Abstract
Purpose of Review
Although somewhat rare, upper extremity compressive neuropathies can occur in the pediatric and adolescent populations due to various etiologies. Some of the most common conditions seen include thoracic outlet syndrome, supracondylar process syndrome, cubital tunnel syndrome with subluxation of the ulnar nerve, and carpal tunnel syndrome. This review will focus on these diagnoses and how to address them in the pediatric and adolescent populations.
Recent Findings
Due to the rarity of upper extremity compressive neuropathies in the pediatric and adolescent populations, substantial advancement in the literature does not routinely occur. However, recent literature has found a difference in the rate of various subtypes of thoracic outlet syndrome in children versus adults. Additionally, cubital tunnel syndrome associated with ulnar nerve subluxation/instability has recently been found to have better outcomes following surgical decompression of the ulnar nerve and transposition than those with stable ulnar nerves.
Summary
In summary, this review provides the most recent knowledge surrounding upper extremity compressive and entrapment neuropathies in the pediatric and adolescent populations.
Electronic supplementary material
The online version of this article (10.1007/s12178-020-09666-4) contains supplementary material, which is available to authorized users.
Keywords: Thoracic outlet syndrome, Cubital tunnel syndrome, Supracondylar process, Carpal tunnel syndrome, Mucopolysaccharidoses, Lipofibromatous hamartoma
Introduction
Although rare, upper extremity compressive neuropathies can and do occur in the pediatric and adolescent populations due to various etiologies. Some of the most common conditions seen in these populations include thoracic outlet syndrome, supracondylar process syndrome, cubital tunnel syndrome with subluxation of the ulnar nerve, and carpal tunnel syndrome which is typically associated with mucopolysaccharidoses or overgrowth conditions. Much of the available literature has substantial overlap with adult patients. However, the timing of presentation as well as differences in signs and symptoms do exist.
Thoracic Outlet Syndrome
Thoracic outlet syndrome (TOS) is an infrequent diagnosis in the pediatric and adolescent populations, with approximately 20% of all TOS cases occurring in patients under the age of 25 [1]. The general population incidence is between 0.3–2% [1]. TOS typically presents in middle-aged adults and is more common in females than males, with an approximate 3:1 ratio [1]. In the pediatric and adolescent population, TOS more commonly presents in female patients with incident ratios reported to range from approximately 9:5 to 4:1 female to male ratios [2••, 3••, 4, 5•, 6, 7]. The vast majority of literature available for TOS is based on the adult population. However, in recent years, there have been multiple pediatric-based studies exploring how TOS manifests itself and the long-term outcomes of interventions in the pediatric and adolescent populations [2••, 3••, 4, 5•, 6–8].
Presentation
TOS occurs due to compression of the nervous, arterial, and/or venous structures in the thoracic outlet, often secondary to anatomic abnormalities and/or repetitive stress or trauma.
The presentation of TOS in the pediatric and adolescent populations is somewhat different from that in the adult population. In the pediatric and adolescent populations, venous TOS (vTOS) is the most common subtype followed by neurogenic TOS (nTOS), the most common subtype in adults, and then arterial TOS (aTOS). Arterial TOS is the rarest TOS subtype in both the pediatric and adolescent population as well as in the adult population.
Risk factors for vTOS include recurrent activity, prolonged shoulder abduction, trauma, the presence of a cervical rib (Fig. 1), thrombophilia, smoking, sports-related injuries, hypercoagulability, playing a musical instrument that requires abduction of the arm (i.e., violin), illicit drug use, and oral contraceptive medication in females [2••, 7]. nTOS risk factors include a cervical rib, an elongated C7 transverse process, deformity of the first rib, overuse with repetitive overhead motion, high energy trauma, low energy trauma, substantial participation in sports activities (particularly sports that utilize the upper extremity substantially such as volleyball, baseball, etc.), a sports-related injury, hypercoagulability, and playing a musical instrument in an abducted position (i.e., violin) [2••, 3••, 5•, 6, 9]. aTOS mainly presents secondary to the presence of a cervical rib but can also result from a sports-related injury [2••]. Tumors can lead to any type of TOS and must be in the differential diagnosis of etiologies. For example, an osteochondroma from a rib can lead to compression of any of the structures in the thoracic outlet.
Fig. 1.
14-year-old female with pain and right thoracic outlet syndrome attributed to cervical rib. (Courtesy of Shriners Hospital for Children, Philadelphia). a. Decreased right arm abduction secondary to pain. b. X-ray demonstrates cervical rib with intervening synchondrosis. c. CT scan better delineates cervical rib and synchondrosis. d. Supraclavicular approach with brachial plexus tented over cervical rib. e. Exposure and resection of cervical rib. f. Resected cervical rib and synchondrosis. g. Resolution of pain and full shoulder motion
The clinical presentation of TOS varies based on the subtype of TOS. vTOS commonly presents with upper extremity swelling, discoloration of the extremity, tenderness, pain, and/or edema [2••, 4, 7, 8]. vTOS may also present with concurrent neurologic symptoms, such as arm paresthesias, pain with arm elevation, or both [2••, 7]. nTOS commonly presents with upper extremity pain, particularly of the neck, shoulder, arm, and hand. The pain is often exacerbated with arm elevation and/or abduction. Additionally, patients may experience numbness, weakness, muscle atrophy, tenderness to palpation over the brachial plexus, and/or tingling [2••, 4, 6]. nTOS can present with concurrent vTOS symptoms of superficial mottling and/or diffuse swelling [6]. aTOS presents with upper extremity fatigue, coldness of the hand or fingers, shoulder pain, and/or weakness and dark coloration of the hand [2••, 4].
Examination
A thorough history consistent with reproducible symptoms is imperative in aiding to obtain an accurate diagnosis of TOS. Provocative maneuvers performed during the physical examination can also greatly assist in determining the diagnosis. nTOS commonly has a positive Roos’ maneuver, positive Tinel’s sign in the posterior triangle, positive upper limb tension test, a positive Wright’s test, and a positive Adson’s test (Table 1) [3••, 4, 5•, 6]. aTOS typically has a positive Adson’s test and Wright’s test. An upper extremity arterial duplex ultrasound and a computed tomography (CT) angiogram or arteriographic evaluation are typically obtained to confirm the diagnosis [2••, 4, 9]. If vTOS is suspected based on the clinical presentation, an upper extremity venous duplex ultrasound is the first conformational test followed by a targeted venography evaluation [2••].
Table 1.
