Abstract
Background
Monoclonal non-specific suppressor factor β (MNSFβ) is a ubiquitously expressed member of the ubiquitin-like family. It functions as a regulator of cell apoptosis and a potential tumor suppressor, playing a vital role in the processes of immune cell function and apoptosis.
Methods
The present study constructed GFP-pMNSFβ swine umbilical vein endothelial cell (SUVEC) lines and investigated the function of porcine MNSFβ (pMNSFβ) in apoptosis, as well as its interactions with pBCL-G. Results revealed that stably expressed pMNSFβ protein in SUVEC lines significantly enhanced staurosporine (STS)-induced apoptosis. pMNSFβ proteins interacted with pBCL-G proteins and the expression of these interacting proteins synergized to further enhance STS-induced apoptosis.
Results
GFP-pMNSFβ stably expressed SUVEC lines through transient transfection and neomycin screening methods. Over 90% of the SUVEC cultures expressed GFP signals, and 41.5 kDa GFP-pMNSFβ proteins were detected with western blotting methods. Annexin V-PE/PI staining and flow cytometry analyses showed that overexpression of pMNSFβ proteins significantly elevated STS-induced apoptosis rates. Co-immunoprecipitation methods revealed an interaction between pMNSFβ and pBCL-G proteins. BCL-G is a proapoptotic member of the BCL-2 family that has been shown to be misexpressed in human systemic lupus erythematosus, as well as mammary and prostate cancers. Here, we demonstrated that the co-expression and potential conjugation of pMNSFβ and pBCL-G proteins synergistically enhanced STS-induced apoptosis.
Conclusions
The present study was the first to characterize the function of MNSFβ in porcine cells, and to clarify the function of MNSFβ in apoptosis. These results reveal that pMNSFβ is a potential molecular model for future investigations of diseases related to human MNSFβ dysfunction.
Keywords: Ubiquitin-like protein, porcine, BCL-G, swine umbilical vein endothelial cell (SUVEC), apoptosis
Introduction
Monoclonal non-specific suppressor factor β (MNSFβ) is synthesized by T cells and is involved in various biological processes (1). MNSFβ is highly conserved, sharing a 91% homology and <0.05 evolutionary distance across 18 common species (2). The gene is approximately 414 bp in length, and contains one exon encoding a 133-amino-acid product (3,4). Structurally, MNSFβ protein is a fusion protein consisting of an ubiquitin-like domain fused with the ribosomal protein S30 at its terminus (5,6).
MNSFβ has been demonstrated to function as a non-specific suppressor of lymphokine in immune responses, and also participates in regulating eukaryotic processes, such as stress reaction, cell division, cell apoptosis, and nuclear transport (7-10). Other studies have shown that MNSFβ binds to specific lysine residues of target proteins. One well-characterized MNSFβ target is BCL-G, a pro-apoptotic member of the BCL-2 family, that was shown to regulate the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) signal cascade in mouse macrophage (Raw264.7) cells lines (11). Given the important roles of MNSFβ proteins, it has been studied extensively in human and mouse models (5,12). However, porcine models may better serve the scientific research community because, compared to mice, they are more similar to humans in both physiology and anatomy (13-15). Thus far, the physiological significance of porcine MNSFβ (pMNSFβ) activity in porcine models has remained elusive. Thus, studies characterizing pMNSFβ gene products are critical for understanding the role of MNSFβ in human diseases.
In this study, we constructed GFP-pMNSFβ swine umbilical vein endothelial cell (SUVEC) lines and investigated the function of pMNSFβ in apoptosis, as well as its interactions with porcine BCL-G (pBCL-G). Results revealed that stably expressed pMNSFβ protein in SUVEC lines significantly enhanced staurosporine (STS)-induced apoptosis. pMNSFβ proteins interacted with pBCL-G proteins and the expression of these interacting proteins synergized to further enhance STS-induced apoptosis. In this study, we characterized pMNSFβ, and our data suggested that MNSFβ is a potential new target for disease intervention. Additionally, results from this study can be added to the growing body of literature exploring the role of human MNSFβ in related diseases. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/atm-20-6348).
