Abstract
Natural killer (NK) cells play a pivotal role in controlling cancer. Multiple extracellular receptors and internal signaling nodes tightly regulate NK activation. Cyclin Dependent Kinases of the mediator complex (CDK8 and CDK19) were described as a signaling intermediates in NK cells. Here we report for the first time the development and use of CDK8/19 inhibitors to suppress phosphorylation of STAT1S727 in NK cells and to augment the production of the cytolytic molecules perforin and granzyme B (GZMB). Functionally, this resulted in enhanced NK cell mediated lysis of primary leukemia cells. Treatment with the CDK8/19 inhibitor BI-1347 increased the response rate and survival of mice bearing melanoma and breast cancer xenografts. In addition, CDK8/19 inhibition augmented the anti-tumoral activity of anti-PD-1 antibody and SMAC mimetic therapy, both agents that promote T-cell mediated antitumor immunity. Treatment with the SMAC mimetic compound BI-8382 resulted in an increased number of NK cells infiltrating EMT6 tumors. Combination of the CDK8/19 inhibitor BI-1347, that augment the amount of degranulation enzymes, with the SMAC mimetic BI-8382 resulted in increased survival of mice carrying the EMT6 breast cancer model. The observed survival benefit was dependent on an intermittent treatment schedule of BI-1347, suggesting the importance of circumventing a hypo-responsive state of NK cells. These results suggest that CDK8/19 inhibitors can be combined with modulators of the adaptive immune system to inhibit the growth of solid tumors, independent of their activity on cancer cells, but rather through promoting NK cell function.
Keywords: CDK8 inhibitors, CDK19 inhibitors, NK cells, PD-1, SMAC mimetics, AML
Introduction
Natural killer (NK) cells play critical roles in host immunity against infection and cancer. In contrast to T cells, NK cells rapidly kill infected, damaged and transformed cells without prior immunization or major histocompatibility complex (MHC) restriction. NK cell activation depends on the net balance of inhibitory and activating signals from different cytokines and receptors (1–3). Stronger signals from activating receptors or reduced signals from inhibitory receptors result in enhanced activation of NK cells. Interference with the inhibitory pathway in NK cells leads to increased NK effector function and improved tumor immune-surveillance that translates into increased patient survival (4). Signal Transducer and Activator of Transcription (STAT1) has been described as an important regulator of NK cell activity (5,6) and its function is stimulated by JAK-mediated phosphorylation of STAT1Y701, resulting in dimerization and nuclear translocation. In contrast, phosphorylation of STAT1S727 has been linked to decreased NK cell activity as reflected by lower expression of granzyme B and perforin (7,8). The connection between STAT1S727 phosphorylation and NK cell activity is based on several lines of evidence such as point mutation of a single phosphorylation site (STAT1S727A) which was described to enhance NK cell cytotoxicity and delayed disease onset in NK cell surveilled tumor models (7,8). Nevertheless the molecular basis for how S727 phosphorylation negatively impacts NK cell function is not fully understood.”
Members of the cyclin dependent kinase family coordinate regulatory events during the cell cycle as well as transcription. Cyclin dependent kinase 8 (CDK8) and CDK19 regulate transcription via interaction with the mediator complex and by phosphorylation of transcription factors (9–13). Since the first description of CDK8 as putative colorectal cancer oncogene in 2008 (9), several CDK8/19 inhibitors have been developed as targeted agents (9–13). However, tolerability issues in preclinical animal models have limited the application of these agents in daily dosing regimens (14).
CDK8 has been shown to phosphorylate STAT1S727 in response to stimulation with interferon beta (IFNβ) or other cytokines (7,15). Knockdown of CDK8 in natural killer cells enhances cytotoxicity against tumor cells where the CDK8 paralog CDK19 may partially compensate for CDK8 with regards to STAT1 phosphorylation (7,8). We have developed the highly selective small molecule inhibitors of CDK8/19, Compound 2 and BI-1347 that potently decrease the phosphorylation of STAT1S727. This augments the production of the cytolytic molecules perforin and granzyme B resulting in increased anti-tumor activity and survival in in vivo murine tumor models.
Enhancing and prolonging NK cell activation and tumor infiltration remain major challenges for NK cell-based immunotherapies for cancer patients as chronic activation of NK cells was described to induce a hypo-responsive state (16–18). These challenges could be overcome by using an intermittent treatment schedule and rational combination therapies that promote NK cell tumor infiltration and engagement of the adaptive immune system. As SMAC mimetics (19) were recently described to promote both T cell-mediated (20) and NK cell-mediated (21) antitumor immunity, we hypothesized that a combination of SMAC mimetic with CDK8/19i therapy may result in synergistic effects. Indeed, we show that the SMAC mimetic BI-8382 increases the number of NK cells and T-cells and the combination with the CDK8/19 inhibitor BI-1347 enhances the NK cell cytotoxicity results in a strong synergistic antitumor efficacy in the murine syngeneic EMT6 breast cancer model. Our data support the application of intermittent dosing of CDK8/19 inhibitors for cancer immunotherapy.
Materials and methods
Compounds and reagents
Compound 1 (Comp. 1), Compound 2 (Comp. 2), BI-1347 (CDK8/19 inhibitors) and BI-8382 (SMAC mimetic) were synthesized at Boehringer Ingelheim. Synthesis details for the CDK8/19 inhibitors can be found in the patent application US20140323468 (compound 1 = 159) and WO2017202719 (Compound 2 = I-015, BI-1347 = I-003). BI-1347 is available through the open innovation portal (https://opnme.com/molecules/cdk8-bi-1347) of Boehringer Ingelheim. The CD33 Fc-engineered monoclonal antibody (mAb) BI 836858 was produced by Boehringer Ingelheim (22). IL-2 (Peprotech, Rocky Hill, NJ), IL-12 and IFNβ (R&D Systems/Biomedica) were used for cell culture experiments. Annexin-V FITC and propidium iodide (BD Biosciences) were used for viability assays. Antibodies against human CD45, CD33, CD34, CD3, CD56, CD16, granzyme B, and perforin (Biolegend) were used for flow cytometry experiments. A rat IgG2a monoclonal anti-mouse PD-1 antibody RMP1–14 (Bio X cell) was used in the syngeneic MC-38 mouse study.
CDK8/CycC LanthaScreen Eu Kinase Binding Assay
The LanthaScreen Eu Kinase Binding assay was used to identify compounds that competitively interact with the ATP binding pocket of CDK8. All assay reagents for this assay including CDK8/CycC (PR7261B), Biotin anti-His Tag Antibody (PV6090), LanthaScreen Eu-Streptavidin (PV6025) and Kinase Tracer 236 (PR9078A) were obtained from Thermo Fisher Scientific (details in supplement).
SelectScreen Kinase Profiling Services
The effect of selected compounds on the activity of various kinases was evaluated by measurement in the SelectScreen® Kinase Profiling Service (Thermo Fisher Scientific). Single point measurements in the assays were performed using 1 μM of the test compounds and the indicated ATP concentration. IC50 measurements of compounds were performed in assays employing a 20 μM start concentration with 10 subsequent 10-fold dilution steps.
Protein production and structural analysis
CDK8 (1–403) containing an N-terminal His6-tag followed by a TEV cleavage site was co-expressed with cyclin C (CycC, 1–283) containing an N-terminal GST-tag and a TEV cleavage site in Trichoplusia ni cells (BaculoDirect, ThermoFisher). The purification and crystallization of the complexes is described in the supplementary section. The CDK8/CycC structure in complex with compound 1 was obtained as previously described (23). Structures have been deposited in the Protein Data Bank (pdb IDs: 6R3S; 6QTJ, 6QTG, data and refinement statistics in Supplementary Table S1).
