Abstract
Coronavirus disease 2019 (COVID-19) is still a global epidemic. Several studies of individuals with severe COVID-19 regard convalescent plasma (CP) transfusion as an effective therapy. However, no significant improvements are found in randomized clinical trials of CP treatment. Until now, data for individuals with mild COVID-19 transfused CP were lacking. This study recruited eight individuals with mild COVID-19 who received at least one dose of CP transfusion. After CP therapy, the clinical symptoms of all individuals improved. Lymphocyte counts tended to increase, and lactate dehydrogenase, creatine kinase and aspartate aminotransferase tended to decrease. However, C-reactive protein increased transiently in three individuals. The median time for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid test to become negative was 2.5 days after CP transfusion. The study shows the potential benefits of CP. Meanwhile, CP probably enhances the inflammatory response to SARS-CoV-2 temporarily in people with insufficient antiviral immunity. However, the effects of CP are not permanent.
Keywords: Convalescent plasma, coronavirus disease 2019, immunity, severe acute respiratory syndrome coronavirus 2, treatment
Background
As of 23 July 2020, the coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), had infected almost 15 million people with more than 600 000 deaths all over the world. SARS-CoV-2 belongs to the coronavirus family and spreads more quickly than SARS-CoV from person to person [1]. COVID-19 is primarily an acute viral pneumonia and can cause respiratory failure [2], though some individuals with COVID-19 are asymptomatic. Currently, there is no approved antiviral agent that can effectively target this novel virus. As vaccines and antiviral medicines are unavailable, the treatment is mainly experimental or empirical.
Convalescent plasma (CP) refers to plasma that is collected from individuals with resolution of infection and development of antibodies. CP was employed successfully as post-exposure prophylaxis and/or treatment for SARS-CoV, Middle East respiratory syndrome (MERS) and Ebola [3,4]. This study screened 40 individuals who had recovered from COVID-19, among which 39 displayed neutralizing antibody with titres ≥160 (97.5%) [5]. Although studies of individuals with severe COVID-19 have demonstrated the potential efficacy of CP therapy [6,7], there is a lack of sufficient data for individuals with less severe disease treated with transfusion of convalescent plasma. For this reason, we performed this pilot study with cases from multiple centres (four hospitals) in Jiangsu China to explore the effect of CP therapy for individuals with mild COVID-19.
Materials and methods
Patients
From February 2020 to March 2020, a total of eight individuals were recruited from the Affiliated Hospital of Xuzhou Medical University and four other hospitals in Jiangsu province and Anhui province, China. All participants underwent testing for SARS-CoV-2 via real-time RT-PCR and were diagnosed as having mild COVID-19 according to the WHO Interim Guidance and the Guideline of Diagnosis and Treatment of COVID-19 of the National Health Commission of China (version 7.0). Chest CT scans of all participants showed ground-glass opacity or pulmonary parenchymal consolidation, predominantly subpleural, in the lungs. The study protocol was approved by the ethics committee of the Affiliated Hospital of Xuzhou Medical University (XYFY2020-KL002-01).
Donors for convalescent plasma transfusion
All donors recovered from COVID-19 and were from Jiangsu province. The criteria of the donors were as follows: (a) two consecutively negative results of sputum for SARS-CoV-2 by RT-PCR assay for more than 1 week, (b) normality of body temperature for more than 1 week, (c) resolution of respiratory tract symptoms and (d) more than 3 weeks after onset of illness. Donors met the blood donor eligibility criteria for plasma donation, including age, weight and reasonable-sized antecubital veins.
Plasma preparation procedure and quality control
Convalescent plasma for treatment was collected from 25 donors. A 200-mL to 400-mL ABO-compatible plasma sample was harvested from each donor and was divided and stored as 250-ml aliquots at 4°C. Before being stored, the CP was tested for SARS-CoV-2 RNA by RT-PCR assay; results of SARS-CoV-2 RNA detections were negative. Serology screening for hepatitis B virus and hepatitis C virus, human immunodeficiency virus and syphilis spirochetes was negative.
