Abstract
Women’s health concerns are generally underrepresented in basic and translational research, but reproductive health in particular has been hampered by a lack of understanding of basic uterine and menstrual physiology. Menstrual health is an integral part of overall health because between menarche and menopause, most women menstruate. Yet for tens of millions of women around the world, menstruation regularly and often catastrophically disrupts their physical, mental, and social well-being. Enhancing our understanding of the underlying phenomena involved in menstruation, abnormal uterine bleeding, and other menstruation-related disorders will move us closer to the goal of personalized care. Furthermore, a deeper mechanistic understanding of menstruation—a fast, scarless healing process in healthy individuals—will likely yield insights into a myriad of other diseases involving regulation of vascular function locally and systemically. We also recognize that many women now delay pregnancy and that there is an increasing desire for fertility and uterine preservation. In September 2018, the Gynecologic Health and Disease Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development convened a 2-day meeting, “Menstruation: Science and Society” with an aim to “identify gaps and opportunities in menstruation science and to raise awareness of the need for more research in this field.” Experts in fields ranging from the evolutionary role of menstruation to basic endometrial biology (including omic analysis of the endometrium, stem cells and tissue engineering of the endometrium, endometrial microbiome, and abnormal uterine bleeding and fibroids) and translational medicine (imaging and sampling modalities, patient-focused analysis of menstrual disorders including abnormal uterine bleeding, smart technologies or applications and mobile health platforms) to societal challenges in health literacy and dissemination frameworks across different economic and cultural landscapes shared current state-of-the-art and future vision, incorporating the patient voice at the launch of the meeting. Here, we provide an enhanced meeting report with extensive up-to-date (as of submission) context, capturing the spectrum from how the basic processes of menstruation commence in response to progesterone withdrawal, through the role of tissue-resident and circulating stem and progenitor cells in monthly regeneration—and current gaps in knowledge on how dysregulation leads to abnormal uterine bleeding and other menstruation-related disorders such as adenomyosis, endometriosis, and fibroids—to the clinical challenges in diagnostics, treatment, and patient and societal education. We conclude with an overview of how the global agenda concerning menstruation, and specifically menstrual health and hygiene, are gaining momentum, ranging from increasing investment in addressing menstruation-related barriers facing girls in schools in low- to middle-income countries to the more recent “menstrual equity” and “period poverty” movements spreading across high-income countries.
Key words: abnormal uterine bleeding, adenomyosis, endometrium, fibroids, menstrual health, microbiome, pelvic health menstrual effluent, period poverty, stem cells, tissue engineering, uterus
Related editorial, page 617.
Introduction
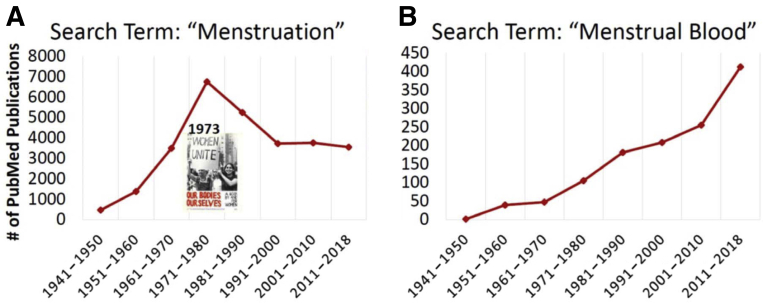
Twenty-five years have passed since the National Institutes of Health (NIH) mandated that women and minorities be included in all government-funded clinical studies unless their exclusion could be justified. Clearly, this policy has led to numerous women’s health research programs. However, women and women’s health concerns continue to be underrepresented in research. Most recently, the 2019–2023 Trans-NIH Strategic Plan for Women’s Health Research was initiated to improve the health of women by advancing rigorous research relevant to advancing women’s health, including sexual and reproductive health (SRH). Despite focused initiatives such as these, diagnostic development for improving women’s reproductive health has been hampered by a lack of understanding of basic uterine and menstrual physiology. A PubMed search of the term “menstruation” yielded less the 1000 publications between 1941 and 1950, followed by a peak of more than 6000 publications between 1971 and 1980 (note: Our Bodies, Ourselves, a book addressing women’s health topics, including menstruation and birth control, was published in 1973), and then a stable trough with less the 4000 publications per decade over the past 3 decades spanning 1991 through 2019 (Figure 1, A). By contrast, a PubMed search of the term “menstrual blood” yielded 1 publication during 1941–1950, followed by a steady increase over time to more than 400 publications in the last decade (Figure 1, B). For reference, PubMed searches of “peripheral blood” and “semen” yielded almost 100,000 and 15,000 publications, respectively, over the past decade.
Glossary of terms.
16S rRNA gene: Encodes a component of the 30S small subunit of a prokaryotic ribosome. 16S rRNA gene sequencing is used for phylogenetic studies because its presence is highly conserved among bacteria, but its sequence is species-specific.
Aromatase: An enzyme that transforms androgens into estrogens.
AUB: Abnormal uterine bleeding.
Biomass: Amount of living biological organisms in a given niche or ecosystem at a given time. The upper genital tract has a significantly lower amount of bacterial DNA than other human microbiomes and is therefore considered a low biomass microbiota.
BMP-2: Bone morphogenetic protein 2.
COEIN: Coagulopathy, Ovulatory, Endometrial, Iatrogenic, Not otherwise classified.
Community state types (CST): Profile that defines the total bacterial community of a given body site based on the relative abundances of each bacterium. The human vaginal microbial communities were classified into 5 groups. Specifically, CSTs I, II, III, and V are dominated by L. crispatus, L. gasseri, L. iners, and L. jensenii, respectively, whereas CST IV has higher proportions of strictly anaerobic organisms.
DCE-MRI: Dynamic contrast enhanced-MRI.
Diversity (Beta diversity): Refers to the change in the number of taxa detected in 2 or more ecosystems. It is usually expressed as the total number of species that are unique to each of the ecosystems being compared.
Dysbiosis: Shift in the physiologic microbiota resulting in an imbalance between commensal and pathogenic bacteria. Changes in microbial composition owing to the gain or loss of the community members or changes in the relative abundance of microbes may contribute to the initiation and/or persistence of many diseases.
Epigenetics: Heritable phenotype changes without changes in genotype (DNA).
Estrobolome: Represents the aggregate of enteric bacterial genes whose products are capable of metabolizing estrogens. Microbes in the estrobolome produce beta-glucuronidase, an enzyme that deconjugates estrogens into their active forms, which are capable of binding to estrogen receptors and influencing estrogen-dependent physiological processes.
FIGO: International Federation of Gynecology and Obstetrics.
GaP: Genotype and Phenotype Registry (registry of normal/control research subjects).
GnRH: Gonadotrophin releasing hormone.
Growth factor: A substance capable of stimulating cell growth, proliferation, and differentiation.
Gut-brain axis: Consists of bidirectional neural processing of information between the central nervous system and digestive system. Recent research indicates that gut microbiota is a crucial part of the gut-brain network and communicates with the brain through the microbiota-gut-brain axis.
HIF: Hypoxia inducible factor.
HMB: Heavy menstrual bleeding.
Hologenome: Theory that maintains that the physiology of any macroscopic organism derives from the integrated activities of the individual genomes contributing to the organism (holobiont).
LNG-IUS: Levonorgestrel-releasing intrauterine system
ME: Menstrual effluent.
ME-SFCs: Menstrual effluent derived stromal fibroblast cells.
mHealth: Mobile health.
Microbiota and Microbiome: The human microbiota encompasses the group of microorganisms that live in association with the human body. Conversely, the microbiome refers to the genes and genomes of this microbiota as well as their products within the host environment.
micro-RNA: Small noncoding RNA molecule regulating posttranscriptional gene expression.
MRI: Magnetic resonance imaging.
MSCs: Mesenchymal stem cells.
MT-MRI: Magnetization transfer-MRI.
Multipotent stem cell: A cell that can self-renew by division and can develop into multiple differentiated cell types.
Natural killer (NK) cell: A type of lymphocyte that can bind to certain tumor cells and virus-infected cells without the stimulation of antigens and can kill them by the insertion of granules containing perforin.
PA: Plasminogen activator.
PAEC: Progesterone receptor-modulator-associated endometrial changes.
PAI: Plasminogen activator inhibitor.
PALM: Polyps, Adenomyosis, Leiomyoma, Malignancy.
Paracrine signaling: Signaling involving hormone that has an effect only in the vicinity of the cell secreting it.
PCOS: Polycystic ovary syndrome.
Richness (Alpha diversity): Refers to the diversity within a particular area or ecosystem. It is usually expressed by the number of species (species richness) in a unique niche.
ROSE: Research OutSmarts Endometriosis (research program dedicated to studying endometriosis).
SPRM: Selective progesterone receptor modulator.
TGF-β3: Transforming growth factor-beta 3.
T2W: T-2 weighted.
t-PA: Tissue plasminogen activator.
uNK cells: Uterine natural killer cells.
u-PA: Urokinase plasminogen activator.
Figure 1.
PubMed publications, 1941–2018
A, Search term “Menstruation.” B, Search term “Menstrual Blood.”.
Critchley. Menstruation: science and society. Am J Obstet Gynecol 2020.
In September 2018, the Gynecologic Health and Disease Branch (GHDB) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) convened a 2-day meeting to “identify gaps and opportunities in menstruation science and to raise awareness of the need for more research in this field.” Leaders in the field with expertise in endometrial biology, omic analysis of the endometrium and menstrual effluent, new imaging or sampling modalities, smart technologies or applications (apps) and mobile health (mHealth) platforms, menstrual health, and health literacy and dissemination frameworks were invited to participate as speakers and discussants to critique and summarize new discoveries and avenues of future research surrounding menstruation. This meeting encompassed normal menstrual health and endometrial function and the potential of diagnostics for abnormal functioning and disease. To provide a broad perspective on menstruation science, this meeting included investigators and stakeholders across multiple disciplines, including population health and public health sectors, and carefully considered the broader societal implications of menstrual health. This manuscript summarizes the presentations and discussions that took place at the 2018 “Menstruation: Science and Society” meeting hosted by GHDB, NICHD.
1. Toward a Better Understanding of Menstrual Health: Menstrual Health Literacy and Communication
Kristen A. Matteson, MD, MPH; Missy Lavender, MBA; Erica E. Marsh, MD, MSCI; Kami Silk, PhD
I. Introduction
According to the World Health Organization, “health” is “a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity.”1 For women, menstrual health is an integral part of overall health because, between menarche and menopause, most women menstruate and menstruation can have a significant impact on the physical, mental, and social well-being.2 Normal menstruation is currently defined as cyclic bleeding that occurs from the uterine corpus between menarche and menopause. It can be described in terms of 4 simple domains: how frequently the woman has episodes of bleeding, the regularity or predictability of these episodes, the duration of bleeding episodes, and the volume or heaviness of bleeding.3, 4, 5 Not all women experience “normal” menstrual bleeding; up to 30% of women will experience alterations in the volume or pattern of menstrual blood flow, which is defined as the symptom of abnormal uterine bleeding (AUB), which in turn can be caused by multiple etiologies and sometimes more than 1 etiology at the same time.3,6 In addition, many women will have other symptoms such as pain, dysmenorrhea, anxiety, depression, and fatigue associated with their menstrual cycle that require attention for them to achieve early diagnosis of reproductive health issues such as endometriosis, premenstrual syndrome, and premenstrual dysphoric disorder and attain optimal health. In research and in clinical care, a better understanding of what the norms of menstrual health are and how a “lack” of menstrual health affects women’s quality of life is needed. Furthermore, for positive health and well-being outcomes, everyone—men and women, as well as clinicians—need to understand menstrual cycles and menstrual health, which can be achieved through menstrual health literacy initiatives and improved health communication.
Menstrual health and menstrual health literacy are extremely broad topics with multiple stakeholders and diverse areas of active investigation and contributors. Adapted from the broader health literacy definition, menstrual health literacy refers to the level of capacity a person has to obtain, process, and understand basic information about menstruation so they can make appropriate health decisions.7 This section of the manuscript summarizes the presentations and discussion that took place related to menstrual health and menstrual health literacy at the 2018 “Menstruation: Science and Society” meeting hosted by the GHDB of the NICHD. We summarize only areas of menstrual health and literacy that were part of the presentations and active discussions at the NICHD GHDB meeting, which were largely focused on the bleeding aspect of menstrual health.
II. Progress in menstrual health terminology and menstrual health literacy and communication
Progress in menstrual health terminology
Standard terminologies related to menstrual bleeding, and specifically AUB, represent real progress for clinical care and research. Ill-defined terminologies to describe symptoms, signs, and diagnoses associated with AUB led to communication challenges in clinical care, difficulty interpreting populations included in published literature, and lost opportunities for multisite research collaboration for clinical research on treatments for AUB.
In 2005, the Menstrual Disorders Working Group of the International Federation of Gynecology and Obstetrics (FIGO) embarked on a worldwide consensus-building process to generate and disseminate a simple symptom description system and a classification system for the etiologies associated with AUB.8,9 The first system, “Terminologies and Definitions,” includes standard definitions for bleeding symptoms domains, which include regularity, frequency, duration, and volume.3 The second system, “Classification of Causes of AUB in the Reproductive Years,” commonly referred to as the polyps/adenomyosis/leiomyoma/malignancy (PALM)–coagulopathy/ovulatory/endometrial/iatrogenic/not otherwise classified (COEIN) system, includes a list of etiologies that can be associated with AUB (polyps, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory, endometrial, iatrogenic, and not otherwise classified). Results of this work were published in peer-reviewed publications and were used by the American College of Obstetricians and Gynecologists (ACOG) during their process standardizing terminologies used across gynecologic specialties.4,5 ACOG and the members of the Women’s Health Registry alliance convened the revitalize Gynecology Data Definitions initiative in December 2013 to develop standardized data elements and definitions in gynecology. Throughout this process, ACOG engaged a broad range of stakeholders to identify priority topics and definitions and then worked with a core group of contributors to generate a total of 119 data elements, including 7 pain-related and 7 bleeding-related definitions.4,5 Although there is more work to be done in terms of evaluating these definitions across diverse populations of women, these standardized terminologies represent positive first steps to facilitate research data collection, collaboration for study participant recruitment, and identification of study cohorts with similar etiologies when investigating the prevalent symptom of AUB.
Another area of progress has been increased emphasis on the patient experience of bleeding, quality of life, and related symptoms associated with the menstrual cycle in both research and national guidelines. The National Institute for Health and Clinical Excellence (NICE) Clinical Guidelines on Heavy Menstrual Bleeding (HMB) published a patient-centric definition of the symptom of HMB, which they define as “excessive menstrual blood loss that interferes with a woman’s physical, social, emotional, and/or material quality of life.”10 Qualitative and quantitative research with women to learn about their experiences with and knowledge of menstrual bleeding, fibroid-related symptoms, pain, and other associated symptoms has begun to inform research priorities, educational tools, and the need for outcome measures for AUB and uterine fibroids.11, 12, 13, 14 Several studies have suggested that patient-reported outcome measures (PROMs), which include standardized interviews, questionnaires, charts, and surveys that assess the patient’s own evaluation of her health and symptoms, are the key to assessing the impact of illness and symptomatology among women with reproductive health issues including AUB.2,15, 16, 17, 18, 19
Progress in menstrual health literacy and communication
There has been recent progress in the areas of menstrual health literacy, advocacy, and communication, in part facilitated by the rapid acceptance of mHealth apps, the use of mobile technologies to provide health-related services (tracking information and providing information or education to support an individual’s achievement of health objectives).20,21 At present, there are more than 300 reproductive health mHealth products in the IOS and Google stores, with the majority of the apps focused on women of childbearing age. The apps vary in their depth of pelvic health information, with the majority including cycle and fertility trackers. Two of the larger apps as far as global reach, CLUE and FLO, have expanded both their tracking (CLUE) or daily notifications and information (FLO) to include facts or daily tips, which, though brief, may be useful to assist in identifying women with the symptom of AUB. Additional data are needed to better understand the demographics of and reasons why women use these apps.
III. Significant conceptual, practical, or technical challenges in the field of menstrual health research and menstrual health literacy
The progress in the field of menstrual health outlined previously is remarkable given the multiple challenges and obstacles in the field of menstruation science. Menstruation is a physiological process that is experienced almost universally across cultures from the ages of menarche to menopause. What makes menstrual health and menstrual health literacy challenging to study is that for many, it is a normal process that is not associated with any distress or disability, but for some it can be associated with a significantly negative impact on the quality of life. Collecting data on a nearly universal process will require collaboration across the spectrum of disciplines and careful consideration of “who” to collect data from, “what” data elements to collect, and “how” to best collect data. Furthermore, the normalization of women’s pain and stigma surrounding menstrual bleeding and reproductive health represent significant barriers to women’s care seeking, diagnosis, and ability to conduct research in this area.22 Menstrual health and menstrual health literacy research is further complicated by a lack of standardization of tools and access to those tools, the multiple different etiologies of HMB, the multidimensional symptom complex surrounding bleeding, lack of clear diagnostic tests for reproductive health disorders that affect menstrual health, suboptimal norms for menstrual health and bleeding across the life span, and insufficient information related to cultural perceptions related to menstrual bleeding and health.
Although awareness of the importance of patient experience with menstrual bleeding and menstrual symptoms has increased in research and clinical care, sustained reliance on “objective” laboratory measures for outcomes related to menstrual health represents an additional conceptual barrier to progress in this area. To provide an AUB-specific example, traditionally in research, bleeding was measured by volume of menstrual blood lost (>80 mL) as measured by the collection of used sanitary products and quantified using the alkaline hematin method.23 However, research has highlighted that most women who seek treatment for HMB do not meet the objective mean blood loss criteria for heavy bleeding and clinical care objectively measured blood loss is not feasible.19 As a result of these studies and others, NICE, the National Health Services (NHS) in the United Kingdom, stated in 2018 that “From the woman’s point of view objective reduction in mean blood loss are poor indicators of treatment effectiveness for heavy menstrual bleeding.”10 This lack of consistency between what has been prioritized as a measurement for research and what women prioritize in terms of desired outcomes represents a current obstacle for high-quality research and synergy between research on HMB, clinical care, and patient-centered care delivery.
IV. Critical gaps in menstrual health literacy, advocacy, or communication and how they can be addressed to optimize women’s menstrual health
Conceptual, practical, and technical challenges related to research on menstrual health and menstrual health literacy and communication have led to several critical gaps in the evidence base in this area. During the meeting, several gaps in the evidence base and opportunities to improve women’s health by addressing these gaps with high-quality research were discussed.
Data to inform “norms” that hold across populations and span from menarche to menopause
Generation of standard terminologies related to norms for uterine bleeding among adult women represents significant progress in the field of menstrual health, and there has been significant progress especially in describing symptom expectations in the later reproductive life stages and during the menopausal transition.24, 25, 26, 27 To ensure that menstrual bleeding norms represent bleeding patterns and other menstrual health symptoms across a racially and ethnically diverse and contemporary population (relative to comorbid conditions and body mass index) of women of all ages, further research is needed.28 Prospective longitudinal cohort data on menstrual bleeding, menstrual symptoms, and reproductive health diagnoses could fill this critical gap.
Developments in mHealth could also be used to inform norms and measure the personal impact menstruation and menstrual symptoms have on women across the life cycle. For population-based data outside of clinical care, data collected from mHealth and mobile device apps are starting to enable the analyses of population-level longitudinal menstrual symptom and cycle data.29 In addition, these mHealth data could facilitate investigation into cultural differences, knowledge, attitudes, and behaviors. By partnering with mHealth and app platforms, researchers, clinicians, and industry could generate data collection mechanisms and assist in generating research programs and interventions that could aid women in identifying when they are having a problem and address stigma and perceptions related to menstrual disorders, delays in diagnosis of reproductive health disorders, and delays in care seeking.
Standardizing data collection in research, clinical care, and mobile health technologies to promote consistency and optimize comparative effectiveness research
A shift in research to focus on measuring patient experiences with symptoms and chronic health problems, including reproductive health and menstrual health issues, represents significant progress in the arena of women’s health. However, although there are several validated PROMs for AUB, there is no single high-quality PROM that is considered “standard of care” or “standard for use across studies.”17,30,31 This translates into hundreds of outcome measures, of varying quality, used across studies and an inability to combine data across studies to summarize patient experience. In a systematic review of patient-reported outcomes used across studies of AUB, authors found 80 studies that used at least 1 PROM and 77 different PROMs were used across studies.31 The Society of Gynecologic Surgeons, in a systematic review comparing treatments for HMB, identified that 114 different outcomes were collected and reported across 79 distinct clinical trials.17 The end result was that, because the method of assessing outcomes differed from study to study, data could not be combined or summarized for these outcomes (such as quality of life and bleeding-related quality of life), which prohibited the group from generating consensus on treatment effectiveness relative to patient-reported outcomes.17 Researchers across disciplines of menstrual health research have expressed challenges describing the menstrual symptom phenotype of patients involved in clinical research because of a lack of standardized structured menstrual history data elements. Finally, discussions at this meeting also highlighted the importance of a broader view of menstrual health that goes beyond bleeding to include other associated symptoms, which will need additional research and standardized data elements.
The research community can collaborate to address this challenge and standardize outcomes and data elements for research and quality assessments. For example, the Core Outcomes in Women’s and Newborn Health, an international initiative led by journal editors and is endorsed by more than 80 peer-reviewed journals in women’s health, is working to stimulate the development of outcome sets that can be used across studies to ensure consistent outcome reporting, thereby improving the interpretability of study results and the feasibility of combining data across studies.32,33 Efforts to standardize data elements from a structured menstrual history describing frequency, regularity, duration, and patient-quantified volume of bleeding along with other associated menstrual symptoms are needed to facilitate consistent descriptions of populations in studies on menstrual health, AUB, uterine fibroids, and other reproductive health issues.34
V. Additional future directions in menstrual health research
Transforming comparative effectiveness research by incorporating patient-reported outcome measures into electronic health records
Looking to the future, standardizing and harnessing the potential of patient-based outcomes assessment could transform comparative effectiveness research. Emerging technology developments may be paving the way to have PROM collection integrated into electronic health records, which would promote patient-centered comparative effectiveness research.35,36 Researchers, policy makers, and professional societies are currently working out best practices for integrating PROMs and electronic health records.35,36 This integration could mean substantially greater capabilities for patient-relevant comparative effectiveness research and health services research, which often relies on electronic health record or administrative datasets that rarely incorporated patient-reported data elements, particularly on reproductive health problems that affect the quality of life.
Incorporating PROM collection into clinical care encounters may represent major opportunities to evaluate processes of healthcare delivery. Future research opportunities include assessing whether or not incorporating PROMs into electronic health records and clinical encounters for menstrual health disorders can improve physician-patient interactions and be used to monitor patient symptoms or progress over time. On the population level, incorporating PROMs into clinical care can assist with clinical care quality assessment and population surveillance. For example, in the UK NHS, PROMs are collected before and after certain surgical interventions to determine the quality of care delivery and to facilitate counseling for patients on what to anticipate in terms of the personal impact of the surgery.37
Partnerships across diverse disciplines and stakeholder groups
Innovative solutions to address comprehensive menstrual health across the life span will require collaboration across scientific disciplines, social science disciplines, and involvement of patient- and person-facing organization to ensure the relevance and success of these solutions for addressing the needs of the population. Menstrual health research in the future could be enhanced by developing collaborative interdisciplinary teams to investigate comprehensive menstrual health premenarche to menopause. In addition, including patient-facing groups in study design and beta testing of programs from the beginning and partnering with patient groups and advocacy groups to create and disseminate communication platforms and menstrual health educational initiatives could enhance the fields of menstrual health, menstrual health literacy, and menstrual health communication.
VI. Conclusion
Each year, 4.5 million women in the United States experience at least 1 gynecologic health problem, and many of these problems are related to menstrual health.6 Although significant progress has been made in menstrual health research in terms of emphasizing patient experience, standardizing terminologies related to menstrual bleeding, and use of PROMs for menstrual disorders, more work and research are needed to standardize data collection, generate longitudinal data on contemporary norms of menstrual bleeding and related symptoms, and optimize use of new technologies and educational interventions. Health communication strategies that are accessible to groups with low literacy and address potential stigma associated with menstruation will help to address barriers as well. Increasing the evidence base on menstrual health and menstrual health literacy will aid in the evolution of contemporary clinical care that meets the unique needs of women. Bringing women and advocacy groups to the table and bringing data collection and information directly to women through innovative technologies, smartphone apps, and mHealth has the potential to move the field of menstruation science away from treating problems and toward optimizing women’s overall health, and more specifically menstrual health. Continuing the recent momentum on patient-focused menstrual health research to sustain progress in the field of menstrual health, literacy, and communication has the potential to have a substantial impact on the lives of women.
2. The Evolutionary History of Menstruation
Günter P. Wagner, PhD
Menstruation and its associated diseases such as HMB and AUB are a significant burden on women of reproductive age (see section Menstruation and abnormal uterine bleeding below), which raises the question of why women menstruate at all. This question is particularly pertinent given the fact that menstruation is dispensable for mammalian reproduction (see below). Answers require a review of the evolutionary history of mammalian reproduction, given that humans and great apes, that is, species that menstruate, evolved from ancestors that did not menstruate. What are the advantages menstruation affords humans and other primates that, from a biological point of view, could make the origin and biological role of menstruation understandable?
I. Menstruation is rare among animals
Menstruation is defined as the shedding of the upper (the so-called “functionalis”) layer of the uterine lining after the luteal phase of the ovarian cycle. Although menstruation is a normal part of the life of a woman during her fertile years, it is only found in a small minority of animals. Because menstruation is a function of the female reproductive organs, one would expect to find menstruation in animals with a similar mode of reproduction as humans, that is, the so-called placental mammals (technically called “eutherian mammals”). Eutherian mammals are all the species that descended from the most recent common ancestor of humans and elephants, meaning all the mammals that we are most familiar with: apes, monkeys, farm animals, cats, dogs, seals, hedgehogs, and others (Figure 2). All of these animals have a placenta and a gestational period that is longer than their ovarian cycle, so-called trans-cyclic gestation,38 with the exception of animals that have pseudopregnancy in the absence of fertilization, such as the dog.39
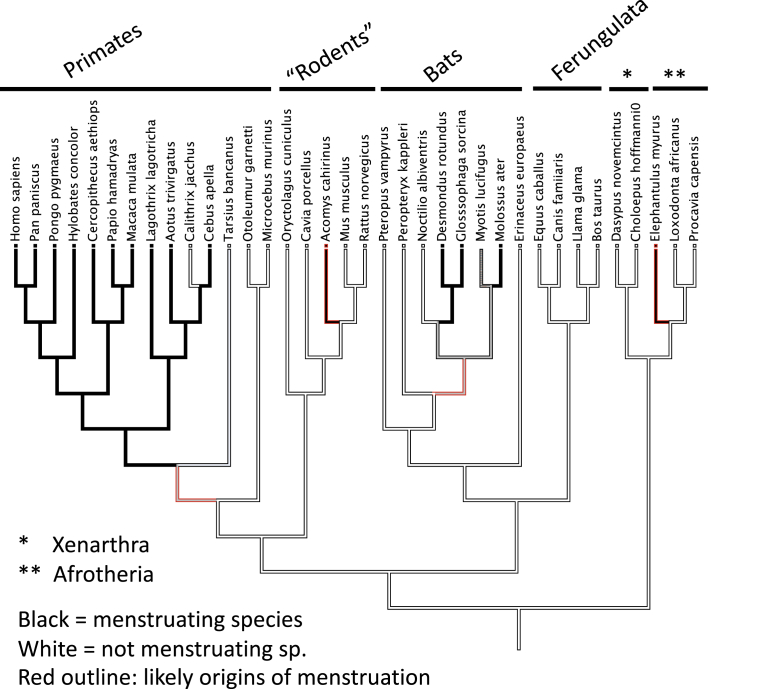
Figure 2.
Phylogenetic distribution of menstruating species among eutherian mammals
Lineages in black are from menstruating species, and lineages in white from nonmenstruating species. The lineages with red outline are the lineages where menstruation originated. Note that there are at least 4 independent originations of menstruation.
Critchley. Menstruation: science and society. Am J Obstet Gynecol 2020.
