Abstract
Purpose of Review
Interbody implants allow for fusion of the anterior column of the spine between vertebral body endplates. As rates of spinal fusion surgery have increased over the past several years, significant research has been devoted to optimizing both the mechanical and biologic properties of the interbody implant in order to promote bony fusion. The first interbody implants used decades ago were fashioned from cortical autograft. Currently, titanium alloy and polyetheretherketone (PEEK) are the most widely used and studied materials for this purpose. This review focuses on recent innovations in material modification and surface treatment techniques for both titanium and PEEK implants to maximize fusion rates in spinal surgery.
Recent Findings
Titanium has an elastic modulus much higher than native bone and however has better osseointegrative properties than PEEK. PEEK, however, has an elastic modulus closer to that of bone without any of the advantageous biologic properties that titanium has. Increasing porosity and surface roughness of titanium implants have been shown to improve the mechanical properties of titanium implants, while the biologic properties of PEEK have been enhanced using surface coating technology, either with titanium or with hydroxyapatite (HA).
Summary
Techniques such as increasing porosity, surface roughening, and surface coating are just some of the recent innovations aimed at optimizing both mechanical and biologic properties of interbody implants to promote spinal fusion. The future of interbody implant design will rely on continued improvements of PEEK and titanium implants as well as exploring new implant materials altogether.
Keywords: Interbody, Titanium, PEEK, Porosity, Coating
Introduction
Rates of spinal fusion surgery have been steadily increasing over the last several decades, and achieving adequate fusion is critical to patient outcomes [1, 2]. The use of an interbody implant in spinal surgery facilitates fusion between vertebral bodies anteriorly, often in conjunction with a posterior fusion allowing for circumferential bony growth. The role of the interbody implant is twofold—to provide a mechanical strut between the two endplates and to facilitate bony growth between the two vertebral bodies. Initially introduced in the 1930s, the first interbody implant used was cortical autograft—this evolved over time to stainless steel, titanium alloy, polyetheretherketone (PEEK), and more recently tantalum and silicon nitride [3]. Over the past several years, significant attention has been given in optimizing the balance between mechanical and biologic properties for interbody implants; this review focuses on recent advances of surface treatments and material modifications to both titanium and PEEK implants in order to improve fusion rates.
Implant Material
The first stage in developing an interbody implant is selection of the implant material itself. An interbody cage must have several key characteristics: first, the material must have sufficient mechanical strength to resist the compressive forces across the interbody space as it will be a load-bearing device, especially in the lumbar spine. This must be balanced, however, with the ability to resist shear forces as well—as such, a solid material is not necessarily ideal as it may be too brittle. Additionally, early stable fixation and bone growth is critical to resisting shear forces. Secondly, the material should have an elastic modulus similar to that of the bone. If the elastic modulus is too high, stress shielding may occur leading to subsidence and interspace collapse [4, 5••, 6]. The elastic modulus of cortical bone is 18 GPa; titanium has an elastic modulus closer to 110 GPa. Neat PEEK has an elastic modulus around 4 GPa and however can be engineered to more closely resemble that of the bone with the addition of other materials [6]. Lastly, the implant material must have osseointegrative properties that allow for adequate fusion; early and sufficient bony growth also aids in resisting shear stress.
The two main materials currently in use for interbody spacers are titanium alloy and PEEK, as they excel in different aspects. The Ti-6Al-4 V alloy has been the most widely used alloy for spinal spacers [7] (Fig. 1). Titanium is advantageous in that it can easily withstand compressive forces. By forming TiO2, titanium implants are able to enhance bony on-growth as well – TiO2 generates hydroxide ions which can bind Ca2+ and PO43− to form apatite similar to bone, promoting osteoblastic activity [3, 8, 9]. Due to its high elastic modulus, however, titanium implants are susceptible to stress shielding and may result in subsidence.
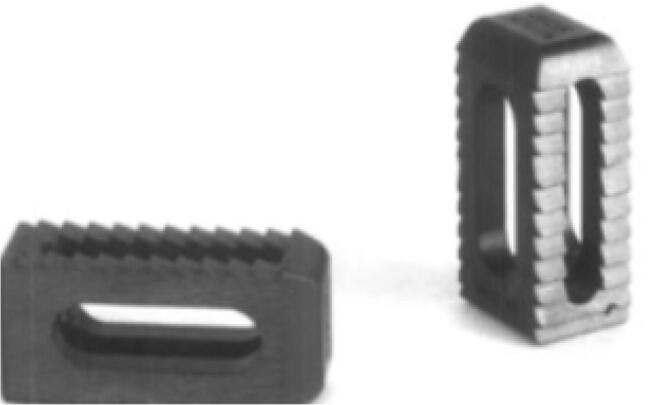
Fig. 1.
