Abstract
Background
Several hematological indices including subtypes of leukocytes populations have been associated with cardiovascular outcome. Takotsubo syndrome (TTS) is a form of acute heart failure syndrome featured by several in‐hospital complications (IHCs).
Hypothesis
Hematological indices at admission may predict IHCs in TTS patients.
Methods
One hundred and sixty consecutive patients with TTS were enrolled in a multicenter prospective registry. Clinical data, admission hemogram, and IHCs were recorded.
Results
Incidence of IHCs was 37%, including pulmonary edema 9%, cardiogenic shock 9%, need of invasive ventilation 10%, death 8%, stroke 2.5%, and left ventricular thrombi 6%.
Patients with IHCs were older, more frequently male, with physical stressor‐induced TTS, lower left ventricular ejection fraction at admission. Neutrophil/lymphocyte ratio (NLr) (12 ± 12 vs 7 ± 8, P = .002) and white blood cells/mean platelet volume ratio (1.2 ± 0.5 vs 1.0 ± 0.5, P = .03) at admission were significantly higher in patients with IHCs.
NLr values were predictor of IHCs (Odds ratios [OR] 1.07, 95% CI 1.03‐1.11, P < .01). When stratified according to NLr into tertiles, the rate of IHCs was from first to third tertile was, respectively, 22%, 31%, and 58%. NLr values in the higher tertile were independent predictors of IHCs even at multivariate analysis (OR 3.7, 95% CI 1.5‐9.4, P < .01).
NLr values higher than 5 were able to predict IHCs with a sensitivity of 82% and specificity of 58%; negative predictive power was 84% (area under the ROC curve 0.73).
Conclusions
NLr is an independent predictor of IHCs in patients admitted with TTS. Admission hemogram may represent a potential tool for prediction of IHCs in TTS.
Keywords: apical ballooning syndrome, complications, hemogram, neutrophil/lymphocyte ratio, Takotsubo syndrome
1. INTRODUCTION
Takotsubo syndrome (TTS) is a form of acute heart failure featured by transient left ventricular dysfunction that can mimic acute myocardial infarction. 1 It mostly affects postmenopausal women after either a physical or an emotional stress. Recent studies showed that TTS is featured by high rate of in‐hospital complications (IHCs), mainly cardiovascular 2 and adverse events at long term. 3
Several algorithms based on the use of clinical and echocardiographic parameters have been proposed for in‐hospital risk stratification in TTS patients 2 ; less is known, however, on the prognostic role of hematological indices in TTS.
In previous studies, the neutrophil/lymphocyte ratio (NLr) has been related with the occurrence of in‐hospital cardiovascular complications and mayor cardiovascular adverse events in patients with acute coronary syndrome (ACS) 4 . 5 Aim of this study was therefore to evaluate whether the assessment of a hematological index, such as NLr at admission could be useful to predict clinical outcomes during hospitalization in TTS patients.
2. METHODS
2.1. Study population
The study included 160 consecutive patients with TTS enrolled from April 2009 to May 2018 in two Italian hospitals (Riuniti University Hospital of Foggia, Apulia, Italy and Bonomo Hospital, Andria, Apulia, Italy). The entire study population fulfilled the 2008 revised Mayo Clinic diagnostic criteria: (a) transient hypokinesis, dyskinesis, or akinesis of the left ventricular (LV) apical and/or midventricular or basal segments extending beyond a single epicardial vessel distribution territory; (b) absence of significant obstructive coronary artery disease explaining the extent of wall motion abnormalities or angiographic evidence of acute plaque rupture; (c) new electrocardiogram (ECG) abnormalities (either ST‐segment elevation and/or T‐wave inversion) or modest elevation in the cardiac troponin levels; (d) absence of pheochromocytoma or myocarditis or intracranial hemorrhage. 6
The study was held according to declaration of Helsinki and approved by local ethics committees; all participants provided a written informed consent.
2.2. Clinical examination and echocardiography
All patients underwent a clinical examination and a detailed anamnesis was collected including: age, gender, and kind of triggering stressor. Medical history, including history of neurologic disorders (cerebrovascular accidents, neurodegenerative disorders, and epilepsy), was also recorded.