Despite the substantial similarities, with respect to female reproductive biology, between humans and all other eutherian mammals (eg, compared with reptiles and birds), menstruation only occurs in a small minority of eutherian species. The largest cluster of menstruating species is found among our closest relatives, the primates. In particular, apes, old world monkeys, and most but not all of new world monkeys have menstruation. More basally diverging primate lineages do not (lemurs and tarsiers, where in the latter conflicting evidence has been reported, summarized in the study by Emera et al40). Outside the primates, menstruating species are rare. Among the rodents, only one species has been described as menstruating: the spiny mouse, Acomys cahirinus.41 This is surprising, given the large number of rodent species (2277 species). Then there is a small number of bat species belonging to 2 groups of bats, 1 molossid bats and 3 phyllostomid bats.42 Most distantly related menstruating species to humans is the elephant shrew (Elephantulus myurus43,44) related to elephants and other afrotherian mammals. These menstruating species add up to 84 species, or about 1.6% of the 5149 recognized extant eutherian species. This estimate could be a slight undercount because it is not easy to diagnose menstruation in species that have not been kept in laboratories or zoos and have been closely monitored.
If we put the menstruating species on the phylogeny of mammals (Figure 2), we see a rather dispersed distribution. Clearly, all the primate species that menstruate are relatively closely related, but the spiny mouse, bats, and the elephant shrew are not. The conclusion that follows from these facts is that menstruation must have evolved at least 4 times independently during the evolutionary history of mammals. This conclusion is also supported, for instance, by differences in the exact location and nature of the endometrial changes in the elephant shrew (summarized in the study by Carter45). The rarity and repeated evolution of menstruation raise the question about its biological role. Menstruation is clearly not necessary for a mammal because it is rare, but it might have a specific role, rather being there accidentally, because it originated at least 4 times independently.
Before we turn our attention to the question of why some mammals menstruate and others do not, we should mention that not every case of vaginal bleeding by a healthy animal is menstruation. The best known example is the vaginal bleeding of the dog, which is not a sign of menstruation.39 The main difference between what is happening in dogs and in menstruating species is that the vaginal bleeding in dogs happens in proestrus, that is, in preparation for mating, rather than after the fertile phase is over, as it is the case in women. The bleeding in dogs is caused by extravasation during the growth of the uterine lining, which can break through the epithelium leading to a vaginal efflux.
II. Why did menstruation evolve?
The fact that menstruation plays a major role in the life of a woman and that it is rare among animals has inspired many scientists, anthropologists, and medical researchers to speculate about its biological role.46, 47, 48, 49, 50, 51, 52 This is not the place to review all the ideas that have been proposed to explain the evolution of menstruation but note that the most honest and shortest answer to this question is “we do not know.”53 Nevertheless, there has been some progress in reframing the question that points to two plausible answers.
An important breakthrough in understanding the evolution of menstruation was the realization that menstruation itself may not be the direct biological trait that was shaped by natural selection, but rather that menstruation could be a secondary consequence of an underlying biological trait: spontaneous decidualization.52
Decidualization is the process by which the uterine lining prepares for pregnancy. This is a complex process including proliferation of the endometrial stroma, the traffic of various kinds of white blood cells into the endometrium, and the differentiation of the endometrial fibroblasts into so-called decidual stromal cells (DSCs).54 Decidualization in the narrow sense refers to the differentiation of DSC, rather than to the whole organ-level process. In most animals, decidualization occurs in the estrogen- and progesterone-primed uterus in response to the presence of the embryo. This is induced decidualization. However, in humans, decidualization occurs even in the absence of an embryo and is therefore called spontaneous decidualization. It turns out that all menstruating species undergo spontaneous decidualization,40,41,52 suggesting that the evolved trait is not menstruation per se, but spontaneous decidualization. In humans, it has been shown that the proximate cause for menstruation (see section on Menstruation and abnormal uterine bleeding) is the decrease in progesterone levels owing to the degeneration of the corpus luteum. An experimental model of artificial decidualization in a nonmenstruating species, the mouse, Mus musculus, shows that in fact progesterone withdrawal after decidualization is sufficient to cause menstruation-like symptoms, that is, degeneration of part of the endometrium and vaginal bleeding.55, 56, 57, 58
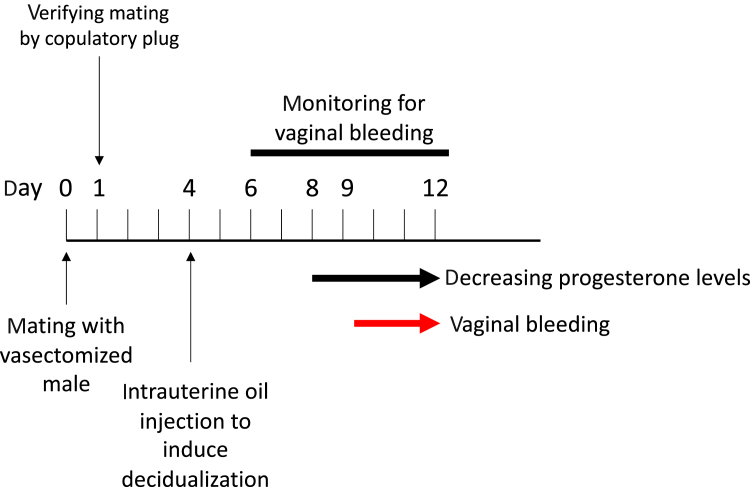
There are several versions of this experiment but the cleanest model is the one published by Rudolph et al56 in 2012: intact female mice were mated with sterile, vasectomized males, which in mice causes pseudopregnancy, meaning that the female maintains a high level of progesterone even though no fetus is developing in her uterus. After copulation, the pseudopregnant mice were injected with a small droplet of oil into the lumen of the uterus. It is known that this treatment causes the uterine lining of the mouse to decidualize, leading to a so-called “deciduoma,” which is a condition that, in many respects, mimics fetus-induced decidualization. The key observation of this experiment then was that as progesterone levels were decreasing toward the end of the pseudopregnancy, menstruation ensued. This result supports a model according to which menstruation is an inevitable consequence of spontaneous decidualization if fertilization and pregnancy do not occur (Figure 3).
Figure 3.
Schematic outline of the experiment by Rudolph et al56 testing the idea that menstruation is a secondary consequence of spontaneous decidualization
The experiment is conducted with the laboratory mouse, which is a species that under normal conditions is neither decidualizing nor menstruating. In this species, clitoral or vaginal stimulation during copulation leads to the maintenance of the corpus luteum even if no pregnancy ensued, leading to pseudopregnancy, as is the case by copulation with a vasectomized male. Furthermore, it is known that injection of a small droplet of oil into the uterine lumen causes decidualization. The experiment starts with mating a female to a vasectomized male to induce a pseudopregnancy. At the morning of the following day, the females are checked for a copulatory plug to verify that copulation has taken place. Then at day 4 after copulation, a small droplet of oil is injected into the uterus to induce decidualization. Day 4 is the normal day of implantation in mice. Then the mice are monitored for their level of progesterone and signs of vaginal bleeding. Progesterone starts to decrease after day 7, and bleeding ensues at about day 9. This experiment shows that differentiation of the endometrium (decidualization) is sufficient to cause menstruation-like symptoms in a species that normally does not menstruate.
Critchley. Menstruation: science and society. Am J Obstet Gynecol 2020.
Both, the comparative evidence, namely, the association between menstruation and spontaneous decidualization among mammals, and the experimental evidence with artificial decidualization lead to the conclusion that the real question thus is not “why some species menstruate?” but “why do some species show spontaneous decidualization and menstruate as a consequence?” There are 2 plausible answers, but no definite consensus on this issue has been reached.
One model assumes that spontaneous decidualization is a protective device for the mother against an aggressive fetus.40,47,52 This model is based on the observation that the degree of invasiveness of the placenta varies between species. This is even the case among species with so-called hemochorial placentation, that is, where the fetus is destroying not only the uterine luminal epithelium but also some of the uterine blood vessels so that the placenta is in direct contact with maternal blood. For instance, great apes have extravillous trophoblast cells, which invade the maternal blood vessels (spiral arterioles), the stroma, and even the muscular layer of the uterus (myometrium).59 Clinical observations have also revealed cases where a placenta embeds too deeply into the uterus, a condition called “placenta accreta” or “placenta percreta” depending on the depth of invasion. These conditions can threaten the life of the mother after birth because of massive uterine bleeding.60 Finally, one of the roles of the decidual cells is to both enable and limit the invasion of the placenta and thus regulate the depth of implantation even though the mechanisms are still unclear. Hence, it seems plausible that spontaneous decidualization is ensuring that a conceptus finds an environment that is prepared to allow and at the same time limit the degree of placental invasion. To our knowledge, no formal test of this model has been attempted. In particular, one would need a way to measure invasiveness of the conceptus in various animals, many of which are not laboratory models and thus hard to work with. Furthermore, we have no information whether and which close relatives of menstruating species are also menstruating to test for a correlation between menstruation and depth of placental invasion.
The second model to explain the evolutionary origin of menstruation assumes that spontaneous decidualization is an adaptation to allow the female to “test” the viability of the conceptus before definite pregnancy ensues.61, 62, 63, 64 This model is inspired by the observation that decidual cells have the ability to sense the vitality of the embryo and react with a stress reaction when the embryo is of inferior quality. The idea is that this ability of DSCs helps the mother avoid investing resources in an ultimately unsuccessful pregnancy and thus increases the reproductive fitness of the female by allowing her to achieve pregnancy sooner. This idea is supported by the fact that humans have a rate of pregnancy loss of 10% to 25%65 (higher estimates found in the literature seem to be spurious) and that spontaneous decidualization is primarily found in animals with a small litter size, that is, one or two neonates per pregnancy and thus with correspondingly higher investment into each offspring. The recently described, yet not fully evaluated, spiny mouse is somewhat an exception because its litter size is usually 2 or 3 but can be as high as 6.41 Again, there is a dearth of comparative data to fully test this idea, given that we do not know the rate of pregnancy loss in most animals, and whether it is different between closely related species that differ in the presence or absence of spontaneous decidualization.
III. An evolutionary argument for the validity of menstruation as a diagnostic tool
In a later section, the utility of menstrual efflux as a diagnostic tool will be discussed in detail. Here, we review an evolutionary argument that supports the idea that menstruation may be predictive of pregnancy complications in the future.
In the evolution of spontaneous decidualization, the decidualization process becomes independent of the actual initiation of pregnancy. Nevertheless, it is uncontroversial that the process of spontaneous decidualization is homologous to the process of embryo-induced decidualization as the former evolved from the latter.40 The only difference is the mode in which the decidualization is triggered, either by maternal hormones as in spontaneous decidualization (as in women) or by the embryo as in induced decidualization (as in the mice or rodents). This is the reason why experimental work on mice is a valid approach toward understanding human decidualization even though the mode of decidualization is different between these two species.
At the end of the ovarian cycle, menstruation is caused by the withdrawal of the supportive function of progesterone for the decidua. As a consequence, menstruation has substantial mechanistic similarities with the processes that initiate labor.66 Birth is also associated either with a systemic progesterone withdrawal through the degeneration of the corpus luteum (luteolysis) or by functional progesterone withdrawal caused by inhibition of progesterone signaling.67,68 Hence, it is likely that the mechanisms deployed in the uterus during menstruation are homologous to those during parturition.66 If in fact menstruation and the uterine manifestations of parturition are homologous, it is likely that defects that affect the maintenance pregnancy or the initiation of parturition could also manifest themselves as aberrations in menstruation. Pavlicev and Norwitz66 therefore suggest that substantial research effort should be dedicated toward testing whether biomarkers expressed during menstruation are associated with pregnancy complications that could be useful as preconception diagnosis of likely pregnancy complications.
3. Menstruation in Humans
3A. Menstruation and abnormal uterine bleeding
Hilary O.D. Critchley, MD; Jacqueline A. Maybin, PhD
I. The impact of menstrual bleeding complaints
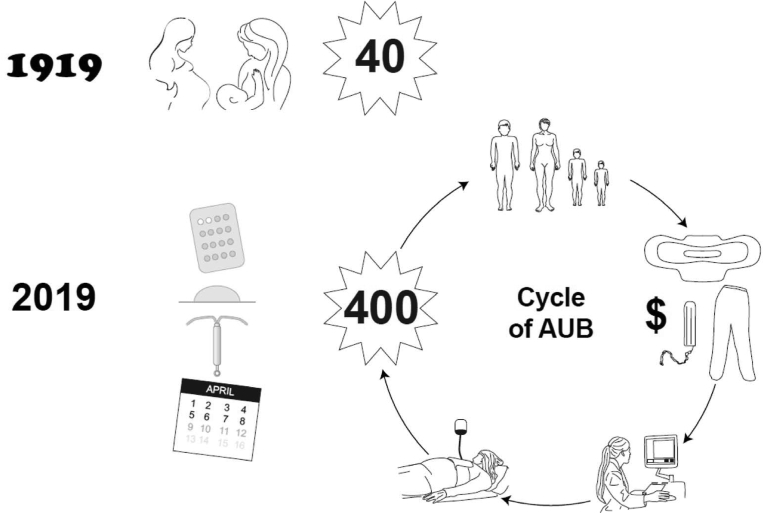
Understanding the mechanisms underpinning the pivotal human event of menstruation is critical to our understanding of AUB. AUB, which includes the symptom of HMB,3 is a chronic complaint that affects the quality of life and well-being of 1 in 4 women of reproductive age (Figure 4).69 Previously, women experienced menstruation approximately 40 times owing to pregnancy and lactation amenorrhea, whereas in developed economies today, women can expect up to 400 menses in their lifetime.70 Therefore, AUB is becoming more common and problematic for women and society. In contemporary society, women are delaying having children for a variety of reasons such as personal choice, prioritization of career, and other factors that impose a delay in childbearing. Therefore, these women wish to preserve their uterus alongside their fertility. As a consequence, surgical options are not always appropriate because these end fertility and may also involve higher risks than medical management alternatives. In a recent systematic review relevant to the United States, it was conservatively estimated that annual direct and indirect economic costs of menstrual bleeding complaints were in the order of $1 billion and $12 billion, respectively.71 Leiomyoma (uterine fibroids) are common, present in 70% to 80% of women by the age of 50 years,72 and associated with AUB or HMB. Among women in their 30s and 40s, leiomyomas are often the underlying cause of AUB, anemia, and iron deficiency anemia. When the presence of uterine fibroids is considered along with complaints of AUB, the annual estimated direct costs of this complaint in the United States, when surgery, hospital attendances, outpatient visits, and prescribed medications are taken into account, are as high as $4.1 billion to $9.4 billion. Furthermore, lost work hours resulted in costs ranging from $1.55 billion to $17.2 billion.71
Figure 4.
The modern effect of menstruation
Previously, women experienced menstruation approximately 40 times in their lifetime, owing to pregnancy and lactational amenorrhea. Women may now expect to have more than 400 episodes of menstruation, mainly as a result of fertility management. Therefore, AUB is increasingly common. Women may experience significant anemia resulting in a poor physical quality of life. A negative financial effect occurs because of the cost of managing their blood loss and an inability to work outside the home. These costs, alongside a loss of caring ability, will have a negative effect on the wider family. The cost to society through loss of work days and healthcare costs is significant.
AUB, abnormal uterine bleeding.
Critchley. Menstruation: science and society. Am J Obstet Gynecol 2020.
II. A classification system for abnormal uterine bleeding
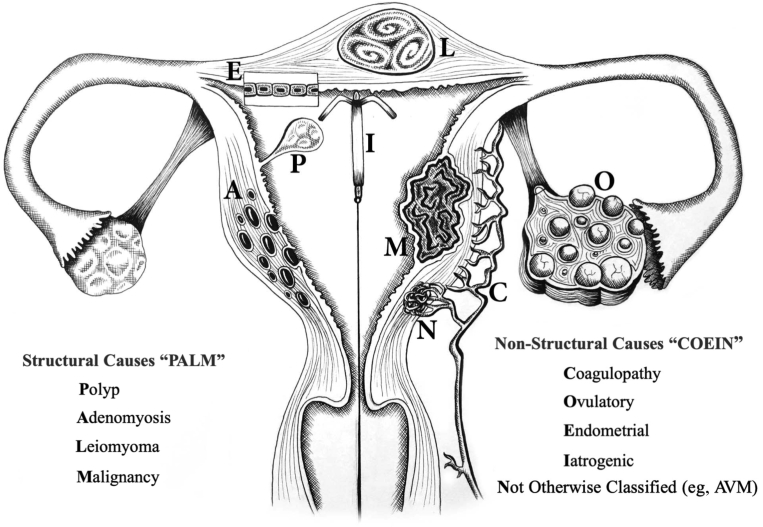
To provide diagnostic precision and specific treatment of AUB, classification of causes of uterine bleeding is crucial. The FIGO Menstrual Disorders Committee has led on the classification systems for causes of chronic AUB in the reproductive years.3,73 As already mentioned, there are 2 systems: the first system focuses on terminology with an encouragement for the removal of ill-defined terminologies such as “menorrhagia” and “dysfunctional uterine bleeding,” and the second system focuses on the underlying causes of AUB, using the acronym PALM-COEIN3,73 for structural and nonstructural causes, respectively (Figure 5). It is hoped that these 2 FIGO systems will be used globally to improve the management of women with AUB.
Figure 5.
The PALM-COEIN classification for abnormal uterine bleeding in the reproductive years illustrating the structural (PALM) and nonstructural causes (COEIN) and as described in Munro et al3,73
Critchley. Menstruation: science and society. Am J Obstet Gynecol 2020.
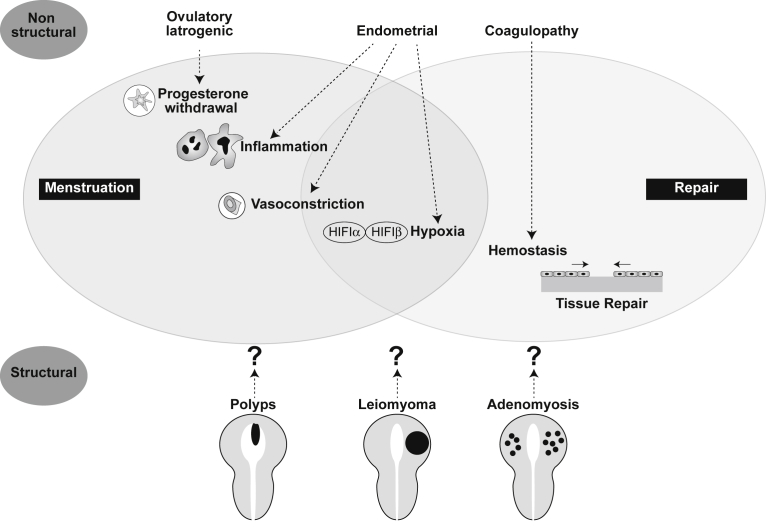
In the absence of any other features, for example, leiomyoma or a coagulopathy,3 bleeding from the endometrium may represent a “primary endometrial disorder” (AUB-E). In the presence of structural features such as leiomyoma, polyp, and adenomyosis,3 it is not known whether the presence of myometrial structural entities such as AUB-L (leiomyoma) or AUB-A (adenomyosis) actually result in a “secondary endometrial disorder” (Figure 6). There remains a true lack of knowledge about the phenotype of the endometrium when adenomyosis and leiomyomas are present.
Figure 6.
Potential mechanisms of “primary” and “secondary” endometrial AUB
As the corpus luteum regresses in the absence of pregnancy, progesterone levels fall. This occurs irregularly in those with ovulatory or iatrogenic AUB. Progesterone withdrawal causes a local inflammatory response in the endometrium and may be increased in those with primary endometrial AUB. An increase in vasoactive factors results in intense vasoconstriction of spiral arterioles to limit blood loss; this may be decreased in primary endometrial AUB. Vasoconstriction may induce transient tissue hypoxia and stabilization of HIF-1, the master regulator of the cellular response to hypoxia, to coordinate endometrial repair. There is evidence that this is less intense in those with endometrial AUB. Efficient hemostasis limits menstrual blood loss at menstruation and this is defective in women with coagulopathy AUB. Structural and nonstructural pathologies have the potential to disrupt endometrial physiology at menstruation, leading to abnormal uterine bleeding; these mechanisms remain undefined.
AUB, abnormal uterine bleeding; HIF-1, hypoxia-inducible factor 1.
Critchley. Menstruation: science and society. Am J Obstet Gynecol 2020.
This exciting area merits substantial research and many questions remain. What is the aberration in women with AUB-E? Do leiomyomas and adenomyosis contribute to the genesis of AUB or HMB? If so, is it because they directly affect the molecular mechanisms of endometrial hemostasis? Do leiomyomas actually need to be adjacent to the endometrium to cause AUB?74 To answer these important questions, we need to fully understand endometrial physiology and pathology.
III. Methods for the study of menstruation
Identification of aberrations in endometrial function necessitates study of human endometrial tissue. Women must have a detailed clinical history and examination and undergo investigation to determine if structural disorders are present. For research purposes, women should have measurement of their menstrual blood loss to enable categorization as having heavy or normal menstrual bleeding. An objective measurement of blood loss may be obtained using the alkaline hematin method and total menstrual volume by using a menstrual cup.23,75 Alternatively, a pictorial menstrual blood loss assessment chart has been validated to assess menstrual blood loss volume and duration.76 In addition, tissue must be carefully classified to determine the correct stage of the menstrual cycle.
Studies in women are often limited to generation of observational data. For more incisive functional studies, animal models of simulated menstruation have been developed.55,77, 78, 79 The nonhuman primate (rhesus macaque) has been studied extensively and provides an excellent model of the human menstrual cycle.80,81 More recently, attention has focused on refinement of the mouse model of simulated menstruation.55,57,77,79
A detailed study of the cellular and histologic events occurring in the mouse endometrium during simulated menstruation has been reported to recapitulate several of the local events that occur in the human endometrium at the time of menstruation, these being apoptosis preceding cytokine and chemokine expression and extensive neutrophil influx into the endometrium.82 There is an interesting recent discovery of a previously unrecognized menstruating rodent, the spiny mouse, which may provide another tool in the study of menstruation.41
The combination of observational data generated from well-categorized human endometrial tissue and mechanistic studies in validated animal models will facilitate definitive experiments to determine menstrual physiology and pathology.
IV. Initiation of menstruation
The human endometrium is a highly dynamic multicellular structure. Its physiological functions are preparation for implantation and, in the absence of pregnancy, menstruation. The regulation of normal menstruation is governed by sequential exposure to circulating sex steroids, estrogen, and then estrogen and progesterone followed by corpus luteum demise causing a fall in both circulating estrogen and progesterone. Progesterone withdrawal is the trigger for menstruation.53,83
Menstruation involves a remarkable sequence of endometrial cell proliferation, differentiation, shedding, and regeneration that may occur as many as 400 times across the reproductive life course.70 The mechanisms underpinning menstruation still remain poorly understood. There are crucial interactions between the endocrine system and the immune system.53,83 These cellular interactions, which are dependent on the menstrual cycle phase, involve epithelial and stromal cells along with an influx of innate immune cells and differentiation of the endometrial vasculature (spiral arterioles). The local endometrial events at the time of menses resemble those of an inflammatory event. There is an increase in endometrial blood vessel permeability and fragility, tissue breakdown, and an influx of innate immune cells into the endometrium, particularly neutrophils and macrophages.82,84,85
V. Cessation of menstruation
The cessation of menstrual bleeding and endometrial repair require 3 closely related events: these being vasoconstriction of the highly specialized spiral arterioles, local endometrial hemostasis, and reepithelialization of the injured endometrial mucosa (Figure 6). After menstruation, the restoration of the injured mucosal surface is a rapid event and the role of endometrial stem cells is addressed in the following contribution concerning endometrial regeneration. Endometrial repair of the denuded epithelial surface after menstruation has been described by Garry et al,86 using hysteroscopy, histology, and scanning electron microscopy. These imaging techniques detail the temporal repair of the epithelial surface, which occurs in a piecemeal fashion adjacent to actively menstruating tissue.86 The regulation of this endometrial repair process is not fully defined. There is a recent interesting interpretation of the link between human menstruation and separation of the placenta after delivery. Both are underpinned by progesterone withdrawal and critically involve uterine spiral arterial function.87
In women with complaints of HMB, impaired vascular differentiation caused by impaired spiral arteriole maturation has been described88, 89, 90 along with exposure to an imbalance of locally generated vasoconstrictors and vasodilators.91, 92, 93 An increase in blood vessel radius will affect the resistance to blood flow (Poiseuille’s equation).91 The pivotal role for vasoconstriction after progesterone withdrawal was described nearly 80 years ago.94 The study of autologous transplants of rhesus macaque endometrium into the anterior chamber of the eye and visualization of the events of menstruation through a slit-lamp ophthalmoscope revealed transient and intense vasoconstriction 4 to 24 hours before menstruation in response to steroid withdrawal. Authors proposed that this vasoconstriction was consistent with local tissue hypoxia. The presence and role of endometrial hypoxia in the process of menstruation have been debated. There is now experimental support for a pivotal role for transient physiological hypoxia because it has been reported to occur in the menstruating endometrium.58 The stabilization of hypoxia-inducible factor 1 (HIF-1; a marker for hypoxia) results in the generation of local repair factors to “heal” the injured mucosal surface (menstruating endometrium).58
Women with HMB have decreased endometrial HIF-1α at the time of menstruation, and these women also experience prolonged menstrual bleeding episodes. These observational data have been recapitulated in a mouse model of simulated menstruation in which physiological endometrial hypoxia is also reported to occur at the time of endometrial bleeding.58
Fibrinolysis is an important component of regulation of normal endometrial bleeding. The human endometrium contains tissue plasminogen activator (t-PA) and urokinase plasminogen activator (u-PA), along with plasminogen activator inhibitor (PAI) (inhibits fibrinolytic activity) and the u-PA receptor. Women complaining of HMB have raised levels of t-PA activity on the second day of bleeding when compared with those with normal menstrual blood loss, consistent with an overactive fibrinolytic system.95,96 Tranexamic acid, a popular nonhormonal treatment for HMB in many countries, targets the overactivation of the fibrinolytic system with a reported 58% reduction in menstrual blood loss.97
VI. Endometrial pathology in structural abnormal uterine bleeding
In the presence of structural pathologies (AUB-P/A/L), a secondary endometrial disorder has been proposed (Figure 6). The current literature presents lines of evidence to support the concept that there may be an element of resistance to normal progesterone-regulated events.98,99 The latter is based on descriptive data and may certainly be implicated given the often reported poor response to many progestin-based therapies, for example, the levonorgestrel-releasing intrauterine system and oral, implant, or injectable progestins.69
VII. Therapies targeting the progesterone receptor
Progestins have long been used to modulate endometrial bleeding either through their action on ovarian function or abolition of ovulation along with a direct effect on the endometrium to reduce bleeding. All progestins, when delivered orally, systemically, or through an intrauterine route, improve menstrual experience in many women; however, there remains a consistent 20% who experience unscheduled endometrial bleeding and spotting. This is often a reason for the discontinuation of use of progestin therapies. The mechanisms underpinning this unscheduled bleeding still remain elusive despite studies focusing on many candidate pathways.100, 101, 102, 103, 104 To date, there has been no reliable preventative intervention, albeit there are strategies to stop or reduce a heavy bleeding episode in users of progestin-only preparations.105
Selective progesterone receptor modulators (SPRMs) reduced endometrial bleeding in women with uterine leiomyomas.106,107 SPRMs inhibit ovulation in 90% of women and also affect the endometrium and many women experience amenorrhea.108 The mechanisms of action of SPRMs on the endometrium in women with abnormal bleeding and uterine leiomyoma still remain poorly understood. SPRMs have an interesting antiproliferative effect, and the study of cell-to-cell interactions within the endometrium in women exposed to this class of drug is a current topic of investigation.109 SPRM administration is associated with an unusual morphologic effect on the endometrium known as progesterone receptor–associated endometrial changes (PAECs). These morphologic features are associated with alterations in expression and localization of sex steroid receptors.109, 110, 111, 112 The fact that circulating estradiol levels remain consistently in midfollicular range has raised concerns among clinicians about the risks of hyperplasia and endometrial cancer. However, no studies to date that have explored in detail the endometrial impact have reported increases in either hyperplasia or endometrial cancer.113,114 Moreover, a recent systematic review reporting the endometrial effects of SPRM (ulipristal acetate [UPA]) use in 10 studies involving 1450 women supports the current view that PAEC is essentially a benign endometrial morphology that is reversible on discontinuation of UPA use.115
VIII. Summary comment
Understanding the pathology underlying AUB is essential to improve treatments for this common symptom that has a significant negative impact on women and society. Progesterone and progesterone receptor interactions play essential roles in uterine physiology and reproduction. Progesterone withdrawal remains the major trigger for the onset of endometrial bleeding. Menstruation itself involves repeated episodes of physiological “injury and repair” and a detailed knowledge of endometrial function is essential for understanding how disturbances in the endometrial function play a role in AUB. A particular gap is the understanding of endometrial function in women with myometrial structural features such as leiomyoma and adenomyosis and whether this represents a “primary or secondary endometrial disorder.” There is without doubt utility and validity of mouse models of simulated menstruation, particularly when used alongside human studies. Ligands for the progesterone receptor, that is, progestins and SPRMs, may reduce endometrial bleeding and modulate endometrial form and function. Identification of novel targets for the treatment of AUB is vital to address the significant personal and societal burden of this common disorder.