Methods
Materials
Staurosporine was purchased from Sigma-Aldrich (St. Louis, MO, USA). Mouse monoclonal antibodies against HA and myc were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA). Horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary antibodies were obtained from Sigma-Aldrich. Both rabbit polyclonal GFP and horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibodies were obtained from CWBiotech (Beijing, China). All experiments were conducted in compliance with current Chinese ethical legislation.
Reverse transcription-polymerase chain reaction (RT-PCR)
pMNSFβ and pBCL-G genes were cloned from porcine spleen tissue by RT-PCR. The primers used to detect pMNSFβ were as follows: PF1: 5'-AGAGATCTATGCAGCTCTTTGTCCG-3' (underlining indicates the BglII site); PR1: 5'-TAGGGCCCTTAAGAGTTGGCATT-3' (underlining indicates the ApaI site). The primer for pBCL-G were published by Jiang et al. (16). All gene products (nucleic acid and proteins) were analyzed to verify their identities with DNAStar and Blast (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Plasmid constructions and transfections
The pMNSFβ gene sequence was subcloned into the pCMV-HA and pEGFP-c1 vector to construct the recombinant plasmid pCMV-HA-pMNSFβ and pEGFP-MNSFβ. pCMV-myc-pBCL-G recombinant constructs were previously produced in our laboratory (16). SUVEC lines were maintained in six-well plates in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) at 37 °C with 5% CO2. When cell densities neared 80%, mediums were changed to pure DMEM, incubated for 1 hour (hr), and then DNA transfection was performed with 4 µg of the recombinant plasmids per well using Lipofectamine 2000 (Invitrogen, US) according to the manufacturer’s protocols.
Establishment of SUVEC lines stably expressing GFP-pMNSFβ proteins
Twenty-four hours after transfection of pEGFP-pMNSFβ in SUVEC lines, the culture media were changed to growth medium supplemented with 800 µg/mL G418. After 72 hr incubation, cells were inspected for GFP fluorescence, and the expressing cultures were trypsin (0.25%) digested and transferred to 10 cm petri dishes at a density of 5×105. Every 2 days, fresh culture medium containing G418 was added. After 10–14 days of incubation, drug-resistance clonal cell populations were observed under fluorescence microscopes. Any cell populations that did not express GFP and were located near the expressing clones were removed with a 10 µL pipette tip. Incubation of the clonal cultures in G418 medium continued until the fluorescent clone density reached up to 50%. Fluorescence-positive clones were then transferred to 24-well plates and expanded. The cloned colonies were additionally subjected to single-cell cloning using limiting dilution methods. Finally, the clonal population exhibiting the highest fluorescence expression was selected from the first and second round of cloning for evaluation.
Immunofluorescence (IF) assays
To detect the expression of HA-tagged pMNSFβ (HA-pMNSFβ), SUVEC lines were seeded into 6-well plates and transfected with pCMV-HA-pMNSFβ plasmids, using the methodologies described above. Approximately 24 hr after transfection, the cells were fixed with 4% paraformaldehyde for 15 minutes (min) at room temperature and permeabilized with 0.2% Triton X-100/PBS for 5 min. Next, cells were incubated with mouse anti-HA monoclonal antibodies (1:1,000) for 1 hr at room temperature, followed by incubation with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobin G (IgG) secondary antibodies (Sigma-Aldrich, 1:100 dilution) for 1 hr at room temperature. After being rinsed with phosphate-buffered saline (PBS), cells were observed under a fluorescence microscope.
