ABSTRACT
Background:
Recent reports on catheter ablation for premature ventricular complex (PVC) or ventricular tachycardia in the context of cardiomyopathy suggest that ablation can improve cardiac function and decrease the number of PVCs. However, reports on exercise tolerance after catheter ablation for PVC are few.
Case:
A 56-year-old woman consulted her primary care doctor presenting with palpitations and fatigue on exertion. Her left ventricular systolic function had been normalized with medications after a diagnosis of dilated cardiomyopathy 5 years previously. Electrocardiography showed sinus rhythm and ventricular bigeminy. Holter electrocardiography revealed a total of 34,867 PVCs. The highest number of consecutive PVCs recorded was three. In the cardiopulmonary exercise test, the peak oxygen consumption (VO2) was markedly reduced to 14 ml/kg/min. The patient was referred to our hospital for catheter ablation because pharmacotherapy was ineffective. PVCs originated from the left ventricular outflow tract and were successfully eliminated by ablation at the non-coronary cusp of the aortic valve using three-dimensional activation mapping with a CARTO system. The patient’s symptoms on exertion improved immediately after ablation. Postoperative Holter electrocardiography revealed that the number of PVCs had decreased to one per day. Peak VO2 had markedly improved to 22 ml/kg/min 2 months after catheter ablation therapy.
Discussion:
The elimination of frequent PVCs contributed to improved exercise tolerance.
Keywords: cardiomyopathy, left ventricular dysfunction, peak oxygen consumption, cardiopulmonary exercise
INTRODUCTION
Frequent isolated premature ventricular complexes (PVCs) can cause cardiac systolic dysfunction and PVC-induced cardiomyopathy.1) Recent reports on ablation treatment for PVC or ventricular tachycardia (VT) in patients with cardiomyopathy suggest that ablation can improve cardiac function and decrease the frequency of PVCs.2) However, reports on exercise tolerance after catheter ablation (CA) for PVC are few. This case report describes a patient with dilated cardiomyopathy who demonstrated improvement in exercise tolerance after CA for PVC.
CASE
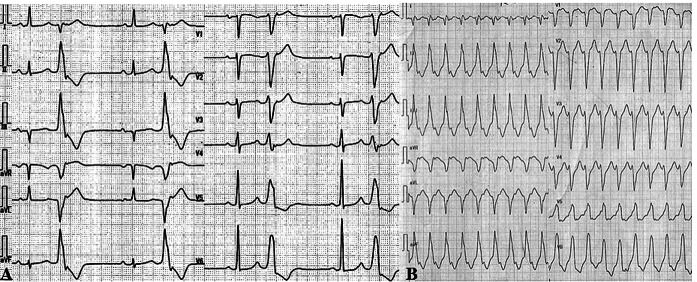
A 56-year-old woman had been admitted for the treatment of dilated cardiomyopathy 5 years previously, and 2.5 mg/day of bisoprolol was administered. Bisoprolol was recently discontinued in this patient because of normalization of the left ventricular ejection fraction (LVEF) (65%) with no recorded arrhythmic events. One month after bisoprolol cessation, the patient complained of palpitations and dyspnea on exertion and consulted a primary care doctor. Electrocardiography (ECG) revealed sinus rhythm and ventricular bigeminy (left bundle branch block pattern and inferior axis morphology) (Fig. 1A).
Fig. 1.
(A) Electrocardiograph shows sinus rhythm and PVC bigeminy. PVC shows complete left bundle branch block and inferior-axis morphology. (B) Medications were discontinued for an electrophysiological study, and ventricular tachycardia was induced. The morphology was similar to clinical PVC.
The patient was admitted to hospital for medical management. Despite the administration of flecainide 100 mg/day and titration of bisoprolol up to 3.75 mg/day, the number of PVCs did not decrease.
