Abstract
Purpose of Review
As immersive learning outside of the operating room is increasingly recognized as a valuable method of surgical training, virtual reality (VR) and augmented reality (AR) are increasingly utilized in orthopedic surgical training. This article reviews the evolving nature of these training tools and provides examples of their use and efficacy. The practical and ethical implications of incorporating this technology and its impact on both orthopedic surgeons and their patients are also discussed.
Recent Findings
Head-mounted displays (HMDs) represent a possible adjunct to surgical accuracy and education. While the hardware is advanced, there is still much work to be done in developing software that allows for seamless, reliable, useful integration into clinical practice and training.
Summary
Surgical training is changing: AR and VR will become mainstays of future training efforts. More evidence is needed to determine which training technology translates to improved clinical performance. Volatility within the HMD industry will likely delay advances in surgical training.
Keywords: Virtual reality, Augmented reality, Medical education, Orthopedic surgery, Surgical simulation
Introduction
Orthopedic surgical training is currently in a state of rapid change. Halsted’s apprenticeship model, where residents and fellows learned their craft under the supervision of a senior trainee or surgeon, drove surgical training into the modern era [1]. In this model, characterized by repetition and graduated responsibility, the patient acted as the primary training modality. However, contemporary surgical trainees face multiple challenges that threaten this paradigm. The increasing complexity of procedures, new technology and techniques, more stringent work hour restrictions, an increasingly litigious legal environment, and increased scrutiny of trainees’ roles in patient care have emphasized the need for innovative education and training techniques. In addition to curriculum standardization and an emphasis on competency-based advancement, the push to provide skills acquisition and assessment in nonclinical environments is an exciting new frontier in surgical training.
Many authors argue that surgical training should mirror pilot training, which leverages the power of simulation before exposing trainees to live situations. Some argue that surgical trainees have “an ethical obligation” to be exposed to all clinical scenarios that can be “reasonably well simulated” before experiencing them with patients. Proponents argue that this technique transfers both instructors’ and learners’ focus to knowledge and skill acquisition and relieves them of the pressure of balancing patients’ safety [2]. This ethical argument is also applied to practicing surgeons who wish to acquire new skills. Over the past 20 years, there has been an explosion of new minimally invasive techniques which often require new skills and tools. These evolving procedures and techniques often impose a learning curve on practicing surgeons that affect early outcomes and complication rates. [3–5] Further, these minimally invasive procedures decrease the viewing field in surgery for learners and educators. The surgeon’s best view is often available to the attending or the resident/fellow, but often not simultaneously [6–9]. This optimal view is critical for goal of graduated autonomy in surgical education. The learner must see as critical portions of the case are performed. The educator must see as it becomes the learner’s time to perform the procedure. With traditional open exposure and arthroscopic procedures on the other end of the spectrum, simultaneous visualization of the surgeon’s best view is possible. It is the middle ground, minimally/less-invasive procedures, where new technology can help bridge these training challenges. It is also possible that technology can help facilitate learning and mastery of new techniques through realistic simulation that can be performed in a nonclinical setting minimizing harm to patients.
Successful surgical skill development though simulation has been well documented. Simulation has been shown to accelerate learning curves for surgical trainees in multiple specialties, including neurosurgery, gynecology, general surgery, and orthopedics [10–13]. Cadaveric models, computer simulation, synthetic models, and animal models can all reliably assess surgical skill [10, 12–15]. While both low- and high-fidelity bench simulations have shown utility and efficacy in orthopedics, they do not allow for the gradual increase in autonomy that surgical trainees need to become proficient and confident. This article provides a narrative review of virtual reality (VR) and augmented reality (AR), two modalities being used to facilitate teaching and assess surgical skills [11].
Mixed Reality
According to Milgram’s theory, mixed reality consists of a continuum existing between our interaction with the real world and a completely virtual world [16, 17]. Virtual reality (VR) is an “immersive, completely artificial computer-simulated image and environment with real-time interaction.” [18]. This mode of simulation can involve any of the senses and is not limited to sight and touch. In contrast, augmented reality (AR) lies between a completely virtual environment and the real world. AR is a broad category that superimposes virtual data on a real-world image.
Mixed reality devices are becoming increasingly ubiquitous in many facets of life. Applications such as Google Translate and Amazon Shop recognize and relay information about objects and words in real time. In healthcare, the mixed reality industry was projected to be worth $641 million by 2018 and $2.4 billion by 2024 [19, 20]. While this is clearly a growing industry, the utility of mixed reality in surgical training has yet to be well characterized.
Appraising Surgical Simulators
The utility and benefits of surgical simulators are best assessed based on face, content, construct, and concurrent validity [21]. Face and content validity are subjective measures of the appropriateness of the simulator’s psychomotor fidelity and of the variables measured, respectively. Construct validity is an objective measure of the simulator’s ability to distinguish novices from experts. Concurrent validity describes the correlation between the variables measured and real-world performance [21].
Virtual Reality
Since the first VR knee arthroscopy simulator was introduced in the 1990s [12], numerous orthopedic simulators and task trainers have been introduced and studied. Modern simulators allow trainees to practice skills such as fracture reduction, sawing, drilling, and arthroscopy. VR-based simulators allow trainees to home diagnostic skills, pre-operative planning techniques, intraoperative decision-making, and surgical techniques outside of the operating room [14].One of the key benefits of VR simulation over real-world simulation is that novices can get immediate constructive feedback and assistance on their performance without the need for face-to-face expert guidance [14].
Ruikar’s review of orthopedic simulators for psychomotor skill and surgical procedure training split orthopedic VR simulators into 3 groups: non-interactive simulators, interactive simulators with visual feedback, and interactive simulators with visio-haptic (tactile) feedback. Non-interactive simulators are defined by their ability to help visualize anatomy and volumetric data. They are primarily used to aid in diagnosis and help to plan and predict surgical outcomes. 3D pre-operative total hip arthroplasty planning is a common application of a non-interactive simulator. While these devices do not improve manual skills, they may help trainees plan a more successful surgery [14].
Interactive simulators are defined by their ability to simulate entire procedures, guiding trainees through key steps. Trainees utilize a mouse, keyboard, or other optical trackers to work through these simulations. Examples of interactive VR simulators range from simple smartphone applications such as ImmersiveTouch Surgery (Yoo, Chicago, Illinois) to intraoperative guidance systems such as Hip Navigator (HipNav, Pittsburgh, Pennsylvania). Applications such as Immersive Touch, which can be downloaded as an app on a smart phone or tablet, can be used by trainees from any location at any time. (This confers an important benefit over visio-haptic simulators, which require a dedicated lab space and can only be used by one trainee at a time.) The few studies assessing the use of interactive simulators with visual feedback in orthopedics demonstrate that there is construct validity when distinguishing novice, intermediate, and expert knowledge in carpal tunnel releases [22] as well as construct, face, and content validity in intramedullary nailing of femur fractures [23]. One downside, however, is that while interactive simulators show benefit in learning about procedures in general, many do not account for the tactile feedback that is inherent in higher-fidelity simulation [14, 24, 25]. Companies such as Fundamental Surgery (FundamentalVR, Boston, Massachusetts) have worked in earnest to partner with academic programs and incorporate VR into surgical training.
