Abstract
Purpose of Review
Particularly common in collision sports such as American football or rugby, stingers are a traumatic transient neuropraxia of a cervical nerve root(s) or brachial plexus that may last anywhere from minutes to hours. This review summarizes the knowledge on the diagnosis and management of stingers in college and professional collision athletes by providing an overview of their epidemiology and pathophysiology, followed by a discussion on current treatment guidelines and return-to-play recommendations.
Recent Findings
Despite modifications to tackling technique, increasing awareness, and various equipment options, American football continues to have a high rate of cervical spine injuries, the majority of which occur in preseason and regular season competition settings. The incidence of stingers has slowly increased among collision athletes, and nearly half of all players report sustaining at least one stinger in their career. Recent studies have shown certain anatomical changes in the cervical spine are related to acute and reoccurring stingers. Most players who experience stingers do not miss practices or games.
Summary
Despite their prevalence, literature highlighting the impact of stingers on college and professional collision athletes is limited. Advances in imaging modalities and novel radiographic parameters have provided tools for screening athletes and can guide return-to-play decisions. Future research regarding appropriate screening practices for athletes with reoccurring stingers, use of protective equipment, and rehabilitation strategies are needed to identify predisposing factors, mitigate the risk of injury, and restore full functional strength and ability.
Keywords: Stinger, Burner, Brachial plexopathy, Spinal cord injury, Sport injuries, Team physician, Return-to-play
Introduction
Athletes participating in collision sports, such as rugby and American football, are at an increased risk for cervical spine trauma, including transient neurological injury [1, 2]. After forceful contact to the neck or upper extremities, stingers (also called “burners”) may occur—typically due to injury to cervical nerve roots or the brachial plexus—resulting in temporary sensory and motor deficits of one upper extremity lasting anywhere from seconds to hours [2–6]. In the competition setting, stingers are predominantly subclinical and are initially evaluated by sideline medical staff. Although their transient nature means stingers often resolve quickly, they may also be associated with prolonged muscle weakness and other long-term sequelae, which could necessitate additional medical therapy. It is therefore imperative to understand the nature of stingers to appropriately treat and prevent chronic ramifications. Accordingly, this review discusses the etiology, epidemiology, presentation, and treatment of stingers in elite competitive collision and contact athletes with a particular focus on college and professional American football players.
Epidemiology
Although the probability of sustaining a stinger injury is especially high among rugby and American football players, athletes participating in boxing, gymnastics, hockey, and weightlifting are also at an increased risk [7]. Nevertheless, due to the injury’s transient nature and playing-time concerns, and potential career implications, athletes are prone to self-underreport stingers [7–9]. Together, these factors make it difficult to ascertain the true incidence of these injuries in collision athletics [3••, 8].
Various studies suggest American football has the highest incidence of nerve injuries and accounts for the most cervical sprains [9, 10]. Stingers have been shown to be almost four times more likely to occur in games than during practice in National Collegiate Athletic Association (NCAA) athletes [11•]. From 2009 to 2015, the NCAA Injury Surveillance Program reported an injury rate of 2.04 stingers per 10,000 athlete-exposures among American football players [3••]. More recently, it was reported that stingers are the most common cervical injury among NCAA American football players, with an injury rate of 1.87 per 10,000 athlete-exposures [12]. Furthermore, a separate study of NCAA American football players found that the preseason practice injury rate was more than two times higher than the in-season practice injury rate [13]. The two possible reasons for the high preseason prevalence of stingers include (1) athletes’ lower fitness levels and (2) athlete’ unwillingness to report injuries during the regular season due to playing-time concerns and related consequences [13].
A positive correlation between stinger frequency and increased level of athletic competition has been shown in the literature [14]. One study found that 23% of incoming Division I college American football players reported a lifetime prevalence of stinger injuries, while another study revealed 50.4% of existing college American football players had experienced multiple stingers throughout their careers [15, 16]. Relatedly, a history of stinger injuries and increasing years played are also associated with recurrent stingers [16]. For instance, a study of prospective American football players at the National Football League (NFL) Scouting Combine found that 17.4% of players had a history of cervical spine injury—62.2% of which were previous stingers [17•]. Moreover, a 2014 survey of 304 American football athletes (including high school, college, and professional players) found 50.3% of players reported sustaining at least one stinger in their career [18]. Similarly, a survey of 569 high school and college rugby players found that 30–40% of players reported a history of at least one stinger [19]. These athletes experienced disproportional recovery periods—80% of players recovered in less than a day and 6% of players required at least 2 weeks for full neurologic recovery [19]. Most athletes were not forced to miss playing time since the majority of symptoms resolved within a relatively short time frame [19].
Mechanisms of Injury
Per the Seddon and Sunderland classification system, acute stinger severity can be categorized into one of three grades of peripheral nerve injury [20]. A grade 1 injury represents neurapraxia, which is characterized by preservation of axonal integrity, transient motor or sensory loss, and remyelination lasting days to weeks [20]. A grade 2 injury represents axonotmesis, which is characterized by axonal damage and Wallerian degeneration, peaking around 7 days after injury, with preservation of Schwann cells, endoneurium, perineurium, and epineurium. It is of note, however, that it may take up to 3 weeks for axonal damage to appear on electromyography (EMG) [20–22]. Lastly, a grade 3 injury represents neurotmesis, which is permanent damage or complete transection of the nerve [20].
Due to the fact that each “grade” requires different remedial measures, it is imperative to correctly categorize a stinger’s severity. In this regard, determining the mechanism of injury is crucial to identifying the appropriate severity classification. There are three proposed mechanisms by which stingers can occur: (1) direct nerve compression from a hit to the brachial plexus at Erb’s point; (2) a traction injury to the brachial plexus due to an increased neck-shoulder angle; and (3) cervical nerve root compression caused by extreme flexion or extension of the neck [23–28]. Fully comprehending these mechanisms is essential to executing a quick diagnosis and treatment plan. Figure 1 depicts the postulated mechanisms of stingers.
Fig. 1.
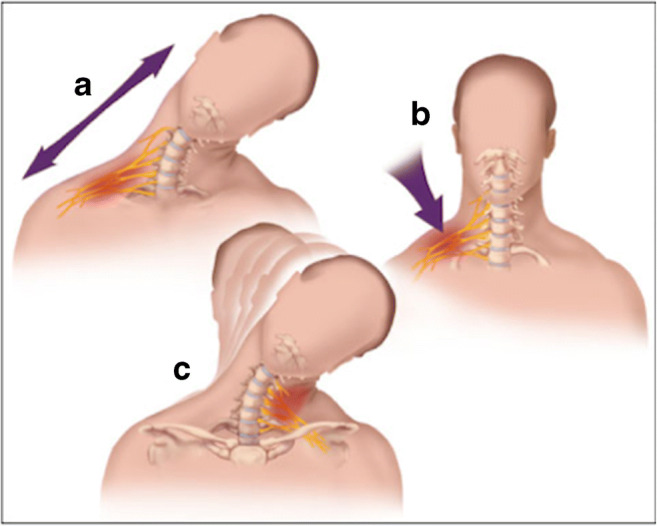
Mechanisms of stingers. Traction injury to the brachial plexus from ipsilateral shoulder depression and contralateral lateral neck flexion (a). Direct compression injury to the brachial plexus from contact to Erb’s point (b). Cervical nerve root or brachial plexus compression injury from ipsilateral lateral flexion and hyperextension (c). Reproduced with permission from [29] by Kuhlman and McKeag
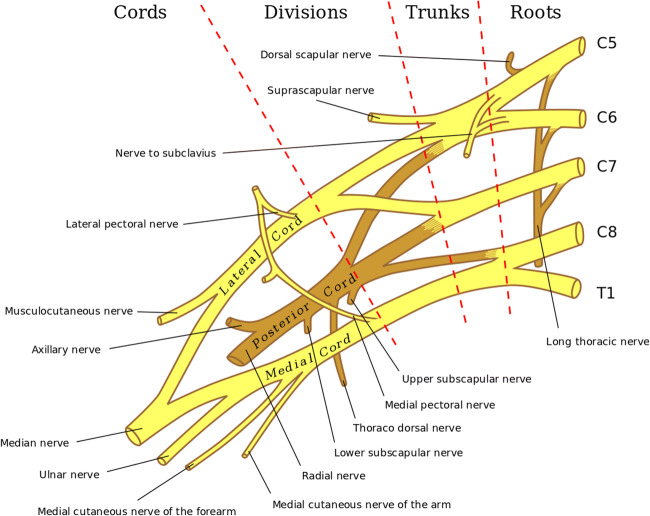
The direct compression mechanism is particularly common among American football players in advanced levels of competition, such as college and professional leagues [30]. Direct compression injuries result from forceful contact to Erb’s point (otherwise known as the nerve point of the neck). The anatomical location of Erb’s point is superior to the clavicle where the C5 and C6 nerve roots join to form a common bundle that branches off into the suprascapular nerve, nerve to the subclavius, and terminal branches stemming from the anterior and posterior divisions of the brachial plexus (Fig. 2) [27, 31]. Direct hits to Erb’s point, the most superficial location of the brachial plexus, commonly injures the C5 and part of the C6 nerve roots in the upper trunk [31]. The resulting affected muscles generally include the deltoid, supraspinatus, and biceps [32]. However, variations have been observed, with Clancy et al. reporting a case where the brachioradialis, pronator teres, and supinator were also affected [33].