Treatments
All patients received antiviral therapy and other supportive care; some received antibiotic treatment and oxygen support. One dose of 200–400 mL of inactivated CP was transfused into the patients within 4 h following the WHO blood transfusion protocol. One individual received a total of 625 mL CP from three CP transfusions and the others received one transfusion of 200–400 mL.
Data collection and statistical analysis
Clinical information on all enrolled participants was retrieved from the hospital electronic history system, including the baseline demographic data, duration of illness (in days), presenting symptoms, and different examinations, and methods of treatment. SARS-CoV-2 RNA from the serum sample was monitored during treatment. SPSS 20.0 (IBM, Armonk, NY, USA) and GraphPad Prism 7.0 (GraphPad, San Diego, CA, USA) were applied to conduct statistical analysis and to plot graphs.
Results
General characteristics of patients treated with CP
Eight individuals with mild COVID-19 (seven men and one woman) were enrolled and received CP transfusion. Their ages ranged from 44.00 to 80.00 years and median age was 69.50 years (interquartile range (IQR) 52.00–76.50 years). Three participants had no co-morbidities, one had hypertension and four had both hypertension and diabetes. The main symptoms were cough (8/8), shortness of breath (4/8), fever (3/8) and fatigue (2/8). The median Spo2 of the participants without oxygen therapy was 97% (IQR 92%–99%). Details of clinical characteristics are shown in Table 1. Three individuals had a combined bacterial infection. One participant did not receive anti-SARS-CoV-2 treatment, the others were treated with arbidol hydrochloride, lopinavir ritonavir, hydroxychloroquine sulphate, ribavirin singly or in combination (see Supplementary material, Table S1, for details). The median time from onset of symptoms to hospital admission and to CP transfusion were 8.00 days (IQR 7.00–11.25 days) and 20.00 days (IQR 11.00–22.75 days), respectively. The baseline examination data 3 days before CP transfusion was shown in Table 2.
Table 1.
Clinical characteristics of patients treated with convalescent plasma
| Patient no. | Age (years) | Male/female | Clinical symptoms | Co-morbidities | Spo2 on admission % |
Days of admission from symptom onset | Days of CP therapy from symptom onset |
|---|---|---|---|---|---|---|---|
| 1 | 44 | F | Cough and fatigue | Hypertension | 97 | 9 | 14 |
| 2 | 73 | M | Fever, shortness of breath | — | 97 | 2 | 22 |
| 3 | 66 | M | Cough and shortness of breath | Hypertension and diabetes | 90 | 8 | 18 |
| 4 | 75 | M | Cough | — | 99 | 19 | 24 |
| 5 | 49 | M | Cough and fatigue | — | 90 | 8 | 9 |
| 6 | 77 | M | Fever, cough and shortness of breath | Hypertension and diabetes | 99 | 12 | 22 |
| 7 | 62 | M | Fever, cough and shortness of breath | Hypertension and diabetes | 97 | 7 | 23 |
| 8 | 80 | M | Fever and cough | Hypertension | 97 | 7 | 10 |
Abbreviations: CP, convalescent plasma; Spo2, oxygen saturation.
Table 2.
Baseline clinical indicators before convalescent plasma transfusion
| Patient no. | WBC (×109/L) | Lym. (×109/L) | AST (U/L) | LDH (U/L) | CK (U/L) | Creatinine (umol/L) | CRP (mg/L) | CT presentation of lungs | Range of lesions in lungs |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.79 | 1.62 | 22 | 187 | 40 | 57 | 0.66 | Ground-glass opacity | <50% |
| 2 | 14.79 | 0.74 | 23 | 931 | 33 | 69 | 123.8 | Ground-glass opacity | <50% |
| 3 | 2.74 | 0.67 | 37 | 269 | 33 | 70 | 3.23 | Pulmonary parenchymal consolidation, predominantly subpleural | <50% |
| 4 | 4.48 | 1.27 | 26 | 199 | 48 | 66 | 1.7 | Ground-glass opacity | >50% |
| 5 | 6.69 | 0.98 | 58 | 362 | 62 | 67 | 113.3 | Ground-glass opacity | <50% |
| 6 | 8.29 | 1.14 | 15 | 291 | 25 | 56 | 32.6 | Ground-glass opacity | <50% |
| 7 | 10.31 | 0.74 | 28 | 305 | 56 | 150 | 92.5 | Ground-glass opacity | <50% |
| 8 | 6.10 | 0.51 | 51 | 428 | 94 | 59 | 68.3 | Ground-glass opacity | <50% |
Abbreviations: AST, aspartate aminotransferase; CK, creatine kinase; CP, convalescent plasma; CRP, C-reactive protein; LDH, lactate dehydrogenase; Lym., lymphocyte count; WBC, white blood cell count.