Maneuvers and tests for the physical examination of suspected thoracic outlet syndrome
| Test/maneuver | Description of test | Thoracic outlet syndrome subtype tested |
|---|---|---|
| Roos’ maneuver | Have the patient abduct their arms to 90° and externally rotate the shoulder with the elbow flexed into a football goal shape. Have the patient repeatedly open and close their hands in this position. Any signs of fatigue, paresthesia, change in radial pulse, or inability to maintain the arms in that shape is considered a positive result. | Neurogenic |
| Tinel’s sign in posterior triangle | With the patient seated, have their arms at the side of their body. Tap the supraclavicular fossa with a reflex hammer or the index and middle fingers. If the patient reports tenderness or reproduction of symptoms, the sign is considered positive. | Neurogenic |
| Upper limb tension test | With the patient in a supine position, depress the shoulder and abduct the arm to 110° and flex the elbow to 90°. Laterally rotate the patient’s shoulder, extend the wrist and fingers, and then slowly extend the elbow. A reproduction of symptoms is considered a positive result. | Neurogenic |
| Wright’s test | With the patient seated, palpate the radial pulse and then abduct and externally rotate the shoulder to 90–100° with the elbow flexed at 90°. A change in the radial pulse is a positive result of the test. | Neurogenic, Arterial |
| Adson’s test | With the patient seated, palpate the radial pulse and then abduct to ~ 30° with slight extension. With the arm in this position, have the patient hyperextend their neck and turn their head toward the affected (and abducted) arm. A change in the radial pulse is a positive result of the test. | Neurogenic, Arterial |
Treatment
Conservative treatment is the initial treatment modality unless there is an anatomic variant compressing the structures in the thoracic outlet, such as a cervical rib or tumor. This includes the incorporation of physical/occupational therapy, limiting overhead motions, and rest for at least 6 months prior to any surgical interventions. However, patients with anatomic variants and those who fail conservative treatment may greatly benefit from surgical intervention to resolve their symptoms (Fig. 1). The most common operative intervention for all three subtypes of TOS is a first rib resection with or without a scalenectomy and/or a brachial plexus neurolysis [2••]. This procedure, as well as the majority of the operative interventions listed below, is typically carried out through a supraclavicular incision.
The interventions that are performed in addition to the first rib resection vary based on the TOS subtype. vTOS treatment includes endovascular interventions such as the creation of an arteriovenous fistula during the first rib resection procedure, thrombolysis and thrombectomy pre-first rib resection, and/or balloon angioplasty post-first rib resection [2••]. aTOS treatment may include a subclavian arterial bypass using a graft, such as a saphenous vein or prosthetic graft [2••]. nTOS has a larger range of treatment options which typically are centered around removing any compressive tissue present abutting the nerves in the thoracic outlet. This can include resection of a tumor, removal of a cervical rib (Fig. 1), resection of a first rib, resection of prominent transverse processes, division of Sibson’s fascia, scalenectomy of the middle and/or anterior scalene muscles, pectoralis minor release (which utilizes a secondary incision into the subcoracoid space), coracocostal ligament excision (which utilizes an incision into the subcoracoid space), and/or exploration of the infraclavicular brachial plexus (which utilizes an incision into the subcoracoid space) [2••, 3••, 5•, 6].
Outcomes
The long-term outcomes following treatment of TOS are somewhat conflicting. Matos et al. reported on 68 pediatric and adolescent patients and noted 100% resolution in arterial cases at 3 and 6 months [2••]. The vTOS patients also had excellent outcomes with 87% and 95% of patients having resolution of symptoms at 3 and 6 months, respectively. The authors noted resolution rates of 71% and 90% in nTOS patients at 3 and 6 months, respectively. Overall, 94% of the cohort had complete resolution of their symptoms at 1-year post-operatively. All patients that experienced TOS secondary to sports-related injuries were able to resume their sport at a competitive level, and three of the four musicians in the cohort were able to return to playing their musical instrument [2••].
Hong et al. reported on 12 pediatric and adolescent patients and found that patients that underwent surgical intervention for nTOS had more modest results [6]. The authors noted that only 43% of patients had complete resolution, 14% had more than 90% resolution, 36% had more than 50% resolution, and 7% had only 50% resolution of pain. Tingling and numbness symptoms resolved completely in only half of the patients and were decreased in an additional 4%. Fourteen percent of patients noted that numbness and tingling were only present with heavy activity [6].
Ransom et al. examined a study population of 54 adolescents and reported their outcomes from surgical treatment of nTOS [3••]. The authors found that only 44% of patients were able to return to full activities without limitations with another 28% able to return to activities with some limitations. The authors did note, however, that the Visual Analog Score (VAS) and Single Assessment Numeric Evaluation (SANE) scores were improved following operative interventions [3••].
Shakarchi et al. also reported a series of nTOS cases in 14 pediatric and adolescent patients. Three patients improved clinically with conservative treatment [5•]. Of the patients that underwent an operative intervention, all but one experienced complete resolution of their symptoms. The one patient with persistent symptoms did experience partial resolution of his symptoms and was able to return to day-to-day activities. The authors noted that a late recurrence of symptoms occurred in five of the nTOS cases at 2, 2.5, 3, 4, and 10 years post-operatively, all of which were managed and treated with a course of physical therapy [5•].
Complications
Complications following surgical intervention of TOS can occur and can be life-threatening. These include pneumothorax, major bleeding, deep venous thrombosis formation, and additional nerve or vessel damage. Additionally, Horner syndrome and recurrence can occur. Patients with nTOS have higher complication rates, particularly associated with an increase in post-operative pain [2••, 3••, 5•, 6].
Supracondylar Process Syndrome
The supracondylar process is a bony anomaly that presents as a spur over the anteromedial aspect of the distal humerus, approximately 5 cm proximal to the medial epicondyle. The spur typically points downward and has a fibrous, ligamentous connection to the medial epicondyle, termed the ligament of Struthers. The brachial artery and median nerve may course beneath the ligament of Struthers, leading to compression.
The presence of a symptomatic supracondylar process is rare with an incidence rate of 0.1–2.7% in the general population, with an unknown rate for the isolated pediatric and adolescent populations [10]. The finding of a supracondylar process in the pediatric and adolescent populations is often incidental during radiographs obtained for a traumatic injury. In the recent literature, there have been two case reports of 18-year-old athletes with supracondylar process syndrome. One pertains to a professional-level tennis player and the other a fast-pitch softball player [11, 12]. When the supracondylar process is found with associated symptoms, it is termed supracondylar process syndrome. Opanova et al. summarized the literature pertaining to supracondylar process syndrome in 43 cases, 37 of which were treated operatively for symptom relief [13]. The authors found that the majority of cases presented with a palpable supracondylar process, symptoms of median nerve compression, and pain and tenderness to palpation over the medial aspect of the elbow [13].