Traditional solid titanium cage with central space to place autograft/allograft within the cage
PEEK, first used in the spine in the 1990s [10], is a biocompatible polymer that can be engineered to possess varying biomechanical properties [6] (Fig. 2). Although PEEK has a native elastic modulus lower than that of the bone (4 GPa), the addition of carbon fiber allows for an elastic modulus that closely resembles the bone [6, 11, 12]. The material, however, is relatively biologically inert due to its hydrophobic surface chemistry [13–15]. Lastly, one of the benefits of PEEK is the fact that it is radiolucent, aiding in assessing fusion results [16]. As such, significant effort has been put to improve on these shortcomings in both titanium and PEEK cages in order to optimize their use as an interbody implant.
Fig. 2.
Traditional PEEK cage of various designs
Other materials that have more recently been proposed for interbody implants. Tantalum is a metal that has an elastic modulus similar to cancellous bone, and a porous design can allow for bony ingrowth [17]. In vitro studies have also shown that tantalum can stimulate osteoblast proliferation [18]. A prospective randomized control trial in cervical surgery found no radiologic or clinical differences at 24 months for 61 anterior cervical discectomy and fusion (ACDF) patients with tantalum spacers compared with autologous iliac bone graft and plating [19]. Of these patients, 89.3% had a radiographic fusion by 6 months with the tantalum implant [19]. Fernandez-Fairen et al. followed patients for 11 years postoperatively with tantalum implants for anterior cervical spine surgery and determined that there were no significant differences long-term when compared with tri-cortical autograft [20•]. In a goat model study performed by Sinclair et al., porous tantalum implants also had significantly higher bone volume at the bone-implant interface versus neat PEEK implants [21].
Silicon nitride (Si3N4) is a radiolucent ceramic material that has also been found to have osseointegrative properties. Silicon nitride has high mechanical wear properties and is resistant to fracture as well [22]. Silicon nitride implants have recently been studied in anterior cervical surgery—Smith et al. looked at 58 patients who underwent ACDF and found 96.83% had achieved fusion at ≥ 12 month follow-up postoperatively with silicon nitride implants. Time to fusion was significantly faster as well in the silicon nitride group with an average subsidence significantly lower in the silicon nitride group compared to an allograft spacer group [23].
Titanium Alloy Modifications
Porosity
Given the excellent biological properties of titanium and its wide use in many areas of orthopedic surgery, significant effort has been given to optimize its use in spinal surgery. A major advancement in this area came with adjusting the material’s porosity (Fig. 3). There are several benefits to a porous titanium implant: decreased elastic modulus closer to that of native bone as well as space for bony in-growth (in addition to bony on-growth). Studies have shown increased osteoblast adhesion and differentiation in porous titanium cages [24, 25], and it has been shown this porous structure resembling trabecular bone allows for osteoblast migration [26]. Developing the ideal porosity, however, is not as simple. While increasing pore size and porosity allows for decreased elastic modulus and more surface for bony in-growth, it results inevitably in decreased mechanical strength and resistance to compressive forces. One must also