A two‐dimensional Doppler Echocardiographic examination at admission, at third day, and at discharge was performed. The left ventricular ejection fraction (LVEF) was calculated using the Simpson method from the apical four‐chamber and two‐chamber view. Coronary angiography was performed in all patients at admission.
2.3. Blood tests
The complete blood cell count was performed after blood samples with K2EDTA tubes (Terumo Europe NV, Leuven, Belgium), which were always collected at the time of admission. The analysis was performed with the fully automated hematological analyzer Advia 2120 (Siemens Healthcare Diagnostics, Tarrytown NY). The local reference ranges are 150 to 400 × 109/L for the platelet count, 4.3 to 10.0 × 109/L for total white blood cells, 2.0 to 7.0 × 109/L for neutrophils, and 0.95 to 4.5 × 109/L for lymphocytes. The entire investigation was carried out using the same analyzer and the quality of results was validated throughout the study period by regular internal quality control procedures and participation in an external quality assessment scheme.
The values of NLr, white blood cell count/mean platelet volume (MPV) ratio, MPV/platelets ratio, and platelet count/lymphocyte ratio were, respectively, calculated by dividing the absolute number of each variable obtained in the same blood sample collected at admission.
2.4. Outcomes
The primary clinical endpoint was occurrence of IHCs including overall mortality, pulmonary oedema, need of invasive ventilation, cardiogenic shock, stroke, and LV thrombosis.
2.5. Statistical analysis
Continuous variables were expressed as mean ± SD and compared with Student t test or Mann‐Whitney U test as required. Categorical variables were presented as percentages and compared with χ 2 or Fisher test as required. The Kolmogorov‐Smirnov test was used to identify variables with normal distribution.
Logistic regression analysis was used to identify predictors of outcome. Forward multivariable logistic regression analysis was used for correcting for principal confounders. Predictors at univariable logistic regression with a significance value ≤0.01 were included in multivariable analysis. Odds ratios (OR) with 95% confidence interval (CI) were calculated. Linear correlations were determined by measuring the Pearson's correlation coefficient.
A P < .05 was considered as statistically significant.
3. RESULTS
3.1. Patient characteristics and in‐hospital complications
Mean age was 74 ± 12 years, mean LVEF 36 ± 8%, 11% (17 patients) were male (Table 1). Rate of IHCs occurrence was 37%, including pulmonary edema 9%, cardiogenic shock 9%, need of invasive ventilation 10%, death 8%, stroke 2.5%, and LV thrombi 6%.
TABLE 1.
Baseline features according to occurrence of IHCs
| Variable | IHCs | No IHCs | P value |
|---|---|---|---|
| Age | 78 ± 9 | 73 ± 13 | .01 |
| Male | 18% | 6% | .01 |
| Hypertension | 72% | 80% | .22 |
| Dyslipidemia | 52% | 45% | .39 |
| Obesity | 28% | 32% | .64 |
| Smoker | 15% | 13% | .71 |
| Diabetes | 34% | 21% | .07 |
| History of previous neurological disorders | 40% | 17% | .01 |
| History of rheumatic disorders | 5% | 8% | .53 |
| COPD | 35% | 27% | .27 |
| Clinical presentation | |||
| No chest pain | 42% | 25% | .02 |
| Typical chest pain | 32% | 53% | .01 |
| Atypical chest pain | 10% | 22% | .06 |
| Dyspnoea | 41% | 41% | .96 |
| Triggering stressor | |||
| Emotional stressor | 17% | 30% | .06 |
| Physical stressor | 61% | 42% | .02 |
| Infectious/inflammatory | 26% | 12% | .03 |
| Non‐infectious/inflammatory | 35% | 30% | .49 |
| No stressor | 22% | 28% | .40 |
| Echocardiographic features | |||
| LVEF at admission | 33% | 37% | .01 |
| Apical ballooning | 98% | 92% | .09 |
| Midventricular ballooning | 2% | 8% | .09 |
| Basal ballooning | 0 | 0 | — |
| ECG features at admission | |||
| Negative T waves | 48% | 50% | .83 |
| ST elevation | 52% | 43% | .28 |
| Long QT interval | 42% | 41% | .85 |
Abbreviations: IHC, in‐hospital cardiovascular complications; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; ECG, electrocardiogram.