Presentation
Symptoms associated with supracondylar process syndrome include paresthesias, weakness, and muscle atrophy [11, 13, 14, 15••, 16••, 17]. Pain can also be present and is most commonly localized to the medial aspect of the elbow joint [11, 13, 15••]. Vascular symptoms, such as ischemic pain and claudication in the forearm, may occur [14]. Symptoms typically worsen when there is local pressure applied or with repetitive activities. Maneuvers that create compression at the supracondylar process, such as elbow extension and forearm pronation, can also provoke symptoms. The onset and worsening of symptoms are gradual in the vast majority of cases [11, 13, 15••, 17].
While the majority of patients with supracondylar process syndrome have symptoms related to compression of the median nerve, some patients have symptoms due to compression or irritation of the ulnar nerve. These symptoms include numbness and pain along the ulnar aspect of the forearm and into the ring and small fingers [16••, 17].
Thompson et al. reported a case of an 18-year-old fast-pitch softball player who experienced 2 days of cyanosis of the left index finger [12]. The affected left hand was the patient’s catching hand. The patient experienced pain and numbness over the pad of the left index finger in addition to cyanosis but demonstrated normal motor function. All pulses, including the brachial, radial, and ulnar arteries, were equal and present with no history of vasospastic symptoms. Arteriography revealed a brachial artery thrombus under the supracondylar process, and photoplethysmographic waveforms revealed monophasic waveforms only in the affected digit [12].
In the distal brachial and radial arteries, arteriography showed a non-occluding embolus, while the digital arteries of the affected left index finger could not be visualized. The patient was treated with thrombolytic therapy to resolve the arterial symptoms and subsequently underwent a resection of the supracondylar process. During the excision, impingement was noted on the brachial artery particularly during elbow extension and wrist supination and pronation, which were surmised to be the movements associated with the repetitive motion of catching a softball [12].
Examination
Patients with supracondylar process syndrome often have a positive Tinel’s sign just distal to the supracondylar process, as well as tenderness to palpation in this region [13, 14, 15••, 16••]. The supracondylar process is, in most cases, palpable through the skin, particularly in young children and adolescents [11, 14, 15••, 17]. Provocative maneuvers, in particular maneuvers of supination, pronation, and flexion, can reproduce the patient’s symptoms and assist in ruling out any other sources of nerve entrapment or compression around the elbow [11, 13, 14, 17].
The presence of a supracondylar process can be confirmed on plain radiographs of the elbow or humerus [13, 14, 15••, 16••, 17]. Electrodiagnostic studies, as well as advanced imaging with a magnetic resonance imaging (MRI), CT, or ultrasound, can help to determine if the median nerve is underneath the supracondylar process and the ligament of Struthers [11, 14, 15••, 16••].
Treatment
Conservative treatment of supracondylar process syndrome involves splint immobilization of the affected extremity, activity modifications, and physical/occupational therapy [13, 14, 17]. If there is no resolution of symptoms with these measures, operative intervention should be considered.
Operative management involves excision of the supracondylar process and the ligament of Struthers. Typically, the procedure is performed utilizing an open incision to assess the median nerve, which usually demonstrates a normal appearance [11–13, 15••, 16••, 17]. Bain et al. have proposed a minimally invasive procedure utilizing endoscopic techniques in order to resect the supracondylar process and the ligament of Struthers [14]. This minimally invasive method involves a small incision just distal to the supracondylar process followed by blunt dissection to the level of the deep fascia. After the deep fascia is incised, an arthroscope is introduced into the surgical site, and the supracondylar process and ligament of Struthers are identified and resected utilizing Kerrison rongeurs and scissors, respectively. Post-operatively, the patient is only immobilized in a sling [14].
Outcomes
Outcomes following the surgical management of supracondylar process syndrome are typically excellent with complete resolution of symptoms in the vast majority of cases [11, 13, 15••, 16••, 17]. Pedret et al. reported on a patient who was able to return to playing professional tennis 2 months post-operatively, and Tzaveas et al. reported complete symptom resolution in their case report at 2 days post-operatively [11, 17].
Excision of the supracondylar process and ligament of Struthers has also been shown to relieve all ulnar nerve paresthesia symptoms. However, persistent weakness may still occur [13, 16••, 17].
Complications
Complications following operative intervention for supracondylar process syndrome are somewhat rare but can include persistence of symptoms including pain and paresthesias. Complete excision of the supracondylar process and ligament of Struthers can decrease the chance of persistent symptomatology.
Cubital Tunnel Syndrome
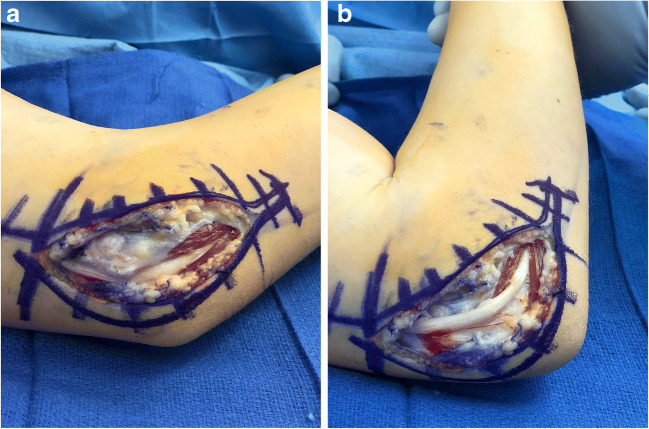
Cubital tunnel syndrome (CuTS) is typically regarded as the result of compression of the ulnar nerve at the elbow at different locations. However, irritation of the nerve leading to symptoms can also occur due to subluxation of the ulnar nerve, typically over the medial epicondyle (Fig. 2 and Video 1).
Fig. 2.
