Abstract
Purpose of Review
The decreased contact area, edge loading, and increased stress in the adjacent area cartilage resulting from chondral defects are believed to predispose this tissue to degenerative changes that have significant economic implications, especially when considering its progression to osteoarthritis of the knee. Growth factors are considered therapeutic possibilities to enhance healing of chondral injuries and modify the progression to degenerative arthritis. Thus, the purposes of this review are to first to summarize important points for defect preparation and recent advances in techniques for marrow stimulation and second, and to identify specific growth factors and cytokines that have the capacity to advance cartilage regeneration and the treatment of osteoarthritis in light of recent laboratory and clinical studies.
Recent Findings
TGF-β, BMP-2, BMP-7, IGF-1, as IL-1 receptor antagonist, and recombinant human FGF-18 are some of the promising growth factor/cytokine treatments with pioneering and evolving clinical developments. The bulk of the review describes and discusses these developments in light of fundamental basic science. It is crucial to also understand the other underlying advances made in the surgical management of cartilage defects prior to onset of OA. These advances are in techniques for defect preparation and marrow stimulation, a common cartilage repair procedure used in combination with growth factor/cytokine augmentation.
Summary
Multiple growth factor/cytokine modulation therapies are currently undergoing clinical trial investigation including Invossa (currently in phase III study), Kineret (currently in phase I study), and Sprifermin (currently in phase II study) for the treatment of symptomatic osteoarthritis.
Keywords: Growth factor, Cytokine, Cartilage repair, Osteoarthritis
Introduction
The treatment of articular cartilage defects of the knee remains an enduring struggle facing an orthopedic surgeon. These lesions are widespread with studies of consecutive knee arthroscopies demonstrating an incidence of chondral defects ranging from 60 to 66% [1–3]. Articular cartilage, devoid of vascularity, relies on diffusion to obtain nutrients and oxygen, thus making intrinsic repair of defects remarkably difficult in vivo [4]. Untreated cartilage injuries (> 12 months duration of symptoms) may create an unfavorable chemical environment for later cartilage repair [5]. Early surgical intervention for articular cartilage injury is particularly important in the athlete’s knee for the successful return to sports participation [6]. Defects in the weight-bearing portion of the femoral condyle results in increased rim stress concentration and chondral wear at the lesion rim [7]. The decreased contact area, edge loading, and increased stress in the adjacent area cartilage resulting from full-thickness chondral defects are believed to predispose this tissue to degenerative changes. [8] Lesions in articular cartilage can cause considerable musculoskeletal morbidity with significant economic implications, especially when considering its progression to osteoarthritis (OA) of the knee [9].
Growth factors are being considered as therapeutic possibilities to enhance healing of chondral injuries and modify the progression to degenerative arthritis. Multiple studies have confirmed that growth factors and chemotactic cytokines promote the migration of pluripotent mesenchymal stromal cells (MSCs) (nonhematopoietic adult cell population with a capability to differentiate into different lineages) from the marrow into the defect [10–12]. The goal of growth factor application is to stimulate differentiation of MSCs to create a phenotype that is closer in appearance to normal articular cartilage with similar biomechanical properties [8]. Some suggest that cartilage repair is exclusively mediated by the proliferation and differentiation of mesenchymal cells [13, 14]. Most commonly, marrow substrates including MSCs, growth factors, and cytokines are introduced through marrow stimulation (MS) [15], although newer techniques have utilized bone marrow aspirate concentrate (BMAC) and adipose tissue (ADSC). Given the vast collection of growth factors that are required for proper cartilage expansion and homeostasis, it is doubtful that any single growth factor will lead to complete cartilage repair, but rather a multifaceted course will be required.
Exciting developments have been made in the field of growth factors, and it is crucial to also understand the other underlying advances made in the surgical management of cartilage defects prior to onset of OA. These advances are in techniques for defect preparation and marrow stimulation, a common cartilage repair procedure used in combination with growth factor/cytokine augmentation. Thus, the purposes of this review are to first to summarize important points for defect preparation and recent advances in marrow stimulation and second, to identify specific growth factors and cytokines that have the capacity to advance cartilage regeneration and OA treatment in light of recent laboratory and clinical studies.
Defect Preparation
There is importance in the individual surgical steps of defect preparation with critical technical details that help optimize clinical results [16, 17]. Many factors have been suspected for the limited potential of vertical and horizontal integration of the peripheral healthy cartilage into the repair tissue. Chondrocyte immobility in the extracellular matrix has been speculated to be a significant limiting factor in the repair capacity [18], but chondrocytes are motile and can move through the extracellular matrix once adequate signals are present [19]. Incision through the cartilage tissue has been shown to induce chondrocyte motility producing neocartilage around the edges [20•]. Bos et al. [20•] reported that the deep zone of articular cartilage contains chondrocytes capable of proliferation while retaining their cartilage phenotype. These cells are able to form new cartilage tissue [20•] and possibly improve the peripheral integration of the repair tissue. These studies highlight the importance of the creation of healthy, vertical walls at the defect edge [21] to maximize the tissue response [22]. A second critical step of defect preparation is debridement of the calcified cartilage which may allow the ingrowth of bone marrow-derived cells [23]. In vitro studies have shown that successful removal of the calcified cartilage layer results in improved neocartilage integration [24•]. Yet, significant variability has been shown in a surgeon’s ability to reliably remove the calcified cartilage layer [25••]. However, care must be taken as excessive debridement of the calcified cartilage may stimulate subchondral bone overgrowth, which can be associated with clinical failure after MS [26•].
Marrow Stimulation Innovations: Technique and Instrumentation
Marrow stimulation (common cartilage repair procedure in combination with growth factor/cytokine augmentation) improvements have been developed to increase the concentration of MSCs as well as increase the amount of growth factors and chemotactic cytokines [27]. Basic science principles elucidated in recent years, namely (1) depth of subchondral penetration, (2) diameter of awl, and (3) number of subchondral perforations, are thought to help solve the subchondral bone structure/overgrowth and MSC access issues. First, depth of subchondral perforation influences the outcomes of cartilage repair and repairing/remodeling of subchondral bone (6 mm > 2 mm). [28, 29•] Improvement in overall tissue repair quantity (defect fill) and quality (histologic parameters) was found to be significantly better with deep (6 mm) versus shallow (2 mm) microdrilling [29•]. Second, diameter of awl used in MS has been correlated with repair tissue quality. Eldracher et al. [30•] demonstrated the application of 1.0-mm diameter subchondral drill holes (compared with 1.8-mm drill holes) led to significantly improved histological matrix staining, cellular morphological characteristics, subchondral bone reconstitution, and average total histological score. Third, increased number of subchondral perforations may improve MSC access. A thin and sharp awl has been shown to produce a statistically higher percentage for marrow access than a beveled tip awl [31]. Min et al. [32] demonstrated the number of MSCs increased with an increase in the number of perforations in the defect (3 or 5 versus 1 perforation; p < 0.05) and concluded as the size of the total exposed area increased, so did the number of MSCs obtained [32].