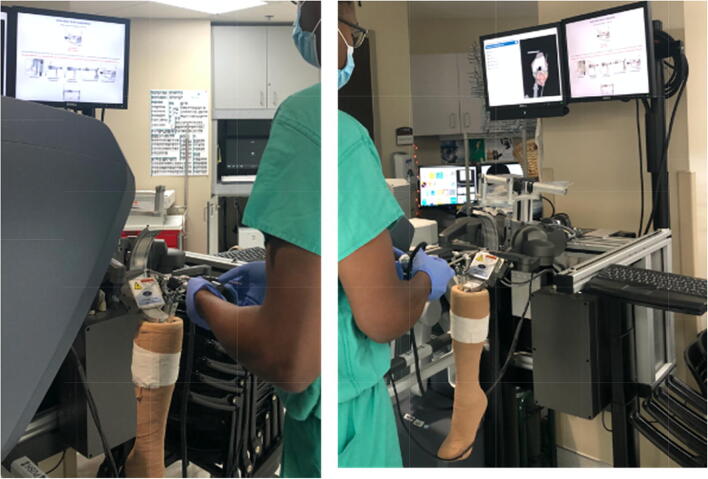
Visio-haptic simulators, or those that apply tactile feedback, include shoulder, knee (Fig. 1), and hip arthroscopy simulators, fracture fixation simulators, and orthopedic drilling simulators [21, 25–29]. While haptic simulators demonstrate construct validity and are able to improve performance of specific tasks among both experts and novices [21, 30–34], whether these skills translate to improvements in clinical practice remains an area of active study. Kalun and colleagues evaluated four studies that attempted to answer this question [30, 35–38]. Three of the four studies used a high-fidelity visio-haptic arthroscopic simulator and assessed trainees on both procedural checklists and an arthroscopic global rating scale (GRS). In two of the three studies, training with the haptic simulator led to better knowledge of the surgical steps [36, 37], but only one showed significant improvement on the GRS compared to controls [36]. The authors concluded that the heterogeneity of training protocols, tools, and outcome measures makes it difficult to assess true transfer validity.
Fig. 1.
ArthroSim (TolTech, Aurora, Colorado) visio-haptic knee arthroscopy simulator
Bartlett’s review of VR in orthopedics identified multiple studies showing construct validity of simulators including knee, hip, and shoulder arthroscopy along with hip and intraarticular fracture fixation [21]. However, the authors concluded that while the simulators showed ability to distinguish between novices and experts, many lacked in their ability to differentiate subtler expertise differences between intermediate and expert learners. These findings may limit the utility of these tools in real-world assessment and training for more advanced surgeons [21]. They also noted the heterogeneity of the outcomes measures used in the studies. Overall, they supported the use of knee and shoulder arthroscopy simulators that have been validated but believe that more investigation is needed before their widespread use can be fully supported [21].
While there is evidence supporting the validity of haptic arthroscopic simulators in orthopedic training, haptic VR simulators for drilling and fracture fixation do not offer a clear benefit over lower-fidelity alternatives, which are usually cheaper and more widely available [39–44]. Low-fidelity techniques for teaching basic orthopedic skills such as avoidance of drill plunging [45], cortical screw tightening [46•], and fracture reduction and fixation [31, 43, 44] have shown evidence of transfer and content validity. Several studies in the general surgery realm have shown no additional benefit of high-fidelity simulation compared to low-fidelity simulation, with both methods demonstrating increased skill acquisition compared to textbook review alone [47, 48]. However, head-to-head studies of high- and low-fidelity simulation for these orthopedic surgery tasks have not been performed.
Augmented Reality
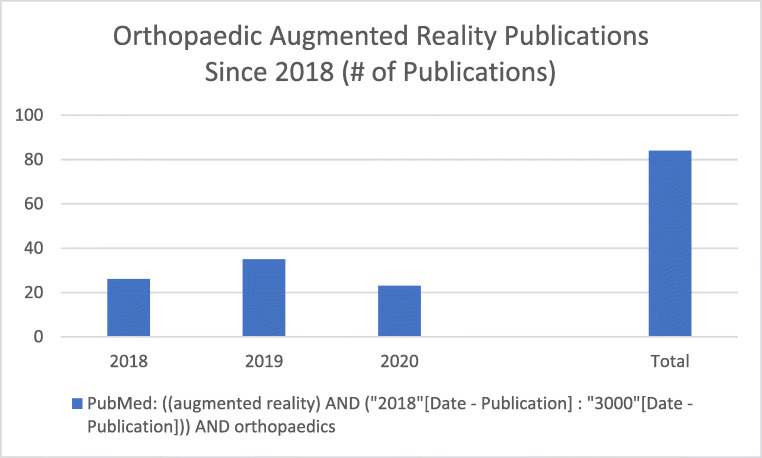
AR in orthopedics is a fairly recent but rapidly developing area of study, with over 50 publications on the topic in the past 2 years (Fig. 2). AR allows supplemental data to be incorporated into the surgeon’s real-world sensory inputs and has been integrated into orthopedic procedures as well as surgical training [18, 49–54]. This commonly includes overlaying useful visual data such as relevant imaging into the surgeon’s field-of-view but can also include auditory or sensory feedback, intraoperative navigation, and telementoring or guidance [18, 49–54].
Fig. 2.
Orthoapedic AR publications since 2018 referenced in PubMed
AR in the Operating Room
Blackwell and colleagues first described the use of AR in orthopedics in 1998. [49] The authors postulated that AR could be used for intraoperative guidance (for positioning components and avoiding critical structures), surgical training, and simulation. Since that time, multiple advancements have made AR implementation a reality in many operating rooms. Despite these advancements, there are still many barriers to the widespread use of AR in orthopedics, including integration into the operating room, interface comfort and reliability, and equipment comfort.
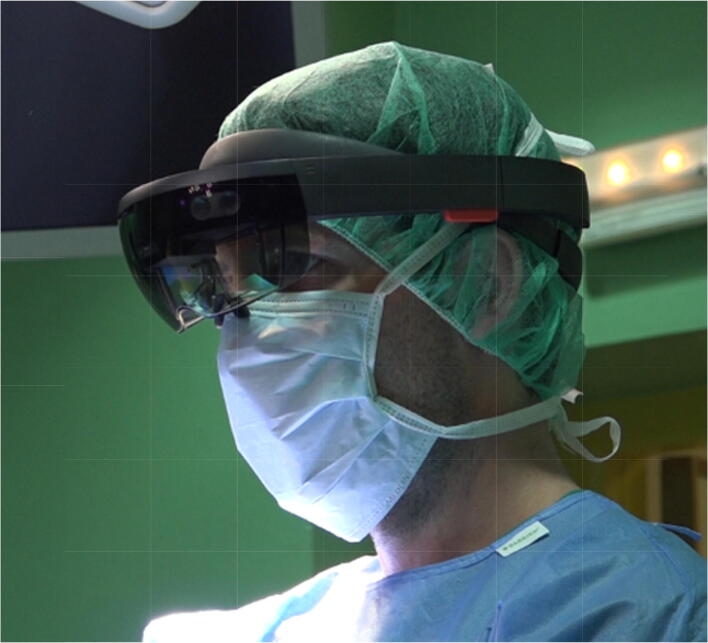
AR devices require a display, a position tracking system, and software that transforms and incorporates data [49]. The display can take the form of either a traditional monitor or a head-mounted device (HMDs). HMDs range from simple opaque displays that rest in front of one of the surgeon’s eyes such as the Vuzix M300 (Vuzix, Rochester, New York) (Fig. 3) to semitransparent displays like the Microsoft HoloLens (Redmond, Washington) that overlay information into the extended visual field (Fig. 4). While most HMDs include cameras, videos, and accelerometers that track the wearer’s head position, some also track hand gestures and/or the user’s eye motion [53, 55, 56].