Ulnar nerve subluxation. a. Note the nerve is posterior to the medial epicondyle lying in the groove when the elbow is somewhat extended. b. Note the nerve has subluxated to lie anterior to the medial epicondyle upon flexion of the elbow. (Courtesy of Joshua M. Abzug, MD)
Presentation
The clinical presentation of CuTS in the pediatric and adolescent population is most commonly pain at the medial elbow, numbness, and paresthesias in the ulnar nerve distribution [18–20]. The presence and severity of pain, numbness, and tingling symptoms can vary with different activities and level of activity from person to person [21••]. While prolonged driving with the elbows flexed is a common activity leading to symptoms in adults, children and adolescents more often complain of symptoms related to excessive reading or video gameplay, both of which also require substantial elbow flexion. Hand weakness has also been reported as a symptom of CuTS in the pediatric population. [21••, 22•]
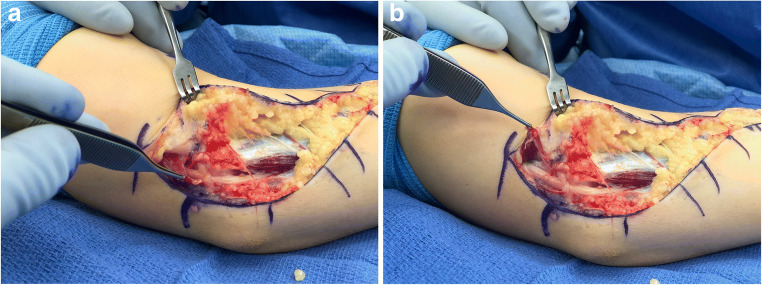
CuTS in children and adolescents is often idiopathic but may also be secondary to sports-related overuse of the upper extremity, which occurs most commonly in overhead repetitive motion or throwing athletes. Traumatic injury, such as a medial epicondyle fracture or elbow dislocation can also contribute to its onset [18, 20, 21••, 22•, 23]. Children and adolescents may also complain of ulnar nerve symptoms due to progressive deformity about the elbow, as seen in tardy ulnar nerve syndrome associated with cubitus valgus. CuTS can also be associated with ulnar nerve subluxation, where patients commonly complain of a “snapping” or “popping” sensation about the medial aspect of the elbow [20, 22•] (Fig. 2 and Video 1). Lastly, anatomical variants, such as the presence of an anconeous epitrochlearis, can cause compression of the ulnar nerve, leading to a clinical presentation analogous to CuTS (Fig. 3).
Fig. 3.
14-year-old female with complaints of numbness and tingling in the ulnar nerve distribution. a. Intraoperative photograph of an anconeus epitrochlearis muscle compressing the ulnar nerve. b. Resection of the anconeus epitrochlearis. (Courtesy of Joshua M. Abzug, MD)
Examination
CuTS often presents on examination with a positive Tinel’s sign about the ulnar nerve at the elbow and a positive elbow flexion compression test [18–20, 21••, 22•]. The physical examination should also assess the elbow for any cubitus varus or cubitus valgus deformities as well as for ulnar nerve subluxation or instability [18–20, 22•] (Video 1).
Electrodiagnostic testing, including electromyography and nerve conduction studies assessing the ulnar nerve, should be obtained, but it is important to note that the studies are frequently false-negative, particularly in the pediatric and adolescent populations [18, 20, 21••, 22•, 23]. Radiographic studies, including advanced imaging, can be obtained to evaluate bony anatomy if there is a prior trauma history or to assess for anatomical variants, such as anconeus epitrochlearis. Dynamic ultrasound is particularly useful to assess for subluxation or instability of the ulnar nerve.
Treatment
Conservative treatment of CuTS should include activity modification, anti-inflammatory medication, elbow immobilization in a partially extended position, and physical/occupational therapy [20, 21••]. Operative intervention should begin with an in situ decompression as long as the ulnar nerve does not subluxate during a passive range of elbow motion. If subluxation occurs, an anterior subcutaneous ulnar nerve transposition should be performed [18, 20, 21••, 22•]. If an anconeus epitrochlearis is visualized, it should be excised (Fig. 3).
Outcomes
The majority of pediatric and adolescent patients diagnosed with CuTS have excellent outcomes following treatment. Quinn et al. reported the outcomes of seven patients following surgical management, ages 15 to 18 years old [21••]. Five patients reported 100% satisfaction on the Michigan Hand Questionnaire, while the other two reported 83% and 75% satisfaction following their surgeries, for an overall average satisfaction of 94% [21••]. Furthermore, five patients were able to return to their sport at the same level after surgical intervention. One was able to continue with baseball but switched from being a pitcher to a second baseman following surgery, and one patient was unable to return to his sport after an anterior subcutaneous transposition procedure [21••].
Stuz et al. treated nine extremities non-operatively from a cohort of 39 patients: three went on to complete symptom resolution, one had persistent symptoms, and five were followed with serial clinical exams [20]. Four of these five patients ultimately opted for operative intervention. The other 30 patients in the cohort underwent surgical intervention, and the vast majority reported excellent outcomes without complication. Following operative intervention, the number of persistent positive Tinel’s signs and positive elbow flexion compression tests decreased, and both the Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and VAS scores were significantly improved. The authors did note that four patients had developed worsening symptoms post-operatively and were treated with a second surgery [20].
Henn et al. found that patients between the ages of 12 and 30 who presented with ulnar nerve subluxation/instability were significantly less likely to experience persistent symptoms following an anterior nerve transposition [22•]. These patients were also found to have a significantly higher VAS score than their stable ulnar nerve counterparts following operative management of CuTS [22•].
Complications
Most patients treated for CuTS do not have complications. However, persistent or worsening symptoms may occur for patients that have an incomplete release, persistent subluxation, or continued compression of the nerve due to failure to recognize anatomic variants such as an anconeous epitrochlearis.
Carpal Tunnel Syndrome
Carpal tunnel syndrome (CTS) is the most common compressive neuropathy in the adult population [24]. In contrast, CTS is fairly rare in the pediatric and adolescent populations. While the majority of adult cases of CTS are idiopathic in nature, the vast majority of pediatric and adolescent cases are secondary to an anatomic anomaly (ganglion cyst, accessory muscle, or bony anomaly), trauma, or a congenital condition such as a metabolic or vascular disorder [25–27, 28••]. In a study of 20 pediatric patients with CTS by Batdorf et al., 50% of patients developed CTS due to overuse from playing a musical instrument, 35% had an idiopathic diagnosis, and 15% developed CTS due to sports participation [29]. Other studies have documented pediatric and adolescent CTS associated with diabetes mellitus type 1, brachial plexus birth injuries, camptodactyly, hypothyroidism, and Klippel-Feil syndrome [25, 26].
The primary metabolic disorder that results in pediatric CTS includes mucopolysaccharidoses (MPS). The various forms of MPS are X-linked, inherited metabolic conditions that are the result of lysosomal enzyme deficiencies during the breakdown of glycosaminoglycan (GAG) [30]. The MPS forms are classified as subtypes I (also known as Hurler, Hurler-Scheie, and Scheie syndrome), II (Hunter syndrome), III (Sanfilippo syndrome), IV (Morquio syndrome), VI (Maroteaux-Lamy syndrome), VII (Sly syndrome), and IX (Natowicz syndrome) with each subtype being the result of a different enzyme deficiency (Table 2). The accumulation of GAG without a catabolic method of removal due to the enzyme deficiency can result in central nervous system impairment, thickened skin, skeletal abnormalities, respiratory problems, cardiac issues, and mental impairment [30–32]. Children with an MPS diagnosis have an incidence of CTS of nearly 100% in MPS subtypes I (Hurler, Hurler-Scheie, Scheie), II (Hunter), and VI (Marotraux-Lamy), all three of which cause an accumulation of dermatan sulfate and either heparan sulfate or chondroitin sulfate as a result of their enzyme deficiency. These buildups cause thickened fascicles in nerves and lead to CTS development [32, 33••, 34].