Cell lines
A detailed list of all cell lines and their source is listed in Supplementary Table S2.
Primary AML, B-lymphoma and NK Cells
Primary leukemic and lymphoma cells were obtained from patients following written informed consent under an institutional review board approved protocol of Ohio State University or the Hospital Universitario Central de Asturias, Spain, according to the Declaration of Helsinki. Normal NK cells from leukopacks purchased from blood bank were isolated using RosetteSep™ Human NK Cell Enrichment Cocktail (Stemcell Technologies) and Ficoll-Paque Plus centrifugation (Amersham/GE Healthcare Bio-Sciences). Cells were cultured in RPMI 1640 (Life Technologies) with 10–20% fetal bovine serum (FBS) (Sigma-Aldrich); 2 mmol/l L-glutamine; penicillin (100 U/ml); streptomycin (100 μg/ml) (Life Technologies). Acute myeloid leukemia (AML) cells were supplemented with 10 ng/ml GM-CSF and SCF (R&D Systems) overnight.
Viability Assays
Cells were plated in 96-well flat-bottom culture plates and CDK8/19 inhibitors were added at various concentrations. Depending on the growth of the cell lines, compounds were added at either 72 hours or 120 hours and cultured overnight in an incubator at 37°C with a humidified atmosphere containing 5% CO2. The CellTiter-Glo® luminescent cell viability assay (Promega) or AlamarBlue® assay (Thermo Fischer Scientific) were used to determine the activity of compounds in proliferation assays. For AML primary cells, the HL-60 cell line and primary NK cells, viability was determined by staining with annexin V-FITC and propidium iodide followed by flow cytometry analysis.
Perforin release assay
The NK cell line NK92MI (CRL-2408) was seeded at 40.000 cells/well with test compounds at various concentrations in 96 well culture plates. IL-15 (50 ng/ml) treated cells were used as a positive control. The plates were incubated at 37°C in an incubator as described above. After 24 h, the amount of secreted perforin was measured in the supernatants using an ELISA assay (Mabtech AB).
Immunoblotting
To analyze the effect of CDK8/19 inhibitors on the phosphorylation of STAT1, NK92MI cells were incubated with test compounds at various concentrations for 6 h. Thereafter, 100 U/ml IFN-β (R&D systems/Biomedica) was added and the cells incubated for an additional 1 h. Cells were then lysed and the lysate was analyzed by Western blotting using a phospho STAT1S727 antibody (Cell Signaling Technology #9177S), pSTAT1Tyr701, STAT1 (Santa Cruz Biotechnology #sc-592), and STAT3Tyr705 (Cell Signalling Technology #7649), STAT3 (Cell Signalling Technology #9134) and STAT5 (Cell Signalling Technology #9363). Human primary NK cells purified from healthy donors (1 million cell/well in 6 well plates) were treated with CDK8/19 inhibitors and incubated for 6 h followed by addition of IL-2 (10 ng/ml) and IFNβ (100 U/ml) for 1 h. Cell lysates were analyzed for STAT1S727 phosphorylation using Western Blotting. Alternatively, primary NK cells from healthy donors (2 million cells/ml) were treated simultaneously with CDK8/19 inhibitors and either IL-2 (10 ng/ml), IL-2 and IL-12 (5 ng/ml) or IL-2 and IFN-β (100 U/ml) for 16 h prior to Western blot analysis of phospho STAT1S727 in cell lysates. The effect of 24 hours treatment of NK92MI cells with either the CDK8 inhibitor BI-1347 or the SMAC mimetic BI-8382 was analyzed by Western Blotting using a CDK8/19 specific antibody from Cell Signaling (#4101S).
Modulation of granzyme B in mouse splenocytes
Splenocytes were cultured in RPMI1640 supplemented with 10% FBS, 10 mmol/l HEPES, 15 mmol/l sodium pyruvate, 1% penicillin/streptomycin and 0.57 μmol/l β-mercaptoethanol and treated with either 2.5 ng/ml IL-15, 150 nmol/l BI-1347 or the combination thereof and incubated for 44 h in an incubator as described above. Protein Transport Inhibitor Cocktail (eBioscience) was added for the last 4 h at 37°C. Separate aliquots of the cells were analyzed by Western blotting and flow cytometry using the following antibodies: CD3epsilon (eFluor 450), NK1.1 (PerCP-Cy5.5) and granzyme B (PE) (eBioscience). Intracellular staining was performed using fixation and permeabilization buffer (eBioscience). Stained cells were collected and analyzed with a FACS Canto II instrument.
Flow cytometry
1–2 million NK cells were stained in ice-cold flow buffer (PBS with 5% FBS and 0.1% sodium azide) containing a live/dead near-IR stain (Life Technologies) and relevant antibodies prior to analysis with a BD LSR Fortessa flow cytometer (BD Biosciences). For intracellular staining, cells were fixed and permeabilized before staining using the Fixation/Permeabilization Solution Kit (BD Biosciences). Flow data were analyzed using Kaluza software 1.5a (Beckman coulter). For further details see supplement.
Antibody dependent cellular cytotoxicity (ADCC) Assays
ADCC was measured using the previously described chromium-51 (51Cr) release assay (22). HL-60 target cells were labelled with 51Cr, washed and incubated with anti-CD33 antibody (BI 836858) or isotype control (BI 836847) at 5 μg/ml for 30 min. Effector cells (normal NK cells from healthy donors), pretreated overnight with vehicle or CDK8/19 inhibitor, were added to target cells at effector:target (E:T) ratios of 6:1 and 3:1. Supernatants were collected after 4 h and counted in a gamma counter. Percent lysis was calculated by (ER-SR)/(MR-SR), where ER, SR, and MR represent experimental release (ER), spontaneous release (SR) and maximum release (MR), respectively. For CD20-mediated ADCC, primary leukemia cells and autologous PBMCs obtained from chronic lymphocytic leukemia patients were stimulated with CDK8/19 inhibitors and rituximab (10 μg/ml) for 48 h. The depletion of leukemia cells was analyzed by flow cytometry using PKH26 reference microbeads (Sigma-Aldrich). For ADCC of leukemia cells, purified NK cells from healthy donors pretreated with vehicle or CDK8/19 inhibitor for 48 hours were added to purified chronic lymphocytic leukemia (CLL) cells from 4 patients in the presence of vehicle or rituximab at E:T ratio of 1:1 for 4 hours. Leukemia cells pretreated with vehicle or CDK8/19 inhibitor for 48 hours were also incubated with vehicle or rituximab in absence of NK cells for 4 hours. Leukemia cell death was measured by flow cytometry.
Physiochemical Properties and Pharmacokinetics
Caco-2 parameters were determined as described previously (24). Aqueous solubilities were determined from 1 mg/mL solid compound dispensed either into aqueous McIlvaine buffer (pH 4.5 or 6.8) or dissolved in acetonitrile/water (1:1) as reference. Dissolved concentrations were determined with an Agilent 1200 HPLC/DAD-UV system.
Plasma protein binding (PPB) of compounds was determined by equilibrium dialysis. Plasma was spiked with a given compound and dialyzed against Soerensen buffer (pH 7.4) for 3 h at 37°C. PPB was calculated by quantifying the concentration of the compound in plasma and buffer by HPLC/MS-MS. In vivo hepatic clearance (CL) was predicted by in vitro incubation of compounds with cryopreserved hepatocytes (Celsis IVT) followed by quantitation of compound depletion over 240 min using LC/MS/MS and the well-stirred model.
