Abstract
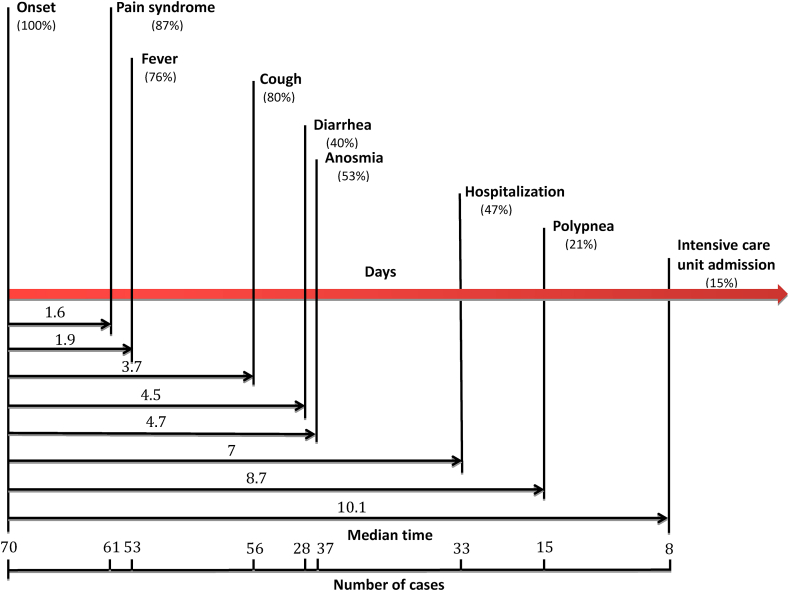
We retrospectively reconstituted the history of evolution and onset of the main symptoms of COVID-19 in 70 patients ([29 males, 41%] with a mean age of 56.7 ± 19.3 [19–96] years). Firstly, pain syndrome defined by headache and/or myalgia and/or arthralgia (87%, n = 61) appeared as the first manifestation 1.6 day after the onset of the illness. Secondly, fever (76%, n = 53), followed by cough (80%, n = 56) and diarrhea (40%, n = 28). Thirty three patients (47.1%) were hospitalized on day 7 (±3) with a mean duration of hospitalization of 6.9 (±5.8 [1–21]) days. Twenty-three patients (32.9%) required oxygen therapy 6.7 (±4.1 [1–13]) days from illness onset. Fifteen patients had a respiratory rate ≥22/min on day 9 (±0.8) and only 8 patients (15%) were admitted or transferred in an ICU on day 10 (±2.7) with a mean duration of hospitalization in ICU of 7.9 (±6.6 [2–21]) days.
Keywords: Clinical features, coronavirus, COVID-19, evolution, history, symptoms, timeline, wave
Countries across Europe, and especially France, are seeing a resurgence in coronavirus disease 2019 (COVID-19) cases after successfully slowing down outbreaks earlier in 2020.
We recently published a study comparing clinical features of COVID-19 and seasonal influenza A/B [1]. In front of the second wave of COVID-19, we want to focus only in the natural history of COVID-19, so we here present a retrospective and observational study in Nord Franche-Comté Hospital a major French cluster of COVID-19 cases that began on 26 February 2020 in the nearby Grand-Est region. Between 26 February and 14 March, we enrolled all adult patients (≥18 years) with confirmed COVID-19. Diagnosis was confirmed by real-time reverse transcriptase PCR on respiratory samples, mainly nasopharyngeal swabs, sputum, bronchial aspirates or bronchoalveolar lavage fluids, for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
We reconstituted the history of evolution and onset of the main symptoms of COVID-19 in 70 patients (29 male, 41%; mean ± SD age, 56.7 ± 19.3 (range, 19–96) years). The mean incubation period (time from contact symptomatic case to illness onset) was 6 ± 2.1 (range, 1–12) days.
First is a pain syndrome, which is defined by some combination of headache, myalgia or arthralgia (87%, n = 61); it appears as the first manifestation 1.6 days after the onset of illness (Fig. 1). Second is fever (76%, n = 53), followed by cough (80%, n = 56) and diarrhoea (40%, n = 28). In fifth position is anosmia, which was described in 37 patients (53%) and began 4.7 ± 1.9 (range, 1–8) days after onset of infection. In our patients, all symptoms persisted for 10 ± 4.9 (range, 3–27) days, and the duration of fever was 5.5 ± 4.4 (range, 1–19) days; however, the mean duration of cough and anosmia was respectively 7.7 ± 4.3 (range, 1–18) days and 7.3 ± 5 (range, 1–19) days.