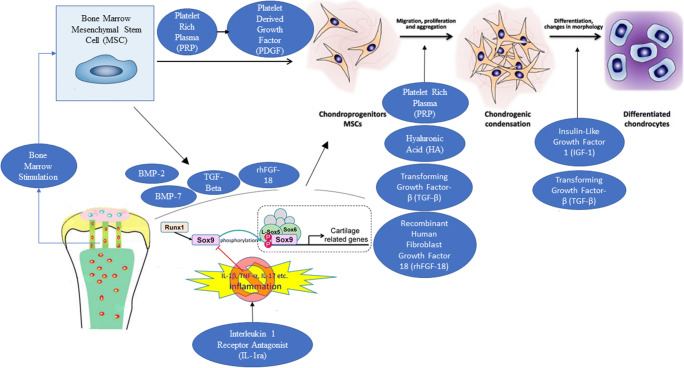
Growth Factor and Cytokine Augmentation (Pictorial Representation in Fig. 1)
Fig. 1.
Pictorial representation of biologic augmentation options for cartilage repair. After marrow stimulation, various biologic options influence (1) chondrogenic differentiation of mesenchymal stem cells (MSCs), (2) Sox-9 expression in MSCs, (3) cartilaginous extracellular matrix (ECM) production in MSCs, (4) chondrocyte proliferation, and (5) synthesis and retention of ECM within articular cartilage. Terms: bone morphogenetic protein 7 (BMP-7) and bone morphogenetic protein 2 (BMP-2)
TGF-β
The transforming growth factor-β (TGF-β) superfamily comprises over 30 structurally related members including TGF-β1, 2, and 3 as well as bone morphogenetic proteins (BMPs) 2, 4, and 7 [33]. TGF-β1, 2, and 3 have been shown to induce Sox-9 expression and increase cartilaginous extracellular matrix (ECM) production in MSCs [34–36]. Additionally, when TGF-β signaling is blocked (inhibition of the TGF-β receptor 1 kinase), autonomous formation of cartilage-like tissue from expanded chondrocytes was shown to be diminished [37]. In an in vitro study, a scaffold crosslinked with TGF-β3 was shown to facilitate MSC proliferation and abundant ECM production on the scaffold, while demonstrating chondrogenic differentiation of MSCs in an animal model [38]. In a rabbit chondral defect model, polylactide-co-glycolide/fibrin gel scaffolds loaded with MSCs plus TGF-β1 resulted in higher gene expression profiles of type II collagen, aggrecan, and Sox-9 versus scaffolds with MSCs alone. [39]
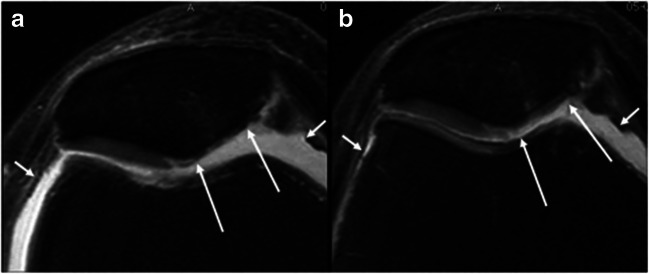
Recently, a novel concept utilizing genetically engineered chondrocytes virally transduced with TGF-β1 (Invossa (TissueGene-C); TissueGene Inc., Rockville, MD, USA) has been introduced. Pre-clinical studies suggest the promotion of type II collagen deposition and hyaline cartilage formation [40••]. Furthermore, Ahn et al. [41] demonstrated TGF-β1-transduced chondrocytes can be employed arthroscopically to treat cartilage defects using atelocollagen, fibrinogen, and thrombin in a 3D knee model. Phase I trials established an injectable treatment for knee OA as safe with no severe adverse events [42]. Results of a multicenter, double-blinded, placebo-controlled, and randomized study of adults with Kellgren-Lawrence grade III knee OA demonstrated significant improvements in IKDC and VAS at 104 weeks with less progression of cartilage damage on MRI at 12 months when compared with the placebo cohort (Fig. 2) [43••, 44]. Currently, a phase III study is under way in the USA (ClinicalTrials.gov Identifier: NCT03203330).
Fig. 2.
Structural effects of intra-articular Invossa (TissueGene-C (TissueGene Inc., Rockville, MD, USA)) in moderate to advanced knee osteoarthritis. Axial intermediate-weighted fat-suppressed MRI in treated patient at baseline (a) and 12 months (b) follow-up show improvement of cartilage focal defect and thickness of the medial patella (long arrows). Also note the decrease in volume of the joint effusion (small arrows). Guermazi, A., Kalsi, G., Niu, J. et al. Structural effects of intra-articular TGF-β1 in moderate to advanced knee osteoarthritis: MRI-based assessment in a randomized controlled trial. BMC Musculoskelet Disord 18, 461 (2017). 10.1186/s12891-017-1830-8. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited
BMP-2
Chondrogenic effects similar to TGF-β have been observed for several bone morphogenetic proteins molecules (BMPs). With an ability to induce both cartilage and bone formation, BMPs, particularly bone morphogenetic protein 2 (BMP-2), are attractive growth factors for the regeneration of the osteochondral tissue unit [45]. BMP-2 is expressed throughout the entire chondrogenic process, from proliferation to calcification [34]. In vitro, MSCs encapsulated in gellan hydrogels plus BMP-2 leads to increased ECM production and increased expression of Sox-9 versus MSCs alone [46]. In a rabbit model, defects treated with BMP-2 had higher average histological grading scores compared to untreated defects at 24 weeks [47]. Additionally, chondral defects treated with BMP-2 demonstrated features of subchondral bone regeneration versus untreated defects [48]. Furthermore, one study’s data suggests the capability of BMP-2 to reverse chondrocyte dedifferentiation as indicated by an increase in synthesis of cartilage-specific collagen type IIB in dedifferentiated/OA chondrocytes [49]. Yang et al. [50•] demonstrated chondral defects in a rabbit model treated with microfracture (MFx) plus BMP-2 delivered with heparin-conjugated fibrinogen had a higher percentage of defect surface area covered with repaired tissue versus MFx alone. Additionally at 8 weeks, the MFx plus long term BMP-2 group had higher histological scores and higher relative values of glycosaminoglycan and type II collagen versus MFx alone [50•].