In vivo efficacy studies
All animal studies were approved by the internal ethics committee and the local governmental committee. Group size in efficacy studies have been selected after performing power analysis. In vivo imaging and treatment of subcutaneous tumors was performed as described previously (25). Female CIEA-NOG mice (NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac; Taconic, Denmark) were engrafted intravenously with 10 million pLPNIG-transduced MV-4–11 AML cells. Compound treatment (treatment groups of 10 mice) was initiated after randomization based on body weight on day 8 following tumor cell inoculation (Fig. S1). Female C57BL/6N mice (Taconic, Denmark) were engrafted intravenously with 100 thousand B16-F10-luc2 cells (PerkinElmer) and treatment was initiated after randomization based on body weight on day 5 following tumor cell inoculation (10 mice per group). Female C57BL/6N mice (Taconic) were engrafted subcutaneously with 100 thousand MC38 cells and treatment started after randomization based on body weight on day 3 (5 mice per group). Female BALB/cJBomTac mice (Taconic) were engrafted subcutaneously with 1 million EMT6 cells suspended in Matrigel and randomization performed after 9 d (8–10 mice per group) at which time tumors reached a volume of 90 and 140 mm3. Tumor growth inhibition (TGI) was calculated to compare the treatment groups. This was done by using this equation: TGI = [1-((treated(final) – treated(Day1 treatment)) /( control(final) – control(Day1))] * 100%. A TGI <100 % describes reduced tumor growth compared to the control group and > 100% describes tumor regression.
To deplete NK cells, an antiserum containing anti-asialo-GM1 antibody (Wako, 200 μl of 1:10 in 1xPBS diluted antiserum) was injected intraperitoneal on two consecutive days and then subsequently applied twice weekly. For depletion of CD8+ T cells, the anti-mouse CD8 antiserum (clone 2.43, BioXCell) was applied (i.p., 0.25 mg in 200 μL PBS) on three consecutive days and then subsequently every third day. Depletion of NK or CD8+ T cells was initiated 4 d prior to randomization and confirmed by flow cytometry analysis of blood samples. All biomarker experiments including explanted tissue (e.g. splenocytes or tumour) were performed with 3–6 mice per group. Any of the presented experiments were at least performed twice with different CDK8i or SMAC mimetic from the same compound class with very similar results.
Statistical analysis
Cell proliferation assay data were fitted by iterative calculation using a sigmoidal curve analysis program (Prism version 3.0; Graph Pad, La Jolla, CA) with variable hill slope for determination of EC50 values. In case treatment groups were compared one-sided non-parametric Mann-Whitney-Wilcoxon U-tests were applied. The level of significance was fixed at α = 5%. An (adjusted) p value of less than 0.05 was considered to show a statistically significant difference between the groups and differences were seen as indicative whenever 0.05 ≤ p-value < 0.10.
In case several groups were compared p-values were adjusted for multiple comparisons according to Bonferroni–Holm. Kaplan-Meier survival curves were calculated using the curve analysis program Graph Pad. Data are represented as dot plots with bar graphs for mean ± standard deviation (s.d.).
Results
Identification of potent and highly selective CDK8/19 inhibitors
To identify favorable starting points for lead optimization, a library of 1850 kinase inhibitors was screened against CDK8 in a biochemical kinase assay. This identified compound 1 as the most promising hit. Compound 1 inhibited the enzymatic activity of CDK8 with an IC50 of 910 nmol/L. Analysis of the x-ray structure of compound 1 in CDK8 (Fig. 1A) revealed three areas of the compound where structural changes could lead to an improvement in potency (Fig. 1B). After several rounds of optimization, two compounds (Compound 2 and BI-1347) were identified that displayed high potency and selectivity and attractive drug-like properties, thus validating the initial design strategy. Both compounds showed similarly high potency with IC50 values in the CDK8 kinase assay of 1.8 and 1.4 nmol/L, respectively. BI-1347 displayed higher metabolic stability in vitro and in vivo (Fig. 1B). Both compounds displayed exquisite selectivity for CDK8 with no inhibition of other CDKs tested (e.g. CDK1, CDK2, CDK4, CDK6, CDK7 and CDK9) (Supplementary Table S4). Only one additional kinase was inhibited out of 326 kinases tested (at IC50 values < 1.0 μmol/L), namely CDK19, the close homologue of CDK8. A selectivity ratio of > 300-fold was observed towards all other kinases tested (Supplementary Table S4).
Figure 1.
Small molecule CDK8/19 inhibitors. A, x-ray of compound 1 in CDK8. B, Starting point of the medicinal chemistry optimization campaign for discovery of CDK8/19 inhibitors: Compound 1. CDK8/19 inhibitors: Compound 2 and BI-1347.
CDK8/19 inhibitors decrease phosphorylation of STAT1S727 and activate NK cells
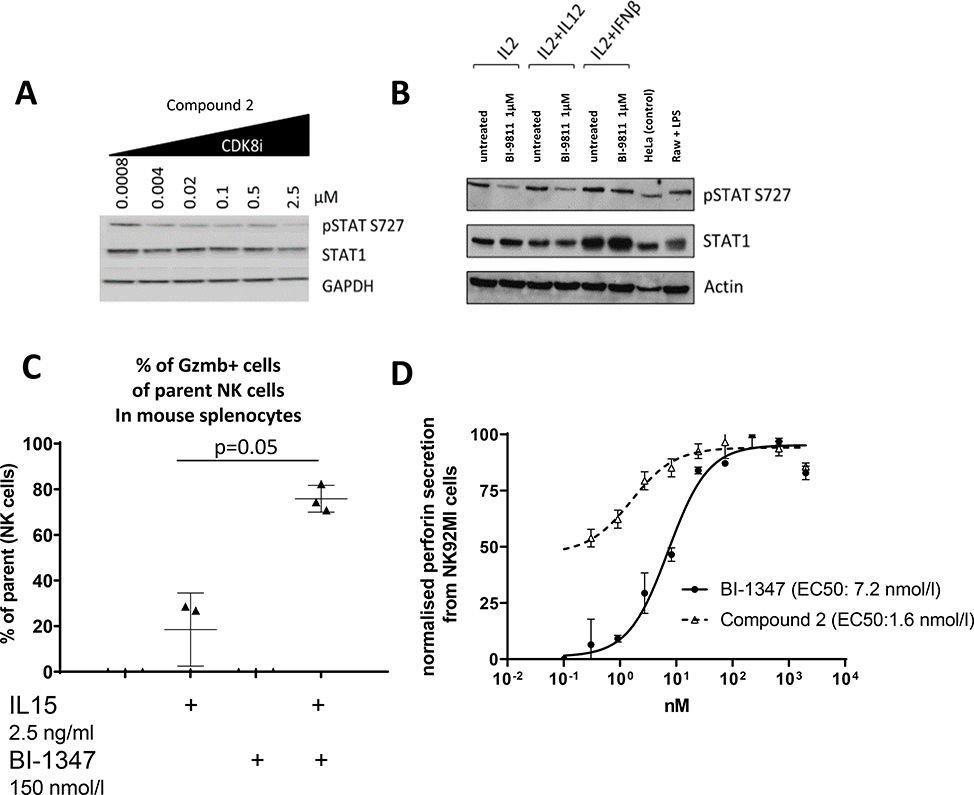
We next evaluated the ability of the CDK8/19 inhibitors to modulate STAT1S727 phosphorylation and NK cell activity. Compound 2 treatment inhibited STAT1S727 phosphorylation in the NK92MI cell line (Fig. 2A) and in IL2 and IL12 activated primary human NK cells isolated from healthy donors (Fig. 2B). Similar effects were seen on murine NK cells (as discussed below in Fig 4). We then assessed the effect of CDK8/19 inhibitors on granzyme B-positive murine spleen-derived NK cells that had been activated with IL-15 in vitro. BI-1347 treatment increased the proportion of granzyme B-positive NK cells by approximately 4-fold (Fig. 2C). This indicated that CDK8/19 inhibition resulted in NK cell activation. In order to test if secretion of perforin was impacted following treatment with CDK8/19 inhibitors, human NK92MI cells were treated with either Compound 2 or BI-1347. While proliferation of NK92MI cells was not affected by treatment with Compound 2 (Supplementary Table S3), the treatment increased perforin levels in the supernatant samples (Fig. 2D). Importantly in NK92MI cells, neither CDK8 protein levels nor phosphorylation of STAT1Tyr701 and STAT3Tyr705, which is mediated by the Janus kinases (JAK1 and 2), was affected by treatment with the CDK8/19 inhibitors (Supplementary Fig S3).