Fig. 1.
Timeline of coronavirus disease 2019 (COVID-19) after onset of illness.
Of 70 patients, 28 (40%) had diarrhoea. Diarrhoea began 4.5 ± 1.8 (range, 1–7) days after infection onset. Only two patients (2.8%) had a history of inflammatory bowel disease. Of those patients with diarrhoea, 25 (89.3%) had at least one simultaneous gastrointestinal symptom other than diarrhoea. Twenty-two patients (31.4%) had nausea, 14 patients (20%) had abdominal pain and two (2.8%) had vomiting.
Concerning the assessment of this disease, hospitalization and clinical aggravation appeared in the second week. Thirty-three patients (47.1%) were hospitalized on day 7 ± 3, with a mean duration of hospitalization of 6.9 ± 5.8 (range, 1–21) days. At admission, the mean oxygen saturation was 93.16 ± 3.46% (range, 85–98%). In our case study, 23 patients (32.9%) required oxygen therapy for 6.7 ± 4.1 (range, 1–13) days after illness onset. The recommended target saturation range for these patients was 94 ± 2%, and 94 ± 2% in patients with chronic obstructive pulmonary disease.
Fifteen patients had a respiratory rate of ≥22 breaths per minute on day 9 ± 0.8; only eight patients (15%) were admitted or transferred in an intensive care unit on day 10 ± 2.7, with a mean duration of hospitalization in the intensive care unit of 7.9 ± 6.6 (range, 2–21) days. The mean oxygen therapy flow was 10.5 L/min in patients admitted to the intensive care unit; all of them required invasive mechanical ventilation.
Among patients with COVID-19, general symptoms appeared first, followed by respiratory, rhinolaryngologic and gastrointestinal symptoms [1]. In addition, most reports so far have associated anosmia (new loss of smell in COVID-19) with neurologic symptoms rather than rhinolaryngologic symptoms [2]. Mucosal inflammation due to SARS-CoV-2 with acute rhinosinusitis can participate in the pathogenesis of anosmia. However, in anosmia related to COVID-19, a neurotropism of SARS-CoV-2 should be proven; assumptions include an invasion of the olfactory receptors or damage of the first cranial nerves in the nasal cavity cell membrane and/or central lesion.
The timeline of symptom onset is explained by the pathophysiology of this coronavirus. The entry of SARS-CoV-2 into human host cells is mediated mainly by cellular receptor angiotensin-converting enzyme 2, which is expressed in human airway epithelia and lung parenchyma, and secondarily in small intestine cells, sensory receptors and neural system [3].
In a systematic review and meta-analysis, Cheung et al. [4] showed that SARS-CoV-2 RNA was detected in stool samples of 48% patients, even in stool samples collected after respiratory samples tested negative, leading to the conclusion that stool samples indicate that patients with COVID-19 are highly contagious even during recovery.
Finally, medical publications have reported a so-called second-week crash among COVID-19 patients. SARS-CoV-2 induces excessive and prolonged cytokine/chemokine responses in some infected individuals, known as a cytokine storm [5]. The severe deterioration of these patients requires monitoring during the second week of COVID-19's course.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Due to the retrospective nature of the study, the Ethics Committee of Nord Franche-Comté Hospital determined that patient consent was not required. We make sure to keep patient data confidential and compliance with the Declaration of Helsinki.
Conflict of interest
All authors declare no competing interests.
References
- 1.Zayet S., Kadiane-Oussou N.J., Lepiller Q., Zahra H., Royer P.Y., Toko L. Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte cluster. Microbe Infect. 2020 Oct;22(9):481–488. doi: 10.1016/j.micinf.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zahra S.A., Iddawela S., Pillai K., Choudhury R.Y., Harky A. Can symptoms of anosmia and dysgeusia be diagnostic for COVID-19? Brain Behav. 2020 Nov;10(11):e01839. doi: 10.1002/brb3.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with COVID-19. N Engl J Med. 2020 Apr;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung K.S., Hung I.F., Chan P.P., Lung K.C., Tso E., Liu R. 2020 Jul. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 159(1):81-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020 Jun;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]