Abstract
Background
In the current opioid epidemic, opioid addiction and overdose deaths are a public health crisis. Researchers have uncovered other concerning findings related to opioid use, such as the association between prescribed opioids and respiratory infection, including pneumonias. Potential mechanisms include the immunosuppressive effects of certain opioids, respiratory depression, and cough suppression. We conducted a systematic review assessing whether prescribed opioid receipt is a risk factor for community-acquired pneumonia (CAP).
Methods
A systematic literature search of published studies was conducted using Ovid MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, Web of Science, AMED, and CINAHL from database inception through March 11, 2020. We included any clinical trial, cohort, or case–control study that reported an association between prescribed opioid receipt and CAP in adults. Two reviewers independently performed data extraction and quality assessment using the Newcastle-Ottawa Quality Assessment Scale. The risk of CAP from prescribed opioid receipt was studied by pooling studies using random effects meta-analysis.
Results
We identified 3229 studies after removing duplicates. After detailed selection, 33 articles were reviewed in full and eight studies (representing 567,472 patients) met inclusion criteria. The pooled effect for the four case–control studies and three cohort studies showed a significant increase in the risk of CAP requiring hospitalization among those with prescribed opioid receipt compared with those without opioid prescribed receipt (OR 1.57 [95% CI (1.34, 1.84)]; HR 1.18 [95% CI (1.00, 1.40)]).
Conclusion
The findings suggest prescribed opioid receipt is a risk factor for CAP. The included studies examined post-operative patients and patients with chronic medical conditions. Further research is needed to examine the impact of opioids on the incidence of CAP in an otherwise healthy population.
Electronic supplementary material
The online version of this article (10.1007/s11606-020-06155-9) contains supplementary material, which is available to authorized users.
KEY WORDS: opioids, pneumonia
INTRODUCTION
Community-acquired pneumonia (CAP) is common, with greater than 1.5 million adults hospitalized annually, and is among the most common infectious causes of death in the USA.1–4 Known risk factors for CAP are older age, current smoking, comorbid respiratory disease, cardiovascular disease, stroke, dementia, and alcohol use.5 Attention has turned to identify additional risk factors for CAP and develop preventive strategies to combat the burden of this disease.6
While opioid addiction and overdose are a major concern during the current opioid epidemic, emerging data also suggest that opioids may increase risk of infectious complications7, 8 including pneumonia. There are plausible reasons to believe opioid use may increase the risk of pneumonia, as opioids have immunosuppressive effects, disrupt gut homeostasis, suppress cough and breathing, prevent the secretion of bronchial mucus, cause sedation that can lead to aspiration, and inhibit neutrophil response to Streptococcus pneumoniae.8–14
There have been emerging epidemiologic data raising concern that prescribed opioids may have clinically significant effects on risk of pneumonia. To summarize the published results examining the association of prescribed opioid receipt and risk for CAP, we conducted a systematic review.
METHODS
The Meta-analysis of Observational Studies in Epidemiology (MOOSE)15 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)16 statements for reporting systematic reviews were used for our study. The study protocol was registered at Research Registry at www.researchregistry.com (Protocol # reviewregistry885).
Data Sources and Search Strategy
With the assistance of a medical research librarian (AB), we conducted a literature search of published studies using Ovid MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, Web of Science, AMED, and CINAHL from database inception through March 11, 2020 (Appendix Table 1). Search terms included “opioid,” “pneumonia,” “lung inflammation,” “opiate alkaloid,” and combinations of these terms.
Study Selection
The Patient-Intervention-Comparator-Outcome-Study Design (PICO) criteria were used to determine eligibility of the articles based on the type of study design, type of population, and type of exposure and outcome. Prescribed opioid receipt was the exposure and CAP was the outcome. We included all comparative study designs (cohort, case–control, and cross sectional) that assessed the association between prescribed opioid receipt and the risk of CAP in adult populations. We included studies that reported risk using either odds ratio or hazard ratio. We only included studies in English. We excluded studies that only examined children and did not present original data, such as narrative reviews, and abstracts that only included minimal study information about the methods and results. Two co-authors (CS, LB) independently screened all title and abstracts for inclusion. Abstracts included by either reviewer underwent full-text review. The same authors then reviewed selected full-text manuscripts for ultimate inclusion. Disagreements were reviewed by a third author (MS).