BMP-7
Bone morphogenetic protein 7 (BMP-7), also known as osteogenic protein-1, is a growth factor found in normal articular cartilage [51] that has been shown to stimulate chondrocyte proliferation, differentiation, and enhanced matrix assembly. Multiple in vitro studies demonstrated BMP-7 treatment stimulates cartilage differentiation while promoting the synthesis and retention of ECM within articular cartilage [52, 53]. In a sheep model where BMP-7 was delivered via a mini–osmotic pump, Jelic et al. [54] demonstrated good defect fill with the regenerated cartilage displaying high levels of proteoglycans and type II collagen at 6 months in the BMP-7 groups. In a rabbit model evaluating MFx plus BMP-7, Kuo et al. [55•] found that BMP-7 alone increased the amount of repair tissue without affecting the quality of repair tissue compared to untreated control. But when BMP-7 (adsorbed onto a type I collagen sponge) was combined with MFx, both the quality (based on ICRS scores for superior matrix and superior cell distribution) and quantity of repair tissue were increased versus MFx alone [55•]. This study suggests a synergistic reaction with BMP-7 and MFx, likely related to the ability of the BMP-7 to act directly on the pluripotent MSCs introduced into the chondral defect by penetration of the subchondral bone plate [8].
IGF-1
Insulin-like growth factor 1 (IGF-1) is considered to be a major anabolic factor in articular cartilage [56]. The anabolic effects of IGF-1 are demonstrated by its ability to increase both proteoglycan and collagen synthesis in vitro [56–58] and inhibit the rate of matrix degradation by regulating the transcription of degradative enzymes such as the matrix metalloproteinases [59]. In an in vitro study, Davies et al. [60] observed significant increases in collagen amounts when compared to untreated controls in response to treatment with IGF-1 of bovine articular chondrocytes. Madry et al. [61] used chondrocytes transfected with plasmid vectors containing human IGF-1 in a hydrogel to treat osteochondral defects in a rabbit model which at 14 weeks demonstrated significantly better histological grading (filling of defect, integration, and cell morphology) versus the control. In a horse model, Fortier et al. [62] showed that the addition of IGF-1 to chondrocyte-fibrin composites enhanced chondrogenesis in cartilage defects with improved gross defect filling and a significant increase in mean type-II collagen (%) in repair tissue versus control chondrocyte-fibrin composites alone at 8 months. IGF-1 has also been shown to work well in combination with other growth factor/cytokine modulations. Chondrogenic differentiation of MSCs is enhanced when IGF-I and TGF-β are used in combination [60, 63] through a mechanism of increased production of cartilage matrix components such as proteoglycan as well as upregulation of the expression of type II collagen and aggrecan genes in chondrocytes [56, 64]. In an in vitro study, An et al. [65•] suggests that IGF-1 and BMP-2 directed ADSCs toward chondrogenesis as demonstrated by chondrocyte-like cells with type II collagen and a reduced production of MMP-3.
IL-1ra
Multiple animal models have shown inflammatory cytokine inhibitors such as IL-1 receptor antagonist (IL-1ra) may decrease proteoglycan breakdown in articular cartilage [66, 67]. An in vitro study showed IL-1-exposed cartilage was depleted of proteoglycan, and this catabolic state was fully reversed by adenovirus IGF-I and IL-1Ra-transduced synovial cocultures [68]. Morisset et al. [69] combined MFx with IL-1ra and IGF-1 and post MFx, joints were injected with an equine IL-1ra/IGF-1 adenoviral preparation or control solution. In gene therapy-treated joints, staining for aggrecan and type II collagen was more intense in all layers of repair tissue compared to MFx alone and control joints [69].
Kineret [Anakinra (Swedish Orphan Biovitrum AB, Sweden)] is an IL-1ra developed as a chondroprotective and anti-inflammatory agent (Fig. 3). In a randomized, controlled pilot trial, Kraus et al. [70••] described patients with acute ACL tear who had received a single intra-articular injection of anakinra had substantially greater improvement in KOOS scores over 14 days providing proof of concept for the hypothesis that higher IL-1 in synovial fluid after injury will predict greater symptomatic response to IL-1ra (decreasing cartilage breakdown). There is an active phase I study evaluating the safety of intra-articular Anakinra in subjects with moderate OA of the knee (ClinicalTrials.gov Identifier: NCT02790723).
Fig. 3.
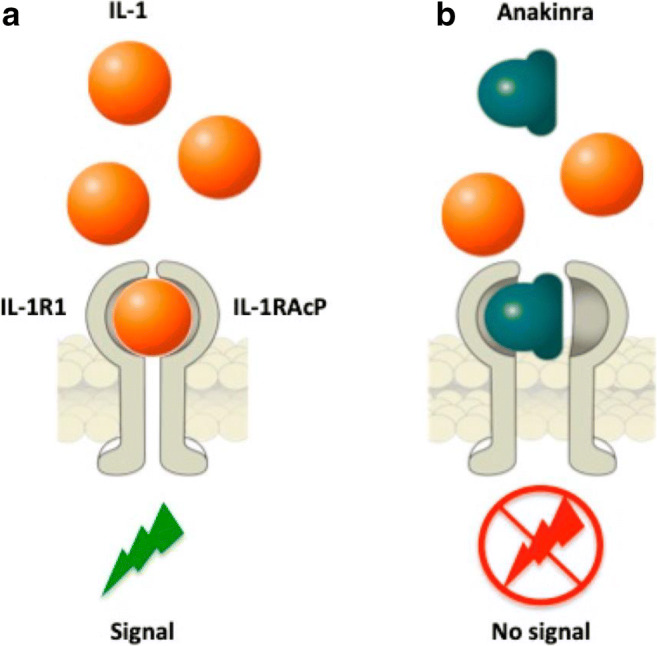
Strategies for IL-1 blockade with Kineret (Anakinra (Swedish Orphan Biovitrum AB, Sweden)). a Interleukin (IL)-1 binds to type 1 IL-1 receptor (IL-1R1) and to the adaptor protein, IL-1RAcP, in order to trigger signal transduction. b The recombinant human IL-1R1 antagonist, Anakinra, directly competes with IL-1 for binding to the IL-1R1, blocking the biological activity of IL-1. Bettiol A, Lopalco G, Emmi G, et al. Unveiling the Efficacy, Safety, and Tolerability of Anti-Interleukin-1 Treatment in Monogenic and Multifactorial Autoinflammatory Diseases. Int J Mol Sci. 2019;20(8):1898. Published 2019 Apr 17. doi:10.3390/ijms20081898. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited
rhFGF-18
Recombinant human fibroblast growth factor 18 (rhFGF-18) is a heparin-binding growth factor that belongs to the FGF family, which play a central role in regeneration of a variety of tissues. rhFGF-18 mainly activates the FGF receptor 3 (FGFR3) on the surface of the chondrocytes [71], and in vitro, rhFGF-18 stimulates both chondrocyte proliferation and Sox-9 expression while strongly decreasing type I collagen expression (Fig. 4) [72]. Furthermore, in an in vitro repair model, mechanical testing and biochemical analysis showed greater adhesive strength and larger contact areas between core and annular cartilage in the rhFGF-18-treated group suggesting enhanced defect healing and lateral cartilage-cartilage integration [73]. In an ovine model, defects treated with MFx plus intra-articular rhFGF-18 (administered as 3 once weekly injections) showed a statistically significant improvement in ICRS tissue repair score and tissue infill score and in the modified O’Driscoll score at 6 months suggesting an increase in hyaline cartilage-like tissue formed in the MFx plus rhFGF-18 treated groups versus MFx alone [74].