Figure 2.
Pharmacodynamics modulation of biomarkers following treatment with a CDK8/19 inhibition. A, Modulation of STAT1S727 phosphorylation in NK92MI cells by the CDK8/19 inhibitor Compound 2. B, Modulation of STAT1S727 phosphorylation in primary human NK cells from healthy donors by Compound 2. C, BI-1347 enhances granzyme B (GZMB+) production in mouse splenic NK cells (gating: viability dye, CD3−, NK1.1+, GZMB+). Levels of GZMB+ were below baseline in absence of IL-15 (plus/minus addition of BI- 1347). Gating strategy is presented in Supplementary Fig. S8 D, Perforin secretion from NK92MI cells after CDK8/19 inhibitor treatment measured in the supernatant by a commercial ELISA.
Figure 4.
Lysis of B-chronic lymphocytic leukemia cells in presence of Rituximab and the CDK8/19 inhibitor BI-1347. A, The CDK8/19 inhibitor BI-1347 enhances the ADCC mediated depletion of primary leukemia cells derived from chronic lymphocytic leukemia patients by rituximab in presence of PBMCs. B-cell counts is shown normalized to mean of DMSO treated controls (n=5). B, Purified NK cells from healthy donors pretreated with vehicle or CDK8/19 inhibitor for 48 hours were added to purified chronic lymphocytic leukemia cells from 4 patients in the presence of vehicle or rituximab at E:T ratio of 1:1 for 4 hours. Percent of specific lysis of primary leukemia cells derived from chronic lymphocytic leukemia patients in presence or absence of purified NK cells (n=4). (*≤0.05 ** < 0.01, *** < 0.001, two sided Student’s t-test).
CDK8/19 inhibition impacts the growth of a subset of hematological cancer cells lines, whilst solid tumor cell lines are unaffected
CDK8/19 inhibitors have been previously developed by other groups with the intention to directly target tumor cells (26). We used Compound 2 and BI-1347 to investigate the intrinsic dependency of a panel of solid and hematological cancer cell lines on CDK8/19 function. Compound-2 treatment resulted in cell growth inhibition (IC50 < 1 μM) in only 5 (OCI-Ly3, HBL-1, MV-4–11B, KG1 and MM1R) of 51 hematological lines tested. None of the 49 solid cancer cell lines, including the two mouse cell lines MC-38 and EMT6, were affected by Compound 2 (Supplementary Table S4). Similar effects were observed with BI-1347 in a smaller cell panel. The in vitro sensitivity of these cell lines (e.g. MV-4–11B) correlated with in vivo single-agent efficacy of BI-1347, as exemplified shown by MV-4–11B tumors treated with BI-1347 (Supplementary Fig. S1).
Since two of the five sensitive cell lines were AML-derived models (KG-1 and MV4–11), we tested the effect of Compound 2 on primary patient-derived human AML. However, no change in viability was observed in these primary AML samples in the presence of Compound 2 (Fig. 3A). No change in viability was also observed in primary human NK cells after 24 hours of incubation with two different doses of Compound-2 (Supplementary Fig. S6).
Figure 3.
The CDK8/19 inhibition inhibitor Compound 2 (Comp. 2), enhances NK cell mediated lysis of AML cells. The CDK8/19 inhibitor Compound 2 did not affect primary AML cell viability as assessed by absence of PI and Annexin staining (A) and CD33 surface expression (B). CD33 expression was measured by flow cytometry. Mean fluorescence intensity (MFI) of CD33 gated on viable myeloid population (CD45 low Side Scatter intermediate-high) is shown. C, Compound 2 did not affect the viability or CD33 expression of HL-60 cells. HL-60 cells were cultured with indicated concentrations of Compound 2 for 24 h prior to assessment of viability and CD33 expression by flow cytometry. D, Compound 2 enhanced the ADCC activity of NK cells against the AML cell line HL-60. Effector NK cells (n=2 healthy donors) were treated with Compound 2 overnight prior to incubation with radiolabeled HL-60 target cells that had been pretreated with the CD33 mAb BI 836858 or isotype control antibody. The percentage lysis of HL-60 cells normalized to that mediated by vehicle-treated NK cells is shown.
CDK8/19 inhibitor Compound 2 enhances ADCC-mediated tumor cell lysis by NK cells
NK cells are important mediators of ADCC, through the interaction of target bound antibodies with the Fc-receptor CD16 on NK cells (27–31). We therefore investigated if CDK8/19 inhibition is able to enhance the ADCC activity of BI 836858; an Fc-engineered anti-CD33 mAb demonstrating ADCC activity (32,33). CD33 is a common myeloid antigen expressed in more than 80% of AML cases. In this assay we used the human leukemia cell line HL-60 cells that have been previously demonstrated to express CD33 (32). We first confirmed that CDK8/19 inhibitor treatment had no effect on the viability or CD33 cell surface expression on primary AML cells and HL-60 cells (Fig. 3B and Fig. 3C). We next evaluated whether CDK8/19 inhibitor treatment could enhance CD33 antibody-dependent NK cell-mediated cytotoxicity by performing radio-labelled HL-60 target cell lysis assays using BI 836858 (32,33) and human primary NK cells isolated from healthy donors. Prior to performing the ADCC assay we confirmed that CDK8/19 inhibitor treatment had no effect on the viability of primary NK cells (Supplementary Fig. S6). Treatment with the CD33 antibody and simultaneous inhibition of CDK8/19 by Compound 2 in presence of NK cells resulted in 25–75% enhanced lysis of HL-60 cells versus control (Fig. 3D) or a 7–17% increased cell lysis compared to cells treated with the antibody alone (Supplementary Fig. S7). This effect was observed with two effector to tumor ratios and using NK cells from two healthy donors. In the ADCC assays no effect was observed on the activation marker CD107a following treatment with Compound 2 (Table S5 and Supplementary Fig. S9). The result suggests that the effects of CDK8/19 inhibition on STAT1 phosphorylation and expression of perforin and granzyme B as described above, might translate into an increased killing capacity of NK cells.
ADCC was studied in a second assay using primary tumor cells obtained from chronic lymphocytic leukemia (CLL) patients. Here again the CDK8/19 inhibitors enhanced the ADCC-mediated activity of the CD20 antibody Rituximab, as assessed by a reduction of primary leukemia B-cells in the presence of PBMC (Fig. 4A) or NK cells purified from healthy donors (Fig. 4B). This enhanced ADCC effect mediated by BI-1347 was not observed in absence of NK cells (Fig. 4B). We could demonstrate that ADCC of two Fc-engineered antibodies (CD33 and CD20 antibody Rituximab) were enhanced in combination with a CDK8/19 inhibitor.
