Abstract
Cetyltrimethylammonium bromide (CTAB) is the preferred detergent in RNA extraction of oil palm tissues. However, the CTAB-based protocol is time-consuming. In this study, a combination of the CTAB-based method and silica-based purification reduced the extraction time from two days to five hours. Quality of total RNA from 27 different tissues of oil palm was shown to have an RNA integrity number (RIN) value of more than seven. The extracted RNA was evaluated by RT-qPCR using three reference oil palm genes (GRAS, CYP2, and SLU7) and three putative mesocarp-specific transcripts annotated as WRKY DNA-binding protein 70 (WRKY-70), metallothionein (MT) and pentatricopeptide repeat (PPR) genes. Tissue-specific expression profiling across complete developmental stages of mesocarp and vegetative tissues was determined in this study. Overall, the RNA extraction protocol described here is rapid, simple and yields good quality RNAs from oil palm tissues.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02514-9) contains supplementary material, which is available to authorized users.
Keywords: Oil palm, RNA extraction protocol, Cetyltrimethylammonium bromide, Silica column, RT-qPCR
Introduction
Oil palm is a major tropical oil crop worldwide. Global production of palm oil is around 70.5 million metric tons in the marketing year 2018/2019 (Kushairi et al. 2019). The demand for palm oil will be triple in the year 2050, exceeding 250 million metric tons (Zulkifli et al. 2017). About 90% of palm oil is used for industrial frying applications, shortenings, margarine and confectionary fats (Noor Lida et al. 2017). The remaining 10% of palm oil is used for soap and oleochemical manufacturing (Lai et al. 2012). Palm oil is favoured by the food industry because of its competitive price, excellent oxidative stability, high nutritional value, free of trans fatty acids and rich with antioxidant properties (Ong and Goh 2002; Noor Lida et al. 2017). In order to fulfil the strong demand for the palm oil, yield and quality of palm oil are being improved through various efforts including plant biotechnology approach (Parveez et al. 2015a; Masura et al. 2017; Masani et al. 2018).
RNA sequencing (RNA-Seq) is an indispensable tool to analyse the continuously changing cellular transcriptome using next-generation sequencing (NGS) (Hrdlickova et al. 2017). Reverse transcription quantitative real-time PCR (RT-qPCR) is a laboratory-based technique for quantification of gene expression (Wong and Medrano 2005; Die et al. 2016). RT-qPCR is the significant development of PCR technology which can produce an accurate, sensitive and fast measurement of gene expression (Wong and Medrano 2005; Sanders et al. 2014). RNA-Seq and RT-qPCR technological advances provide understanding and insight into the complex transcriptional coordination underlie the developmental, biological and cellular processes (Nwafor et al. 2014; Li et al. 2019). A successful gene expression analysis requires high purity and integrity of RNA. High-quality RNA plays a critical role in the accuracy, reproducibility and relevance of downstream analyses (Patel et al. 2017). Therefore, obtaining abundant, intact and pure RNA molecules is the most critical step for gene profiling analysis.
RNA extraction from oil palm tissues to be used for RT-qPCR is quite challenging due to the high levels of polysaccharides, polyphenolics, lipids, proteins and other secondary metabolites (Qadri et al. 2019; Ong et al. 2019). These macromolecules tend to degrade or co-precipitate with the RNA molecule (Liu et al. 2018). The polyphenolic compound is easily oxidised to quinone by polyphenol oxidase (PPO). This quinone group has a cytotoxic activity which can bind to the nucleic acids and cause the strand to breaks (Bolton and Dunlap 2017). RNA and polysaccharides can co-precipitate during RNA extraction, owing to their similar physical and chemical properties (Sharma et al. 2003). Macromolecular contaminants like lipids, proteins and other secondary metabolites, if not removed, will interfere with the reverse transcription process to generate complementary DNA (cDNA) from RNA template (Kuang et al. 2018). The removal of these contaminating substances effectively is crucial to extract high quality of RNA from oil palm.
Various RNA extraction protocols have been reported in the isolation of high-quality RNA across diverse species using either conventional method or commercial kit reagents (Kałużna et al. 2016; Barbier et al. 2019). Different plants consist of different types of cell wall polysaccharides and variable levels of secondary metabolites which require different methods to extract RNA (Houston et al. 2016). The phospholipid is an amphipathic molecules which is the primary building blocks of all cell membrane (Alberts et al. 2002). It consists of two hydrophobic fatty acid chains linked to a phosphate-containing hydrophilic head group. Phospholipid forms lipid bilayers with the polar heads being attracted to water and nonpolar tails buried in the cell membrane (Cooper and Hausman 2014). Cetyltrimethylammonium bromide (CTAB) is the preferred detergent in RNA extraction buffer system as it can dissolve both polar and nonpolar end groups of the cell membrane (Tan and Yiap 2013). Hemicellulose, cellulose and pectin made up the rigid structure of the plant cell wall. The positive charge of the CTAB molecule allows it to form a complex with these cell wall polysaccharides, denature these compounds and ease the purification process of RNA (Wang and Stegemann 2010).