Fig. 2.
Anatomy of the brachial plexus. Reproduced with modifications from the 1918 edition of Gray’s Anatomy and licensed under Creative Commons Attribution 3.0
Contrastingly, traction injuries can be caused in numerous ways. Typically, a hit sustained in a tackle causes depression of the ipsilateral shoulder and contralateral lateral neck flexion (thereby leading to an excessive stretch of the brachial plexus that results in an injury on the side of contact) [34]. Traction injuries can also occur when a player’s football helmet and facemask are used for blocking, “clothes lining,” and “spear tackling”—each of which have been prohibited in amateur and professional competition [7, 34–36]. Nevertheless, cervical spine injuries have significantly decreased over the past three decades as a result of rules banning spear tackling and tackling with the helmet’s crown [37]. Additionally, efforts have been made to teach tackling forms emphasizing the “head-up” technique wherein the front of the shoulder is the focus of contact [38, 39, 40•]. Notwithstanding these efforts, it should be noted that any tackling form increasing the angle between the neck and shoulder raises the risk of traction nerve injury [8].
Lastly, extreme flexion of the neck can cause cervical nerve root compression, which subsequently leads to stingers. Specifically, extreme neck flexion or extension can result in cervical nerve root compression in the neuroforamina [41–44]. Compression of the fixed brachial plexus between the athlete’s shoulder pad and the superior medial scapula can contribute to this mechanism [27]. Due to the fact that the narrowed foramen and its irritative effects can predispose the nerve roots to injury, hyperflexion and hyperextension injuries tend to be associated with cervical spinal stenosis [42, 45, 46]. Studies have further implicated cervical spinal stenosis in chronic stinger syndrome, which, unlike acute stingers, is often indicative of long-term structural changes in the subaxial cervical spinal canal [47, 48]. Athletes with cervical stenosis often have more stingers and recurrences than athletes without cervical stenosis [44, 46, 49].
While at baseline collision athletes are generally more likely to sustain a stinger injury due to the nature of the sport, older “veteran” collision athletes may be more predisposed due to cervical spine degeneration secondary to aging and repeated microtrauma [50]. Anatomical changes in the supporting structures may make the cervical nerve roots more susceptible to repeat injury than the brachial plexus [48]. Factors contributing to this phenomenon may include (1) insufficient protective epineurium or perineurium; (2) stiffened denticulate ligaments, which generate countertraction forces when the brachial plexus is under tension; (3) local osteophyte formation; and (4) scalene muscle hypertrophy [3, 51]. More specifically, the pathophysiologic narrowing of the vertebral foramen observed in chronic stinger syndrome has been studied and associated with cervical intervertebral disc degeneration (CIDD) [49, 52]. Hakkaku et al. reviewed magnetic resonance imaging (MRI), cervical range of motion, and isometric muscle strength in 49 college American football players to ascertain how CIDD and neck functionality relate to a history of stinger syndrome. Their results showed that the prevalence of CIDD was significantly higher, whereas the cervical ROM was remarkably lower in athletes with a history of stinger syndrome. They also demonstrated that the presence of CIDD and a reduction in cervical extension ROM were independent risk factors for stinger syndrome [52•]. Based on the aforementioned information and considering that the risk of CIDD increases with age, flexion-extension injuries are likely the leading cause of chronic stinger syndrome in aging athletes [53]. Cervical spine imaging of an athlete diagnosed and treated for this condition are shown in Figs. 3 and 4.
Fig. 3.
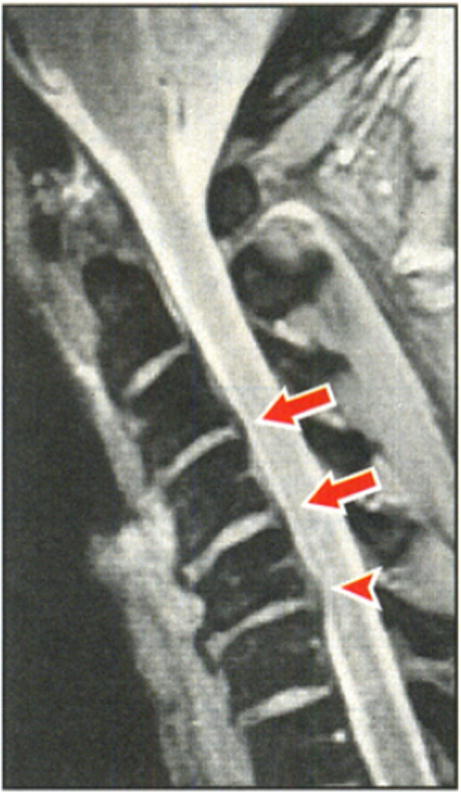
A sagittal MRI of an athlete with chronic stinger syndrome with disk bulges at C3-4 and C4-5 (arrows) and a disk herniation at C5-6 compressing the spinal cord. Reproduced with permission from [49] by Levitz et al.
Fig. 4.

An axial MRI of an athlete with chronic syndrome with narrowing of the intervertebral foramina (arrows). Due to degenerative disk disease, there is decreased space for the nerve root at the C4-5 level. Reproduced with permission from [49] by Levitz et al.
Clinical Presentation
Stingers frequently occur in practice or game settings with injured athletes generally showing a characteristic pattern of symptoms. Sharp pain and reduced range of neck motion are the two most common presenting symptoms [6, 8, 52•, 54]. Patients typically report the pain starting in the supraclavicular area and then transitioning to circumferential, non-dermatomal pain, which radiates down a unilateral arm [55]. Weakness and paresthesias are present in approximately one-third of cases with a majority lasting up to 1 day [19]. A survey of collegiate American football players who experienced stingers found that 77% reported tingling, 61% reported numbness, 44% reported weakness, and 17% reported neck pain [16]. The extent of these motor deficits varies from mild weakness to complete transient paralysis [18, 25, 56–59]. Symptoms may be purely sensory if compression preferentially involves the dorsal root ganglion [41].
The wide display of stinger symptoms can present a diagnostic challenge for the evaluating healthcare provider. Although a variety of presentations exist, stingers are exclusively unilateral. Therefore, if an athlete reports bilateral upper extremity symptoms or any lower extremity symptoms (including weakness, numbness, or tingling), a cervical cord neurapraxia of spinal cord origin should instead be suspected [60, 61]. Drakos et al. reported two cases of NFL players who initially presented with symptoms consistent with a stinger; one presented with persistent pain and difficulty with shoulder elevation and the other with neck range of motion limited by pain. Subsequent imaging of both athletes revealed a subaxial cervical spine fracture [62]. The management of these athletes (non-operative and operative treatment, respectively) therefore highlights the importance of considering fractures when diagnosing and evaluating stingers. It also emphasizes the key principle that no athlete can return to play unless he/she is neurologically normal, and have full painless range of motion of the cervical spine.