3B. Regeneration after menstruation—the role of stem cells
Hugh S. Taylor, MD
I. Introduction
In each monthly menstrual cycle, the endometrium is renewed from the basalis layer.116, 117, 118 This regenerative process recapitulates some features of development and includes production of all components of the endometrium, including glands, stroma, vasculature, and an influx of immune cells. The ability to rapidly and repetitively regenerate this tissue is fundamental to reproduction. Therefore, it is not surprising that there exists a population of cells that serve to replace and maintain the endometrium despite repetitive loss with menstruation.116, 117, 118 These stem cells maintain a reservoir of regenerative cells while simultaneously giving rise to more differentiated cells.
II. Endometrial stem cells
Early research in stem cells centered on the hematopoietic system, because experimental transplants to repopulate bone marrow could be performed using tissue ablation.119 These studies gave rise to the concept that stem cells divide asymmetrically, reproducing the stem cell and giving rise to a more differentiated cell, in contrast to the symmetrical division observed in somatic cells.119 However, translation of this asymmetrical division concept to other tissues and organs has recently become controversial because tremendous plasticity in the fate of epithelial cells in the intestine, liver, and other organs is being uncovered.119 Although specific mechanisms remain debated, stem cells throughout the body maintain the pool of regenerative stem cells for populating each tissue and organ. Most tissues contain a collection of stem and progenitor cells that replace adult cells lost to age or damage. In many organs, stem cells divide only under unusual conditions such as in response to injury. In other organs characterized by rapid turnover, such as the gastrointestinal tract, stem cells regularly divide to replace worn or damaged cells as part of normal tissue homeostasis.119 In the endometrium, the vast majority of cells are lost every month, making the need for frequent stem cell division more acute and essential.
Totipotent, pluripotent, and multipotent stem cells give rise to many different tissues, whereas tissue-specific stem and progenitor cells give rise to a limited set of differentiated cells in a local environment. Tissue-specific stem and progenitor cells may give rise to a single cell type or several types of cells that make up an individual organ. In the endometrium, multiple lines of evidence in mice and humans support the presence of a population of stem and progenitors that give rise to stromal fibroblasts and another population that gives rise to epithelia. Much of the current knowledge on endometrial stem cells comes from the studies in mice, where cell lineages can be traced using molecular tags and reporters, but understanding of the human endometrium is accelerating as more signatures of stem and progenitor cells in other organs are identified, investigated, and validated in the endometrium.116, 117, 118,120,121 Although early and even more recent studies in humans suggest that endometrial stem and progenitor cells are localized to the basalis layer,122, 123, 124 more recent evidence of stem and progenitor cell markers in the luminal region suggests a more complex picture of wider dispersal125 because they are in nonmenstruating species such as the mouse.126 Moreover, recent studies using tracers in the mouse endometrium have identified stem and progenitor cells that give rise to both epithelial glandular and luminal epithelial cells,126, 127, 128 whereas other tissue-resident stem and progenitor cells give rise to stromal cells in the mouse endometrium.128 It is possible that there is a common stem cell that gives rise first to stromal cells and can also differentiate into a distinct bipotential epithelial progenitor cell, as suggested by studies in mice showing gene expression evidence of mesenchymal-epithelial transitions (METs)57 and morphologic evidence of such METs in humans.129 These tissue-resident stem and progenitor cells regenerate the endometrium after menstruation in each menstrual cycle.
III. Bone marrow–derived stem cells
There also exist multipotent stem cells in several tissues that can divide and differentiate into multiple types of cells and are found in many tissue types. Most notably, bone marrow hosts both hematopoietic stem cells, which give rise to circulating white blood cells, red blood cells, and platelets, and mesenchymal stem cells, which give rise to bone, cartilage, and fat.130 Bone marrow hematopoietic and mesenchymal stem cells are found in the circulation, where they can be recruited to sites of injury and contribute to tissue repair in ways that are still incompletely understood in humans.131, 132, 133 In human patients who received bone marrow transplants, allowing donor cells to be tracked through sex chromosomes or human leukocyte antigen type, early studies reporting that bone marrow cells differentiated into hepatocytes or other epithelial tissue types are now mostly attributed to cell fusion or artifactual protocols.133 However, bone marrow fusion to endometrial stromal cells has been characterized in mice and is rare compared with bone marrow cells directly contributing to endometrial cell fates.134 Although the ability of mesenchymal stem cells to transdifferentiate broadly into cells in other tissue types remains controversial,135 convincing evidence from human studies using single-cell sequencing indicates that bone marrow–derived donor cells differentiate into mature adipocytes—a known cell fate for mesenchymal stem cells.136 Studies in mice and humans support the idea that bone marrow–derived stem and progenitor cells also contribute to the reproductive tract, supplementing the resident stem and progenitor cells. In both the mouse model and in humans, bone marrow–derived cells are incorporated into the endometrium where they differentiate into endometrial stromal cells, epithelial cells, and endothelial cells.137,138 The vast majority of bone marrow–derived endometrial cells are stromal cells with epithelial cells differentiating slowly and in smaller numbers. Other groups have subsequently confirmed a bone marrow origin for endothelial cells in the human139 and for stromal and epithelial cells in mouse,140,141 establishing a potential role of bone marrow in endometrial repair in humans and prompting human clinical studies aimed at treating endometrial disorders.118 Perhaps because of the depletion after menstruation, exogenous stem cells may be even more essential in the uterus than in other organs. Furthermore, increased recruitment and engraftment of these cells to the uterus occur in response to injury such as hypoxia or inflammation to aid in repair and regeneration.142
IV. Consequences of stem and progenitor cell loss
Infection and iatrogenic trauma can lead to endometrial destruction and loss of progenitor cells, causing failure to regenerate lost tissue and resulting in permanent damage. Multipotent stem cells circulate to the endometrium and engraft, contributing to the regeneration of damaged endometrium and mitigating endometrial atrophy, thin endometrium, and Asherman’s syndrome.143,144 However, these circulating bone marrow–derived stem cells are found in only very limited numbers in the circulation. In the setting of severe injury, the number of stem cells may prove insufficient to repair the damage. We have shown that augmented numbers of bone marrow cells in the circulation can prevent injury to damaged tissue including the endometrium. Transfer of bone marrow cells to mice after endometrial injury led to subsequently normal fertility, whereas those receiving placebo had severe infertility because of Asherman’s syndrome. Several case reports and nonrandomized trials have explored delivery of endometrial stem cells to women with inadequate endometrial development or Asherman’s syndrome with promising results for this potential novel therapy.145,146 Understanding normal menstruation and endometrial repair may provide insight into several endometrial pathologies.
We also found that the chemokine CXCL12 attracts bone marrow–derived mesenchymal stem cells to the endometrium.147 In a mouse model, we found that the administration of CXCL12 to the damaged uterus can mobilize and recruit stem cells from the bone marrow to the uterus. In a mouse model of Asherman’s syndrome, intrauterine administration of CXCL12 led to restoration of normal fertility.148,149 Similarly, in a mouse model of thin endometrium, treatment with either bone marrow supplementation or CXCL12 administration restores normal endometrial architecture and fertility.144 Future therapy for Asherman’s syndrome may make use of chemokines that mobilize and attract bone marrow cells without the need for bone marrow stem cell transplantation.
V. Menstruation and potential role of endometrial stem cells in endometriosis
Although rapid endometrial regeneration is essential for reproduction in menstruating species, one of the adverse consequences of menstruation and a rapidly regenerating endometrium is endometriosis. Menstruation allows for retrograde menstruation and the possibility of ectopic implantation of endometrial tissue. Continued menstrual flow regularly feeds the endometriosis and allows for lesion expansion. Retrograde menstruation of stem cells in particular contributes to the lesions.150 Furthermore, bone marrow stem cells contribute to the continued growth of endometriosis lesions.138,151 Bone marrow–derived stem cells may be responsible for those rare endometriosis cases outside of the peritoneal cavity such as endometriosis occasionally seen in the lungs or brain. The very processes designed to regenerate and repair the endometrium after menstruation can lead to disease. Here, the circulating stem cells can even lead to endometriosis in areas where endometrial cells cannot reach even through retrograde menstruation.
Although retrograde menstruation is a well-established cause of endometriosis, in reality endometrial cell trafficking is common; we have previously shown that stem cells from endometriosis can be found in the circulation in a mouse endometriosis model.152 Similarly, we have shown that endometrial cells can be identified in very small numbers in multiple organs not typically associated with endometriosis including the brain, lung, spleen, and liver.153 This vast cell migration may explain many of the systemic effects of endometriosis. Women with this disease are more likely to have depression, anxiety, autoimmune disease, and a lower average body mass index.154 The regenerative ability of endometrium and use of circulating stem cells may allow for regeneration after menstruation and enhance fertility; however, it may predispose menstruating animals to endometriosis and associated disease. Endometriosis can be considered a systemic disease in which widespread cell trafficking contributes to the pathophysiology.154
VI. Endometrium, stem cells, and pregnancy
Finally, endometrium has an essential role in the establishment of pregnancy. Indeed, many complications throughout pregnancy have their origin at the time of implantation.155 It is not surprising that stem cells are an important part of endometrial and decidual function in pregnancy. We recently reported that there is a major flux of bone marrow–derived stem cells to the uterus in pregnancy.156 These cells differentiate into endothelial cells and decidual cells that have a functional role in pregnancy. In a mouse model of infertility based on an endometrial receptivity defect, administration of normal bone marrow can restore fertility and successful pregnancy in otherwise infertile animals. This leads to the fascinating conclusion that some instances of infertility or pregnancy loss may be caused by inadequate bone marrow rather than defects in reproductive organs or gametes. Indeed, one can now include the bone marrow as a key reproductive organ!
VII. Beyond the uterus: menstrual blood–derived stem cells in the context of regenerative medicine
The fast, scarless regenerative power of the endometrium, along with the relatively easy access to endometrial stem cells from menstrual effluent (see section 4), has spurred efforts to use menstrual blood–derived endometrial stem and progenitor cells therapeutically for a range of regenerative medicine applications beyond those in the uterus mentioned previously.157,158 Endometrial mesenchymal stem and progenitor cells (MSCs)120,124 share many properties with mesenchymal stem cells derived from the bone marrow, adipose tissue, and other sources.159 Similar to mesenchymal stem cells from these other sources, they can be readily expanded in culture, show features of differentiation into the canonical mesenchymal stem cell connective tissues (bone, cartilage, and fat), and produce a range of immunomodulating cytokines, chemokines, and growth factors.157,159 Reports that mesenchymal stem cells from endometrium and other sources can transdifferentiate into a variety of nonconnective tissues, including liver, pancreatic beta cells, and hepatocytes, both in vitro and in animal models or in studies of human bone marrow transplant patients, have been attributed to experimental artifacts, as described previously and in section 4.
More than 1000 clinical trials involving human mesenchymal stem cells or their products are currently listed on ClinicalTrials.gov as of May, 2020, with many in advanced phase III stages of testing. Of these trials, about 400 are listed as involving autologous cells and about 300 as involving allogeneic cells (others do not specify in a searchable term; they may involve cell products such as matrix or exosomes). Clinical applications listed on ClinicalTrials.gov may broadly be divided into direct, permanent regeneration of connective tissues (bone, cartilage, fat, or related tissues), where autologous cells are required, and immunomodulatory applications that tilt healing toward regeneration rather than fibrotic repair through transient action of the therapeutic cells. For example, a phase III clinical trial is underway at the Cleveland Clinic to treat Crohn’s disease fistulas with allogeneic bone marrow–derived mesenchymal stem cells after a successful phase III trial outside the United States using adipose-derived mesenchymal stem cells.160 Similarly, advanced clinical trials using allogeneic or autologous bone marrow–derived mesenchymal stem cells to modulate inflammation are underway for aplastic anemia,161,162 liver,163 lung,164,165 and many other acute or chronic inflammation pathologies.
Of the mesenchymal stem cell clinical trials listed on ClinicalTrials.gov, only 2 use menstrual blood–derived cells, both taking place at Zhejiang University in Hangzhou, China: 1 for chronic liver disease166 and 1 for type 1 diabetes. However, because menstrual blood–derived mesenchymal stem cells are an attractive source for autologous transplant in regeneration of connective tissues in women,157 especially considering that connective tissue cells exhibit strong sex-based phenotypic differences, several regenerative applications are advancing through large animal studies. Particularly promising is the potential for endometrial mesenchymal stem cells to repair pelvic organ prolapse by seeding cells onto degradable scaffolds.158 These and other connective tissue applications are moving toward human trials. Whether the ease of collection of human menstrual effluent–derived stem cells, or performance factors of these cells, will overcome the established infrastructure that relies on bone marrow, adipose tissue, and other sources for regenerative medicine or other purposes is difficult to predict, but they are in the running.
VIII. Conclusion
In summary, menstruation in humans requires rapid regeneration of endometrium that is facilitated by stem cells. Stem and progenitor cells in the basalis layer are the major source of new endometrium each cycle. Bone marrow stem cells are engaged to repair endometrium after damage and are now known to be functionally important for pregnancy in mice.156 Stem cells play a crucial role in reproduction. With this stem cells flux comes the possibility of disease related to aberrant endometrial growth, namely, endometriosis. Endometriosis is a systemic disease of inappropriate stem cell differentiation. Menstruation is far more complex than a simple loss of endometrium and regrowth—it requires contributions from stem cells both within the uterus and bone marrow. Menstruation also predisposes to endometriosis, which also involves far more than just the immediate surroundings of the uterus where most endometriosis settles.
3C. What does fibroid (leiomyoma) research teach us about endometrial function?
Elnur Babayev, MD; Serdar E. Bulun, MD
I. Pathophysiology of uterine fibroid (leiomyoma) formation and growth
Uterine fibroids are extremely common. More than half of women will develop uterine fibroids by the age of 50 years.72 Patients present with AUB or pressure symptoms such as pelvic discomfort and pain, constipation, or changes in urinary habits. Submucosal fibroids are also associated with infertility and early pregnancy loss.167, 168, 169
Fibroids are benign uterine tumors characterized by disordered monoclonal proliferation of uterine smooth muscle cells embedded in an abundant extracellular matrix. One proposed mechanism of fibroid formation involves genetic and epigenetic changes in multipotent stem cells in the myometrium that lead to abnormal proliferation and differentiation. Physiological fluctuations in sex steroid levels with subsequent growth and involution of myometrial cells during the menstrual cycle make these stem cells vulnerable to mutations or epigenetic changes and fibroid formation. The genetic, epigenetic, molecular, and paracrine mechanisms underlying fibroid pathophysiology are highly diverse, which explains the observed variations in individual tumors’ clinical behavior (growing, stable, or regressing) and response to medications.167,168
Fibroid growth is hormone dependent, and the sex steroids estrogen and progesterone are important regulators of fibroid growth. Most fibroids decrease in size after menopause, whereas pregnancy can lead to an increase in the size of fibroids. Both systemic and local estrogen can stimulate fibroid growth, but local estrogen production through aromatase activity in fibroid tissue seems to play an important role in fibroid pathophysiology.170, 171, 172 Estrogen induces progesterone receptor expression and progesterone responsiveness of the tumor. Progesterone has been shown to be essential for fibroid growth in animal studies.173 Progesterone may regulate fibroid growth indirectly through its action on differentiated smooth muscle cells, which in turn secrete paracrine molecules that stimulate proliferation of multipotent stem cells.174,175
II. The role of vasoactive substances and inflammatory molecules in the pathogenesis of abnormal uterine bleeding secondary to fibroids
Fibroids may interfere with normal endometrial function. In fact, heavy menstrual or irregular bleeding is the most common clinical presentation of fibroids and can affect the physical, social, and emotional well-being of women. The degree of endometrial dysfunction seems to be related to the size and location of the fibroids. Submucosal fibroids located immediately beneath the endometrium are more likely to disrupt endometrial integrity and cause AUB. Subserosal fibroids are less likely to do so. Intramural fibroids represent an intermediate pathology, although large intramural fibroids that distort the endometrial cavity will likely lead to abnormal menstruation.168,169 Dissecting the mechanisms of interaction between fibroids and endometrium can help us understand menstrual biology and may lead to the development of novel therapeutic modalities for AUB.
Fibroids are space-occupying lesions that, depending on their size, can place significant mechanical stress on uterine architecture. Fibroids can increase the amount of bleeding by simply increasing the surface area of the endometrium. In addition, changes in cell shape and stretch can affect gene expression in the myometrium and endometrium.176, 177, 178 Large intramural and submucosal fibroids may interfere with the myometrial contractions that occur during menstruation. These contractions help to evacuate menstruation material from the uterus and decrease blood loss from endometrial vessels under physiological conditions; thus, even a small submucosal fibroid can lead to a significant blood loss in these patients.167
Endothelin 1 (ET-1) is a vasoconstrictor that affects spiral arterioles in the endometrium53 and plays an important role in myometrial contractility.179 Altered expression levels of ET-1 and endothelin receptors (ETA-R and ETB-R) in uterine fibroids may interfere with the normal physiological function of the myometrium during menstruation. Fibroids have higher levels of ETA-R and lower levels of ETB-R than normal myometrium.180,181 Thus, it may be envisioned that altered endothelin biology induced by a uterine fibroid may affect the vascular function of the adjacent endometrium, giving rise to its irregular development or shedding.
Menstruating endometrium is rich in cytokines and prostaglandins. How this inflammatory milieu affects fibroids and vice versa is an active area of investigation. The composition of inflammatory cells is different in areas of the endometrium that overlay fibroids compared with distant sites. Perifibroid endometrium has increased numbers of macrophages in all phases of menstrual cycle; however, the number of uterine natural killer (uNK) cells is decreased in the secretory phase.182 Prostaglandin F2α levels are increased in fibroid uteri, which may explain the disordered contractility and increased blood loss observed in these patients. Moreover, prostaglandin E2 (PGE2), which is produced in the normal menstruating uterus, affects leiomyoma (fibroid) cells. Normal myometrial cells do not show any changes in gene expression in response to PGE2, whereas leiomyoma (fibroid) cells demonstrate increased expression of antiapoptotic microRNAs.183 Celecoxib, a cyclooxygenase 2 inhibitor, reduces the proliferation rate of leiomyomas (fibroids) through a nuclear factor κB–mediated decrease in expression of cytokines and growth factors.184 Our understanding of the role of prostaglandins in fibroid pathogenesis may lead to new therapeutic approaches. Gene expression analysis of endometrial biopsies from women with HMB has also indicated differential expression of antigen processing pathway genes in women with and without fibroids.185 Thus, specific molecular pathways might be responsible for abnormal bleeding associated with fibroids.
III. Growth factors as primary mediators of endometrial dysfunction in fibroid uteri
Fibroid uteri demonstrate rich vascularity and increased venous plexus.168,169 There also seems to be defective vasoconstriction as evidenced by dilated venous spaces and vasocongestion. Increased angiogenesis is also apparent in patients with fibroids,181,186 with altered expression of angiogenic growth factors and their receptors. Variations in the number and type of inflammatory cells, which produce angiogenic factors, in the endometrium of fibroid uteri may contribute to the differences in the expression of these factors. Moreover, angiogenic genes are differentially expressed in fibroids, myometrium immediately adjacent to fibroids, and distant myometrium.168 Fibroids express increased levels of the important angiogenic molecule basic fibroblast growth factor (bFGF) and endometrium associated with fibroids demonstrates increased expression of the bFGF receptor, bFGF receptor 1 (FGFR1).186 Increased activity of bFGF through its receptor represents a possible pathophysiological mechanism underlying increased angiogenesis in fibroids, which may ultimately contribute to HMB.
A paracrine interaction between fibroids and the endometrium exists that is not just localized to the endometrium overlying the fibroid; this interaction has global effects on endometrial function.187 For example, the Wnt/β-catenin pathway plays an important role in fibroid growth. Activation of this pathway leads to increased expression of transforming growth factor β3 (TGF-β3), and fibroids demonstrate increased expression of TGF-β3 compared with normal myometrium. TGF-β3 stimulates smooth muscle cell proliferation and fibronectin expression.188 Moreover, TGF-β3 affects endometrial receptivity and decidualization by altering the expression of bone morphogenetic protein 2 receptors.189 This effect is likely secondary to the decreased expression of homeobox A10 (HOXA10). Removal of intramural, but not submucosal, fibroids seems to reverse the changes observed in HOXA10 levels.190,191 Interestingly, endometrium obtained from patients with fibroids demonstrates decreased levels of PAI-1 and thrombomodulin. Endometrial stromal cells exposed to TGF-β3 in vitro show decreased levels of PAI-1, antithrombin III, and thrombomodulin.74 These experiments suggest that fibroids affect menstruation by altering homeostasis in the endometrium. Changes in clotting factor levels may tip the balance toward anticoagulation, which may at least partially account for the increased bleeding seen in patients with fibroids.
Taken together, fibroids seem to cause abnormal menstruation by interfering with myometrial contractility, paracrine signaling (growth factors, prostaglandins, endothelin, angiogenic factors), and hemostatic regulation (alteration in the expression of clotting factors) in the endometrium. Understanding the mechanisms of AUB secondary to fibroids will shed light on these endometrial functions in normal menstruation physiology and may lead to the development of new therapeutics for women with fibroids.
3D. Microbiome of the endometrium
Inmaculada Moreno, PhD; Iolanda Garcia-Grau, MS; Carlos Simon, MD, PhD
I. Introduction
Humans have always lived in a microorganism-colonized world, in which microbes, especially bacteria, exist in clear symbiosis with humans. The concept of a human microbiota refers to the sum of microorganisms that inhabit the human body, and the number of commensal microbes is estimated to be the same as the number of human cells.192 Human physiology is influenced by the presence of such microorganisms through the expression of microbial genes (of which there are several million in total in contrast to only 23,000 human genes).193 Thus, the human microbiome is considered our second genome and interacts with the host genome creating what is called a hologenome defining a whole complex organism and contributing to genetic diversity.194 The balance between host and bacterial cells has been shaped through evolution, and the microbiota in each body niche has adapted in response to intrinsic (eg, host genetics) and extrinsic or environmental factors (eg, diet). This individual microbiota constitutes a critical component of immunity, and thus, colonization by different bacteria may turn this mutualistic or commensal interaction into a parasitic relationship, predisposing the host to pathologic conditions with variable severity of symptoms.
II. Role of the microbiota in human health and disease
The first evidence that microbes contribute to health and disease comes from the 17th century when it was shown that bacteria from different body niches in the same individual are different, and there are different bacterial communities in the same body site in healthy vs diseased subjects.195 It is now apparent that microorganisms, specifically bacteria, exert functional roles in our body and communicate with host cells by influencing metabolic function, training of the host immune system, and modulating drug interactions.196 It is also known from studies in mice and humans that the profile of microorganisms inhabiting an individual early in life contributes to postnatal development and adulthood by influencing metabolism, respiratory function, bone growth, and immunomodulation.197, 198, 199, 200 Several studies have revealed that the gut microbiota also affects neurodevelopment, thereby affecting an individual’s behavior and cognition and susceptibility to mental disorders through the gut-brain axis.201,202 More recently, the gut microbiome has been linked to the secretion of circulating estrogens, leading to the estrobolome concept (Figure 7). Because estrogens are implicated in numerous biological processes, disequilibrium of the microbiota may subsequently contribute to a large variety of estrogen-modulated conditions, including metabolic disorders (eg, metabolic syndrome, obesity), alterations of female reproductive function, and diseases in women (eg, polycystic ovary syndrome, endometriosis, endometrial hyperplasia).203
Figure 7.
The estrobolome plays a central role in health and disease through the gut microbiota-estrogen axis
Dysbiosis of gut microbiota may induce systemic inflammation and interferes with estrogen metabolism and receptor activation in estrogen-regulated organs, influencing neurocognition, metabolism, and the onset of gynecologic diseases and infertility.
Reprinted from Baker et al.203
Critchley. Menstruation: science and society. Am J Obstet Gynecol 2020.
III. The existence of an endometrial microbiota
Molecular detection of bacterial communities through 16S rRNA gene sequencing has shown that the human microbiota is related to human health and welfare, as symbiotic microorganisms colonize every human organ, including the reproductive tissues.204 In adult women, the vaginal microbiota contributes to 9% of the total bacterial load and is characterized by a high stability with low richness and diversity indexes.205,206
Highly sensitive detection techniques, for example, the latest sequencing technology applied to microbiology, permit the study of microbial communities at the molecular level, providing ecological information about the microbiota of low biomass samples that have traditionally been considered sterile owing to the inefficiency of culture-dependent methods for isolating some types of bacteria under standard laboratory conditions.207 It must be noted that low biomass samples are susceptible to being masked by background bacterial DNA contained in laboratory reagents and equipment.208 For this reason, the analysis of such samples requires extra caution during handling and manipulation and the simultaneous analysis of blank controls for monitoring potential contamination.209 The endometrial microbiota is considered a low biomass microbiota because the total amount of bacteria colonizing the uterine cavity is 102 to 104 times lower than the total bacterial load in the vagina.210,211
The microbiota of the upper reproductive tract was identified by studies applying molecular techniques, such as quantitative polymerase chain reaction or parallel sequencing, to endometrial, fallopian, and peritoneal samples (review by Koedooder et al, 2019).212 Recently, the existence of a microbiota continuum throughout the reproductive tract has also been described. Lactobacillus spp. are the most frequently identified bacteria in the lower reproductive tract of asymptomatic reproductive-age women, but the abundance and structure of the microbiota change progressively toward the upper tract.211 To date, several studies have analyzed the composition of the endometrial microbiome of reproductive-age women using culture-independent methods. Comparative studies have reported that the endometrial and vaginal microbiota are similar but not identical in every woman.210,213,214 Routes of endometrial seeding have been proposed,212 with the most likely route being the ascent of bacteria from the vagina, as supported by the resemblance of the microbiota in consecutive spatial niches and the identification of Gardnerella vaginalis biofilms in the endometrial walls of women with bacterial vaginosis (BV).215 Most studies analyzing the endometrial microbiota agree on reporting Lactobacillus as the most common bacteria detected in studies using culture-independent techniques, whereas other genera, from the Bifidobacteriaceae, Comamonadaceae, and Streptococcaceae families, are also commonly found in the uterine cavity of healthy and fertile women.211, 212, 213,216, 217, 218, 219, 220 However, a recently published paper shows that although 60% of the analyzed endometrial samples present a detectable microbiota compared with background controls and different from that in the vagina, rectum, and oral cavity, Lactobacillus was rarely abundant in this type of sample.221 Because each study used different designs, types of samples, and sequencing platforms, defining the core endometrial microbiota is challenging; no consensus has been reached so far regarding the molecular signature of the uterine cavity. In addition to the investigation of the core endometrial microbiota under physiological conditions, the role of endometrial microbiota in the origin and maintenance of several gynecologic diseases, including pelvic inflammatory disease, endometriosis, and cancer, is currently under study.211,222, 223, 224, 225
IV. The impact of the endometrial microbiome on reproductive health outcomes, fertility, and pregnancy
Fertility problems can be related to microbial imbalance in the reproductive tract. The cervicovaginal microbiota of infertile women is more diverse and has lower levels of Lactobacilli (specifically Lactobacillus iners) and higher levels of BV-associated bacteria (Atopobium vaginae, G vaginalis, Ureaplasma spp., Leptotrichia, Sneathia) than the microbiota of fertile women.226, 227, 228, 229, 230 Moreover, the abundance of Lactobacillus spp. in vaginal and endometrial samples of infertile patients undergoing assisted reproductive technology (ART) is significantly lower than that in samples from fertile volunteers.217
Pregnancy success is affected by the endometrial microbiota as indicated by conventional culture techniques showing that isolation of bacterial pathogens from the tip of catheters used for embryo transfers associates with poor reproductive outcomes,231, 232, 233, 234, 235 but the effect of bacteria on human reproduction is not restricted to the uterine cavity. Microbial culture of ovarian and follicular fluid showed that isolation of dysbiotic bacteria correlates with higher embryo discard rates and adverse ART success after in vitro fertilization (IVF), whereas isolation of Lactobacillus spp. associates with better pregnancy outcomes.236
Our research group has used 16S rRNA sequencing to prospectively investigate the microbiota of endometrial fluid samples collected from patients undergoing IVF with repeated implantation failure in relation to their clinical results after embryo transfer.213 Lactobacillus was more abundant in patients with successful pregnancy compared with those with cycle failure. Interestingly, high Lactobacillus abundance in endometrial samples was a significant variable for predicting the reproductive success of the patients. In contrast, low abundance of Lactobacilli together with specific pathogens was associated with poor reproductive outcomes resulting in implantation failure, biochemical pregnancy, or clinical miscarriage.213
In addition, as an incidental finding, we were able to compare, at the taxonomical and functional level, the human endometrial microbiota present in a successful fourth-week pregnancy to that of a previous eighth-week spontaneous clinical miscarriage in the same patient with euploid embryos. Bacterial diversity was lower and Lactobacillus abundance higher (L iners was the only bacterium found) during the healthy pregnancy.237 These novel observations may profoundly affect our understanding and possible clinical translation of the microbiome in relation to healthy or pathologic human pregnancy.