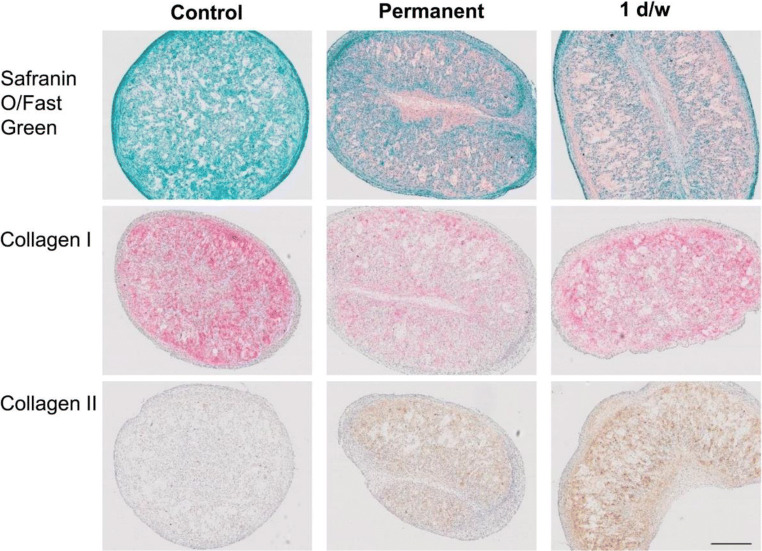
Fig. 4.
Sprifermin ((rhFGF18); (EMD Serono Inc., Rockland MA, subsidiary of Merck KGaA, Germany)] enables proliferation of chondrocytes producing a hyaline cartilage matrix. The histology results correspond well to the biochemical and gene expression results. In the 3D constructs cultured in the absence of sprifermin, no Safranin O or type II collagen staining was visible, but the constructs were positive for type I collagen, illustrating that these chondrocytes were not able to produce a cartilage-like extracellular matrix. In the presence of both permanent and 1 day/week sprifermin, a Safranin O and type II collagen-positive matrix was observed, while type I collagen staining was less intense in comparison to the control. Gigout A, Guehring H, Froemel D, et al. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthritis Cartilage. 2017;25(11):1858–1867. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited
Sprifermin (EMD Serono Inc., Rockland MA, subsidiary of Merck KGaA, Germany) is a rhFGF-18 currently in phase II testing. In a RCT with 168 patients with symptomatic OA, Lohmander et al. [75] demonstrated a dose-related reduction of cartilage loss with intra-articular sprifermin across the total tibiofemoral joint and in the lateral tibiofemoral compartment at 12 months. Furthermore, in a recent multicenter, randomized, placebo-controlled trial published in JAMA, the intra-articular administration of 100 μg of sprifermin resulted in a significant improvement in total femorotibial joint cartilage thickness after 2 years vs placebo. [76••] However, there was no significant differences in change from baseline in total WOMAC scores for any sprifermin group vs placebo. More information about the effectiveness and durability of sprifermin is forthcoming as data from the remaining 3 years of this study is evaluated (ClinicalTrials.gov Identifier: NCT01919164).
PRP and BMAC
Platelet-rich plasma (PRP) and bone marrow aspirate concentrate (BMAC) preparations are methods of combined growth factor delivery that have been extensively studied and are not the focus of this review, thus will only be covered briefly. PRP is defined to contain more than 1 million platelets per mL of serum [77••] or 5 times the amount of baseline platelets [78]. Platelets contain alpha granules that are rich in several growth factors, such as platelet-derived growth factor (PDGF), TGF-β, insulin-like growth factor, and vascular endothelial growth factor, which play key roles in tissue repair mechanisms [78]. PDGF can stimulate chondrogenic differentiation of bone marrow stem cells as well as enhance chondrocyte proliferation and metabolism [79]. In vitro studies show PRP stimulates in vitro chondrocyte proliferation and chondrogenic differentiation of MSCs [80, 81]. In a 2-year randomized control trial of 49 patients, Lee et al. [82] demonstrated significant decreased visual analog scores in the PRP enhanced MFx group versus MFx alone at final follow-up. Additionally, 55% of patients in the study group had normal IKDC activity scores at final follow-up, which was significantly more than the MFx alone group. PRP was injected around microfractured holes under arthroscopic view at the time of surgery after all joint fluid was removed [82]. It is important to note that there is wide variation of blood components, including platelets, red blood cells, leukocytes, pH, and glucose in different methods of PRP extractions [83••]. The high concentrations of cells may be important, as the white blood cell has frequently been considered insignificant [83••]. Further, there is variation between different patients as well as within the same patient as different blood draws has been shown to contain different concentrations of platelets [84]. The lack of standardization of PRP preparation for clinical use has contributed at least in part to the varying clinical efficacy in PRP use [83••].