Fig. 3.
Vuzix M300. Simple opaque display
Fig. 4.
The Microsoft Hololens is an example of a head-mounted display that has been utilized in the operating room as an example of augmented reality
The Google Glass (GG, Mountain View, California) HMD was the first commercially available HMD and has been most studied HMD device in the orthopedic literature over the years [12, 18, 57–60]. Many researchers adopted Google Glass because it was lightweight, had a high-resolution video camera, and connected to wireless internet [18]. However, production ceased on Google Glass in 2015 and multiple issues that continue to plague newer HMDs were found including battery life, image quality, line of sight, and network authentication [18, 57].
Multiple other commercially available AR HMDs have been developed since GG was released. The simplest designs, like the Vuzix M300, consist of an opaque viewfinder that sits on glasses frames and uses an Android-based operating system (OS). The Osterhaut Design Group (ODG; San Francisco, California) R7 HMD (Fig. 5) is a popular non-wired HMD with a semitransparent display, an Android-based OS, head gestures, and voice control. There are multiple reports of its promise in intraoperative image guidance, data display, and education [56, 61, 62]. However, ODG recently collapsed following a failed acquisition and the device is no longer available [63].
Fig. 5.
ODG R7 HMD—non-wired HMD with a semitransparent display, Android-based OS, head gestures, and voice control
The Microsoft HoloLens was, until recently, the most advanced commercially available HMD. It has a large wireless semitransparent display that runs on a proprietary OS. Its eye tracking and head and hand gesture-based commands set it apart from its competitors [61, 64]. A 2017 comparison of the HoloLens and the ODG R7 concluded that its contrast perception, text readability, frame rate, and limited system lag made the HoloLens more suited for professional use [61].
As of August 2018, the Magic Leap One HMD was developed and released for use. It is a semitransparent HMD with a wired “lightpack” that handles processing, head and hand gesture controls, and eye tracking [65]. There are no published reports on its use in orthopedics or healthcare. However, reports that the company has partnered with multiple healthcare companies and surgeon training companies suggest that its use may become more widespread in the future [66].
In spine surgery, AR is primarily used to facilitate intraoperative navigation [67]. Elmi-Terander and colleagues used a heads-up display monitor coupled with intraoperative cone-beam CT (CBCT) and an AR surgical navigation system to place thoracic and lumbosacral pedicle screws in 20 patients [68••]. Several bench and cadaveric studies found that HoloLens-assisted pedicle screw placement had up to 97% accuracy when compared to the gold standard technique [69•, 70]. Edstrom and colleagues proposed that AR-assisted surgical navigation of pedicle screw placement decreases radiation exposure when used in conjunction with CBCT [71]. AR has also been used as an adjunct for osteotomy guidance. Kosterhon used a proprietary navigation system to overlay planned osteotomies on a surgical microscope’s field of view, helping surgeons identify the correct osteotomy planes [72].
AR-assisted tumor resection has made headway in orthopedic oncology as well. AR-based navigation systems have been used to resect bone and soft tissue tumors with excellent adherence to surgical margins [73–75], less blood loss, and shorter operative time [76]. While this technology is promising, whether these techniques provide a definitive benefit over the current standard of care remains to be seen [77].
AR has been useful in fracture management, where image overlay or projection has been used to simulate guidewire placement [78•], facilitate intramedullary nail insertion [79–81], and insert sacroiliac screws [82, 83]. Projecting fluoroscopic images into the surgeon’s visual field was shown to improve tip-apex distance, shorter radiation exposure time, and shorter intramedullary nail total insertion time, likely related to time saved by the surgeon not having to move their eyes to look at a fluoroscopy monitor [79].
In adult reconstruction, AR has been used primarily for intraoperative navigation, anatomic referencing, and intraoperative imaging [84–86, 87•, 88••]. Multiple bench studies have demonstrated accuracy with AR-assisted acetabular cup placement [85, 86]. Logishetty and colleagues found no difference accurate placement of the acetabular cup accuracy between groups of novices randomized to surgeon supervision versus HoloLens assistance [87•]. Fallavollita devised a novel c-arm augmented with a camera which allowed for 3 fluoroscopic images to be constructed together to make an intraoperative mechanical axis view with no parallax errors [84]. Finally, Lei and colleagues successfully used the Hololens HMD, 3D printing, and anatomic referencing to perform a total hip arthroplasty on a patient with prior hip arthrodesis [88••]. The HMD and preop scans were overlayed on the patient intraoperatively to assist in placement of the 3D-printed implant [88••].
AR in Education and Training
HMDs have been used in medical [24] and surgical education [62]; however, AR has not demonstrated a clear advantage in terms of skill or knowledge retention when compared to conventional training or VR. However, they did find the VR group was most likely to have adverse effects such as headache, dizziness, and blurred vision [24]. Until the benefit of AR to learners is better understood, caution that should be observed when implementing AR into surgical education, particularly due to its high cost.
AR in TELEMONITORING
Telemonitoring, or remote surgical guidance, is another area where AR and HMDs show much promise. Remote guidance from an experienced surgeon benefits trainees as well as experienced surgeons learning a new skill [89]. Outside of the operating room, telemonitoring can be used to assess post-operative wounds [90, 91] or for remote consultation among colleagues.
The University of Alabama-Birmingham Orthopedic and Neurosurgical departments developed a remote surgical assistance model known as Virtual Interactive Presence and Augmented Reality (VIPAR) [50–52, 91–93]. Their initial proprietary design consisted of screen that combined images from a “local” (intraoperative) camera and a remote camera [52]. This allowed a remote surgeon to provide visual assistance to an operating surgeon [52]. Orthopedic faculty successfully used this system to provide virtual assistance to resident surgeons during shoulder arthroscopy [50]. Both residents and attendings believed it to be a safe and useful adjunct to teaching in the operating room [50]. Later iterations of this technology transitioned to using an iPad as the display and eventually to using the Google Glass HMD [51, 93]. Using the Google Glass VIPAR system, a total shoulder arthroplasty case was performed in Birmingham, AL with remote assistance from a surgeon in Atlanta, GA [93]. While they considered it a successful procedure, users reported secure network connection issues, a limited battery life, and divergent lines of sight between the surgeon and the camera [93].
A similar set-up used the ODG R7 HMD to guide trainees through temporal bone dissections [56••]. The attending physicians uploaded imaging, annotated relevant anatomy in the trainees’ field of view, and provided audible guidance during the procedure [56••]. Users cited poor connectivity, limited line of sight, lack of magnification, and the need for a headlight as factors that limited the practicality of this tool [56••].
Concerns with HMDS
While HMDs have shown promise for surgical navigation and telemonitoring, concerns about battery life, line of sight, secure network access, cost, and HIPAA compliance persist [56, 93]. Furthermore, fragmentation of hardware and software development (i.e., the development of Microsoft HoloLens and Magic Leap One, backed by Microsoft and Google respectively and run off two separate operating systems) may slow the development of useful applications [55, 65].
Another unintended consequence of HMDs is inattentional blindness, or failure to see an object located within one’s visual field because it did not engage the viewer’s attention [94]. There is early evidence that HMDs use may lower a surgeon’s ability to notice foreign bodies intraoperatively [94, 95]. Differences in inattentional blindness between tasks may be affected by cognitive load [94]. Further study is needed to determine whether this phenomenon has sustained or adverse impact among users.
Multiple studies have demonstrated that HMDs can cause side effects such as nausea, headaches, and vertigo [24, 49, 96]. While there is some evidence that these effects are more pronounced in VR simulations compared to AR, a better understanding of potential adverse effects is needed as HMDs become more prevalent in the operating room [24].