When assessing trauma to the cervical spine, the injured athlete should also be evaluated for potential vascular injuries. Even though blunt trauma-induced injury to the carotid arteries or vertebral arteries is associated with a minority of cervical trauma cases and is commonly asymptomatic, reports of headache, dizziness, nausea and vomiting, altered consciousness, dysarthria, and changes in vision are cautionary signs of vascular damage [63]. In cases of severe blunt trauma with arterial transection, clinical signs can range from asymptomatic to vertebrobasilar insufficiency and posterior circulation ischemia [64]. Rarely, injury to the carotid and vertebral arteries can result in autonomic instability, presenting with characteristic changes in heart rate, blood pressure, and temperature [65, 66].
Immediate Management
Although a stinger is often managed safely without sequelae by the sideline medical staff, it is important to always remember that assessing any traumatic cervical spine injury should follow a standardized methodology of care. Initial management of stingers in collision sport athletes is the same as managing any trauma patient with a potential cervical spine injury. The first objective is to ensure the athlete did not sustain any life-threatening traumatic injuries [67]. The initial evaluation therefore begins by ensuring airway patency, regular breathing, and adequate circulation as outlined by the Advanced Trauma Life Support (ATLS) algorithm [68]. The subsequent evaluation should follow the protocol used when assessing any member of the general population with a possible cervical spine injury [67]. Prompt remedial action by the sideline medical personnel is essential to prevent acute cervical trauma from causing precipitous neurological deterioration [68, 69]. Understanding the mechanism of injury is also essential, as it can generate an appropriate diagnosis and guide the evaluation.
Once the initial trauma assessment is completed, a thorough examination is necessary. The physical exam begins with an inspection of the neck and shoulder region to identify apparent deformities and asymmetry indicative of possible fractures or dislocations. Palpation of the cervical paraspinal muscles or the shoulder girdle may reveal stinger-induced tenderness or muscle spasm. However, because this examination technique by itself does not rule out subtler fractures or subluxation, it is imperative to conduct a detailed neurovascular examination with particular attention to the C5-T1 nerve root distributions if a stinger injury is suspected. With the contralateral upper extremity as baseline, a complete motor exam should be conducted including the following muscles: (1) supraspinatus; (2) infraspinatus; (3) deltoid; (4) biceps; (5) brachioradialis; (6) triceps; (7) serratus anterior; (8) wrist flexors; (9) wrist extensors; and (10) grip strength [70]. Any perfusion abnormalities noticed during initial examination should be further evaluated in a hospital setting with a comprehensive vascular assessment entailing blood pressure, Doppler ultrasonography, and brachial-brachial index [48]. Once major injuries have been ruled out, within pain limitations, range of motion of the upper extremities and cervical spine should be evaluated on the field. Weakness lifting the arms above shoulder-level may be indicative of a stinger, considering the shoulder stabilizers are innervated by commonly affected nerves [70]. Cervical spine range of motion includes lateral rotation, lateral flexion, forward flexion, and extension. Spurling’s test (concomitant lateral flexion with hyperextension) can irritate the nerve root in the neuroforamen and may reproduce the player’s symptoms [71]. Associated, recurrent neck pain with range of motion testing is suggestive of cervical spine injury and warrants further evaluation [48]. A negative Spurling test with a lack of neck pain is suggestive of brachial plexus stretch, a mechanism commonly seen in younger athletes [48]. The injured athlete should be asked about the location, quality, and duration of their symptoms, and a review of systems including pain, numbness, tingling, and weakness should be re-assessed in follow-up.
Athletes should be removed from competition and referred for further workup if symptoms persist and should be prohibited from returning to competition until cleared by a healthcare professional. An example would be a stinger associated with persistent weakness, neck pain, or when there is a clinical suspicion of a major injury. These symptoms would require immediate spinal imaging and close follow-up [48]. For athletes with prolonged symptoms of a stinger, imaging is warranted to rule out other acute or structural causes of nerve compression that can resemble a stinger, such as a cervical or high thoracic disc herniation [72].
Imaging and Electrodiagnostic Studies
Modalities
The medical community has yet to reach a consensus regarding the use of cervical spine imaging for a stinger injury [73, 74]. Athletes who experience a first-time, isolated stinger with immediate symptom resolution within 5 min of injury do not require cervical spine imaging or electrodiagnostic testing [48]. Cervical spine radiographs are necessary if symptoms persist longer than 1 h, symptoms localize to one nerve root, and when neck pain accompanies normal cervical spine range of motion [8]. Radiographs are also indicated in athletes who experience their second or more stinger episode [75]. This evaluation allows physicians to determine the extent of injury, structures involved, and prognosis.
The first step in imaging evaluation of a diagnosed stinger includes obtaining plain radiographs consisting of anteroposterior, lateral flexion and extension, and odontoid views of the cervical spine. This is then followed by an MRI due to its optimal resolution of visualizing the cervical spinal cord and brachial plexus [76–78]. A recent survey of expert spine surgeons regarding the use of imaging after a stinger injury revealed 69.9% would not obtain an MRI if symptoms resolved quickly after a first event; however, any subsequent stinger would require MRI [79•]. In cases where patients cannot undergo MRI, computed tomography (CT) imaging is an acceptable alternative. The advantages of CT imaging include increased sensitivity for central and foraminal stenosis [75]. Computed tomography and single-photon emission computed tomography (SPECT) scans can also expedite diagnosis if an occult cervical spine fracture is suspected [31].
Electromyography (EMG) can be used to precisely localize a site of injury and further aid in the identification of neuromuscular abnormalities if initial screening is negative [80]. EMG can be performed as early as 7 days following the onset of symptoms. However, it may take 2 to 4 weeks for axonal degeneration to occur and appear as positive sharp waves or fibrillation potentials on the EMG measurement [70]. In the context of clinical weakness, the presence or absence of denervation can aid in discrimination between neurapraxia and more severe neurological injury. Common findings on an EMG include delayed conduction and signal latencies [26, 81]. EMG can also be used to evaluate the paraspinal musculature for conduction abnormalities, which help distinguish a preganglionic nerve root avulsion injury from a postganglionic injury to the nerves of the brachial plexus [48].
Imaging as a Screening Tool
The prevalence of cervical spinal stenosis among collegiate and professional American football players further increases their already heightened stinger susceptibility [82, 83]. In fact, relative spinal stenosis has been previously reported as a pathophysiologic contributor in a young athlete with recurrent stingers [84•]. Initially, cervical spinal stenosis was defined as a Torg-Pavlov ratio of less than 0.8 [85]. In reviewing radiographs of American football players with recurrent stingers, Levitz et al. found 53% had congenital cervical canal stenosis and 93% had evidence of disk degeneration with narrowing of the intervertebral foramina [49]. Relatedly, Kelly et al. used the Torg-Pavlov ratio and foramen/intervertebral body ratio in a similar population of athletes and found a significantly higher risk of cervical spinal canal and foraminal stenosis [42]. Even though the Torg-Pavlov ratio has been found to be highly sensitive (93%), its use for screening is limited by its low positive predictive value (0.2%) [31].
Advanced imaging modalities have also provided improved screening parameters. The mean subaxial cervical space available for the spinal cord (MSCSAC) on an MRI has been shown to be a reliable tool to screen for the risk of chronic stingers [44, 47]. The MSCSAC is determined by subtracting the sagittal diameter of the spinal cord from the disc-level sagittal diameter of the spinal canal at levels C3 through C6, and calculating the average of these values. Presciutti et al. found a threshold value of 5.0 mm provides a sensitivity of 80% as a screening tool for chronic stingers [47]. A cutoff value of 4.3 mm for the MSCSAC can serve as a confirmatory test, with a specificity of 96% and 13.3 positive likelihood ratio for stingers [47]. Additionally, the MSCSAC was 20% more accurate in this application than the traditional Torg-Pavlov ratio because it accounts for chronic stinger syndrome as a process that involves the entire subaxial cervical spine [47]. Measurement of the Torg-Pavlov ratio and MSCSAC are depicted in Fig. 5.