consider pore interconnectivity to promote bony in-growth [27]. Prior studies have shown optimal pore sizes to be ~100–400um [27]. A study by Fujibayashi et al. looked at titanium implants that had on average 60% porosity and a 250 μm pore size [28]. These implants were found to be mechanically stable (withstanding cyclic loading of 10,000 N at 4hz for 1,000,000 cycles) [28]. They were implanted prospectively in 5 patients, and by 3 months postoperatively they showed a solid bony construct without abnormal motion or radiolucency and bony union in all cases on radiological assessment by 6 months. CT imaging showed bony in-growth on the porous surface with no subsidence at final follow-up at 12 months as well [28]. Wu et al. similarly developed a porous titanium interbody cage using electron beam melting (EBM) with average porosity of 68% and a slightly larger pore size of 710 μm with full interconnectivity throughout [29]. The implants were placed in a sheep cervical spine model. Wu et al. found their porous titanium implant to have an elastic modulus similar to that of natural bone, with fast bony in-growth on histomorphometrical analysis and subsequent decreased micro-motion relative to PEEK cages [29]. A study by McGilvray et al. compared 3D-printed porous titanium cages, PEEK cages, and plasma-sprayed porous titanium-coated PEEK (PSP) cages in an ovine lumbar spine model [5••]. Radiographic analysis found that the 3D-printed porous titanium interbody cages had significantly lower range of motion, increased bone in-growth profile, and increased construct stiffness compared with the PEEK and PSP cages at both 8 and 16 weeks following implantation [5••]. Li et al. similarly looked at 3D-printed porous titanium cages relative to solid titanium and PEEK cages implanted in a sheep cervical model. The 3D-printed porous titanium cage showed compressive strength similar to that of the bone, with stiffness just slightly higher than that of PEEK [30••]. The 3D-printed porous titanium cage demonstrated a continuous mineralized trabecular structure throughout the implant at 3 months postoperatively, unlike the solid titanium cage [30••]. They found more fibrous tissue on histological analysis at the bone-implant interface at the 3-month mark in the PEEK cage group compared with the porous titanium cage group; by 6 months postoperatively, the bone contact rate of the porous Ti cage also was significantly higher at 7 times that of the control PEEK cage [30••].
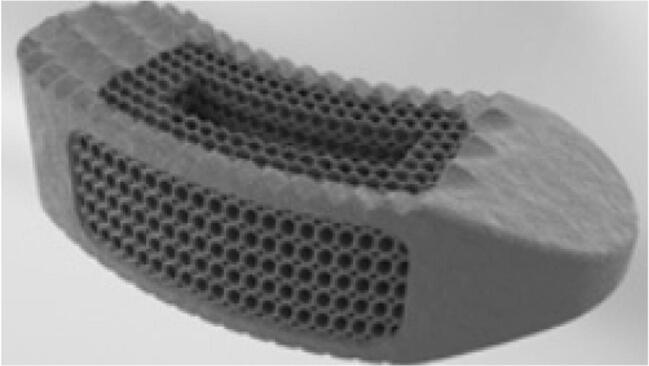
Fig. 3.
Porous titanium cage design to promote bony in-growth
Surface Roughness
Another method of increasing osseointegration that has been implemented in titanium implants is the use of surface roughness. Again, there is both a mechanical and biological advantage to increased surface roughness (Fig. 4). First, a rough surface simply creates more friction, decreasing the likelihood of implant dislodgement [31]. This friction allows for decreased micro-motion and may be the difference between bony growth and undesirable fibrous growth [32]. Increased roughness has also been shown to increase protein binding and fibronectin, while smooth surfaces preferentially bind albumin [33]. An in vitro study by Olivares et al. found that BMP2 and BMP4 was also upregulated with roughened titanium implants versus smooth implants when osteoblast-like cells were cultured on the implant surfaces [34]; various other in vitro studies have also found increased cell attachment with roughened surfaces [35, 36].
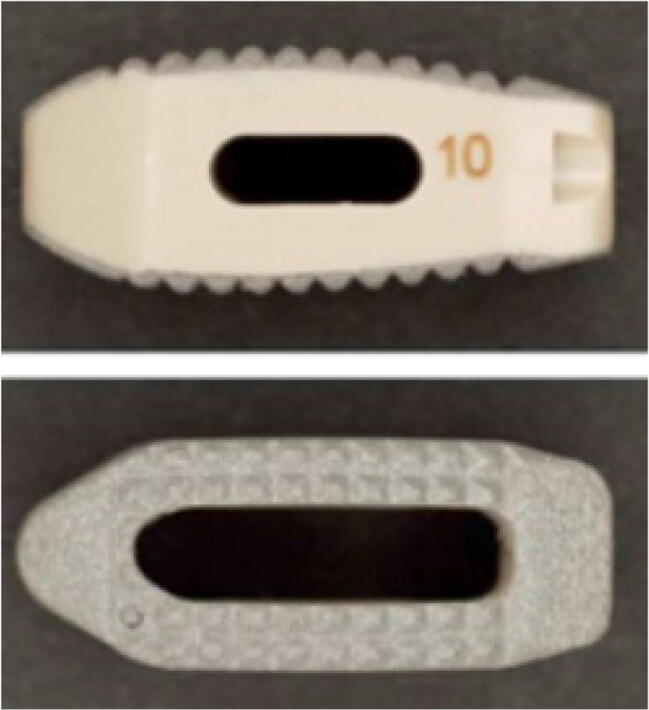
Fig. 4.
