Abstract
Objectives
To provide an overview of the spectrum, characteristics and outcomes of neurologic manifestations associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Methods
We conducted a single-centre retrospective study during the French coronavirus disease 2019 (COVID-19) epidemic in March–April 2020. All COVID-19 patients with de novo neurologic manifestations were eligible.
Results
We included 222 COVID-19 patients with neurologic manifestations from 46 centres in France. Median (interquartile range, IQR) age was 65 (53–72) years and 136 patients (61.3%) were male. COVID-19 was severe or critical in 102 patients (45.2%). The most common neurologic diseases were COVID-19–associated encephalopathy (67/222, 30.2%), acute ischaemic cerebrovascular syndrome (57/222, 25.7%), encephalitis (21/222, 9.5%) and Guillain-Barré syndrome (15/222, 6.8%). Neurologic manifestations appeared after the first COVID-19 symptoms with a median (IQR) delay of 6 (3–8) days in COVID-19–associated encephalopathy, 7 (5–10) days in encephalitis, 12 (7–18) days in acute ischaemic cerebrovascular syndrome and 18 (15–28) days in Guillain-Barré syndrome. Brain imaging was performed in 192 patients (86.5%), including 157 magnetic resonance imaging (70.7%). Among patients with acute ischaemic cerebrovascular syndrome, 13 (22.8%) of 57 had multiterritory ischaemic strokes, with large vessel thrombosis in 16 (28.1%) of 57. Brain magnetic resonance imaging of encephalitis patients showed heterogeneous acute nonvascular lesions in 14 (66.7%) of 21. Cerebrospinal fluid of 97 patients (43.7%) was analysed, with pleocytosis found in 18 patients (18.6%) and a positive SARS-CoV-2 PCR result in two patients with encephalitis. The median (IQR) follow-up was 24 (17–34) days with a high short-term mortality rate (28/222, 12.6%).
Conclusions
Clinical spectrum and outcomes of neurologic manifestations associated with SARS-CoV-2 infection were broad and heterogeneous, suggesting different underlying pathogenic processes.
Keywords: COVID-19, Nervous system, Neurologic manifestations, Registry, SARS-CoV-2
Introduction
Coronavirus disease 2019 (COVID-19), the disease linked to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an emerging infectious disease, with the first cases reported in China in December 2019 [1,2]. The virus has continued to spread since then, and on 11 March 2020, the World Health Organization characterized COVID-19 as a pandemic. Common manifestations of the disease include respiratory tract and associated systemic manifestations, but neurologic manifestations including headaches, dizziness, anosmia, encephalopathy and stroke have been reported in cohort studies [3,4]. However, the potential pathogenesis of SARS-CoV-2 in the central nervous system remains unclear [5], and the range of neurologic disorders associated with COVID-19 is not fully defined.
The present study aimed to provide a comprehensive overview of neurologic manifestations associated with SARS-CoV-2 infection and to describe the clinical course and outcomes of COVID-19 patients with neurologic manifestations.
Methods
Study design
We conducted a retrospective single-centre observational study to collect neurologic manifestations associated with COVID-19 in 46 hospitals in France. A case report form (CRF) was sent from 16 March to 27 April 2020 to French neurologists, infectious diseases specialists and intensivists. The study complied with French Commission Nationale de l’Informatique et des Libertés (CNIL; no. 2217844) and ethics committee (RCB 2020-A01300-39) requirements. The local institutional review board approved the study (no. 2020-0602 COVID).
Patients and data collection
We included adult COVID-19 patients with any neurologic manifestations occurring 5 days before to 35 days after the first symptoms of COVID-19. A confirmed case of COVID-19 was defined as a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) real-time reverse transcriptase PCR (RT-PCR) assay result on a nasopharyngeal sample or positive SARS-CoV-2 serology. As RT-PCR analysis and serology were unavailable in some centres, some cases were considered COVID-19 if the clinical history and the chest computed tomographic (CT) scan were typical of the disease according to the referring clinicians. We excluded patients with no diagnosis of COVID-19, patients with neurologic signs that were not time related to COVID-19, patients with incomplete data on the CRF and patients with exacerbations of chronic neurologic diseases. We defined COVID-19 illness severity as mild, moderate, severe or critical according to the criteria of the US National Institutes of Health [6]. The follow-up for each patient was recorded up to the completion of the CRF by clinicians.
Classification of neurologic manifestations
Neurologic manifestations were identified as either related to the central nervous system (CNS) or the peripheral nervous system (PNS), then classified into categories as follows.
Stroke
Stroke was considered in patients with sudden neurologic deficit related to an acute vascular lesion on cerebral magnetic resonance imaging (MRI) or CT scan, in patients with transient focal deficit and normal MRI (transient ischaemic attack) or in patients with cerebral venous thrombosis.
Encephalitis
Encephalitis was defined as an altered mental status lasting ≥24 hours along with one of the following criteria: white blood cell count (WBC) in cerebrospinal fluid (CSF) < 5/mm³; or presence of compatible acute lesion on brain MRI. All patients with encephalitis had CSF examination [7,8].
Encephalopathy
Encephalopathy was defined by an altered mental status lasting ≥24 hours that could be associated with seizure and/or focal neurologic signs in the absence of criteria for encephalitis [8]. We identified COVID-19–associated encephalopathy (CAE) if encephalopathy could not be accounted for by another cause, such as toxic or metabolic factors, according to the reporting clinician.
Guillain-Barré syndrome
Guillain-Barré syndrome (GBS) was defined according to standard diagnostic criteria [9].