Fig. 5.
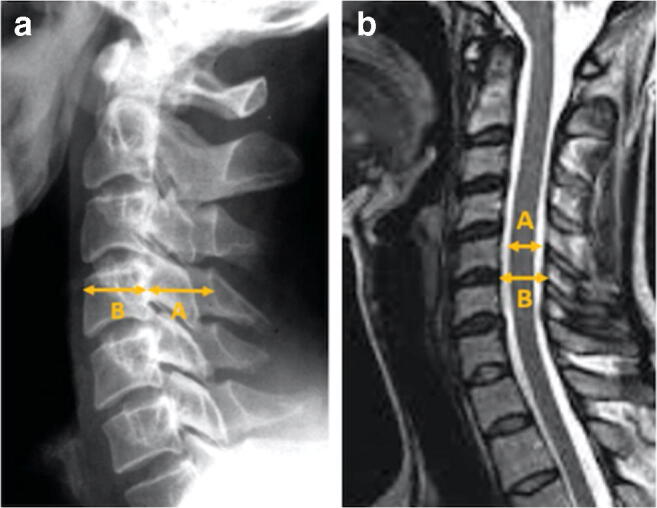
Lateral spine radiograph (a) illustrating calculation of the Torg-Pavlov ratio as the AP diameter of the spinal canal (A) divided by the AP diameter of the vertebral body (B). Sagittal T2 MRI (b) depicting the mean subaxial cervical space available for the cord as the AP diameter of the spinal canal at the disk level (A) minus the AP diameter of the spinal cord (B). Reproduced with permission from [48] by Ahearn et al.
MRI has also been explored for assessing cervical stenosis, particularly as a predictive tool for risk and severity of traumatic spinal cord injury after minor trauma. More recently, Takao et al. measured the sagittal cerebrospinal fluid column diameter on cervical spine MRIs and defined a column diameter of less than 8 mm as cervical stenosis. They found the relative risk of SCI in cervical stenosis was 124.5 times higher compared with control subjects without CSCS [86]. In another study looking at spinal cord injury, Ruegg et al. obtained three axial MRI measurements: the transverse spinal canal and cord area, the transverse and sagittal cord diameter, and the sagittal canal diameter of the cervical spine [87]. The authors calculated the cord to canal area ratio by dividing the transverse cord area by the transverse canal area, the space available for the cord by subtracting the sagittal canal diameter from the sagittal cord diameter, and the compression ratio by dividing the transverse cord diameter by its sagittal diameter. They found that the cord to canal area ratio (> 0.8) and the space available for the cord (< 1.2 mm) reliably identified patients at risk for cervical spinal cord injury after minor trauma [87]. However, consistent with previous studies, their analysis did not find any measurements that correlated significantly with injury severity [87, 88]. Nevertheless, MRI can contribute valuable insight for athletes with a history of stingers, and novel radiographic parameters such as these can be implemented in risk factor surveillance.
Current Management Practices
Non-operative Management
Stinger treatment is contingent upon the mechanism and severity of the injury. Although physical therapy can begin with the sideline physician, remedial treatment is typically monitored and modified as the athlete progresses towards recovery. Hypermobility in the mid-cervical spine can promote early degenerative changes, and a substantial portion of athletes show such changes on their MRI or CT imaging [49]. Therapy should generally emphasize postural correction and normalization of flexibility and strength imbalances [70]. An example of a specific therapy technique includes targeted myofascial stretch (including myofascial release and massage), which promotes lengthening of shortened and weakened musculature [70, 89]. Likewise, the anterior and posterior shoulder girdle can benefit from balanced exercises of its musculature including the capital and cervical flexors, middle and lower trapezius, rhomboids, thoracic extensors, and latissimus dorsi [70]. While targeting specific muscle groups can improve recovery, a structured muscle-strengthening program also contributes to the prevention of recurrent stingers [50].
Cervical collars and neck rolls may minimize the risk of reinjury, particularly in athletes with recurrent stingers, by decreasing cervical extension and lateral flexion; however, research demonstrating their effectiveness is contentious [78, 84•, 90, 91]. The decision to use a football collar is case-specific and should be based on the mechanism of injury because placing the athlete’s head in a more flexed position could increase the risk of severe cervical spine injury [84•]. Alternatively, cowboy collars incorporate additional padding, which may be protective against direct compression injuries [92]. Use of a neck collar is shown in Fig. 6. Other available methods of non-operative management are limited, although there may be situations where they are appropriate. For instance, Leung et al. reported cervical transforaminal epidural steroid injection as a successful treatment modality in a collegiate American football player with a history of recurrent stingers and persistent upper extremity symptoms [93]. The authors highlighted the importance of counseling to maintain optimal cervical posture and incorporating strengthening exercises of the cervical, thoracic, scapular, and core stabilizers [93]. Their study also illustrated the importance of case-specific rehabilitation programs designed to prevent stinger recurrence.
Fig. 6.
Common American football protective equipment. a Standard shoulder pads. b Shoulder pads with an added neck collar. Reproduced with permission from Kerr Sports, LLC
Operative Management
Even though the primary management of stingers is non-operative, individuals with residual deficits due to irreparable nerve root damage may require surgery [94]. For cases in which permanent or residual deficits are present, the primary surgical objective is to restore mobility of those muscles controlled by the injured nerves. For example, in a C5/C6 nerve root injury, restoration of elbow flexion and glenohumeral joint movement (including shoulder abduction and external rotation) would be the primary goal. Moreover, while C5 and C6 nerve roots are the most commonly injured, other nerve roots of the brachial plexus are also at risk of damage [95]. In this regard, current literature on surgical repairs to C5/C6 nerve root injuries strongly favors dual nerve transfer over traditional nerve grafting for reconstruction [96, 97]. Nevertheless, because surgery is an atypical recourse for stingers, operative intervention should be considered on an individual basis, and early consultation with a specialist is advised for timely and effective treatment.
Return-to-Play
Approximately 85% of collision athletes who experience stingers do not miss subsequent practices or games [7]. Although most stingers resolve within seconds to 24 h without any intervention, symptoms can last longer than 2 weeks [98, 99]. While there are no standardized return-to-play protocols, some timelines have been proffered as acceptable return-to-play guidelines [7, 31, 100, 101]. Traditionally, an athlete who is asymptomatic, retains full strength, and whose imaging—when it is indicated and acquired as previously outlined—shows no evidence of instability or spinal cord parachymal changes, can return-to-play [31, 80, 99]. Muscle weakness occurs in nearly a third of cases and can take five or 6 days to completely resolve [19]. In the context of sideline management, athletes may return to play within the same competition if they demonstrate a full spectrum of recovery, which includes (1) absent or minimal pain; (2) completely intact neurovascular function; (3) full cervical spine and shoulder range of motion; and (4) a negative Spurling Test [7, 41, 102]. Cervical spine stiffness or bilateral upper extremity deficits both suggest the player should be prohibited from immediate return-to-play absent further clinical evaluation, including imaging studies [31].
Athletes with persistent stinger symptoms benefit from electrodiagnostic testing to assess the affected nerves and consolidate a safe return-to-play plan. In the context of clinical weakness, an EMG that reveals moderate fibrillation potentials justifies maintaining the injured athlete away from competition and repeating an EMG [70]. However, because electromyographic changes can persist for prolonged periods after the resolution of symptoms, abnormal EMG findings alone should not be used as an exclusion criterion for return-to-play [50]. Accordingly, return-to-play is permitted when follow-up testing demonstrates (1) absence of spontaneous potentials or only scattered positive waves, and (2) polyphasic potentials without a significant increase in the motor unit firing rate, therefore suggestive of reinnervation [70]. Nonetheless, EMG studies should be considered in the broader diagnostic picture and used in the context of other clinical indicators to support the return-to-play decision.