Patients with IHCs were older (78 ± 9 vs 72 ± 13 years, P < .01), more frequently male (24% vs 6%, P = .01), more frequently presented a physical stressor (64% vs 43%, P = .02), with higher prevalence of infective/inflammatory triggers (26 vs 12% P = .03) or absence of chest pain (42% vs 25%, P = .01), and a lower LVEF at admission (33 ± 7% vs 37 ± 8%, P = .01) (Table 1).
3.2. Hemogram at admission
Patients with IHCs presented higher values of white blood cells (13 ± 5 vs 11 ± 5 103/μL, P = 0.01), neutrophils (13 ± 15 vs 9 ± 9 103/μL, P = .02), and lower values of eosinophils (0.03 ± 0.1vs 0.08 ± 0.1 103/μL P = <.01). NLr (12 ± 12 vs 7 ± 8; P = .002) and white blood cells/MPV ratio (1.2 ± 0.5 vs 1.0 ± 0.5, P = .03) were significantly higher in patients with IHCs (Table 2).
TABLE 2.
Admission hemogram parameters according to occurrence of IHCs
| Variable | IHCs | No IHCs | P value |
|---|---|---|---|
| RBC × 106/μL | 4.5 ± 1.5 | 4.4 ± 0.7 | .47 |
| HB, g/dL | 12 ± 2 | 12 ± 2 | .98 |
| HCT, % | 38 ± 6 | 37 ± 5 | .50 |
| MCV, fL | 88 ± 6 | 87 ± 8 | .18 |
| MCH, pg | 29 ± 3 | 29 ± 3 | .58 |
| MCHC, g/dL | 33 ± 2 | 33 ± 1 | .09 |
| RDW, % | 15 ± 1 | 15 ± 2 | .33 |
| PLT 103/μL | 247 ± 87 | 254 ± 124 | .72 |
| MPV, fL | 11 ± 1 | 11 ± 1 | .35 |
| PCT, % | 0.27 ± 0.09 | 0.27 ± 0.12 | .86 |
| PDW, fL | 15 ± 3 | 14 ± 3 | .56 |
| NEUTR × 103/μL | 13 ± 15 | 9 ± 9 | .02 |
| LYN × 103/μL | 1.7 ± 1.6 | 1.8 ± 1.2 | .63 |
| EOS × 103/μL | 0.03 ± 0.1 | 0.08 ± 0.1 | <.01 |
| BAS × 103/μL | 0.07 ± 0.34 | 0.02 ± 0.02 | .19 |
| WBC × 103/μL | 13 ± 5 | 11 ± 5 | .01 |
| WBC/MPV ratio | 1.16 ± 0.48 | 0.97 ± 0.48 | .03 |
| NEUT/LYN ratio | 12 ± 12 | 7 ± 8 | .01 |
| MPV/PLT ratio | 0.05 ± 0.02 | 0.05 ± 0.03 | .93 |
| PLT/LYN ratio | 267 ± 222 | 208 ± 266 | .14 |
Abbreviations: BAS, basophil; EOS, eosinophil; HB, hemoglobin; HCT, hematocrit; IHC, in‐hospital cardiovascular complications; Lyn, lymphocyte; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; Neutr, neutrophil; PDW, platelet distribution width; PLT, platelet; RBC, red blood cell; RDW, red cells distribution width; WBC, white blood cell.
3.3. Predictive value of NLr
NLr values were significant predictors of IHCs (OR 1.07, 95% CI 1.03‐1.11, P < .01) and significantly correlated with hospital stay duration (r = 0.34, P < .001) (Figure 1). When stratified according NLr tertiles, the rate of IHCs occurrence was 22%, 31%, and 58% (Figure 2). NLr values in the higher tertile were independent predictors of IHCs at multivariate forward stepwise logistic regression analysis (OR 3.7, 95% CI 1.5‐9.4, P < .01) (Table 3).
FIGURE 1.