Acute meningitis
Acute meningitis was defined as meningeal syndrome (head stiffness, headache, fever) without encephalitic course and CSF WBC counts of <5/mm³.
Other
Neurologic manifestations that did not meet any of these criteria were categorized as other.
Results
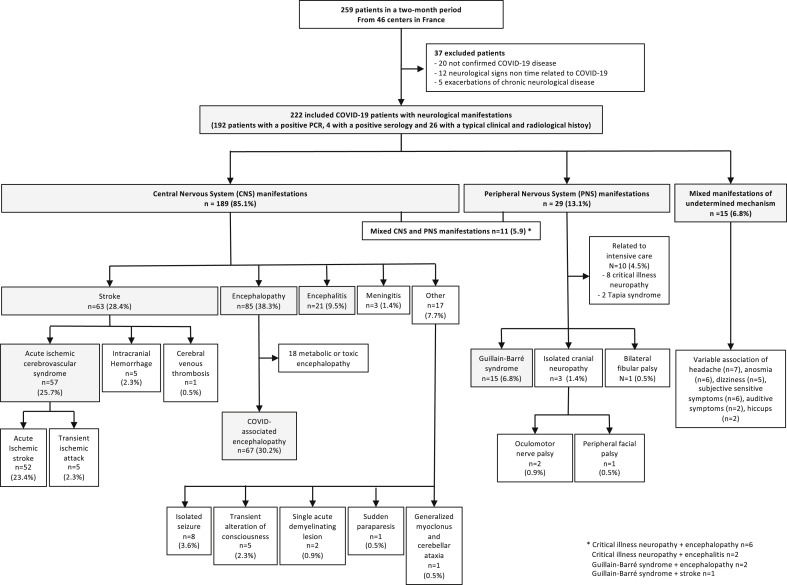
The study population comprised 259 patients, which included 222 hospitalized COVID-19 patients with neurologic manifestations from 46 centres in all regions of continental France and overseas (Fig. 1 , Supplementary Appendix S2). Participating physicians were neurologists (146/222, 65.8%), infectious diseases or internal medicine specialists (43/222, 19.4%), intensivists (14/222, 6.3%) or other specialists (19/222, 8.6%). The prevalence of neurologic manifestations among COVID-19 patients was estimated in one centre to be 8.8%: 43 patients with neurologic manifestations were reported from a total of 490 patients hospitalized with COVID-19.
Fig. 1.
Study population of coronavirus disease 2019 (COVID-19) patients with neurologic manifestations.
General characteristics of COVID-19 patients with neurologic manifestations
Median (interquartile range, IQR) age was 65 (53–72) years and 136 patients (61.3%) were male (Table 1 ). Forty-seven patients (21.2%) had a neurologic history, mostly prior stroke (20, 9.0%) and neurodegenerative disease (17, 7.7%). The diagnosis of COVID-19 was confirmed by a positive SARS-CoV-2 PCR result in 192 patients (86.5%) and by serology in four patients (1.8%). Twenty-six patients (11.7%) had a diagnosis that was based on a typical clinical course and imaging. COVID-19 severity was severe or critical in 102 patients (45.2%). The most common neurologic symptom was altered mental status (117, 52.4%). Neurologic assessment mostly included brain MRI (157, 70.7%) and CSF examination (97, 43.7%). SARS-CoV-2 PCR was performed on CSF samples in 75 patients (33.8%) and was negative in 73 (97.3%) of them. The median (IQR) follow-up was 24 (17–34) days. Twenty-eight patients (12.6%) died, mostly following acute respiratory distress syndrome (n = 17, 7.7%) or stroke (2.3%).
Table 1.
General characteristics of 222 COVID-19 patients with neurologic manifestations
| Characteristic | Value |
|---|---|
| Age (years), median (IQR) | 65 (53–72) |
| Male | 136 (61.3) |
| Neurologic comorbidities | 47 (21.2) |
| Prior stroke | 20 (9.0) |
| Neurodegenerative disease | 17 (7.7) |
| Epilepsy | 5 (2.3) |
| Other | 5 (2.3) |
| Diagnosis of COVID-19 | |
| Positive SARS-CoV-2 nasopharyngeal PCR | 192 (86.5) |
| Positive SARS-CoV-2 serology | 4 (1.8) |
| Typical clinical course and chest CT | 26 (11.7) |
| Severity of COVID-19a | |
| Mild | 55 (24.8) |
| Moderate | 65 (29.3) |
| Severe | 46 (20.7) |
| Critical | 56 (25.2) |
| Occurrence of neurologic manifestations | |
| Neurologic manifestations occurring as first symptoms | 45 (20.3) |
| Neurologic manifestation occurring after first COVID-19 symptoms | 141 (63.5) |
| Time (days) between first symptoms and neurologic manifestation, median (IQR) | 7 (1–12) |
| Neurologic manifestation after withholding sedation in ICU | 36 (16.2) |
| Neurologic symptoms | |
| Altered mental status | 117 (52.4) |
| Focal central neurologic symptoms | 97 (43.7) |
| Peripheral limb weakness | 26 (11.7) |
| Headache | 24 (10.8) |
| Seizure | 21 (9.5) |
| Cranial neuropathy | 10 (4.5) |
| Movement disorder | 8 (3.6) |
| Anosmia | 7 (3.2) |
| Dizziness | 5 (2.3) |
| Ageusia | 4 (1.8) |
| Neurologic assessment | 205 (92.3) |
| Brain imaging | 192 (86.5) |
| Brain MRI | 157 (70.7) |
| Brain CT scan | 35 (15.8) |
| Presence of acute lesion, n/N (%) | 85/192 (44.3) |
| Spine MRI | 6 (2.7) |
| Cerebrospinal fluid examination | 97 (43.7) |
| WBC count >5/mm³, n/N (%) | 18/97 (18.6) |
| SARS-CoV-2 PCR in cerebrospinal fluid | 75 (33.8) |
| Positive, n/N (%) | 2/75 (2.7) |
| Electroencephalogram | 74 (33.3) |
| Electroneuromyography | 19 (8.6) |
| Follow-up (days), median (IQR) | 24 (17–34) |
| Death | 28 (12.6) |
| Acute respiratory distress syndrome | 17 (7.7) |
| Stroke | 5 (2.3) |
| Other | 6 (2.7) |
Data are presented as n (%) unless otherwise indicated. COVID-19, coronavirus disease 2019; CT, computed tomography; ICU, intensive care unit; IQR, interquartile range; MRI, magnetic resonance imaging; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WBC, white blood cell.