BMAC is a source of MSCs [85–87]. Bone marrow aspirate is also a rich source of growth factors (including PDGF and TGF-β) [88] secreted by MSCs, which are important for inducing effective chondrogenesis [86, 89]. In an equine model, Fortier et al. [90] showed BMAC plus thrombin placed into defects post MFx demonstrated statistically significant higher macroscopic and mean ICRS histological scores at 8 months versus MFx alone. Additionally, there was statistically significant quantitative increase of defect fill on MRI. Hannon et al. [91] described MFx enhanced with BMAC for the treatment of chondral defects in 34 patients and reported significantly higher MOCART scores in the BMAC-enhanced MFx group with a fewer percentage of patients with fissuring and fibrillation of the articular cartilage surface compared to MFx alone. However, there was no difference in Foot and Ankle Outcome Score pain subscale and the short form 12 (SF-12) general health questionnaire physical component summary score at a mean follow-up of 48.3 months between MFx and MFx plus BMAC groups [91].
BMAC has increased in popularity as an option that uses MSCs while avoiding the potential subchondral bone effects associated with 1st generation MS (i.e., lack of subchondral bone repair and osseous overgrowth). However, bone marrow harvesting is an invasive procedure with donor site morbidities and risk for wound infections [92]. Combination therapies using BMAC have been introduced, namely activated BMAC plus HA scaffold (Hyalofast). Activated BMAC with Hyalofast was used by Gobbi et al. [93•] to treat 50 patients (mean age 45) with ICRS grade IV chondral lesions. A level 2 cohort study at the endpoint of 5 years showed BMAC plus HA scaffold treated patients maintained a statistically significant improved Tegner, IKDC objective, KOOS–Pain, and KOOS–sports scores compared with MFx [93•].
Conclusion
In summary, the study of growth factors and cytokines promoting the differentiation of MSCs to create a phenotype closer to articular cartilage and as a model for an anti-arthritic environment has enhanced our grasp of cartilage biology. Lesions in articular cartilage can cause considerable musculoskeletal morbidity including pain and loss of function. With that comes significant economic implications, especially when considering its progression to OA. The ambition of a growth factor/cytokine therapy aims to bring about a repair without the need for further long-term surgery. Furthermore, execution and improvement of OA treatment protocols with emphasis on the utilization of growth factors and cytokines could lead to the production of more robust and functional cartilage. Bearing this in mind, we have highlighted the ongoing research encompassing novel treatments that are being developed and employed for cartilage repair and the treatment of OA utilizing growth factors and cytokines.
Compliance with Ethical Standards
Conflict of Interest
Sarav S. Shah declares that he has no conflict of interest.
Kai Mithoefer declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Investigation performed at New England Baptist Hospital, Boston, MA 02120, USA
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sarav S. Shah, Email: saravshah1@gmail.com
Kai Mithoefer, Email: kmithoefer1@gmail.com.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456–460. doi: 10.1016/S0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 2.Arøen A, Løken S, Heir S, Alvik E, Ekeland A, Granlund OG, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211–215. doi: 10.1177/0363546503259345. [DOI] [PubMed] [Google Scholar]
- 3.Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18(7):730–734. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- 4.Richter W. Mesenchymal stem cells and cartilage in situ regeneration. J Intern Med. 2009;266(4):390–405. doi: 10.1111/j.1365-2796.2009.02153.x. [DOI] [PubMed] [Google Scholar]
- 5.Blevins FT, Steadman JR, Rodrigo JJ, Silliman J. Treatment of articular cartilage defects in athletes: an analysis of functional outcome and lesion appearance. Orthopedics. 1998;21(7):761–767. doi: 10.3928/0147-7447-19980701-05. [DOI] [PubMed] [Google Scholar]
- 6.Mithoefer K, Gill TJ, Cole BJ, Williams RJ, Mandelbaum BR. Clinical outcome and return to competition after microfracture in the athlete’s knee: an evidence-based systematic review. Cartilage. 2010;1(2):113–120. doi: 10.1177/1947603510366576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Convery FR, Akeson WH, Keown GH. The repair of large osteochondral defects. An experimental study in horses. Clin Orthop Relat Res. 1972;82:253–262. doi: 10.1097/00003086-197201000-00033. [DOI] [PubMed] [Google Scholar]
- 8.Strauss EJ, Barker JU, Kercher JS, Cole BJ, Mithoefer K. Augmentation strategies following the microfracture technique for repair of focal chondral defects. Cartilage. 2010;1(2):145–152. doi: 10.1177/1947603510366718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson DW, Simon TM, Aberman HM. Symptomatic articular cartilage degeneration: the impact in the new millennium. Clin Orthop Relat Res. 2001;(391 Suppl):S14–25. [PubMed]
- 10.Endres M, Neumann K, Häupl T, Erggelet C, Ringe J, Sittinger M, Kaps C. Synovial fluid recruits human mesenchymal progenitors from subchondral spongious bone marrow. J Orthop Res. 2007;25(10):1299–1307. doi: 10.1002/jor.20394. [DOI] [PubMed] [Google Scholar]
- 11.Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 12.Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, Kaps C, Sittinger M. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101(1):135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 13.Solheim E, Hegna J, Strand T, Harlem T, Inderhaug E. Randomized study of long-term (15-17 years) outcome after microfracture versus mosaicplasty in knee articular cartilage defects. Am J Sports Med. 2017;363546517745281:826–831. doi: 10.1177/0363546517745281. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75(4):532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 16.Mithoefer K, Williams RJ, Warren RF, Potter HG, Spock CR, Jones EC, et al. Chondral resurfacing of articular cartilage defects in the knee with the microfracture technique. Surgical technique. J Bone Joint Surg Am. 2006;88(Suppl 1 Pt 2):294–304. doi: 10.2106/JBJS.F.00292. [DOI] [PubMed] [Google Scholar]