The CTAB-based method is an inexpensive and consistent protocol for RNA extraction from plant with a high content of polysaccharide and polyphenol component (Inglis et al. 2018). Conventional CTAB protocol can be divided into five main steps which are: lysis of the cell wall by CTAB buffer; separation of the RNA from the other organelles using chloroform; precipitation of the RNA by lithium chloride (LiCl); purification of extracted RNA by 70% alcohol; and dissolving the RNA by Tris–EDTA buffer. RNA precipitation step using LiCl is time-consuming, where the 10 M of LiCl was added into the supernatant containing RNA and incubated overnight at 4 °C (Barman et al. 2017). The whole process of the extraction time using the conventional CTAB-based method takes two days to complete. The result from the previous study has shown that the precipitation step using LiCl could be reduced to one to four hours; however, overnight precipitation results in better yield of RNA (Barman et al. 2017). It is thus necessary to develop CTAB-based protocol which is not time-consuming and labour-intensive for RNA extraction from oil palm tissues.
The purpose of this study is to improve CTAB-based protocol which could reduce the time of extraction without compromising the quality and yield of RNA extracted from oil palm tissues. In this study, the CTAB-based method combined with a silica column of purification kit was employed to obtain high-quality RNA from 27 various tissue culture materials. The suitability of RNA extracted for the downstream application was determined using RT-qPCR.
Materials and methods
Plant materials
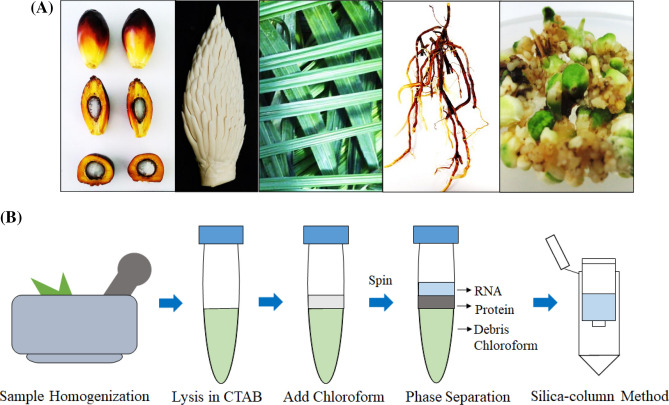
A total of 27 different tissues of oil palm including mesocarp tissues from five weeks after anthesis (WAA) to 20 WAA, 10 WAA kernel, male inflorescence, female inflorescence, spear leaf, mature leaf (frond-17), primary roots, lateral roots, whitish roots and oil palm tissue culture materials (embryogenic callus, polyembryoid and plantlet) were obtained from commercial tenera planting materials (Fig. 1a). All samples were frozen in liquid nitrogen and stored at − 80 °C prior to RNA extraction.
Fig. 1.
RNA extraction from oil palm tissues. a Different tissues of oil palm were used in this study. From left to right: 20 WAA mesocarp, male inflorescence, mature leaf, primary root and polyembroid tissue. b Overview of the total RNA extraction protocol using a combination of CTAB-based method and silica column
Total RNA extraction
About 0.5 g of frozen tissue of each oil palm sample was ground in liquid nitrogen using mortar and pestle. The tissue was ground into fine powder. After that, the fine powder was transferred from the mortar into a 50-ml falcon tube. Then, 2.5 ml of pre-heated (65 °C) RNA extraction buffer contained 2% (w/v) CTAB, 100 mM Tris–HCL (pH 8), 2 M sodium chloride (NaCl), 0.1% (w/v) spermidine trihydrochlorate, 2% (w/v) polyvinylpyrrolidone (PVP-40), 2% (v/v) β-Mercaptoethanol and 1.5% (w/v) sodium dodecyl sulfate (SDS) was added into the falcon tube. The mixture was vortexed vigorously and incubated at 65 °C for 30 min. To aid tissue disruption process, the sample was mixed by vortexing briefly every 5 min. After incubation, an equal volume of chloroform: isoamyl alcohol (24:1) was added to each sample, vortexed for 30 s and centrifuged at 5000 × g, 4 °C, for 15 min. The upper aqueous phase was carefully transferred into a new falcon tube. The mixing step with chloroform: isoamyl alcohol was repeated again. Without touching the white layer, the supernatant (1.0 ml) was transferred into 1.5-ml tube added with 0.5 ml of 100% ethanol. The sample was then applied to the RNA binding column of RNeasy Mini Kit (Qiagen) and spun at 10 000 × g for 30 s at room temperature. The extra supernatant–ethanol mixture was loaded again onto the same RNA binding column and centrifuged like the previous step. Manufacturer’s recommendations were followed to purify the total RNA further and to get rid of the genomic contamination through DNase treatment. Finally, RNA was eluted from the column using 30 µl of RNase-free water and stored at − 80 °C. The yield, purity and integrity of the RNA samples were determined using a spectrophotometer Nanodrop ND-1000 at two wavelength ratios of A260/280 nm and A260/230 nm. The integrity of total RNA was analysed using an Agilent Bioanalyzer 2100 (Agilent Technologies, USA).