According to National Institute of Health guidelines.
Clinical spectrum of neurologic manifestations associated with SARS-CoV-2 infection
CNS manifestations
One hundred eighty-nine patients (85.1%) had CNS manifestations, mostly encephalopathy (85/222, 38.3%), stroke (63/222, 28.4%) and encephalitis (21/222, 9.5%) (Fig. 1). The distribution of stroke was as follows: acute ischaemic cerebrovascular syndromes (AICS, 57/63) including 52 acute ischaemic strokes and five transient ischaemic attacks; intracranial haemorrhage (5/63, 7.9%) (Fig. 2 (P–S)); and cerebral venous thrombosis (1/63, 1.6%). Among patients with encephalopathy, 67 (78%) of 85 were classified as CAE. The 18 remaining patients had other factors accounting for encephalopathy, as follows: acute kidney injury (n = 10), medication (n = 6) and complications of alcohol withdrawal (n = 4). The other CNS manifestations (Supplementary Appendix S1) were isolated de novo seizures (8/222, 3.6%), transient loss of consciousness (5/222, 2.3%), acute benign lymphocytic meningitis (3/222, 1.4%) with mild or moderate COVID-19, single acute demyelinating lesion (2/222, 0.9%), paraparesis (1/222, 0.5%) and generalized myoclonus with cerebellar ataxia (1/222, 0.5%).
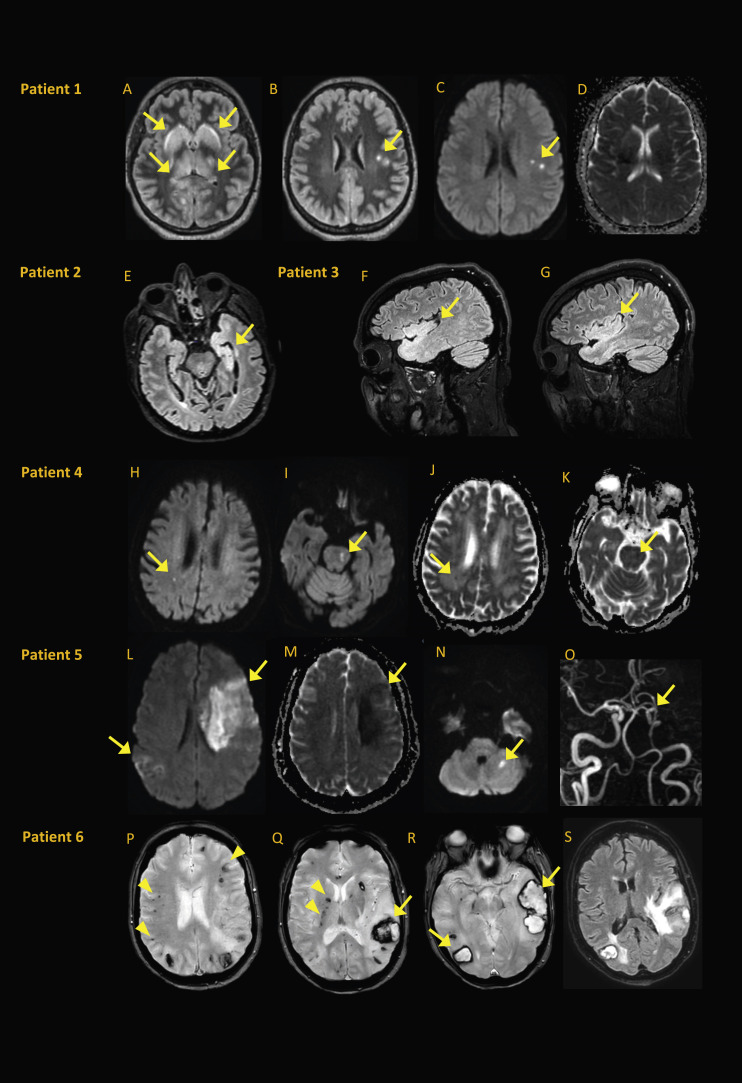
Fig. 2.