- 17.Steadman JR, Miller BS, Karas SG, Schlegel TF, Briggs KK, Hawkins RJ. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg. 2003;16(2):83–86. [PubMed] [Google Scholar]
- 18.Bardos T, Vancsodi J, Farkas B, Fazekas A, Nagy SA, Bogner P, Vermes C, Than P. Pilot study of cartilage repair in the knee joint with multiply incised chondral allograft. Cartilage. 2015;6(2):73–81. doi: 10.1177/1947603514563596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theodoropoulos JS, De Croos JN, Park SS, Pilliar R, Kandel RA. Integration of tissue-engineered cartilage with host cartilage: an in vitro model. Clin Orthop Relat Res. 2011;469(10):2785–2795. doi: 10.1007/s11999-011-1856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bos PK, Kops N, Verhaar JA, van Osch GJ. Cellular origin of neocartilage formed at wound edges of articular cartilage in a tissue culture experiment. Osteoarthr Cartil. 2008;16(2):204–211. doi: 10.1016/j.joca.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Asik M, Ciftci F, Sen C, Erdil M, Atalar A. The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: midterm results. Arthroscopy. 2008;24(11):1214–1220. doi: 10.1016/j.arthro.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Benthien JP, Behrens P. Reviewing subchondral cartilage surgery: considerations for standardised and outcome predictable cartilage remodelling: a technical note. Int Orthop. 2013;37(11):2139–2145. doi: 10.1007/s00264-013-2025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelse K, Riedel D, Pachowsky M, Hennig FF, Trattnig S, Welsch GH. Limited integrative repair capacity of native cartilage autografts within cartilage defects in a sheep model. J Orthop Res. 2015;33(3):390–397. doi: 10.1002/jor.22773. [DOI] [PubMed] [Google Scholar]
- 24.Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824–1831. doi: 10.1177/0363546506289882. [DOI] [PubMed] [Google Scholar]
- 25.Yanke AB, Lee AS, Karas V, Abrams G, Riccio ML, Verma NN, et al. Surgeon ability to appropriately address the calcified cartilage layer: an in vitro study of arthroscopic and open techniques. Am J Sports Med. 2019;47(11):2584–2588. doi: 10.1177/0363546519859851. [DOI] [PubMed] [Google Scholar]
- 26.•.Mithoefer K, Venugopal V, Manaqibwala M. Incidence, degree, and clinical effect of subchondral bone overgrowth after microfracture in the knee. Am J Sports Med. 2016;44(8):2057–2063. doi: 10.1177/0363546516645514. [DOI] [PubMed] [Google Scholar]
- 27.Bark S, Piontek T, Behrens P, Mkalaluh S, Varoga D, Gille J. Enhanced microfracture techniques in cartilage knee surgery: fact or fiction? World J Orthop. 2014;5(4):444–449. doi: 10.5312/wjo.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, Chevrier A, Hoemann CD, Sun J, Ouyang W, Buschmann MD. Characterization of subchondral bone repair for marrow-stimulated chondral defects and its relationship to articular cartilage resurfacing. Am J Sports Med. 2011;39(8):1731–1740. doi: 10.1177/0363546511403282. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Hoemann CD, Sun J, Chevrier A, McKee MD, Shive MS, et al. Depth of subchondral perforation influences the outcome of bone marrow stimulation cartilage repair. J Orthop Res. 2011;29(8):1178–1184. doi: 10.1002/jor.21386. [DOI] [PubMed] [Google Scholar]
- 30.Eldracher M, Orth P, Cucchiarini M, Pape D, Madry H. Small subchondral drill holes improve marrow stimulation of articular cartilage defects. Am J Sports Med. 2014;42(11):2741–2750. doi: 10.1177/0363546514547029. [DOI] [PubMed] [Google Scholar]
- 31.Hoemann CD, Gosselin Y, Chen H, Sun J, Hurtig MB, Carli A, Stanish W. Characterization of initial microfracture defects in human condyles. J Knee Surg. 2013;26(5):347–355. doi: 10.1055/s-0033-1341580. [DOI] [PubMed] [Google Scholar]
- 32.Min BH, Choi WH, Lee YS, Park SR, Choi BH, Kim YJ, Jin LH, Yoon JH. Effect of different bone marrow stimulation techniques (BSTs) on MSCs mobilization. J Orthop Res. 2013;31(11):1814–1819. doi: 10.1002/jor.22380. [DOI] [PubMed] [Google Scholar]
- 33.Horbelt D, Denkis A, Knaus P. A portrait of transforming growth factor beta superfamily signalling: background matters. Int J Biochem Cell Biol. 2012;44(3):469–474. doi: 10.1016/j.biocel.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Santo VE, Gomes ME, Mano JF, Reis RL. Controlled release strategies for bone, cartilage, and osteochondral engineering--part I: recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Eng B Rev. 2013;19(4):308–326. doi: 10.1089/ten.TEB.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cals FL, Hellingman CA, Koevoet W, Baatenburg de Jong RJ, van Osch GJ. Effects of transforming growth factor-beta subtypes on in vitro cartilage production and mineralization of human bone marrow stromal-derived mesenchymal stem cells. J Tissue Eng Regen Med. 2012;6(1):68–76. doi: 10.1002/term.399. [DOI] [PubMed] [Google Scholar]
- 36.Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tekari A, Luginbuehl R, Hofstetter W, Egli RJ. Transforming growth factor beta signaling is essential for the autonomous formation of cartilage-like tissue by expanded chondrocytes. PLoS One. 2015;10(3):e0120857. doi: 10.1371/journal.pone.0120857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan H, Tao H, Wu Y, Hu Y, Yan Y, Luo Z. TGF-beta3 immobilized PLGA-gelatin/chondroitin sulfate/hyaluronic acid hybrid scaffold for cartilage regeneration. J Biomed Mater Res A. 2010;95(4):982–992. doi: 10.1002/jbm.a.32899. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Li B, Yang J, Xin L, Li Y, Yin H, Qi Y, Jiang Y, Ouyang H, Gao C. The restoration of full-thickness cartilage defects with BMSCs and TGF-beta 1 loaded PLGA/fibrin gel constructs. Biomaterials. 2010;31(34):8964–8973. doi: 10.1016/j.biomaterials.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Noh MJ, Copeland RO, Yi Y, Choi KB, Meschter C, Hwang S, Lim CL, Yip V, Hyun JP, Lee HY, Lee KH. Pre-clinical studies of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 (TG-C) Cytotherapy. 2010;12(3):384–393. doi: 10.3109/14653240903470639. [DOI] [PubMed] [Google Scholar]