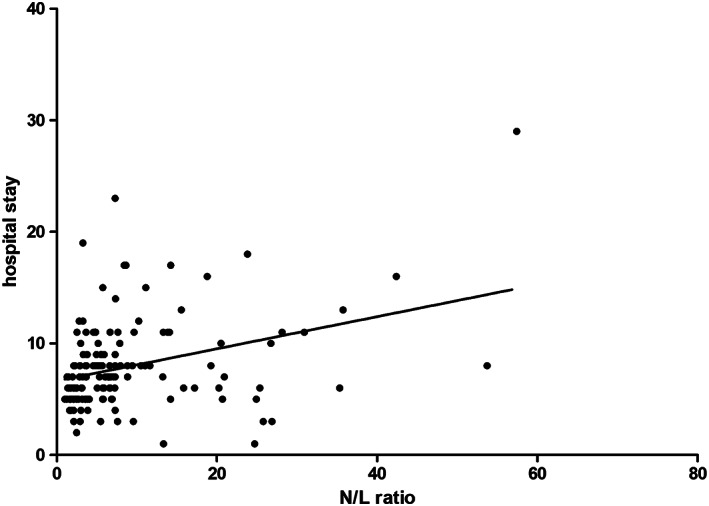
Linear correlation between NLr and hospital stay duration (days). N/Lr, neutrophil/lymphocyte ratio
FIGURE 2.
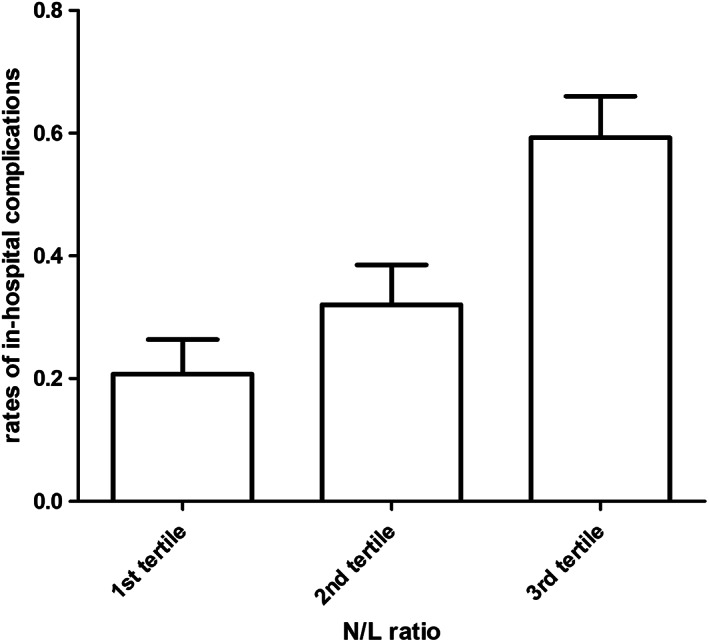
In‐hospital cardiovascular events stratified according to neutrophils/lymphocytes ratio in tertiles
TABLE 3.
Univariate and multivariate forward stepwise logistic regression analysis: predictors of in‐hospital cardiovascular complications
| Univariate OR | 95% CI | P | Multivariate OR | 95% CI | P | |
|---|---|---|---|---|---|---|
| NLr (upper tertile vs lower) | 5.4 | 2.3‐12.7 | .0001 | 3.7 | 1.5‐9.4 | .0051 |
| Systolic pressure at admission (mm Hg) | 0.96 | 0.94‐0.98 | <.0001 | 1.0 | 0.9‐1.0 | .0029 |
| Neurologic disease | 3.3 | 1.6‐7.0 | .0015 | |||
| Age (y) | 1.05 | 1.01‐1.09 | .0050 | |||
| Male gender | 3.5 | 1.2‐10.0 | .0203 | |||
| Eosinophils (103/μL) | 0.0003 | <0.0001‐0.1 | .0063 | |||
| LVEF at admission | 0.0022 | <0.0001‐0.2 | .0059 | |||
| WBC (103/μL) | 1.1 | 1.02‐1.17 | .0146 | |||
| Typical chest pain | 0.4 | 0.2‐0.8 | .0118 |
Abbreviations: LVEF, left ventricular ejection fraction; NLr, neutrophil/lymphocyte ratio; OR, odds ratio; WBC, white blood cells.