The effect of CP transfusion on clinical characteristics
All patients already had no fever before CP treatment. The strength and appetite of four patients was improved 1 day after CP transfusion. By 7 days after CP transfusion, all patients had reduced cough. Meanwhile, the chest CT scans of three patients showed the disappearance of ground-glass opacity and other patients had a reduction of pulmonary lesions.
The effect of CP transfusion on laboratory indicators
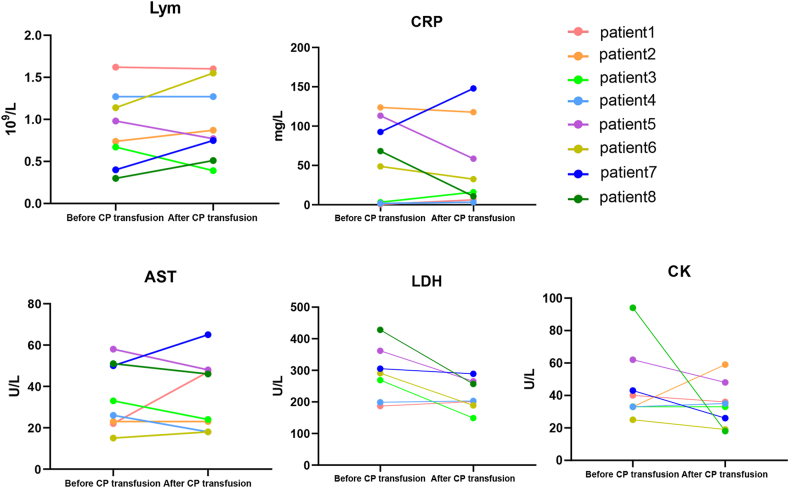
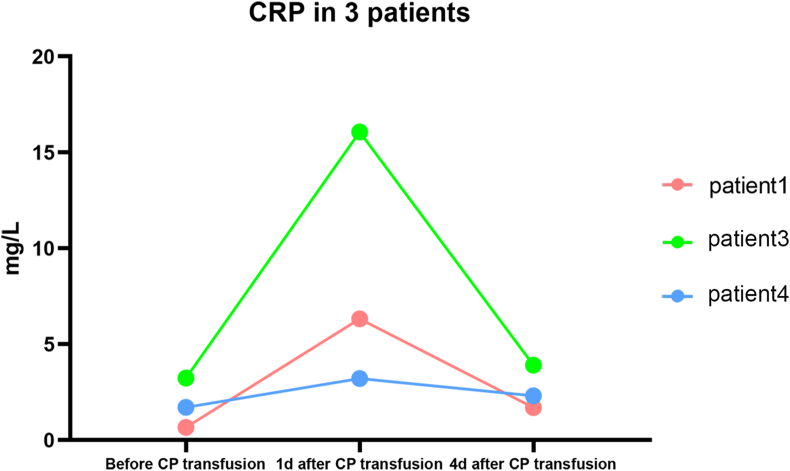
The levels of lymphocyte counts, C-reactive protein (CRP), aspartate aminotransferase (AST), lactate dehydrogenase (LDH) and creatine kinase (CK) in patients were compared with the corresponding levels 1 day after CP transfusion (Fig. 1). These indicators are known to be closely related to the severity of inflammation and viral infection. Lymphocyte counts of four patients increased after CP transfusion. In total, lymphocyte counts after CP transfusion were higher than before but the difference did not reach statistical significance (mean ± SD 0.89 ± 0.16 × 109/L versus 0.96 ± 0.16 × 109/L, p 0.75). CRP tended to be improved after CP transfusion (mean ± SD 56.52 ± 18.03 mg/L versus 49.13 ± 19.52 mg/L, p 0.79). Baseline CRP of five patients was abnormal, but improved in four of them after CP transfusion. However, among the other three patients with normal CRP, the levels of CRP were elevated 1 day after CP transfusion and returned to normal in 4 days (Fig. 2). LDH, CK and AST had downward trends after CP transfusion (mean ± SD 291.57 ± 32.28 U/L versus 221.86 ± 18.76 U/L, p 0.09; 45.38 ± 7.96 U/L versus 34.25 ± 4.95 U/L, p 0.26; and 39.32 ± 19.49 U/L versus 38.00 ± 6.85 U/L, p 0.87, respectively).