Reverse transcription quantitative real-time PCR (RT-qPCR)
Applied Biosystem High Capacity cDNA kit (Applied Biosystems, USA) was used to generate cDNA from total RNA (2 μg). RT-qPCR was used for verification of the gene expression of three putative mesocarp-specific transcripts using gene-specific primers listed in Table S1. The RT-qPCR reactions were conducted using CFX Connect™ Real-Time PCR Detection System (Biorad) with thermal profile as follows: 3 min at 95 °C and 40 cycles of 5 s at 95 °C, 30 s at 50 °C and followed by melting curve analysis at 65 °C to 95 °C with 0.5 °C per 5 s increments to determine the specificity of the PCR product. Twenty µl reaction mixtures contained 16 ng of cDNA template, 500 nM of each primer and 1 × iTaq Universal SYBR Green Supermix (Biorad). For each total RNA sample, a no reverse transcriptase control (NRT) and a no template control (NTC) were included. All reactions were prepared in three technical replicates for each cDNA sample. PCR amplification efficiencies and R2 values of reference gene primers and gene of interest were determined across six different pools of cDNA at five different concentrations (1, 2, 4, 8 and 16 ng). The six pools of cDNA were mesocarp (16 different developing stages from 5 to 20 WAA), kernel (10 WAA), inflorescence (male inflorescence and female inflorescence), leaf (spear leaf and mature leaf frond-17), root (primary roots, lateral roots and whitish roots) and oil palm tissue culture materials (embryogenic callus, polyembryoid and plantlet). The expression data of the putative mesocarp-specific transcript were normalised to three reference genes, namely Cyclophilin 2 (CYP2), Pre-mRNA splicing factor SLU7 (SLU7), Gibberellin-responsive protein 2 (GRAS) using Bio-Rad CFX Manager Software (ΔΔCq analysis mode) based on the method described by Livak and Schmittgen (2001). The relative expression of the putative mesocarp-specific transcripts was calculated by using 16WAA mesocarp as the calibrator.
Results and discussion
RNA extraction using CTAB-based method and silica column of purification kit
A rapid and optimised RNA extraction method was developed to extract RNA from various tissues of oil palm. This simplified method combined the CTAB-based method and silica column of a commercial RNA purification kit (Fig. 1b). After the grinding process, fine powder was added to the pre-warmed CTAB extraction buffer. Composition of the CTAB extraction buffer is crucial to prevent cellular contaminants from co-precipitating with RNA and ease the extraction process. Components of the CTAB extraction buffer were selected based on their specific properties. CTAB is a cationic detergent which functions to facilitate the separation of polysaccharide (Tümmler and Stanke 2018). PVP act as a polyphenol removal (Inglis et al. 2018), spermidine and SDS protect the nucleic acid by inhibiting the RNase (Katz et al. 2017), and β-mercaptoethanol is a potent reducing agent which can remove tannins and other polyphenols (Liao et al. 2004).
For tissue disruption purpose, sample added with CTAB extraction buffer was incubated for 30 min at 65 °C and vortexed for every five min. The lysed cells were then mixed with chloroform: isoamyl alcohol mixture. The function of chloroform is to denature the protein and facilitate the separation of the aqueous and organic phases, while the isoamyl alcohol acts to reduce foaming during the extraction process (Xia et al. 2019). Three distinct phases, namely aqueous phase, interphase and organic phase, were formed after the centrifugation step. RNA and other contaminants such as salts and sugars remain in the upper aqueous phase, which is less dense than the organic phase (chloroform: isoamyl alcohol). The lipid and cellular debris partition into the organic phase, while proteins remain at the interphase. The acidity of the mixture caused the DNA to precipitate into the organic phase.
The conventional CTAB-based extraction method is widely used for isolation of high-quality RNA from oil palm tissues (Parveez et al. 2015b; Qadri et al. 2019; Ong et al. 2019; Hanin et al. 2020). However, the CTAB-based method is time-consuming because of the LiCl precipitation step that required at least overnight incubation at 4 °C. Therefore by replacing the LiCl precipitation step with a silica column in this study, the extraction time can be reduced from two days to about five hours. Combination of the CTAB-based method with silica column has been used for isolation of RNA from immature seeds of Jatropha curcas L, which the extraction time was reduced from two days to about three hours (Sangha et al. 2010). Extraction and purification of total RNA using commercial kit alone might cause the silica column to be more susceptible to clogging by viscous extracts. Blockage of the silica columns can be prevented by using CTAB extraction buffer, which consists of the CTAB, PVP, SDS and β-mercaptoethanol that can efficiently remove the interfering cellular contaminants. Therefore, the combination of advantage of the CTAB-based method and convenience of the silica column of commercial kit can expedite the RNA extraction process.
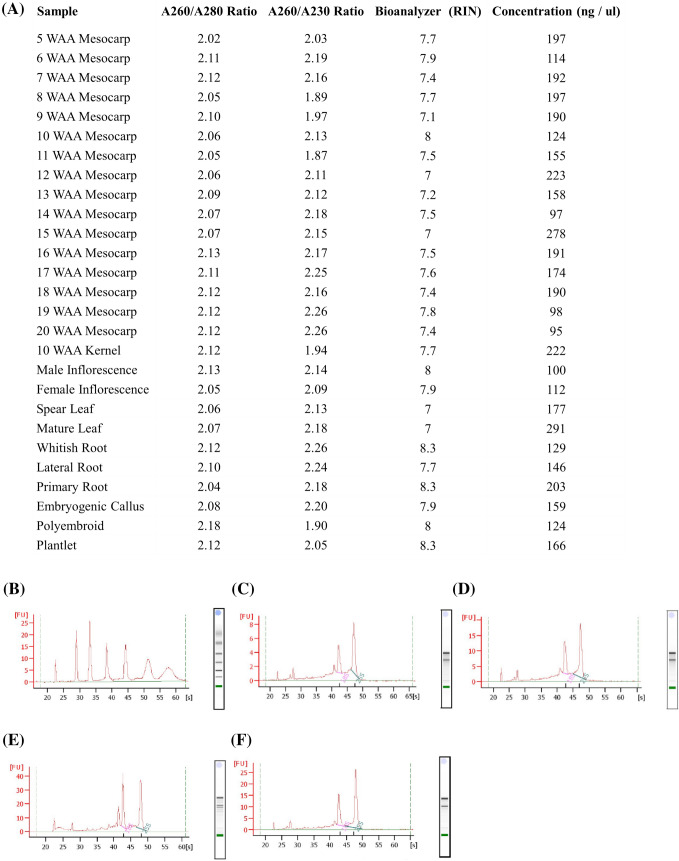
Various methods have been exploited to extract RNA from plant tissues. The efficiency of a specific method to extract RNA from the distinct plant varied because of the differences in chemical composition and structural features of plant tissue. Oil palm tissues contain a high amount of polysaccharides, polyphenols and secondary metabolites. Extraction intact RNA from oil palm is challenging due to the similar physical and chemical properties between polysaccharides and nucleic acids. In this study, total RNA from 27 different tissues of oil palm was extracted using the CTAB-based method and silica column of commercial kit. The results of spectrophotometric absorbance showed that the ratio of A260/A280 and A260/A230 is higher than 2.00 and 1.80, respectively, for all the extracted samples indicating minimal protein and polysaccharide contamination (Fig. 2a). Thus, a combination of the CTAB-based method and silica column, which takes 5 h for extraction time, can produce good purity of total RNA as compared to the traditional CTAB-based method.