NLr values >5 were able to predict the occurrence of IHCs with a sensitivity of 82% and a specificity of 58%; negative predictive power was 84%, positive predictive power 54%, accuracy 63%. NLr area under the ROC curve was 0.73 (95% CI 0.65‐0.79, P < .01) (Figure 3).
FIGURE 3.
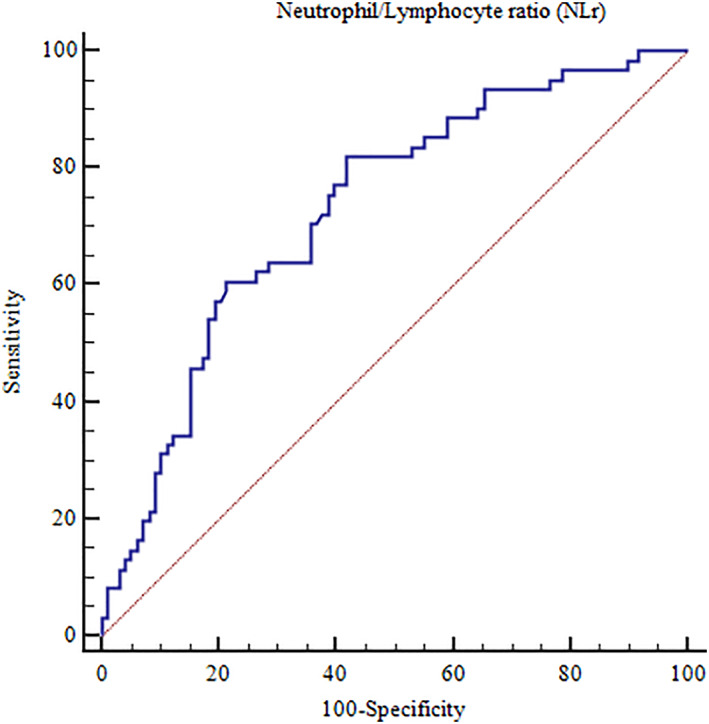
Area under the ROC curve of neutrophils/lymphocytes ratio and occurrence of in‐hospital complications in patients with Takotsubo Syndrome
4. DISCUSSION
To the best of our knowledge, this is one of the first studies showing possible correlations between hemogram parameters and IHCs in TTS. Main findings of the study are the followings:
1) Patients that experienced IHCs presented higher values of white blood cells and neutrophils at admission;
2) NLr is an independent predictor of IHCs;
3) When stratified according to NLr tertiles, values in the higher tertile were independent predictors of IHCs.
TTS has been for a long time considered a benign disease, because of the completely reversible nature of the distinct wall motion abnormalities. However, several clinical studies have shown that TTS is a form of acute heart failure that can be associated with life‐threatening complications. 7 Therefore, an early risk stratification with simple and fast clinical tools is crucial for clinical management of these patients.
The role of hemogram parameters in cardiovascular diseases has been extensively studied and a relationship between several inflammatory markers and cardiovascular diseases has been established mainly in the context of coronary artery disease. 8 Recent studies found that several subtypes of leukocytes compared with the total leukocyte count and specific subtype, including neutrophils, lymphocytes, and monocytes, have a better predictive value for cardiovascular events. 4 , 5 , 9 Lymphocytes regulate the inflammatory response and have an anti‐atherosclerotic role in which regulatory T‐cell, a subclass of lymphocyte, may have an inhibitory effect on atherosclerosis. 10 Lymphocyte populations and population's ratios may be associated with early clinical presentation of coronary artery disease 11 or poor clinical outcome. 12
Neutrophils represent the first type of white cells that is generally observed, following damage, in myocardial tissue. 13 They also play an important role in the process of destabilization of the atherosclerotic plaque. Neutrophil count, however, is easily affected by individual variables, such as blood volume. Neutrophils secrete inflammatory mediators that can lead to vascular wall degeneration. 14 Moreover, in an experimental study, Kim et al 15 found that catecholamines may increase the neutrophil (PMN)‐dependent inflammatory response to cell damage. Indeed, prolonged systemic exposure of epinephrine resulted in persistent PMN trafficking.