For athletes with otherwise normal radiographic imaging, MRI, and electrodiagnostic testing, cervical spinal stenosis by itself is not a contraindication for return-to-play [84•, 103]. Functional spinal stenosis, defined as a cervical spinal canal lacking on MRI the protective cushion of the cerebrospinal fluid or, in extreme cases, causing deformity of the spinal cord, has been used by some clinicians as a contraindication for return-to-play [41, 104]. This however is not generally accepted by many physicians caring for collegiate and professional athletes. In the athlete with persistent neurologic symptoms, cervical stenosis of any degree is a reason to prohibit further participation in collision sports [50].
Return-to-play decisions should take into account the persistence of symptoms, and players with rapid resolution may proceed without cervical spine imaging [8]. Similarly, a second stinger occurring in a separate season with rapid resolution and examination within normal limits supports the athlete’s to return-to-play in the same game where the injury occurred [8]. Comparatively, when multiple stingers occur in one game, the athlete is generally prohibited from return-to-play even if the symptoms resolve [8]. Regardless of timing in the season, three or more stingers precludes return-to-play without medical intervention [30, 31, 105]. These athletes should sit out the remainder of the competition and undergo cervical spine imaging to rule out potential congenital anomalies, foraminal cervical stenosis, or further cord compromise [2, 106]. As previously mentioned, athletes with recurrent stingers generally have a high prevalence of cervical stenosis, which places them at increased risk for future spinal injuries [44, 46, 49]. Athletes with severe or persistent symptoms may also warrant exclusion from practice and competition for the remainder of the season. Accordingly, spine surgeons should engage athletes in informed discussions regarding the quality, duration, and severity of symptoms, as well as associated functional consequences, as multiple stingers may preclude a recommendation to return to contact sports, even if symptoms eventually resolve [106]. A synthesis of return-to-play recommendations from the literature are summarized in Table 1.
Table 1.
Return-to-play recommendations
| Absolute contraindications | Level of evidence* |
| Clinical | |
| Second stinger in the same game | IV |
| Persistent neurological deficits on clinical exam | IV |
| Symptoms in bilateral or multiple extremities | IV |
| Suspicion of neck injury | IV |
| Continued neck pain or discomfort | IV |
| Lack of full cervical range of motion | IV |
| Radiologic | |
| Cervical spine fracture | V |
| Ligamentous laxity or injury | V |
| Cervical spinal cord edema or abnormality | V |
| Neural compression | V |
| Evidence of spear-tackler’s spine | V |
| Multi-level fusion from Klippel-Feil syndrome | V |
| Ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis | IV |
| Evidence of rheumatoid arthritis | IV |
| Relative contraindications | Level of evidence* |
| Persistence of symptoms beyond 1 h | IV |
| Second stinger in the same season | V |
| Three or more prior episodes of stingers with full return to baseline cervical range of motion and neurological status and without an increase in baseline neck discomfort | V |
| No contraindications | Level of evidence* |
| First-time stinger that is transient (less than 1 h) with full resolution of symptoms | V |
| Second-time stinger not in the same game or season that is transient (less than 1 h) with full resolution of symptoms | V |
| Less than three previous episodes of stingers lasting less than 24 h, with full cervical range of motion and no evidence of neurological deficit | V |
| Single-level Klippel-Feil anomaly, without involvement of the C0-C1 articulation | IV |
| Spina bifida occulta | IV |
| Torg ratio < 0.8 and asymptomatic | IV |
Synthesis of return-to-play recommendations [8, 32, 43, 63, 70, 82, 106]. The level of evidence of a systematic review and meta-analyses reflects the ranking of the studies included in the review
*Based on the JBJS levels of evidence [107], level I indicates good evidence for or against recommending intervention (i.e., randomized clinical trials); level II indicates fair evidence for or against recommending intervention (i.e., prospective cohort studies); level III indicates conflicting or poor-quality evidence for or against recommending intervention (i.e., retrospective cohort and case-control studies); level IV indicates insufficient or conflicting evidence for or against intervention (i.e., case series/reports); and level V is mechanism-based reasoning, often from expert opinion (i.e., surveys)
Conclusion
Stingers, although likely underreported, are the most common cervical spine injuries in collision sports. These athletes generally show characteristic unilateral upper extremity symptoms, the majority of which spontaneously resolve with conservative treatment. Advanced imaging should be used in cases of reoccurring stingers or when symptoms persist. Decisions permitting return-to-play are contingent upon symptom resolution; a benign physical exam; and, in cases of recurrent stingers, imaging within normal limits. A history of three or more stingers is an indication to remove an athlete from competition until further evaluation and interventions can be pursued.
Author’s Contributions
All authors significantly contributed to the document and have reviewed the final manuscript.
Compliance with Ethical Standards
Conflict of Interest
There are no relevant disclosures.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Feinberg JH. Burners and stingers. Phys Med Rehabil Clin N Am. 2000;11(4):771–784. [PubMed] [Google Scholar]
- 2.Schroeder GD, Vaccaro AR. Cervical spine injuries in the athlete. J Am Acad Orthop Surg. 2016;24(9):e122–e133. doi: 10.5435/JAAOS-D-15-00716. [DOI] [PubMed] [Google Scholar]
- 3.Green J, Zuckerman SL, Dalton SL, Djoko A, Folger D, Kerr ZY. A 6-year surveillance study of “stingers” in NCAA American football. Res Sports Med. 2017;25(1):26–36. doi: 10.1080/15438627.2016.1258642. [DOI] [PubMed] [Google Scholar]
- 4.Cunnane M, Pratten M, Loughna S. A retrospective study looking at the incidence of ‘stinger injuries in professional rugby union players. Br J Sports Med. 2011;45(15):A19 LP-A19. [Google Scholar]
- 5.Toth C. Peripheral nerve injuries attributable to sport and recreation. Phys Med Rehabil Clin N Am. 2009;20(1):77–100. doi: 10.1016/j.pmr.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Shannon B, Klimkiewicz JJ. Cervical burners in the athlete. Clin Sports Med. 2002;21(1):29–35. doi: 10.1016/s0278-5919(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 7.Tosti R, Rossy W, Sanchez A, Lee SG. Burners, stingers, and other brachial plexus injuries in the contact athlete. Oper Tech Sports Med. 2016;24(4):273–277. [Google Scholar]
- 8.Carr JB, Dines JS. In: Transient brachial plexopathy (stingers/burners) BT-spinal conditions in the athlete: a clinical guide to evaluation, management and controversies. Hsu WK, Jenkins TJ, editors. Cham: Springer International Publishing; 2020. pp. 109–121. [Google Scholar]
- 9.Meron A, McMullen C, Laker SR, Currie D, Comstock RD. Epidemiology of cervical spine injuries in high school athletes over a ten-year period. PM R. 2018;10(4):365–372. doi: 10.1016/j.pmrj.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 10.DePasse JM, Durand W, Palumbo MA, Daniels AH. Sex- and sport-specific epidemiology of cervical spine injuries sustained during sporting activities. World Neurosurg. 2019;122:e540–e545. doi: 10.1016/j.wneu.2018.10.097. [DOI] [PubMed] [Google Scholar]
- 11.Deckey DG, Makovicka JL, Chung AS, Hassebrock JD, Patel KA, Tummala SV, et al. Neck and cervical spine injuries in National College Athletic Association Athletes: a 5-year epidemiologic study. Spine (Phila Pa 1976) 2020;45(1):55–64. doi: 10.1097/BRS.0000000000003220. [DOI] [PubMed] [Google Scholar]
- 12.Chung AS, Makovicka JL, Hassebrock JD, Patel KA, Tummala SV, Deckey DG, Hydrick TC, Rubel NC, Chhabra A. Epidemiology of cervical injuries in NCAA football players. Spine (Phila Pa 1976) 2019;44(12):848–854. doi: 10.1097/BRS.0000000000003008. [DOI] [PubMed] [Google Scholar]
- 13.Steiner ME, Berkstresser BD, Richardson L, Elia G, Wang F. Full-contact practice and injuries in college football. Sports Health. 2016;8(3):217–223. doi: 10.1177/1941738115626689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vereschagin KS, Wiens JJ, Fanton GS, Dillingham MF. Burners. Phys Sportsmed. 1991;19(9):96–106. [Google Scholar]
- 15.Sarac N, Haynes W, Pedroza A, Kaeding C, Borchers J. Lifetime prevalence of injuries in incoming division I collegiate football players. Phys Sportsmed. 2017;45(4):458–462. doi: 10.1080/00913847.2017.1386068. [DOI] [PubMed] [Google Scholar]
- 16.Charbonneau RME, McVeigh SA, Thompson K. Brachial neuropraxia in Canadian Atlantic University sport football players: what is the incidence of “stingers”? Clin J Sport Med. 2012;22(6):472–477. doi: 10.1097/JSM.0b013e3182699ed5. [DOI] [PubMed] [Google Scholar]
- 17.• Beaulieu-Jones BR, Rossy WH, Sanchez G, Whalen JM, Lavery KP, McHale KJ, et al. Epidemiology of injuries identified at the NFL scouting combine and their impact on performance in the national football league: evaluation of 2203 athletes from 2009 to 2015. Orthop J Sport Med. 2017;5(7) Highlights the prevalence of existing cervical spine injuries, including a predomianance of stingers, in professional athletes. In this study, performance in the NFL tended to worsen with injury history. [DOI] [PMC free article] [PubMed]
- 18.Starr HM, Anderson B, Courson R, Seiler JG. Brachial plexus injury: a descriptive study of American football. J Surg Orthop Adv. 2014;23(2):90–97. doi: 10.3113/jsoa.2014.0090. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki T, Ota C, Yoneda T, Maki N, Urayama S, Nagao M, Nagayama M, Kaketa T, Takazawa Y, Kaneko K. Incidence of stingers in young rugby players. Am J Sports Med. 2015;43(11):2809–2815. doi: 10.1177/0363546515597678. [DOI] [PubMed] [Google Scholar]
- 20.Chhabra A, Ahlawat S, Belzberg A, Andreseik G. Peripheral nerve injury grading simplified on MR neurography: as referenced to Seddon and Sunderland classifications. Indian J Radiol Imaging. 2014;24(3):217–224. doi: 10.4103/0971-3026.137025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armantrout E. Chapter 18-nerve conduction studies and needle electromyography. In: Placzek JD, Boyce DABT-OPTS (Third E, editors. Elsevier; 2017. p. 135–139.