Porous titanium cage combined with surface roughening
Hydroxyapatite Coating
Another option to optimize the biology of osseointegration while taking advantage of the mechanical properties of titanium is to use a hydroxyapatite (HA) coating. Hydroxyapatite can be sintered at high temperatures, which can form an apatite layer similar to the bone, allowing for chemical integration when implanted [37]. Sintered HA may be applied to titanium implants using a plasma spray [38]. The efficacy of HA-coated titanium implants was demonstrated in-vivo first in 2005 by Hasegawa et al., using HA-coated Ti pedicle screws in dogs. Titanium screws were implanted in the lumbar spine with either no coating or with an HA-coating and the HA-coated screws were found to have significantly increased pullout resistance postoperatively compared with the non-coated screws [39]. A similar study was performed in 2015 by Jing et al. in beagles assessed titanium screws coated with HA using micro-arc oxidation. They similarly showed the bone-to-implant contact was significantly higher in the HA-coated implant group, as well as significantly higher mechanical strength at the bone-implant interface [40].
PEEK Modifications
PEEK Composites
PEEK cages, similar to titanium, have been used widely in other fields of orthopedic surgery. Unlike titanium, PEEK has excellent mechanical properties but lacks in osseointegrative properties. One key advantage of PEEK is its radiolucency, allowing for accurate assessment of fusion postoperatively. As such, several solutions have been proposed to enhance bony growth with PEEK implants. The two main methods that have been explored are the development of PEEK composite implants with a more biologically active material, as well as various biologically active coatings for PEEK implants. A study in 2012 by Wu et al. created a n-TiO2/PEEK composite and found that there was significantly more bone volume produced compared with cages made of PEEK alone [41]. McGilvray et al. also analyzed PEEK-titanium composite implants in an ovine lumbar model. The PEEK-titanium composite resulted in a significant decrease in range of motion following implantation, as well as increased stiffness. MicroCT also showed significantly increased bone formation both at the fusion site and in-growth into the implant itself16.
PEEK has also been combined with other materials such as HA in order to more closely simulate a bone environment [42]. A recent study by Ma et al. had implanted various PEEK and HA-PEEK composite implants (of varying proportions) in rabbit tibias. They found that the HA-PEEK composites had increased push-out forces and interfacial shear strength relative to those of pure PEEK implants; the optimal amount determined by this study was 5 wt.% of HA (relative to higher concentrations of HA) [43] .
PEEK Coating
The use of various coatings using bioactive material has also been applied to PEEK implants. Titanium can be used to coat PEEK through various methods such as electron beam deposition (Fig. 5). Han et al. used this method to coat PEEK implants and found increased osteoblast proliferation of more than twofold in vitro relative to pure PEEK implants [44]. In vivo experiments in rabbits showed a significantly higher bone-in-contact ratio in the Ti-coated PEEK implants versus pure PEEK implants [44]. Interestingly, they also used PEEK screws that were only half-coated in titanium and showed gaps between the bone and implant surface versus much tighter contact on the coated half of the screw [44]. Hoppe et al. used vacuum plasma spray to apply a titanium coating to PEEK cages; they performed a retrospective review on 42 patients who underwent a transforaminal lumbar interbody fusion (TLIF) using Ti-coated PEEK implants and had no patients with evidence of pseudarthrosis or radiolucency around the cage. At 24-month follow-up, they found 93.6% of patients had a G1 fusion rate (per Bridwell classification), and 90.4% of patients were pain-free with high satisfaction at final follow-up [45]. Lastly, Makino et al. evaluated 24 patients who underwent 1- or 2- level posterior lumbar interbody fusion (PLIF) surgery using Ti-coated PEEK cages. They implanted two cages at every level and using color CT mapping found that 134 of 248 (54%) bone-implant surfaces demonstrated bony on-growth, although this study’s main focus was on the efficacy of color CT mapping as a means to assess fusion [46•].
Fig. 5.