CDK8/19 inhibitors Compound 2 and BI-1347 modulate STAT1S727 phosphorylation and show anti-tumor efficacy in vivo
Pharmacokinetic analysis of BI-1347 revealed a low clearance (14% QH) after i.v. administration. Excellent exposure and bioavailability (F) was observed after p.o. dosing. Concentration of BI-1347 24 h after dosing at 25 mg/kg p.o. was around 100-fold the CDK8 IC50 suggesting complete target coverage might be achieved by daily dosing (Fig. 1B and 5A). Compound 2 displayed a similar clearance in mice (10 %QH) compared to BI-1347, but a reduced bioavailability after p.o. dosing (Fig. 1B). Thus, BI-1347 was used for subsequent in vivo efficacy experiments. We next examined the ability of CDK8/19 Inhibitors to affect STAT1 phosphorylation in vivo. C57BL/6 mice were treated once with 75 mg/kg of Compound 2 and spleens were harvested 6 h later. A significant reduction in STAT1S727 phosphorylation was observed in purified NK1.1-positive splenocytes, with recovery of the signal after 24 h (Fig 5B). Similar effects were observed in splenocytes upon treatment with a reduced dose of 3 mg/kg of either Compound 2 or BI-1347 (Fig. 5C).
Figure 5.
CDK8/19 inhibition enhanced tumor surveillance by NK cells in the murine B16-F10-luc2 melanoma model. A, Mouse pharmacokinetics (PK) analysis. C57BL/6 mice (n=3 per group) were dosed with BI-1347 orally (25 mg/kg) or intravenously (1 mg/kg). The plasma concentrations were measured at the indicated time points using LC-MS. B, C57BL/6 mice (n=6 per group) were treated either with 0.5% Natrosol or 75 mg/kg of Compound 2 formulated in 0.5 % Natrosol by oral gavage. Modulation of STAT1S727 phosphorylation was analyzed in murine splenocytes and NK1.1+ NK cells following treatment with Compound 2 at two time points (6 hours and 24 hours (n=3) C, Modulation of STAT1S727 phosphorylation in murine splenocytes following treatment with 3 mg/kg of BI-1347 (n=3) or control (n=2). D, In vivo efficacy of BI-1347 in the murine B16-F10-luc2 melanoma model measured by luciferase mediated in vivo imaging and detection of the average radiance (n=10 mice per group). The inlay figure shows median body weight change observed in the treated (grey) and control (black) groups. E, Data from in vivo efficacy in B-16-F10-luc2 melanoma tumor model plotted as survival curve. Mice were sacrificed immediately once they showed first signs of breathing difficulties F, Tumor burden of mice on treatment day 23 in the B16-F10-luc2 melanoma model.
Since there is functional evidence for a role of CDK8 in STAT1S727 phosphorylation in the B16-F10-luc2 syngeneic melanoma model (5), we treated B16-F10-luc2 tumor bearing mice with the CDK8/19 inhibitor BI-1347. A daily oral dose of 10 mg/kg was selected that reduced phosphorylation of STAT1S727 for at least 6 h by 60%. At this dose, BI-1347 was well tolerated with minimal effect on body weight (Fig. 5D). Compound treatment resulted in tumor growth inhibition (TGI) of 94% on day 23 and 88% on day 29 and a median survival advantage of 4 days compared to vehicle-treated animals (Fig. 5E). The sensitivity of B16-F10-luc2 tumors to BI-1347 treatment in vivo contrasts with the resistance of these cells to CDK8/19 inhibitors in vitro (Supplementary Table S2). All control mice were sacrificed due to tumor burden by day 32, whilst in the BI-1347-treated group, 2/10 mice survived until day 42. In vivo imaging revealed lower tumor burden in the BI-1347 treated group both on day 23 and 29, compared to the control group (Fig. 5F). These observed effects of BI-1347 are consistent with previously published observations using genetic ablation of CDK8 in the B16-F10-luc2 tumor model (5).
Combining a CDK8/19 inhibitor with an anti PD-1 antibody or SMAC mimetic increases the anti-tumor efficacy.
We next evaluated whether CDK8 inhibitor-mediated activation of the innate immune response could potentiate anti-tumor effects of modulators of the adaptive immune system, such as PD-1 inhibitors or SMAC mimetics. In order to test this hypothesis, we first combined CDK8/19 inhibitors with a murine anti-PD-1 antibody (RMP1–14) to promote activation of T cells in the murine colon adenocarcinoma MC38 syngeneic tumor model. The combination of RMP1–14 with BI-1347 led to a significant (P = 0.048) increase in efficacy (TGI = 97%) compared to BI-1347 monotherapy (TGI = 56%) (Table 1). Despite the benefit of the combination, tumor regression was not observed in the MC38 model.
Table 1.
Efficacy of combining daily oral treatment of the CDK8/19 inhibitor BI-1347 (10 mg/kg) with the anti-murine PD-1 antibody (10 mg/kg, intraperitoneal, twice weekly) in the MC-38 murine syngeneic mouse model. Combination of anti-PD-1 plus BI-1347 leads to significant enhanced anti-tumor response compared to both BI-1347 (p=0.0317) and anti-PD-1 (p=0.0476) in monotherapy (Mann Whitney, one tailed, non-adjusted).
| CR [@ end of study, number of mice] | Response [@ d20, number of mice] | TGI [%,@ d20] | |
|---|---|---|---|
| Control | 0/5 | 0/5 | - |
| BI-1347 | 0/5 | 2/5 | 56 |
| Anti-PD-1 | 0/5 | 4/5 | 84 |
| Anti-PD-1 + BI-1347 | 1/5 | 5/5 | 97 |
We next combined the CDK8/19 inhibitor BI-1347 with the previously described Smac mimetic BI-8382 in the murine mammary carcinoma model EMT6 (20). This rational combination was guided by previous findings describing the intrinsic sensitivity of EMT6 tumor cells to SMAC mimetics (19) as well as the promotion of T cell-mediated (20) and NK cell-mediated (21) antitumor immunity by SMAC mimetics. In keeping with these previously reports, treatment of EMT6 mammary carcinoma-bearing mice with BI-8382 resulted in an increase in the number of tumor-residing CD4+ or CD8+ T cells as well as NKp46+ NK cells (Supplementary Fig S3).
Daily oral treatment of the CDK8/19 inhibitor BI-1347 in monotherapy (10 mg/kg) did not significantly increase the median survival (22.5 days) of EMT6 tumor-bearing mice compared to the control group (22 days). 5 days on / 5 days off intermittent scheduling cycle of BI-1347 resulted in median survival of 26 days. Monotherapy with the SMAC mimetic BI-8382 resulted in a medium survival of 32 d, which corresponded to a 10 d increase compared to the control group. During continuous treatment with the SMAC mimetic, most tumors continued to grow (8/10 mice). Notably, the combination of BI-8382 with BI-1347 (applied daily) did not increase efficacy compared to BI-8382 alone (Fig. 6B). Since permanent activation of NK cells was described to induce a hypo-responsive state (16–18) we examined the antitumor efficacy of intermittent scheduling of the CDK8/19 inhibitor BI-1347. Combination of continuous treatment of BI-8382 with an intermittent schedule of BI-1347 increased efficacy compared to each monotherapy. In the combination group, the median survival was 45 days, whilst complete regression (CR) of tumors was demonstrated in 5/10 mice in the mammary carcinoma EMT6 model (Fig. 6A–C). This significant survival increase in the combination (p=0.0004) compared favorably to a median survival of 32 days and 2/10 mice showing CRs with SMAC mimetic in monotherapy.