- 22.Jain S, Gupta R. chapter 32-neural blockade with neurolytic agents. In: Waldman SD, Bloch JIBT-PM, editors. Philadelphia: W.B. Saunders; 2007. p. 343–348.
- 23.Sallis RE, Jones K, Knopp W. Burners. Phys Sportsmed. 1992;20(11):47–55. doi: 10.1080/00913847.1992.11947521. [DOI] [PubMed] [Google Scholar]
- 24.Prentice WE. Rehabilitation techniques in sports medicine. St. Louis: Mosby; 1994. [Google Scholar]
- 25.Hershman E. Brachial plexus injuries. Clin Sports Med. 1990;9(2):311–329. [PubMed] [Google Scholar]
- 26.Di Benedetto M, Markey K. Electrodiagnostic localization of traumatic upper trunk brachial plexopathy. Arch Phys Med Rehabil. 1984;65(1):15–17. [PubMed] [Google Scholar]
- 27.Markey KL, Di Benedetto M, Curl WW. Upper trunk brachial plexopathy. The stinger syndrome. Am J Sports Med. 1993;21(5):650–655. doi: 10.1177/036354659302100503. [DOI] [PubMed] [Google Scholar]
- 28.Watkins RG. Neck injuries in football players. Clin Sports Med. 1986;5(2):215–246. [PubMed] [Google Scholar]
- 29.Kuhlman GS, McKeag DB. The ‘burner’: a common nerve injury in contact sports. Am Fam Physician. 1999;60(7):2035–2040. [PubMed] [Google Scholar]
- 30.Chao S, Pacella MJ, Torg JS. The pathomechanics, pathophysiology and prevention of cervical spinal cord and brachial plexus injuries in athletics. Sport Med. 2010;40(1):59–75. doi: 10.2165/11319650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Vaccaro AR, Klein GR, Ciccoti M, Pfaff WL, Moulton MJR, Hilibrand AJ, Watkins B. Return to play criteria for the athlete with cervical spine injuries resulting in stinger and transient quadriplegia/paresis. Spine J. 2002;2(5):351–356. doi: 10.1016/s1529-9430(02)00202-4. [DOI] [PubMed] [Google Scholar]
- 32.Quong WL, Hynes SL, Arneja JS. Pediatric stinger syndrome: acute brachial plexopathy after minor trauma. Plast Reconstr Surg Glob Open. 2015;3(11):1–3. doi: 10.1097/GOX.0000000000000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clancy WG, Brand RL, Bergfield JA. Upper trunk brachial plexus injuries in contact sports. Am J Sports Med. 1977;5(5):209–216. doi: 10.1177/036354657700500508. [DOI] [PubMed] [Google Scholar]
- 34.Kewalramani LS, Krauss JF. Cervical spine injuries resulting from collision sports. Spinal Cord. 1981;19(5):303–312. doi: 10.1038/sc.1981.58. [DOI] [PubMed] [Google Scholar]
- 35.Koffler KM, Kelly JD., IV Neurovascular trauma in athletes. Orthop Clin. 2002;33(3):523–534. doi: 10.1016/s0030-5898(01)00004-9. [DOI] [PubMed] [Google Scholar]
- 36.Benjamin HJ, Lessman DS. Sports-related cervical spine injuries. Clin Pediatr Emerg Med. 2013;14(4):255–266. [Google Scholar]
- 37.Torg JS, Vegso JJ, O’Neill MJ, Sennett B. The epidemiologic, pathologic, biomechanical, and cinematographic analysis of football-induced cervical spine trauma. Am J Sports Med. 1990;18(1):50–57. doi: 10.1177/036354659001800109. [DOI] [PubMed] [Google Scholar]
- 38.Funk FJ, Wells RE. Injuries of the cervical spine in football. Clin Orthop Relat Res. 1975;No. 109:50–8. [DOI] [PubMed]
- 39.Heck JF, Clarke KS, Peterson TR, Torg JS, Weis MP. National Athletic Trainers’ association position statement: head-down contact and spearing in tackle football. J Athl Train. 2004;39(1):101–111. [PMC free article] [PubMed] [Google Scholar]
- 40.Schussler E, Jagacinski RJ, White SE, Chaudhari AM, Buford JA, Onate JA. The effect of tackling training on head accelerations in youth American football. Int J Sports Phys Ther. 2018;13(2):229–237. [PMC free article] [PubMed] [Google Scholar]
- 41.Cantu RC, Li YM, Abdulhamid M, Chin LS. Return to play after cervical spine injury in sports. Curr Sports Med Rep. 2013;12(1):14–17. doi: 10.1249/JSR.0b013e31827dc1fb. [DOI] [PubMed] [Google Scholar]
- 42.Kelly JD, Aliquo D, Sitler MR, Odgers C, Moyer RA. Association of burners with cervical canal and foraminal stenosis. Am J Sports Med. 2000;28(2):214–217. doi: 10.1177/03635465000280021201. [DOI] [PubMed] [Google Scholar]
- 43.Kurian PA, Light DI, Kerr HA. In: Burners, stingers, and cervical cord neurapraxia/transient quadriparesis BT-head and neck injuries in young athletes. O’Brien M, Meehan WP III, editors. Cham: Springer International Publishing; 2016. pp. 129–141. [Google Scholar]
- 44.Greenberg J, Leung D, Kendall J. Predicting chronic stinger syndrome using the mean subaxial space available for the cord index. Sports Health. 2011;3(3):264–267. doi: 10.1177/1941738111403866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qureshi SA, Hecht AC. Burner syndrome and cervical cord neuropraxia. Semin Spine Surg. 2010;22(4):193–197. [Google Scholar]
- 46.Meyer SA, Schulte KR, Callaghan JJ, Albright JP, Powell JW, Crowley ET, el-Khoury GY. Cervical spinal stenosis and stingers in collegiate football players. Am J Sports Med. 1994;22(2):158–166. doi: 10.1177/036354659402200202. [DOI] [PubMed] [Google Scholar]
- 47.Presciutti SM, DeLuca P, Marchetto P, Wilsey JT, Shaffrey C, Vaccaro AR. Mean subaxial space available for the cord index as a novel method of measuring cervical spine geometry to predict the chronic stinger syndrome in American football players: clinical article. J Neurosurg Spine. 2009;11(3):264–271. doi: 10.3171/2009.3.SPINE08642. [DOI] [PubMed] [Google Scholar]
- 48.Ahearn BM, Starr HM, Seiler JG. Traumatic brachial plexopathy in athletes: current concepts for diagnosis and management of stingers. J Am Acad Orthop Surg. 2019;27(18):677–684. doi: 10.5435/JAAOS-D-17-00746. [DOI] [PubMed] [Google Scholar]
- 49.Levitz CL, Reilly PJ, Torg JS. The pathomechanics of chronic, recurrent cervical nerve root neurapraxia: the chronic burner syndrome. Am J Sports Med. 1997;25(1):73–76. doi: 10.1177/036354659702500114. [DOI] [PubMed] [Google Scholar]