Data Extraction and Quality Assessment
Two co-authors (CS, LB) extracted data from published reports into evidence tables; additional co-authors over-read evidence tables (CG, MS). For included studies, data were extracted on study populations, interventions, comparators, outcomes, quality, and applicability. For analysis, opioids were categorized as immunosuppressive and non-immunosuppressive. Immunosuppressive opioids included codeine, morphine, fentanyl, diamorphine, dihydrocodeine, sufentanil, and methadone. Non-immunosuppressive opioids included hydrocodone, buprenorphine, hydromorphone, oxycodone, oxycodone/naloxone, oxymorphone, and tramadol. Two co-authors (CS, LB) independently rated risk of bias using the Newcastle-Ottawa Quality Assessment Scale (NOS) for case–control and cohort studies17 and assigned a numerical score out of a possible 9 points. Disagreements were adjudicated by obtaining a third author’s (MS) opinion.
Data Synthesis and Statistical Analysis
When at least three studies were available with comparable study designs and outcomes, we performed random effects meta-analyses and estimated pooled ORs with 95% confidence intervals (CI) as described by DerSimonian and Laird.18 When studies reported multiple models, we used the most adjusted model. We evaluated heterogeneity visually and with the I2 statistic. I2 values of 25%, 50%, and 75% were considered low, medium, and high heterogeneity.19 Subgroup analysis comparing immunosuppressive opioids to non-immunosuppressive opioids was done using meta-regression. The Knapp–Hartung variance estimator and associated t test was used to calculate p values for the comparison.20 Statistical analysis was performed using Stata/MP, version 15.1 (StataCorp, College Station, Texas).
RESULTS
Study Selection
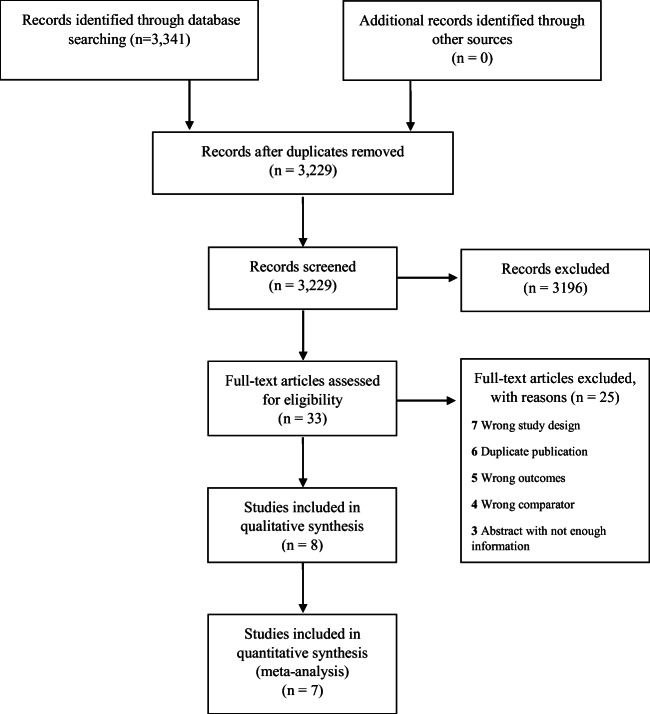
The electronic literature search identified 3341 studies, of which 112 were duplicates. Of the remaining 3229 studies, 33 were reviewed in full and eight met study inclusion criteria (Fig. 1).10, 21–27 We included one study that examined children because the proportion of the pediatric population was very small (3.6%) (Table 1).24Common reasons for exclusion after full-text review included review articles without original data, the wrong exposure such as the diagnosis of opioid use disorder, the wrong outcome such as hospital-acquired (i.e., nosocomial) pneumonia, abstracts with scant information, duplicate publications using the same data, and the outcome not including a diagnosis of pneumonia (Fig. 1).
Figure 1.
Evidence search and selection.
Table 1.