High levels of white blood cells have been associated with an increased risk of mortality in patients with ACS. 16 In any case, the total number of circulating leukocytes is the result of the combination of several variables, including genetics, gender, and age. A previous study also showed that a low lymphocyte count served as an early marker of physiologic stress and systemic collapse secondary to myocardial ischemia mediated by cortisol release. 17 Increased cortisol and catecholamine levels result in a reduction in the relative level of lymphocytes. 18 , 19
Platelets levels in term of platelet counts and MPV may be higher in ACS as a result and a precipitating factor of inflammatory response. Megakaryocyte could be stimulated by several inflammatory mediators and present accelerated proliferation and platelet‐production. On the other hand, platelets can release mediators that promote the progression of atherosclerosis 20 . 21 As a matter of fact, platelet/lymphocyte ratio and white blood cells/MPV ratio have been found to be predictors of in‐hospital adverse events during ACS 22 . 23 In TTS patients, Pirzer el al found that expression of CD62P on platelets at hospital admission was lower in TTS patients vs acute myocardial infarction 24 ; Nunez‐Gil et al, however, found no differences regarding platelet reactivity between the two populations. 25 In the present study white blood cells/MPV ratio was statistically higher in TTS patients with IHCs, while no differences were found in term of platelet/lymphocyte ratio.
A systemic inflammatory activation can be observed in TTS as well as in ACS. We previously found that, in the acute and sub‐acute phase of TTS, serum levels of anti‐inflammatory interleukins (IL‐2, IL‐4, IL‐10) are higher when compared with ACS patients, 26 suggesting a potential chronic inflammation among these patients. However, during the acute phase of TTS, increased levels of catecholamines through IL‐6 mechanism may result into persistent neutrophil inflammatory response to cell damage and relative reduction of lymphocytes levels. 27
Murakami et al, 28 in a Japanese registry of 107 patients with TTS, previously found that white blood cell count and brain natriuretic peptide were independent predictors of IHCs. NT‐pro‐BNP levels higher than median were found to be associated with 30‐day major adverse cardiovascular events. 29 Additional clinical and echocardiographic parameters associated with IHCs in previous TTS cohorts were right ventricular involvement, LVEF, male sex, history of neurological disorder, and diabetes. 2 , 30 Recently, physical triggers have also been associated with a worse in and out‐of‐hospital outcome, especially in case of inflammatory response. 31 These data are in line with the present study where patients with IHCs had a higher prevalence of physical triggers, mainly inflammatory.
When compared to other parameters, NLr predicted adverse events in ACS. In a meta‐analysis on 10 245 patients with ST‐elevation acute myocardial infarction after percutaneous angioplasty, Zhang et al 32 found a significant association between NLr and both hospitalization and adverse events including angina pectoris, heart failure, and major adverse cardiovascular events. The ratio between neutrophil and lymphocyte may thus reflect this inflammatory mechanism and represent a simple tool for clinicians for risk stratification of IHCs.
The question whether lymphocyte anomalies are induced by catecholamine surge or myocardial dysfunction, however, still remains unresolved, deserving further future investigations.
5. LIMITATIONS
These are preliminary data to be confirmed in larger cohorts of patients. Further and more adequately powered prospective studies are warranted to clarify the assay standardization, the optimal cutoff, and the prognostic value of NLr in association with other biomarkers and clinical scores.
Prior to admission hemograms were unknown and a comparison with admission hemograms was not possible; baseline conditions associated with immunomodulation or chronic leukocytosis were not ruled out.
6. CONCLUSIONS
NLr is an independent predictor of IHCs in patients admitted with TTS. Admission hemogram may be an easy and fast tool for risk stratification of IHCs in TTS.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHORS CONTRIBUTION
All authors have read and approved the manuscript.
Santoro F, Guastafierro F, Zimotti T, et al. Neutrophil/lymphocyte ratio predicts in‐hospital complications in Takotsubo syndrome. Results from a prospective multi‐center registry. Clin Cardiol. 2020;43:1294–1300. 10.1002/clc.23442
Contributor Information
Francesco Santoro, Email: dr.francesco.santoro.it@gmail.com.