- 50.Torg JS. Cervical spine injuries and the return to football. Sports Health. 2009;1(5):376–383. doi: 10.1177/1941738109343161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCarthy MM, Hannafin JA. The mature athlete: aging tendon and ligament. Sports Health. 2014;6(1):41–48. doi: 10.1177/1941738113485691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hakkaku T, Nakazato K, Koyama K, Kouzaki K, Hiranuma K. Cervical intervertebral disc degeneration and low cervical extension independently associated with a history of stinger syndrome. Orthop J Sport Med. 2017;5(11):2325967117735830. doi: 10.1177/2325967117735830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiler C, Schietzsch M, Kirchner T, Nerlich AG, Boos N, Wuertz K. Age-related changes in human cervical, thoracal and lumbar intervertebral disc exhibit a strong intra-individual correlation. Eur Spine J. 2012;21(SUPPL. 6):810–818. doi: 10.1007/s00586-011-1922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nissen SJ, Laskowski ER, Rizzo TD. Burner syndrome. Phys Sportsmed. 1996;24(6):57–64. doi: 10.3810/psm.1996.06.1378. [DOI] [PubMed] [Google Scholar]
- 55.Bateman JE. Nerve injuries about the shoulder in sports. J Bone Jt Surg Am. 1967;49(4). [PubMed]
- 56.Olson DE, McBroom SA, Nelson BD, Broton MS, Pulling TJ. Unilateral cervical nerve injuries: brachial plexopathies. Curr Sports Med Rep. 2007;6(1):43–49. doi: 10.1007/s11932-007-0011-1. [DOI] [PubMed] [Google Scholar]
- 57.Patel DR, Greydanus DE. Neurologic considerations for adolescent athletes. Adolesc Med. 2002;13(3):569–578. [PubMed] [Google Scholar]
- 58.Wegener V, Stäbler A, Jansson V, Birkenmaier C, Wegener B. Lumbar burner and stinger syndrome in an elderly athlete. Korean J Pain. 2018;31(1):54–57. doi: 10.3344/kjp.2018.31.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Safran MR. Nerve injury about the shoulder in athletes, part 2: long thoracic nerve, spinal accessory nerve, burners/stingers, thoracic outlet syndrome. Am J Sports Med. 2004;32(4):1063–1076. doi: 10.1177/0363546504265193. [DOI] [PubMed] [Google Scholar]
- 60.Paulus S, Kennedy DJ. Return to play considerations for cervical spine injuries in athletes. Phys Med Rehabil Clin. 2014;25(4):723–733. doi: 10.1016/j.pmr.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Puvanesarajah V, Qureshi R, Cancienne JM, Hassanzadeh H. Traumatic sports-related cervical spine injuries. Clin Spine Surg. 2017;30(2):50–56. doi: 10.1097/BSD.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 62.Drakos MC, Feeley BT, Barnes R, Muller M, Burruss TP, Warren RF. Lower cervical posterior element fractures in the national football league: a report of 2 cases and a review of the literature. Neurosurgery. 2011;68(6):E1743–E1748. doi: 10.1227/NEU.0b013e31821815af. [DOI] [PubMed] [Google Scholar]
- 63.De Souza RM, Crocker MJ, Haliasos N, Rennie A, Saxena A. Blunt traumatic vertebral artery injury: a clinical review. Eur Spine J. 2011;20(9):1405–1416. doi: 10.1007/s00586-011-1862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maloney E, Lehnert B, McNeeley MF. Vertebral artery transection in nonpenetrating trauma: a series of 4 patients. Ann Vasc Surg. 2015;29(7):1450.e11–1450.e16. doi: 10.1016/j.avsg.2015.03.061. [DOI] [PubMed] [Google Scholar]
- 65.Evans C, Chaplin T, Zelt D. Management of major vascular injuries: neck, extremities, and other things that bleed. Emerg Med Clin North Am. 2018;36(1):181–202. doi: 10.1016/j.emc.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 66.Wang J, Pearse DD. Therapeutic hypothermia in spinal cord injury: the status of its use and open questions. Int J Mol Sci. 2015;16(8):16848–16879. doi: 10.3390/ijms160816848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeyamohan S, Harrop JS, Vaccaro A, Sharan AD. Athletes returning to play after cervical spine or neurobrachial injury. Curr Rev Musculoskelet Med. 2008;1(3–4):175–179. doi: 10.1007/s12178-008-9034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blatz D, Ross B, Dadabo J. Cervical spine trauma evaluation. In: Handbook of clinical neurology. Elsevier B.V.; 2018. p. 345–351. [DOI] [PubMed]
- 69.Andersen JC, Courson RW, Kleiner DM, McLoda TA. National Athletic Trainers’ Association position statement: emergency planning in athletics. J Athl Train. 2002;37(1):99–104. [PMC free article] [PubMed] [Google Scholar]
- 70.Weinstein SM. Assessment and rehabilitation of the athlete with a “stinger”. A model for the management of noncatastrophic athletic cervical spine injury. Clin Sports Med. 1998;17(1):127–135. doi: 10.1016/s0278-5919(05)70067-3. [DOI] [PubMed] [Google Scholar]
- 71.Tong HC, Haig AJ, Yamakawa K. The Spurling test and cervical radiculopathy. Spine (Phila Pa 1976) 2002;27(2):156–159. doi: 10.1097/00007632-200201150-00007. [DOI] [PubMed] [Google Scholar]
- 72.Kuzma SA, Doberstein ST, Rushlow DR. Postfixed brachial plexus radiculopathy due to thoracic disc herniation in a collegiate wrestler: a case report. J Athl Train. 2013;48(5):710–715. doi: 10.4085/1062-6050-48.5.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torg JS, Guille JT, Jaffe S. Injuries to the cervical spine in American football players. J Bone Jt Surg Am. 2002;84(1):112–122. doi: 10.2106/00004623-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 74.Herzog RJ, Wiens JJ, Dillinghamd MF, Sontag MJ. Normal cervical spine morphometry and cervical spinal stenosis in asymptomatic professional football players: plain film radiography, multiplanar computed tomography, and magnetic resonance imaging. Spine (Phila Pa 1976) 1991;16(6S):S178–S186. doi: 10.1097/00007632-199106001-00001. [DOI] [PubMed] [Google Scholar]
- 75.Lewno, A, Maxwell, M, Kahn, S, Xu R. Stingers and burners. In: Musculoskeletal sports and spine disorders. Springer International Publishing; 2017.