Table 2.
Mucopolysaccharidosis subtypes, syndrome names, and associated enzymes
| Subtype of MPS | Syndrome name | Accumulation of: | Associated enzyme |
|---|---|---|---|
| MPS I | Hurler, Hurler-Scheie, Scheie | Dermatan sulfate and/or heparan sulfate | α-L-iduronidase |
| MPS II | Hunter | Dermatan sulfate and/or heparan sulfate | Iduronate sulfatase |
| MPS III | Sanfillipo | Heparan sulfate | N-sulfoglucosamine sulfohydrolase |
| MPS IV | Morquio | Chondroitin sulfate | Arylsulfatase B |
| MPS VI | Marotraux-Lamy | Dermatan sulfate and/or chondroitin sulfate | Arylsulfatase |
| MPS VII | Sly | Dermatan sulfate, heparan sulfate, and/or chondroitin sulfate | β-glucuronodase |
| MPS IX | Natowicz | Hyaluronic acid | Hyaluronidase |
An additional condition that can cause a pediatric or adolescent patient to develop CTS is the presence of a lipofibromatous hamartoma (LFH) (Fig. 4). A LFH is a rare tumor that affects the peripheral nerves, most often in the upper extremity, and is most often benign [35–40]. LFH typically presents as a slowly growing, non-tender mass in the distribution of the affected nerve, most commonly the median nerve, which results in carpal tunnel syndrome [35–40]. The condition is more commonly seen in females than males and is rarely seen outside of the pediatric and adolescent populations [36, 38]. The presence of a LFH can be concomitant with the presence of macrodactyly, another overgrowth disorder [35–39] (Fig. 5). LFH causes the overgrowth of fat and fibrous tissues which leads to the peripheral nerve enlargement [35–40]. There are two main suspected etiologies of LFH, including repetitive microtrauma to the median nerve from the transverse carpal ligament and congenital deformity causing the formation of a LFH [35–39].
Fig. 4.
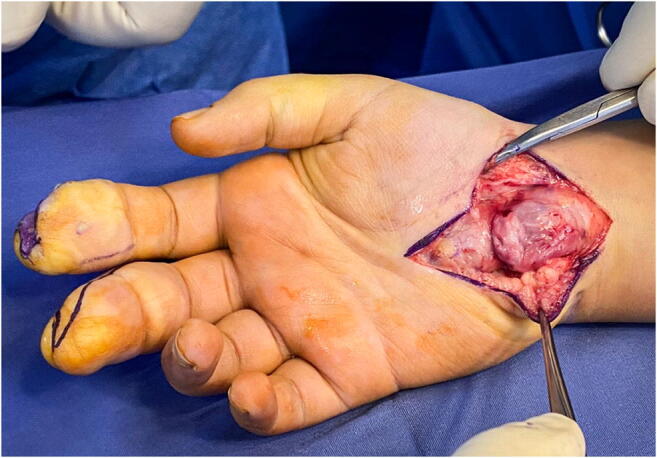
Lipofibromatous hamartoma of the median nerve. (Courtesy of Shriners Hospital for Children, Philadelphia)
Fig. 5.

Lipofibromatous hamartoma of the median nerve associated with macrodactyly. (Courtesy of Shriners Hospital for Children, Philadelphia)
Presentation
The clinical presentation of CTS in the pediatric and adolescent populations without a metabolic disorder or LFH is often analogous to the adult population and includes symptoms of numbness, paresthesias, pain, weakness of the thenar muscles, nocturnal symptoms, sensory loss to touch and pinprick, and hand fatigue [25, 26, 29]. Young children may bypass the involved digits or bite on them due to a lack of sensation.
The clinical presentation of CTS in children with MPS is frequently more subtle, likely in part due to the often-limited communication ability of the patient. CTS in these cases often presents bilaterally with an asymmetrical degree of severity [41]. Some symptoms that have been reported in the literature include irritability and crying when the hands are being touched, pain, clumsiness, gnawing of the hands, frequent bouts of crying (particularly at night), general agitation, a positive Tinel’s sign at the wrist, withdrawing of the hands, alteration in the child’s playing pattern, increased difficulty with fine motor skills, picking up hot items without pain, claw-like appearance of the hand, and stiffness of the fingers [33••, 41, 42]. Due to the nature of MPS, it is often the caregiver that must identify these symptoms and any changes in these symptoms. The age of onset of the symptoms and the diagnosis of CTS can vary depending on the MPS subtype. Patel et al. found that MPS I (Hurler, Hurler-Scheie, Scheie) and MPS II (Hunter) were diagnosed at an average of 5.3 years of age, MPS IV (Morquio) was diagnosed at an average of 9.5 years of age, and MPS VI (Marotraux-Lamy) was diagnosed at an average of 7.4 years of age [33••].
The clinical presentation of LFH can vary from an asymptomatic and non-tender mass, most likely on the volar aspect of the hand, to presenting with the typical symptoms of CTS including pain, paresthesias, thenar muscle weakness, and numbness [35–38, 40]. The most telling diagnostic aspect of LFH comes from the clinical presentation of the enlarged nerve and/or hand/digits.
Examination
The physical examination in older children and adolescents should include the provocative tests performed in adults, such as Phalen’s test, carpal compression test (Durkan’s test), and Tinel’s sign [29, 33••]. Two-point discrimination and monofilament testing, quite reliable at ages 6 and up for two-point discrimination and uniformly reliable for ages 5 and up for monofilament testing, should be performed but has been found to remain normal despite the presence of CTS [29, 43]. One should also always assess for the presence of thenar muscle atrophy [25, 26, 29].
Electrodiagnostic testing can provide more insight into the impairment of both the motor and sensory functions of the nerve. However, the results can be inconclusive or difficult to obtain as the examination is not well tolerated by children, particularly young children [28••, 29]. Ultrasound has emerged as an ideal imaging modality for the diagnosis of CTS in the pediatric population due to its non-invasive nature and accuracy.
In comparison to electromyography and nerve conduction studies, ultrasound performed in the adult population has been shown to have a comparable accuracy in diagnosing CTS with a sensitivity of 65–97%, a specificity of 73–98%, and a positive predictive value of 79–97% [28••, 44]. The cross-sectional area of the median nerve at the proximal inlet is the most accurate measurement point. However, the sensitivity of ultrasound increases by 10–20% when the entire carpal tunnel is scanned. Ultrasound can also be utilized to visualize anatomic anomalies, such as ganglion cysts, accessory muscles, or bony anomalies that may be contributing to the development of CTS [28••].