Fig. 2.
Total RNA extracted from various oil palm tissues. a The purity and integrity of total RNA were analysed using Nanodrop ND-1000 and Agilent Bioanalyzer 2100. b Electropherograms of ladder, c 5 WAA mesocarp, d male inflorescence, e spear leaf and f primary root are provided as examples
Assessment of RNA integrity
Quality of starting RNA influences the accuracy of gene expression evaluation. High quality of RNA is crucial for molecular and diagnostic applications (Fleige and Pfaffl 2006; Vermeulen et al. 2011). In this study, the integrity of RNA was analysed using an Agilent Bioanalyzer 2100 (Fig. 2c–f). This high innovative lab-on-chip capillary gel-electrophoresis is the most convenient and widely used to assess the quality of large RNA samples (Davies et al. 2016). In this method, RNA samples are load on the Agilent chip and electrophoretically separated through microchannels (Schroeder et al. 2006). Before separation, RNA are labelled with a fluorescent dye, and these complexes are then detected by laser-induced fluorescence. The use of an RNA ladder (Fig. 2b) as a set of size standard allows determining the size and the concentration of the sample peaks (Schroeder et al. 2006). RNA integrity number (RIN) is an algorithm for assigning integrity values for RNA measurements. RIN assigns an electropherogram a value of 1–10, with ten being the most intact. The RIN values observed in this study ranged from 7.0 to 8.3 (Fig. 2a). The highest RIN values were observed in the whitish root, primary root and oil palm tissue culture derived from plantlet. Samples with RIN values greater or equal to 7 are generally suitable for gene expression profiling such as RNA seq, RT-qPCR and cDNA library construction (Padhi et al. 2018). Overall, these results showed that this method is suitable for obtaining high-quality RNA from various oil palm tissues.
Gene expression analysis using extracted total RNA
RT-qPCR is the method of choice for gene expression analysis because of its high sensitivity, good reproducibility and broad quantification range (Kuang et al. 2018; Taylor et al. 2019). The quality of extracted RNA from 27 different tissues of oil palm was further examined using RT-qPCR. Reliability of RT-qPCR can be improved by including internal controls in the assay to correct for sample to sample dissimilarities caused by various factors such as quality of extracted RNA, the efficiency of reverse transcription and inaccurate pipetting (Kozera and Rapacz 2013). The normalisation of raw expression data using reliable reference genes is pivotal for accurate measurement of mRNA transcription level (Piazza et al. 2017).
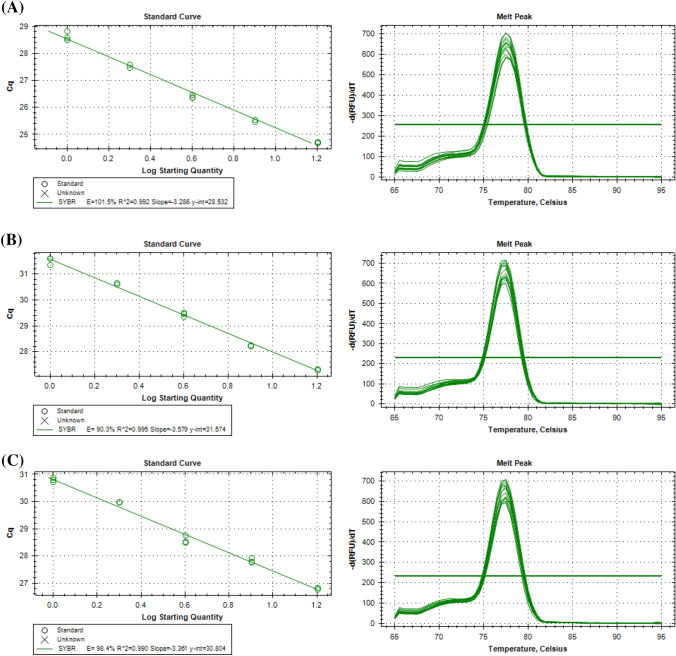
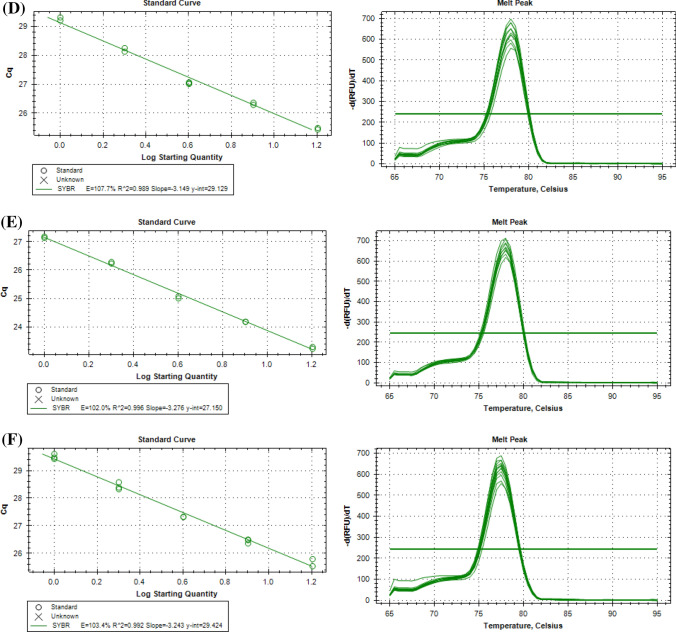