Physical examination and laboratory test results were normal, except for brain natriuretic peptide levels (BNP; 23.6 pg/ml). ECG revealed left ventricular systolic dysfunction (using the biplane Simpson’s method, LVEF was 44% with a left ventricular end-diastolic volume [LVEDV] of 128 ml and a left ventricular end-systolic volume [LVESV] of 72 ml) (Table 1). In the Holter electrocardiogram, the number of PVCs was 34,867 (40.4% of the total heart beats) and no sustained VT (sVT) was recorded. PVCs continued to appear daily. However, a cardiopulmonary exercise test (CPET) revealed that peak oxygen consumption was reduced to 14 ml/kg/min (Table 2). During CPET, the number of PVCs decreased during increased workload and disappeared with a load ≥54 W. Nevertheless, following exercise cessation, PVCs began appearing, initially as trigeminy and subsequently as bigeminy. The CPET end point was determined by symptoms such as leg fatigue.
Table 1. Follow-up echocardiography and laboratory data.
| Before ablation | 3 months after ablation | 6 months after ablation | |
| LVDd/LVDs (mm) | 53/37 | 52/39 | 49/35 |
| LVEF (%) | 44 | 47 | 51 |
| LVEDV/LVESV (ml) | 128/72 | 121/67 | 83/41 |
| LAD (mm) | 34 | 30 | 32 |
| LAVi (ml/m2) | 53 | 40 | |
| E/e’ ratio | 17.9 | 17.8 | 16.7 |
| MR | Moderate | Moderate | Mild |
| BNP (pg/ml) | 23.6 | 26.8 | 5.8 |
LAD, left atrium dimension; LAVi, left atrium volume index; LVDd, left ventricular diastolic dimension; LVDs, left ventricular systolic dimension; MR, mitral regurgitation.
Table 2. Follow-up data of cardiopulmonary exercise test .
| Before ablation | 2 months after ablation | 6 months after ablation | |||||||
| Rest | AT | Peak | Rest | AT | Peak | Rest | AT | Peak | |
| VO2 (ml) | 208 | 696 | 911 | 218 | 700 | 1415 | 273 | 846 | 1340 |
| VO2 weight correction value (ml/kg/min) | 3.2 | 10.7 | 14.0 | 3.4 | 12.2 | 22.2 | 4.0 | 11.4 | 19.7 |
| Respiratory exchange ratio | 0.81 | 0.99 | 1.09 | 0.9 | 0.9 | 1.11 | 0.86 | 0.9 | 1.03 |
| HR (bpm) | 60 | 73 | 81 | 55 | 77 | 104 | 61 | 86 | 110 |
| BP (mmHg) | 91/43 | - | - | 130/61 | 148/68 | 156/65 | 127/84 | 138/81 | 148/88 |
| Mets | 0.91 | 3.06 | 4 | 0.98 | 3.48 | 6.36 | 1.15 | 3.56 | 5.63 |
| Load (W) | 0 | 53 | 83 | 0 | 44 | 92 | 0 | 45 | 86 |
| VE vs. VCO2 slope | 33.0 | 27.6 | 28.3 | ||||||
| Peak VO2/HR | 11.3 | 13.6 | 12.2 | ||||||
AT, anaerobic threshold; BP, blood pressure; HR, heart rate; VE vs. VCO2, ventilatory equivalent versus carbon dioxide.
Before the planned CA procedure to treat PVC, medications were discontinued. In an electrophysiological study, sVT was induced for the first time (Figure 1B). The QRS morphology was similar to that of clinically documented PVCs. The patient was conscious during sVT. Three-dimensional mapping for PVC was performed using the CARTO system (Biosense Webster, Diamond Bar, CA, USA). Radiofrequency CA was applied to the left ventricular outflow and was delivered around the supra-noncoronary cusp of the aortic valve, where deflection of the local potential preceded the QRS wave of PVC by 30 ms; PVCs were eliminated within a few seconds after energy delivery. VT and PVC were not inducible by electrical stimulation after intravenous infusion of isoproterenol. Bisoprolol 3.75 mg/day was continued after CA. The patient’s symptoms on exertion improved shortly after the CA session.
No PVCs were observed on the Holter electrocardiogram recorded 1 month after the CA procedure. CPET showed a marked improvement in peak oxygen consumption to 22 ml/kg/min (Table 2) 3 months after the CA procedure, with no significant change in LVEF (47%), LVEDV (121 ml), or LVESV (67 ml) (Table 1). It was surmised that PVC had aggravated the patient’s reduced exercise tolerance.
The patient provided written informed consent for the treatment and publication of this report.