For patients with MPS, there is some disagreement in the literature as to when testing and monitoring for CTS should occur. Patel et al. found that a systematic review of the literature resulted in five of six papers recommending clinical screening for CTS starting at the time of MPS diagnosis [33••]. The recommended frequency of subsequent screening also varies from every 3 months, every 6 months, every year, and every 2 years. Screening should include a physical examination, electrodiagnostic testing if the child can tolerate it, and an ultrasound [33••, 41]. Ultrasound scanning has been found to have a sensitivity of 92% in pediatric patients with MPS types I, II, and VI [28••, 32]. Electromyography timing is debated as it is often not well tolerated by young children [33••, 41]. Patel et al. found that four of six papers recommended the initiation of electromyography screening at 4–5 years of age, while two papers suggested screening starting at the time of MPS diagnosis. Subsequent evaluations should occur with a frequency of every 1–2 years after the initial screening, as suggested by five of the seven papers, or every 3–7 years after the initial screening, as suggested by the other two papers [33••]. Our practice is to screen early and re-evaluate often as early detection and treatment of CTS is ideal in order to prevent permanent nerve damage [45].
If there is a very high suspicion that a patient is presenting with a mass that could be LFH, the most effective diagnostic tool is an MRI. The nerve enlargement from the LFH causes the nerve to present with a coaxial cable appearance in the axial plane and a spaghetti appearance in the coronal and sagittal planes [35–40]. In addition to the utilization of an MRI for diagnosis, ultrasound visualization of the nerve can also be utilized to confirm LFH [35, 37–40]. When macrodactyly is involved, radiographs should be obtained in order to visualize the bony structures [35, 38].
Treatment
In pediatric and adolescent patients that have CTS unrelated to MPS or overgrowth conditions, the treatment is analogous to the adult population. Conservative care should be the initial treatment consisting of activity modification, physical/occupational therapy, anti-inflammatory medication, and the utilization of orthoses. Corticosteroid injections can be utilized to confirm the diagnosis and treat it. A carpal tunnel release, utilizing any method, should be performed if conservative treatments are not successful. However, the surgeon must be aware of the potential for anatomical variations. Therefore, one may consider a technique that provides optimal visualization instead of a less invasive approach [29].
Patients with an MPS or overgrowth diagnosis that have CTS are most often treated with an open carpal tunnel release. It is important to note that MPS patients often present with concomitant trigger fingers which can be treated with A1 and/or A3 pulley release in conjunction with their carpal tunnel release [45, 46].
Due to the rarity of LFH cases, a consensus on the best method of treatment has yet to be determined. Conservative treatment is used for asymptomatic patients and involves ongoing observation in the clinical setting [35–37]. One of the most common procedures for the management of LFH is a carpal tunnel release in order to alleviate any CTS symptoms caused by the presence of the LFH [35, 36, 38, 40]. In addition to a carpal tunnel release, operative management can include a biopsy of the LFH or excision of the tumor with or without excision of the affected section of the nerve [35–39]. If macrodactyly is present in conjunction with an LFH, debulking is often pursued as the operative management for both macrodactyly and the LFH [35, 36, 39].
Outcomes
Batdorf et al. reported on pediatric and adolescent patients that had CTS unrelated to MPS or overgrowth diagnoses [29]. Of the 31 wrists studied, 30 were initially treated conservatively with orthoses [29]. Of the conservatively treated wrists, nine had complete symptom resolution, 18 had partial resolution, and three had no resolution [29]. In those that experienced incomplete resolution of their CTS with orthoses, 11 wrists underwent a steroid injection which resulted in complete resolution of symptoms for five, partial resolution of symptoms for five, and no resolution of symptoms in one [29]. Ten wrists that were not successfully treated with orthoses and/or steroid injections were managed surgically, with five patients achieving complete symptom resolution and five achieving partial symptom resolution. This study also sent a long-term follow-up survey, including the Boston Carpal Tunnel Questionnaire, to patients that were treated in their study. The results of the follow-up survey showed that there was no development of carpal tunnel syndrome in the contralateral wrist, and only one patient experienced a recurrence of their carpal tunnel syndrome. The responses to the Boston Carpal Tunnel Questionnaire pertaining to symptom severity revealed that 27% were asymptomatic, 18% had mild symptoms, 45% had moderate symptoms, and 9% had severe symptoms. Regarding the results of functional severity, 27% were asymptomatic, 45% were mild, and 45% were moderate [29].
There is limited data regarding the outcomes of MPS patients treated for CTS. Williams et al. found that in eight patients treated with carpal tunnel release and concomitant trigger finger releases, four were able to return to tasks and activities with no difficulty, two had occasional difficulty, and two noted frequent difficulties. Caregivers reported that patients had spontaneous hand use and increased dexterity as compared with preoperatively [45]. The ultrasound findings have been found to be unchanged between the pre- and post-operative periods [32]. However, electromyography is typically improved from pre-operative results [32, 41]. It is also important to note that enzyme replacement therapy has been shown to have no effect on the development of CTS in patients with MPS (positive or negative effect), and many who received enzyme replacement therapy required operative management of their CTS [41].
The rare nature of LFH and the variability of treatment options provide very little in the way of outcomes of LFH and its treatment. Among operative treatment options for LFH, the least recommended option is excision of the tumor with the affected segment of the nerve due to the neural deficits associated with this procedure [35–38]. Treatment with carpal tunnel release has shown to help treat the CTS symptoms of LFH with far less neural risk than excision [35, 36, 38, 40]. Decompression of the nerve with microsurgical resection/debulking of LFH while preserving and protecting the median nerve has demonstrated good results in alleviating symptoms and decreasing the size of LFH [35–37, 39].
Complications
Persistence and recurrence of symptoms can occur, particularly, in patients that have an inadequate release and those that do not modify their activities. Optimal visualization of the median nerve, as is present during an open procedure, may limit the risk of incompletely releasing the transverse carpal ligament or missing an anatomical variant which can cause compression of the median nerve.
Conclusions
Compressive neuropathies affecting the upper extremity, though somewhat rare in the pediatric and adolescent populations, do occur and warrant our attention and understanding. These aforementioned neuropathies do not always present themselves in the same manner as they would present in the adult population. Therefore, it is important to know how to assess for these diagnoses in the pediatric and adolescent populations. Lastly, the treatment may be different in the pediatric and adolescent populations. This must be recognized, as failure to treat the condition appropriately may lead to inadequate treatment or early recurrence.