PEEK cage with titanium coating to promote osseointegration
An alternative to using a titanium coating is the use specifically of a TiO2 coating, part of the reason why titanium implants are biologically active [3, 9]. A study by Tsou et al. implanted TiO2-coated PEEK implants in rabbit femurs in vivo and found more lamellar bone formation versus fibrous tissue seen in the PEEK-only implants on histological analysis [9]. Biomechanical testing also showed greater than twofold shear strength at the bone-implant interface of the TiO2-coated implant versus the pure PEEK implant (6.51 MPa versus 2.54 MPa, respectively).
HA-coated PEEK implants have also been explored—in vivo studies by Barkamo et al. in rabbit femurs found improved osseointegration based on bone-to-implant contact in nanocrystalline-HA-coated PEEK implants versus pure PEEK implants [47]. Johansson et al. implanted HA-coated PEEK screws in rabbits in 2016 and found that the HA-coated screws had higher bone-in-contact ratios as well [48]. Johansson’s group subsequently performed a biomechanical analysis of HA-coated PEEK screws inserted into the tibia and femur of rabbit models—here they found that the removal torque was significantly higher in HA-coated PEEK screw implants [49••].
One of the mechanical disadvantages of coating PEEK cages is the possibility of wear debris and shearing [50]. Several studies by Kienle et al. used polyurethane foam blocks to emulate the interbody space. They found that the Ti-coated PEEK implants deposited wear debris with coating abrasion on 26% of the implant’s teeth after simulating implant insertion [50]. A study in 2019 by Kienle et al. additionally looked at PEEK cages coated with calcium phosphate as well and found that Ti-coated cages had the greatest amount of weight loss following impaction [51]. Though they did not observe full delamination in their studies, one must consider potential adverse effects of titanium debris following implantation such as an inflammatory or immune response.
Conclusion
The advent of the spinal interbody implant has allowed for circumferential spinal fusion for many decades. Implant design has come a long way since autograft was used initially, as both biologic and mechanical properties have been enhanced since. Recent advancements have resulted in significant improvements in the two most widely used implant materials today, titanium and PEEK. Porosity and surface roughness have provided both mechanical and biologic advantages to titanium implants; meanwhile the development of composites and surface coatings have greatly enhanced the biologic properties of PEEK implants. These modifications are constantly changing, and new results are coming out often; additionally new materials altogether are being explored with the ultimate goal of optimizing osseointegration and fusion in spinal surgery.
Compliance with Ethical Standards
Conflict of Interest
Paul J. Park, MD declares he has no conflict of interest to declare related to this publication.
Ronald A. Lehman, MD declares he has no conflict of interest to declare related to this publication.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Updates In Spine Surgery—Techniques, Biologics, and Non-Operative Management
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Adogwa O, Parker SL, Shau D, Mendelhall SK, Cheng J, Aaronson O, Devin CJ, McGirt MJ. Long-term outcomes of revision fusion for lumbar pseudarthrosis: clinical article. J Neurosurg Spine. 2011;15:393–398. doi: 10.3171/2011.4.SPINE10822. [DOI] [PubMed] [Google Scholar]
- 2.Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726–733. doi: 10.1097/01.brs.0000119398.22620.92. [DOI] [PubMed] [Google Scholar]
- 3.Rao PJ, Pelletier MH, Walsh WR, Mobbs RJ. Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop Surg. 2014;6:81–89. doi: 10.1111/os.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niu C-C, Liao J-C, Chen W-J, Chen L-H. Outcomes of interbody fusion cages used in 1 and 2-levels anterior cervical discectomy and fusion: titanium cages versus polyetheretherketone (PEEK) cages. J Spinal Disord Tech. 2010;23:310–316. doi: 10.1097/BSD.0b013e3181af3a84. [DOI] [PubMed] [Google Scholar]