- 76.Shindle M, Urquhart M, Frassica FJ, Sponseller PD, Wilckens J. 5 minute orthopedic consult. 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 46–48. [Google Scholar]
- 77.Sola RJ, Christmas AB, Thomas BW, Fischer PE, Eubanks GC, Raynor NE, et al. Do not waste your time: straight to magnetic resonance imaging for pediatric burners and stingers. Am J Emerg Med. 2016;34(8):1442–1445. doi: 10.1016/j.ajem.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 78.Gorden JA, Straub SJ, Swanik CB, Swanik KA. Effects of football collars on cervical hyperextension and lateral flexion. J Athl Train. 2003;38(3):209–215. [PMC free article] [PubMed] [Google Scholar]
- 79.• Schroeder GD, Canseco JA, Patel PD, Hilibrand AS, Kepler CK, Mirkovic SM, Watkins III RG, Dossett A, Hecht AC VA. Updated return-to-play recommendations for collision athletes after cervical spine injury: a modified Delphi consensus study with the Cervical Spine Research Society. Neurosurgery. 2020. Summary of consensus understanding of returning to play after cervical spine injuries including clinical concerns for recurrent stingers and guidelines for the use of imaging. [DOI] [PubMed]
- 80.Huang P, Anissipour A, McGee W, Lemak L. Return-to-play recommendations after cervical, thoracic, and lumbar spine injuries: a comprehensive review. Sports Health. 2016;8(1):19–25. doi: 10.1177/1941738115610753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poindexter DP, Johnson EW. Football shoulder and neck injury: a study of the “stinger.” Arch Phys Med Rehabil 1984;65(10):601—602 [PubMed]
- 82.Smith MG, Fulcher M, Shanklin J, Tillett ED. The prevalence of congenital cervical spinal stenosis in 262 college and high school football players. J Ky Med Assoc. 1993;91(7):273–275. [PubMed] [Google Scholar]
- 83.Odor JM, Watkins RG, Dillin WH, Dennis S, Saberi M. Incidence of cervical spinal stenosis in professional and rookie football players. Am J Sports Med. 1990;18(5):507–509. doi: 10.1177/036354659001800510. [DOI] [PubMed] [Google Scholar]
- 84.Zaremski JL, Horodyski MB, Herman DC. Recurrent stingers in an adolescent American football player: dilemmas of return to play. A case report and review of the literature. Res Sport Med. 2017;25(3):384–390. doi: 10.1080/15438627.2017.1314297. [DOI] [PubMed] [Google Scholar]
- 85.Torg JS, Naranja RJ, Pavlov H, Galinat BJ, Warren R, Stine RA. The relationship of developmental narrowing of the cervical spinal canal to reversible and irreversible injury of the cervical spinal cord in football players. An epidemiological study. J Bone Jt Surg Am. 1986;78(9):1308–1314. doi: 10.2106/00004623-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 86.Takao T, Morishita Y, Okada S, Maeda T, Katoh F, Ueta T, Mori E, Yugue I, Kawano O, Shiba K. Clinical relationship between cervical spinal canal stenosis and traumatic cervical spinal cord injury without major fracture or dislocation. Eur Spine J. 2013;22(10):2228–2231. doi: 10.1007/s00586-013-2865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rüegg TB, Wicki AG, Aebli N, Wisianowsky C, Krebs J. The diagnostic value of magnetic resonance imaging measurements for assessing cervical spinal canal stenosis. J Neurosurg Spine. 2015;22(3):230–236. doi: 10.3171/2014.10.SPINE14346. [DOI] [PubMed] [Google Scholar]
- 88.Aebli N, Wicki AG, Rüegg TB, Petrou N, Eisenlohr H, Krebs J. The Torg-Pavlov ratio for the prediction of acute spinal cord injury after a minor trauma to the cervical spine. Spine J. 2013;13(6):605–612. doi: 10.1016/j.spinee.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 89.Hartley RA, Kordecki ME. Rehabilitation of chronic brachial plexus neuropraxia and loss of cervical extension in a high school football player: a case report. Int J Sports Phys Ther. 2018;13(6):1061–1072. [PMC free article] [PubMed] [Google Scholar]
- 90.Hovis WD, Limbird TJ. An evaluation of cervical orthoses in limiting hyperextension and lateral flexion in football. Med Sci Sports Exerc. 1994;26(7):872–876. [PubMed] [Google Scholar]
- 91.Stuber K. Cervical collars and braces in athletic brachial plexus injury and excessive cervical motion prevention: a review of the literature. J Can Chiropr Assoc. 2005;49(3):216–222. [PMC free article] [PubMed] [Google Scholar]
- 92.Concannon LG, Harrast MA, Herring SA. Radiating upper limb pain in the contact sport athlete. Curr Sports Med Rep. 2012;11(1):28–34. doi: 10.1249/JSR.0b013e318240dc3f. [DOI] [PubMed] [Google Scholar]
- 93.Leung D, Greenberg JS, Henning PT, Chiodo AE. Cervical Transforaminal epidural injection in the management of a stinger. PM R. 2012;4(1):73–77. doi: 10.1016/j.pmrj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 94.Daly CA, Payne SH, Seiler JG. Severe brachial plexus injuries in American football. Orthopedics. 2016;39(6):e1188–e1192. doi: 10.3928/01477447-20160721-03. [DOI] [PubMed] [Google Scholar]
- 95.Fan YL, Othman MI Bin, Dubey N, Peh WCG. Magnetic resonance imaging of traumatic and non-traumatic brachial plexopathies. Singap Med J 2016;57(10):552–560. [DOI] [PMC free article] [PubMed]
- 96.Yang LJS, Chang KWC, Chung KC. A systematic review of nerve transfer and nerve repair for the treatment of adult upper brachial plexus injury. Neurosurgery. 2012;71(2):417–429. doi: 10.1227/NEU.0b013e318257be98. [DOI] [PubMed] [Google Scholar]
- 97.Garg R, Merrell GA, Hillstrom HJ, Wolfe SW. Comparison of nerve transfers and nerve grafting for traumatic upper plexus palsy: a systematic review and analysis. J Bone Jt Surg Am. 2011;93(9):819–829. doi: 10.2106/JBJS.I.01602. [DOI] [PubMed] [Google Scholar]
- 98.John TS, Fishman F, Sharkey MS, Carter CW. Current concepts review: peripheral neuropathies of the shoulder in the young athlete. Phys Sportsmed. 2019;9:1–11. doi: 10.1080/00913847.2019.1676136. [DOI] [PubMed] [Google Scholar]
- 99.Rosenthal BD, Boody BS, Hsu WK. Return to play for athletes. Neurosurg Clin. 2017;28(1):163–171. doi: 10.1016/j.nec.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 100.Speer KP, Bassett FH., 3rd The prolonged burner syndrome. Am J Sports Med. 1990;18(6):591–594. doi: 10.1177/036354659001800606. [DOI] [PubMed] [Google Scholar]
- 101.Cantu RC. The cervical spinal stenosis controversy. Clin Sports Med. 1998;17(1):121–126. doi: 10.1016/s0278-5919(05)70066-1. [DOI] [PubMed] [Google Scholar]
- 102.Araujo D. Burner. Basics Phys Sportsmed. 1998;26(4):24. [Google Scholar]
- 103.Schroeder GD, Lynch TS, Gibbs DB, Chow I, Labelle MW, Patel AA, et al. The impact of a cervical spine diagnosis on the careers of national football league athletes. Spine (Phila Pa 1976) 2014;39(12):947–952. doi: 10.1097/BRS.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 104.Cantu RC. Functional cervical spinal stenosis: a contraindication to participation in contact sports. Med Sci Sports Exerc. 1993;25(3):316–317. [PubMed] [Google Scholar]
- 105.Torg JS, Ramsey-Emrhein JA. Cervical spine and brachial plexus injuries. Phys Sportsmed. 1997;25(7):60–88. doi: 10.3810/psm.1997.07.1487. [DOI] [PubMed] [Google Scholar]
- 106.Kepler CK, Vaccaro AR. Injuries and abnormalities of the cervical spine and return to play criteria. Clin Sports Med. 2012;31(3):499–508. doi: 10.1016/j.csm.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 107.Marx RG, Wilson SM, Swiontkowski MF. Updating the assignment of levels of evidence. J Bone Jt Surg Am. 2015;97(1):1–3. doi: 10.2106/JBJS.N.01112. [DOI] [PubMed] [Google Scholar]