V. Reproductive tract microbiome before, during, and after reproductive age
Understanding endometrial microbiota fluctuations during the life cycle and in response to different stimuli will help to anticipate dysbiotic shifts from the Lactobacillus-dominated physiological state and will allow the design of novel interventional strategies to restore the endometrial microbial profile. However, because sampling the endometrium is invasive, longitudinal studies have not yet been published describing the stability of the endometrial microbiota in the life cycle of healthy, diseased, and infertile subjects. In contrast, the microbial profile of the vagina, which is easily sampled, has been temporally analyzed. Estrogen levels are the most critical variable driving vaginal microbiota changes occurring over a life span. Estrogen modulates the availability of glycogen in the vaginal epithelium and the subsequent growth of Lactobacilli (Figure 8).238 Because Lactobacillus spp. produce lactic acid, the dominance of the vaginal niche by Lactobacilli entails the acidification of the niche (where Lactobacillus have a growth advantage), creating a hostile environment that impedes the growth of pathogens.
Figure 8.
The vaginal microbiome during the life cycle
Bacterial populations inhabiting the vagina change in response to estrogen levels, modulating glycogen availability in the vaginal epithelium and subsequently the growth of bacteria based on the physicochemical features of the niche at each phase of the lifecycle.
Reprinted from Muhleisen et al.254
Mod, moderate.
Critchley. Menstruation: science and society. Am J Obstet Gynecol 2020.
In children, the vaginal microbiota is mainly colonized by common aerobic bacteria (ie, Enterobacteria, Streptococcus, Staphylococcus) and other gram-positive (ie, Actinomyces, Peptostreptococcus) and gram-negative (ie, Veillonella, Bacteroides) anaerobes. Interestingly, Lactobacillus, G vaginalis, and Prevotella bivia, some of the most representative reproductive tract bacteria in adults, are absent from the vagina during this period.239,240 Then, coinciding with the estrogen rise at the onset of puberty, the bacterial profile is reshaped to resemble that of adult women, with increased abundance of Lactobacillus detected in premenarcheal adolescents. After menarche, the vaginal microbiota is definitively stabilized and obtains the reproductive-age microbiota profile with dominance of Lactobacillus clusters in the majority of studied subjects. Interestingly, G vaginalis levels also rise during puberty in some subjects even before their first sexual contact.241
The vaginal microbiome of healthy women can be classified in different community state types (CSTs) based on the structure of bacteria identified. Four CSTs are characterized by the dominance of Lactobacillus spp., namely, Lactobacillus crispatus (CST I), Lactobacillus gasseri (CST II), L iners (CST III), and Lactobacillus jensenii (CST V). These 4 clusters associate with vaginal health, whereas a non-Lactobacilli microbiota abundant in reproductive tract pathogens, such as G vaginalis, A vaginae, Dialister, Megasphaera, Prevotella, and Sneathia, is classified as CST IV and associates with BV.242
Colonization and maintenance of microbial populations in the vagina of premenopausal women may be affected by many factors (eg, age, hormonal milieu, hygiene, menstruation, use of contraceptives, sexual activity, ethnicity), leading to potential CST shifts over short periods of time or even within 1 menstrual cycle.242, 243, 244 During pregnancy, the richness and diversity of the vaginal microbiota tend to decrease, accompanied by increased Lactobacillus, which is consistent with higher levels of estrogens.245, 246, 247, 248 However, dominance of vaginal microbiota by G vaginalis, Ureaplasma, Prevotella, or other pathogenic taxa during pregnancy associates with complications, mainly preterm birth.249, 250, 251 After delivery, vaginal bacterial diversity increases and may generally shift to CST IV for up to 1 year postpartum even for women with high Lactobacillus abundance during pregnancy.248
During menopause, estrogen levels drop, and Lactobacillus spp. levels fall to become 10- to 100-fold less than in premenopausal women. This occurs with a concomitant increase in Prevotella, Gardnerella, Atopobium, Ureaplasma, and anaerobic bacteria belonging to CST IV. Interestingly, postmenopausal women receiving hormone replacement therapy have levels of Lactobacillus similar to those observed before the menopause.252, 253, 254
VI. Influence of menstruation on the reproductive tract microbiota
Hormonal changes within the menstrual cycle are proposed major regulators of the reproductive tract microbiota. During the menstrual cycle, circulating estrogens and progesterone positively correlate with community constancy, whereas during menses, the microbiota is more prone to bacterial changes.243 There are different community trends during the menstrual cycle with some communities remaining stable across the whole cycle, whereas others experience CST shifts in response to menses243 and shift back after menstruation. A stable pattern is observed in some women colonized by L crispatus,255 whereas the majority of women undergo microbial population changes with menses, entailing transitions from microbiota dominated by Lactobacillus to microbiota with L iners, G vaginalis, gram-positive cocci, or other dysbiotic bacteria.256,257
L iners and G vaginalis levels may rise in the vagina during menses because of their capacity to grow under adverse conditions. For example, G vaginalis cannot grow in iron-limiting conditions but is able to secrete vaginolysin to lyse host cells (ie, erythrocytes) to gather iron. In addition, some Lactobacillus strains have protective mechanisms enabling them to grow in the presence of iron. For example, L crispatus encodes an iron transport system. Similarly, L iners synthesizes a unique iron–sulfur protein cluster that confers the ability to sequester iron from menstrual blood, providing L iners with an advantage over L gasseri and L jensenii, within the vaginal niche during menses.255,258,259
At the functional level, fluctuations of the cervicovaginal microbiota have been associated with innate immunity, HIV acquisition, inflammatory status, and epithelial barrier function.260 For example, women with G vaginalis showed a sharper decrease of the epithelial barrier protein repetin from the ovulatory to the luteal phase than women with a Lactobacillus-dominated microbiota.260 However, no studies have identified yet the relevance of the microbiota for menstrual function or ascertained if the observed changes are driven by the cycling sex hormone levels, the microbiota profile, other causing agents, or a combination. To shed some light on the role of bacterial taxa on menstruation, a recent study has characterized the endometrial and cervical microbiota of women with AUB at different phases of the menstrual cycle.261 This study has revealed significant differences in the endometrial microbiota between women presenting with HMB and dysmenorrhea. Although the endometrial samples of women with dysmenorrhea presented an increased abundance of Acinetobacter spp., facultative anaerobic genera were increased in endometrial samples of patients with dysmenorrhea, suggesting a potential contribution of microbial communities to these menstrual symptoms, although the cause-consequence analysis has yet to be undertaken.261
VII. Mechanisms for bacterial-host interaction
How bacterial cells communicate with their hosts is still under investigation, but several mechanisms have been proposed. Bacteria can synthesize small molecules (eg, short-chain fatty acids, proteins, oligosaccharides, vitamins, short noncoding RNAs, neurotransmitters) that may interact with host cells in several ways, including regulation of the physicochemical conditions of a niche, epigenetic regulation through proteins interacting with the host transcriptional machinery, or binding to host receptors (see reviews207,262,263) (Figure 9). Of note, amines produced by gut bacteria can, owing to their chemical and structural similarity to human endogenous ligands, effectively bind G protein–coupled receptors (GPCRs), indicating how microbial metabolites might regulate host functions.264 GPCRs comprise the largest family of receptors in humans and are responsible for a wide variety of intracellular processes in response to extracellular signals mainly mediated by hormones, neurotransmitters, or other stimuli. During reproduction, GPCRs are responsible for responding to neuropeptides and essential hormones, such as gonadotropin-releasing hormone (GnRH), luteinizing hormone, follicle-stimulating hormone, prostanoids, and others, in the hypothalamus-pituitary-gonadal axis.265 This type of host-microbial interaction is of outstanding relevance in pathologic processes because GPCRs are pharmacologic targets of 35% of approved drugs, and the composition of the microbiota and its derived products could interfere with drug efficacy. Conversely, up to 25% of nonantibiotic pharmaceutical drugs designed to target human cells, including antidiabetics, antidepressants, antipsychotics, and some anti-inflammatory drugs, present antimicrobial activity or alter the composition of the indigenous microbiota, leading to potential side effects and increasing resistance to antibiotics.202
Figure 9.
Potential mechanisms of interaction between endometrial cells and the uterine microbiome
Reprinted from Baker et al.207
AMP, adenosine monophosphate; ROS, reactive oxygen species; SCFA, short-chain fatty acid; TLR, toll-like receptor.
Critchley. Menstruation: science and society. Am J Obstet Gynecol 2020.
VIII. Conclusion
The reproductive tract microbiome is currently considered a pivotal player in women’s health. Further investigation of the underlying mechanisms of host-bacterial interactions is needed to better understand both physiological and pathologic conditions. Translational implementation of this knowledge might allow us to shape the microbiome to promote global health using alternative methods and thereby avoid antibiotic abuse.
4. Menstruation as an Investigative Tool and Diagnostic Resource
Christine N. Metz, PhD; Ridhi Tariyal, MBA, SM; Ji-Yong Julie Kim, PhD; Aoife Kilcoyne, MBBCh, BAO; Peter K. Gregersen, MD
I. Introduction
The process of menstruation produces a natural tissue biopsy that is arguably underappreciated as a potential source of rich information on the health status of the endometrium. Growing awareness among patient populations about menstrual disorders and the advances in mHealth apps, data science, and the ever-decreasing costs of sequencing are driving new opportunities to characterize normal and pathologic menstrual functions. Interest in the endometrium as a model of fast, scarless healing for a variety of regenerative medicine applications further motivates the study of both normal and pathologic menstrual shedding and regeneration, using both analysis of shed menses and tissue engineering approaches to capture complex interactions among epithelia, stromal, immune, and other cell types present in the uterus. Complementing these approaches, insight into the behaviors of the endometrium in the context of the uterus in health and disease is achieved with recent advances in imaging technologies.
II. Using menstrual effluent to aid diagnosis of menstruation-associated conditions
Menstrual discharge contains shed endometrium, comprising endometrial epithelial cells, stromal cells, endothelial cells, and other nonimmune and immune cells together with microbial species present in the uterus (see section 3D on the microbiome) and vaginal tract along with a vast array of proteins, RNA, DNA, and metabolites. It is distinctly different from peripheral blood, and its composition aligns closely with that of the endometrium.266 Because it is considered a waste product discharged from the body, the term “menstrual effluent” has been used by some investigators (in 41 papers on PubMed as of January 2020), whereas others refer to the discharge as menses or menstrual blood.
Menstrual effluent offers many advantages for investigating uterine health compared with endometrial or uterine tissues collected through surgical biopsies, including noninvasive collection methods, relatively large sample volumes, and opportunities for repeat collections (within and across cycles). Although these advantages have been recognized since menstrual cups first became clinically available in the 1950s,267,268 menstrual effluent remains surprisingly understudied, and until recently, only a few sporadic attempts in small-scale studies have been made to relate properties of the menstrual effluent to disease states or to use it for disease diagnostics.269, 270, 271, 272, 273, 274, 275, 276, 277, 278, 279, 280 Biochemically, in healthy women, menstrual blood has been shown to have comparable concentrations of steroid hormones but higher prolactin levels when compared with peripheral blood,269 and proteomics analysis has revealed more than 300 different proteins in menstrual blood compared with peripheral blood,275 including scores of proteins and molecules involved in wound healing and regeneration.277 Small-scale investigations of platelets and coagulation proteins in menstrual blood compared with peripheral or uterine vein blood have thus far given little insight into potential causes of HMB but revealed that menstrual blood platelets were largely degranulated.270,271 At the cellular level, in healthy women, small studies showed that the NK cell repertoire in menstrual blood is stable over many menstrual cycles and different from peripheral blood,276 and menstrual blood has relatively fewer CD16+ monocytes and more NKT cells compared with peripheral blood.266,279 Extending the immune cell analysis to disease states, a small (38 patients) study reported alterations in immune cell populations in the menstrual blood of women with fertility disorders.280 Viable shed endometrial tissue collected from menstrual cups has also been studied to investigate the role of matrix metalloproteinases in endometriosis, although again the studies were pilot in nature.272, 273, 274
Several current forces support using menstrual effluent for diagnostics. Women are more comfortable with various forms of hygiene products, including menstrual cups, which are becoming more mainstream for managing menstruation, potentially allowing for more reproducible collections. At the same time, women are gravitating toward consumer health and digital recruitment platforms, creating new opportunities for research and diagnostic development.281,282 Greater awareness of menstrual disorders and gynecologic diseases is promoted by widespread reporting of personal suffering by celebrities, along with an explosion of social media platforms engaging patients, thus priming women and adolescents to seek answers for debilitating gynecology problems. Finally, the growing awareness of the shortcomings of blood-based biomarkers in the diagnosis of complex, chronic diseases is driving increased interest in proximal tissue–based approaches.283
Endometriosis affects 6% to 10% of reproductive-age women and is estimated to cost more than $20 billion per year in the United States.284, 285, 286, 287 Endometriosis offers a particularly compelling case for development of minimally invasive menstrual effluent–based diagnostics. Endometriosis is characterized by lesions of endometrial-like glands and stromal cells growing outside of the uterus, which are often associated with debilitating pain and infertility.284,285,288 Although several theories for its etiology involve developmental origins,289,290 Sampson’s theory of reflux menstruation into the peritoneal cavity remains a plausible explanation in light of the clinical presentation observed in most cases of endometriosis.
Numerous factors likely contribute to a diagnostic delay for endometriosis of up to 7 to 10 years.291, 292, 293 Some women and adolescents experience vague symptoms that overlap with other conditions, whereas other women and adolescents have few or no symptoms and are not diagnosed until they present with infertility. In addition, women often experience minimization or dismissal of pain symptoms, more frequent misdiagnoses related to pain, and gender-related disparities in the treatment of pain when compared with men.294,295 Although standard magnetic resonance imaging (MRI) or ultrasound imaging can suggest the presence of endometriomas and a limited number of lesions in other locations, most patients display no detectable lesions on imaging, thus motivating more invasive investigation of symptoms The definitive diagnosis of endometriosis requires invasive surgery, a procedure many women and adolescents delay, avoid, or cannot afford. None of the peripheral blood biomarkers proposed for diagnosing endometriosis exhibit the accuracy required for clinical use.296
Menstrual effluent also offers a particularly attractive noninvasive diagnostic for endometriosis because numerous differences between the eutopic endometrium in women with endometriosis compared with unaffected women have already been cataloged at the cellular and molecular level based on analysis of biopsies.297, 298, 299 These characteristics prompted the launch of a large-scale study to use cells in menstrual effluent as a minimally invasive diagnostic for endometriosis. To date, more than 500 women, with and without endometriosis, have been recruited and enrolled through Research OutSmarts Endometriosis (ROSE; https://feinstein.northwell.edu/institutes-researchers/institute-molecular-medicine/robert-s-boas-center-for-genomics-and-human-genetics/rose-research-outsmarts-endometriosis) and the Genotype and Phenotype Registry,300 respectively. Women consented to provide samples of menstrual effluent and access to their medical records (including the pathology reports documenting their diagnosis) and completed health or lifestyle questionnaires. In early studies, women provided menstrual effluent samples using a reusable menstrual cup (provided by Diva International). Once menstrual effluent collections and processing methods were standardized, the cellular composition of menstrual effluent cells was profiled and the menstrual effluent–derived stromal fibroblast cells were characterized to develop a noninvasive diagnostic for endometriosis.301
As found in previous studies, the menstrual effluent in the ROSE study was observed to be a complex, heterogeneous mixture of numerous cell populations, with a predominance of hematopoietic and immune cells.266,279,301 Although the sample size in the initial published study is relatively small (n=14 controls and 6–8 endometriosis subjects), menstrual effluent samples from endometriosis subjects were characterized by lower numbers of uNK cells when compared with healthy control subjects.301 Within the menstrual effluent, stromal cells (comprising <1% of cells) show numerous phenotypic and functional differences between controls and patients with endometriosis,301 similar to those previously described for stromal cells isolated from endometrial biopsies.297,302, 303, 304 Additional results from examining the genetic and functional characteristics of menstrual blood–derived stromal cells support a dysregulated retinoic acid pathway associated with endometriosis vs controls.301
One of the major barriers of this study was the inability to reliably collect menstrual effluent from women with pelvic pain using the menstrual cup. In response to this challenge, a novel diagnostic menstrual collection sponge is being developed for external use. Although this external collection sponge is still in development to maximize cell yield and collection of noncellular content, the early experience has considerably simplified menstrual effluent collections from all populations, including adolescents. Ongoing studies are focusing on (1) refining assay methods to quickly and noninvasively diagnose endometriosis with reasonable sensitivity and specificity, (2) implementing a prospective study of women who provide menstrual effluent samples before diagnostic surgery and then subsequently undergo laparoscopic surgery to definitively diagnose endometriosis as validation of the predictive power of this diagnostic test, and (3) enrolling adolescents (>9 years old) with symptoms of endometriosis because this patient group may greatly benefit from an early diagnostic. If large enough samples sizes are evaluated, results may identify diagnostic phenotypes or stratify endometriosis subtypes for treatment. Repeated sampling of menstrual effluent may allow treatment responses to be assessed, if correlates with treatment response can be identified.
Collection and analysis of live cells in menstrual effluent offer potential for discrimination of patient subgroups based on the analysis of cell identities and phenotype responses to various stimulations ex vivo that promote decidualization, proliferation, or progesterone responsiveness that can potentially provide insight into patient responses to therapies. However, cell isolation, characterization, and culture are a resource-intensive approach, similar to that employed for amniocentesis or chronic villus sampling. This method is feasible for research studies but may be challenging to translate into routine clinical practice. By contrast, stabilization of the molecular constituents in menstrual effluent to allow sample storage and batch processing offers the possibility of lower-cost, high-information content data regarding cell types present through highly standardized sequencing approaches. Genomic sequencing data on well-controlled patient populations are rapidly becoming available not only for the microbiome (see section 3D) but also at single-cell resolution of the endometrium characterization for better disease genotyping.305,306
To both take advantage of and contribute to the increasing availability of genomics data focus on the endometrial microenvironment, the company NextGen Jane developed a Smart Tampon system to provide facile access to menstrual effluent for diagnostic assessment of women’s reproductive health using granular genomic analysis and bioinformatic deconvolution. The Smart Tampon may also be used on nonbleeding days for sampling the vaginal tract, allowing for a natural enrichment of the various cell types found in the reproductive tract, depending on day of cycle (ovarian and fallopian tube cells, cervical or endometrial cells, and vaginal microbiome).
NextGen Jane studies found that transcriptional analysis of menstrual fluid has specific genomic characteristics that are unique from cervicovaginal and venous blood samples. Analysis of menstrual fluid has shown that the genomic profile of menstrual blood varies greatly by day of cycle, with nearly 800 genes that are differentially expressed in menstrual blood on heavy flow day (day 2) compared with venous blood. Day 1 of the menstrual cycle shows little variability to venous blood, compared with day 2 where the greatest differential expression may be observed. In the future, it is hoped that the optimal Smart Tampon will allow analysis of the genetics, epigenetics, microbiome, and transcriptome at scale. Methylation sequencing, transcriptomics, small RNA sequencing, microbiome analysis, and exome sequencing can produce up to 35 gigabytes of data. This platform has the potential to help fulfill the promise of machine learning and precision medicine for malignant and nonmalignant conditions in women’s health.
Finally, analysis of menstrual effluent at either cell or genomic levels offers potential to improve clinical therapies by pointing to new mechanisms that might stratify patients into subgroups for different therapies. In many cancers, patients are stratified according to molecular markers that are related to the disease mechanism, prognosis, and response to therapy. For diseases as common as endometriosis, adenomyosis, and others, it is likely that there are subtypes of patients with different molecular features that might respond to different therapies.307,308 Compared with cancer, where somatic mutations guide targeted therapies, the molecular features in endometriosis and adenomyosis are harder to identify because the presence of somatic mutations is still not well established.309 Menstrual effluent provides both molecular and cellular materials and, hence, may improve diagnosis and patient stratification toward a particular therapy.
III. Tissue engineering and microfluidic approaches to study menstruation phenomena
Paradoxically, one of the most well-studied potential applications of menstrual effluent over the past 30 years is as a source of MSCs for various nonreproductive tract tissue engineering applications. Although early reports that endometrial MSCs could transdifferentiate into insulin-producing islets,310 cardiac tissue,311 and other differentiated tissue have not borne out, applications in reconstructing connective tissues in the reproductive tract still hold promise.312,313 Tissue engineering of the endometrium as a target of learning about menstruation—defined as growing three-dimensional (3D) models with at least stromal and epithelial cells present—has percolated at a low level for decades, hindered in part by the incredible difficulty in expanding and cryopreserving human primary endometrial epithelial cells compared with the relative ease of growing human primary endometrial stromal cells (even from menstrual effluent). The landscape changed dramatically in 2017 with publication of 2 papers reporting robust expansion of human primary endometrial epithelial cells as organoids in basement membrane Matrigel,314,315 using modifications of protocols established by the Clevers group for expansion of human intestinal epithelial cells.316 Recently, scaffold-free endometrial organoids comprising both epithelial and stromal cells from endometrial tissue were established, providing yet another 3D model of the endometrium to study important paracrine actions between 2 important cell types in response to menstrual cycle hormones.317,318 These protocols enable creation of tissue banks comprising all the major endometrial cell types and lay the foundation for an explosion of activity in building models of the menstrual cycle.
Efforts to grow the endometrium and cells from the endometrium as a means to investigate its pathophysiology date back almost 100 years, with the earliest efforts targeted at trying to understand whether Sampson’s hypothesis for retrograde menstruation as a cause for endometriosis could be substantiated.319 The difficulty of growing epithelial cells—they reportedly grew poorly unless stroma was abundant, and epithelial cells grew as a sheet to cover the explant—was noted in these early explant cultures.319 The first 3D coculture of primary human endometrial epithelial and stromal cells, comprising stroma embedded in a collagen gel, overcoated with basement membrane Matrigel seeded with epithelia, resulted in a well-differentiated confluent epithelial monolayer with a basement membrane with ciliated (luminal) and secretory epithelia and was tailored to study blastocyst implantation.320 This model, which recapitulates hormone receptor expression and morphology, also revealed the changes in uterine receptivity that occurred with mifepristone compared with levonorgestrel.321 An alternate model employing decellularized human endometrium reseeded with stromal cells and epithelial glands showed hormone responsiveness over a 28-day cycle by secreting prolactin and IGFBP1, but it was unclear whether a monolayer with endometrial epithelial morphology was achieved.322 The creation of multicellular endometrial organoids with polarized epithelial cells surrounding stromal cells provided a model to study paracrine interactions between 2 important cell types of the endometrium in response to hormones.317,318 Although several implantation and cell cross talk models have been developed with endometrial cell lines,323, 324, 325, 326, 327 the profound differences in production of cytokines and growth factors by cell lines and primary cells call into question the utility of such models.328 However, a cell line–based model comprised of stromal cells embedded in hormone cues: degradation and breakdown of tissue were observed in response to the withdrawal of decidual levels of progesterone.329 The intricate cross talk between endometrial stromal and epithelial cells in driving hormone responses during menstruation has prompted efforts to create synthetic extracellular matrices for the coculture of endometrial stromal and epithelial cells in 3D. These matrices allow gentle dissolution of the extracellular matrix to release local cytokines and growth factors into the local pericellular environment and formation of confluent, stable epithelial monolayer in coculture with an underlying stroma.326,328 Although these approaches have not yet directly been applied to menstrual tissues, they are poised for this application.
A crucial missing element in 3D culture models of the endometrium needed for menstruation is microvasculature, which provides initial signals for decidualization330 and regulates oxygenation cues important for tissue breakdown and repair.58 Such models are on the horizon, as several microfluidic culture models of microvascular networks have been developed for studies of immune cell-microvascular interactions, tumor cell extravasation and growth, and blood-brain barrier.331, 332, 333, 334, 335 Recently, approaches to using these models as foundations for mucosal barriers have been described. Apart from the intrinsic interest in menstruation, the interest in endometrium as a model of fast scarless healing and tissue repair277 has created momentum for applying these types of models to menstruation, in hopes of gaining broader insights into regenerative processes.
Finally, microfluidic approaches allow the integration of multiple so-called “microphysiological systems” (MPSs) or 3D models representing part of a tissue or organ on a microscale. Integrated systems allow the investigation of systemic effects, including hormonal and other factors that might influence menstruation. An enabling technology for such integration is a now-commercialized onboard microfluidic pump, first used to drive long-term culture of 3D liver tissue336,337 and adapted to study gut-liver interactions338,339 and ultimately an integrated platform supporting 10 different interconnected MPSs communicating in a common culture medium for a month,340 including a 3D endometrium.328 This platform pumping technology was also adapted to build a model of interconnected 3D units of ovarian, fallopian, uterine, cervical, and liver tissues integrated into a single communicating fluidic system,341 allowing the assessment of up to 5 different types of tissues at a time over a menstrual cycle mimic. These cultured MPSs are responsive to ovarian hormones, and when combined with other tissues, hormones responses were amplified.341 As observed in other interacting-MPS studies, paracrine actions between tissues allowed the use of 1 universal medium without compromising the viability of the tissues during this study. Microfluidic technologies are evolving quickly as the need for user-friendly and affordable systems becomes evident for the research community. Microfluidics will change the way in vitro studies are conducted and will allow for new discoveries that will deepen our understanding of uterine biology and menstruation in a systematic way.
IV. Next-generation uterine imaging
Uterine imaging has been employed to allow for noninvasive methods for diagnosing women’s health symptoms. Imaging may be used to noninvasively assess conditions of pregnancy and aspects of uterine health and pelvic health, including endometriosis.342, 343, 344
The indication for pelvic imaging varies by patient age and clinical presentation. Common indications in premenopausal patients include evaluation for focal endometrial or myometrial lesions (eg, uterine leiomyoma [fibroids]) in patients with symptoms of AUB and pelvic pain. In postmenopausal patients, endometrial imaging is often performed to evaluate the endometrium in patients with postmenopausal bleeding.
Currently, diagnosis relies primarily on anatomic imaging using both ultrasound and MRI that allows for direct visualization of the endometrium, which is complementary to the previously described techniques, similarly noninvasive, but allowing for direct visualization of the endometrium in situ, rather than sloughed endometrial tissue. Ultrasound and MRI may evaluate for the presence of endometrial thickening and the presence of focal endometrial lesions or polyps. In the evaluation of suspected endometriosis, transvaginal ultrasound may be used to assess for deeply infiltrating endometrial implants.345 MRI is useful to map endometrial implants throughout the pelvis, including extrauterine locations, and confers advantages in terms of the larger field of view, multiplanar capabilities, and excellent contrast resolution.346,347 A systematic review and meta-analysis (based on the results of 6 studies) compared the accuracy of transvaginal ultrasound with MRI for diagnosing deep infiltrating endometriosis.343 The detection of deep infiltrating endometriosis by both MRI and transvaginal ultrasound methods indicated similar sensitivities–between 0.59 and 0.85 depending on the site, with greater sensitivity for detection in the rectosigmoid segment over rectovaginal, uterosacral, and rectovaginal septum locations.343 The specificities of MRI and transvaginal ultrasound were similar and, similar to sensitivities, showed a wide range depending on the location.343 It is expected that imaging methods will continue to improve and are likely to be used in the diagnostic workup for women experiencing symptoms of endometriosis.