- 5.McGilvray KC, Easley J, Seim HB, Regan D, Berven SH, Hsu WK, Mroz TE, Puttlitz CM. Bony ingrowth potential of 3D-printed porous titanium alloy: a direct comparison of interbody cage materials in an in vivo ovine lumbar fusion model. Spine J. 2018;18:1250–1260. doi: 10.1016/j.spinee.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enders JJ, Coughlin D, Mroz TE, Vira S. Surface technologies in spinal fusion. Neurosurg Clin N Am. 2020;31:57–64. doi: 10.1016/j.nec.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Leong JC, Chow SP, Yau AC. Titanium-mesh block replacement of the intervertebral disk. Clin Orthop Relat Res. 1994:52–63. [PubMed]
- 9.Tsou H-K, Chi M-H, Hung Y-W, Chung C-J, He J-L. In vivo Osseointegration performance of titanium dioxide coating modified polyetheretherketone using arc ion plating for spinal implant application. Biomed Res Int. 2015;2015:328943. doi: 10.1155/2015/328943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine. 1993;18:2106–2107. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 11.Ramakrishna S, Mayer J, Wintermantel E, Leong KW. Biomedical applications of polymer-composite materials: a review. Compos Sci Technol. 2001;61:1189–1224. [Google Scholar]
- 12.Steinberg EL, Rath E, Shlaifer A, Chechik O, Maman E, Salai M. Carbon fiber reinforced PEEK optima--a composite material biomechanical properties and wear/debris characteristics of CF-PEEK composites for orthopedic trauma implants. J Mech Behav Biomed Mater. 2013;17:221–228. doi: 10.1016/j.jmbbm.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Walsh WR, Bertollo N, Christou C, Schaffner D, Mobbs RJ. Plasma-sprayed titanium coating to polyetheretherketone improves the bone-implant interface. Spine J. 2015;15:1041–1049. doi: 10.1016/j.spinee.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Torstrick B, Evans N, Stevens H, Gall K, Guldberg R. Do surface porosity and pore size influence mechanical properties and cellular response to PEEK? Clin Orthop Relat Res. 2016;474:2373–2383. doi: 10.1007/s11999-016-4833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noiset O, Schneider YJ, Marchand-Brynaert J. Fibronectin adsorption or/and covalent grafting on chemically modified PEEK film surfaces. J Biomater Sci Polym Ed. 1999;10:657–677. doi: 10.1163/156856299x00865. [DOI] [PubMed] [Google Scholar]
- 16.McGilvray KC, Waldorff EI, Easley J, Seim HB, Zhang N, Linovitz RJ, Ryaby JT, Puttlitz CM. Evaluation of a polyetheretherketone (PEEK) titanium composite interbody spacer in an ovine lumbar interbody fusion model: biomechanical, microcomputed tomographic, and histologic analyses. Spine J. 2017;17:1907–1916. doi: 10.1016/j.spinee.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Hanc M, Fokter SK, Vogrin M, Molicnik A, Recnik G. Porous tantalum in spinal surgery: an overview. Eur J Orthop Surg Traumatol. 2016;26:1–7. doi: 10.1007/s00590-015-1654-x. [DOI] [PubMed] [Google Scholar]
- 18.Sagomonyants KB, Hakim-Zargar M, Jhaveri A, Aronow MS, Gronowicz G. Porous tantalum stimulates the proliferation and osteogenesis of osteoblasts from elderly female patients. J Orthop Res. 2011;29:609–616. doi: 10.1002/jor.21251. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Fairen M, Sala P, Dufoo M, Ballester J, Murcia A, Merzthal L. Anterior cervical fusion with tantalum implant: a prospective randomized controlled study. Spine. 2008;33:465–472. doi: 10.1097/BRS.0b013e3181657f49. [DOI] [PubMed] [Google Scholar]
- 20.Fernández-Fairen M, Alvarado E, Torres A. Eleven-Year Follow-Up of Two Cohorts of Patients Comparing Stand-Alone Porous Tantalum Cage Versus Autologous Bone Graft and Plating in Anterior Cervical Fusions. World Neurosurg. 2019;122:e156–e167. doi: 10.1016/j.wneu.2018.09.160. [DOI] [PubMed] [Google Scholar]
- 21.Sinclair SK, Konz GJ, Dawson JM, Epperson RT, Bloebaum RD. Host bone response to polyetheretherketone versus porous tantalum implants for cervical spinal fusion in a goat model. Spine. 2012;37:E571–E580. doi: 10.1097/BRS.0b013e318240f981. [DOI] [PubMed] [Google Scholar]