Characteristics of Included Studies
| Study and year | Study design | Patient population and database | Study participants | Follow-up period | Prescribed opioid receipt definition | Pneumonia definition |
|---|---|---|---|---|---|---|
| Dublin et al., 2011 | Nested case–control (matched by age, sex, diagnosis date, and calendar year) | Patients aged 65–94 from national integrated health care system, USA | 1039 CAP cases vs. 2022 matched controls | NR | Current use defined as opioid prescription fill between 5 and 60 days prior to index | ICD-9 codes (480-487.0 or 507.0) validated with chest radiograph reports and medical record review, excluded HAP |
| Vozoris et al., 2016 | Retrospective cohort of patients with COPD aged > 65 years | Patients > 65 years with COPD. Used administrative database, Ontario, Canada | 89,224 COPD pts with new opioid use vs. 41,930 COPD controls with non-use | Within 30 days of incident opioid use | New opioid use defined by pharmacy fill, excluded buprenorphine and methadone | ICD-10 codes (J09–J18, J20–J22 or J40) |
| Velly et al., 2017 | Nested case–control study with up to 2 controls matched with incident cases of CAP on sex, age, general practice, cohort entry, date of CAP diagnosis | Patients ≥ 18 who were new opioid users between April 1, 1998 and March 31, 2014 in Montreal Canada | 18,445 CAP cases vs. 35,088 matched controls | NR | Recent opioid use defined as a prescription 30 to 10 days before CAP diagnosis | CAP requiring hospitalization |
| Wiese et al., 2018 | Nested case–control (matched by age, index date, and county) | Patients enrolled in Tennessee Medicaid and aged ≥ 5 years (96.4% were ≥ 18 years) | 1233 invasive pneumococcal disease cases and 24,399 matched controls; broad age range | Cases identified in cohort 1995–2014 | Current use defined as opioid prescription fill overlapping index date, excluded buprenorphine | Isolation of Streptococcus pneumoniae from a normally sterile site |
| Edelman et al., 2019 | Nested case–control (matched by age, sex, race/ethnicity, length of observation, and HIV status) | Patients from the Veterans Aging Cohort Study (VACS), composed of HIV-positive veterans matched with non-HIV group | 4246 of CAP cases requiring hospitalization vs. 21,146 controls; mean age 55 | Median follow-up was 3 years | Current use defined as opioid prescription fill between 5 and 60 days prior to index, excluded buprenorphine and methadone | ICD-9 codes (480–487.0 or 507.0) |
| Hamina et al., 2019 | Nested case–control (matched by age, sex, and time since Alzheimer’s diagnosis) | Patients from the Medication use and Alzheimer’s Disease (MEDALZ) cohort, Finland | 5623 Alzheimer’s disease participants with new opioid use vs. 5623 non-use; mean age 83 | 180-day follow-up after incident opioid prescription | New opioid use defined by pharmacy fill | ICD-10 codes (J10.0–J16, J18 or J69.0) |
| Kim et al., 2019 | Retrospective cohort of patients undergoing total knee replacement (TKR) | Patients were Medicare enrollees who underwent TKR between 2010 and 2014 in the USA | 316,593 undergoing TKR and pre-operative opioid use as follows: 22,895 (7.2%) continuous opioid users, 161,511 (51.0%) intermittent opioid users, and 132,817 opioid naïve (41.7%) | Within 30 days post-TKR | Based on opioid dispensing in 360 days prior to TKR, continuous opioid users defined as ≥ 1 prescription in each of 12 months; intermittent opioid users defined as any prescription but not continuous and opioid naive | ICD-9 codes for pneumonia 30 days post-TKR (specific pneumonia codes not defined) |
| Shah et al., 2019 | Retrospective cohort of patients undergoing craniotomy | Patients undergoing craniotomy identified from January 1, 2013 to October 1, 2018 in the USA | Among 861 craniotomy cases, preoperative opioid milligram morphine equivalent was examined with postoperative pneumonia | Within 90 days of hospital discharge | Preoperative MME determined from electronic medical record using the Oregon Health Authority online calculator | Postoperative pneumonia recorded within 90 days of hospital discharge |
USA United States of America, CAP community-acquired pneumonia, ICD International Classification of Diseases, COPD chronic obstructive pulmonary disease, HIV human immunodeficiency virus
Quality Appraisal
Study quality was based on three main elements: selection, comparability, and outcome. Overall, the studies were of high quality (Table 2). Of the case–control studies, two scored all nine points and three scored eight points (downgraded one point under the category of selection for using hospital controls). The three cohort studies scored eight out of nine points (downgraded one point under the category of selection for not being representative of the general population). The main reason that studies had a decrease in points was for how the study population was selected.