A study reported by Yeap et al. (2014) showed that Gibberellin-responsive protein 2 (GRAS), Cyclophilin 2 (CYP2) and Pre-mRNA splicing factor SLU7 (SLU7) are most suitable reference genes for all oil palm tissues and vegetative tissues. A standard curve for three candidate reference genes, GRAS (Fig. 3), CYP2 (Fig. S1) and SLU7 (Fig. S2) was generated across six different pools of cDNA, namely mesocarp, kernel, flower, leaf, root and oil palm tissue culture materials. The estimated PCR amplification efficiencies (Ex) of these genes ranged from 86 to 108% (Table 1). PCR efficiency is highly dependent on the primers used, and precise qPCR assays are correlated with high PCR efficiency. Meanwhile, the correlation coefficient (R2) values represent how well the experimental data fit the regression line. Most of the genes have R2 values higher than 0.99 (Table 1), which indicate a strong correlation between the amplification efficiency and starting template material. A single peak was observed in the melting curve generated from GRAS, CYP2 and SLU7. These results indicated the specificity of the amplified PCR products.
Fig. 3.
Determination of PCR amplification efficiencies (Ex), correlation coefficient (R2) and melting curve generated for GRAS across six different pools of cDNA samples: a mesocarp, b kernel, c flower, d leaf, e root and f oil palm tissue culture materials
Table 1.
PCR amplification efficiencies (Ex) and correlation coefficient (R2) values of oil palm reference genes
| Tissue | GRAS | CYP2 | SLU7 |
|---|---|---|---|
| Mesocarp |
Ex = 101.5% R2 = 0.992 |
Ex = 98.2% R2 = 0.986 |
Ex = 105.6% R2 = 0.985 |
| Kernel |
Ex = 90.3% R2 = 0.995 |
Ex = 86.3% R2 = 0.993 |
Ex = 97.5% R2 = 0.996 |
| Flower |
Ex = 98.4% R2 = 0.990 |
Ex = 98.0% R2 = 0.992 |
Ex = 104.8% R2 = 0.996 |
| Leaf |
Ex = 107.7% R2 = 0.989 |
Ex = 98.5% R2 = 0.988 |
Ex = 105.8% R2 = 0.986 |
| Root |
Ex = 102.0% R2 = 0.996 |
Ex = 100.6% R2 = 0.996 |
Ex = 100.1% R2 = 0.992 |
| Polyembroid |
Ex = 103.4% R2 = 0.992 |
Ex = 108.0% R2 = 0.992 |
Ex = 107.8% R2 = 0.987 |
A total of 41 transcripts that specifically expressed in the mesocarp tissue of oil palm were identified through in silico analysis of multiple transcriptomic datasets (Badai et al. 2019). Expression profiling of three putative mesocarp-specific transcripts annotated as WRKY DNA-binding protein 70 (WRKY-70), metallothionein (MT) and pentatricopeptide repeat (PPR) genes was validated using extracted total RNA from 27 different tissues of oil palm. The PCR amplification efficiency and correlation coefficient (R2) values of these three putative mesocarp-specific transcripts are summarised in Table S2, with melting curve showing a single peak for the amplified PCR product (Fig S3). Based on the expression results, the transcript level of WRKY-70 was up-regulated in all tested mesocarp tissue (5 WAA—20 WAA), lateral roots and in all oil palm tissue culture materials. Expression of WRKY-70 gene was down-regulated in 10 WAA kernel, male inflorescence, female inflorescence, mature leaf and primary roots (Fig. 4a). The expression pattern of MT gene was increased around 30-fold in mesocarp at 20 WAA, 19-folds in mesocarp at 18 WAA, and threefold in kernel and primary root tissue. Low expression of MT gene was observed in male flower, female flower, mature leaf, spear leaf, root hairs, whitish root and tissue culture materials (Fig. 4b). The transcript level of PPR gene was up-regulated to around 2.3-fold in 18 WAA and 8 WAA mesocarp tissues, and 1.6-fold in female flower, 14 WAA and 9 WAA mesocarp as compared to the calibrator (Fig. 4c).
Fig. 4.