Fig. 1.
The change of laboratory indicators before and one day after convalescent plasma (CP) transfusion. AST, aspartate aminotransferase; CK, creatine kinase; CRP, C-reactive protein; LDH, lacate dehydrogenase; Lym, lymphocyte count.
Fig. 2.
The change in C-reactive protein (CRP) in three patients with normal CRP before and after convalescent plasma (CP) transfusion.
The effect of CP transfusion on the negative conversion of SARS-CoV-2 nucleic acid
Participants were positive for SARS-CoV-2 nucleic acid for a median of 13.5 days. After CP transfusion, patients remained SARS-CoV-2 nucleic acid-positive for a mean of 2.5 days (Table 3). Patient 2 became negative for SARS-CoV-2 nucleic acid 1 day after CP transfusion, but the next day had returned to positive and was finally negative 2 days later. The longest period of being SARS-CoV-2 nucleic acid-positive, after CP transfusion, was 17 days. This patient was 80 years old with hypertension, cerebral infarction and pulmonary bacterial infection.
Table 3.
Time of the negative conversion of SARS-CoV-2 nucleic acid
| Patient no. | CP transfusion date | First SARS-CoV-2 nucleic acid negative date | Time positive for SARS-CoV-2 nucleic acid (days) | Time positive for SARS-CoV-2 nucleic acid after CP transfusion (days) |
|---|---|---|---|---|
| 1 | 19 February 2020 | 21 February 2020 | 7 | 2 |
| 2 | 15 February 2020 | 16 February 2020 | 20 | 1 |
| 3 | 24 February 2020 | 26 February 2020 | 13 | 2 |
| 4 | 21 February 2020 | 24 February 2020 | 8 | 3 |
| 5 | 24 February 2020 | 4 March 2020 | 10 | 9 |
| 6 | 23 February 2020 | 1 March 2020 | 14 | 7 |
| 7 | 20 February 2020 | 22 February 2020 | 17 | 2 |
| 8 | 14 February 2020 | 2 March 2020 | 22 | 17 |
Abbreviations: CP, convalescent plasma; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The patient treated three times with CP transfusion
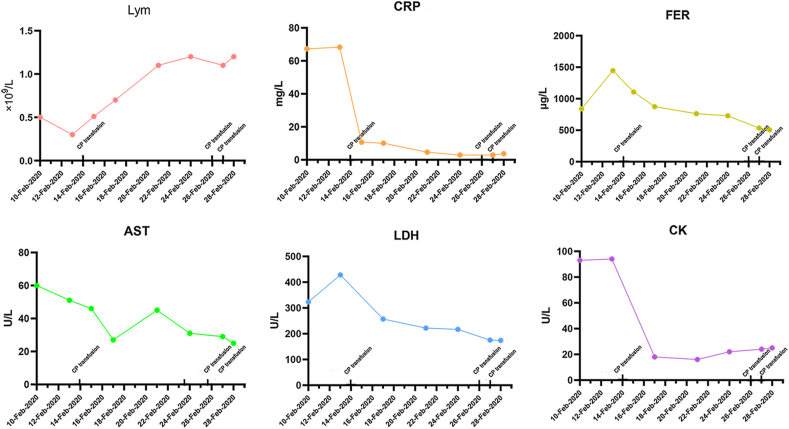
Patient 8, with hypertension, cerebral infarction and hemiplegia, received three CP transfusions. The condition of the patient continued to progress after admission. Levels of CRP, ferritin, LDH and CK increased and lymphocyte counts decreased (Fig. 3). After CP transfusion, all the indicators improved and the lesions in the lungs were also reduced (Fig. 3, Fig. 4). However, this individual remained positive for SARS-CoV-2 nucleic acid for 17 days after CP transfusion and was positive for a total of 22 days (9 February 2020 to 2 March 2020). After 18 days (20 March 2020), the individual again became positive for SARS-CoV-2 nucleic acid and 5 days later (25 March 2020) became negative.