Western blot analyses
Whole-cell lysates were generated using lysis buffer (50 mM Tris-HCl, 5 mM EDTA150 mM NaCl, 0.1% NP-40, 0.5% deoxycholic acid, 1 mM sodium orthovanadate, 100 µg/mL PMSF) and protease inhibitors (20 µg/mL leupeptin, 10 µg/mL pepstatin A and 10 µg/mL aprotinin). Protein concentrations of cell lysates were determined with BCA Protein Assay Kits (Pierce, USA). Equal amounts of lysates were boiled in sodium dodecyl sulfate (SDS) sample buffer for 10 min, proteins were separated by SDS-polyacrylamide gels, and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, USA). Membranes were incubated overnight at 4 °C in a tris-buffered saline solution containing 5% milk to block nonspecific binding sites, followed by HRP-conjugated secondary antibodies at room temperature for 1 hr. Protein bands were visualized using Luminata Classico Western HRP Substrate according to the manufacturer’s instructions (Millipore, USA).
Co-immunoprecipitations
Co-immunoprecipitations were performed using Mammalian c-Myc Tag IP/Co-IP Kits (Pierce) according to the manufacturer’s instructions. Briefly, SUVEC lines were transiently transfected with a mixture of pCMV-myc-pBCL-G and pCMV-HA-pMNSFβ vectors, while control experiments were transfected with either a mixture of pCMV-myc-pBCL-G and pCMV-HA empty vectors, or a mixture of pCMV-HA-pMNSFβ and pCMV-myc empty vectors. Thirty-six hours post-transfection, proteins were extracted using Mammalian Protein Extraction Reagent (MPER). Samples were then incubated overnight at 4 °C in the presence of 10 µL of anti-c-Myc agarose slurry. The agarose slurries were washed three times with Tris Buffered Saline plus 0.05% Tween-20, and immunoprecipitates were released from the agarose slurries by boiling in 25 µL of non-reducing sample buffer for 5 min. Western blots were carried out with anti-HA or anti-myc antibodies to detect binding partners.
Statistical analyses
Annexin V-PE/PI staining and flow cytometry method (FCM) was used to discern between apoptotic and necrotic cells death, according to the instructions. Expressions of stained cells were analyzed using double flow cytometry parameters. Resulting data were analyzed with LMD and Motilcycle software packages. Calculations of means, standard deviations (SD), and all statistical analyses were performed with SPSS (version 13.0). Differences between test and control groups were assessed using independent samples t-tests, with P values <0.05 considered statistically significant and <0.01 considered highly statistically significant.
Results
Transfection of GFP-pMNSFβ protein was stably expressed in SUVEC lines
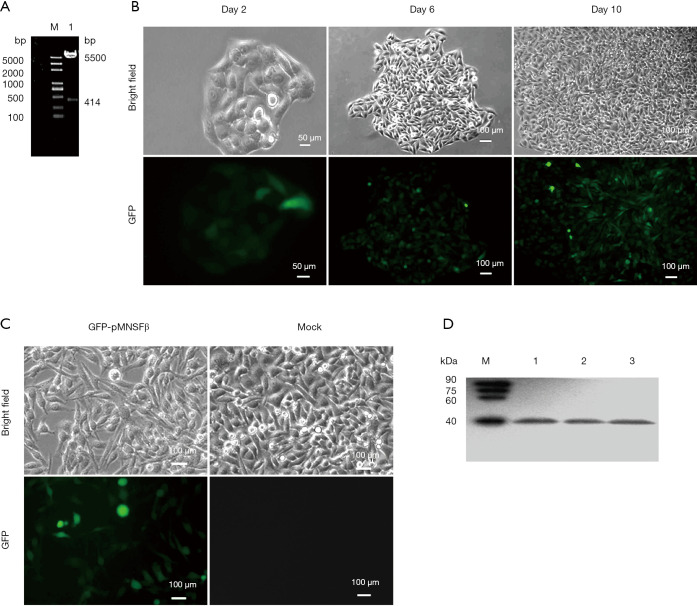
GFP-pMNSFβ that highly expressed the clonal cell line was obtained using limiting dilution methods and G418 selection in SUVEC lines that had been transfected with the successfully constructed plasmid, pEGFP-pMNSFβ, which was verified by sequencing and digestion (Figure 1A). The cloned cells expressing GFP-PMNSF protein obtained by limiting dilution continued to proliferate steadily under the treatment of G418. Figure 1B shows the cell and GFP-pMNSFβ protein condition during continuous cell proliferation. As shown in Figure 1C, clonal cell lines stably expressing GFP-pMNSFβ proteins were successfully sub-cultured for more than 40 passages during a 4-month period. The expression of GFP-pMNSFβ proteins was analyzed by western blot at passages of 5, 10, and 40 (Figure 1D).