Gene expression analysis of a WRKY-70, b MT and c PPR across 27 different oil palm tissues using RT-qPCR. The expression level is presented in log2 (relative expression). The relative expression of the putative mesocarp-specific transcripts was calculated by using 16WAA mesocarp as the calibrator. Standard deviations are shown by vertical bars
The studies presented thus far provide evidence that WRKY-70 has a pivotal role in signalling crosstalk between salicylic acid (SA) and jasmonic acid (JA) during plant response against biotrophic/hemibiotrophic and necrotrophic pathogens (Li et al. 2004, 2017). WRKY-70, in collaboration with WRKY46 and WRKY54, involved in regulating brassinosteroid (BR) signalling but negatively modulates drought tolerance (Chen et al. 2017). Brassinosteroids (BRs) are a class of steroidal plant hormones that play important roles in diverse processes such as cell differentiation, cell expansion, photomorphogenesis, reproduction and responding to various biotic and abiotic stresses (Bishop and Koncz 2002). All these data suggest that WRKY-70 is involved in modulating various phytohormones signalling pathways (Ülker et al. 2007; Chen et al. 2017). Metallothioneins (MTs) are low-molecular-weight cysteine-rich proteins that play important roles in detoxification of heavy metals like cadmium, copper, silver and mercury and homeostasis of essential metal ions such as Zn2+, Fe2+ and Cu2+ (Zhou et al. 2006). PPR protein is one of the largest protein families in land plant and can be defined by the presence of tandem arrays of a degenerate 35–amino acid motifs (Alice and Ian 2014). PPR protein plays an essential role in RNA processing and translation in mitochondria and chloroplast (Lurin et al. 2004). For example, Arabidopsis organelle transcript processing 43 (otp43) PPR mutants are defective in seed development, severely reduced germination rates and poor growth of the plant (De Longevialle et al. 2007).
Conclusion
In conclusion, our results demonstrated that a combination of the CTAB-based method and silica column is capable of extracting high quality and intact total RNA from various oil palm tissues. Compared to the traditional CTAB method, extraction time can be reduced from 2 days to 5 h without compromising the quality and yield of RNA samples. Total RNA extracted using this protocol was suitable for downstream analyses. This was exemplified by the analysis of expression level of genes, WRKY-70, MT and PPR, in different stages of mesocarp development and in various tissues of oil palm.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the MPOB management for permission to publish this work. We would also like to acknowledge the members of Transgenic Technology Group (TTG) for their technical assistance. We thank Breeding and Tissue Culture Unit for providing mesocarp tissues from five weeks after anthesis (WAA) to 20 WAA and oil palm tissue culture materials (embryogenic callus, polyembryoid and plantlet).
Author contributions
BSS established the protocol and drafted the manuscript. ROA, PGKA and MMYA initiated and guided the project, and revised the manuscript.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. 4. New York: Garland Science; 2002. [Google Scholar]
- Alice B, Ian S. Pentatricopeptide repeat proteins in plants. Annu Rev Plant Biol. 2014;65:415–442. doi: 10.1146/annurev-arplant-050213-040159. [DOI] [PubMed] [Google Scholar]
- Badai SS, Chan KL, Chan PL, Parveez GKA, Rasid OA. Identification of genes preferentially expressed in mesocarp tissue of oil palm using in silico analysis of transcripts. J Oil Palm Res. 2019;31:540–549. [Google Scholar]
- Barbier FF, Chabikwa TG, Ahsan MU, Cook SE, Powell R, Tanurdzic M, Beveridge CA. A phenol/chloroform-free method to extract nucleic acids from recalcitrant, woody tropical species for gene expression and sequencing. Plant Methods. 2019;15:62. doi: 10.1186/s13007-019-0447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman P, Choudhary AK, Geeta R. A modified protocol yields high-quality RNA from highly mucilaginous dioscorea tubers. 3 Biotech. 2017;7:150. doi: 10.1007/s13205-017-0775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Koncz C. Brassinosteroids and plant steroid hormone signaling. Plant Cell. 2002;14:97–110. doi: 10.1105/tpc.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Dunlap T. Formation and biological targets of quinones: cytotoxic versus cytoprotective effects. Chem Res Toxicol. 2017;30:13–37. doi: 10.1021/acs.chemrestox.6b00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Nolan T, Ye H, Zhang M, Tong H, Xin P, Chu J, Chu C, Li Z, Yin Y. Arabidopsis WRKY46, WRKY54 AND WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought response. Plant Cell. 2017;29:1425–1439. doi: 10.1105/tpc.17.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GM, Hausman RE. The cell: a molecular approach. Yale J Biol Med. 2014;87:603–604. [Google Scholar]
- Davies J, Denyer T, Hadfield J. Bioanalyser chips can be used interchangeably for many analyses of DNA or RNA. Biotechniques. 2016;60:197–199. doi: 10.2144/000114403. [DOI] [PubMed] [Google Scholar]