- 22.Bal BS, Rahaman MN. Orthopedic applications of silicon nitride ceramics. Acta Biomater. 2012;8:2889–2898. doi: 10.1016/j.actbio.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 23.Smith MW, Romano DR, McEntire BJ, Bal BS. A single center retrospective clinical evaluation of anterior cervical discectomy and fusion comparing allograft spacers to silicon nitride cages. J Spine Surg. 2018;4:349–360. doi: 10.21037/jss.2018.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olivares-Navarrete R, Gittens RA, Schneider JM, Hyzy SL, Haithcock DA, Ullrich PF, Schwartz Z, Boyan BD. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012;12:265–272. doi: 10.1016/j.spinee.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng A, Humayun A, Cohen DJ, Boyan BD, Schwartz Z. Additively manufactured 3D porous Ti-6Al-4V constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication. 2014;6:045007. doi: 10.1088/1758-5082/6/4/045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assad M, Jarzem P, Leroux MA, Coillard C, Chernyshov AV, Charette S, Rivard C-H. Porous titanium-nickel for intervertebral fusion in a sheep model: part 1. Histomorphometric and radiological analysis. J Biomed Mater Res Part B Appl Biomater. 2003;64:107–120. doi: 10.1002/jbm.b.10530. [DOI] [PubMed] [Google Scholar]
- 27.Otsuki B, Takemoto M, Fujibayashi S, Neo M, Kokubo T, Nakamura T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials. 2006;27:5892–5900. doi: 10.1016/j.biomaterials.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Fujibayashi S, Takemoto M, Neo M, Matsushita T, Kokubo T, Doi K, Ito T, Shimizu A, Nakamura T. A novel synthetic material for spinal fusion: a prospective clinical trial of porous bioactive titanium metal for lumbar interbody fusion. Eur Spine J. 2011;20:1486–1495. doi: 10.1007/s00586-011-1728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu S-H, Li Y, Zhang Y-Q, Li X-K, Yuan C-F, Hao Y-L, Zhang Z-Y, Guo Z. Porous titanium-6 aluminum-4 vanadium cage has better osseointegration and less micromotion than a poly-ether-ether-ketone cage in sheep vertebral fusion. Artif Organs. 2013;37:E191–E201. doi: 10.1111/aor.12153. [DOI] [PubMed] [Google Scholar]
- 30.Li P, Jiang W, Yan J, et al. A novel 3D printed cage with microporous structure and in vivo fusion function. J Biomed Mater Res A. 2019;107:1386–1392. doi: 10.1002/jbm.a.36652. [DOI] [PubMed] [Google Scholar]
- 31.Shirazi-Adl A, Dammak M, Paiement G. Experimental determination of friction characteristics at the trabecular bone/porous-coated metal interface in cementless implants. J Biomed Mater Res. 1993;27:167–175. doi: 10.1002/jbm.820270205. [DOI] [PubMed] [Google Scholar]
- 32.Jasty M, Bragdon C, Burke D, O’Connor D, Lowenstein J, Harris WH. In vivo skeletal responses to porous-surfaced implants subjected to small induced motions. J Bone Joint Surg Am. 1997;79:707–714. doi: 10.2106/00004623-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Deligianni DD, Katsala N, Ladas S, Sotiropoulou D, Amedee J, Missirlis YF. Effect of surface roughness of the titanium alloy Ti–6Al–4V on human bone marrow cell response and on protein adsorption. Biomaterials. 2001;22:1241–1251. doi: 10.1016/s0142-9612(00)00274-x. [DOI] [PubMed] [Google Scholar]
- 34.Olivares-Navarrete R, Hyzy SL, Pan Q, Dunn G, Williams JK, Schwartz Z, Boyan BD. Osteoblast maturation on microtextured titanium involves paracrine regulation of bone morphogenetic protein signaling. J Biomed Mater Res A. 2015;103:1721–1731. doi: 10.1002/jbm.a.35308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosa AL, Beloti MM. Effect of cpTi surface roughness on human bone marrow cell attachment, proliferation, and differentiation. Braz Dent J. 2003;14:16–21. doi: 10.1590/s0103-64402003000100003. [DOI] [PubMed] [Google Scholar]