Electronic Supplementary Material
Ulnar nerve subluxation. Note that elbow flexion causes the nerve to subluxate anterior to the medial epicondyle, while elbow extension causes the nerve to reduce into its normal anatomic position, posterior to the medial epicondyle. (Courtesy of Joshua M. Abzug, MD) (MOV 1243 kb)
Compliance with Ethical Standards
Conflict of Interest
Casey M. Codd and Joshua M. Abzug declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Compressive Neuropathies in the Upper Extremity
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Casey M. Codd, Email: ccodd@som.umaryland.edu
Joshua M. Abzug, Email: jabzug@som.umaryland.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Roos DB. Overview of thoracic outlet syndromes. In: Machleder HI, editor. Vascular disorders of the upper extremity. Mount Kisco, NY: Futura; 1989. pp. 155–177. [Google Scholar]
- 2.Matos JM, Gonzalez L, Kfoury E, Echeverria A, Bechara CF, Lin PH. Outcomes following operative management of thoracic outlet syndrome in pediatric patients. Vascular. 2018;26(4):410–417. doi: 10.1177/1708538117747628. [DOI] [PubMed] [Google Scholar]
- 3.•• Ransom EF, Minton HL, Young BL, He JK, Ponce BA, McGwin G, Meyer RD, Brabston EW 3rd. Intermediate and long-term outcomes following surgical decompression of neurogenic thoracic outlet syndrome in an adolescent patient population. Hand (N.Y.). 2020 [Epub ahead of print]. 10.1177/1558944719901. Outcomes following surgical intervention for neurogenic thoracic outlet syndrome in a large pediatric and adolescent cohort, both short and long term, were noted to be favorable in relieving symptoms. No statistical difference was found in quantitative measures between pre- and post-operative VAS and SANE scores. [DOI] [PMC free article] [PubMed]
- 4.Rehemutula A, Zhang L, Chen L, Chen D, Gu Y. Managing pediatric thoracic outlet syndrome. Ital J Pediatr. 2015;41:22. doi: 10.1186/s13052-015-0128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Shakarchi J, Jaipersad A, Morgan R, Pherwani A. Early and late outcomes of surgery for neurogenic thoracic outlet syndrome in adolescents. Ann Vasc Surg. 2020;63:332–335. doi: 10.1016/j.avsg.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Hong J, Pisapia JM, Ali ZS, Heuer AJ, Alexander E, Heuer GG, Zager EL. Long-term outcomes after surgical treatment of pediatric neurogenic thoracic outlet syndrome. J Neurosurg Pediatr. 2018;21(1):54–64. doi: 10.3171/2017.7.PEDS17257. [DOI] [PubMed] [Google Scholar]
- 7.Trenor CC, 3rd, Fisher JG, Fa K, Sparks EA, Duzan J, Harney K, Dillon B, Menard M, Modi BP. Paget-Schroetter syndrome in 21 children: outcomes after multidisciplinary care. J Pediatr. 2015;166(6):1493–7.e1. doi: 10.1016/j.jpeds.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 8.Arthur LG, Teich S, Hogan M, Caniano DA, Smead W. Pediatric thoracic outlet syndrome: a disorder with serious vascular complications. J Pediatr Surg. 2008;43(6):1089–1094. doi: 10.1016/j.jpedsurg.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 9.Funakoshi T, Furushima K, Kusano H, Itoh Y, Miyamoto A, Sugawara M, Itoh Y. First-rib stress fracture in overhead throwing athletes. J Bone Joint Surg Am. 2019;101(10):896–903. doi: 10.2106/JBJS.18.01375. [DOI] [PubMed] [Google Scholar]
- 10.Camerlinck M, Vanhoenacker FM, Kiekens G. Ultrasound demonstration of Struthers’ ligament. J Clin Ultrasound. 2010;38(9):499–502. doi: 10.1002/jcu.20700. [DOI] [PubMed] [Google Scholar]
- 11.Pedret C, Balius R, Alomar X, Vilaró J, Ruiz-Cotorro A, Minoves M. Stress fracture of the supracondylar process of the humerus in a professional tennis player. Clin J Sport Med. 2015;25(1):e20–e22. doi: 10.1097/JSM.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 12.Thompson JK, Edwards JD. Supracondylar process of the humerus causing brachial artery compression and digital embolization in a fast-pitch softball player. A case report. Vasc Endovasc Surg. 2005;39(5):445–448. doi: 10.1177/153857440503900510. [DOI] [PubMed] [Google Scholar]
- 13.Opanova MI, Atkinson RE. Supracondylar process syndrome: case report and literature review. J Hand Surg Am. 2014;39(6):1130–1135. doi: 10.1016/j.jhsa.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 14.Bain G, Gupta P, Phadnis J, Singhi PK. Endoscopic excision of supracondylar humeral spur for decompression of the median nerve and brachial artery. Arthrosc Tech. 2016;5(1):e67–e70. doi: 10.1016/j.eats.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shon HC, Park JK, Kim DS, Kang SW, Kim KJ, Hong SH. Supracondylar process syndrome: two cases of median nerve neuropathy due to compression by the ligament of Struthers. J Pain Res. 2018;11:803–807. doi: 10.2147/JPR.S160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.May-Miller P, Robinson S, Sharma P, Shahane S. The supracondylar process: a rare case of ulnar nerve entrapment and literature review. J Hand Microsurg. 2019;11(Suppl S1):S06–S10. doi: 10.1055/s-0038-1642067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzaveas AP, Dimitriadis AG, Antoniou KI, Pazis IG, Paraskevas GK, Vrettakos AN. Supracondylar process of the humerus: a rare case with compression of the ulnar nerve. J Plast Surg Hand Surg. 2010;44(6):325–326. doi: 10.3109/02844310903123320. [DOI] [PubMed] [Google Scholar]
- 18.Boero S, Sénès FM, Catena N. Pediatric cubital tunnel syndrome by anconeus epitrochlearis: a case report. J Shoulder Elb Surg. 2009;18(2):e21–e23. doi: 10.1016/j.jse.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura M, Itsubo T, Horii E, Hayashi M, Uchiyama S, Kato H. Tardy ulnar nerve palsy caused by chronic radial head dislocation after Monteggia fracture: a report of two cases. J Pediatr Orthop B. 2016;25(5):450–453. doi: 10.1097/BPB.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 20.Stutz CM, Calfee RP, Steffen JA, Goldfarb CA. Surgical and nonsurgical treatment of cubital tunnel syndrome in pediatric and adolescent patients. J Hand Surg Am. 2012;37(4):657–662. doi: 10.1016/j.jhsa.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Quinn DP, Gu A, Greenberg JA, Fischer TJ, Merrell GA. Surgical treatment of cubital tunnel in pediatric athletes. J Hand Microsurg. 2018;10(2):82–85. doi: 10.1055/s-0038-1626685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henn CM, Patel A, Wall LB, Goldfarb CA. Outcomes following cubital tunnel surgery in young patients: the importance of nerve mobility. J Hand Surg Am. 2016;41(4):e1–e7. doi: 10.1016/j.jhsa.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Djordjevic N, Micic I, Pawaskar A, Jeon IH. Intra-articular entrapment of the ulnar nerve after acute elbow dislocation: a rare cause of flexion contracture. J Orthop Sci. 2015;20(2):418–421. doi: 10.1007/s00776-013-0459-1. [DOI] [PubMed] [Google Scholar]