Other potential future clinical applications of uterine imaging techniques include early endometrial cancer detection, distinguishing between leiomyoma and leiomyosarcoma, and assessing cancer response to treatment. Uterine and pelvic imaging may be combined with the cellular and molecular assessment of menstrual effluent to help aid in the diagnosis of uterine pathology and for improving the diagnosis of endometriosis through noninvasive methods.
MRI and ultrasound are modalities currently used for monitoring and predicting response to therapies offered to reduce menstrual bleeding or achieve amenorrhea before surgical interventions for the management of AUB. For example, GnRH analogs are used to reduce leiomyoma volume and perfusion. Contrast-enhanced MRI is used clinically to assess suitability of patients with uterine leiomyoma for uterine artery embolization and to indicate reductions in perfusion after treatment.348 Applications of T2-weighted MRI for estimation of uterine and fibroid volume may be augmented with dynamic contrast-enhanced (DCE) MRI for the assessment of tissue perfusion and permeability, and magnetization transfer (MT) MRI to assess changes in fibrosis and macromolecular content. Such approaches have been explored extensively in other organs.349,350 There has been limited application of DCE-MRI and MT-MRI in the assessment of uterine leiomyomas. DCE-MRI has been reported to be sensitive to vascular changes considered to accompany successful GnRH analog treatment of leiomyomas.351 Future development of MRI capabilities may offer complementary noninvasive modes to assess treatment responses for menstrual complaints. Furthermore, evolving MRI techniques during pregnancy that can track fetal motion and evaluate glucose and oxygen transport across the placenta may provide anatomic and functional information regarding placental health and fetal well-being.352,353
V. Conclusion
The analysis of menstrual effluent in combination with other new modalities for the understanding of uterine biology is in the very early stages of development. It is highly likely that the application of new technologies of genomic and cellular analysis of menstrual effluent and uterine tissues, including single-cell approaches, will yield a deeper understanding of uterine pathophysiology and new and less invasive methods of diagnosis, including developments for body imaging. These new technologies may be applied to a variety of uterine health and female reproductive disorders, including endometriosis, uterine leiomyoma, adenomyosis, and uterine-factor infertility and will thereby aid management strategies for the symptom of AUB. We hope that these exciting scientific opportunities will catalyze a new era of collaborative investigation that will correct the past deficit of attention to female reproductive health and biology.
5. Addressing Menstruation Globally: Progress and Gaps
Marni Sommer, DrPH, MSN; Sandy Clark, MPA
I. Introduction
The global agenda to address menstruation, and specifically menstrual health and hygiene, has gained significant momentum in recent years, ranging from increasing investment in addressing the menstruation-related barriers girls in schools in low- and middle-income countries are facing to the more recent “menstrual equity” and “period poverty” movements spreading across high-income countries. Although there is growing recognition of menstruation as a relevant issue within public health globally,354 there still exist many gaps in the evidence for informing program and policy. Reviewing how the menstruation agenda has shifted in the last 15 years provides useful insights into how efforts have evolved and what remains to be done.
II. Shift in menstrual agenda over the last 15 years
In reviewing how the global menstruation agenda has evolved, we explore shifts in the population of interest, the research and programs underway, the variation in activities by country income status, and the milestones achieved. There emerge from the analysis 5 periods of time during which distinct efforts were underway.
Earlier than 2004-2005
Before 2005, multiple efforts were underway exploring or addressing menstruation within global health. The population of interest included adult women of reproductive age, and in high-income countries, an interest in the declining age of menarche among girls. Interventions addressing adult women’s menstruation-related needs were primarily within the clinical realm, such as a focus on reproductive health and disorders355,356 and the promotion of family planning.357 Although the latter did not address menstruation as a life course issue, there was attention to the challenges of unscheduled, breakthrough bleeding among other contributors to contraceptive discontinuation.358. There also existed a rich literature on menstruation within the social sciences, primarily derived from anthropologists documenting menstrual traditions and rituals, and its relationship to girls’ and women’s roles within society. In the 1980s and 1990s, in high-income countries in particular, researchers explored girls’ maturation experiences,359, 360, 361 examining the psychological effects of menarche, and the associations of early menarche with girls’ engagement in risky behaviors, such as increased vulnerability to early sexual initiation,362,363 depression,364,365 and substance use.366 Overall, the focus in high-income countries remained on the individual and the clinical aspects of menstruation.
In contrast, in low- and middle-income countries, there began to emerge a public health lens on menstruation. Alongside the family planning agenda, there were burgeoning efforts within the water, sanitation, and hygiene (WASH) field to address menstruation as a challenge faced by girls in school.354 United Nations Children’s Fund (UNICEF) hosted a roundtable event in Oxford aimed at bringing attention to “menstrual hygiene management” (MHM), a newly coined concept focused on addressing menstrual management within WASH,367 and the Rockefeller Foundation supported a series of case studies on sexual maturation in schools in Africa.368 In humanitarian contexts, United Nations High Commissioner for Refugees recognized the provision of sanitary pads to refugees as part of one of its core mandates,369,370 providing important recognition of menstruation as a key response aspect.
2005–2011
This window of time brought an increased focus on girls as a population of interest, with a growing public health approach to menstruation in low- and middle-income countries. More specifically, important formative research was conducted with girls in and out of school, exploring their first menstrual experiences, their levels and sources of knowledge about menstruation, and how the onset of menstruation and puberty might be influencing girls’ education.371, 372, 373 The studies, conducted primarily in Africa and Asia, suggested that many girls were experiencing their first menstrual period with no previous information or support, thus feeling confusion, shame, and embarrassment and, for some, a significant fear that they were ill or dying.374,375 Multiple studies highlighted ongoing taboos, restrictions, and stigma around menstruation and how menstrual onset and its management negatively affected girls’ abilities to engage and participate in school.374,376,377 Social and physical barriers included, for example, inadequate toilets, water and disposal within school grounds, insufficient guidance and support around managing their menstrual periods, and, for some, a lack of effective menstrual products and underwear.378,379 In response, a number of interventions emerged, such as the WASH in Schools (WinS) agenda that focused on addressing MHM in schools,380 puberty books developed for girls in low-income countries that included content on MHM,381 new social entrepreneurs developing improved locally produced menstrual products for girls,382,383 and public-private partnerships by global sanitary pad companies focused on improving access to products.384
2012–2015
Over the next few years, menstruation gained traction as a public health issue for girls in particular. Although in high-income countries it remained within the clinical realm for girls and women, in low- and middle-income countries, research documentation of the MHM barriers faced by girls continued, and pilot trials began to be funded, primarily by the UK Government Medical Research Council, exploring MHM interventions for adolescent girls in school.385,386 A pilot trial in Kenya included, for example, the provision of sanitary pads, menstrual cups, and reproductive health information, examining the impact on the rates of sexually transmitted infections and on reproductive tract infections (BV), unintended pregnancy, and school attendance and performance.385 A case-control study in India examined women’s vulnerability to reproductive tract infections in relation to the menstrual cloths or products they used, with the sample drawn from hospitals.387 Systematic reviews analyzed, for example, the psychosocial and educational effects of addressing menstruation,388 and a small number of studies explored the impact of early menarche on rates of infection with herpes simplex virus and HIV and AIDS.389,390 These growing efforts, particularly those emerging from the water and sanitation arena, contributed to a decision to include MHM in the lobbying related to the new sustainable development goals (SDGs), with the aim of having targets and indicators addressing MHM included in the SDGs. This led to the development of a formal definition for MHM (Box).367
Box.
Definition of MHM (JMP, 2012)
| Women and adolescent girls are using a clean menstrual management material to absorb or collect menstrual blood, that can be changed in privacy as often as necessary for the duration of a menstrual period, using soap and water for washing the body as required, and have access to facilities to dispose of used menstrual management materials. They understand the basic facts linked to the menstrual cycle and how to manage it with dignity and without discomfort or fear. |
Critchley. Menstruation: science and society. Am J Obstet Gynecol 2020.
During this period, additional donors began to support projects related to MHM. The Canadian Government provided funding to UNICEF and the UN Girls Education Initiative to partner with Emory University on a 14-country WinS for Girls project, which focused on conducting formative MHM research and developing intervention packages addressing MHM in schools.380 The UK government supported research on MHM in emergencies, providing funds to the International Federation of the Red Cross to assess beneficiary preferences around the types of menstrual products (disposable vs reusable) in differing emergency contexts.391 A new platform arose for sharing learning with the launching of an annual virtual conference co-organized by the UNICEF and Columbia University showcasing research, practice, and policy on MHM in schools.392 Funding from the Canadian government also enabled the creation of the “MHM in Ten” agenda led by the UNICEF and Columbia University, which brought together WASH, education, SRH, gender, and adolescent health experts to develop a 10-year agenda (2014–2024) aimed at transforming schools for menstruating girls.393 Additional social entrepreneurs focused on developing affordable menstrual products, and advocacy campaigns grew around “breaking the silence” on menstruation, including support for WASH United from the Bill & Melinda Gates Foundation and other donors to launch an annual global Menstrual Hygiene Day on May 28.394
In addition, new publications began to call attention to the overdue need to explore additional ways in which menstruation affects girls’ lives, such as the need for data on the average age of menarche in countries,395 the potential for menarche to be a window of opportunity for engaging girls, their parents or caregivers, and teachers on health as a step toward subsequent conversations on SRH, including family planning,396 and for women, their MHM experiences in the workplace.397 A study conducted in India explored associations between the use and management of menstrual cloths and disposal pads and reproductive tract infections.387,398 The first resource guidance on MHM, Menstrual Hygiene Matters, was published with support from the UK government (DFID), recommending approaches for addressing MHM in development and emergency contexts,399 and UNESCO, with support from Procter & Gamble, published a puberty policy document including attention to menstruation and MHM as a key component of puberty and comprehensive sexuality education.400 There also emerged a stronger articulation of menstruation as an issue of health and human rights.401
2016–2018
During these years, there has been an exponential growth in attention to the menstruation agenda in global health. This included increasing resources and attention focused on research and interventions in low- and middle-income countries, along with a growing awareness that high-income countries were overdue to address the menstruation-related needs of girls in particular. The population of interest expanded around the world, with an ongoing focus on girls in and out of school (ages 10–19 years) but growing recognition that menstruation presents challenges for women and all individuals who menstruate, such as those with differing gendered identities. The Bill & Melinda Gates Foundation funded FSG to conduct a global landscape in 2016, An Opportunity to Address Menstrual Health and Gender Equity, which examined the existing research links between MH and broader health outcomes, social norms, and education.402 The UK government (Enhanced Learning and Research for Humanitarian Assistance funding/DFID and Wellcome Trust) supported the International Rescue Committee and Columbia University to build the evidence on MHM in humanitarian contexts403 and develop the MHM in Emergencies Toolkit; the latter was launched in 2017, with 27 copublishing humanitarian response organizations. In 2018, United States Agency for International Development (USAID)/Office of Foreign Disaster Assistance provided additional funding to the joint team to focus on the menstrual product disposal, waste management, and laundering needs of displaced populations with the aim of developing additional evidence and a compendium of practice. There also emerged a growing social and mainstream media attention. Newsweek and other major outlets published significant stories on menstruation, and the Period Poverty and Menstrual Equity campaigns emerged, focusing on removing taxes on sanitary products.404 This growing global movement also introduced new conceptualizations and terminology in relation to menstruation, which sought to broaden the issue beyond that of the focus on water and sanitation, such as menstrual health, menstrual health and hygiene, and others. Medical Research Council, DFID, and Wellcome Trust Joint Global Health Trials have also supported both feasibility pilot and full-scale trial evaluating potential effect of menstrual support on schoolgirls’ SRH and schooling outcomes.385,386
Research, programming, and policy all expanded during this period. A small number of pilot and full-scale quantitative studies continued or were initiated in Africa evaluating MHM interventions in schools,375,385,405 with findings generated on new measures for addressing menstruation. Menstrual health policies were drafted in multiple countries, such as India, Zambia, and Kenya,385,386,405,406 and in high-income countries, new legislation began to emerge, such as the Dignity Acts in the United States, which improve access to menstrual products for incarcerated individuals, and policies focused on improving access to products in homeless shelters and public schools.407, 408, 409 Despite these important legislative efforts, limited evidence exists from the United States and other high-income countries on the actual experiences, including barriers faced, of managing menstruation among girls and the incarcerated and homeless individuals. However, a small body of evidence is emerging, particularly around the menstrual management needs of low-income populations in the United States.410 In addition, this window of time brought an explosion of attention to the provision of menstrual products, with Grand Challenges Canada, the Case for Her, and other donors supporting the scaling of social entrepreneurial efforts in this arena such as AfriPads, BeGirl, and others411,412; the launching of new global advocacy and networking organizations, such as the Menstrual Health Hub, the Menstrual Health Alliance, and the UNFPA-supported African Coalition on Menstrual Health Management; and new regional research capacity building initiatives, such as the UK Government Global Challenges Research Fund supporting an East African research group.385,386
Two challenges that remained included the lack of support from sectors beyond WASH, including limited attention to menstruation and its relevancy within SRH, education, gender, and other key sectors, and the limited funding available for furthering the measurement aspects of the menstruation-related agenda that would enable demonstration of the range of effects of addressing menstruation.
2019 onward
Already in 2019, the evidence base and action are growing, with new publications examining what is known about MHM among populations with disabilities,413 proposed revisions to the MHM definition to broaden the concept and its measurement beyond the original WASH origins,414 additional systematic reviews,415,416 and ongoing menstrual equity campaigns, global advocacy, and intervention trials. In an effort to move forward the existing menstruation measurement–related challenges, including the lack of uptake among other key sectors, a “Monitoring Menstruation” meeting was hosted by the Columbia University in March 2019 with support from the Water Supply and Sanitation Collaborative Council that brought together key global monitoring and measurement experts from WASH, gender, education, and health (sexual and reproductive, psychosocial) to review and find areas of alignment between the priority outcome and impact measures of these sectoral areas with the progress being made on menstruation.414 Importantly, USAID provided new funding to explore and pilot interventions addressing menstruation and women’s economic empowerment.417 However, overall, resources still remain limited globally to support systematic coverage of all menstruation components, including access to information, water and sanitation infrastructure, supplies, and related clinical aspects, such as engagement with healthcare workers well trained on regular and irregular bleeding.
III. Evidence on menstruation globally
As described previously, the early years of the menstruation agenda included the use of primarily qualitative research methods, given the need for formative research on a sensitive topic about which there was little documentation from a public health perspective. In recent years, there has been a shift toward intervention trials, which have brought a rigorous quantitative approach to examining the impact of select menstruation-related interventions for girls in school in development contexts. Research in emergency contexts has primarily also been qualitative in nature, including feasibility pilots of guidance and programmatic response approaches. Funding has remained limited for larger-scale intervention trials that include attention to water and sanitation in schools, to longitudinal associations between inadequate and adequate attention to menstruation and SRH and education outcomes, and to the relationship between menstruation and women’s economic productivity and empowerment in the workplace. In addition, there has been a growth in national-level data, such as the PMA2020 national surveys incorporating questions around menstrual management,418, 419, 420 and the inclusion of questions on MHM within UNICEF’s Multiple Indicator Cluster Surveys in select countries.421 Finally, there exists limited evidence on the menstruation-related needs and experiences of girls growing up today in high-income contexts and the MHM challenges faced by low-income and other vulnerable populations in such contexts.
IV. Status of menstruation-related programming and policy
There exists a broad range of menstruation-related programming around the world. This includes, for example, nongovernmental organizations providing sanitary products, reproductive health or MHM information, and improvement of water and sanitation facilities in schools, both in development and emergency contexts. Many national governments, such as South Africa, India, and Kenya, have also begun subsidizing the provision of sanitary pads (reusable and disposable) to girls in school. In addition, new innovations are emerging in humanitarian contexts, such as effort by Medicins Sans Frontieres to build female-friendly washrooms with disposal mechanisms in the health clinics they run in the refugee camps in Bangladesh hosting Rohingya populations.422 Social entrepreneurs, such as Days for Girls, Sustainable Health Enterprises, BeGirl, and AfriPads, continue to develop and evaluate the production and distribution of menstrual products, ranging from reusable pads made by local populations to period underwear.
As mentioned previously, there has been a growth in menstrual policies around the world. The Uganda National Bureau of Standards passed one of the first national standards for reusable sanitary pads in Africa.423 A recent analysis of the existing higher-level education policies in low-income countries indicated that education sector plans and policies still lack inclusion of attention to menstruation and its proxies (such as the provision of gender-segregated toilets), which has implications for the inclusion of budget line items to address the issue in schools424; WASH in Schools–focused menstrual policies provide important guidance on what interventions are needed but lack sectoral buy-in and financial support. The Philippines provides an important example with recent policy being incorporated into the country’s Education Monitoring Information System, providing local-level incentive to include, for example, improved toilets in schools and the provision of supplies of sanitary pads for emergencies.425 However, as the “Monitoring Menstruation” meeting held in Geneva in March 2019 indicated, menstruation has yet to be taken up by other key sectoral programming and policy, such as within SRH, which could improve attention to anemia in adolescent girls, or the potential for the onset of menstruation to trigger child marriage.
V. Current and future pathways
Moving forward, there is much left to be done, including addressing the menstruation-related issues faced by all those who menstruate, such as transmasculine and other populations; menstrual barriers faced in workplace contexts; recognition that more evidence is needed on vaginal bleeding across the life course, including the implications for the provision of water, sanitation, supplies, and access to healthcare and information; improved engagement of healthcare workers on the issue of menstruation in low- and middle-income countries in particular; the important intersection of menstruation and family planning; and improved measures for monitoring and assessing the impact of menstruation-focused interventions along with cost-effectiveness studies. Funding support has thus far been limited for addressing this broad spectrum of issues and for essential intervention and measures-related work that are needed to demonstrate critical associations between menstruation and population health more broadly. There is an urgent need for a strong funding stream to assure this impactful work can be done.
Acknowledgments
We thank Sheila Milne for assistance with manuscript preparation and Ronnie Grant for assistance with figure preparation. We thank Helena Ward, Bachelor of Arts with Honors in Illustration and Animation (First Class), for preparation of Figure 5 in section 3A, and Sheila M. Cherry, PhD, ELS, President and Senior Editor from Fresh Eyes Editing LLC, for editing section 3D.
We also wish to acknowledge Diana Bianchi, Lisa Halvorson, and Candace Tingen for recognizing the global importance of this topic and for convening and facilitating the meeting in Bethesda and for encouragement with preparation of this manuscript.
Footnotes
H.O.D.C. has clinical research support for laboratory consumables and staff from Bayer AG and provides consultancy advice (but with no personal remuneration) for Bayer AG, PregLem SA, Gedeon Richter, Vifor Pharma UK Ltd, AbbVie Inc, and Myovant Sciences GmbH. H.O.D.C. receives royalties from UpToDate for article on abnormal uterine bleeding. A.K. receives royalties from UpToDate, Wolters Kluwer for work on the topic hysterosalpingography. E.E.M. consults for Myovant Sciences. K.A.M. is coinvestigator for Bayer Essure longitudinal research study and clinical trial (all funds for this research go to the site of research [W.I.H.] – no personal compensation). K.A.M. is scientific advisor for Myovant (advises on patient questionnaires related to AUB—compensation goes to employer [C.N.E.M.G.]—no personal compensation). K.A.M. has received honoraria from ABOG, ACOG, and NIH for participating in working groups and meetings. K.A.M. is HHS Office of Population Affairs Title X Grant Reviewer (received honorarium). I.M. is employee of Igenomix R&D. C.S. is Head of the Igenomix Scientific Advisory Board. The other authors report no conflict of interest.
Some of the data herein were derived from research grants funded by the Medical Research Council (G0000066, G0500047, G0600048, MR/J003611/1), Wellcome Trust (083908/Z/07/Z), and NIHR Efficacy and Mechanism Evaluation Programme (12/206/52). This work has been supported by the NIH grants P01-HD57877 and R37-HD38691 and by Research Evaluation and Commercialization Hub (REACH), Center for Biotechnology’s NIH award entitled “Establishing a Long Island Bioscience Hub,” and National Heart, Lung, and Blood Institute of the National Institutes of Health-Award Number U01HL127522; The Endometriosis Foundation of America (EFA). J.J.K. is a recipient of NIEHS/NIH/NCATS UG3 (ES029073) and NIH/NCI R01CA243249. J.A.M. is a recipient of Wellcome Trust grant (100646/Z/12/Z), Academy of Medical Sciences (SGCL13). G.P.W. is a recipient of John Templeton Foundation grant 61329; NIH U54-CA209992; WSU19073. L.G.G. received support from NIH U01EB029132-01, The John and Karine Begg Fund, and the Manton Foundation.
References
- 1.World Health Organization Frequently asked questions. 2019. https://www.who.int/about/who-we-are/frequently-asked-questions Available at: Accessed July 20, 2020.