Figure 1.
The expression of GFP-pMNSFβ protein in SUVEC cells was detected. (A) The products of pEGFP-MNSFβ was digested by ApaIand BglII. (B) GFP-pMNSFβ protein expression in clonal cell line under G418 selection after 2, 4, and 10 days of incubation observed by inverted fluorescence microscopy. (C) Expression of GFP-pMNSFβ in clonal cells at passage 40. (D) Western blot analyses of GFP-pMNSFβ fusion protein expression levels in stably expressing GFP-pMNSFβ SUVEC lines using anti-GFP tag antibodies. Lanes 1, 2, and 3 represent 5, 10, and 40 passages, respectively. GFP, green fluorescent protein; pMNSFβ, porcine monoclonal non-specific suppressor factor β; EGFP, enhanced green fluorescent protein.
pMNSFβ overexpression enhanced STS-induced apoptosis in SUVEC lines
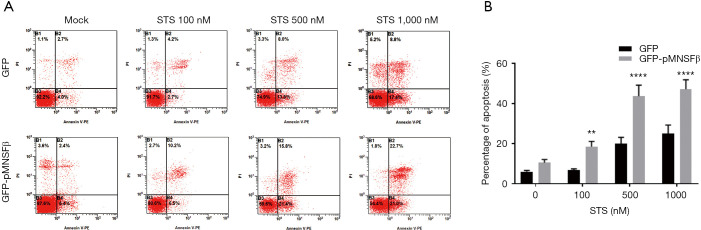
We explored the role of pMNSFβ in apoptotic pathways. Apoptosis rates were quantified in stable GFP-pMNSFβ SUVEC lines induced to undergo apoptosis by exposure to STS (concentrations: 0, 100, 500, and 1,000 nM). Annexin V-PE/PI staining in combination with FCM were used to discern between apoptotic and necrotic cells. Early apoptotic events were visualized in the lower right quadrant of FCM diagrams, whereas late apoptotic events were found in the upper right quadrant (Figure 2A). Statistical analyses of apoptosis rates, as calculated by FCM, clearly demonstrated that overexpression of pMNSFβ significantly enhanced apoptosis compared to controls (Figure 2B). These results suggest that overexpressed pMNSFβ may have a similar role in apoptosis as pBCL-G, a canonical proapoptotic factor.
Figure 2.
Quantification of apoptosis was enhanced by overexpression of GFP-pMNSFβ. (A) Representative FCM diagrams of SUVEC lines stably expressing GFP-vector or GFP-pMNSFβ were treated for 18 hrs with the STS doses indicated on the x-axis. (B) Annexin V-positive cells were quantified. All experiments were performed in triplicate, and data are expressed as mean ± SD (n=3). Error bars represent standard deviations of replicate data points. **, P<0.01 and ****, P<0.0001 versus the control groups (cells stably expressing GFP-vector proteins). FCM, flow cytometry method; SUVEC, swine umbilical vein endothelial cell; STS, staurosporine.
pMNSFβ co-immunoprecipitate with pBCL-G
In our studies, MNSFβ proteins were demonstrated to bind with BCL-G proteins. Specifically, residues in the MNSFβ n-terminal region physically interacted with BCL-GL in humans cell lines. Previous findings indicated porcine BCL-G to be highly conserved and structurally similar to human BCL-GL, with 71% identity between species (2). In the present study, co-immunoprecipitations were performed to determine whether pMNSFβ interacts with pBCL-G. HA tags fused to pMNSFβ and myc-BCL-G proteins were detected using western blotting techniques (Figure 3). Additionally, HA-pMNSFβ and myc-pBCL-G were overexpressed in SUVEC lines, and expression patterns were observed by indirect IF and western blotting analyses. FITC-conjugated secondary antibody signals were detected in cells expressing either myc-pBCL-G or HA-pMNSFβ proteins above background expression in parent SUVEC lines (data not shown). Furthermore, when myc-pBCL-G and HA-pMNSFβ were overexpressed in SUVEC cells, and lysates were pulled down with myc beads, HA-pMNSFβ could be detected by western blotting analyses. Conversely, HA-pMNSFβ was not detected in the immunoprecipitated product when myc-pBCL-G and HA-tag control vectors were co-expressed. Similarly, co-expressions of myc-tag empty vectors and HA-pMNSFβ yielded negative results by myc pull down methods (Figure 3). These results indicated that overexpressed pMNSFβ proteins indirectly interacted with pBCL-G proteins.
