Shoulder Elb Surg. 2017;26(6):939–947. doi: 10.1016/j.jse.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 89.Pellegrini A, Legnani C, Macchi V, Meani E. Management of periprosthetic shoulder infections with the use of a permanent articulating antibiotic spacer. Arch Orthop Trauma Surg. 2018;138(5):605–609. doi: 10.1007/s00402-018-2870-8. [DOI] [PubMed] [Google Scholar]
- 90.McFarland EG, Rojas J, Smalley J, Borade AU, Joseph J. Complications of antibiotic cement spacers used for shoulder infections. J Shoulder Elb Surg. 2018;27(11):1996–2005. doi: 10.1016/j.jse.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 91.Hsu JE, Gorbaty JD, Whitney IJ, Matsen FA., 3rd Single-stage revision is effective for failed shoulder arthroplasty with positive cultures for Propionibacterium. J Bone Joint Surg Am. 2016;98(24):2047–2051. doi: 10.2106/JBJS.16.00149. [DOI] [PubMed] [Google Scholar]