Table 2.
Study Quality Assessment Using the Newcastle-Ottawa Quality Assessment Scale
| Study and year | Selection | Comparability | Outcome | NOS score | Limitations; items missing from NOS |
|---|---|---|---|---|---|
| Case–control studies | |||||
| Dublin et al., 2011 | **** | ** | *** | 9/9 | Older adult population ≥ 65 |
| Velly et al., 2017 | *** | ** | *** | 8/9 | CAP requiring hospitalization; selection: hospital controls |
| Wiese et al., 2018 | *** | ** | *** | 8/9 | Outcome was invasive pneumococcal disease, 74% with pneumonia; selection: hospital controls |
| Edelman et al., 2019 | *** | ** | *** | 8/9 | 59% of patients were HIV positive; selection: hospital controls |
| Hamina, et al. 2019 | **** | ** | *** | 9/9 | Patients with Alzheimer’s disease |
| Cohort studies | |||||
| Vozoris et al., 2016 | *** | ** | *** | 8/9 | All patients had COPD and outcome was hospitalization with pneumonia or COPD; selection: representativeness |
| Kim et al., 2019 | *** | ** | *** | 8/9 | All patients underwent total knee replacement; selection: representativeness |
| Shah et al., 2019 | *** | ** | *** | 8/9 | All patients underwent craniotomy; selection: representativeness |
Asterisk indicated item achieves one point on the NOS
NOS Newcastle Ottawa Scale, HIV human immunodeficiency virus, COPD chronic obstructive pulmonary disease
Characteristics of Included Studies
Among the eight included studies, five studies used a case–control design10, 21, 22, 24, 27 and three used a cohort design23, 25, 26 (Table 1). Geographically, the studies were varied with five of the studies from the United States (USA),10, 21, 24–26 one study from Finland,22 and two studies from Canada.23, 27 Six studies examined the association of prescribed opioids and pneumonia in a specific population of patients including human immunodeficiency virus (HIV),10 chronic obstructive pulmonary disease (COPD),23 Alzheimer’s disease,22 those undergoing craniotomy26 or total knee replacement,25 and older adults over 65 years old.21
With regard to type of opioid exposure, all studies reported pharmacy fill/refill data to assess prescribed opioid receipt. The reference group for these studies comprised of patients who had not received prescribed opioids or had in the past. Dose, duration, and long-acting versus short-acting opioid receipt were examined by five studies (Table 3). Wiese reported high dose (≥ 90 mg morphine equivalent daily dose (MEDD)) and long-acting opioids to have higher risk for pneumonia.24 Dublin found highest risk for CAP if prescribed medium dose opioids (20–49 MME), in the first 14 days of opioid receipt and on long-acting opioids.21 Edelman reported highest risk for CAP among patients with current and high dose (> 50 mg MEDD) and immunosuppressive opioid receipt.10 Vozoris found the highest risk for CAP or COPD hospitalization for those patients prescribed long-acting opioid formulations.23 Hamina found the highest risk for CAP in the first 2 months of opioid receipt and among higher doses (≥ 50 MME) of prescribed opioids.22, 25
Table 3.