- De Longevialle AF, Meyer EH, Andrés C, Taylor NL, Lurin C, Millar AH, Small ID. The pentatricopeptide repeat gene OTP43 is required for trans-splicing of the mitochondrial nad1 Intron 1 in Arabidopsis thaliana. Plant Cell. 2007;19:3256–3265. doi: 10.1105/tpc.107.054841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Die JV, Roman B, Flores F, Rowland LJ. Design and sampling plan optimisation for RT-qPCR experiments in plants: a case study in blueberry. Front Plant Sci. 2016;7:271. doi: 10.3389/fpls.2016.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006;27:126–139. doi: 10.1016/j.mam.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Hanin AN, Parveez GKA, Rasid OA, Masani MYA. Biolistic-mediated oil palm transformation with alfalfa glucanase (AGLU1) and rice chitinase (RCH10) genes for increasing oil palm resistance towards Ganoderma boninense. Ind Crops Prod. 2020;144:112008. [Google Scholar]
- Houston K, Tucker MR, Chowdhury J, Shirley N, Little A. The plant cell wall: a complex and dynamic structure as revealed by the responses of genes under stress conditions. Front Plant Sci. 2016;7:984. doi: 10.3389/fpls.2016.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdlickova R, Toloue M, Tian B. RNA-Seq methods for transcriptome analysis. Wiley Interdiscip Rev RNA. 2017;8:1364. doi: 10.1002/wrna.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis PW, Pappas MD, Resende LV, Grattapaglia D. Fast and inexpensive protocols for consistent extraction of high quality DNA and RNA from challenging plant and fungal samples for high-throughput SNP genotyping and sequencing applications. PLoS ONE. 2018;13:e0206085. doi: 10.1371/journal.pone.0206085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kałużna M, Kuras A, Mikiciński A, Puławska J. Evaluation of different RNA extraction methods for high-quality total RNA and mRNA from Erwinia amylovora in planta. Eur J Plant Pathol. 2016;146:893–899. [Google Scholar]
- Katz AM, Tolokh IS, Pabit SA, Baker N, Onufriev AV, Pollack L. Spermine condenses DNA, but not RNA duplexes. Biophys J. 2017;112:22–30. doi: 10.1016/j.bpj.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozera B, Rapacz M. Reference genes in real-time PCR. J Appl Genet. 2013;54:391–406. doi: 10.1007/s13353-013-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J, Yan X, Genders AJ, Granata C, Bishop DJ. An overview of technical considerations when using quantitative real-time PCR analysis of gene expression in human exercise research. PLoS ONE. 2018;13:e0196438. doi: 10.1371/journal.pone.0196438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushairi A, Loh SK, Azman I, Hishamuddin E, Ong-Abdullah M, Mohd Noor Izuddin ZB, Razmah G, Subramaniam V, Sundram S, Parveez GKA. Oil palm economic performance in malaysia and R and D progress in 2018. J Oil Palm Res. 2019;31:165–194. [Google Scholar]
- Lai OM, Tan CP, Akoh CC. Palm oil: production, processing, characterisation, and uses. Amsterdam: Elsevier; 2012. [Google Scholar]
- Li J, Brader G, Palva ET. The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell. 2004;16:319–331. doi: 10.1105/tpc.016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhong R, Palva ET. WRKY70 and its homolog WRKY54 negatively modulate the cell wall-associated defenses to necrotrophic pathogens in Arabidopsis. PLoS ONE. 2017;12:e0183731. doi: 10.1371/journal.pone.0183731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Yang JL, Hao B, Lu YC, Qian ZL, Li Y, Ye S, Tang JR, Chen M, Long GQ, Zhao Y, Zhang GH, Chen JW, Fan W, Yang SC. Comparative transcriptome and metabolome analyses provide new insights into the molecular mechanisms underlying taproot thickening in Panax notoginseng. BMC Plant Biol. 2019;19:451. doi: 10.1186/s12870-019-2067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Chen M, Guo L, Gong Y, Tang F, Sun X, Tang K. Rapid isolation of high-quality total RNA from taxus and ginkgo. Prep Biochem Biotechnol. 2004;34:209–214. doi: 10.1081/PB-200026790. [DOI] [PubMed] [Google Scholar]
- Liu L, Han R, Yu N, Zhang W, Xing L, Xie D, Peng D. A method for extracting high-quality total RNA from plant rich in polysaccharides and polyphenols using Dendrobium huoshanense. PLoS ONE. 2018;13:e0196592. doi: 10.1371/journal.pone.0196592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyère C, Caboche M, Debast C, Gualberto J, Hoffmann B, Lecharny A, Le Ret M, Martin-Magniette ML, Mireau H, Peeters N, Renou JP, Szurek B, Taconnat L, Small I. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell. 2004;16:2089–2103. doi: 10.1105/tpc.104.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masani MYA, Izawati AM, Rasid OA, Parveez GKA. Biotechnology of oil palm: current status of oil palm genetic transformation. Biocatal Agric Biotechnol. 2018;15:335–347. [Google Scholar]
- Masura SS, Tahir NI, Rasid OA, Ramli US, Othman A, Masani MYA, Parveez GKA, Kushairi A. Post-genomic technologies for the advancement of oil palm research. J Oil Palm Res. 2017;29:469–486. [Google Scholar]
- Noor Lida HMD, Abd Hamid R, Kanagaratnam S, Awg Isa WR, Mohd Hassim NA, Ismail NH, Omar Z, Mat Sahri M. Palm oil and palm kernel oil: versatile ingredients for food applications. J of Oil Palm Res. 2017;29:487–511. [Google Scholar]