Brain MRI from patients with encephalitis or atypical strokes. (A–D) Patient 1∗, a 56-year-old woman with encephalitis, experienced headache, confusion, facial palsy, ophthalmoparesis, refractory status epilepticus and pleocytosis. SARS-CoV-2 PCR results were positive in respiratory sample but negative in CSF. Bilateral basal ganglia and thalami exhibited FLAIR hyperintensity (A), with small subcortical white matter FLAIR hyperintensities (B) visible in diffusion (C) with normal ADC map (D). (E) Patient 2, a 58-year-old man with encephalitis, was found to be SARS-CoV-2 PCR positive in nasopharyngeal swab sample and negative in CSF sample. Pleocytosis and left mesiotemporal and temporopolar hyperintensity were evident on axial FLAIR (E). (F, G) Patient 3, a 49-year-old man with encephalitis, experienced psychomotor agitation and inattention after withdrawal of sedation. SARS-CoV-2 PCR was positive in nasopharyngeal swab sample and negative in CSF. Bilateral temporal and insular hyperintensities were evident on sagittal FLAIR (F, G). (H–K) Patient 4, a 76-year-old man with encephalopathy, had altered mental status 14 days after severe respiratory symptoms. SARS-CoV-2 PCR results were positive in nasopharyngeal sample and negative in CSF; no pleocytosis was noted. Small diffusion hyperintensities were evident in right periventricular white matter (H) and left side of pons (I). Both lesions had decreased ADC (J, K) consistent with small acute ischaemic lesions that did not explain encephalopathy. (L–O) Patient 5, a 60-year-old woman with acute ischaemic stroke, experienced sudden right haemiparesis 11 days after severe respiratory symptoms. SARS-CoV-2 PCR results were positive in nasopharyngeal sample; assessment was negative for stroke and vascular risk factors. Diffusion hyperintensities were evident in left frontal and right parietal areas (L) and in left cerebellum (N), with decreased ADC (M) and left middle cerebral artery occlusion on time-of-flight magnetic resonance angiography (O). (P–S) Patient 6†, a 60-year-old woman with multiple intracranial haemorrhages, experienced sudden right haemiparesis and aphasia after withdrawal of sedation. SARS-CoV-2 PCR results were positive in nasopharyngeal sample. Multiple hypointensities on axial gradient echo T2-weighted images were consistent with cortical microhemorrhages (arrowheads in P), deep microhemorrhages (arrowheads in Q) and haematoma in left parietal lobe (arrow in Q) occipital and temporal lobes (arrows in R) with perilesional oedema on axial FLAIR (S). ADC, apparent diffusion coefficient; CSF, cerebrospinal fluid; FLAIR, fluid-attenuated inversion recovery; MRI, magnetic resonance imaging; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. ∗Image courtesy of Dr F.Bruneel, Intensive Care Unit, Versailles Hospital; †Image courtesy of Dr L.Dubuc, Neurology, Saint-Lo Hospital.
PNS manifestations
Twenty-nine patients (13.1%) had PNS manifestations, mostly GBS (15/222, 6.8%). Ten other patients had peripheral complications of intensive care unit management, either critical illness neuropathy (8/222, 3.6%) or Tapia syndrome (hypoglossal and pneumogastric nerve palsy following orotracheal intubation) (2/222, 0.9%). The remaining PNS manifestations were cranial neuropathy (3/222, 1.4%), including two oculomotor nerve palsy and one facial peripheral nerve palsy; and bilateral fibular nerve palsy (1/222, 0.5%).
Mixed manifestations
Eleven patients (5.9%) had both CNS and PNS manifestations (Fig. 1).
Fifteen patients (6.8%) had mixed manifestations of undetermined mechanisms, including headache, dizziness, anosmia, auditory symptoms and subjective sensitive symptoms.
Main neurologic manifestations associated with COVID-19
Neurologic manifestations occurred as the first symptoms of COVID-19 in 14 (24.6%) of 57 patients with AICS and in 15 (22.4%) of 67 patients with CAE (Table 2 ). The remaining patients exhibited neurologic manifestations several days after the first COVID-19 symptoms, with a median (IQR) delay of 6 (3–8) days and 7 (5–10) days respectively in CAE and encephalitis patients, and 12 days (IQR 7–18) in AICS and 18 days (IQR 15–28) in GBS.
Table 2.
Baseline and clinical characteristics of COVID-19 patients with acute ischaemic cerebrovascular syndrome, encephalopathy, encephalitis and GBS
| Characteristic | Acute ischaemic cerebrovascular syndrome (n = 57) | Encephalitis (n = 21) | COVID-19–associated encephalopathy (n = 67) | GBS (n = 15) |
|---|---|---|---|---|
| Age (years), median (IQR) | 65 (55–78) | 67 (51–70) | 68 (61–75) | 59 (53–65) |
| Male | 34 (59.6) | 15 (71.4) | 41 (60.3) | 13 (86.7) |
| Medical history | ||||
| Prior stroke | 8 (14.0) | 0 | 4 (6.0) | 1 (6.7) |
| Neurodegenerative disease | 1 (1.8) | 1 (4.8) | 20 (29.9) | 0 |
| Vascular comorbiditiesa | 43 (75.4) | NA | NA | NA |
| Severity of COVID-19 | ||||
| Mild | 21 (36.8) | 4 (19) | 6 (9.0) | 7 (46.7) |
| Moderate | 16 (28.1) | 7 (33.3) | 16 (23.9) | 3 (20.0) |
| Severe | 13 (22.8) | 3 (14.3) | 16 (23.9) | 1 (6.7) |
| Critical | 7 (12.3) | 7 (33.3) | 29 (43.3) | 4 (26.7) |
| Neurologic manifestations occurrence | ||||
| Neurologic manifestations occurring as first symptoms | 14 (24.6) | 1 (4.8) | 15 (22.4) | 0 |
| Neurologic manifestation occurring after first COVID-19 symptoms | 40 (70.2) | 14 (66.7) | 32 (47.8) | 12 (80.0) |