Future
Despite the popularity of AR for surgical navigation, the intraoperative use of HMDs and their role in clinical care has not been firmly established. While pilot studies have demonstrated their utility in bench and cadaveric models and in limited clinical studies, surgeons must navigate the practical and ethical challenges associated with implementing this technology in patient care. If positive outcomes over the usual practice are shown in larger clinical studies, HMDs may soon be used to display pertinent information, images, and even fluoroscopy images in the surgeon’s field of view. To increase these devices’ utility in the operating room, more advanced tracking software must be developed. While optical tracking is an accurate way of tracking position intraoperatively, it does suffer from the need to have line of sight, which can limit the surgeon’s freedom in the operating room. One solution is electromagnetic tracking, which overcomes the need for line of sight; however, current technology is limited by interference from metal tools [81, 96–98]. As HMD tracking and spatial registration systems become more advanced, these devices could conceivably replace (instead of augment) bulky surgical navigation systems [96] [49, 54, 78, 96, 99].
Our Experience
The lead author’s institution recently acquired 9 HMDs (Vuzix M300 ×3, ODG R7 ×3, Google Glass ×3) courtesy of funding from an AOTrauma grant to assess the deployability and sustainability of AR in orthopedic training. In our short time with the HMDs, we found multiple issues consistent with those described in the literature, including callibration of the field of view alignment, technical glitches obscuring the surgeon’s field of view, and poor connectivity (Table 1). We also struggled to find an affordable, efficient software to record and stream the surgeon’s best view to both learners and instructors. We found that the Vuzix M300 and ODG R7 HMDs were easiest to use due to being based on an open source Android operating system. The ODG R7 HMDs showed the most promise; however, their parent company went out of business before completion of our study thus highlighting the challenges of volatile product markets when trying to study a new technology.
Table 1.
Experience with three head-mounted displays for use in orthopedic training
| HMD Type | |||
|---|---|---|---|
| ODG R7 glasses ×3 | Google Glass X ×3 | Vuzix M300 glasses ×3 | |
| Pros | – Easiest interface to use and set up (touchpad with mouse, finger remote, head gestures) | - Lightweight, unobstrusive | - Lightweight, easy to set up with wifi network because Android based. |
| - Multiple software applications to use, and can communicate with other android devices. | |||
| - While FOV is obscured, it is transparent. | |||
| Cons | - Camera field of view is slightly off from human field of view. Need to have glasses sit tilted downwards for view to overlap. |
- Cannot set up on protected hospital network - Interface is least intuitive to use. |
- Opaque viewfinder, so blocks field of vision. |
| - Uniocular (some dizziness with testing) | |||
| - Camera field of view difficult to line up with viewer field of view. | |||
| - Heavy with entire field of view affected | |||
Conclusions
Virtual and augmented reality are taking an increasingly important role in surgical education as well as in surgical care. Numerous studies have demonstrated that VR improves skill mastery outside the operating room, though translation of these skills to the clinical environment is difficult to assess. AR is primarily being used for simulated and intraoperative navigation; however, as the devices’ ability to replicate the surgical environment improves, these tools are likely to become more prevalent in both training and patient care. HMDs in particular show promise in surgical training; however, given the volatility within the HMD industry, more study is needed before a clear leader can emerge in terms of both the HMD and the accompanying software.
Funding Information
Dr. McKnight, Dr. Hsu, and Dr. Hwang received funding from the AOTrauma North America Fellows Research Support grant.
Compliance with Ethical Standards
Conflict of Interest
The other authors declare that they have no conflict of interest.
Joseph Hsu is a paid presenter or speaker for Smith & Nephew.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on The Use of Technology in Orthopaedic Surgery—Intraoperative and Post-Operative Management
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Bass BL. Fundamental changes in general surgery residency training. Am Surg. 2007;73:109–113. doi: 10.1177/000313480707300204. [DOI] [PubMed] [Google Scholar]
- 2.Ziv A, Small SD, Wolpe PR. Patient safety and simulation-based medical education. Med Teach. 2000;22:489–495. doi: 10.1080/01421590050110777. [DOI] [PubMed] [Google Scholar]
- 3.Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am [Internet] 2010;92:2643–2652. doi: 10.2106/JBJS.I.01477. [DOI] [PubMed] [Google Scholar]
- 4.Shervin N, Rubash HE, Katz JN. Orthopaedic procedure volume and patient outcomes: A systematic literature review. Clin Orthop Relat Res. Lippincott Williams and Wilkins; 2007. p. 35–41. [DOI] [PubMed]
- 5.Gates EA. New surgical procedures: can our patients benefit while we learn? Am J Obstet Gynecol [Internet] 1997;176:1293–1298. doi: 10.1016/S0002-9378(97)70348-X. [DOI] [PubMed] [Google Scholar]
- 6.Grogan BF, Blair JA, Blease RE, Cho MS, Hsu JR. Exposure of the distal humerus using a triceps hemi-peel approach. Orthopedics [Internet]. 2014 [cited 2017 May 25];37:e455–9. Available from: http://www.healio.com/doiresolver?doi=10.3928/01477447-20140430-56 [DOI] [PubMed]
- 7.Bedigrew KM, Blair JA, Possley DR, Kirk KL, Hsu JR. Comparison of calcaneal exposure through the extensile lateral and sinus tarsi approaches. Foot Ankle Spec. 2018;11:142–147. doi: 10.1177/1938640017713616. [DOI] [PubMed] [Google Scholar]
- 8.Beltran MJ, Blair JA, Huh J, Kirby JM, Hsu JR. Articular exposure with the swashbuckler versus a “mini- swashbuckler” approach. Injury. 2013;44:189–193. doi: 10.1016/j.injury.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Blair JA, Stinner DJ, Kirby JM, Gerlinger TL, Hsu JR. Quantification of femoral neck exposure through a minimally invasive Smith-Petersen approach. J Orthop Trauma. 2010;24:355–358. doi: 10.1097/BOT.0b013e3181c675d0. [DOI] [PubMed] [Google Scholar]
- 10.Larsen CR, Soerensen JL, Grantcharov TP, Dalsgaard T, Schouenborg L, Ottosen C, et al. Effect of virtual reality training on laparoscopic surgery: randomised controlled trial. BMJ [Internet]. BMJ Publishing Group; 2009 [cited 2017 Jul 12];338:b1802. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19443914 [DOI] [PMC free article] [PubMed]