- 2.Matteson K.A., Raker C.A., Clark M.A., Frick K.D. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the Medical Expenditures Panel Survey. J Womens Health (Larchmt) 2013;22:959–965. doi: 10.1089/jwh.2013.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munro M.G., Critchley H.O.D., Fraser I.S., Committee FIGO Menstrual Disorders Committee The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018;143:393–408. doi: 10.1002/ijgo.12666. [DOI] [PubMed] [Google Scholar]
- 4.Sharp H.T., Johnson J.V., Lemieux L.A., Currigan S.M. Executive summary of the reVITALize initiative: standardizing gynecologic data definitions. Obstet Gynecol. 2017;129:603–607. doi: 10.1097/AOG.0000000000001939. [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists reVITALize: gynecology data definitions. https://www.acog.org/practice-management/health-it-and-clinical-informatics/revitalize-gynecology-data-definitions Available at:
- 6.Kjerulff K.H., Erickson B.A., Langenberg P.W. Chronic gynecological conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. Am J Public Health. 1996;86:195–199. doi: 10.2105/ajph.86.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratzan S.C., Parker R.M. Introduction. In: Selden C.R., Zorn M., Ratzan S.C., Parker R.M., editors. National Library of Medicine current bibliographies in medicine: health literacy. NLM Pub. No. Christoffel Blinden Mission 2000–1. National Institutes of Health; Bethesda, MD: 2000. [Google Scholar]
- 8.Woolcock J.G., Critchley H.O., Munro M.G., Broder M.S., Fraser I.S. Review of the confusion in current and historical terminology and definitions for disturbances of menstrual bleeding. Fertil Steril. 2008;90:2269–2280. doi: 10.1016/j.fertnstert.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 9.Munro M.G., Critchley H.O., Fraser I.S., FIGO Menstrual Disorders Working Group The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95(2204–8):2208.e1–2208.e3. doi: 10.1016/j.fertnstert.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence (NICE) Heavy menstrual bleeding: assessment and management. 2018. https://www.nice.org.uk/guidance/ng88 Available at: [PubMed]
- 11.Matteson K.A., Scott D.M., Raker C.A., Clark M.A. The Menstrual Bleeding Questionnaire: development and validation of a comprehensive patient-reported outcome instrument for heavy menstrual bleeding. BJOG. 2015;122:681–689. doi: 10.1111/1471-0528.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matteson K.A., Clark M.A. Questioning our questions: do frequently asked questions adequately cover the aspects of women’s lives most affected by abnormal uterine bleeding? Opinions of women with abnormal uterine bleeding participating in focus group discussions. Women Health. 2010;50:195–211. doi: 10.1080/03630241003705037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghant M.S., Sengoba K.S., Recht H., Cameron K.A., Lawson A.K., Marsh E.E. Beyond the physical: a qualitative assessment of the burden of symptomatic uterine fibroids on women’s emotional and psychosocial health. J Psychosom Res. 2015;78:499–503. doi: 10.1016/j.jpsychores.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Marsh E.E., Brocks M.E., Ghant M.S., Recht H.S., Simon M. Prevalence and knowledge of heavy menstrual bleeding among African American women. Int J Gynaecol Obstet. 2014;125:56–59. doi: 10.1016/j.ijgo.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardi L.A., Ghant M.S., Andrade C., Recht H., Marsh E.E. The association between subjective assessment of menstrual bleeding and measures of iron deficiency anemia in premenopausal African-American women: a cross-sectional study. BMC Womens Health. 2016;16:50. doi: 10.1186/s12905-016-0329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weldring T., Smith S.M. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs) Health Serv Insights. 2013;6:61–68. doi: 10.4137/HSI.S11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahn D.D., Abed H., Sung V.W. Systematic review highlights difficulty interpreting diverse clinical outcomes in abnormal uterine bleeding trials. J Clin Epidemiol. 2011;64:293–300. doi: 10.1016/j.jclinepi.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark T.J., Khan K.S., Foon R., Pattison H., Bryan S., Gupta J.K. Quality of life instruments in studies of menorrhagia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2002;104:96–104. doi: 10.1016/s0301-2115(02)00076-3. [DOI] [PubMed] [Google Scholar]
- 19.Warner P.E., Critchley H.O., Lumsden M.A., Campbell-Brown M., Douglas A., Murray G.D. Menorrhagia II: is the 80-mL blood loss criterion useful in management of complaint of menorrhagia? Am J Obstet Gynecol. 2004;190:1224–1229. doi: 10.1016/j.ajog.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization Global Observatory for eHealth mHealth: new horizons for health through mobile technologies: second global survey on eHealth. 2011. https://apps.who.int/iris/bitstream/handle/10665/44607/9789241564250_eng.pdf?sequence=1 Available at:
- 21.Radbron E., Wilson V., McCance T., Middleton R. The use of data collected from mHealth apps to inform evidence-based quality improvement: an integrative review. Worldviews Evid Based Nurs. 2019;16:70–77. doi: 10.1111/wvn.12343. [DOI] [PubMed] [Google Scholar]
- 22.As-Sanie S., Black R., Giudice L.C. Assessing research gaps and unmet needs in endometriosis. Am J Obstet Gynecol. 2019;221:86–94. doi: 10.1016/j.ajog.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Hallberg L., Högdahl A.M., Nilsson L., Rybo G. Menstrual blood loss—a population study. Variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand. 1966;45:320–351. doi: 10.3109/00016346609158455. [DOI] [PubMed] [Google Scholar]
- 24.Harlow S.D., Lin X., Ho M.J. Analysis of menstrual diary data across the reproductive life span applicability of the bipartite model approach and the importance of within-woman variance. J Clin Epidemiol. 2000;53:722–733. doi: 10.1016/s0895-4356(99)00202-4. [DOI] [PubMed] [Google Scholar]
- 25.Harlow S.D., Mitchell E.S., Crawford S. The ReSTAGE Collaboration: defining optimal bleeding criteria for onset of early menopausal transition. Fertil Steril. 2008;89:129–140. doi: 10.1016/j.fertnstert.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paramsothy P., Harlow S.D., Greendale G.A. Bleeding patterns during the menopausal transition in the multi-ethnic Study of Women’s Health Across the Nation (SWAN): a prospective cohort study. BJOG. 2014;121:1564–1573. doi: 10.1111/1471-0528.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dasharathy S.S., Mumford S.L., Pollack A.Z. Menstrual bleeding patterns among regularly menstruating women. Am J Epidemiol. 2012;175:536–545. doi: 10.1093/aje/kwr356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harlow S.D., Gass M., Hall J.E. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Fertil Steril. 2012;97:843–851. doi: 10.1016/j.fertnstert.2012.01.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bull J.R., Rowland S.P., Scherwitzl E.B., Scherwitzl R., Danielsson K.G., Harper J. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit Med. 2019;2:83. doi: 10.1038/s41746-019-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matteson K.A. Menstrual questionnaires for clinical and research use. Best Pract Res Clin Obstet Gynaecol. 2017;40:44–54. doi: 10.1016/j.bpobgyn.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Matteson K.A., Boardman L.A., Munro M.G., Clark M.A. Abnormal uterine bleeding: a review of patient-based outcome measures. Fertil Steril. 2009;92:205–216. doi: 10.1016/j.fertnstert.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan K.S., Romero R. Chief Editors of Journals participating in CROWN Initiative. The CROWN initiative: journal editors invite researchers to develop core outcomes in women’s health. Am J Obstet Gynecol. 2014;211:575–576. doi: 10.1016/j.ajog.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 33.CROWN Initiative Core Outcomes in Women’s and Newborn Health. http://www.crown-initiative.org/14-2/about/ Available at:
- 34.Matteson K.A., Munro M.G., Fraser I.S. The structured menstrual history: developing a tool to facilitate diagnosis and aid in symptom management. Semin Reprod Med. 2011;29:423–435. doi: 10.1055/s-0031-1287666. [DOI] [PubMed] [Google Scholar]
- 35.Gensheimer S.G., Wu A.W., Snyder C.F. PRO-EHR Users’ Guide Steering Group, PRO-EHR Users’ Guide Working Group. Oh, the places we’ll go: patient-reported outcomes and electronic health records. Patient. 2018;11:591–598. doi: 10.1007/s40271-018-0321-9. [DOI] [PubMed] [Google Scholar]
- 36.Wu A.W., Kharrazi H., Boulware L.E., Snyder C.F. Measure once, cut twice—adding patient-reported outcome measures to the electronic health record for comparative effectiveness research. J Clin Epidemiol. 2013;66(8 Suppl):S12–S20. doi: 10.1016/j.jclinepi.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder C.F., Jensen R.E., Segal J.B., Wu A.W. Patient-reported outcomes (PROs): putting the patient perspective in patient-centered outcomes research. Med Care. 2013;51(8 Suppl 3):S73–S79. doi: 10.1097/MLR.0b013e31829b1d84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chavan A.R., Bhullar B.A., Wagner G.P. What was the ancestral function of decidual stromal cells? A model for the evolution of eutherian pregnancy. Placenta. 2016;40:40–51. doi: 10.1016/j.placenta.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Kowalewski M.P., Gram A., Kautz E., Graubner F.R. The dog: nonconformist, not only in maternal recognition signaling. Adv Anat Embryol Cell Biol. 2015;216:215–237. doi: 10.1007/978-3-319-15856-3_11. [DOI] [PubMed] [Google Scholar]
- 40.Emera D., Romero R., Wagner G. The evolution of menstruation: a new model for genetic assimilation: explaining molecular origins of maternal responses to fetal invasiveness. Bioessays. 2012;34:26–35. doi: 10.1002/bies.201100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellofiore N., Ellery S.J., Mamrot J., Walker D.W., Temple-Smith P., Dickinson H. First evidence of a menstruating rodent: the spiny mouse (Acomys cahirinus) Am J Obstet Gynecol. 2017;216:40.e1–40.e11. doi: 10.1016/j.ajog.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 42.Rasweiler J.J., Badwaik N.K. Anatomy and physiology of the female reproductive tract. In: Crichton E.G., Krutzsch P.H., editors. Reproductive biology of bats. Academic Press; London: 2000. pp. 157–219. [Google Scholar]
- 43.van der Horst C., Gillman J. The menstrual cycle in Elephantulus. S Afr J Med Sci. 1941;6:27–42. [Google Scholar]
- 44.van der Horst C. Elephantulus going into anoestrus; menstruation and abortion. Phil Trans R Soc Lond B. 1954;238:27–61. [Google Scholar]
- 45.Carter A.M. Classics revisited: C. J. van der Horst on pregnancy and menstruation in elephant shrews. Placenta. 2018;67:24–30. doi: 10.1016/j.placenta.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Ramos-Goyette S., Craton L.G. Evolution of the menstrual cycle. In: Gosselin M., editor. Menstrual cycle: signs and symptoms, psychological/behavioral changes and abnormalities. NOVA Biomedical; Hauppauge, New York: 2013. pp. 1–34. [Google Scholar]
- 47.Brosens J.J., Parker M.G., McIndoe A., Pijnenborg R., Brosens I.A. A role for menstruation in preconditioning the uterus for successful pregnancy. Am J Obstet Gynecol. 2009;200:615.e1–615.e6. doi: 10.1016/j.ajog.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 48.Jarrell J. The significance and evolution of menstruation. Best Pract Res Clin Obstet Gynaecol. 2018;50:18–26. doi: 10.1016/j.bpobgyn.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Alvergne A., Högqvist Tabor V. Is female health cyclical? Evolutionary perspectives on menstruation. Trends Ecol Evol. 2018;33:399–414. doi: 10.1016/j.tree.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Strassmann B.I. The evolution of endometrial cycles and menstruation. Q Rev Biol. 1996;71:181–220. doi: 10.1086/419369. [DOI] [PubMed] [Google Scholar]
- 51.Bellofiore N., Cousins F., Temple-Smith P., Dickinson H., Evans J. A missing piece: the spiny mouse and the puzzle of menstruating species. J Mol Endocrinol. 2018;61:R25–R41. doi: 10.1530/JME-17-0278. [DOI] [PubMed] [Google Scholar]
- 52.Finn C.A. Menstruation: a nonadaptive consequence of uterine evolution. Q Rev Biol. 1998;73:163–173. doi: 10.1086/420183. [DOI] [PubMed] [Google Scholar]
- 53.Maybin J.A., Critchley H.O. Menstrual physiology: implications for endometrial pathology and beyond. Hum Reprod Update. 2015;21:748–761. doi: 10.1093/humupd/dmv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gellersen B., Brosens J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35:851–905. doi: 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- 55.Finn C.A., Pope M. Vascular and cellular changes in the decidualized endometrium of the ovariectomized mouse following cessation of hormone treatment: a possible model for menstruation. J Endocrinol. 1984;100:295–300. doi: 10.1677/joe.0.1000295. [DOI] [PubMed] [Google Scholar]
- 56.Rudolph M., Döcke W.D., Müller A. Induction of overt menstruation in intact mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cousins F.L., Murray A., Esnal A., Gibson D.A., Critchley H.O., Saunders P.T. Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maybin J.A., Murray A.A., Saunders P.T.K., Hirani N., Carmeliet P., Critchley H.O.D. Hypoxia and hypoxia inducible factor-1α are required for normal endometrial repair during menstruation. Nat Commun. 2018;9:295. doi: 10.1038/s41467-017-02375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pollheimer J., Vondra S., Baltayeva J., Beristain A.G., Knöfler M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front Immunol. 2018;9:2597. doi: 10.3389/fimmu.2018.02597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Booker W., Moroz L. Abnormal placentation. Semin Perinatol. 2019;43:51–59. doi: 10.1053/j.semperi.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 61.Teklenburg G., Salker M., Heijnen C., Macklon N.S., Brosens J.J. The molecular basis of recurrent pregnancy loss: impaired natural embryo selection. Mol Hum Reprod. 2010;16:886–895. doi: 10.1093/molehr/gaq079. [DOI] [PubMed] [Google Scholar]
- 62.Teklenburg G., Salker M., Molokhia M. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brosens J.J., Salker M.S., Teklenburg G. Uterine selection of human embryos at implantation. Sci Rep. 2014;4:3894. doi: 10.1038/srep03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macklon N.S., Brosens J.J. The human endometrium as a sensor of embryo quality. Biol Reprod. 2014;91:98. doi: 10.1095/biolreprod.114.122846. [DOI] [PubMed] [Google Scholar]
- 65.Jarvis G.E. Early embryo mortality in natural human reproduction: what the data say. F1000Res. 2016;5:2765. doi: 10.12688/f1000research.8937.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pavlicev M., Norwitz E.R. Human parturition: nothing more than a delayed menstruation. Reprod Sci. 2018;25:166–173. doi: 10.1177/1933719117725830. [DOI] [PubMed] [Google Scholar]
- 67.Csapo A.I. The ’see-saw’ theory of parturition. Ciba Found Symp. 1977;47:159–210. [PubMed] [Google Scholar]
- 68.Romero R., Espinoza J., Kusanovic J.P. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Royal College of Obstetricians and Gynaecologists National heavy menstrual bleeding audit. Final report. July 2014. https://www.rcog.org.uk/globalassets/documents/guidelines/research--audit/national_hmb_audit_final_report_july_2014.pdf Available at:
- 70.Short R.V. The evolution of human reproduction. Proc R Soc Lond B Biol Sci. 1976;195:3–24. doi: 10.1098/rspb.1976.0095. [DOI] [PubMed] [Google Scholar]
- 71.Cardozo E.R., Clark A.D., Banks N.K., Henne M.B., Stegmann B.J., Segars J.H. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206:211.e1–211.e9. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baird D.D., Dunson D.B., Hill M.C., Cousins D., Schectman J.M. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 73.Munro M.G., Critchley H.O., Broder M.S., Fraser I.S., FIGO Working Group on Menstrual Disorders FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynecol Obstet. 2011;113:3–13. doi: 10.1016/j.ijgo.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 74.Sinclair D.C., Mastroyannis A., Taylor H.S. Leiomyoma simultaneously impair endometrial BMP-2-mediated decidualization and anticoagulant expression through secretion of TGF-β3. J Clin Endocrinol Metab. 2011;96:412–421. doi: 10.1210/jc.2010-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Donoso M.B., Serra R., Rice G.E. Normality ranges of menstrual fluid volume during reproductive life using direct quantification of menses with vaginal cups. Gynecol Obstet Invest. 2019;84:390–395. doi: 10.1159/000496608. [DOI] [PubMed] [Google Scholar]
- 76.Higham J.M., O’Brien P.M., Shaw R.W. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol. 1990;97:734–739. doi: 10.1111/j.1471-0528.1990.tb16249.x. [DOI] [PubMed] [Google Scholar]
- 77.Brasted M., White C.A., Kennedy T.G., Salamonsen L.A. Mimicking the events of menstruation in the murine uterus. Biol Reprod. 2003;69:1273–1280. doi: 10.1095/biolreprod.103.016550. [DOI] [PubMed] [Google Scholar]
- 78.Menning A., Walter A., Rudolph M., Gashaw I., Fritzemeier K.H., Roese L. Granulocytes and vascularization regulate uterine bleeding and tissue remodeling in a mouse menstruation model. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cousins F.L., Kirkwood P.M., Saunders P.T., Gibson D.A. Evidence for a dynamic role for mononuclear phagocytes during endometrial repair and remodelling. Sci Rep. 2016;6:36748. doi: 10.1038/srep36748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brenner R.M., Rudolph L., Matrisian L., Slayden O.D. Non-human primate models; artificial menstrual cycles, endometrial matrix metalloproteinases and s.c. endometrial grafts. Hum Reprod. 1996;11(Suppl 2):150–164. doi: 10.1093/humrep/11.suppl_2.150. [DOI] [PubMed] [Google Scholar]
- 81.Brenner R.M., Nayak N.R., Slayden O.D., Critchley H.O., Kelly R.W. Premenstrual and menstrual changes in the macaque and human endometrium: relevance to endometriosis. Ann N Y Acad Sci. 2002;955:60–74. doi: 10.1111/j.1749-6632.2002.tb02766.x. discussion 86–8, 396–406. [DOI] [PubMed] [Google Scholar]
- 82.Armstrong G.M., Maybin J.A., Murray A.A. Endometrial apoptosis and neutrophil infiltration during menstruation exhibits spatial and temporal dynamics that are recapitulated in a mouse model. Sci Rep. 2017;7:17416. doi: 10.1038/s41598-017-17565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Critchley H.O., Kelly R.W., Brenner R.M., Baird D.T. The endocrinology of menstruation—a role for the immune system. Clin Endocrinol (Oxf) 2001;55:701–710. doi: 10.1046/j.1365-2265.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- 84.Thiruchelvam U., Dransfield I., Saunders P.T., Critchley H.O. The importance of the macrophage within the human endometrium. J Leukoc Biol. 2013;93:217–225. doi: 10.1189/jlb.0712327. [DOI] [PubMed] [Google Scholar]
- 85.Salamonsen L.A., Woolley D.E. Menstruation: induction by matrix metalloproteinases and inflammatory cells. J Reprod Immunol. 1999;44:1–27. doi: 10.1016/s0165-0378(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 86.Garry R., Hart R., Karthigasu K.A., Burke C. A re-appraisal of the morphological changes within the endometrium during menstruation: a hysteroscopic, histological and scanning electron microscopic study. Hum Reprod. 2009;24:1393–1401. doi: 10.1093/humrep/dep036. [DOI] [PubMed] [Google Scholar]
- 87.Thomas V.G. The link between human menstruation and placental delivery: a novel evolutionary interpretation: menstruation and fetal placental detachment share common evolved physiological processes dependent on progesterone withdrawal. Bioessays. 2019;41 doi: 10.1002/bies.201800232. [DOI] [PubMed] [Google Scholar]
- 88.Abberton K.M., Healy D.L., Rogers P.A. Smooth muscle alpha actin and myosin heavy chain expression in the vascular smooth muscle cells surrounding human endometrial arterioles. Hum Reprod. 1999;14:3095–3100. doi: 10.1093/humrep/14.12.3095. [DOI] [PubMed] [Google Scholar]
- 89.Biswas Shivhare S., Bulmer J.N., Innes B.A., Hapangama D.K., Lash G.E. Altered vascular smooth muscle cell differentiation in the endometrial vasculature in menorrhagia. Hum Reprod. 2014;29:1884–1894. doi: 10.1093/humrep/deu164. [DOI] [PubMed] [Google Scholar]
- 90.Biswas Shivhare S., Bulmer J.N., Innes B.A., Hapangama D.K., Lash G.E. Endometrial vascular development in heavy menstrual bleeding: altered spatio-temporal expression of endothelial cell markers and extracellular matrix components. Hum Reprod. 2018;33:399–410. doi: 10.1093/humrep/dex378. [DOI] [PubMed] [Google Scholar]
- 91.Maybin J.A., Critchley H.O., Jabbour H.N. Inflammatory pathways in endometrial disorders. Mol Cell Endocrinol. 2011;335:42–51. doi: 10.1016/j.mce.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 92.Marsh M.M., Malakooti N., Taylor N.H., Findlay J.K., Salamonsen L.A. Endothelin and neutral endopeptidase in the endometrium of women with menorrhagia. Hum Reprod. 1997;12:2036–2040. doi: 10.1093/humrep/12.9.2036. [DOI] [PubMed] [Google Scholar]
- 93.Smith O.P., Jabbour H.N., Critchley H.O. Cyclooxygenase enzyme expression and E series prostaglandin receptor signalling are enhanced in heavy menstruation. Hum Reprod. 2007;22:1450–1456. doi: 10.1093/humrep/del503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Markee J.E. Menstruation in intraocular transplants in the rhesus monkey. Contrib Embryol. 1940;28:219–308. [Google Scholar]
- 95.Gleeson N., Devitt M., Sheppard B.L., Bonnar J. Endometrial fibrinolytic enzymes in women with normal menstruation and dysfunctional uterine bleeding. Br J Obstet Gynaecol. 1993;100:768–771. doi: 10.1111/j.1471-0528.1993.tb14272.x. [DOI] [PubMed] [Google Scholar]
- 96.Nordengren J., Pilka R., Noskova V. Differential localization and expression of urokinase plasminogen activator (uPA), its receptor (uPAR), and its inhibitor (PAI-1) mRNA and protein in endometrial tissue during the menstrual cycle. Mol Hum Reprod. 2004;10:655–663. doi: 10.1093/molehr/gah081. [DOI] [PubMed] [Google Scholar]
- 97.Gleeson N.C., Buggy F., Sheppard B.L., Bonnar J. The effect of tranexamic acid on measured menstrual loss and endometrial fibrinolytic enzymes in dysfunctional uterine bleeding. Acta Obstet Gynecol Scand. 1994;73:274–277. doi: 10.3109/00016349409023453. [DOI] [PubMed] [Google Scholar]
- 98.Benagiano G., Brosens I., Habiba M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum Reprod Update. 2014;20:386–402. doi: 10.1093/humupd/dmt052. [DOI] [PubMed] [Google Scholar]
- 99.Patel B.G., Rudnicki M., Yu J., Shu Y., Taylor R.N. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96:623–632. doi: 10.1111/aogs.13156. [DOI] [PubMed] [Google Scholar]
- 100.Guttinger A., Critchley H.O. Endometrial effects of intrauterine levonorgestrel. Contraception. 2007;75(6 Suppl):S93–S98. doi: 10.1016/j.contraception.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 101.Weisberg E., Hickey M., Palmer D. A randomized controlled trial of treatment options for troublesome uterine bleeding in Implanon users. Hum Reprod. 2009;24:1852–1861. doi: 10.1093/humrep/dep081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Warner P., Guttinger A., Glasier A.F. Randomized placebo-controlled trial of CDB-2914 in new users of a levonorgestrel-releasing intrauterine system shows only short-lived amelioration of unscheduled bleeding. Hum Reprod. 2010;25:345–353. doi: 10.1093/humrep/dep377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rogers P.A., Martinez F., Girling J.E. Influence of different hormonal regimens on endometrial microvascular density and VEGF expression in women suffering from breakthrough bleeding. Hum Reprod. 2005;20:3341–3347. doi: 10.1093/humrep/dei239. [DOI] [PubMed] [Google Scholar]
- 104.Jain J.K., Nicosia A.F., Nucatola D.L., Lu J.J., Kuo J., Felix J.C. Mifepristone for the prevention of breakthrough bleeding in new starters of depo-medroxyprogesterone acetate. Steroids. 2003;68:1115–1119. doi: 10.1016/s0039-128x(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 105.Faculty of Sexual and Reproductive Healthcare FSRH clinical guidance: problematic bleeding with hormonal contraception. 2015. https://www.fsrh.org/documents/ceuguidanceproblematicbleedinghormonalcontraception/ Available at: Accessed July 4, 2019.
- 106.Donnez J., Tatarchuk T.F., Bouchard P. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409–420. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- 107.Donnez J., Tomaszewski J., Vázquez F. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421–432. doi: 10.1056/NEJMoa1103180. [DOI] [PubMed] [Google Scholar]
- 108.Lakha F., Ho P.C., Van der Spuy Z.M. A novel estrogen-free oral contraceptive pill for women: multicentre, double-blind, randomized controlled trial of mifepristone and progestogen-only pill (levonorgestrel) Hum Reprod. 2007;22:2428–2436. doi: 10.1093/humrep/dem177. [DOI] [PubMed] [Google Scholar]
- 109.Whitaker L.H., Murray A.A., Matthews R. Selective progesterone receptor modulator (SPRM) ulipristal acetate (UPA) and its effects on the human endometrium. Hum Reprod. 2017;32:531–543. doi: 10.1093/humrep/dew359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Williams A.R., Bergeron C., Barlow D.H., Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int J Gynecol Pathol. 2012;31:556–569. doi: 10.1097/PGP.0b013e318251035b. [DOI] [PubMed] [Google Scholar]
- 111.Wagenfeld A., Saunders P.T., Whitaker L., Critchley H.O. Selective progesterone receptor modulators (SPRMs): progesterone receptor action, mode of action on the endometrium and treatment options in gynecological therapies. Expert Opin Ther Targets. 2016;20:1045–1054. doi: 10.1080/14728222.2016.1180368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mutter G.L., Bergeron C., Deligdisch L. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol. 2008;21:591–598. doi: 10.1038/modpathol.2008.19. [DOI] [PubMed] [Google Scholar]
- 113.Stewart E.A., Diamond M.P., Williams A.R.W. Safety and efficacy of the selective progesterone receptor modulator asoprisnil for heavy menstrual bleeding with uterine fibroids: pooled analysis of two 12-month, placebo-controlled, randomized trials. Hum Reprod. 2019;34:623–634. doi: 10.1093/humrep/dez007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kannan A., Bhurke A., Sitruk-Ware R. Characterization of molecular changes in endometrium associated with chronic use of progesterone receptor modulators: ulipristal acetate versus mifepristone. Reprod Sci. 2018;25:320–328. doi: 10.1177/1933719117746764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Milliano I., Van Hattum D., Ket J.C.F., Huirne J.A.F., Hehenkamp W.J.K. Endometrial changes during ulipristal acetate use: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;214:56–64. doi: 10.1016/j.ejogrb.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 116.Du H., Taylor H.S. Stem cells and female reproduction. Reprod Sci. 2009;16:126–139. doi: 10.1177/1933719108329956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gargett C.E., Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod. 2010;16:818–834. doi: 10.1093/molehr/gaq061. [DOI] [PubMed] [Google Scholar]
- 118.Santamaria X., Mas A., Cervelló I., Taylor H., Simon C. Uterine stem cells: from basic research to advanced cell therapies. Hum Reprod Update. 2018;24:673–693. doi: 10.1093/humupd/dmy028. [DOI] [PubMed] [Google Scholar]
- 119.Post Y., Clevers H. Defining adult stem cell function at its simplest: the ability to replace lost cells through mitosis. Cell Stem Cell. 2019;25:174–183. doi: 10.1016/j.stem.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 120.Gargett C.E., Schwab K.E., Deane J.A. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22:137–163. doi: 10.1093/humupd/dmv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tempest N., Maclean A., Hapangama D.K. Endometrial stem cell markers: current concepts and unresolved questions. Int J Mol Sci. 2018;19:3240. doi: 10.3390/ijms19103240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Valentijn A.J., Palial K., Al-Lamee H. SSEA-1 isolates human endometrial basal glandular epithelial cells: phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum Reprod. 2013;28:2695–2708. doi: 10.1093/humrep/det285. [DOI] [PubMed] [Google Scholar]
- 123.Hapangama D.K., Drury J., Da Silva L. Abnormally located SSEA1+/SOX9+ endometrial epithelial cells with a basalis-like phenotype in the eutopic functionalis layer may play a role in the pathogenesis of endometriosis. Hum Reprod. 2019;34:56–68. doi: 10.1093/humrep/dey336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gargett C.E., Schwab K.E., Zillwood R.M., Nguyen H.P., Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tempest N., Baker A.M., Wright N.A., Hapangama D.K. Does human endometrial LGR5 gene expression suggest the existence of another hormonally regulated epithelial stem cell niche? Hum Reprod. 2018;33:1052–1062. doi: 10.1093/humrep/dey083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jin S. Bipotent stem cells support the cyclical regeneration of endometrial epithelium of the murine uterus. Proc Natl Acad Sci USA. 2019;116:6848–6857. doi: 10.1073/pnas.1814597116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Syed S.M., Kumar M., Ghosh A. Endometrial Axin2+ cells drive epithelial homeostasis, regeneration, and cancer following oncogenic transformation. Cell Stem Cell. 2020;26:64–80.e13. doi: 10.1016/j.stem.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 128.Yin M., Zhou H.J., Lin C. CD34+KLF4+ stromal stem cells contribute to endometrial regeneration and repair. Cell Rep. 2019;27:2709–2724.e3. doi: 10.1016/j.celrep.2019.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garry R., Hart R., Karthigasu K.A., Burke C. Structural changes in endometrial basal glands during menstruation. BJOG. 2010;117:1175–1185. doi: 10.1111/j.1471-0528.2010.02630.x. [DOI] [PubMed] [Google Scholar]
- 130.Crane G.M., Jeffery E., Morrison S.J. Adult haematopoietic stem cell niches. Nat Rev Immunol. 2017;17:573–590. doi: 10.1038/nri.2017.53. [DOI] [PubMed] [Google Scholar]
- 131.Zhang M., Malik A.B., Rehman J. Endothelial progenitor cells and vascular repair. Curr Opin Hematol. 2014;21:224–228. doi: 10.1097/MOH.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fadini G.P., Ciciliot S., Albiero M. Concise review: perspectives and clinical implications of bone marrow and circulating stem cell defects in diabetes. Stem Cells. 2017;35:106–116. doi: 10.1002/stem.2445. [DOI] [PubMed] [Google Scholar]
- 133.Myerson D., Parkin R.K. Donor-derived hepatocytes in human hematopoietic cell transplant recipients: evidence of fusion. Virchows Arch. 2019;474:365–374. doi: 10.1007/s00428-018-2497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tal A., Tal R., Shaikh S., Gidicsin S., Mamillapalli R., Taylor H.S. Characterization of cell fusion in an experimental mouse model of endometriosis†. Biol Reprod. 2019;100:390–397. doi: 10.1093/biolre/ioy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Robey P. ”Mesenchymal stem cells”: fact or fiction, and implications in their therapeutic use. F1000Res. 2017;6:F1000. doi: 10.12688/f1000research.10955.1. Faculty Rev-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Arner P., Rydén M. The contribution of bone marrow-derived cells to the human adipocyte pool. Adipocyte. 2017;6:187–192. doi: 10.1080/21623945.2017.1306158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taylor H.S. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292:81–85. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 138.Du H., Taylor H.S. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25:2082–2086. doi: 10.1634/stemcells.2006-0828. [DOI] [PubMed] [Google Scholar]