DISCUSSION
PVC-induced cardiomyopathy is an established cause of systolic dysfunction. However, few reports on the relationships between frequent PVCs and reduced exercise tolerance have been published.
In the current patient, the frequency of PVCs was >30/hour. Niwano et al.3) reported that frequent PVCs are related to decreased LVEF and an enlarged left ventricle; therefore, the frequency of PVCs is an important prognostic factor. Peak oxygen consumption (VO2)/heart rate, which is an index of stroke volume, improved after CA, and the increase in maximum heart rate after the disappearance of PVCs contributed to improvement in the peak oxygen consumption.
LVEF was mildly reduced, and exercise tolerance was markedly impaired in this patient. The peak oxygen consumption during the CPET improved markedly from 14 ml/kg/min (59%) to 22 ml/kg/min (93%) 2 months after ablation.
Exercise tolerance is determined not only by cardiac function but also by skeletal muscle and lung function. However, it is unlikely that skeletal muscle or pulmonary function contributed to the rapid improvement in peak oxygen consumption in the current case. Insufficient BNP elevation and relevant physical signs suggested that heart failure was not primarily responsible for the impairment of exercise tolerance.
In terms of LV diastolic function, elevation of the left ventricular end-diastolic pressure was also a factor associated with exercise intolerance, because E/e’ showed a moderately high level throughout the clinical course. However, diastolic function is unlikely to have contributed to the improvement of exercise capacity at 2 months because there was no improvement in E/e’.
The negative inotropic effect of flecainide prescribed to treat frequent PVCs may have contributed to exercise intolerance in this patient. A previous report described the negative inotropic effects of flecainide, which showed no significant change at 2 or 4 mg/kg compared with a control group, but showed a significant negative inotropic effect at 8 mg/kg.4) The QRS wave width did not change during flecainide administration, and it remained unchanged 1 month after cessation (both 100 ms). The adverse effects of flecainide may have been less significant than those of frequent PVCs, because the patient was administered a low dose of flecainide. Increases in the peak heart rate also contributed to the improvement of peak oxygen consumption; this increase resulted from the discontinuation of flecainide, which has a negative chronotropic effect.
Non-ischemic cardiomyopathy is reportedly associated with increased risk of PVC recurrence.5) Serial follow-ups would be necessary to confirm whether this applies in the present case.
We often experience patients with frequent PVCs, and their cardiac function is taken into account when considering CA; however, there are no standard guidelines on exercise tolerance in this context. This case suggested that frequent PVCs may affect exercise tolerance.
Footnotes
CONFLICTS OF INTEREST: The authors have no conflicts of interest to declare for this article.
REFERENCES
- 1.Chugh SS,Shen WK,Luria DM,Smith HC: First evidence of premature ventricular complex-induced cardiomyopathy: a potentially reversible cause of heart failure. J Cardiovasc Electrophysiol 2000;11:328–329. 10.1111/j.1540-8167.2000.tb01802.x [DOI] [PubMed] [Google Scholar]
- 2.Takemoto M,Yoshimura H,Ohba Y,Matsumoto Y,Yamamoto U,Mohri M,Yamamoto H,Origuchi H: Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol 2005;45:1259–1265. 10.1016/j.jacc.2004.12.073 [DOI] [PubMed] [Google Scholar]
- 3.Niwano S,Wakisaka Y,Niwano H,Fukaya H,Kurokawa S,Kiryu M,Hatakeyama Y,Izumi T: Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009;95:1230–1237. 10.1136/hrt.2008.159558 [DOI] [PubMed] [Google Scholar]
- 4.Hoffmeister HM,Hepp A,Seipel L: Negative inotropic effect of class-I-antiarrhythmic drugs: comparison of flecainide with disopyramide and quinidine. Eur Heart J 1987;8:1126–1132. 10.1093/oxfordjournals.eurheartj.a062178 [DOI] [PubMed] [Google Scholar]
- 5.Lee D,Hoffmayer KS,Hsu JC,Schricker A,Birgersdotter-Green U,Raissi F,Feld GK,Krummen DE: Long-term mode and timing of premature ventricular complex recurrence following successful catheter ablation. J Interv Card Electrophysiol 2019;55:153–160. 10.1007/s10840-019-00520-3 [DOI] [PubMed] [Google Scholar]