Study Outcomes of Risk of Pneumonia Including Subgroups
| Study and year | Immuno-suppressive opioids* vs. non-use, aOR (95% CI) | MEDD dose, aOR (95% CI) | Recency of use, aOR (95% CI) | Long and short-acting, vs. non-use, aOR (95% CI) | Overall risk, current use vs. non-use, aOR (95% CI) |
|---|---|---|---|---|---|
| Dublin et al., 2011 |
IS = 1.88 (1.26–2.79) NIS = 1.23 (0.89–1.69) |
< 20 mg = 1.05 (0.71–1.56) 20-49 mg = 2.30 (1.10–4.83) ≥ 50 mg = 1.37 (0.64–2.92) |
5–14 d = 3.24 (1.64–6.39) 15–30 d = 1.28 (0.72–2.29) 31–90 d = 1.24 (0.78–1.99) > 90 d = 1.27 (0.91–1.77) |
Short = 1.27 (0.98–1.64) Long = 3.43 (1.44–8.21) |
aOR = 1.38 (1.08, 1.76) |
| Vozoris et al., 2016 | NR | < 30 mg similar to ≥ 30 mg dosing | NR |
Short = 1.50 (1.26–1.79) Long = 1.86 (1.23–2.81) |
aHR† = 1.08 (0.97–1.21) |
| Velly et al., 2017 |
IS = 1.90 (1.72–2.11) NIS = 1.80 (1.58–2.06) Tramadol = 2.04 (1.65–2.53) Oxycodone = 5.55 (2.17–14.2) Morphine = 4.84 (3.12–7.51) Fentanyl = 2.52 (1.55–4.11) Codeine = 1.65 (1.48–1.85) Hydrocodone = 1.34 (1.11–1.62) |
NR | NR | NR | aOR = 1.83 (1.68–1.99) |
| Wiese et al., 2018 |
IS = 1.74 (1.20–2.53) NIS = 1.55 (1.27–1.88) |
< 50 mg, 1.54 (1.26–1.88) 50–90 mg, 1.71 (1.22–2.39) ≥ 90 mg, 1.75 (1.33–2.29) |
New use = 2.44(1.49–4.00) Recent past use = 1.03(0.87–1.21) |
Short = 1.58 (1.32–1.90) Long = 1.87 (1.24–2.82) |
aOR = 1.62 (1.36–1.92) |
| Edelman et al., 2019 |
IS = 1.42 (1.21–1.67) NIS = 1.24 (1.09–1.40) |
IS: < 20 mg = 1.35 (1.07–1.70) 20–50 mg = 2.07 (1.59–2.71) > 50 mg = 3.18 (2.44–4.14) NIS: < 20 mg = 1.23 (1.03–1.48) 20–50 mg = 1.35 (1.13–1.62) > 50 mg = 2.07 (1.50–2.86) |
NR | NR |
aOR = 1.42 (1.31–1.54) |
| Hamina et al., 2019 |
IS = 1.68 (1.20–2.36) NIS = 2.91 (1.94–4.34 |
< 50 mg = 1.36 (1.13–1.62) ≥ 50 mg 2.86 (1.72–4.72) |
< 60 d = 2.58 (1.87–3.55) 61–180 d = 1.42 (1.00–2.02) 181–365 = 0.91 (0.62–1.33) > 365 = 0.90 (0.65–1.25) |
NR | aHR = 1.34 (1.14–1.57) |
| Kim et al., 2019 | NR | NR | NR | NR |
aHR = 1.14 (0.70–1.84) for continuous use aHR = 0.81 (0.60–1.09) for intermittent use |
| Shah et al., 2019 | NR | NR | NR | NR | NR |
Non-immunosuppressive opioids include hydrocodone, buprenorphine, hydromorphone, oxycodone, oxycodone/naloxone, tramadol
IS immunosuppressive, NIS non-immunosuppressive, MEDD morphine equivalent daily dose, d days, NR not reported, aHR adjusted hazard ratio
*Immunosuppressive opioids include codeine, morphine, fentanyl, diamorphine, dihydrocodeine, and sufentanil
†Vozoris reports risk of hospitalization for pneumonia or COPD exacerbation
Only a few studies examined the effect of buprenorphine (a partial opioid agonist) and methadone (a synthetic opioid agonist) on risk of CAP. Three studies excluded buprenorphine10, 23, 24 and two excluded methadone.10, 23 One study separately reported the risk of pneumonia with one of these medications and reported that compared with no buprenorphine receipt, buprenorphine receipt was associated with increased risk of CAP in the unadjusted model, but not in the adjusted model (aHR 1.20, 95% CI 0.83, 1.76).23