- 139.Mints M., Jansson M., Sadeghi B. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum Reprod. 2008;23:139–143. doi: 10.1093/humrep/dem342. [DOI] [PubMed] [Google Scholar]
- 140.Morelli S.S., Rameshwar P., Goldsmith L.T. Experimental evidence for bone marrow as a source of nonhematopoietic endometrial stromal and epithelial compartment cells in a murine model. Biol Reprod. 2013;89:7. doi: 10.1095/biolreprod.113.107987. [DOI] [PubMed] [Google Scholar]
- 141.Gil-Sanchis C., Cervelló I., Khurana S., Faus A., Verfaillie C., Simón C. Contribution of different bone marrow-derived cell types in endometrial regeneration using an irradiated murine model. Fertil Steril. 2015;103:1596–1605.e1. doi: 10.1016/j.fertnstert.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 142.Du H., Naqvi H., Taylor H.S. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev. 2012;21:3324–3331. doi: 10.1089/scd.2011.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Alawadhi F., Du H., Cakmak H., Taylor H.S. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman’s syndrome. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yi K.W., Mamillapalli R., Sahin C., Song J., Tal R., Taylor H.S. Bone marrow-derived cells or C–X-C motif chemokine 12 (CXCL12) treatment improve thin endometrium in a mouse model. Biol Reprod. 2019;100:61–70. doi: 10.1093/biolre/ioy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Santamaria X., Cabanillas S., Cervelló I. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31:1087–1096. doi: 10.1093/humrep/dew042. [DOI] [PubMed] [Google Scholar]
- 146.Singh N., Mohanty S., Seth T., Shankar M., Bhaskaran S., Dharmendra S. Autologous stem cell transplantation in refractory Asherman’s syndrome: a novel cell based therapy. J Hum Reprod Sci. 2014;7:93–98. doi: 10.4103/0974-1208.138864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang X., Mamillapalli R., Mutlu L., Du H., Taylor H.S. Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res. 2015;15:14–22. doi: 10.1016/j.scr.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sahin Ersoy G., Zolbin M.M., Cosar E., Moridi I., Mamillapalli R., Taylor H.S. CXCL12 promotes stem cell recruitment and uterine repair after injury in Asherman’s syndrome. Mol Ther Methods Clin Dev. 2017;4:169–177. doi: 10.1016/j.omtm.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moridi I., Mamillapalli R., Cosar E., Ersoy G.S., Taylor H.S. Bone marrow stem cell chemotactic activity is induced by elevated CXCL12 in endometriosis. Reprod Sci. 2017;24:526–533. doi: 10.1177/1933719116672587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cousins F.L., O D.F., Gargett C.E. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;50:27–38. doi: 10.1016/j.bpobgyn.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 151.Figueira P.G., Abrão M.S., Krikun G., Taylor H.S. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2011;1221:10–17. doi: 10.1111/j.1749-6632.2011.05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li F., Alderman M.H., 3rd, Tal A. Hematogenous dissemination of mesenchymal stem cells from endometriosis. Stem Cells. 2018;36:881–890. doi: 10.1002/stem.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Samani E.N., Mamillapalli R., Li F. Micrometastasis of endometriosis to distant organs in a murine model. Oncotarget. 2017;10:2282–2291. doi: 10.18632/oncotarget.16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alderman M.H., 3rd, Yoder N., Taylor H.S. The systemic effects of endometriosis. Semin Reprod Med. 2017;35:263–270. doi: 10.1055/s-0037-1603582. [DOI] [PubMed] [Google Scholar]
- 155.Vannuccini S., Clifton V.L., Fraser I.S. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update. 2016;22:104–115. doi: 10.1093/humupd/dmv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tal R., Shaikh S., Pallavi P. Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen L., Qu J., Cheng T., Chen X., Xiang C. Menstrual blood-derived stem cells: toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem Cell Res Ther. 2019;10:406. doi: 10.1186/s13287-019-1503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Emmerson S., Mukherjee S., Melendez-Munoz J. Composite mesh design for delivery of autologous mesenchymal stem cells influences mesh integration, exposure and biocompatibility in an ovine model of pelvic organ prolapse. Biomaterials. 2019;225:119495. doi: 10.1016/j.biomaterials.2019.119495. [DOI] [PubMed] [Google Scholar]
- 159.Alcayaga-Miranda F., Cuenca J., Luz-Crawford P. Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2015;6:32. doi: 10.1186/s13287-015-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Panés J., García-Olmo D., Van Assche G. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 161.Pang Y., Xiao H.W., Zhang H. Allogeneic bone marrow-derived mesenchymal stromal cells expanded in vitro for treatment of aplastic anemia: a multicenter phase II trial. Stem Cells Transl Med. 2017;6:1569–1575. doi: 10.1002/sctm.16-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fouillard L., Bensidhoum M., Bories D. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia. 2003;17:474–476. doi: 10.1038/sj.leu.2402786. [DOI] [PubMed] [Google Scholar]
- 163.Forbes S.J., Newsome P.N. New horizons for stem cell therapy in liver disease. J Hepatol. 2012;56:496–499. doi: 10.1016/j.jhep.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 164.Behnke J., Kremer S., Shahzad T. MSC based therapies-new perspectives for the injured lung. J Clin Med. 2020;9:682. doi: 10.3390/jcm9030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wilson J.G., Liu K.D., Zhuo H. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cen P.P., Fan L.X., Wang J., Chen J.J., Li L.J. Therapeutic potential of menstrual blood stem cells in treating acute liver failure. World J Gastroenterol. 2019;25:6190–6204. doi: 10.3748/wjg.v25.i41.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bulun S.E. Uterine fibroids. N Engl J Med. 2013;369:1344–1355. doi: 10.1056/NEJMra1209993. [DOI] [PubMed] [Google Scholar]
- 168.Ikhena D.E., Bulun S.E. Literature review on the role of uterine fibroids in endometrial function. Reprod Sci. 2018;25:635–643. doi: 10.1177/1933719117725827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hapangama D.K., Bulmer J.N. Pathophysiology of heavy menstrual bleeding. Womens Health (Lond) 2016;12:3–13. doi: 10.2217/whe.15.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Imir A.G., Lin Z., Yin P. Aromatase expression in uterine leiomyomata is regulated primarily by proximal promoters I.3/II. J Clin Endocrinol Metab. 2007;92:1979–1982. doi: 10.1210/jc.2006-2482. [DOI] [PubMed] [Google Scholar]
- 171.Ishikawa H., Reierstad S., Demura M. High aromatase expression in uterine leiomyoma tissues of African-American women. J Clin Endocrinol Metab. 2009;94:1752–1756. doi: 10.1210/jc.2008-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sumitani H., Shozu M., Segawa T. In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology. 2000;141:3852–3861. doi: 10.1210/endo.141.10.7719. [DOI] [PubMed] [Google Scholar]
- 173.Ishikawa H., Ishi K., Serna V.A., Kakazu R., Bulun S.E., Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151:2433–2442. doi: 10.1210/en.2009-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ono M., Qiang W., Serna V.A. Role of stem cells in human uterine leiomyoma growth. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mas A., Cervelló I., Gil-Sanchis C. Identification and characterization of the human leiomyoma side population as putative tumor-initiating cells. Fertil Steril. 2012;98:741–751.e6. doi: 10.1016/j.fertnstert.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 176.Rogers R., Norian J., Malik M. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198:474.e1–474.e11. doi: 10.1016/j.ajog.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Payson M., Malik M., Siti-Nur Morris S., Segars J.H., Chason R., Catherino W.H. Activating transcription factor 3 gene expression suggests that tissue stress plays a role in leiomyoma development. Fertil Steril. 2009;92:748–755. doi: 10.1016/j.fertnstert.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Norian J.M., Owen C.M., Taboas J. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31:57–65. doi: 10.1016/j.matbio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Masaki T. Endothelins: homeostatic and compensatory actions in the circulatory and endocrine systems. Endocr Rev. 1993;14:256–268. doi: 10.1210/edrv-14-3-256. [DOI] [PubMed] [Google Scholar]
- 180.Pekonen F., Nyman T., Rutanen E.M. Differential expression of mRNAs for endothelin-related proteins in human endometrium, myometrium and leiomyoma. Mol Cell Endocrinol. 1994;103:165–170. doi: 10.1016/0303-7207(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 181.Farrer-Brown G., Beilby J.O., Tarbit M.H. Venous changes in the endometrium of myomatous uteri. Obstet Gynecol. 1971;38:743–751. [PubMed] [Google Scholar]
- 182.Kitaya K., Yasuo T. Leukocyte density and composition in human cycling endometrium with uterine fibroids. Hum Immunol. 2010;71:158–163. doi: 10.1016/j.humimm.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 183.Kim H.G., Jung G.Y., Park S.B., Cho Y.J., Han M. Assessment of the effects of prostaglandins on myometrial and leiomyoma cells in vitro through microRNA profiling. Mol Med Rep. 2018;18:2499–2505. doi: 10.3892/mmr.2018.9160. [DOI] [PubMed] [Google Scholar]
- 184.Park S.B., Jee B.C., Kim S.H., Cho Y.J., Han M. Cyclooxygenase-2 inhibitor, celecoxib, inhibits leiomyoma cell proliferation through the nuclear factor κB pathway. Reprod Sci. 2014;21:1187–1195. doi: 10.1177/1933719114542010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Girling J.E., Lockhart M.G., Olshansky M. Differential gene expression in menstrual endometrium from women with self-reported heavy menstrual bleeding. Reprod Sci. 2017;24:28–46. doi: 10.1177/1933719116648217. [DOI] [PubMed] [Google Scholar]
- 186.Anania C.A., Stewart E.A., Quade B.J., Hill J.A., Nowak R.A. Expression of the fibroblast growth factor receptor in women with leiomyomas and abnormal uterine bleeding. Mol Hum Reprod. 1997;3:685–691. doi: 10.1093/molehr/3.8.685. [DOI] [PubMed] [Google Scholar]
- 187.Rackow B.W., Taylor H.S. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010;93:2027–2034. doi: 10.1016/j.fertnstert.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Arici A., Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73:1006–1011. doi: 10.1016/s0015-0282(00)00418-0. [DOI] [PubMed] [Google Scholar]
- 189.Doherty L.F., Taylor H.S. Leiomyoma-derived transforming growth factor-β impairs bone morphogenetic protein-2-mediated endometrial receptivity. Fertil Steril. 2015;103:845–852. doi: 10.1016/j.fertnstert.2014.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Matsuzaki S., Canis M., Darcha C., Pouly J.L., Mage G. HOXA-10 expression in the mid-secretory endometrium of infertile patients with either endometriosis, uterine fibromas or unexplained infertility. Hum Reprod. 2009;24:3180–3187. doi: 10.1093/humrep/dep306. [DOI] [PubMed] [Google Scholar]
- 191.Unlu C., Celik O., Celik N., Otlu B. Expression of endometrial receptivity genes increase After myomectomy of intramural leiomyomas not distorting the endometrial cavity. Reprod Sci. 2016;23:31–41. doi: 10.1177/1933719115612929. [DOI] [PubMed] [Google Scholar]
- 192.Sender R., Fuchs S., Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 193.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The Human Microbiome Project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rosenberg E., Koren O., Reshef L., Efrony R., Zilber-Rosenberg I. The role of microorganisms in coral health, disease and evolution. Nat Rev Microbiol. 2007;5:355–362. doi: 10.1038/nrmicro1635. [DOI] [PubMed] [Google Scholar]
- 195.Leeuwenhoek A. An abstract of a letter from Mr. Anthony Leevvenhoeck at Delft, dated Sep. 17. 1683. Containing some microscopical observations, about animals in the scurf of the teeth, the substance call'd worms in the nose, the cuticula consisting of scales. Philos Trans R Soc Lond. 1684;14:568–574. [Google Scholar]
- 196.Grice E.A., Segre J.A. The human microbiome: our second genome. Annu Rev Genomics Hum Genet. 2012;13:151–170. doi: 10.1146/annurev-genom-090711-163814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cho I., Yamanishi S., Cox L. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cox L.M., Yamanishi S., Sohn J. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Azad M.B., Bridgman S.L., Becker A.B., Kozyrskyj A.L. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes (Lond) 2014;38:1290–1298. doi: 10.1038/ijo.2014.119. [DOI] [PubMed] [Google Scholar]
- 200.Arrieta M.C., Stiemsma L.T., Dimitriu P.A. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aab2271. 307ra152. [DOI] [PubMed] [Google Scholar]
- 201.Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 202.Codagnone M.G., Spichak S., O’Mahony S.M. Programming bugs: microbiota and the developmental origins of brain health and disease. Biol Psychiatry. 2019;85:150–163. doi: 10.1016/j.biopsych.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 203.Baker J.M., Al-Nakkash L., Herbst-Kralovetz M.M. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 204.Funkhouser L.J., Bordenstein S.R. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;11 doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sirota I., Zarek S.M., Segars J.H. Potential influence of the microbiome on infertility and assisted reproductive technology. Semin Reprod Med. 2014;32:35–42. doi: 10.1055/s-0033-1361821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Baker J.M., Chase D.M., Herbst-Kralovetz M.M. Uterine microbiota: residents, tourists, or invaders? Front Immunol. 2018;9:208. doi: 10.3389/fimmu.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Salter S.J., Cox M.J., Turek E.M. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014;12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kim D., Hofstaedter C.E., Zhao C. Optimizing methods and dodging pitfalls in microbiome research. Microbiome. 2017;5:52. doi: 10.1186/s40168-017-0267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mitchell C.M., Haick A., Nkwopara E. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol. 2015;212 doi: 10.1016/j.ajog.2014.11.043. 611.e1-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen C., Song X., Wei W. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun. 2017;8:875. doi: 10.1038/s41467-017-00901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Koedooder R., Mackens S., Budding A. Identification and evaluation of the microbiome in the female and male reproductive tracts. Hum Reprod Update. 2019;25:298–325. doi: 10.1093/humupd/dmy048. [DOI] [PubMed] [Google Scholar]
- 213.Moreno I., Codoñer F.M., Vilella F. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 2016;215:684–703. doi: 10.1016/j.ajog.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 214.Miles S.M., Hardy B.L., Merrell D.S. Investigation of the microbiota of the reproductive tract in women undergoing a total hysterectomy and bilateral salpingo-oopherectomy. Fertil Steril. 2017;107:813–820.e1. doi: 10.1016/j.fertnstert.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 215.Swidsinski A., Verstraelen H., Loening-Baucke V., Swidsinski S., Mendling W., Halwani Z. Presence of a polymicrobial endometrial biofilm in patients with bacterial vaginosis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Franasiak J.M., Werner M.D., Juneau C.R. Endometrial microbiome at the time of embryo transfer: next-generation sequencing of the 16S ribosomal subunit. J Assist Reprod Genet. 2016;33:129–136. doi: 10.1007/s10815-015-0614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kyono K., Hashimoto T., Nagai Y., Sakuraba Y. Analysis of endometrial microbiota by 16S ribosomal RNA gene sequencing among infertile patients: a single-center pilot study. Reprod Med Biol. 2018;17:297–306. doi: 10.1002/rmb2.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tao X., Franasiak J.M., Zhan Y. Characterizing the endometrial microbiome by analyzing the ultra-low bacteria from embryo transfer catheter tips in IVF cycles: next generation sequencing (NGS) analysis of the 16S ribosomal gene. Hum Microbiome J. 2017;3:15–21. [Google Scholar]
- 219.Hashimoto T., Kyono K. Does dysbiotic endometrium affect blastocyst implantation in IVF patients? J Assist Reprod Genet. 2019;36:2471–2479. doi: 10.1007/s10815-019-01630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kitaya K., Nagai Y., Arai W., Sakuraba Y., Ishikawa T. Characterization of microbiota in endometrial fluid and vaginal secretions in infertile women with repeated implantation failure. Mediators Inflamm. 2019;2019:4893437. doi: 10.1155/2019/4893437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Winters A.D., Romero R., Gervasi M.T. Does the endometrial cavity have a molecular microbial signature? Sci Rep. 2019;9:9905. doi: 10.1038/s41598-019-46173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Takebayashi A., Kimura F., Kishi Y. The association between endometriosis and chronic endometritis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Khan K.N., Fujishita A., Masumoto H. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2016;199:69–75. doi: 10.1016/j.ejogrb.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 224.Tai F.W., Chang C.Y., Chiang J.H., Lin W.C., Wan L. Association of pelvic inflammatory disease with risk of endometriosis: a nationwide cohort study involving 141,460 individuals. J Clin Med. 2018;7:379. doi: 10.3390/jcm7110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Walther-António M.R., Chen J., Multinu F. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016;8:122. doi: 10.1186/s13073-016-0368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kasprzykowska U., Elias J., Elias M., Mączyńska B., Sobieszczańska B.M. Colonization of the lower urogenital tract with Ureaplasma parvum can cause asymptomatic infection of the upper reproductive system in women: a preliminary study. Arch Gynecol Obstet. 2014;289:1129–1134. doi: 10.1007/s00404-013-3102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Babu G., Singaravelu B.G., Srikumar R., Reddy S.V., Kokan A. Comparative study on the vaginal flora and incidence of asymptomatic vaginosis among healthy women and in women with infertility problems of reproductive age. J Clin Diagn Res. 2017;11:DC18–DC22. doi: 10.7860/JCDR/2017/28296.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Campisciano G., Florian F., D’Eustacchio A. Subclinical alteration of the cervical-vaginal microbiome in women with idiopathic infertility. J Cell Physiol. 2017;232:1681–1688. doi: 10.1002/jcp.25806. [DOI] [PubMed] [Google Scholar]
- 229.Wee B.A., Thomas M., Sweeney E.L. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust N Z J Obstet Gynaecol. 2018;58:341–348. doi: 10.1111/ajo.12754. [DOI] [PubMed] [Google Scholar]
- 230.Graspeuntner S., Bohlmann M.K., Gillmann K. Microbiota-based analysis reveals specific bacterial traits and a novel strategy for the diagnosis of infectious infertility. PLoS One. 2018;13 doi: 10.1371/journal.pone.0191047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Egbase P.E., al-Sharhan M., al-Othman S., al-Mutawa M., Udo E.E., Grudzinskas J.G. Incidence of microbial growth from the tip of the embryo transfer catheter after embryo transfer in relation to clinical pregnancy rate following in-vitro fertilization and embryo transfer. Hum Reprod. 1996;11:1687–1689. doi: 10.1093/oxfordjournals.humrep.a019470. [DOI] [PubMed] [Google Scholar]
- 232.Fanchin R., Harmas A., Benaoudia F., Lundkvist U., Olivennes F., Frydman R. Microbial flora of the cervix assessed at the time of embryo transfer adversely affects in vitro fertilization outcome. Fertil Steril. 1998;70:866–870. doi: 10.1016/s0015-0282(98)00277-5. [DOI] [PubMed] [Google Scholar]
- 233.Moore D.E., Soules M.R., Klein N.A., Fujimoto V.Y., Agnew K.J., Eschenbach D.A. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil Steril. 2000;74:1118–1124. doi: 10.1016/s0015-0282(00)01624-1. [DOI] [PubMed] [Google Scholar]
- 234.Salim R., Ben-Shlomo I., Colodner R., Keness Y., Shalev E. Bacterial colonization of the uterine cervix and success rate in assisted reproduction: results of a prospective survey. Hum Reprod. 2002;17:337–340. doi: 10.1093/humrep/17.2.337. [DOI] [PubMed] [Google Scholar]
- 235.Selman H., Mariani M., Barnocchi N. Examination of bacterial contamination at the time of embryo transfer, and its impact on the IVF/pregnancy outcome. J Assist Reprod Genet. 2007;24:395–399. doi: 10.1007/s10815-007-9146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pelzer E.S., Allan J.A., Waterhouse M.A., Ross T., Beagley K.W., Knox C.L. Microorganisms within human follicular fluid: effects on IVF. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Moreno I., Garcia-Grau I., Bau D. The first glimpse of the endometrial microbiota in early pregnancy. Am J Obstet Gynecol. 2020;222:296–305. doi: 10.1016/j.ajog.2020.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cruickshank R. The conversion of the glycogen of the vagina into lactic acid. J Pathol. 1934;39:213–219. [Google Scholar]
- 239.Ranđelović G., Mladenović V., Ristić L. Microbiological aspects of vulvovaginitis in prepubertal girls. Eur J Pediatr. 2012;171:1203–1208. doi: 10.1007/s00431-012-1705-9. [DOI] [PubMed] [Google Scholar]
- 240.Huang B., Fettweis J.M., Brooks J.P., Jefferson K.K., Buck G.A. The changing landscape of the vaginal microbiome. Clin Lab Med. 2014;34:747–761. doi: 10.1016/j.cll.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hickey R.J., Zhou X., Settles M.L. Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. mBio. 2015;6 doi: 10.1128/mBio.00097-15. e00097–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ravel J., Gajer P., Abdo Z. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gajer P., Brotman R.M., Bai G. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4:132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kroon S.J., Ravel J., Huston W.M. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil Steril. 2018;110:327–336. doi: 10.1016/j.fertnstert.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 245.Aagaard K., Riehle K., Ma J. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Romero R., Hassan S.S., Gajer P. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2:4. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hyman R.W., Fukushima M., Jiang H. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci. 2014;21:32–40. doi: 10.1177/1933719113488838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.MacIntyre D.A., Chandiramani M., Lee Y.S. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep. 2015;5:8988. doi: 10.1038/srep08988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Stout M.J., Conlon B., Landeau M. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol. 2013;208 doi: 10.1016/j.ajog.2013.01.018. 226.e1-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.DiGiulio D.B., Callahan B.J., McMurdie P.J. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A. 2015;112:11060–11065. doi: 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Fettweis J.M., Serrano M.G., Brooks J.P. The vaginal microbiome and preterm birth. Nat Med. 2019;25:1012–1021. doi: 10.1038/s41591-019-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Pabich W.L., Fihn S.D., Stamm W.E., Scholes D., Boyko E.J., Gupta K. Prevalence and determinants of vaginal flora alterations in postmenopausal women. J Infect Dis. 2003;188:1054–1058. doi: 10.1086/378203. [DOI] [PubMed] [Google Scholar]
- 253.Brotman R.M., Shardell M.D., Gajer P. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014;21:450–458. doi: 10.1097/GME.0b013e3182a4690b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Muhleisen A.L., Herbst-Kralovetz M.M. Menopause and the vaginal microbiome. Maturitas. 2016;91:42–50. doi: 10.1016/j.maturitas.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 255.Srinivasan S., Liu C., Mitchell C.M. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Santiago G.L., Cools P., Verstraelen H. Longitudinal study of the dynamics of vaginal microflora during two consecutive menstrual cycles. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Santiago G.L., Tency I., Verstraelen H. Longitudinal qPCR study of the dynamics of L. crispatus, L. iners, A. vaginae, (sialidase positive) G. vaginalis, and P. bivia in the vagina. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hallberg L., Högdahl A.M., Nilsson L., Rybo G. Menstrual blood loss and iron deficiency. Acta Med Scand. 1966;180:639–650. doi: 10.1111/j.0954-6820.1966.tb02880.x. [DOI] [PubMed] [Google Scholar]
- 259.Petrova M.I., Reid G., Vaneechoutte M., Lebeer S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017;25:182–191. doi: 10.1016/j.tim.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 260.Bradley F., Birse K., Hasselrot K. The vaginal microbiome amplifies sex hormone-associated cyclic changes in cervicovaginal inflammation and epithelial barrier disruption. Am J Reprod Immunol. 2018;80 doi: 10.1111/aji.12863. [DOI] [PubMed] [Google Scholar]
- 261.Pelzer E.S., Willner D., Buttini M., Huygens F. A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie Van Leeuwenhoek. 2018;111:933–943. doi: 10.1007/s10482-017-0992-6. [DOI] [PubMed] [Google Scholar]
- 262.Stilling R.M., Bordenstein S.R., Dinan T.G., Cryan J.F. Friends with social benefits: host-microbe interactions as a driver of brain evolution and development? Front Cell Infect Microbiol. 2014;4:147. doi: 10.3389/fcimb.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cryan J.F., Clarke G., Dinan T.G., Schellekens H. A microbial drugstore for motility. Cell Host Microbe. 2018;23:691–692. doi: 10.1016/j.chom.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 264.Cohen L.J., Esterhazy D., Kim S.H. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549:48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Satake H., Matsubara S., Aoyama M., Kawada T., Sakai T. GPCR heterodimerization in the reproductive system: functional regulation and implication for biodiversity. Front Endocrinol (Lausanne) 2013;4:100. doi: 10.3389/fendo.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.van der Molen R.G., Schutten J.H., van Cranenbroek B. Menstrual blood closely resembles the uterine immune micro-environment and is clearly distinct from peripheral blood. Hum Reprod. 2014;29:303–314. doi: 10.1093/humrep/det398. [DOI] [PubMed] [Google Scholar]
- 267.Liswood R. Internal menstrual protection; use of a safe and sanitary menstrual cup. Obstet Gynecol. 1959;13:539–543. [PubMed] [Google Scholar]
- 268.Pena E.F. Menstrual protection. Advantages of the menstrual cup. Obstet Gynecol. 1962;19:684–687. [PubMed] [Google Scholar]
- 269.Zhou J.P., Fraser I.S., Caterson I. Reproductive hormones in menstrual blood. J Clin Endocrinol Metab. 1989;69:338–342. doi: 10.1210/jcem-69-2-338. [DOI] [PubMed] [Google Scholar]
- 270.Rees M.C., Cederholm-Williams S.A., Turnbull A.C. Coagulation factors and fibrinolytic proteins in menstrual fluid collected from normal and menorrhagic women. Br J Obstet Gynaecol. 1985;92:1164–1168. doi: 10.1111/j.1471-0528.1985.tb03031.x. [DOI] [PubMed] [Google Scholar]
- 271.Rees M.C., Demers L.M., Anderson A.B., Turnbull A.C. A functional study of platelets in menstrual fluid. Br J Obstet Gynaecol. 1984;91:667–672. doi: 10.1111/j.1471-0528.1984.tb04828.x. [DOI] [PubMed] [Google Scholar]
- 272.Koks C.A., Dunselman G.A.J., de Goeij A.F., Arends J.W., Evers J.L. Evaluation of a menstrual cup to collect shed endometrium for in vitro studies. Fertil Steril. 1997;68:560–564. doi: 10.1016/s0015-0282(97)00250-1. [DOI] [PubMed] [Google Scholar]
- 273.Koks C.A., Groothuis P.G., Slaats P., Dunselman G.A., de Goeij A.F., Evers J.L. Matrix metalloproteinases and their tissue inhibitors in antegradely shed menstruum and peritoneal fluid. Fertil Steril. 2000;73:604–612. doi: 10.1016/s0015-0282(99)00566-x. [DOI] [PubMed] [Google Scholar]
- 274.Ahrens C.C., Chiswick E.L., Ravindra K.C. Development and application of the metalloprotease activity multiplexed bead-based immunoassay (MAMBI) Biochemistry. 2019;58:3938–3942. doi: 10.1021/acs.biochem.9b00584. [DOI] [PubMed] [Google Scholar]
- 275.Yang H., Zhou B., Prinz M., Siegel D. Proteomic analysis of menstrual blood. Mol Cell Proteomics. 2012;11:1024–1035. doi: 10.1074/mcp.M112.018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ivarsson M.A., Stiglund N., Marquardt N., Westgren M., Gidlöf S., Björkström N.K. Composition and dynamics of the uterine NK cell KIR repertoire in menstrual blood. Mucosal Immunol. 2017;10:322–331. doi: 10.1038/mi.2016.50. [DOI] [PubMed] [Google Scholar]
- 277.Evans J., Infusini G., McGovern J. Menstrual fluid factors facilitate tissue repair: identification and functional action in endometrial and skin repair. FASEB J. 2019;33:584–605. doi: 10.1096/fj.201800086R. [DOI] [PubMed] [Google Scholar]
- 278.Altmäe S., Esteban F.J., Stavreus-Evers A. Guidelines for the design, analysis and interpretation of ’omics’ data: focus on human endometrium. Hum Reprod Update. 2014;20:12–28. doi: 10.1093/humupd/dmt048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Hosseini S., Shokri F., Tokhmechy R. Menstrual blood contains immune cells with inflammatory and anti-inflammatory properties. J Obstet Gynaecol Res. 2015;41:1803–1812. doi: 10.1111/jog.12801. [DOI] [PubMed] [Google Scholar]
- 280.Hosseini S., Shokri F., Pour S.A., Khoshnoodi J., Jeddi-Tehrani M., Zarnani A.H. Diminished frequency of menstrual and peripheral blood NKT-like cells in patients With unexplained recurrent spontaneous abortion and infertile women. Reprod Sci. 2019;26:97–108. doi: 10.1177/1933719118766261. [DOI] [PubMed] [Google Scholar]
- 281.Smith K.S., Eubanks D., Petrik A., Stevens V.J. Using web-based screening to enhance efficiency of HMO clinical trial recruitment in women aged forty and older. Clin Trials. 2007;4:102–105. doi: 10.1177/1740774506075863. [DOI] [PubMed] [Google Scholar]
- 282.Gorman J.R., Roberts S.C., Dominick S.A., Malcarne V.L., Dietz A.C., Su H.I. A diversified recruitment approach incorporating social media leads to research participation Among young adult-aged female cancer survivors. J Adolesc Young Adult Oncol. 2014;3:59–65. doi: 10.1089/jayao.2013.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Baird A.L., Westwood S., Lovestone S. Blood-based proteomic biomarkers of Alzheimer’s disease pathology. Front Neurol. 2015;6:236. doi: 10.3389/fneur.2015.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Eskenazi B., Warner M.L. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 285.Giudice L.C., Clinical practice Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Meuleman C., Vandenabeele B., Fieuws S., Spiessens C., Timmerman D., D’Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. 2009;92:68–74. doi: 10.1016/j.fertnstert.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 287.Hickey M., Ballard K., Farquhar C. Endometriosis. BMJ. 2014;348:g1752. doi: 10.1136/bmj.g1752. [DOI] [PubMed] [Google Scholar]