Figure 6.
The SMAC mimetic BI-8382 enhances CDK8/19 inhibitor (BI-1347)-mediated NK cell tumor lysis and survival in the EMT6 murine breast cancer model (8–10 mice per group). A, Daily application of the BI-1347 at 10 mg/kg resulted in a median survival (MS.) of 22.5 days, 5 days on / 5 days off intermittent scheduling cycle of BI-1347 resulted in MS. of 26 days whilst the control group demonstrated a MS. of 22 days. Daily administration of the SMAC mimetic BI-8382 at 50 mg/kg resulted in MS. of 32 days. B, The SMAC mimetic was combined with intermittent scheduling cycle of BI-1347 at 10 mg/kg (5 days on / 5 days off). Treatment with the CDK8/19 inhibitor was not initiated until day 10 after randomization. C and D, Individual relative growth curves of the control (red) and treated tumors (blue) are shown. SMAC mimetic monotherapy (C) resulted in complete responses (CR) in 2/10 animals, whilst combination (D) of the SMAC mimetic with the CDK8/19 inhibitor resulted in 4 CRs and 1 partial response as well as the survival of all 5 mice until day 45. E, Depletion of CD8+ T cells in the combination group resulted in efficacy comparable to intermittent scheduling cycle of BI-1347 in monotherapy resulting in a MS. of 23 days compared to 45 days without T-cell depletion. F, Depletion of NK cells in the combination (MS. of 34 days) resulted in an efficacy that is comparable to SMAC mimetic in monotherapy. The end-point was defined as sacrifice criteria once animals reached a tumor size of 1500 mm3.
Depletion of NK cells erased the effect of the CDK8/19 inhibitor BI-1347 in the SMAC mimetic combination.
We next interrogated whether the SMAC mimetic-CDK8/19 inhibitor combination effect observed in the EMT6 model was linked to NK cell function. To this end we depleted either CD8+ T cells or NK cells to explore the impact of these immune cell populations on efficacy (Supplementary Fig S4). Depletion of NK cells with the GM1 anti–asialo glycoprotein antibody reduced the number of NK cells but did not affect the number of CD8+ T-cells (Fig. 6F, S4A and S5). Depletion of NK cells prevented the ability of the CDK8/19 inhibitor to increase efficacy in combination with the SMAC mimetic (median survival 34 days). This suggests that CDK8/19 inhibition contributes to antitumor efficacy, when applied in combination with a SMAC mimetic by employing a mechanism dependent on NK cells. Depletion of CD8+ T cells in the group treated with intermittent scheduling (5 days on and 5 days off) of the CDK8/19 inhibitor BI-1347 plus the SMAC mimetic BI-8382 reduced the efficacy to that observed with the CDK8/19 inhibitor in monotherapy (Fig 6E and S4B). This indicates that while the antitumor effect of the SMAC mimetic is mediated predominately by T-cells. The mechanism underlying the antitumor effects of the CDK8/19 inhibitor efficacy is dependent on NK cell function.
Thus, we conclude that treatment with a CDK8/19 inhibitor that leads to activation of NK cells can be combined with either an immune checkpoint inhibitor that mechanistically releases the inhibition of T cells or with a SMAC mimetic for which CDK8/19 inhibitor-dependent efficacy was found to be dependent on an intermittent treatment schedule and on the presence of NK cells.
Discussion
Although immunotherapies are highly effective in a growing number of cancer indications (40–42), only a minority of patients benefit from these agents. Most approved immunotherapies focus on the adaptive immune system, e.g. activating T-cells with checkpoint inhibitors targeting the PD-1/PD-L1 axis or CTLA4 (34–36). The low response rates to checkpoint inhibitors could potentially be explained by low mutational burden and immunogenicity of certain tumors, overexpression of PD-L1, or heterogeneity of the origin and number of tumor-associated immune cells (37,38). The innate immune system also plays a relevant role in anti-cancer immunity, as the presence of tumor residing innate immune cells has been shown to correlate with cancer patient survival (39). Direct NK cell mediated tumor lysis represents a therapeutic option and combination of drugs that activate T cells and NK cells could potentially further improve outcomes in cancer patients. Adoptive NK cell-mediated immunotherapy is currently under clinical investigation in patients with hematological malignancies and solid tumors (4), however, these agents have not yet been approved.
We present here novel, potent and selective inhibitors of CDK8, which inhibit STAT1S727 phosphorylation at comparable IC50 values in mouse and human NK cells. The only kinase that was equipotently inhibited by these compounds was CDK19; a paralog of CDK8 with overlapping and compensatory functions in the mediator complex (40–42). CDK8/19 inhibition with BI-1347 resulted in lower tumor burden and longer median survival in the B16-F10-luc2 syngeneic melanoma model, which is poorly immunogenic for T cells and therefore is not responding following treatment with a PD-1 antibody (43). Our results matched the observed anti-tumor efficacy of a genetic model mimicking CDK8 inhibition, which suggests that CDK8 and not CDK19 is the predominant target underlying the efficacy of BI-1347 (5).
In contrast to most other CDK8 inhibitors (9–12,14,44), which displayed anti-proliferative effects on diverse cancer cell lines, Compound 2 and BI-1347 selectively reduced the proliferation of only a small subset of hematological cancer cell lines, whilst solid tumor cell lines were unaffected. This may be due to the exquisite selectivity of Compound 2 and BI-1347 for CDK8 and CDK19 compared to other published compounds; a hypothesis consistent with the restricted anti-proliferative activity of another highly selective CDK8/19 inhibitor (13). Despite the anti-proliferative activity of our CDK8/19 inhibitors in AML cell lines, we did not observe similar effects in a limited number of primary human AML samples. This might be explained by the possibility that only subsets of AML cells are susceptible to CDK8/19 inhibitors when used as monotherapy. It is also possible that STAT1 signalling is not critical for the viability of primary AML cells under the ex vivo culture conditions used in our study. The murine solid tumor cell lines that we used for in vivo studies (e.g. MC-38, B16-F10 and EMT6 cells) were resistant to Compound 2 and BI-1347 in vitro. As such, it is likely that the in vivo activity of our CDK8/19 inhibitors is predominantly mediated via tumor extrinsic mechanism, such as the activation of antitumor immunity.
The observed effects of the CDK8/19 inhibitor BI-1347 were comparable to those previously published for a model involving genetic targeting of CDK8 (5). However, as the observed antitumor effects of BI-1347 were not durable, it was of high interest to examine the therapeutic utility of drug combinations. One strategy of high interest was to combine NK cell activating agents such as CDK8/19 inhibitors and Fc-engineered antibodies to promote NK cell recruitment via binding of CD16 (27–31). We could demonstrate that CDK8/19 inhibition enhanced ADCC of the Fc-engineered anti-CD33 antibody BI 836858 towards the AML cell line HL-60. Furthermore, the combination of CDK8/19 inhibitors with the CD20 antibody rituximab enhanced ADCC towards primary leukemia B-cells. These novel findings suggest that inhibition of CDK8/19 may have therapeutic value in patients with hematological malignancies when used in combination with ADCC-promoting drugs.