Seven studies examined a hospital admission for CAP, while one study included hospitalizations for combined COPD or pneumonia.23 Seven studies used ICD codes (ICD9 or ICD 10) to define pneumonia while Dublin validated codes with chest radiograph reports and hospital records.21 Wiese documents laboratory confirmed invasive Streptococcus pneumoniae, of which 74% were pneumonia.24
Opioid Receipt and CAP
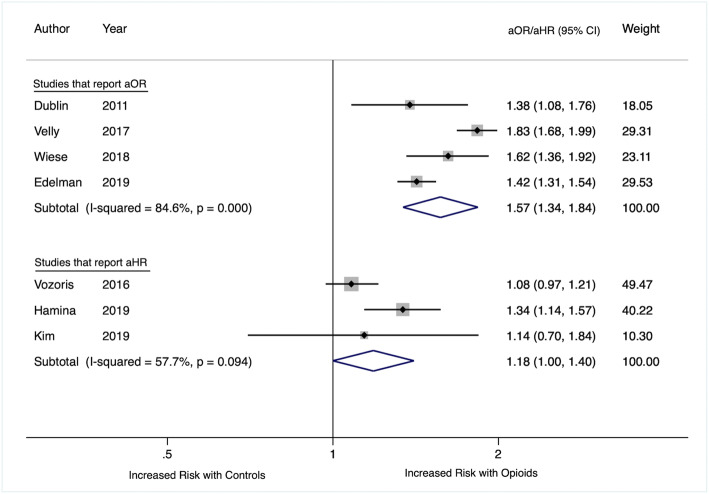
A total population of 567,472 patients were included in our review. Five studies found an increased risk of CAP for patients with prescribed opioid receipt compared with those without any receipt in the specified time period with a range of adjusted odds ratios or hazard ratios from 1.34 to 1.8310, 21, 22, 24, 27 (Table 3). Two cohort studies did not find an increased risk of CAP associated with opioid receipt (aHR = 1.08 (95% CI, 0.97, 1.21)23 and aHR = 1.14 (95% CI, 0.70, 1.84).25 The four studies that used case–control methods and reported adjusted odds ratios were pooled using random effects meta-analysis. The pooled odds ratio was 1.57 (95% CI 1.34, 1.84) (Fig. 2). The three studies reporting adjusted hazard ratios were pooled using random effects meta-analysis and the pooled hazard ratio was 1.18 (95% CI, 1.00, 1.40). I2 was 84.6% for studies reporting odds ratios and 57.7% for those reporting hazard ratios.
Figure 2.
Forest plot of included studies by opioid receipt.
Immunosuppressive Status and CAP
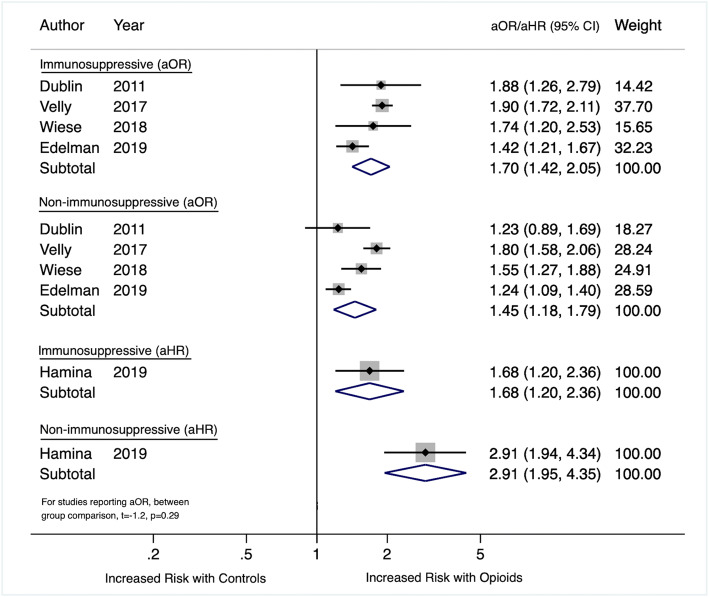
Five studies separately reported the effect of immunosuppressive opioids and non-immunosuppressive opioids compared with no opioid receipt on the risk for CAP. Four studies reported higher risk for CAP with immunosuppressive opioids.10, 21, 24, 27 The other study reported a higher risk for CAP among the group prescribed non-immunosuppressive opioids.22 None of the individual studies directly compared the risk of CAP between immunosuppressive and non-immunosuppressive opioids. We pooled the risk of immunosuppressive opioids and non-immunosuppressive opioids for the four studies that reported adjusted odds ratios and used case–control designs. All four studies reported higher rates of CAP with immunosuppressive opioids compared with no opioid receipt. Compared with no receipt, the pooled risk for CAP among patients prescribed immunosuppressive opioids was 1.70 (95% CI, 1.42, 2.05) compared with 1.45 (95% CI, 1.18, 1.79) for non-immunosuppressive opioids and the difference was not statistically significant (Fig. 3). We also report the results of the one study that measured adjusted hazard ratios in Figure 3.22
Figure 3.
Forest plot of included studies by immunosuppressive status.