- 41.Ahn J, Kim SA, Kim KW, Oh JH, Kim SJ. Optimization of TGF-beta1-transduced chondrocytes for cartilage regeneration in a 3D printed knee joint model. PLoS One. 2019;14(5):e0217601. doi: 10.1371/journal.pone.0217601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ha CW, Noh MJ, Choi KB, Lee KH. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy. 2012;14(2):247–256. doi: 10.3109/14653249.2011.629645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee B, Parvizi J, Bramlet D, Romness DW, Guermazi A, Noh M, et al. Results of a phase II study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-beta1. J Knee Surg. 2020;33(2):167–172. doi: 10.1055/s-0038-1676803. [DOI] [PubMed] [Google Scholar]
- 44.Guermazi A, Kalsi G, Niu J, Crema MD, Copeland RO, Orlando A, Noh MJ, Roemer FW. Structural effects of intra-articular TGF-beta1 in moderate to advanced knee osteoarthritis: MRI-based assessment in a randomized controlled trial. BMC Musculoskelet Disord. 2017;18(1):461. doi: 10.1186/s12891-017-1830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam J, Lu S, Kasper FK, Mikos AG. Strategies for controlled delivery of biologics for cartilage repair. Adv Drug Deliv Rev. 2015;84:123–134. doi: 10.1016/j.addr.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan J, Gong Y, Ren L, Varshney RR, Cai D, Wang DA. In vitro engineered cartilage using synovium-derived mesenchymal stem cells with injectable gellan hydrogels. Acta Biomater. 2010;6(3):1178–1185. doi: 10.1016/j.actbio.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 47.Sellers RS, Peluso D, Morris EA. The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1997;79(10):1452–1463. doi: 10.2106/00004623-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Lopiz-Morales Y, Abarrategi A, Ramos V, Moreno-Vicente C, Lopez-Duran L, Lopez-Lacomba JL, et al. In vivo comparison of the effects of rhBMP-2 and rhBMP-4 in osteochondral tissue regeneration. Eur Cell Mater. 2010;20:367–378. doi: 10.22203/eCM.v020a30. [DOI] [PubMed] [Google Scholar]
- 49.Gouttenoire J, Valcourt U, Ronziere MC, Aubert-Foucher E, Mallein-Gerin F, Herbage D. Modulation of collagen synthesis in normal and osteoarthritic cartilage. Biorheology. 2004;41(3–4):535–542. [PubMed] [Google Scholar]
- 50.Yang HS, La WG, Bhang SH, Kim HJ, Im GI, Lee H, et al. Hyaline cartilage regeneration by combined therapy of microfracture and long-term bone morphogenetic protein-2 delivery. Tissue Eng A. 2011;17(13–14):1809–1818. doi: 10.1089/ten.TEA.2010.0540. [DOI] [PubMed] [Google Scholar]
- 51.Chubinskaya S, Merrihew C, Cs-Szabo G, Mollenhauer J, McCartney J, Rueger DC, Kuettner KE. Human articular chondrocytes express osteogenic protein-1. J Histochem Cytochem. 2000;48(2):239–250. doi: 10.1177/002215540004800209. [DOI] [PubMed] [Google Scholar]
- 52.Klein-Nulend J, Louwerse RT, Heyligers IC, Wuisman PI, Semeins CM, Goei SW, et al. Osteogenic protein (OP-1, BMP-7) stimulates cartilage differentiation of human and goat perichondrium tissue in vitro. J Biomed Mater Res. 1998;40(4):614–620. doi: 10.1002/(SICI)1097-4636(19980615)40:4<614::AID-JBM13>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 53.Nishida Y, Knudson CB, Kuettner KE, Knudson W. Osteogenic protein-1 promotes the synthesis and retention of extracellular matrix within bovine articular cartilage and chondrocyte cultures. Osteoarthr Cartil. 2000;8(2):127–136. doi: 10.1053/joca.1999.0281. [DOI] [PubMed] [Google Scholar]
- 54.Jelic M, Pecina M, Haspl M, Kos J, Taylor K, Maticic D, McCartney J, Yin S, Rueger D, Vukicevic S. Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth Factors. 2001;19(2):101–113. doi: 10.3109/08977190109001079. [DOI] [PubMed] [Google Scholar]
- 55.Kuo AC, Rodrigo JJ, Reddi AH, Curtiss S, Grotkopp E, Chiu M. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthr Cartil. 2006;14(11):1126–1135. doi: 10.1016/j.joca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 56.McQuillan DJ, Handley CJ, Campbell MA, Bolis S, Milway VE, Herington AC. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J. 1986;240(2):423–430. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Osch GJ, van den Berg WB, Hunziker EB, Hauselmann HJ. Differential effects of IGF-1 and TGF beta-2 on the assembly of proteoglycans in pericellular and territorial matrix by cultured bovine articular chondrocytes. Osteoarthr Cartil. 1998;6(3):187–195. doi: 10.1053/joca.1998.0111. [DOI] [PubMed] [Google Scholar]
- 58.Sah RL, Chen AC, Grodzinsky AJ, Trippel SB. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308(1):137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- 59.Hui W, Rowan AD, Cawston T. Modulation of the expression of matrix metalloproteinase and tissue inhibitors of metalloproteinases by TGF-beta1 and IGF-1 in primary human articular and bovine nasal chondrocytes stimulated with TNF-alpha. Cytokine. 2001;16(1):31–35. doi: 10.1006/cyto.2001.0950. [DOI] [PubMed] [Google Scholar]
- 60.Davies LC, Blain EJ, Gilbert SJ, Caterson B, Duance VC. The potential of IGF-1 and TGFbeta1 for promoting “adult” articular cartilage repair: an in vitro study. Tissue Eng A. 2008;14(7):1251–1261. doi: 10.1089/tea.2007.0211. [DOI] [PubMed] [Google Scholar]
- 61.Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, Menger MD, Kohn D, Trippel SB. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12(15):1171–1179. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- 62.Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg (Br) 2002;84(2):276–288. doi: 10.1302/0301-620X.84B2.0840276. [DOI] [PubMed] [Google Scholar]
- 63.Longobardi L, O'Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res. 2006;21(4):626–636. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- 64.Yaeger PC, Masi TL, de Ortiz JL, Binette F, Tubo R, McPherson JM. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997;237(2):318–325. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]
- 65.•.An C, Cheng Y, Yuan Q, Li J, et al. Ann Biomed Eng. 2010;38(4):1647–1654. doi: 10.1007/s10439-009-9892-x. [DOI] [PubMed] [Google Scholar]
- 66.Roessler BJ, Hartman JW, Vallance DK, Latta JM, Janich SL, Davidson BL. Inhibition of interleukin-1-induced effects in synoviocytes transduced with the human IL-1 receptor antagonist cDNA using an adenoviral vector. Hum Gene Ther. 1995;6(3):307–316. doi: 10.1089/hum.1995.6.3-307. [DOI] [PubMed] [Google Scholar]