Beyond combination with ADCC eliciting antibodies, we evaluated additional rational combinations that could be enhanced by CDK8/19 inhibitor treatment. It was recently shown that in addition to T cells, activated NK cells can be inhibited by the PD-1/PD-L1 axis (45). We hypothesized that the combination of an NK cell activating CDK8/19 inhibitor plus a PD-1 blocking antibody might result in more durable antitumor responses. This hypothesis was supported by our findings that the combination of inhibitors of PD-1 and CDK8/19 increased survival in a syngeneic MC-38 murine colon adenocarcinoma model, which contains both NK cells and T cells in the tumor microenvironment.
Small molecule SMAC mimetics, that induce degradation of Inhibitor of Apoptosis Proteins (IAPs), have been recently shown to enhance T cell-mediated antitumor immunity via the induction of immunogenic cell death (46–49). Treatment with the SMAC mimetic BI-8382 in the EMT6 mouse model resulted not only in an increase in the number of intra-tumoral CD4+ and CD8+ T cells, but also in a strong increase in NKp46+ NK cell infiltration (Supplementary Fig. S2). Therefore, we anticipated a synergistic antitumor effect in combination with a CDK8/19 inhibitor. However, the combination of a CDK8/19 inhibitor on a continuous schedule with a SMAC mimetic did not increase efficacy compared to each monotherapy. One explanation is that chronic activation of NK cells has been described to induce a hypo-responsive state (16–18) that results in deactivation of NK cells. We hypothesized that intermittent scheduling of a CDK8/19 inhibitor might overcome NK cell hypo-responsiveness and result in improved efficacy. The combination of a SMAC mimetic with intermittent CDK8/19 inhibitor treatment resulted in synergistic efficacy; an effect that was dependent on the presence of NK cells. Also, the threshold of activation, which is regulated according to the rheostat model of NK cell activation, might contribute to the increased activation of NK cells upon CDK8/19 inhibition (50). We propose a model in which the efficacy of this combination is mediated by the promotion of NK cell tumor infiltration by a SMAC mimetic and the activation of NK cell antitumor immunity by CDK8/19 inhibition (Supplementary Fig S2 and Fig. 2).
The inhibition of CDK8/19 kinases has been associated with selected toxicities in preclinical animal studies such as adverse effects on stem cell compartments of the intestine, bone and immune system (14). These observations were made in multiple species when CDK8/19 inhibitors were applied on daily treatment regimens over 14 days. Our observation, that intermittent scheduling of CDK8/19 inhibitors is associated with comparable or even improved antitumor efficacy compared to daily treatment suggests that the therapeutic index of these agents may be improved by less frequent application. Future nonclinical safety studies are warranted to realize the potential of CDK8/19 inhibitors on intermittent schedules.
In summary, we have identified highly selective CDK8/19 inhibitors, which are able to activate NK cell-mediated antitumor activity. The intermittent combination of CDK8/19 inhibitors with drugs activating other immune cells (e.g. T cells) further increased efficacy. We propose new strategies for the development of CDK8/19 inhibitors by: (i) using CDK8/19 inhibitors as mediators of NK cell activity against solid cancers, as opposed to as tumor-directed agents; (ii) combining CDK8/19 inhibitors with ADCC-enhancing antibodies or T cell activating agents such as SMAC mimetics and PD-1/PDL1 axis modulators and (iii) applying intermittent scheduling in order to avoid NK cell hypo-responsiveness and to potentially increase the therapeutic index of CDK8/19 inhibitors. The selective and potent inhibitors described here can also serve as useful tools in the future to dissect the molecular mechanisms underlying the regulation of NK cell activity by CDK8/19.
Supplementary Material
Acknowledgments
The authors are thankful to the AML patients, the healthy blood donors who contributed to this study, the OSU Comprehensive Cancer Center Leukemia Tissue Bank Shared Resource (P30 CA016058) and the Lauber Funds for Immunotherapy in AML: (R01 CA159296).
This study was supported and funded by Boehringer Ingelheim, the Lauber Funds for Immunotherapy in AML and the Spanish grant of Instituto de Salud Carlos III (PI19/01353). We thank Melanie Gschaider, Astrid Jeschko, Sabine Kallenda, Stefan Fischer, Reiner Meyer, Markus Reschke, Katharina Rudisch, Sandra Strauss, Harald Studensky, Martina Sykora and Elisabeth Zier for technical support. We thank Mark P. Petronczki for reviewing the manuscript. We thank Veronika Sexl from the Institute of Pharmacology and Toxicology, Department of Biomedical Science, University of Veterinary Medicine Vienna for scientific discussions.
N.M. and S. Gonzalez received research funding from Boehringer Ingelheim.
Footnotes
Additional information
Conflict of interest: All authors except R.M., G.R., S.G., S.V, S, Gonzalez, and N.M. are employees of Boehringer Ingelheim. M.K. was an employee of Boehringer Ingelheim and is currently working for Astra Zeneca. M.P.S. was an employee of Boehringer Ingelheim and is now working for Merck Healthcare KGaA. J.M. was an employee of Boehringer Ingelheim and is currently working for Sanofi Vitry.
References
- 1.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev 2006;214:73–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caligiuri MA. Human natural killer cells. Blood 2008;112:461–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science 2011;331:44–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Childs RW, Carlsten M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat Rev Drug Discov 2015;14:487–98 [DOI] [PubMed] [Google Scholar]
- 5.Putz EM, Majoros A, Gotthardt D, Prchal-Murphy M, Zebedin-Brandl EM, Fux DA, et al. Novel non-canonical role of STAT1 in Natural Killer cell cytotoxicity. Oncoimmunology 2016;5:e1186314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CK, Rao DT, Gertner R, Gimeno R, Frey AB, Levy DE. Distinct requirements for IFNs and STAT1 in NK cell function. J Immunol 2000;165:3571–7 [DOI] [PubMed] [Google Scholar]
- 7.Putz EM, Gotthardt D, Hoermann G, Csiszar A, Wirth S, Berger A, et al. CDK8-mediated STAT1-S727 phosphorylation restrains NK cell cytotoxicity and tumor surveillance. Cell Rep 2013;4:437–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witalisz-Siepracka A, Gotthardt D, Prchal-Murphy M, Didara Z, Menzl I, Prinz D, et al. NK Cell-Specific CDK8 Deletion Enhances Antitumor Responses. Cancer Immunol Res 2018;6:458–66 [DOI] [PubMed] [Google Scholar]
- 9.Firestein R, Bass AJ, Kim SY, Dunn IF, Silver SJ, Guney I, et al. CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature 2008;455:547–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter DC, Farmaki E, Altilia S, Schools GP, West DK, Chen M, et al. Cyclin-dependent kinase 8 mediates chemotherapy-induced tumor-promoting paracrine activities. Proc Natl Acad Sci U S A 2012;109:13799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelish HE, Liau BB, Nitulescu II, Tangpeerachaikul A, Poss ZC, Da Silva DH, et al. Mediator kinase inhibition further activates super-enhancer-associated genes in AML. Nature 2015;526:273–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dale T, Clarke PA, Esdar C, Waalboer D, Adeniji-Popoola O, Ortiz-Ruiz MJ, et al. A selective chemical probe for exploring the role of CDK8 and CDK19 in human disease. Nat Chem Biol 2015;11:973–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rzymski T, Mikula M, Zylkiewicz E, Dreas A, Wiklik K, Golas A, et al. SEL120–34A is a novel CDK8 inhibitor active in AML cells with high levels of serine phosphorylation of STAT1 and STAT5 transactivation domains. Oncotarget 2017;8:33779–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke PA, Ortiz-Ruiz MJ, TePoele R, Adeniji-Popoola O, Box G, Court W, et al. Assessing the mechanism and therapeutic potential of modulators of the human Mediator complex-associated protein kinases. Elife 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bancerek J, Poss ZC, Steinparzer I, Sedlyarov V, Pfaffenwimmer T, Mikulic I, et al. CDK8 kinase phosphorylates transcription factor STAT1 to selectively regulate the interferon response. Immunity 2013;38:250–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonsson AH, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv Immunol 2009;101:27–79 [DOI] [PubMed] [Google Scholar]