Figure 3.
Interaction of HA-pMNSFβ with myc-pBCL-G was detected by co-immunoprecipitation. (A) Detection of HA-pMNSFβ and myc-pBCL-G fusion protein expressed in cells by western blot; line 1, 2, and 3 represent SUVEC cells transiently transfected with pCMV-HA-pMNSFβ plasmid, SUVEC cells transiently transfected with pCMV-myc-pBCL-G plasmid, and SUVEC cells, respectively. (B) The individual and co-expressions of HA-pMNSFβ and myc-pBCL-G proteins in transfected SUVEC cells were examined by western blot analyses. HA, HA-tagged.
Co-expression of pMNSFβ and pBCL-G significantly enhanced STS-induced apoptosis
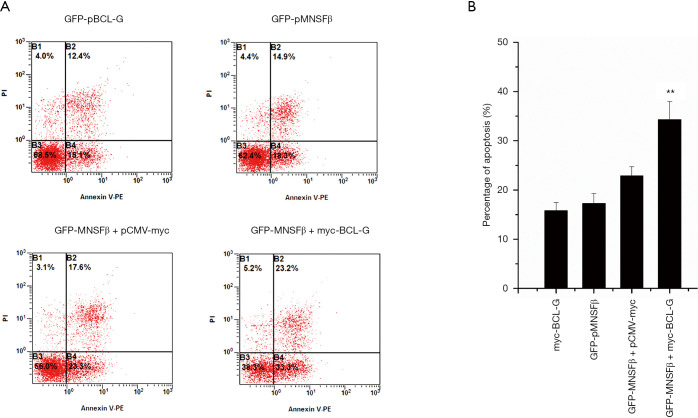
Our previous studies demonstrated that pBCL-G overexpression enhanced STS-induced apoptosis (16). Given that pBCL-G and pMNSFβ proteins indirectly interacted, we explored whether STS-induced apoptosis would be influenced by co-transfection of pMNSFβ and pBCL-G plasmids. Our previous work demonstrated that early apoptotic events induced by 500–1,000 nM STS were significantly increased when cells stably expressed GFP-pBCL-G (16). SUVEC cells stably expressing GFP-pBCL-G were transfected with pCMV-HA-pMNSFβ or pCMV-HA empty vectors. Twenty-four hours after transfection, cells were treated with 500 nM STS and incubated for 18 hr. Using the same FCM methods as described above, cell apoptosis was detected and quantified. Apoptosis rates were significantly elevated in cells overexpressing pBCL-G and pMNSFβ, when compared with cells overexpressing pBCL-G and the empty vector alone (Figure 4).
Figure 4.
GFP-pMNSFβ and myc-pBCL-G co-overexpression enhanced STS-induced apoptosis rates. Representative FACS diagrams were given for STS-treated (500 nM) SUVEC lines that stably expressed GFP-pMNSFβ and were then co-transfected with either myc-pBCL-G or empty vectors. Parent SUVEC lines either transiently expressed myc-BCL-G proteins, or stably expressed GFP-pMNSFβ and were treated with STS (500 nM) and analyzed as controls. (B) Quantification of Annexin V-positive cells: data, gathered in triplicate, are expressed as the mean ± SD. Cells co-expressing GFP-pMNSFβ and myc-BCL-G had significantly more Annexin V-positive events than controls (**, P<0.01). FACS, fluorescence-activated cell sorting.