Fig. 3.
The change of laboratory indicators in patient 8 before and after three convalescent plasma (CP) transfusions. FER, ferritin.
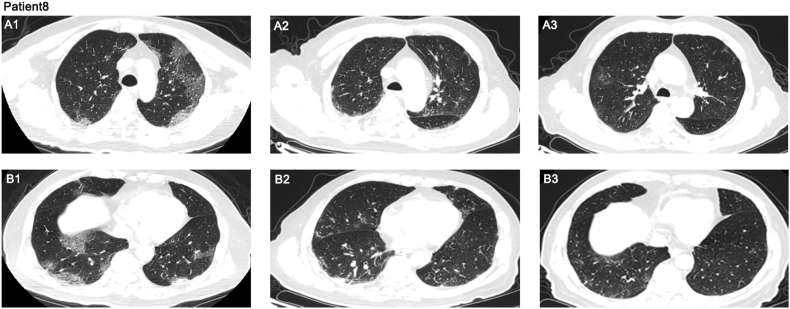
Fig. 4.
Chest CTs of patient 8 showed ground-glass opacity and pulmonary parenchymal consolidation, predominantly subpleural in middle lung field (a) and lower lung field (b). (a1, b1) CT images obtained on 11 February 2020 (3 days before convalescent plasma (CP) transfusion), (a2, b2) CT images obtained on 20 February 2020 (6 days after first CP transfusion), (a3, b3) CT images obtained on 3 March 2020 (5 days after third CP transfusion).
Discussion
In our study, all participants were treated with at least one CP transfusion. The symptoms of all patients improved after CP treatments and the ranges of pulmonary lesions in all patients were reduced within 7 days after CP treatments. Convalescent plasma contains antibodies, anti-inflammatory cytokines, albumin, proteins C and S, anti-clotting factors and so on. The immunoglobulin in CP can blockade autoreactive recipient antibodies and then reduce autoinflammation [8,9]. An important part of the pathogenesis of COVID-19 is an over-activation of the immune system, probably with systemic hyper-inflammation or ‘cytokine storm’ [10,11]. In this sense, CP treatment may reduce over-activation of the immune system and inhibit complement cascade. The results of the study in patients with severe COVID-19 showed that CP therapy improved the clinical symptoms of nine out of ten patients [6]. Meanwhile, the patients treated with CP in the early stages of COVID-19 showed a rapid increase in lymphocyte counts and a decrease of CRP. However, in patients with severe COVID-19, CP therapy added to standard treatment, compared with standard treatment alone, did not result in a statistically significant improvement in time to clinical improvement within 28 days in the randomized clinical trial [12]. In our research, the lymphocyte counts of half of the participants increased after CP treatment. In the same situation, the levels of LDH, CK and AST tended to decline. More interestingly, patients with normal CRP levels had a transient increase in CRP after CP transfusion, but patients with abnormal CRP levels showed a decrease in CRP. Hence, the immunomodulatory effects of CP in COVID-19 may be bidirectional. IgG in CP can activate Fcγ receptors [13], which are found in almost all immune cells. These receptors are critical factors in modulating or inhibiting activity of immune cells, including lymphocytes. Meanwhile, CP transfusion may enhance anti-inflammatory properties of dendritic cells and then reduce antigen presentation by T cells [14]. So CP therapy can reduce the excessive inflammatory stimuli in patients with COVID-19 [15]. More importantly, CP can provide neutralizing antibodies to promote virus clearance, so CP can enhance antiviral immunity in people with COVID-19 who have insufficient antiviral immunity. This is probably the reason that the patients with normal CRP showed a transient increase in CRP.