| Time between first symptoms and neurologic manifestation, median (IQR), day | 12 (7–18) | 7 (5–10) | 6 (3–8) | 18 (15–28) |
| Neurologic manifestation after withholding sedation in ICU | 3 (5.3) | 6 (28.6) | 20 (29.9) | 3 (20.0) |
| Neurologic symptoms | ||||
| Headache | 2 (3.5) | 3 (14.3) | 6 (9.0) | 0 |
| Altered mental status | 8 (14.0) | 21 (100) | 67 (100) | 3 (20.0) |
| Seizure | 1 (1.8) | 2 (9.5) | 7 (10.4) | 0 |
| Focal central neurologic symptoms | 56 (98.2) | 12 (57.1) | 13 (19.4) | 2 (13.3) |
| Motor or sensitive deficit | 42 (73.7) | 2 (9.5) | 1 (1.5) | 1 (6.7) |
| Cerebellar ataxia | 6 (10.5) | 6 (28.6) | 9 (13.4) | 0 |
| Pyramidal syndrome | NA | 6 (28.6) | 4 (6.0) | 0 |
| Central oculomotor syndrome | 6 (10.5) | 1 (4.8) | 1 (1.5) | 1 (6.7) |
| Movement disorder | 0 | 6 (28.6) | 3 (4.5) | 1 (6.7) |
| Peripheral limb weakness | 1 (1.8) | 2 (9.5) | 7 (10.4) | 11 (73.3) |
| Cranial neuropathy | 0 | 1 (4.8) | 2 (3.0) | 4 (26.7) |
| Follow-up (days), median (IQR) | 24 (16–32) | 21 (18–29) | 28 (19–37) | 18 (14–29) |
| Resolution of neurologic symptoms | 21 (36.8) | 10 (47.6) | 34 (50.7) | 1 (6.7) |
| Death | 9 (15.8) | 1 (4.8) | 10 (14.9) | 0 |
Data are presented as n (%) unless otherwise indicated. COVID-19, coronavirus disease 2019; GBS, Guillain-Barré syndrome; ICU, intensive care unit; IQR, interquartile range; NA, not applicable.
Vascular comorbidities were only collected for patients with stroke. Data collected included hypertension, diabetes, obesity and cardiovascular diseases.
Acute ischaemic cerebral syndrome
Median (IQR) age was 65 (55–78) years. Eight patients (14.0%) had a history of stroke and 43 (75.4%) of 57 had known cardiovascular risk factors: 34 had hypertension, 15 had diabetes, 13 had dyslipidaemia, seven were obese and five were active smokers. Large vessel infarct (Fig. 2(L–O)) was observed in 46 (88.4%) of 57 patients, with persisting thrombosis noted in 16 patients (16.1%). Thirteen patients (22.8%) experienced multiterritory ischaemic stroke. AICS was cryptogenic (ischaemic stroke for which no probable cause was found despite thorough diagnostic evaluation) in 38 (66.7%) of 57 patients. The mortality rate was 15.8%.
Encephalitis
Median (IQR) age was 67 (51–70) years. More than half of the patients (12/21, 57.1%) exhibited focal neurologic deficit in addition to altered mental status, with predominant cerebellar ataxia and pyramidal syndrome. Six patients (28.6%) also had movement disorders, mostly tremor and myoclonus. Brain MRI was abnormal in 14 (66.7%) of 21 patients with imaging compatible with encephalitis (Table 3 , Fig. 2(A–G)). CSF examination demonstrated lymphocytic pleocytosis, with WBC count from 6 to 77/mm³ in 14 (66.7%) of 21 patients. SARS-CoV-2 PCR results of CSF testing were positive in two patients, both of whom had critical COVID-19 illness. Electroencephalogram was abnormal in 14 (93.3%) of the 15 patients so assessed (Table 3). Ten patients (47.6%) fully recovered, three of whom received corticosteroids. The mortality rate was 4.8%.
Table 3.
Neurologic assessment in COVID-19 patients with acute ischaemic cerebrovascular syndrome, encephalitis, encephalopathy and GBS
| Characteristic | Acute ischaemic cerebrovascular syndrome (n = 57) | Encephalitis (n = 21) | Encephalopathy (n = 67) | GBS (n = 15) |
|---|---|---|---|---|
| Brain imaging | 57 (100) | 21 (100) | 57 (85.1) | 5 (33.3) |
| CT scan | 9 (15.8) | 0 | 12 (17.9) | 0 |
| MRI | 48 (84.2) | 21 (100) | 45 (67.2) | 5 (33.3) |
| Acute ischaemic lesion | 52 (91.7) | 2 (9.5) | 6 (9) | 2 (13.3) |
| Unifocal ischaemic lesion | 39 (68.4) | 1 (4.8) | 1 (1.5) | 1 (6.7) |
| Multifocal ischaemic lesions | 13 (22.8) | 1 (4.8) | 5 (7.5) | 1 (6.7) |
| Large vessel infarct | 46 (88.4)a | 0 | 0 | 1 (6.7) |
| Small vessel infarct | 6 (11.5) | 2 (9.5) | 6 (9) | 1 (6.7) |
| Microhemorrhages | 0 | 2 (9.5) | 2 (3) | 0 |
| Other lesion | 0 | 14 (66.7)b | 1 (1.5)c | 0 |
| Spine MRI | 0 | 0 | 2 (3) | 3 (20) |
| Any lesion | — | — | 0 | 0 |
| Cerebrospinal fluid examination | 3 (5.2) | 21 (100) | 36 (53.7) | 14 (93.3) |
| Normal | 3 (5.2) | 3 (14.3) | 28 (41.8) | 5 (33.3) |
| WBC count >5/mm³ | — | 14 (66.7) | 0 | 1 (6.7) |
| Proteins >0.45 g/L | — | 12 (57.1) | 8 (11.9) | 8 (53.3) |
| Isolated elevated proteins | — | 4 (19.0) | 8 (11.9) | 8 (53.3) |
| Positive SARS-CoV-2 PCR | 0 | 2 (9.5) | 0 | 0 |
| Electroencephalogram | 4 (7.0) | 15 (71.4) | 32 (47.8) | 2 (14.3) |
| Normal | 0 | 1 (4.8) | 6 (9) | 0 |
| Diffuse slowing | 3 (5.3) | 9 (42.9) | 17 (25.4) | 1 (6.7) |
| Anterior slowing | 0 | 2 (9.5) | 5 (7.5) | 1 (6.7) |
| Focal lateralized slowing and/or paroxysm | 1 (1.8) | 4 (19) | 8 (11.9) | 0 |
| Periodic pattern | 0 | 1 (4.8) | 3 (4.5) | 0 |
| Status epilepticus | 0 | 1 (4.8) | 1 (1.5) | 0 |
| Electroneuromyography | 1 (1.8) | 1 (4.8) | 3 (4.5) | 14 (93.3) |
| Abnormal findings | 1 (1.8) | 1 (4.8) | 3 (4.5) | 13 (86.7) |
| Axonal injury | 1 (1.8) | 1 (4.8) | 1 (1.5) | 0 |
| Demyelination | 1 (1.8) | 0 | 2 (3) | 13 (86.7) |
Data are presented as n (%). COVID-19, coronavirus disease 2019; CT, computed tomography; FLAIR, fluid-attenuated inversion recovery; GBS, Guillain-Barré syndrome; MRI, magnetic resonance imaging; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WBC, white blood cell count.