- 67.Hung GL, Galea-Lauri J, Mueller GM, Georgescu HI, Larkin LA, Suchanek MK, Tindal MH, Robbins PD, Evans CH. Suppression of intra-articular responses to interleukin-1 by transfer of the interleukin-1 receptor antagonist gene to synovium. Gene Ther. 1994;1(1):64–69. [PubMed] [Google Scholar]
- 68.Nixon AJ, Haupt JL, Frisbie DD, Morisset SS, McIlwraith CW, Robbins PD, et al. Gene-mediated restoration of cartilage matrix by combination insulin-like growth factor-I/interleukin-1 receptor antagonist therapy. Gene Ther. 2005;12(2):177–186. doi: 10.1038/sj.gt.3302396. [DOI] [PubMed] [Google Scholar]
- 69.Morisset S, Frisbie DD, Robbins PD, Nixon AJ, McIlwraith CW. IL-1ra/IGF-1 gene therapy modulates repair of microfractured chondral defects. Clin Orthop Relat Res. 2007;462:221–228. doi: 10.1097/BLO.0b013e3180dca05f. [DOI] [PubMed] [Google Scholar]
- 70.Kraus VB, Birmingham J, Stabler TV, Feng S, Taylor DC, Moorman CT, 3rd, et al. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: a randomized controlled pilot trial ( NCT00332254) Osteoarthr Cartil. 2012;20(4):271–278. doi: 10.1016/j.joca.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Davidson D, Blanc A, Filion D, Wang H, Plut P, Pfeffer G, Buschmann MD, Henderson JE. Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J Biol Chem. 2005;280(21):20509–20515. doi: 10.1074/jbc.M410148200. [DOI] [PubMed] [Google Scholar]
- 72.Gigout A, Guehring H, Froemel D, Meurer A, Ladel C, Reker D, Bay-Jensen AC, Karsdal MA, Lindemann S. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthr Cartil. 2017;25(11):1858–1867. doi: 10.1016/j.joca.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 73.Sennett ML, Meloni GR, Farran AJE, Guehring H, Mauck RL, Dodge GR. Sprifermin treatment enhances cartilage integration in an in vitro repair model. J Orthop Res. 2018;36:2648–2656. doi: 10.1002/jor.24048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Power J, Hernandez P, Guehring H, Getgood A, Henson F. Intra-articular injection of rhFGF-18 improves the healing in microfracture treated chondral defects in an ovine model. J Orthop Res. 2014;32(5):669–676. doi: 10.1002/jor.22580. [DOI] [PubMed] [Google Scholar]
- 75.Lohmander LS, Hellot S, Dreher D, Krantz EF, Kruger DS, Guermazi A, et al. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2014;66(7):1820–1831. doi: 10.1002/art.38614. [DOI] [PubMed] [Google Scholar]
- 76.Hochberg MC, Guermazi A, Guehring H, Aydemir A, Wax S, Fleuranceau-Morel P, et al. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: The FORWARD Randomized Clinical Trial. JAMA. 2019;322(14):1360–1370. doi: 10.1001/jama.2019.14735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chahla J, Cinque ME, Piuzzi NS, Mannava S, Geeslin AG, Murray IR, et al. A call for standardization in platelet-rich plasma preparation protocols and composition reporting: a systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am. 2017;99(20):1769–1779. doi: 10.2106/JBJS.16.01374. [DOI] [PubMed] [Google Scholar]
- 78.Dhillon RS, Schwarz EM, Maloney MD. Platelet-rich plasma therapy - future or trend? Arthritis Res Ther. 2012;14(4):219. doi: 10.1186/ar3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mizuta H, Kudo S, Nakamura E, Otsuka Y, Takagi K, Hiraki Y. Active proliferation of mesenchymal cells prior to the chondrogenic repair response in rabbit full-thickness defects of articular cartilage. Osteoarthr Cartil. 2004;12(7):586–596. doi: 10.1016/j.joca.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 80.Drengk A, Zapf A, Stürmer EK, Stürmer KM, Frosch KH. Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs. 2009;189(5):317–326. doi: 10.1159/000151290. [DOI] [PubMed] [Google Scholar]
- 81.Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V, Jacobs CR. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng C Methods. 2009;15(3):431–435. doi: 10.1089/ten.tec.2008.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee GW, Son JH, Kim JD, Jung GH. Is platelet-rich plasma able to enhance the results of arthroscopic microfracture in early osteoarthritis and cartilage lesion over 40 years of age? Eur J Orthop Surg Traumatol. 2013;23(5):581–587. doi: 10.1007/s00590-012-1038-4. [DOI] [PubMed] [Google Scholar]
- 83.Fitzpatrick J, Bulsara MK, McCrory PR, Richardson MD, Zheng MH. Analysis of platelet-rich plasma extraction: variations in platelet and blood components between 4 common commercial kits. Orthop J Sports Med. 2017;5(1):2325967116675272. doi: 10.1177/2325967116675272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mazzocca AD, McCarthy MB, Chowaniec DM, Cote MP, Romeo AA, Bradley JP, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308–316. doi: 10.2106/JBJS.K.00430. [DOI] [PubMed] [Google Scholar]
- 85.Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25(7):913–925. doi: 10.1002/jor.20382. [DOI] [PubMed] [Google Scholar]
- 86.Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10(5):223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fortier LA, Nixon AJ, Williams J, Cable CS. Isolation and chondrocytic differentiation of equine bone marrow-derived mesenchymal stem cells. Am J Vet Res. 1998;59(9):1182–1187. [PubMed] [Google Scholar]
- 88.McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27(8):1033–1042. doi: 10.1002/jor.20853. [DOI] [PubMed] [Google Scholar]
- 89.Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320(3):914–919. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 90.Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, Stokol T, Cheetham J, Nixon AJ. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927–1937. doi: 10.2106/JBJS.I.01284. [DOI] [PubMed] [Google Scholar]
- 91.Hannon CP, Ross KA, Murawski CD, Deyer TW, Smyth NA, Hogan MV, et al. Arthroscopic bone marrow stimulation and concentrated bone marrow aspirate for osteochondral lesions of the talus: a case-control study of functional and magnetic resonance observation of cartilage repair tissue outcomes. Arthroscopy. 2016;32(2):339–347. doi: 10.1016/j.arthro.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 92.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 93.Gobbi A, Whyte GP. One-stage cartilage repair using a hyaluronic acid-based scaffold with activated bone marrow-derived mesenchymal stem cells compared with microfracture: five-year follow-up. Am J Sports Med. 2016;44(11):2846–2854. doi: 10.1177/0363546516656179. [DOI] [PubMed] [Google Scholar]