Francesca Guastafierro, Email: f.guastafierro@gmail.com.
Tecla Zimotti, Email: tecla.zim93@gmail.com.
Adriana Mallardi, Email: adriana.mallardi3@gmail.com.
Alessandra Leopizzi, Email: alessandraleopizzi91@gmail.com.
Michele Cannone, Email: cannonecard@alice.it.
Matteo Di Biase, Email: dibiama@gmail.com.
Natale Daniele Brunetti, Email: natale.brunetti@unifg.it.
REFERENCES
- 1. Dias A, Núñez Gil IJ, Santoro F, et al. Takotsubo syndrome: state‐of‐the‐art review by an expert panel–part 1. Cardiovasc Revasc Med. 2019;20(1):70‐79. [DOI] [PubMed] [Google Scholar]
- 2. Santoro F, Núñez Gil IJ, Stiermaier T, et al. Assessment of the German and Italian stress cardiomyopathy score for risk stratification for in‐hospital complications in patients with Takotsubo syndrome. JAMA Cardiol. 2019;4:892 10.1001/jamacardio.2019.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stiermaier T, Moeller C, Oehler K, et al. Long‐term excess mortality in takotsubo cardiomyopathy:predictors, causes and clinical consequences. Eur J Heart Fail. 2016;18:650‐656. [DOI] [PubMed] [Google Scholar]
- 4. Arbel Y, Finkelstein A, Halkin A, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225:456‐460. [DOI] [PubMed] [Google Scholar]
- 5. Dentali F, Nigro O, Squizzato A, et al. Impact of neutrophils to lymphocytes ratio on major clinical outcomes in patients with acute coronary syndromes: a systematic review and meta‐analysis of the literature. Int J Cardiol. 2018;266:31‐37. [DOI] [PubMed] [Google Scholar]
- 6. Madhavan M, Prasad A. Proposed Mayo Clinic criteria for the diagnosis of Tako‐Tsubo cardiomyopathy and long‐term prognosis. Herz. 2010;35:240‐243. [DOI] [PubMed] [Google Scholar]
- 7. Madhavan M, Rihal CS, Lerman A, Prasad A. Acute heart failure in apical ballooning syndrome (TakoTsubo/ stress cardiomyopathy): clinical correlates and Mayo Clinic risk score. J Am Coll Cardiol. 2011;57:1400‐1401. [DOI] [PubMed] [Google Scholar]
- 8. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685‐1695. [DOI] [PubMed] [Google Scholar]
- 9. Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11:55‐59. [DOI] [PubMed] [Google Scholar]
- 10. Simpson E, Cantor H. Regulation of the immune response by subclasses of T lymphocytes. II. The effect of adult thymectomy upon humoral and cellular responses in mice. Eur J Immunol. 1975;5:337‐343. [DOI] [PubMed] [Google Scholar]
- 11. Brunetti ND, D'Antuono C, Rana M, D'Arienzo G, de Gennaro L, di Biase M. Lymphocyte subset characterization in patients with early clinical presentation of coronary heart disease. J Thromb Thrombolysis. 2012;34:475‐482. [DOI] [PubMed] [Google Scholar]
- 12. Brunetti ND. Hot stuff’: inflammatory lymphocyte populations in acute coronary syndrome. Cell Mol Immunol. 2015;12:513‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shen XH, Chen Q, Shi Y, Li HW. Association of neutrophil/lymphocyte ratio with long‐term mortality after ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Chin Med J (Engl). 2010;123:3438‐3443. [PubMed] [Google Scholar]
- 14. Ikeda U, Ikeda M, Oohara T, Kano S, Yaginuma T. Mitogenic action of interleukin‐1 alpha on vascular smooth muscle cells mediated by PDGF. Atherosclerosis. 1990;84:183‐188. [DOI] [PubMed] [Google Scholar]
- 15. Kim MH, Gorouhi F, Ramirez S, et al. Catecholamine stress alters neutrophil trafficking and impairs wound healing by β2‐adrenergic receptor‐mediated upregulation of IL‐6. J Invest Dermatol. 2014;134:809‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Núñez J, Fácila L, Llàcer A, et al. Prognostic value of white blood cell count in acute myocardial infarction: long‐term mortality. Rev Esp Cardiol. 2005;58:631‐639. [PubMed] [Google Scholar]