Among 46 patients with large vessel infarct, 16 had a persisting thrombosis located in internal carotid artery (n = 9) and/or proximal segment of middle cerebral artery (n = 6) or in basilar artery (n = 1).
Basal ganglia FLAIR hyperintensity (n = 3), acute diffuse hemispheric white matter lesions (n = 2), FLAIR hyperintensity of genu of corpus callosum (n = 1), mesiotemporal FLAIR hyperintensity (n = 3) with frontoinsular extension in 2, brainstem and cerebellar peduncular FLAIR hyperintensity (n = 2), cranial nerve FLAIR hyperintensity (n = 1), focal leptomeningeal FLAIR hyperintensity (n = 2).
Lesion in splenium of corpus callosum typical of mild encephalopathy with reversible splenial lesion syndrome.
COVID-19–associated encephalopathy
Median (IQR) age was 68 (61–75) years and 20 (29.9%) of 67 had neurodegenerative disease. The majority of CAE patients experienced severe to critical COVID-19 (46/67, 68.7%). Neuroimaging was unremarkable except for six patients (9%) with acute small cerebral infarcts unrelated to clinical symptoms (Fig. 2(H–K)) and one with a typical reversible lesion of the splenium of corpus callosum. Thirty-four patients (50.7%) recovered spontaneously. Two patients received corticosteroids with partial improvement. The mortality rate was 14.9%.
Guillain-Barré syndrome
Median (IQR) age was 59 (53–65) years and ten (66.7%) of 15 had mild or moderate COVID-19. Fourteen patients had CSF examinations, that demonstrated isolated elevated protein levels in eight (57.1%) of them, ranging from 0.49 to 2.36 g/L. Negative SARS-CoV-2 PCR results were obtained in nine patients tested. Electroneuromyography was performed in 14 patients and was suggestive of demyelination in 13 (92.9%) of them.
Most patients with GBS, 14 (93.3%) of 15, were treated with intravenous immunoglobulin. Two required mechanical ventilation. There was no mortality during follow-up.
Discussion
Our results highlight the broad spectrum of neurologic manifestations associated with SARS-CoV-2 infection: the majority of neurologic manifestations were CAE (67/222, 30%), AICS (57/222, 26%), encephalitis (21/222, 10%) or GBS (15/222, 7%). Neurologic manifestations appeared after the first COVID-19 symptoms after a median delay of 6 days in CAE, 7 days in encephalitis, 12 days in AICS and 18 days in GBS. With a median delay of follow-up of 24 days, our registry found a high rate of short-term mortality in COVID-19 patients with CAE and AICS of around 15% (19/124).
Altered mental status was reported in 52% of patients in our registry. Several cohorts of hospitalized patients with COVID-19 have shown a significant proportion of impaired consciousness, ranging from 7.5% to 20% [1,3,4,10]. Encephalitis represented up to 10% of patients in our registry; more than half had focal neurologic deficit. Ellul et al. [8] suggested case definitions for neurologic associations of COVID-19. COVID-19 encephalitis is considered confirmed in a patient with encephalitis (as defined by Venkatesan et al. [7]) and specific intrathecal antibody or SARS-CoV-2 found in the CSF or the brain (PCR or culture). COVID-19 encephalitis is probable if SARS-CoV-2 is found in a respiratory sample. Following these definitions, we found two confirmed COVID-19 encephalitis and 19 probable COVID-19 encephalitis cases. Brain MRI results were highly heterogeneous, consistent with the published cases of encephalitis: white matter lesion and/or basal ganglia and thalami involvement suggestive of acute disseminated encephalomyelitis [11] or acute necrotizing encephalopathy [[12], [13], [14]], other nonspecific diffuse involvement of white matter [15,16], mesiotemporal lesions [10,17] with possible frontoinsular extension, leptomeningeal abnormalities [4] and brainstem lesions [18]. Only two patients in our registry had a positive SARS-CoV-2 PCR result from a CSF sample. Two other encephalitis patients with positive SARS-CoV-2 PCR results from CSF testing have been reported [11,17].