- 11.Atesok K, MacDonald P, Leiter J, Dubberley J, Satava R, VanHeest A, et al. Orthopaedic education in the era of surgical simulation: still at the crawling stage. World J Orthop [Internet]. Baishideng Publishing Group Inc; 2017 [cited 2017 Jul 14];8:290–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28473955 [DOI] [PMC free article] [PubMed]
- 12.Baker DK, Fryberger CT, Ponce BA. The emergence of augmented reality in orthopaedic surgery and education. Orthop J Harvard Med School. 2015;23:8–16. [Google Scholar]
- 13.Barsom EZ, Graafland M, Schijven MP. Systematic review on the effectiveness of augmented reality applications in medical training. Surg Endosc [Internet]. Springer; 2016 [cited 2017 Aug 30];30:4174–83. Available from: https://link.springer.com/content/pdf/10.1007%252Fs00464-016-4800-6.pdf [DOI] [PMC free article] [PubMed]
- 14.Ruikar DD, Ravindra HS, Santosh KC. A systematic review on orthopedic simulators for psycho-motor skill and surgical procedure training. J Med Syst [Internet]. 2018 [cited 2019 Nov 15];42:168. Available from. 10.1007/s10916-018-1019-1. [DOI] [PubMed]
- 15.Hodgins JL, Veillette C. Arthroscopic proficiency: methods in evaluating competency. BMC Med Educ [Internet]. 2013; [cited 2017 Jul 11];13:61. Available from: http://bmcmededuc.biomedcentral.com/articles/10.1186/1472-6920-13-61. [DOI] [PMC free article] [PubMed]
- 16.Milgram P, Takemura H, Utsumi A, Kishino F. Augmented reality: a class of displays on the reality-virtuality continuum [Internet]. 282 / SPIE. 1994. Available from: http://vered.rose.utoronto.ca
- 17.Hamacher A, Kim SJ, Cho ST, Pardeshi S, Lee SH, Eun S-J, et al. Application of virtual, augmented, and mixed reality to urology. Int Neurourol J [Internet]. Korean Continence Society; 2016 [cited 2018 Mar 24];20:172–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27706017 [DOI] [PMC free article] [PubMed]
- 18.Khor WS, Baker B, Amin K, Chan A, Patel K, Wong J. Augmented and virtual reality in surgery-the digital surgical environment: applications, limitations and legal pitfalls. Ann Transl Med [Internet]. AME Publications; 2016 [cited 2017 Aug 29];4:454. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28090510 [DOI] [PMC free article] [PubMed]
- 19.Wood L. Global Augmented Reality &; Virtual reality in healthcare industry worth USD 641 million by 2018 - analysis, technologies & Forecasts 2013–2018 - Key Vendors: Hologic Inc, Artificial Life Inc, Aruba Networks - Research and Markets | Business Wire [Internet]. Bus. Wire. 2016 [cited 2018 Apr 6]. Available from: https://www.businesswire.com/news/home/20160316005923/en/Global-Augmented-Reality-Virtual-Reality-Healthcare-Industry
- 20.Mixed Reality in Healthcare Market by Value, Revenue, Segments, Mega trends, Prominent Players and Outlook to 2024 - Reuters [Internet]. [cited 2019 Nov 15]. Available from: https://www.reuters.com/brandfeatures/venture-capital/article?id=144434
- 21.Bartlett JD, Lawrence JE, Stewart ME, Nakano N, Khanduja V. Does virtual reality simulation have a role in training trauma and orthopaedic surgeons? Bone Jt. J. Br Editorial Society of Bone and Joint Surgery; 2018. p. 559–65. [DOI] [PubMed]
- 22.Tulipan J, Miller A, Park AG, Labrum JT, Ilyas AM. Touch surgery: analysis and assessment of validity of a hand surgery simulation “app.” Hand. SAGE Publications Inc.; 2018; [DOI] [PMC free article] [PubMed]
- 23.Sugand K, Mawkin M, Gupte C. Training effect of using Touch SurgeryTM for intramedullary femoral nailing. Injury Elsevier Ltd. 2016;47:448–452. doi: 10.1016/j.injury.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 24.Moro C, Štromberga Z, Raikos A, Stirling A. The effectiveness of virtual and augmented reality in health sciences and medical anatomy. Anat Sci Educ. 2017;10:549–559. doi: 10.1002/ase.1696. [DOI] [PubMed] [Google Scholar]
- 25.Luciano CJ, Banerjee PP, Bellotte B, Lemole GM, Oh M, Charbel FT, et al. Learning retention of thoracic pedicle screw placement using a high-resolution augmented reality simulator with haptic feedback 1. [DOI] [PMC free article] [PubMed]
- 26.Leong JJH, Leff DR, Das A, Aggarwal R, Reilly P, Atkinson HDE, et al. Validation of orthopaedic bench models for trauma surgery. J Bone Jt Surg [Br] [Internet]. 2008 [cited 2017 Jul 11];90:958–65. Available from: http://bjj.boneandjoint.org.uk.libproxy.lib.unc.edu/content/jbjsbr/90-B/7/958.full.pdf [DOI] [PubMed]
- 27.Pollard TCB, Khan T, Price AJ, Gill HS, Glyn-Jones S, Rees JL. Simulated hip arthroscopy skills: learning curves with the lateral and supine patient positions. J Bone Jt Surgery-American Vol [Internet]. 2012 [cited 2017 Jul 12];94:e68–1–10. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00004623-201205160-00023 [DOI] [PubMed]
- 28.Homma Y, Mogami A, Baba T, Naito K, Watari T, Osamu O, et al. Is actual surgical experience reflected in virtual reality simulation surgery for a femoral neck fracture? Eur J Orthop Surg Traumatol [Internet]. 2019 [cited 2019 Nov 13];29:1429–34. Available from. 10.1007/s00590-019-02465-9. [DOI] [PubMed]
- 29.Cho HS, Park YK, Gupta S, Yoon C, Han I, Kim H-S, et al. Augmented reality in bone tumour resection: an experimental study. Bone Joint Res [Internet]. British Editorial Society of Bone and Joint Surgery; 2017 [cited 2018 Mar 19];6:137–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28258117 [DOI] [PMC free article] [PubMed]
- 30.Kalun P, Wagner N, Yan J, Nousiainen M, Sonnadara R. Surgical simulation training in orthopedics: current insights. Adv Med Educ Pract. 2018;9:125–131. doi: 10.2147/AMEP.S138758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayne IP, Brydges R, Moktar J, Murnaghan ML. Development and assessment of a distal radial fracture model as a clinical teaching tool. J BONE Jt Surg [Internet]. 2016 [cited 2017 Jul 14];98:410–6. Available from: http://insights.ovid.com/crossref?an=00004623-201603020-00010 [DOI] [PubMed]
- 32.Goff BA, Lentz GM, Lee D, Fenner D, Morris J, Mandel LS. Development of a bench station objective structured assessment of technical skills. Obstet Gynecol [Internet]. 2001 [cited 2017 Jul 12];98:412–6. Available from: http://ac.els-cdn.com/S0029784401014739/1-s2.0-S0029784401014739-main.pdf?_tid=0614599c-671d-11e7-87f7-00000aab0f6b&acdnat=1499876186_1231ab1b1c7ad1abed53d1909841acaa [DOI] [PubMed]
- 33.Rose K, Pedowitz R. Fundamental arthroscopic skill differentiation with virtual reality simulation. Arthrosc J Arthrosc Relat Surg [Internet]. 2015 [cited 2018 Mar 19];31:299–305. Available from: https://ac-els-cdn-com.libproxy.lib.unc.edu/S0749806314007154/1-s2.0-S0749806314007154-main.pdf?_tid=0258fdc6-d6ae-4cce-b212-fb6e0a12f604&acdnat=1521482765_68d74cd652aad08786da5570b17dc582 [DOI] [PubMed]