- 288.Hummelshoj L., Prentice A., Groothuis P. Update on endometriosis. Womens Health (Lond) 2006;2:53–56. doi: 10.2217/17455057.2.1.53. [DOI] [PubMed] [Google Scholar]
- 289.Puttemans P., Benagiano G., Gargett C., Romero R., Guo S.W., Brosens I. Neonatal uterine bleeding as a biomarker for reproductive disorders during adolescence: a worldwide call for systematic registration by nurse midwife. J Matern Fetal Neonatal Med. 2017;30:1434–1436. doi: 10.1080/14767058.2016.1216540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kulp J.L., Mamillapalli R., Taylor H.S. Aberrant HOXA10 methylation in patients With common gynecologic disorders: implications for reproductive outcomes. Reprod Sci. 2016;23:455–463. doi: 10.1177/1933719116630427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Nnoaham K.E., Hummelshoj L., Webster P. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366–373.e8. doi: 10.1016/j.fertnstert.2011.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Culley L., Law C., Hudson N. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update. 2013;19:625–639. doi: 10.1093/humupd/dmt027. [DOI] [PubMed] [Google Scholar]
- 293.Simoens S., Dunselman G., Dirksen C. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27:1292–1299. doi: 10.1093/humrep/des073. [DOI] [PubMed] [Google Scholar]
- 294.Fillingim R.B., King C.D., Ribeiro-Dasilva M.C., Rahim-Williams B., Riley J.L., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Schäfer G., Prkachin K.M., Kaseweter K.A., Williams A.C. Health care providers’ judgments in chronic pain: the influence of gender and trustworthiness. Pain. 2016;157:1618–1625. doi: 10.1097/j.pain.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 296.Nisenblat V., Bossuyt P.M., Shaikh R. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2016:CD012179. doi: 10.1002/14651858.CD012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Aghajanova L., Tatsumi K., Horcajadas J.A. Unique transcriptome, pathways, and networks in the human endometrial fibroblast response to progesterone in endometriosis. Biol Reprod. 2011;84:801–815. doi: 10.1095/biolreprod.110.086181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Aghajanova L., Velarde M.C., Giudice L.C. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med. 2010;28:51–58. doi: 10.1055/s-0029-1242994. [DOI] [PubMed] [Google Scholar]
- 299.Tamaresis J.S., Irwin J.C., Goldfien G.A. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155:4986–4999. doi: 10.1210/en.2014-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Gregersen P.K., Klein G., Keogh M. The Genotype and Phenotype (GaP) registry: a living biobank for the analysis of quantitative traits. Immunol Res. 2015;63:107–112. doi: 10.1007/s12026-015-8711-8. [DOI] [PubMed] [Google Scholar]
- 301.Warren L.A., Shih A., Renteira S.M. Analysis of menstrual effluent: diagnostic potential for endometriosis. Mol Med. 2018;24:1. doi: 10.1186/s10020-018-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Klemmt P.A., Carver J.G., Kennedy S.H., Koninckx P.R., Mardon H.J. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85:564–572. doi: 10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Yin X., Pavone M.E., Lu Z., Wei J., Kim J.J. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J Clin Endocrinol Metab. 2012;97:E35–E43. doi: 10.1210/jc.2011-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Barragan F., Irwin J.C., Balayan S. Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis. Biol Reprod. 2016;94:118. doi: 10.1095/biolreprod.115.136010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Cancer Genome Atlas Research Network. Kandoth C., Schultz N. et alIntegrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wang W, Vilella F, Alama P, et al. Single cell RNAseq provides a molecular and cellular cartography of changes to the human endometrium through the menstrual cycle. BioRxiv 2019, 10.1101/350538. [DOI]
- 307.Beste M.T., Pfäffle-Doyle N., Prentice E.A. Molecular network analysis of endometriosis reveals a role for c-Jun-regulated macrophage activation. Sci Transl Med. 2014;6:222ra16. doi: 10.1126/scitranslmed.3007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Miller M.A., Meyer A.S., Beste M.T. ADAM-10 and -17 regulate endometriotic cell migration via concerted ligand and receptor shedding feedback on kinase signaling. Proc Natl Acad Sci U S A. 2013;110:E2074–E2083. doi: 10.1073/pnas.1222387110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Anglesio M.S., Papadopoulos N., Ayhan A. Cancer-associated mutations in endometriosis without cancer. N Engl J Med. 2017;376:1835–1848. doi: 10.1056/NEJMoa1614814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Li H.Y., Chen Y.J., Chen S.J. Induction of insulin-producing cells derived from endometrial mesenchymal stem-like cells. J Pharmacol Exp Ther. 2010;335:817–829. doi: 10.1124/jpet.110.169284. [DOI] [PubMed] [Google Scholar]
- 311.Hida N., Nishiyama N., Miyoshi S. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26:1695–1704. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- 312.Gargett C.E., Ye L. Endometrial reconstruction from stem cells. Fertil Steril. 2012;98:11–20. doi: 10.1016/j.fertnstert.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 313.Paul K., Darzi S., McPhee G. 3D bioprinted endometrial stem cells on melt electrospun poly epsilon-caprolactone mesh for pelvic floor application promote anti-inflammatory responses in mice. Acta Biomater. 2019;97:162–176. doi: 10.1016/j.actbio.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 314.Turco M.Y., Gardner L., Hughes J. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19:568–577. doi: 10.1038/ncb3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Boretto M., Cox B., Noben M. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 2017;144:1775–1786. doi: 10.1242/dev.148478. [DOI] [PubMed] [Google Scholar]
- 316.Sato T., Vries R.G., Snippert H.J. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 317.Wiwatpanit T., Murphy A.R., Lu Z. Scaffold-free endometrial organoids respond to excess androgens associated With polycystic ovarian syndrome. J Clin Endocrinol Metab. 2020:105. doi: 10.1210/clinem/dgz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Murphy A.R., Wiwatpanit T., Lu Z., Davaadelger B., Kim J.J. Generation of multicellular human primary endometrial organoids. J Vis Exp. 2019;152 doi: 10.3791/60384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Hellweg G., Shaka J.A. Endometrial Granulocytes; tissue culture studies of endometrium and decidua with special attention to the endometrial granulocytes. Obstet Gynecol. 1959;13:519–529. [PubMed] [Google Scholar]
- 320.Bentin-Ley U., Pedersen B., Lindenberg S., Larsen J.F., Hamberger L., Horn T. Isolation and culture of human endometrial cells in a three-dimensional culture system. J Reprod Fertil. 1994;101:327–332. doi: 10.1530/jrf.0.1010327. [DOI] [PubMed] [Google Scholar]
- 321.Meng C.X., Andersson K.L., Bentin-Ley U., Gemzell-Danielsson K., Lalitkumar P.G. Effect of levonorgestrel and mifepristone on endometrial receptivity markers in a three-dimensional human endometrial cell culture model. Fertil Steril. 2009;91:256–264. doi: 10.1016/j.fertnstert.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 322.Olalekan S.A., Burdette J.E., Getsios S., Woodruff T.K., Kim J.J. Development of a novel human recellularized endometrium that responds to a 28-day hormone treatment. Biol Reprod. 2017;96:971–981. doi: 10.1093/biolre/iox039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wang H., Bocca S., Anderson S. Sex steroids regulate epithelial-stromal cell cross talk and trophoblast attachment invasion in a three-dimensional human endometrial culture system. Tissue Eng C. 2013;19:676–687. doi: 10.1089/ten.TEC.2012.0616. [DOI] [PubMed] [Google Scholar]
- 324.Zambuto S.G., Clancy K.B.H., Harley B.A.C. A gelatin hydrogel to study endometrial angiogenesis and trophoblast invasion. Interface Focus. 2019;9:20190016. doi: 10.1098/rsfs.2019.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Pence J.C., Clancy K.B., Harley B.A. The induction of pro-angiogenic processes within a collagen scaffold via exogenous estradiol and endometrial epithelial cells. Biotechnol Bioeng. 2015;112:2185–2194. doi: 10.1002/bit.25622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Valdez J., Cook C.D., Ahrens C.C. On-demand dissolution of modular, synthetic extracellular matrix reveals local epithelial-stromal communication networks. Biomaterials. 2017;130:90–103. doi: 10.1016/j.biomaterials.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Schutte S.C., James C.O., Sidell N., Taylor R.N. Tissue-engineered endometrial model for the study of cell-cell interactions. Reprod Sci. 2015;22:308–315. doi: 10.1177/1933719114542008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Cook C.D., Hill A.S., Guo M. Local remodeling of synthetic extracellular matrix microenvironments by co-cultured endometrial epithelial and stromal cells enables long-term dynamic physiological function. Integr Biol (Camb) 2017;9:271–289. doi: 10.1039/c6ib00245e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Schutte S.C., Taylor R.N. A tissue-engineered human endometrial stroma that responds to cues for secretory differentiation, decidualization, and menstruation. Fertil Steril. 2012;97:997–1003. doi: 10.1016/j.fertnstert.2012.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Gnecco J.S., Pensabene V., Li D.J. Compartmentalized culture of perivascular stroma and endothelial cells in a microfluidic model of the human endometrium. Ann Biomed Eng. 2017;45:1758–1769. doi: 10.1007/s10439-017-1797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zervantonakis I.K., Hughes-Alford S.K., Charest J.L., Condeelis J.S., Gertler F.B., Kamm R.D. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci U S A. 2012;109:13515–13520. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Pavesi A., Tan A.T., Koh S. A 3D microfluidic model for preclinical evaluation of TCR-engineered T cells against solid tumors. JCI Insight. 2017;2 doi: 10.1172/jci.insight.89762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Spiegel A., Brooks M.W., Houshyar S. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6:630–649. doi: 10.1158/2159-8290.CD-15-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Osaki T., Sivathanu V., Kamm R.D. Vascularized microfluidic organ-chips for drug screening, disease models and tissue engineering. Curr Opin Biotechnol. 2018;52:116–123. doi: 10.1016/j.copbio.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Wang X., Phan D.T., Sobrino A., George S.C., Hughes C.C., Lee A.P. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip. 2016;16:282–290. doi: 10.1039/c5lc01050k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Domansky K., Inman W., Serdy J., Dash A., Lim M.H., Griffith L.G. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip. 2010;10:51–58. doi: 10.1039/b913221j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Inman W., Domansky K., Serdy J., Owens B., Trumper D., Griffith L.G. Design, modeling and fabrication of a constant flow pneumatic micropump. J Micromech Microeng. 2007;17:891–899. [Google Scholar]
- 338.Chen W.L.K., Edington C., Suter E. Integrated gut/liver microphysiological systems elucidates inflammatory inter-tissue crosstalk. Biotechnol Bioeng. 2017;114:2648–2659. doi: 10.1002/bit.26370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Trapecar M., Communal C., Velazquez J. Gut-liver physiomimetics reveal paradoxical modulation of IBD-related inflammation by short-chain fatty acids. Cell Syst. 2020;10:223–239.e9. doi: 10.1016/j.cels.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Edington C.D., Chen W.L.K., Geishecker E. Interconnected microphysiological systems for quantitative biology and pharmacology studies. Sci Rep. 2018;8:4530. doi: 10.1038/s41598-018-22749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Xiao S., Coppeta J.R., Rogers H.B. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun. 2017;8:14584. doi: 10.1038/ncomms14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Fauconnier A., Borghese B., Huchon C. Epidemiology and diagnosis strategy: CNGOF-HAS Endometriosis Guidelines. Gynecol Obstet Fertil Senol. 2018;46:223–230. doi: 10.1016/j.gofs.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 343.Guerriero S., Saba L., Pascual M.A. Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51:586–595. doi: 10.1002/uog.18961. [DOI] [PubMed] [Google Scholar]
- 344.Chamié L.P., Ribeiro D.M.F.R., Tiferes D.A., Macedo Neto A.C., Serafini P.C. Atypical sites of deeply infiltrative endometriosis: clinical characteristics and imaging findings. Radiographics. 2018;38:309–328. doi: 10.1148/rg.2018170093. [DOI] [PubMed] [Google Scholar]
- 345.Chamié L.P., Blasbalg R., Pereira R.M., Warmbrand G., Serafini P.C. Findings of pelvic endometriosis at transvaginal US, MR imaging, and laparoscopy. Radiographics. 2011;31:E77–E100. doi: 10.1148/rg.314105193. [DOI] [PubMed] [Google Scholar]
- 346.Chamié L.P., Blasbalg R., Gonçalves M.O., Carvalho F.M., Abrão M.S., de Oliveira I.S. Accuracy of magnetic resonance imaging for diagnosis and preoperative assessment of deeply infiltrating endometriosis. Int J Gynecol Obstet. 2009;106:198–201. doi: 10.1016/j.ijgo.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 347.Bazot M., Lafont C., Rouzier R., Roseau G., Thomassin-Naggara I., Daraï E. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril. 2009;92:1825–1833. doi: 10.1016/j.fertnstert.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 348.Jha R.C., Ascher S.M., Imaoka I., Spies J.B. Symptomatic fibroleiomyomata: MR imaging of the uterus before and after uterine arterial embolization. Radiology. 2000;217:228–235. doi: 10.1148/radiology.217.1.r00se49228. [DOI] [PubMed] [Google Scholar]
- 349.Bastin M.E., Clayden J.D., Pattie A., Gerrish I.F., Wardlaw J.M., Deary I.J. Diffusion tensor and magnetization transfer MRI measurements of periventricular white matter hyperintensities in old age. Neurobiol Aging. 2009;30:125–136. doi: 10.1016/j.neurobiolaging.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 350.Harry V.N., Semple S.I., Parkin D.E., Gilbert F.J. Use of new imaging techniques to predict tumour response to therapy. Lancet Oncol. 2010;11:92–102. doi: 10.1016/S1470-2045(09)70190-1. [DOI] [PubMed] [Google Scholar]
- 351.Munro K.I., Thrippleton M.J., Williams A.R. Quantitative serial MRI of the treated fibroid uterus. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Luo J., Abaci Turk E., Bibbo C. In vivo quantification of placental insufficiency by BOLD MRI: a human study. Sci Rep. 2017;7:3713. doi: 10.1038/s41598-017-03450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Turk E.A., Luo J., Gagoski B. Spatiotemporal alignment of in utero BOLD-MRI series. J Magn Reson Imaging. 2017;46:403–412. doi: 10.1002/jmri.25585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Sommer M., Hirsch J.S., Nathanson C., Parker R.G. Comfortably, safely, and Without shame: defining menstrual hygiene management as a public health issue. Am J Public Health. 2015;105:1302–1311. doi: 10.2105/AJPH.2014.302525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Slap G.B. Menstrual disorders in adolescence. Best Pract Res Clin Obstet Gynaecol. 2003;17:75–92. doi: 10.1053/ybeog.2002.0342. [DOI] [PubMed] [Google Scholar]
- 356.Albers J.R., Hull S.K., Wesley R.M. Abnormal uterine bleeding. Am Fam Phys. 2004;69:1915–1926. [PubMed] [Google Scholar]
- 357.A cross-cultural study of menstruation: implications for contraceptive development and use World Health Organization Task Force on Psychosocial Research in Family Planning, Special Programme of Research, Development and Research, Training in Human Reproduction. Stud Fam Plann. 1981;12:3–16. [PubMed] [Google Scholar]
- 358.Rosenberg M.J., Waugh M.S., Meehan T.E. Use and misuse of oral contraceptives: risk indicators for poor pill taking and discontinuation. Contraception. 1995;51:283–288. doi: 10.1016/0010-7824(95)00074-k. [DOI] [PubMed] [Google Scholar]
- 359.Brooks-Gunn J., Ruble D.N. The development of menstrual-related beliefs and behaviors during early adolescence. Child Dev. 1982;53:1567–1577. [PubMed] [Google Scholar]
- 360.Ruble D.N., Brooks-Gunn J. The experience of menarche. Child Dev. 1982;53:1557–1566. [PubMed] [Google Scholar]
- 361.Koff E., Rierdan J., Jacobson S. The personal and interpersonal significance of menarche. J Am Acad Child Psychiatry. 1981;20:148–158. doi: 10.1016/s0002-7138(09)60724-x. [DOI] [PubMed] [Google Scholar]
- 362.Koo H.P., Rose A., Bhaskar B., Walker L.R. Relationships of pubertal development Among early adolescents to sexual and nonsexual risk behaviors and caregivers’ parenting behaviors. J Early Adolesc. 2011;31:1–26. doi: 10.1177/0272431611409746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Cavanagh S.E. The sexual debut of girls in early adolescence: the intersection of race, pubertal timing, and Friendship Group Characteristics. J Res Adolesc. 2004;14:285–312. [Google Scholar]
- 364.Williams J.M., Currie C. Self-esteem and physical development in early adolescence: pubertal timing and body image. J Early Adolesc. 2000;20:129–149. [Google Scholar]
- 365.McCabe M.P., Ricciardelli L.A. A longitudinal study of pubertal timing and extreme body change behaviors among adolescent boys and girls. Adolescence. 2004;39:145–166. [PubMed] [Google Scholar]
- 366.Mendle J., Turkheimer E., Emery R.E. Detrimental psychological outcomes associated with early pubertal timing in adolescent girls. Dev Rev. 2007;27:151–171. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Programme WUJM . Meeting Report of JMP Post; Washington DC; 2015: 2012. Global Monitoring Working Group on Hygiene. [Google Scholar]
- 368.Kirumira E.K., Stewart, editors. Life skills, sexual maturation and sanitation: what’s (not) happening in our schools? An exploratory study from Uganda. Women’s Press Law Centre, University of Zimbabwe; Harare, Zimbabwe: 2003. [Google Scholar]
- 369.Sommer M. Menstrual hygiene management in humanitarian emergencies: gaps and recommendations. Waterlines. 2012;31:83–104. [Google Scholar]
- 370.Kuncio T. Pilot study findings on the provision of hygiene kits with reusable sanitary pads. 2018. https://data2.unhcr.org/en/documents/details/69059 Available at: Accessed August 10, 2019.
- 371.Sommer M., Ackatia-Armah N., Connolly S., Smiles D. A comparison of the menstruation and education experiences of girls in Tanzania, Ghana, Cambodia and Ethiopia. Compare J Comp Int Educ. 2015;45:589–609. [Google Scholar]
- 372.McMahon S.A., Winch P.J., Caruso B.A. ‘The girl with her period is the one to hang her head’ Reflections on menstrual management among schoolgirls in rural Kenya. BMC Int Health Hum Rights. 2011;11:7. doi: 10.1186/1472-698X-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Scorgie F., Foster J., Stadler J. “Bitten By Shyness”: menstrual hygiene management, sanitation, and the quest for privacy in South Africa. Med Anthropol. 2016;35:161–176. doi: 10.1080/01459740.2015.1094067. [DOI] [PubMed] [Google Scholar]
- 374.Sommer M., Skolnik A., Ramirez A., Lee J., Rasoazanany H., Ibitoye M. Early adolescence in Madagascar: girls’ transitions through puberty in and out of school. J Early Adolesc. 2020;40:354–376. [Google Scholar]
- 375.Shah V., Nabwera H.M., Sosseh F. A rite of passage: a mixed methodology study about knowledge, perceptions and practices of menstrual hygiene management in rural Gambia. BMC Public Health. 2019;19:277. doi: 10.1186/s12889-019-6599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Mohamed Y., Durrant K., Huggett C. A qualitative exploration of menstruation-related restrictive practices in Fiji, Solomon Islands and Papua New Guinea. PLOS ONE. 2018;13 doi: 10.1371/journal.pone.0208224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Mason L., Nyothach E., Alexander K. We keep it secret so no one should know’—a qualitative study to explore young schoolgirls attitudes and experiences with menstruation in rural western Kenya. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Pillitteri S. School menstrual hygiene management in Malawi: more than toilets. 2011. http://www.shareresearch.org/file/2007/download?token=B7mGZ6wc Available at: Accessed May 15, 2019.
- 379.Jewitt S., Ryley H. It’s a girl thing: menstruation, school attendance, spatial mobility and wider gender inequalities in Kenya. Geoforum. 2014;56:137–147. [Google Scholar]
- 380.United Nations Children’s Emergency Fund WinS4Girls compendium: WASH in Schools for Girls. https://www.unicef.org/wash/schools/files/WinS4Girls_-_Voices_from_the_field.pdf Available at: Accessed May 15, 2019.
- 381.Sommer M. An early window of opportunity for promoting girls’ health: policy implications of the girl’s puberty book project in Tanzania. Int Electron J Health Educ. 2011;14:77–92. [Google Scholar]
- 382.Millington K., Bolton L. Improving access to menstrual hygiene products. 2015. http://www.gsdrc.org/wp-content/uploads/2015/10/HDQ1280.pdf Available at: Accessed May 15, 2019.
- 383.Alpha I. By women, for women: the new economics of menstrual pads in Africa. 2018. https://impactalpha.com/by-women-for-women-the-new-economics-of-menstrual-pads-in-africa-a6e1ace71ba0 Available at: Accessed May 15, 2019.
- 384.Proctor and Gamble Always keeping girls in school. http://www.pg.com/en_ZA/sustainability/social-responsibility/always-keeping-girls-in-school.shtml Available at: Accessed May 15, 2019.
- 385.Phillips-Howard P.A., Nyothach E., Ter Kuile F.O. Menstrual cups and sanitary pads to reduce school attrition, and sexually transmitted and reproductive tract infections: a cluster randomised controlled feasibility study in rural Western Kenya. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-013229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Miiro G., Rutakumwa R., Nakiyingi-Miiro J. Menstrual health and school absenteeism among adolescent girls in Uganda (meniscus): a feasibility study. BMC Womens Health. 2018;18:4. doi: 10.1186/s12905-017-0502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Das P., Baker K.K., Dutta A. Menstrual hygiene practices, WASH access and the risk of urogenital infection in women from Odisha, India. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Hennegan J., Montgomery P. Do menstrual hygiene management interventions improve education and psychosocial outcomes for women and girls in low and middle income countries? A systematic review. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Glynn J.R., Kayuni N., Gondwe L., Price A.J., Crampin A.C. Earlier menarche is associated with a higher prevalence of Herpes simplex type-2 (HSV-2) in young women in rural Malawi. eLife. 2014;3 doi: 10.7554/eLife.01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Glynn J.R., Kayuni N., Floyd S. Age at menarche, schooling, and sexual debut in northern Malawi. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Robinson A., Obrecht A. Improving menstrual hygiene management in emergencies: IFRC’s MHM kit. 2016. https://www.alnap.org/system/files/content/resource/files/main/alnap-ifrc-menstrual-hygiene-case-study-2016.pdf Available at: Accessed July 24, 2019.
- 392.Burgers L., Yamakoshi B., Serrano M. WASH in Schools Empowers Girls’ Education. 2018. https://static1.squarespace.com/static/5988738af9a61e3bd699b5e4/t/5c74056853450acdb8faf98a/1551213840255/2018+virtual+conference+on+MHM+in+schools+report Available at: Accessed July 24, 2019.
- 393.Columbia University, United Nations Children’s Emergency Fund MHM in ten: advancing the MHM agenda in WASH in schools (third annual meeting). 2016. https://www.mhmvirtualconference.com/mhm-in-ten Available at: Accessed July 24, 2019.
- 394.WASH united. 28 May menstrual hygiene day. Available at: https://menstrualhygieneday.org/wp-content/uploads/2017/01/Homeless-Menstruation-Report.pdf. Accessed July 16, 2020.
- 395.Sommer M. Menarche: a missing indicator in population health from low-income countries. Public Health Rep. 2013;128:399–401. doi: 10.1177/003335491312800511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Sommer M., Sutherland C., Chandra-Mouli V. Putting menarche and girls into the global population health agenda. Reprod Health. 2015;12:24. doi: 10.1186/s12978-015-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Sommer M., Chandraratna S., Cavill S., Mahon T., Phillips-Howard P. Managing menstruation in the workplace: an overlooked issue in low- and middle-income countries. Int J Equity Health. 2016;15:86. doi: 10.1186/s12939-016-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Torondel B., Sinha S., Mohanty J.R. Association between unhygienic menstrual management practices and prevalence of lower reproductive tract infections: a hospital-based cross-sectional study in Odisha, India. BMC Infect Dis. 2018;18:473. doi: 10.1186/s12879-018-3384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.House S., Mahon T., Cavill S. Menstrual Hygiene Matters: a resource for improving menstrual hygiene around the world. Reprod Health Matters. 2013;21:257–259. [Google Scholar]
- 400.UNESCO Good policy and practice in health education—Booklet 9, puberty education and menstrual hygiene management. 2014. https://asanteafrica.org/wp-content/uploads/2018/03/UNESCO-Puberty-rpt.pdf Available at: Accessed July 24, 2019.
- 401.Albuquerque C. 2012. Special rapporteur on right to safe water and sanitation issues statement to mark global handwashing day. Geneva; 2012. Available at: https://www.un.org/waterforlifedecade/pdf/15_10_2012_albuquerque_statement_for_ghd.pdf. Accessed July 16, 2020. [Google Scholar]
- 402.Geertz A., Iyer L., Kasen P., Mazzola F., Peterson K. Menstrual health in Kenya: country landscape analysis. 2016. http://www.fsg.org/publications/opportunity-address-menstrual-health-and-gender-equity Available at: Accessed July 24, 2019.
- 403.R2HC Building a cross-sectoral toolkit and Research Foundation for the integration of menstrual hygiene management into emergency response. Research for health in humanitarian crises. 2015. http://www.elrha.org/map-location/irc-menstrual-hygiene-call2/ Available at: Accessed July 24, 2019.
- 404.Jones A. Periods, policy and politics: menstrual equity is the new thing. Newsweek. 2017. http://www.newsweek.com/periods-policy-and-politics-menstrual-equity-new-thing-596027 Available at: Accessed July 24, 2019.
- 405.Wilson E., Reeve J., Pitt A. Education. Period. Developing an acceptable and replicable menstrual hygiene intervention. Dev Pract. 2014;24:63–80. [Google Scholar]
- 406.SHOPS Project ZanaAfrica: empowering women and girls to improve reproductive health grantee at a glance. 2015. https://www.hanshep.org/member-area/programmes/hanshep-health-enterprise-fund/zanaafrica-grantee-profile.pdf Available at: Accessed July 24, 2019.
- 407.Vora S. The experiences of menstruation by homeless women: a preliminary report Breaking taboos around menstruation and sanitation. Empowering Women. 2016. https://menstrualhygieneday.org/wp-content/uploads/2017/01/Homeless-Menstruation-Report.pdf Available at: Accessed July 24, 2019.
- 408.Hinckley S. 2018. California keeps girls in school by providing feminine products. [Google Scholar]
- 409.Evans T., Smith W., Themistocles D. Periods, poverty, and the need for policy: A Report on Menstrual Inequity in the United States. 2018. http://www.law.udc.edu/page/LegislationClinic Available at: Accessed July 24, 2019.
- 410.Sebert Kuhlmann A., Peters Bergquist E., Danjoint D., Wall L.L. Unmet menstrual hygiene needs Among low-income women. Obstet Gynecol. 2019;133:238–244. doi: 10.1097/AOG.0000000000003060. [DOI] [PubMed] [Google Scholar]
- 411.Weinstock L. Empowering women and girls by investing in menstrual health. 2019. https://www.grandchallenges.ca/2016/empowering-women-girls-investing-menstrual-health Available at:
- 412.The case for her The case for menstruation. 2019. http://www.thecaseforher.com/women-deliver-the-case-for-her Available at:
- 413.Wilbur J., Torondel B., Hameed S., Mahon T., Kuper H. Systematic review of menstrual hygiene management requirements, its barriers and strategies for disabled people. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Sommer M., Zulaika G., Schmitt M., Gruer C. Monitoring menstrual health and hygiene: measuring progress for girls on menstruation. 2019. https://www.mailman.columbia.edu/sites/default/files/green_paper_monitoring_menstrual_health_and_hygiene.pdf Available at: Accessed May 15, 2019.
- 415.Hennegan J., Shannon A.K., Rubli J., Schwab K.J., Melendez-Torres G.J. Women’s and girls’ experiences of menstruation in low- and middle-income countries: A systematic review and qualitative metasynthesis. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.van Eijk A.M., Zulaika G., Lenchner M. Menstrual cup use, leakage, acceptability, safety, and availability: a systematic review and meta-analysis. Lancet Public Health. 2019;4:e376–e393. doi: 10.1016/S2468-2667(19)30111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Iris Group WASHPaLS. What we do 2019. https://www.irisgroupinternational.com/washpals Available at:
- 418.Performance Monitoring and Accountability, Johns Hopkins University. Menstrual hygiene management—Kaduna State, Nigeria 2015. Baltimore 2015; 2020. https://www.pma2020.org/sites/default/files/MHM_Brief_Nigeria-Kaduna_050818-Final.pdf Available at: Accessed May 15, 2019.
- 419.Performance Monitoring and Accountability, Johns Hopkins University Menstrual hygiene management—Kenya 2017. Baltimore 2017; 2020. https://www.pma2020.org/mhm-briefs Available at: Accessed May 15, 2019.
- 420.Performance Monitoring and Accountability, Johns Hopkins University Menstrual hygiene management—Uganda. 2020. https://www.pma2020.org/mhm-briefs Available at: Accessed May 15, 2019.
- 421.United Nations Children’s Emergency Fund Multiple indicator cluster surveys; 2019. http://mics.unicef.org/ Available at: Accessed May 15, 2019.
- 422.Clatworthy D., Schmitt M. Integrating MHM into Humanitarian Response: an introduction to the toolkit resource; 2019. https://rescue.webex.com/recordingservice/sites/rescue/recording/playback/15af4e429fea4ec0bc914544aed67bee Available at: Accessed May 15, 2019.
- 423.Uganda National Bureau of Standards Draft Uganda standard: reusable sanitary towels—specification. Kampala 2017. https://members.wto.org/crnattachments/2017/TBT/UGA/17_3724_00_e.pdf Available at: Accessed May 15, 2019.
- 424.Sommer M., Figueroa C., Kwauk C., Jones M., Fyles N. Attention to menstrual hygiene management in schools: an analysis of education policy documents in low- and middle-income countries. Int J Educ Dev. 2017;57:73–82. [Google Scholar]
- 425.Sommer M., Schmitt M., Yamakoshi B., Serrano M. WASH in Schools Empowers Girls’ Education. http://www.unicef.org/wash/schools. Proceedings of the 6th annual virtual conference on Menstrual Hygiene Management in Schools Available at: 2017. Accessed May 15, 2019.