DISCUSSION
Opioids are prescribed for pain management for patients with both acute and chronic pain, but the adverse consequences associated with these medications are still being quantified. Across several high-quality observational studies, prescribed opioid receipt was associated with a 57% increase in odds of CAP. Prior research has suggested that some opioids (e.g., codeine, morphine, methadone, and fentanyl) have immunosuppressive properties and their use may increase the risk for infections.8, 9, 28 The risk of infection in one study was higher for patients who were newly prescribed immunosuppressive opioids.28 Partial agonists like buprenorphine may have a more favorable immune profile.29 Our data do not suggest that immunosuppression is the main mechanism by which opioids increase CAP risk. The mechanism of opioids increasing CAP risk may be more likely due to sedation or other effects.
Several clinical questions regarding the risk of prescribed opioid receipt and CAP remain unanswered. For example, does opioid dosage matter? The studies in this review used different cut-points for high-dose opioids (≥ 30 mg, ≥ 50 mg, and ≥ 90 mg MEDD) and we were unable to pool results to examine this question. Future research should use a standardized definition of high-dose opioids. Another clinical question is whether short-acting versus long-acting formulation impacts the risk of prescribed opioid receipt and CAP. Two case–control and one cohort study examined this question and the findings are inconclusive. Because long-acting formulations of opioids are more likely to be prescribed for chronic use, recency of use would also need to be examined. This question warrants further research.
Our study has important limitations. We only included published studies. Furthermore, studies controlled for different covariates. Although we extracted the adjusted ORs when possible, few studies adjusted for all possible confounders. For example, only two studies included tobacco use as a covariate in the fully adjusted models.10, 24 Hence, bias or confounding could account for some or all of the observed association. Finally, many of the participants in these studies had medical co-morbidities (e.g., those with HIV, Alzheimer’s disease, older adults with COPD and those post-surgery). Further research is needed to examine the impact of opioids on the incidence of CAP in an otherwise healthy population.
Continued research in the realm of opioid use and pneumonia can benefit from standardization in definitions of opioid use and pneumonia. We also need large-scale longitudinal studies evaluating the association between prescribed opioid receipt and pneumonia and examine if there are other subgroups at higher risk for CAP based on their co-morbidities or the type of opioids prescribed. As more patients are being converted to buprenorphine for long-term management, monitoring their risk for CAP is important. In addition, investigation of whether prescribed opioids impact risk of other infectious complications as well as other immunomodulatory effects (e.g., increasing risk of recurrence among patients with cancer) are needed.30 Given the mounting evidence for the hazards associated with prescription opioid receipt, including pneumonia, we need to continue to invest in the development of safer alternatives for treating pain.
CONCLUSIONS
The findings suggest prescribed opioid receipt is a risk factor for CAP. The included studies examined post-operative patients and patients with chronic medical conditions. Further research is needed to examine the impact of opioids on the incidence of CAP in an otherwise healthy population. Clinicians should consider the additional risk of pneumonia when weighing the risk-benefit of prescribing opioids.
Electronic supplementary material
(DOCX 16 kb)
Funding
This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Health Services Research and Development #CIN 13-407.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
Footnotes
Prior Presentations
Presented, in part, at the Northeast Regional SGIM Meeting, Boston MA, November 2, 2019
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet. 2011;377(9773):1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong S, Amand C, Kieffer A, Kyaw MH. Trends in healthcare utilization and costs associated with pneumonia in the United States during 2008-2014. BMC Health Serv Res. 2018;18(1):715. doi: 10.1186/s12913-018-3529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson G, Davis AM. Diagnosis and treatment of adults with community-acquired pneumonia. JAMA. 2020. [DOI] [PubMed]
- 4.Arias E, Xu J, Kochanek KD. United States Life Tables, 2016. Natl Vital Stat Rep. 2019;68(4):1–66. [PubMed] [Google Scholar]