- 34.Moktar J, Popkin CA, Howard A, Murnaghan ML. Development of a cast application simulator and evaluation of objective measures of performance. J Bone Jt Surg - Am Vol [Internet]. 2014 [cited 2017 Jul 11];96:e76.1-e76.6. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00004623-201405070-00017 [DOI] [PubMed]
- 35.Dunn JC, Belmont PJ, Lanzi J, Martin K, Bader J, Owens B, et al. Arthroscopic shoulder surgical simulation training curriculum: transfer reliability and maintenance of skill over time. J Surg Educ [Internet] 2015;72:1118–1123. doi: 10.1016/j.jsurg.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Waterman BR, Martin KD, Cameron KL, Owens BD, Belmont PJ. Simulation training improves surgical proficiency and safety during diagnostic shoulder arthroscopy performed by residents. Orthopedics [Internet] 2016;39:e479–e485. doi: 10.3928/01477447-20160427-02. [DOI] [PubMed] [Google Scholar]
- 37.Cannon WD, Garrett WE, Hunter RE, Sweeney HJ, Eckhoff DG, Nicandri GT, et al. Improving residency training in arthroscopic knee surgery with use of a virtual-reality simulator. A randomized blinded study. J Bone Joint Surg Am [Internet] 2014;96:1798–1806. doi: 10.2106/JBJS.N.00058. [DOI] [PubMed] [Google Scholar]
- 38.Howells NR, Gill HS, Carr AJ, Price AJ, Rees JL. Transferring simulated arthroscopic skills to the operating theatre a randomised blinded study. J Bone Jt Surg [Br] [Internet]. 2008 [cited 2017 Jul 12];90:494–9. Available from: http://bjj.boneandjoint.org.uk.libproxy.lib.unc.edu/content/jbjsbr/90-B/4/494.full.pdf [DOI] [PubMed]
- 39.Tillander B, Ledin T, Nordqvist P, Skarman E, Wahlström O. A virtual reality trauma simulator. Med Teach. 2004;26:189–191. doi: 10.1080/0142159042000192037. [DOI] [PubMed] [Google Scholar]
- 40.Blyth P, Stott NS, Anderson IA. Virtual reality assessment of technical skill using the Bonedoc DHS simulator. Injury. 2008;39:1127–1133. doi: 10.1016/j.injury.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Vankipuram M, Kahol K, McLaren A, Panchanathan S. A virtual reality simulator for orthopedic basic skills: a design and validation study. J Biomed Inform [Internet]. Elsevier Inc.; 2010;43:661–8. Available from: 10.1016/j.jbi.2010.05.016 [DOI] [PubMed]
- 42.Froelich JM, Milbrandt JC, Novicoff WM, Saleh KJ, Allan DG. Surgical simulators and hip fractures: a role in residency training? J Surg Educ [Internet]. Elsevier Inc.; 2011;68:298–302. Available from: 10.1016/j.jsurg.2011.02.011 [DOI] [PubMed]
- 43.LeBlanc J, Hutchison C, Hu Y, Donnon T. Topics in training: a comparison of orthopaedic resident performance on surgical fixation of an ulnar fracture using virtual reality and synthetic models. J Bone Jt Surg - Ser A. 2013;95:1–6. doi: 10.2106/JBJS.K.01558. [DOI] [PubMed] [Google Scholar]
- 44.Akhtar K, Sugand K, Sperrin M, Cobb J, Standfield N, Gupte C. Training safer orthopedic surgeons. Acta Orthop. 2015;86:616–621. doi: 10.3109/17453674.2015.1041083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruder JA, Turvey B, Hsu JR, Scannell BP. Effectiveness of a low-cost drilling module in orthopaedic surgical simulation. [cited 2017 Aug 2]; Available from: https://www-clinicalkey-com.libproxy.lib.unc.edu/service/content/pdf/watermarked/1-s2.0-S193172041630229X.pdf?locale=en_US [DOI] [PubMed]
- 46.• Buck JS, Wally MK, Patt JC, Scannell B, Seymour RB, Hsu JR. Teaching cortical-screw tightening: a simple, affordable, torque-directed training protocol improves resident performance. J Bone Joint Surg Am. NLM (Medline). 2019:101, e51. The authors developed a low-fidelity training protocol to teach residents how to insert cortical screws without stripping the screw-bone interface. Immediately following training, all residents demonstrated improved ability to insert screws without stripping, but at 3-month follow-up, only senior residents retained the benefits of the training protocol. [DOI] [PubMed]
- 47.Anastakis DJ, Regehr G, Reznick RK, Cusimano M, Murnaghan J, Brown M, et al. Assessment of technical skills transfer from the bench training model to the human model. [cited 2017 Jul 13]; Available from: http://ac.els-cdn.com/S0002961098003274/1-s2.0-S0002961098003274-main.pdf?_tid=30e2008c-6800-11e7-b10d-00000aacb361&acdnat=1499973749_2439c3aa32a8900be2a507214ef3a8e6 [DOI] [PubMed]
- 48.Grober ED, Hamstra SJ, Wanzel KR, Reznick RK, Matsumoto ED, Sidhu RS, et al. The educational impact of bench model fidelity on the acquisition of technical skill: the use of clinically relevant outcome measures. Ann Surg [Internet] 2004;240:374–381. doi: 10.1097/01.sla.0000133346.07434.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blackwell M, Morgan F, Di Gioia AM. Augmented reality and its future in orthopaedics. Clin Orthop Relat Res. Lippincott Williams and Wilkins; 1998. p. 111–22. [DOI] [PubMed]
- 50.Ponce BA, Jennings JK, Clay TB, May MB, Huisingh C, Sheppard ED, et al. J Bone Jt Surg - Am. 2014;96:e84(1). doi: 10.2106/JBJS.M.00663. [DOI] [PubMed] [Google Scholar]
- 51.Davis MC, Can DD, Pindrik J, Rocque BG, Johnston JM. Virtual interactive presence in global surgical education: international collaboration through augmented reality. World Neurosurg [Internet]. NIH Public Access; 2016 [cited 2018 Mar 19];86:103–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26342783 [DOI] [PMC free article] [PubMed]
- 52.Shenai MB, Dillavou M, Shum C, Ross D, Tubbs RS, Shih A, et al. Virtual interactive presence and augmented reality (VIPAR) for remote surgical assistance. Neurosurgery [Internet]. 2011 [cited 2017 Aug 31];68:ons200–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21304333 [DOI] [PubMed]
- 53.Rahman R, Wood M. Head-mounted display use in surgery: a systematic review. 2019 [cited 2019 Nov 12]; Available from: 10.1177/1553350619871787
- 54.Ewurum CH, Guo Y, Pagnha S, Feng Z, Luo X. Surgical navigation in orthopedics: workflow and system review. Adv Exp Med Biol. Springer New York LLC; 2018. p. 47–63. [DOI] [PubMed]
- 55.Tepper OM, Rudy HL, Lefkowitz A, Weimer KA, Marks SM, Stern CS, et al. Mixed reality with HoloLens. Plast Reconstr Surg [Internet]. 2017 [cited 2018 Mar 16];140:1066–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29068946 [DOI] [PubMed]
- 56.•• Yong M, Pauwels J, Kozak FK, Chadha NK. Application of augmented reality to surgical practice: a pilot study using the ODG R-7 Smartglasses. Clin Otolaryngol [Internet]. 2019 [cited 2019 Nov 12]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/31610087. Remote attending surgeons ODG R7 HMD to guide trainees through simulated temporal bone dissection. Uploaded imaging and annotations to trainees field of view. Found issues with connectivity, lack of eye protection, no built-in headlight, angle of camera didn't align with user.