Discussion
In this study, stably expressing GFP-pMNSFβ SUVEC lines provided useful and convenient molecular tools. Previous studies have demonstrated MNSFβ to be a natural immunosuppressive factor that is involved in various biological processes (17-19). Furthermore, MNSFβ was found to bind to distinct lysine residues of specific target proteins, including BCL-G; this binding inhibited activation of ERK proteins, which regulate MAPK pathways (5,11). MNSFβ has also been shown to conjugate with endophilin II and to regulate phagocytosis (1,8); it is also known to play a vital role in immune regulation and apoptosis, but until now, studies on MNSFβ function in porcine models have been scant and unclear. Porcine model systems have close phylogenetic relationships and physiological similarities with humans (15), so porcine systems have often been considered preferable to mice for modeling human conditions. In this study, we constructed stable SUVEC lines that overexpressed GFP-pMNSFβ proteins. These lines are now available for use as convenient molecular tools for future experimental studies of pMNSFβ functions.
Studies of MNSFβ in porcine cell lines offer potential molecular models for investigations of human MNSFβ-related diseases. In humans, Watanabe et al. found that MNSFβ and BCL-G proteins interacted; these binding events enhanced lipopolysaccharide (LPS)/interferon c-induced apoptosis in macrophages (20). This study showed that overexpression of GFP-pMNSFβ had proapoptotic effects on SUVEC lines in which apoptosis was induced by varying doses of STS. Together, these previous results and our current study suggest that pMNSFβ and human MNSFβ serve similar functions. Human and porcine MNSFβ share 93.98% protein sequence similarities (2). Furthermore, porcine models have been used to study many human conditions including sepsis (21), abdominal aortic aneurysm (22), severe tension pneumothorax (23), and a variety of cancers (24). Thus, porcine models overall, and, specifically, pMNSFβ-expressing SUVEC lines have potential as a tool for characterizing MNSFβ-related diseases and testing drug targets in humans.
The results presented here suggest that pMNSFβ is an apoptosis regulatory factor and potential tumor suppressor. Watanabe et al. demonstrated that human MNSFβ promotes LPS/interferon γ (IFNγ)-induced apoptosis of Raw264.7 macrophages (20). Statistical analyses of our flow cytometry results demonstrated that overexpression of pMNSFβ had proapoptotic functions, as it significantly enhanced STS-induced apoptosis in SUVEC lines. This is consistent with previous results. It has been previously reported that overexpression of pBCL-G amplifies STS-induced apoptosis in SUVEC lines (16). Taken together, pMNSFβ and pBCL-G proteins may similarly function to regulate SUVEC apoptosis. Therefore, pMNSFβ gene products may have a new role in the apoptotic regulation and potential tumor suppression of porcine umbilical vein endothelial cells.
Exploration of the specific mechanisms of pMNSFβ and pBcl-G activities may offer a drastically new direction for the treatment of human diseases. BCL-G and MNSFβ proteins have been demonstrated to interact (20), while BCL-G was shown to regulate apoptosis due to chemotherapy and was found to be misexpressed in systemic lupus erythematosus and congenital heart disease cases (25-28). In this study, we also found that pMNSFβ interacted with pBCL-G, and co-overexpression synergistically enhanced STS-induced cell apoptosis rates, which was consist with previous studies in humans (20). The present study suggested that pMNSFβ-pBCL-G complex formation may be important for apoptosis regulation. Future studies exploring specific mechanisms of interaction between MNSFβ and BCL-G could provide insights and potential treatment options for BCL-G–related human diseases.
In this study, we constructed stably expressing GFP-pMNSFβ SUVEC lines and stored them in our laboratory. These cell lines were useful and powerful tools for comparative function experiments. We also found that overexpression of pMNSFβ proteins significantly enhanced STS-induced apoptosis rates in SUVEC lines, which was consist with previous foundational studies in humans on MNSFβ function. Additionally, pMNSFβ co-immunoprecipitated with pBCL-G, along with overexpression of pMNSFβ, significantly promoted pBCL-G-induced cell apoptosis. Therefore, pMNSFβ may be a novel drug target for BCL-G-related human diseases, such as cell apoptosis caused by chemotherapy for cancer, and for systemic lupus erythematosus and congenital heart diseases.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All experiments were conducted in compliance with current Chinese ethical legislation.