- 5.Simou E, Britton J, Leonardi-Bee J. Alcohol and the risk of pneumonia: a systematic review and meta-analysis. BMJ Open. 2018;8(8):e022344. doi: 10.1136/bmjopen-2018-022344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanks CW, Musani AI, Hsia DW. Community-acquired pneumonia and hospital-acquired pneumonia. Med Clin North Am. 2019;103(3):487–501. doi: 10.1016/j.mcna.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Risdahl JM, Khanna KV, Peterson PK, Molitor TW. Opiates and infection. J Neuroimmunol. 1998;83(1-2):4–18. doi: 10.1016/s0165-5728(97)00216-6. [DOI] [PubMed] [Google Scholar]
- 8.Roy S, Ninkovic J, Banerjee S, et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol. 2011;6(4):442–465. doi: 10.1007/s11481-011-9292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plein LM, Rittner HL. Opioids and the immune system—friend or foe. Br J Pharmacol. 2018;175(14):2717–2725. doi: 10.1111/bph.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelman EJ, Gordon KS, Crothers K, et al. Association of prescribed opioids with increased risk of community-acquired pneumonia among patients with and without HIV. JAMA Intern Med. 2019;179(3):297–304. doi: 10.1001/jamainternmed.2018.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaefer CP, Tome ME, Davis TP. The opioid epidemic: a central role for the blood brain barrier in opioid analgesia and abuse. Fluids Barriers CNS. 2017;14(1):32–11. doi: 10.1186/s12987-017-0080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson RS, Morjaria JB, Wright CE, Morice AH. Is opiate action in cough due to sedation? Ther Adv Chronic Dis. 2014;5(5):200–205. doi: 10.1177/2040622314543220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Roy S. Gut homeostasis, microbial dysbiosis, and opioids. Toxicol Pathol. 2017;45(1):150–156. doi: 10.1177/0192623316679898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenstein TK. The role of opioid receptors in immune system function. Front Immunol. 2019;10:2904. doi: 10.3389/fimmu.2019.02904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.Wells GA, Shea B, O’Connell D, et al. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 21.Dublin S, Walker RL, Jackson ML, et al. Use of opioids or benzodiazepines and risk of pneumonia in older adults: a population-based case-control study. J Am Geriatr Soc. 2011;59(10):1899–1907. doi: 10.1111/j.1532-5415.2011.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamina A, Taipale H, Karttunen N, et al. Hospital-treated pneumonia associated with opioid use among community dwellers with Alzheimer’s disease. J Alzheimers Dis. 2019;69(3):807–816. doi: 10.3233/JAD-181295. [DOI] [PubMed] [Google Scholar]
- 23.Vozoris NT, Wang X, Fischer HD, et al. Incident opioid drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2016;48(3):683–693. doi: 10.1183/13993003.01967-2015. [DOI] [PubMed] [Google Scholar]
- 24.Wiese AD, Griffin MR, Schaffner W, et al. Opioid analgesic use and risk for invasive pneumococcal diseases: a nested case-control study. Ann Intern Med. 2018;168(6):396–404. doi: 10.7326/M17-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SC, Jin Y, Lee YC, et al. Association of preoperative opioid use with mortality and short-term safety outcomes after total knee replacement. JAMA Netw Open. 2019;2(7):e198061. doi: 10.1001/jamanetworkopen.2019.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah V, Hoang, N., Rodgers, B., Dornbos, D.L., Eaton, R., Pezzutti, D., Hoover, E., Duenas, H., Cua, S., Nimjee, S.M. The impact of preopertive opioids on outcomes in craniotomy patients. Clinical Neurosurgery 2019;66 (Supplement 1):160-161.
- 27.Velly AM, Steel R, Karp I, Morenz E, Moulin D, Morales DR, Landry J, Azoulay L, Moldovan F. The impact of opioids on the incidence of community-acquired pneumonia: a large nested case-control study. Annals of Epidemiology. 2017;27:520. [Google Scholar]
- 28.Wiese AD, Griffin MR, Schaffner W, et al. Long-acting opioid use and the risk of serious infections: a retrospective cohort study. Clin Infect Dis. 2019;68(11):1862–1869. doi: 10.1093/cid/ciy809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacerdote P. Opioid-induced immunosuppression. Curr Opin Support Palliat Care. 2008;2(1):14–18. doi: 10.1097/SPC.0b013e3282f5272e. [DOI] [PubMed] [Google Scholar]
- 30.Boland JW, McWilliams K, Ahmedzai SH, Pockley AG. Effects of opioids on immunologic parameters that are relevant to anti-tumour immune potential in patients with cancer: a systematic literature review. Br J Cancer. 2014;111(5):866–873. doi: 10.1038/bjc.2014.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 16 kb)