In our series, the short-term outcome was generally favourable without any specific treatment, suggesting a parainfectious mechanism rather than direct neuropathogenicity of SARS-CoV-2. In an autopsy study of six COVID-19 patients, von Weyhern et al. [19] highlighted the presence of lymphocytic panencephalitis and meningitis. Another study documented the presence of SARS-CoV-2 in neural cells of a patient with negative SARS-CoV-2 PCR result in a postmortem CSF sample [20]. Twenty percent of patients with encephalitis in our series had microvascular lesions on brain MRI, suggesting a potential implication of COVID-19–associated endotheliitis [21,22] or coagulopathy [23]. In contrast with other studies [[24], [25]], our series did not show any specific electroencephalographic features in patients with encephalitis, that mainly consisted of a nonspecific generalized background slowing.
We described 67 patients with CAE who had severe forms of COVID-19, as previously shown [3,10]. A high proportion of patients with encephalopathy had preexisting neurodegenerative disorders, which may reflect the fact that chronic cognitive impairment is a known risk factor for delirium in patients with an acute illness [26]. CAE patients had a clinical presentation suggestive of septic-associated encephalopathy [27]: advanced age, previous cognitive impairment, illness severity, focal deficit and seizures, tremor, myoclonus and acute vascular lesion on brain MRI [28]. The release of proinflammatory cytokines is a key pathogenic pathway suggested in septic-associated encephalopathy and is thought to play a central role in COVID-19 [29]. AICS was reported in 57 patients (26%). Although 75% of AICS patients had vascular comorbidities, our study highlighted some features already described in published articles: high prevalence of large-vessel stroke [30,31], multiterritory involvement [31], undetermined aetiology [32] and high mortality rate [32]. Several cases of GBS are currently reported in the literature [[33], [34], [35], [36], [37], [38]], and one study has demonstrated an increased incidence of GBS during the COVID-19 epidemic compared to the three previous years [39]. GBS cases reported in this study can be considered to be probably associated with COVID-19, as defined by Ellul et al. [8].
Our study has several limitations. Firstly, this is a retrospective registry analysis, with all the reporting biases inherent in this mode, which means that the different proportions of neurologic manifestations should be interpreted with caution. Hospitals participated in the study on a voluntary basis, and our sample is probably not representative of all health facilities in France. However, our objective was to present a panel of neurologic manifestations associated with SARS-CoV-2 and their clinical description, not estimate the proportion of neurologic diseases among the entire population of COVID-19 patients. We think that with 46 participating centres including general hospitals as well as specialized neurology centres, we have captured a large panel of COVID-19 neurologic manifestations. Secondly, we only included hospitalized patients, so neurologic symptoms or manifestations associated with milder ambulatory forms of COVID-19 are probably underreported. This could explain why a low proportion of patients with dizziness or anosmia were found in this study. Thirdly, this registry focused on the acute phase of COVID-19 with a limited follow-up duration; we did not study long-term symptoms, including neurologic complaints, described in a variable proportion of patients with long COVID-19 [40]. Fourthly, the data are entirely descriptive and are based on the report at a definite time period during the French outbreak. We used a deliberately simplified CRF, given the exceptional workload shouldered by the medical teams; there was no exhaustive collection of medical history other than neurologic comorbidities and vascular comorbidities for AICS; nor did we analyse biological parameters. Fifthly, some neurologic manifestations that we report here may not be specific to SARS-CoV-2 infection, such as critical illness neuropathy or Tapia syndrome. Further studies are needed to study the direct or indirect role of SARS-CoV-2 infection in the different neurologic manifestations exhibited by patients with COVID-19.
Conclusions
Our study highlights the broad spectrum of neurologic manifestations associated with SARS-CoV-2 infection, which is probably related to different pathogenic pathways. Although encephalopathies were the most frequently reported manifestations, possibly linked to sepsis and cytokine storm, encephalitis was described in 10% of cases. A large majority of SARS-CoV-2 PCR results of CSF (73/75, 97.3%) were negative, and the short-term outcome of patients with encephalitis was generally favourable. Ischaemic strokes were also frequently reported, as was GBS, which occurred later in the course of the disease (18 days, compared to 7 days for encephalitis and 12 days for stroke). Further studies are needed to understand the physiopathology of neurologic manifestations in COVID-19 patients.
Transparency declaration
All authors report no conflicts of interest relevant to this article.
Acknowledgements
We acknowledge the support of the National College of General Hospital Neurologists (CNNHG), the French Society for Infectious Diseases (SPILF), the Association of French-Speaking Liberal Neurologists (ANLLF), the Multiple Sclerosis French Society (SF-SEP), REACTing, a French multidisciplinary collaborative network working on emerging infectious diseases, and the CoCo Neurosciences Study Group.
We acknowledge the support of Jérôme Aboab and the Clinical Research Unit of Saint-Denis for obtaining the approval of the national ethics committees.
Editor: M. Paul
Footnotes
Presented in part at the 6th (Virtual) Congress of the European Academy of Neurology (EAN); 23–26 May 2020.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.11.005.