- Nwafor C, Gribaudo I, Schneider A, Wehrens R, Grando M, Costantini L. Transcriptome analysis during berry development provides insights into co-regulated and altered gene expression between a seeded wine grape variety and its seedless somatic variant. BMC Genomics. 2014;15:1030. doi: 10.1186/1471-2164-15-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong AS, Goh SH. Palm oil: a healthful and cost-effective dietary component. Food Nutr Bull. 2002;23:11–22. doi: 10.1177/156482650202300102. [DOI] [PubMed] [Google Scholar]
- Ong PW, Chan PL, Ooi LCL, Singh R. Isolation of high quality total RNA from various tissues of oil palm (Elaeis guineensis) for reverse transcription quantitative real-time PCR (RT-qPCR) J Oil Palm Res. 2019;31:195–203. [Google Scholar]
- Padhi BK, Singh M, Rosales M, Pelletier G, Cakmak S. A PCR-based quantitative assay for the evaluation of mRNA integrity in rat samples. Biomol Detect Quantif. 2018;15:18–23. doi: 10.1016/j.bdq.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveez GKA, Rasid OA, Masani MYA, Sambanthamurthi R. Biotechnology of oil palm: strategies towards manipulation of lipid content and composition. Plant Cell Rep. 2015;34:533–543. doi: 10.1007/s00299-014-1722-4. [DOI] [PubMed] [Google Scholar]
- Parveez GKA, Bahariah B, Ayub NH, Masani MYA, Rasid OA, Tarmizi AH, Ishak Z. Production of polyhydroxybutyrate in oil palm (Elaeis guineensis Jacq.) mediated by microprojectile bombardment of PHB biosynthesis genes into embryogenic calli. Front Plant Sci. 2015;6:598. doi: 10.3389/fpls.2015.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PG, Selvarajah S, Guérard KP, Bartlett JMS, Lapointe J, Berman DM, Okello JBA, Park PC. Reliability and performance of commercial RNA and DNA extraction kits for FFPE tissue cores. PLoS ONE. 2017;12:e0179732. doi: 10.1371/journal.pone.0179732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza V, Bartke A, Miquet J, Sotelo A. Analysis of different approaches for the selection of reference genes in RT-qPCR experiments: a case study in skeletal muscle of growing mice. Int J Mol Sci. 2017;18:e1060. doi: 10.3390/ijms18051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri R, Iqbal A, Wu Y, Li J, Nisar N, Azam M, Yang Y. A modified protocol for total RNA isolation from different oil palm Elaeis guineensis tissues using cetyltrimethylammonium bromide. Curr Sci. 2019;116:479–482. [Google Scholar]
- Sanders R, Mason DJ, Foy CA, Huggett JF. Considerations for accurate gene expression measurement by reverse transcription quantitative PCR when analysing clinical samples. Anal Bioanal Chem. 2014;406:6471–6483. doi: 10.1007/s00216-014-7857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha JS, Gu K, Kaur J, Yin Z. An improved method for RNA isolation and cDNA library construction from immature seeds of Jatropha curcas L. BMC Res Notes. 2010;3:126. doi: 10.1186/1756-0500-3-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AD, Gill PK, Singh P. RNA isolation from plant tissues rich in polysaccharides. Anal Biochem. 2003;314:319–321. doi: 10.1016/s0003-2697(02)00689-9. [DOI] [PubMed] [Google Scholar]
- Tan SC, Yiap BC. Erratum to “DNA, RNA, and protein extraction: the past and the present”. J Biomed Biotechnol. 2013;2013:628968. doi: 10.1155/2009/574398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SC, Nadeau K, Abbasi M, Lachance C, Nguyen M, Fenrich J. The ultimate qPCR experiment: producing publication quality, reproducible data the first time. Trends Biotechnol. 2019;37:761–774. doi: 10.1016/j.tibtech.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Tümmler B, Stanke F (2018) Genomic DNA: purification. John Wiley and Sons Ltd. https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470015902.a0005330.pub3. Accessed 26 January 2020
- Ülker B, Shahid Mukhtar M, Somssich IE. The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta. 2007;226:125–137. doi: 10.1007/s00425-006-0474-y. [DOI] [PubMed] [Google Scholar]
- Vermeulen J, De Preter K, Lefever S, Nuytens J, De Vloed F, Derveaux S, Hellemans J, Speleman F, Vandesompele J. Measurable impact of RNA quality on gene expression results from quantitative PCR. Nucleic Acids Res. 2011;39:e63. doi: 10.1093/nar/gkr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Stegemann JP. Extraction of high quality RNA from polysaccharide matrices using cetlytrimethylammonium bromide. Biomaterials. 2010;31:1612–1618. doi: 10.1016/j.biomaterials.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39:75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- Xia Y, Chen F, Du Y, Liu C, Bu G, Xin Y, Liu B. A modified SDS-based DNA extraction method from raw soybean. Biosci Rep. 2019;39:BSR20182271. doi: 10.1042/BSR20182271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap W, Loo JM, Wong YC, Kulaveerasingam H. Evaluation of suitable reference genes for qRT-PCR gene expression normalisation in reproductive, vegetative tissues and during fruit development in oil palm. Plant Cell Tiss Organ Cult. 2014;116:55–66. [Google Scholar]
- Zhou G, Xu Y, Lingyan JL, Liu YJ. Molecular analyses of the metallothionein gene family in rice (Oryza sativa L) J Biochem Mol Biol. 2006;39:595–606. doi: 10.5483/bmbrep.2006.39.5.595. [DOI] [PubMed] [Google Scholar]
- Zulkifli Y, Norziha A, Naqiuddin MH, Fadila AM, Nor Azwani AB, Suzana M, Samsul KR, Ong-Abdullah M, Singh R, Parveez GKA, Kushairi A. Designing the oil palm of the future. J Oil Palm Res. 2017;29:440–455. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.