- 24.Padua L, Coraci D, Erra C, Pazzaglia C, Paolasso I, Loreti C, Caliandro P, Hobson-Webb LD. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. 2016;15(12):1273–1284. doi: 10.1016/S1474-4422(16)30231-9. [DOI] [PubMed] [Google Scholar]
- 25.Davis L, Vedanarayanan VV. Carpal tunnel syndrome in children. Pediatr Neurol. 2014;50(1):57–59. doi: 10.1016/j.pediatrneurol.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Potulska-Chromik A, Lipowska M, Gawel M, Ryniewicz B, Maj E, Kostera-Pruszczyk A. Carpal tunnel syndrome in children. J Child Neurol. 2014;29(2):227–231. doi: 10.1177/0883073813504458. [DOI] [PubMed] [Google Scholar]
- 27.Chammas M, Boretto J, Burmann LM, Ramos RM, Dos Santos Neto FC, Silva JB. Carpal tunnel syndrome – part I (anatomy, physiology, etiology and diagnosis) Rev Bras Ortop. 2014;49(5):429–436. doi: 10.1016/j.rboe.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mah JK, van Alfen N. Neuromuscular ultrasound: clinical applications and diagnostic values. Can J Neurol Sci. 2018;45(6):605–619. doi: 10.1017/cjn.2018.314. [DOI] [PubMed] [Google Scholar]
- 29.Batdorf NJ, Cantwell SR, Moran SL. Idiopathic carpal tunnel syndrome in children and adolescents. J Hand Surg Am. 2015;40(4):773–777. doi: 10.1016/j.jhsa.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi H. Recent trends in mucopolysaccharidosis research. J Hum Genet. 2019;64(2):127–137. doi: 10.1038/s10038-018-0534-8. [DOI] [PubMed] [Google Scholar]
- 31.Galimerti C, Madeo A, Di Rocco M, Fiumara A. Mucopolysaccharidoses: early diagnostic signs in infants and children. Ital J Pediatr. 2018;44(Suppl 2):133. doi: 10.1186/s13052-018-0550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bäumer T, Bühring N, Schelle T, Münchau A, Muschol N. Nerve ultrasound in clinical management of carpal tunnel syndrome in mucopolysaccharidoses. Dev Med Child Neurol. 2016;58(11):1172–1179. doi: 10.1111/dmcn.13127. [DOI] [PubMed] [Google Scholar]
- 33.Patel P, Antoniou G, Clark D, Ketteridge D, Williams N. Screening for carpal tunnel syndrome in patients with mucopolysaccharidoses. J Child Neurol. 2020;35(6):410–417. doi: 10.1177/0883073820904481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bocsa C, Asavoaie C, Bucerzan S, Nascu I, Brumboiu I, Al-Khzouz C. Ultrasonographic evaluation of the median nerve at the level of the carpal tunnel outlet and mid forearm in patients with type II mucopolysaccharidosis. Med Ultrason. 2016;18(1):36–41. doi: 10.11152/mu.2013.2066.181.cob. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal S, Haase SC. Lipofibromatous hamartoma of the median nerve. J Hand Surg Am. 2013;38(2):392–397. doi: 10.1016/j.jhsa.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 36.Kini JR, Kini H, Rau A, Kamath J, Kini A. Lipofibromatous hamartoma of the median nerve in association with or without macrodactyly. Turk Patoloji Derg. 2018;34(1):87–91. doi: 10.5146/tjpath.2014.01282. [DOI] [PubMed] [Google Scholar]
- 37.Kitridis D, Dionellis P, Xarchas K, Givissi P. Giant median nerve due to hamartoma causing severe carpal tunnel syndrome. J Orthop Case Rep. 2018;8(4):57–60. doi: 10.13107/jocr.2250-0685.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohammed Saeed MA, Dawood AA, Mahmood HM. Lipofibromatous hamartoma of the median nerve with macrodactyly of middle finger. J Clin Orthop Trauma. 2019;10(6):1077–1081. doi: 10.1016/j.jcot.2019.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sirinoglu H, Sönmez A, Sav A, Numanoglu A. Lipofibromatous hamartoma of the median nerve. Ann Plast Surg. 2010;65(2):174–176. doi: 10.1097/SAP.0b013e3181c9c41b. [DOI] [PubMed] [Google Scholar]
- 40.Son ES, Kim DH. Morphological changes of the median nerve after carpal tunnel release in a median nerve lipofibromatous hamartoma. Am J Phys Med Rehabil. 2019;98(3):e24–e26. doi: 10.1097/PHM.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 41.Maincent K, Héron B, Billette de Villemeur T, Mayer M. Early detection of median nerve compression by electroneurography can improve outcome in children with mucopolysaccharidoses. Orphanet J Rare Dis. 2018;13(1):209. doi: 10.1186/s/13023-018-0937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Argenta AE, Davit A. Carpal tunnel syndrome in the setting of mucopolysaccharidosis II (Hunter syndrome) Plast Reconstr Surg Glob Open. 2017;5(8):e1477. doi: 10.1097/GOX.0000000000001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dua K, Lancaster TP, Abzug JM. Age-dependent reliability of Semmes-Weinstein and 2-point discrimination tests in children. J Pediatr Orthop. 2019;39(2):98–103. doi: 10.1097/BPO.0000000000000892. [DOI] [PubMed] [Google Scholar]
- 44.Cartwright MS, Hobson-Webb LD, Boon AJ, Alter KE, Hunt CH, Flores VH, Werner RA, Shook SJ, Thomas TD, Primack SH, Walker FO. Evidence-based guideline: neuromuscular ultrasound for the diagnosis of carpal tunnel syndrome. Muscle Nerve. 2012;46(2):287–293. doi: 10.1002/mus.23389. [DOI] [PubMed] [Google Scholar]
- 45.Williams N, Challoumas D, Eastwood DM. Does orthopaedic surgery improve quality of life and function in patients with mucopolysaccharidoses? J Child Orthop. 2017;11(4):289–297. doi: 10.1302/1863-2548.11.170042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morishita K, Petty RE. Musculoskeletal manifestations of mucopolysaccharidoses. Rheumatology (Oxford) 2011;50(Suppl 5):v19–v25. doi: 10.1093/rheumatology/ker397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ulnar nerve subluxation. Note that elbow flexion causes the nerve to subluxate anterior to the medial epicondyle, while elbow extension causes the nerve to reduce into its normal anatomic position, posterior to the medial epicondyle. (Courtesy of Joshua M. Abzug, MD) (MOV 1243 kb)