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-6348
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-6348
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-6348). The authors have no conflicts of interest to declare.
(English Language Editor: J. Gray)
References
- 1.Nakamura M, Shimosaki S. The ubiquitin-like protein monoclonal nonspecific suppressor factor β conjugates to endophilin II and regulates phagocytosis. FEBS J 2009;276:6355-63. 10.1111/j.1742-4658.2009.07348.x [DOI] [PubMed] [Google Scholar]
- 2.Wang JN, Jiang P, Kang Z, et al. Cloning, eukaryotic expression and spatial expression patterns of porcine MNSFbeta. Yi Chuan 2013;35:1377-83. 10.3724/SP.J.1005.2013.01377 [DOI] [PubMed] [Google Scholar]
- 3.Nakamura M, Xavier RM, Tsunematsu T, et al. Molecular cloning and characterization of a cDNA encoding monoclonal nonspecific suppressor factor. Proc Natl Acad Sci U S A 1995;92:3463-7. 10.1073/pnas.92.8.3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kas K, Michiels L, Merregaert J. Genomic structure and expression of the human fau gene: encoding the ribosomal protein S30 fused to a ubiquitin-like protein. Biochem Biophys Res Commun 1992;187:927-33. 10.1016/0006-291X(92)91286-Y [DOI] [PubMed] [Google Scholar]
- 5.Nakamura M, Tanigawa Y. Characterization of ubiquitin-like polypeptide acceptor protein, a novel pro-apoptotic member of the Bcl2 family. Eur J Biochem 2003;270:4052-8. 10.1046/j.1432-1033.2003.03790.x [DOI] [PubMed] [Google Scholar]
- 6.Mahajan R, Delphin C, Guan T, et al. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 1997;88:97-107. 10.1016/S0092-8674(00)81862-0 [DOI] [PubMed] [Google Scholar]
- 7.Malakhov MP, Kim KI, Malakhova OA, et al. High-throughput Immunoblotting ubiquitin-like protein isg15 modifies key regulators of signal transduction. J Biol Chem 2003;278:16608-13. 10.1074/jbc.M208435200 [DOI] [PubMed] [Google Scholar]
- 8.Raasi S, Schmidtke G, Groettrup M. The ubiquitin-like protein FAT10 forms covalent conjugates and induces apoptosis. J Biol Chem 2001;276:35334-43. 10.1074/jbc.M105139200 [DOI] [PubMed] [Google Scholar]
- 9.Hemelaar J, Lelyveld VS, Kessler BM, et al. A single protease, Apg4B, is specific for the autophagy-related ubiquitin-like proteins GATE-16, MAP1-LC3, GABARAP, and Apg8L. J Biol Chem 2003;278:51841-50. 10.1074/jbc.M308762200 [DOI] [PubMed] [Google Scholar]
- 10.Marx J. Cell biology. SUMO wrestles its way to prominence in the cell. Science 2005;307:836. 10.1126/science.307.5711.836 [DOI] [PubMed] [Google Scholar]
- 11.Nakamura M, Yamaguchi S. The ubiquitin-like protein MNSFβ regulates ERK-MAPK cascade. J Biol Chem 2006;281:16861-9. 10.1074/jbc.M509907200 [DOI] [PubMed] [Google Scholar]
- 12.Nakamura M, Watanabe N, Notsu K. Ubiquitin-like protein MNSFβ covalently binds to cytosolic 10-formyltetrahydrofolate dehydrogenase and regulates thymocyte function. Biochem Biophys Res Commun 2015;464:1096-100. 10.1016/j.bbrc.2015.07.083 [DOI] [PubMed] [Google Scholar]
- 13.Codas R, Badet L, Eugene M, et al. editors. Evaluation of pulsatile perfusion machine RM3 for kidney preservation in a swine model of renal autotransplantation. Transplant Proc 2009;41:3296-8. 10.1016/j.transproceed.2009.08.045 [DOI] [PubMed] [Google Scholar]