- 17. Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol. 1997;79:812‐814. [DOI] [PubMed] [Google Scholar]
- 18. Thomson SP, McMahon LJ, Nugent CA. Endogenous cortisol: a regulator of the number of lymphocytes in peripheral blood. Clin Immunol Immunopathol. 1980;17:506‐514. [DOI] [PubMed] [Google Scholar]
- 19. Cook‐Mills JM, Cohen RL, Perlman RL, Chambers DA. Inhibition of lymphocyte activation by catecholamines: evidence for a non‐classical mechanism of catecholamine action. Immunology. 1995;85:544‐549. [PMC free article] [PubMed] [Google Scholar]
- 20. Aukrust P, Waehre T, Damås JK, Gullestad L, Solum NO. Inflammatory role of platelets in acute coronary syndromes. Heart. 2001;86:605‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furman MI, Benoit SE, Barnard MR, et al. Increased platelet reactivity and circulating monocyte‐platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol. 1998;31:352‐358. [DOI] [PubMed] [Google Scholar]
- 22. Li W, Liu Q, Tang Y. Platelet to lymphocyte ratio in the prediction of adverse outcomes after acute coronary syndrome: a meta‐analysis. Sci Rep. 2017;7:40426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dehghani MR, Rezaei Y, Taghipour‐Sani L. White blood cell count to mean platelet volume ratio as a novel non‐invasive marker predicting long‐term outcomes in patients with non‐ST elevation acute coronary syndrome. Cardiol J. 2015;22:437‐445. [DOI] [PubMed] [Google Scholar]
- 24. Pirzer R, Elmas E, Haghi D, et al. Platelet and monocyte activity markers and mediators of inflammation in Takotsubo cardiomyopathy. Heart Vessels. 2012;27:186‐192. [DOI] [PubMed] [Google Scholar]
- 25. Núñez‐Gil IJ, Bernardo E, Feltes G, et al. Platelet function in Takotsubo cardiomyopathy. J Thromb Thrombolysis. 2015;39:452‐458. [DOI] [PubMed] [Google Scholar]
- 26. Santoro F, Costantino MD, Guastafierro F, et al. Inflammatory patterns in Takotsubo cardiomyopathy and acute coronary syndrome: a propensity score matched analysis. Atherosclerosis. 2018;274:157‐161. [DOI] [PubMed] [Google Scholar]
- 27. Santoro F, Tarantino N, Ferraretti A, et al. Serum interleukin 6 and 10 levels in Takotsubo cardiomyopathy: increased admission levels may predict adverse events at follow‐up. Atherosclerosis. 2016;254:28‐34. [DOI] [PubMed] [Google Scholar]
- 28. Murakami T, Yoshikawa T, Maekawa Y, et al. Characterization of predictors of in‐hospital cardiac complications of takotsubo cardiomyopathy: multi‐center registry from Tokyo CCU network. J Cardiol. 2014;63:269‐273. [DOI] [PubMed] [Google Scholar]
- 29. Stiermaier T, Santoro F, Graf T, et al. Prognostic value of N‐terminal pro‐B‐type natriuretic peptide in Takotsubo syndrome. Clin Res Cardiol. 2018;107:597‐606. [DOI] [PubMed] [Google Scholar]
- 30. Citro R, Bossone E, Parodi G, et al. Takotsubo Italian network investigators. Clinical profile and in‐hospital outcome of Caucasian patients with takotsubo syndrome and right ventricular involvement. Int J Cardiol. 2016;219:455‐461. [DOI] [PubMed] [Google Scholar]
- 31. Uribarri A, Núñez‐Gil IJ, Conty DA, et al. Short‐ and long‐term prognosis of patients with Takotsubo syndrome based on different triggers: importance of the physical nature. J Am Heart Assoc. 2019;8:e013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang S, Diao J, Qi C, et al. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: a meta‐analysis. BMC Cardiovasc Disord. 2018;18:75. [DOI] [PMC free article] [PubMed] [Google Scholar]