- 17.Viant C, Fenis A, Chicanne G, Payrastre B, Ugolini S, Vivier E. SHP-1-mediated inhibitory signals promote responsiveness and anti-tumour functions of natural killer cells. Nat Commun 2014;5:5108. [DOI] [PubMed] [Google Scholar]
- 18.Shifrin N, Raulet DH, Ardolino M. NK cell self tolerance, responsiveness and missing self recognition. Semin Immunol 2014;26:138–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rathore R, McCallum JE, Varghese E, Florea AM, Busselberg D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 2017;22:898–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reschke M, Impagnatiello MA, Reiser U, Scharn D, Spevak W, Savchenko A, et al. BI5: a novel SMAC mimetic that triggers tumor cell death and potentiates PD-1 mediated cancer therapy. Cancer Res Proceedings of the American Association for Cancer Research Annual Meeting 2017;77: [Google Scholar]
- 21.Brinkmann K, Hombach A, Seeger JM, Wagner-Stippich D, Klubertz D, Kronke M, et al. Second mitochondria-derived activator of caspase (SMAC) mimetic potentiates tumor susceptibility toward natural killer cell-mediated killing. Leuk Lymphoma 2014;55:645–51 [DOI] [PubMed] [Google Scholar]
- 22.Vasu S, He S, Cheney C, Gopalakrishnan B, Mani R, Lozanski G, et al. Decitabine enhances anti-CD33 monoclonal antibody BI 836858-mediated natural killer ADCC against AML blasts. Blood 2016;127:2879–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider EV, Bottcher J, Huber R, Maskos K, Neumann L. Structure-kinetic relationship study of CDK8/CycC specific compounds. Proc Natl Acad Sci U S A 2013;110:8081–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luippold AH, Arnhold T, Jorg W, Kruger B, Sussmuth RD. Application of a rapid and integrated analysis system (RIAS) as a high-throughput processing tool for in vitro ADME samples by liquid chromatography/tandem mass spectrometry. J Biomol Screen 2011;16:370–7 [DOI] [PubMed] [Google Scholar]
- 25.Rudolph D, Impagnatiello MA, Blaukopf C, Sommer C, Gerlich DW, Roth M, et al. Efficacy and mechanism of action of volasertib, a potent and selective inhibitor of Polo-like kinases, in preclinical models of acute myeloid leukemia. J Pharmacol Exp Ther 2015;352:579–89 [DOI] [PubMed] [Google Scholar]
- 26.Mallinger A, Schiemann K, Rink C, Stieber F, Calderini M, Crumpler S, et al. Discovery of Potent, Selective, and Orally Bioavailable Small-Molecule Modulators of the Mediator Complex-Associated Kinases CDK8 and CDK19. J Med Chem 2016;59:1078–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frassanito MA, Silvestris F, Cafforio P, Silvestris N, Dammacco F. IgG M-components in active myeloma patients induce a down-regulation of natural killer cell activity. Int J Clin Lab Res 1997;27:48–54 [DOI] [PubMed] [Google Scholar]
- 28.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008;26:1789–96 [DOI] [PubMed] [Google Scholar]
- 29.Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol 2009;27:1122–9 [DOI] [PubMed] [Google Scholar]
- 30.Benson DM Jr., Cohen AD, Jagannath S, Munshi NC, Spitzer G, Hofmeister CC, et al. A Phase I Trial of the Anti-KIR Antibody IPH2101 and Lenalidomide in Patients with Relapsed/Refractory Multiple Myeloma. Clinical cancer research : an official journal of the American Association for Cancer Research 2015;21:4055–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hejazi M, Manser AR, Frobel J, Kundgen A, Zhao X, Schonberg K, et al. Impaired cytotoxicity associated with defective natural killer cell differentiation in myelodysplastic syndromes. Haematologica 2015;100:643–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eksioglu EA, Chen X, Heider KH, Rueter B, McGraw KL, Basiorka AA, et al. Novel therapeutic approach to improve hematopoiesis in low risk MDS by targeting MDSCs with the Fc-engineered CD33 antibody BI 836858. Leukemia 2017;31:2172–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gopalakrishnan B, Cheney C, Mani R, Mo X, Bucci D, Walker A, et al. Polo-like kinase inhibitor volasertib marginally enhances the efficacy of the novel Fc-engineered anti-CD33 antibody BI 836858 in acute myeloid leukemia. Oncotarget 2018;9:9706–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 2018;18:153–67 [DOI] [PubMed] [Google Scholar]
- 36.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zappasodi R, Merghoub T, Wolchok JD. Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies. Cancer cell 2018;33:581–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popovic A, Jaffee EM, Zaidi N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. The Journal of clinical investigation 2018;128:3209–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 2000;356:1795–9 [DOI] [PubMed] [Google Scholar]
- 40.Tsutsui T, Fukasawa R, Tanaka A, Hirose Y, Ohkuma Y. Identification of target genes for the CDK subunits of the Mediator complex. Genes Cells 2011;16:1208–18 [DOI] [PubMed] [Google Scholar]
- 41.Rzymski T, Mikula M, Wiklik K, Brzozka K. CDK8 kinase--An emerging target in targeted cancer therapy. Biochim Biophys Acta 2015;1854:1617–29 [DOI] [PubMed] [Google Scholar]
- 42.Philip S, Kumarasiri M, Teo T, Yu M, Wang S. Cyclin-Dependent Kinase 8: A New Hope in Targeted Cancer Therapy? J Med Chem 2018;61:5073–92 [DOI] [PubMed] [Google Scholar]
- 43.Seaman WE, Sleisenger M, Eriksson E, Koo GC. Depletion of natural killer cells in mice by monoclonal antibody to NK-1.1. Reduction in host defense against malignancy without loss of cellular or humoral immunity. J Immunol 1987;138:4539–44 [PubMed] [Google Scholar]
- 44.McDermott MS, Chumanevich AA, Lim CU, Liang J, Chen M, Altilia S, et al. Inhibition of CDK8 mediator kinase suppresses estrogen dependent transcription and the growth of estrogen receptor positive breast cancer. Oncotarget 2017;8:12558–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. The Journal of clinical investigation 2018;128:4654–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li R, Ruttinger D, Urba W, Fox BA, Hu HM. Targeting and amplification of immune killing of tumor cells by pro-Smac. Int J Cancer 2004;109:85–94 [DOI] [PubMed] [Google Scholar]
- 47.Clancy-Thompson E, Ali L, Bruck PT, Exley MA, Blumberg RS, Dranoff G, et al. IAP Antagonists Enhance Cytokine Production from Mouse and Human iNKT Cells. Cancer Immunol Res 2018;6:25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beug ST, LaCasse EC, Korneluk RG. Smac mimetics combined with innate immune stimuli create the perfect cytokine storm to kill tumor cells. Oncoimmunology 2014;3:e28541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beug ST, Beauregard CE, Healy C, Sanda T, St-Jean M, Chabot J, et al. Smac mimetics synergize with immune checkpoint inhibitors to promote tumour immunity against glioblastoma. Nat Commun 2017;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brodin P, Karre K, Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol 2009;30:143–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.