- 14.Fodor WL, Williams BL, Matis LA, et al. Expression of a functional human complement inhibitor in a transgenic pig as a model for the prevention of xenogeneic hyperacute organ rejection. Proc Natl Acad Sci U S A 1994;91:11153-7. 10.1073/pnas.91.23.11153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozianka J, Kielan W, Waleczek H. Barium peritonitis-a study in pigs. Adv Clin Exp Med 2003;12:569-74. [Google Scholar]
- 16.Jiang P, Li D, Bi L, et al. BCL-G as a new candidate gene for immune responses in pigs: Bioinformatic analysis and functional characterization. Vet Immunol Immunopathol 2012;150:112-7. 10.1016/j.vetimm.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 17.Nakamura M, Tanigawa Y. Ubiquitin-like polypeptide conjugates to acceptor proteins in concanavalin A-and interferon γ-stimulated T-cells. Biochem J 1998;330:683-8. 10.1042/bj3300683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas AL, Ahrens P, Bright P, et al. Interferon induces a 15-kilodalton protein exhibiting marked homology to ubiquitin. J Biol Chem 1987;262:11315-23. [PubMed] [Google Scholar]
- 19.Raasi S, Schmidtke G, Do Giuli R, et al. A ubiquitin-like protein which is synergistically inducible by interferon- and tumor necrosis factor. Eur J Immunol 1999;29:4030-6. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe J, Nakagawa M, Nakamura M, et al. Ubiquitin-like protein MNSFβ covalently binds to Bcl–G and enhances lipopolysaccharide/interferon γ-induced apoptosis in macrophages. FEBS J 2013;280:1281-93. 10.1111/febs.12120 [DOI] [PubMed] [Google Scholar]
- 21.Soerensen KE, Olsen HG, Skovgaard K, et al. Disseminated intravascular coagulation in a novel porcine model of severe Staphylococcus aureus sepsis fulfills human clinical criteria. J Comp Pathol 2013;149:463-74. 10.1016/j.jcpa.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 22.Czerski A, Bujok J, Gnus J, et al. Experimental methods of abdominal aortic aneurysm creation in swine as a large animal model. J Physiol Pharmacol 2013;64:185-92. [PubMed] [Google Scholar]
- 23.Nelson D, Porta C, Satterly S, et al. Physiology and cardiovascular effect of severe tension pneumothorax in a porcine model. J Surg Res 2013;184:450-7. 10.1016/j.jss.2013.05.057 [DOI] [PubMed] [Google Scholar]
- 24.Flisikowska T, Kind A, Schnieke A. The new pig on the block: modelling cancer in pigs. Transgenic Res 2013;22:673-80. 10.1007/s11248-013-9720-9 [DOI] [PubMed] [Google Scholar]
- 25.Benito A, Gutierrez O, Pipaon C, et al. A novel role for proline-and acid-rich basic region leucine zipper (PAR bZIP) proteins in the transcriptional regulation of a BH3-only proapoptotic gene. J Biol Chem 2006;281:38351-7. 10.1074/jbc.M607004200 [DOI] [PubMed] [Google Scholar]
- 26.O'Connor L, Strasser A, O'Reilly LA, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J 1998;17:384-95. 10.1093/emboj/17.2.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin ML, Park JH, Nishidate T, et al. Involvement of maternal embryonic leucine zipper kinase (MELK) in mammary carcinogenesis through interaction with Bcl-G, a pro-apoptotic member of the Bcl-2 family. Breast Cancer Res 2007;9:R17. 10.1186/bcr1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Pan Z, Zhang L, et al. JAB1 accelerates mitochondrial apoptosis by interaction with proapoptotic BclGs. Cellular Signalling 2008;20:230-40. 10.1016/j.cellsig.2007.10.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as