- 57.Albrecht U-V, von Jan U, Kuebler J, Zoeller C, Lacher M, Muensterer OJ, et al. Google Glass for documentation of medical findings: evaluation in forensic medicine. J Med Internet Res [Internet]. 2014 [cited 2017 Aug 29];16:e53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24521935 [DOI] [PMC free article] [PubMed]
- 58.Armstrong DG, Rankin TM, Giovinco NA, Mills JL, Matsuoka Y. A heads-up display for diabetic limb salvage surgery: a view through the Google looking glass. J Diabetes Sci Technol [Internet]. Diabetes Technology Society; 2014 [cited 2017 Aug 29];8:951–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24876445 [DOI] [PMC free article] [PubMed]
- 59.Knight HM, Gajendragadkar PR, Bokhari A. Wearable technology: using Google Glass as a teaching tool. BMJ Case Rep [Internet]. BMJ Publishing Group; 2015 [cited 2017 Aug 31];2015. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25969411 [DOI] [PMC free article] [PubMed]
- 60.Wei NJ, Dougherty B, Myers A, Badawy SM. Using Google glass in surgical settings: systematic review. JMIR mHealth uHealth [Internet]. JMIR Publications Inc.; 2018 [cited 2018 Mar 30];6:e54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29510969 [DOI] [PMC free article] [PubMed]
- 61.Qian L, Barthel A, Johnson A, Osgood G, Kazanzides P, Navab N, et al. Comparison of optical see-through head-mounted displays for surgical interventions with object-anchored 2D-display. Int J Comput Assist Radiol Surg [Internet]. 2017 [cited 2017 Aug 31];12:901–10. Available from: https://link-springer-com.libproxy.lib.unc.edu/content/pdf/10.1007%2Fs11548-017-1564-y.pdf [DOI] [PMC free article] [PubMed]
- 62.Peden RG, Mercer R, Tatham AJ. The use of head-mounted display eyeglasses for teaching surgical skills: a prospective randomised study. 2016 [cited 2017 Aug 31]; Available from: https://www-clinicalkey-com.libproxy.lib.unc.edu/service/content/pdf/watermarked/1-s2.0-S1743919116308500.pdf?locale=en_US [DOI] [PubMed]
- 63.An AR glasses pioneer collapses | TechCrunch [Internet]. [cited 2019 Nov 17]. Available from: https://techcrunch.com/2019/01/10/an-ar-glasses-pioneer-collapses/
- 64.Mahmood F, Mahmood E, Dorfman RG, Mitchell J, Mahmood FU, Jones SB, et al. Augmented reality and ultrasound education: initial experience. J Cardiothorac Vasc Anesth [Internet]. Elsevier Inc.; 2018;1–5. Available from: 10.1053/j.jvca.2017.12.006 [DOI] [PubMed]
- 65.Input Methods Overview | Magic Leap [Internet]. [cited 2019 Nov 17]. Available from: https://creator.magicleap.com/learn/guides/design-input-methods-overview
- 66.Magic Leap sees a bright future in AR healthcare [Internet]. [cited 2019 Nov 17]. Available from: https://www.fastcompany.com/90398573/magic-leap-sees-a-bright-future-in-ar-health-care
- 67.Pelargos PE, Nagasawa DT, Lagman C, Tenn S, Demos J V, Lee SJ, et al. Utilizing virtual and augmented reality for educational and clinical enhancements in neurosurgery. J Clin Neurosci [Internet]. 2017 [cited 2018 Mar 16];35:1–4. Available from: https://www-clinicalkey-com.libproxy.lib.unc.edu/service/content/pdf/watermarked/1-s2.0-S0967586816303162.pdf?locale=en_US [DOI] [PubMed]
- 68.•• Elmi-Terander A, Burström G, Nachabe R, Skulason H, Pedersen K, Fagerlund M et al. Pedicle screw placement using augmented reality surgical navigation with intraoperative 3D imaging: a first in-human prospective cohort study. Spine (Phila Pa 1976). NLM (Medline); 2019;44:517–25. AR assisted in vivo pedicle screw placement. Found 6% of screws breeched 2-4mm. Used display screen monitor system and noninvasive patient monitoring including C-arm mounted 3D cone-beam CT. Found low intraoperative screw revision rate (1.2%). [DOI] [PMC free article] [PubMed]
- 69.• Gibby JT, Swenson SA, Cvetko S, Rao Raj, Javan R, Rao R. Head-mounted display augmented reality to guide pedicle screw placement utilizing computed tomography. Int J Comput Assist Radiol Surg [Internet]. 2019 [cited 2019 Nov 12];14:525–35. Available from: 10.1007/s11548-018-1814-7. Bench model using Hololens to give superimposed CT-based image guidance during pedicle screw placement. Found 97% accuracy. [DOI] [PubMed]
- 70.Müller F, Roner S, Liebmann F, Spirig JM, Furnstahl P, Farshad M. Augmented reality navigation for spinal pedicle screw instrumentation using intraoperative 3D imaging. Spine J [Internet]. 2019 [cited 2019 Nov 12]; Available from. 10.1016/j.spinee.2019.10.012. [DOI] [PubMed]
- 71.Edström E, Burström G, Omar A, Nachabe R, Söderman M, Persson O, et al. Augmented reality surgical navigation in spine surgery to minimize staff radiation exposure. Spine (Phila Pa 1976). Ovid Technologies (Wolters Kluwer Health); 2019;1. [DOI] [PubMed]
- 72.Kosterhon M, Gutenberg A, Kantelhardt SR, Archavlis E, Giese A. Navigation and image injection for control of bone removal and osteotomy planes in spine surgery. Oper Neurosurg (Hagerstown, Md) [Internet] 2017;13:297–304. doi: 10.1093/ons/opw017. [DOI] [PubMed] [Google Scholar]
- 73.Cho HS, Oh JH, Han I, Kim HS. The outcomes of navigation-assisted bone tumour surgery: minimum three-year follow-up. J Bone Jt Surg - Ser B. 2012;94(B):1414–1420. doi: 10.1302/0301-620X.94B10.28638. [DOI] [PubMed] [Google Scholar]
- 74.Choi H, Park Y, Lee S, Ha H, Kim S, Cho HS, et al. A portable surgical navigation device to display resection planes for bone tumor surgery. Minim Invasive Ther Allied Technol. 2017;26:144–150. doi: 10.1080/13645706.2016.1274766. [DOI] [PubMed] [Google Scholar]
- 75.Cho HS, Park MS, Gupta S, Han I, Kim HS, Choi H, et al. Can augmented reality be helpful in pelvic bone cancer surgery? An in vitro study. Clin Orthop Relat Res. 2018;476:1719–1725. doi: 10.1007/s11999.0000000000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laitinen MK, Parry MC, Albergo JI, Grimer RJ, Jeys LM. Is computer navigation when used in the surgery of iliosacral pelvic bone tumours safer for the patient? Bone Jt J. 2017;99-B:261–266. doi: 10.1302/0301-620X.99B2.BJJ-2016-0149.R2. [DOI] [PubMed] [Google Scholar]
- 77.Gerrand C. CORR Insights®. Clin Orthop Relat Res. 2018;476:1726–1727. doi: 10.1097/01.blo.0000533634.13092.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.• van Duren BH, Sugand K, Wescott R, Carrington R, Hart A. Augmented reality fluoroscopy simulation of the guide-wire insertion in DHS surgery: a proof of concept study. Med Eng Phys. Elsevier Ltd; 2018;55:52–9. Augmented and VR combo trainer for inserting guidewires with optical tracking and fake fluoroscopic image overlay using sawbones model. Found accuracy to within 2mm. [DOI] [PubMed]