Contributor Information
the contributors to the NeuroCOVID registry:
Sophie Abgrall, Fanny Alby-Laurent, Thibault Allou, Joséphine Amevigbe, Hanifa Amarguellay, Nabil Alloussi, Guillaume Baille, Mathilde Barbaz, Imen Bekri, Lamia Bencherif, Samia Bensaadi, Guillaume Beraud, Alexandra Bizot, Laure Bottin, Fabrice Bruneel, Jean-Philippe Camdessanche, Jeanne Chauffier, Jean-Philippe Csajaghy, Chloé De Broucker, Thomas De Broucker, Luc Defebvre, Cécile Delorme, Elodie Dembloque, Nathalie Derache, Olivier Dereeper, Céline Derollez, Cécile Descotes-Genon, Virginie Desestret, Mathilde Devaux, Lydie Dubuc, Gilles Edan, Andréa Fickl, Thibault Fraisse, Michel Gugenheim, Karolina Hankiewicz, Yves Hansmann, Geoffroy Hautecloque-Raysz, Carole Henry, Stéphanie Jobard, Fanny Jouan, Arnaud Kwiatkowski, Thibault Lalu, Sophie Landre, Annie Lannuzel, Johan Leguilloux, Camille Lejeune, Clémence Liegeois, Sophie Mahy, Jonathan Marey, Alexandra Maury, Elodie Meppiel, Laure Michel, Rita Mitri, Chloé Moulin, Solène Moulin, Nathan Peiffer-Smadja, Asma Omarjee, Canan Ozsancak, Peggy Perrin, Paul Petitgas, Fernando Pico, Marie Poupard, Valérie Rabier, Camille Rizzato, Caroline Roos, Julien Saison, Naomi Sayre, Nicolas Sedillot, François Sellal, Jérôme Servan, Caroline Storey, Laurent Suchet, Paul Tarteret, Pierre Tattevin, Mathilde Thiebaut, Claudia Vaduva, David Varlan, Adrien Wang, and Virginie Zarrouk
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A. Clinical characteristics of covid-19 in New York City. N Engl J Med. 2020 doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US National Institutes of Health (NIH) COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/overview/management-of-covid-19/ Available at:
- 7.Venkatesan A., Tunkel A.R., Bloch K.C., Lauring A.S., Sejvar J., Bitnun A. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57:1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asbury A.K., Cornblath D.R. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27:S21–S24. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 10.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sanchez-Larsen A., Layos-Romero A., García-García J. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020 doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novi G., Rossi T., Pedemonte E., Saitta L., Rolla C., Roccatagliata L. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e797. doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon L., Varley J., Gontsarova A., Mallon D., Tona F., Muir D. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7:e789. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virhammar J., Kumlien E., Fällmar D., Frithiof R., Jackmann S., Sköld M.K. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020 doi: 10.1212/WNL.0000000000010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kandemirli S.G., Dogan L., Sarikaya Z.T., Kara S., Akinci C., Kaya D. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020 doi: 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brun G., Hak J.F., Coze S., Kaphan E., Carvelli J., Girard N. COVID-19—white matter and globus pallidum lesions: demyelination or small-vessel vasculitis? Neurol Neuroimmunol Neuroinflamm. 2020;7:e777. doi: 10.1212/NXI.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong P.F., Craik S., Newman P., Makan A., Srinivasan K., Crawford E. Lessons of the month 1: a case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin Med (Lond) 2020 doi: 10.7861/clinmed.2020-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020 doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinckernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanafi R., Roger P.A., Perin B., Kuchcinski G., Deleval N., Dallery F. COVID-19 neurologic complication with CNS vasculitis-like pattern. AJNR Am J Neuroradiol. 2020;41:1384–1387. doi: 10.3174/ajnr.A6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vellieux G., Rouvel-Tallec A., Jaquet P., Grinea A., Sonneville R., d’Ortho M.P. COVID-19 associated encephalopathy: is there a specific EEG pattern? Clin Neurophysiol. 2020;131:1928–1930. doi: 10.1016/j.clinph.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vespignani H., Colas D., Lavin B.S., Soufflet C., Maillard L., Pourchet V. Report of EEG finding on critically ill patients with COVID-19. Ann Neurol. 2020 doi: 10.1002/ana.25814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slooter A.J.C., Otte W.M., Devlin J.W., Arora R.C., Bleck T.P., Claassen J. Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med. 2020;46:1020–1022. doi: 10.1007/s00134-019-05907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazeraud A., Righy C., Bouchereau E., Benghanem S., Bozza F.A., Sharshar T. Septic-associated encephalopathy: a comprehensive review. Neurotherapeutics. 2020 doi: 10.1007/s13311-020-00862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 30.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020 doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaghi S., Ishida K., Torres J., Mac Grory B., Raz E., Humbert K. SARS2-CoV-2 and stroke in a New York healthcare system. Stroke. 2020 doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padroni M., Mastrangelo V., Asioli G.M., Pavolucci L., Abu-Rumeileh S., Piscaglia M.G. Guillain-Barré syndrome following COVID-19: new infection, old complication? J Neurol. 2020 doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfefferkorn T., Dabitz R., von Wernitz-Keibel T., Aufenanger J., Nowak-Machen M., Janssen H. Acute polyradiculoneuritis with locked-in syndrome in a patient with Covid-19. J Neurol. 2020 doi: 10.1007/s00415-020-09897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alberti P., Beretta S., Piatti M., Karantzoulis A., Piatti M.L., Santoro P. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e741. doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bigaut K., Mallaret M., Baloglu S., Nemoz D., Morand P., Baicry F. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e785. doi: 10.1212/NXI.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gigli G.L., Bax F., Marini A., Pellitteri G., Scalise A., Surcinelli A. Guillain-Barré syndrome in the COVID-19 era: just an occasional cluster? J Neurol. 2020 doi: 10.1007/s00415-020-09911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Institute for Health Research (NIHR) 2020. Living with Covid-19. A dynamic review of the evidence around ongoing Covid-19 symptoms (often called long Covid)https://evidence.nihr.ac.uk/themedreview/living-with-covid19/ Available at: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.