- 36.Gittens RA, Olivares-Navarrete R, McLachlan T, Cai Y, Hyzy SL, Schneider JM, Schwartz Z, Sandhage KH, Boyan BD. Differential responses of osteoblast lineage cells to nanotopographically-modified, microroughened titanium-aluminum-vanadium alloy surfaces. Biomaterials. 2012;33:8986–8994. doi: 10.1016/j.biomaterials.2012.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H-M, Himeno T, Kokubo T, Nakamura T. Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterials. 2005;26:4366–4373. doi: 10.1016/j.biomaterials.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 38.de Groot K, Geesink R, Klein CP, Serekian P. Plasma sprayed coatings of hydroxylapatite. J Biomed Mater Res. 1987;21:1375–1381. doi: 10.1002/jbm.820211203. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa T, Inufusa A, Imai Y, Mikawa Y, Lim T-H, An HS. Hydroxyapatite-coating of pedicle screws improves resistance against pull-out force in the osteoporotic canine lumbar spine model: a pilot study. Spine J. 2005;5:239–243. doi: 10.1016/j.spinee.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Jing W, Zhang M, Jin L, Zhao J, Gao Q, Ren M, Fan Q. Assessment of osteoinduction using a porous hydroxyapatite coating prepared by micro-arc oxidation on a new titanium alloy. Int J Surg. 2015;24:51–56. doi: 10.1016/j.ijsu.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 41.Wu X, Liu X, Wei J, Ma J, Deng F, Wei S. Nano-TiO2/PEEK bioactive composite as a bone substitute material: in vitro and in vivo studies. Int J Nanomedicine. 2012;7:1215–1225. doi: 10.2147/IJN.S28101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonfield W. Hydroxyapatite-reinforced polyethylene as an analogous material for bone replacement. Ann N Y Acad Sci. 1988;523:173–177. doi: 10.1111/j.1749-6632.1988.tb38510.x. [DOI] [PubMed] [Google Scholar]
- 43.Ma R, Li Q, Wang L, Zhang X, Fang L, Luo Z, Xue B, Ma L. Mechanical properties and in vivo study of modified-hydroxyapatite/polyetheretherketone biocomposites. Mater Sci Eng C Mater Biol Appl. 2017;73:429–439. doi: 10.1016/j.msec.2016.12.076. [DOI] [PubMed] [Google Scholar]
- 44.Han C-M, Lee E-J, Kim H-E, Koh Y-H, Kim KN, Ha Y, Kuh S-U. The electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological properties. Biomaterials. 2010;31:3465–3470. doi: 10.1016/j.biomaterials.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 45.Hoppe S, Albers CE, Elfiky T, Deml MC, Milavec H, Bigdon SF, et al. First results of a new vacuum plasma sprayed (VPS) titanium-coated carbon/PEEK composite cage for lumbar Interbody fusion. J Funct Biomater. 2018;9. 10.3390/jfb9010023. [DOI] [PMC free article] [PubMed]
- 46.Makino T, Kaito T, Sakai Y, Takenaka S, Yoshikawa H. Computed tomography color mapping for evaluation of bone ongrowth on the surface of a titanium-coated polyetheretherketone cage in vivo: a pilot study. Medicine (Baltimore) 2018;97:e12379. doi: 10.1097/MD.0000000000012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barkarmo S, Wennerberg A, Hoffman M, Kjellin P, Breding K, Handa P, Stenport V. Nano-hydroxyapatite-coated PEEK implants: a pilot study in rabbit bone. J Biomed Mater Res A. 2013;101:465–471. doi: 10.1002/jbm.a.34358. [DOI] [PubMed] [Google Scholar]
- 48.Johansson P, Jimbo R, Naito Y, Kjellin P, Currie F, Wennerberg A. Polyether ether ketone implants achieve increased bone fusion when coated with nano-sized hydroxyapatite: a histomorphometric study in rabbit bone. Int J Nanomedicine. 2016;11:1435–1442. doi: 10.2147/IJN.S100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansson P, Barkarmo S, Hawthan M, Peruzzi N, Kjellin P, Wennerberg A. Biomechanical, histological, and computed X-ray tomographic analyses of hydroxyapatite coated PEEK implants in an extended healing model in rabbit. J Biomed Mater Res A. 2018;106:1440–1447. doi: 10.1002/jbm.a.36345. [DOI] [PubMed] [Google Scholar]
- 50.Kienle A, Graf N, Wilke H-J. Does impaction of titanium-coated interbody fusion cages into the disc space cause wear debris or delamination? Spine J. 2016;16:235–242. doi: 10.1016/j.spinee.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 51.Kienle A, Krieger A, Willems K, Wilke H-J. Resistance of coated polyetheretherketone lumbar interbody fusion cages against abrasion under simulated impaction into the disc space. J Appl Biomater Funct Mater. 2019;17:2280800018782854. doi: 10.1177/2280800018782854. [DOI] [PubMed] [Google Scholar]