- 79.Hiranaka T, Fujishiro T, Hida Y, Shibata Y, Tsubosaka M, Nakanishi Y, et al. Augmented reality: the use of the picolinker smart glasses improves wire insertion under fluoroscopy. World J Orthop. 2017;8:891–894.pdf. doi: 10.5312/wjo.v8.i12.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diotte B, Fallavollita P, Wang L, Weidert S, Euler E, Thaller P, et al. Multi-modal intra-operative navigation during distal locking of intramedullary nails. IEEE Trans Med Imaging. 2015;34:487–495. doi: 10.1109/TMI.2014.2361155. [DOI] [PubMed] [Google Scholar]
- 81.Ma L, Zhao Z, Zhang B, Jiang W, Fu L, Zhang X, et al. Three-dimensional augmented reality surgical navigation with hybrid optical and electromagnetic tracking for distal intramedullary nail interlocking. Int J Med Robot Comput Assist Surgvery. John Wiley and Sons Ltd; 2018;14. [DOI] [PubMed]
- 82.Richter PH, Gebhard F, Dehner C, Scola A. Accuracy of computer-assisted iliosacral screw placement using a hybrid operating room. 2016 [cited 2018 Apr 5]; Available from: https://ac-els-cdn-com.libproxy.lib.unc.edu/S0020138315007457/1-s2.0-S0020138315007457-main.pdf?_tid=a69b6021-571c-4240-9dee-b21f037899e0&acdnat=1522947591_d38225497a120d41d0a0e43892726b48 [DOI] [PubMed]
- 83.Wang H, Wang F, Leong APY, Xu L, Chen X, Wang Q. Precision insertion of percutaneous sacroiliac screws using a novel augmented reality-based navigation system: a pilot study. Int Orthop Springer Verlag. 2016;40:1941–1947. doi: 10.1007/s00264-015-3028-8. [DOI] [PubMed] [Google Scholar]
- 84.Fallavollita P, Brand A, Wang L, Euler E, Thaller P, Navab N, et al. An augmented reality C-arm for intraoperative assessment of the mechanical axis: a preclinical study. Int J CARS [Internet]. 2016 [cited 2018 Mar 19];11:2111–7. Available from: https://link-springer-com.libproxy.lib.unc.edu/content/pdf/10.1007%2Fs11548-016-1426-z.pdf [DOI] [PubMed]
- 85.Ogawa H, Hasegawa S, Tsukada S, Matsubara M. A pilot study of augmented reality technology applied to the acetabular cup placement during total hip arthroplasty. 2018 [cited 2018 Mar 16]; Available from: https://www-clinicalkey-com.libproxy.lib.unc.edu/service/content/pdf/watermarked/1-s2.0-S0883540318301050.pdf?locale=en_US [DOI] [PubMed]
- 86.Fotouhi J, Alexander CP, Unberath M, Sing GT, Lee C, Fuerst B, et al. Plan in 2-D, execute in 3-D: an augmented reality solution for cup placement in total hip arthroplasty. Med Imag. 2018;5:21205. doi: 10.1117/1.JMI.5.2.021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.• Logishetty K, Western L, Morgan R, Iranpour F, Cobb JP, Auvinet E. Can an augmented reality headset improve accuracy of acetabular cup orientation in simulated THA? A randomized trial. Clin Orthop Relat Res. Ovid Technologies (Wolters Kluwer Health); 2019;477:1190–9. Looked at accuracy differences in acetabular cup placement between novices trained by a surgeon vs. those trained with AR assistance with Hololens guidance. Found no differences in placement accuracy between groups. [DOI] [PMC free article] [PubMed]
- 88.•• Lei P-F, Su S-L, Kong L-Y, Wang C-G, Zhong D, Hu Y-H. Mixed Reality Combined with three-dimensional printing technology in total hip arthroplasty: an updated review with a preliminary case presentation. 2019; First example of using 3D printing, HMD, and anatomic referencing intraoperatively to perform Total Hip Arthroplasty. The authors developed a low-fidelity training protocol to teach residents how to insert cortical screws without stripping the screw-bone interface. Immediately following training, all residents demonstrated improved ability to insert screws without stripping, but at 3-month follow-up, only senior residents retained the benefits of the training protocol.
- 89.Guraya SY. Using telementoring and augmented reality in surgical specialties. J. Taibah Univ. Med. Sci. Elsevier B.V.; 2019. p. 101–2. [DOI] [PMC free article] [PubMed]
- 90.Kaylor J, Hooper V, Wilson A, Burkert R, Lyda M, Fletcher K, et al. Reliability testing of augmented reality glasses technology. J Wound, Ostomy Cont Nurs [Internet]. 2019 [cited 2019 Nov 12];1. Available from: http://insights.ovid.com/crossref?an=00152192-900000000-99717 [DOI] [PubMed]
- 91.Ponce BA, Brabston EW, Zu S, Watson SL, Baker D, Winn D, et al. Telemedicine with mobile devices and augmented reality for early postoperative care. 2016 38th Annu Int Conf IEEE Eng Med Biol Soc [Internet]. 2016;22031:4411–4. Available from: http://ieeexplore.ieee.org/document/7591705/ [DOI] [PubMed]
- 92.Ponce BA, Menendez ME, Oladeji LO, Fryberger CT, Dantuluri PK. Emerging technology in surgical education: combining real-time augmented reality and wearable computing devices. Orthopedics Slack Incorporated. 2014;37:751–757. doi: 10.3928/01477447-20141023-05. [DOI] [PubMed] [Google Scholar]
- 93.Lindeque BGP, Ponce BA, Menendez ME, Oladeji LO, Fryberger CT, Dantuluri PK. Emerging technology in surgical education: combining real-time augmented reality and wearable computing devices. Orthopedics [Internet]. 2014;37:751–7. Available from: http://www.healio.com/doiresolver?doi=10.3928/01477447-20141023-05 [DOI] [PubMed]
- 94.Dixon BJ, Daly MJ, Chan HHLL, Vescan A, Witterick IJ, Irish JC. Inattentional blindness increased with augmented reality surgical navigation. Am J Rhinol Allergy [Internet]. 2014;28:433–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25198032 [DOI] [PubMed]
- 95.Hughes-Hallett A, Mayer EK, Marcus HJ, Pratt P, Mason S, Darzi AW, et al. Inattention blindness in surgery. Surg Endosc Other Interv Tech [Internet]. 2015 [cited 2018 Mar 19];29:3184–9. Available from: https://link-springer-com.libproxy.lib.unc.edu/content/pdf/10.1007%2Fs00464-014-4051-3.pdf [DOI] [PubMed]
- 96.Vávra P, Roman J, Zonča P, Ihnát P, Němec M, Kumar J, et al. Recent development of augmented reality in surgery: a review. J. Healthc. Eng. Hindawi Limited; 2017. [DOI] [PMC free article] [PubMed]
- 97.Antonini G, Stuflesser W, Crippa C, Touloupakis G. A distal-lock electromagnetic targeting device for intramedullary nailing: Suggestions and clinical experience. Chinese J Traumatol - English Ed Elsevier BV. 2016;19:358–361. doi: 10.1016/j.cjtee.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Veliceasa B, Bandac R, Popescu D, Puha B, Alexa O. Evaluation of TRIGEN SURESHOT® distal targeting system - a new electromagnetic computer-assisted guidance system. 2015 E-Health Bioeng Conf EHB 2015. Institute of Electrical and Electronics Engineers Inc.; 2016.
- 99.Elmi-Terander A, Skulason H, Soderman M, Racadio J, Homan R, Babic D, et al. Surgical navigation technology based on augmented reality and integrated 3D intraoperative imaging a spine cadaveric feasibility and accuracy study. Spine (Phila Pa 1976) 2016;41:E1303–E1311. doi: 10.1097/BRS.0000000000001830. [DOI] [PMC free article] [PubMed] [Google Scholar]