Most of the patients in our study became negative for SARS-CoV-2 nucleic acids within 1 week after CP transfusion. In particular, the viral nucleic acids of all patients with a brief increase in CRP became negative within 3 days after CP transfusion. Hence, CP therapy may be effective for viral removal, particularly in those patients with insufficient antiviral immunity. Infection by SARS-CoV induces IgG antibody production against nucleoprotein that can be detected at day 4 after the onset of disease and with seroconversion at day 14 [16]. Neutralizing antibodies are crucial in virus clearance. In SARS-CoV and MERS it was discovered that neutralizing antibodies bound to the Spike1-receptor binding protein, S1-N-terminal domain and S2, inhibiting their entry and limiting viral amplification [17]. Hence, the presence of neutralizing antibodies in the recipients probably restricts viral infection [5]. The plasma obtained from the donors and transfused into the recipients on the same day led to decreased viral load. CP therapy in our research showed the same effects and seemed to be more effective in those patients with insufficient antiviral immunity.
However, the effects of CP on viral clearance may be different for patients with COVID-19 with different immune status. The oldest patient in our study, who had pulmonary bacterial infection and hypertension, had a long period of viral clearance after CP transfusion. After three CP transfusions, the patient became negative for viral nucleic acids. The result hinted that increasing plasma infusion volume and frequency would be of benefit for SARS-CoV-2 clearance. Meanwhile, the impact of CP on the virus are probably not persistent. The viral nucleic acids of two participants turned from negative to positive briefly after CP therapy in our study. This phenomenon indicated that the seemingly inhibiting effects on SARS-CoV-2 of CP were transient. Repeated CP treatment might solve this condition.
Conclusions
This pilot study shows the potential benefits of CP for treating individuals with mild COVID-19. Meanwhile, CP probably enhances the inflammatory response to SARS-CoV-2 temporarily in people with insufficient antiviral immunity. However, the effects of CP are transient. Hence, repeat CP transfusions are required and would be more beneficial.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 81371867), Jiangsu Provincial Special Programme of Medical Science (BL2014033) and Major National Science and Technology Projects of China (2018ZX10302206-003-010). The Open Access publication charges for this article were paid by the National Natural Science Foundation of China.
Conflict of interest
The authors do not have any conflicts of interest.
Acknowledgment
We thank all participants for their contributions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nmni.2020.100814.
Contributor Information
F. Ji, Email: jifang800410@foxmail.com.
X.-B. Yan, Email: yxbxuzhou@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Therapeutic drugs.
References
- 1.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inciardi R.M., Adamo M., Lupi L., Cani D.S., Di Pasquale M., Tomasoni D. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y., Wong R., Soo Y.O., Wong W.S., Lee C.K., Ng M.H. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arabi Y.M., Hajeer A.H., Luke T., Raviprakash K., Balkhy H., Johani S. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22:1554–1561. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang B., Liu S., Tan T., Huang W., Dong Y., Chen L. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158:e9–e13. doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurez V., Kazatchkine M.D., Vassilev T., Ramanathan S., Pashov A., Basuyaux B. Pooled normal human polyspecific IgM contains neutralizing anti-idiotypes to IgG autoantibodies of autoimmune patients and protects from experimental autoimmune disease. Blood. 1997;90:4004–4013. [PubMed] [Google Scholar]
- 9.Chaigne B., Mouthon L. Mechanisms of action of intravenous immunoglobulin. Transfus Apher Sci. 2017;56:45–49. doi: 10.1016/j.transci.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Liu J., Li S., Liu J., Liang B., Wang X., Wang H. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:1–11. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nimmerjahn F., Ravetch J.V. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 14.Aubin E., Lemieux R., Bazin R. Indirect inhibition of in vivo and in vitro T-cell responses by intravenous immunoglobulins due to impaired antigen presentation. Blood. 2010;115:1727–1734. doi: 10.1182/blood-2009-06-225417. [DOI] [PubMed] [Google Scholar]
- 15.Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev. 2020;19:102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsueh P.R., Huang L.M., Chen P.J., Kao C.L., Yang P.C. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect. 2004;10:1062–1066. doi: 10.1111/j.1469-0691.2004.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Therapeutic drugs.