Abstract
Introduction
Since the epidemic of COVID-19 attracted the attention, reports were surrounding electrocardiographic changes in the infected individuals. We aimed at pinpointing different observed ECG findings and discussing their clinical significance.
Methods
We conducted a systematic search in PubMed, Embase, and Scopus databases. We included eligible original papers, reports, letters to the editors, and case reports published from December 2019 to May 10, 2020.
Results
The team identified 20 articles related to this topic. We divided them into articles discussing drug-induced and non-drug-induced changes. Studies reported an increased risk of QTc interval prolongations influenced by different therapies based on chloroquine, hydroxychloroquine, and azithromycin. Although these medications increased risks of severe QTc prolongations, they induced no arrhythmia-related deaths. In the non-drug-induced group, ST-T abnormalities, notably ST elevation, accounted for the most observed ECG finding in the patients with COVID-19, but their relation with myocardial injuries was under dispute.
Conclusion
This systematic review suggests that identifying ECG patterns that might be related to COVID-19 is vital. Provided that physicians do not recognize these patterns, they might erroneously risk the lives of their patients. Furthermore, important drug-induced ECG changes provide awareness to the health-care workers on the risks of possible therapies.
Keywords: Electrocardiography, COVID-19, SARS-CoV-2
1. Introduction
Coronavirus Disease 2019 (COVID-19) is a disease caused by Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) infection, considered as a “public health emergency of international concern” 1, and causing the current pandemic around the world.2, 3, 4 Facing this challenge requires immediate and strict action. This virus utilizes the Angiotensin-Converting Enzyme 2 (ACE2) as a functional receptor for cellular entry. Research demonstrates that various tissues, including the myocardium of the heart, express ACE2 protein on their cellular surface.5 This protein has described roles in the heart function and pathophysiology of diabetes mellitus (DM) and hypertension. SARS-CoV-2 might use this entry pathway as a trajectory to invade myocardial cells and directly damage them. Related studies suggest other mechanisms for the perceived cardiac injury, including cytokine storms and hypoxemia.6,7 Cardiac injuries correlate with a more detrimental outcome in patients with COVID-19, thus requiring adequate attention. All these effects potentiate cardiac injuries that might be detected as various patterns in an Electrocardiogram (ECG).8,9
Investigators are conducting studies on some medicines with a potential benefit in COVID-19 settings.10,11 Some proposed medications, most notably chloroquine, hydroxychloroquine, and azithromycin, have shown an increased risk of ECG changes in the settings of past diseases, specifically QTc interval prolongations.12, 13, 14, 15 Nevertheless, as every problem needs to be addressed in its specific context, uncertainties still overshadow our clinical knowledge in this area. Therefore, careful studies ought to specifically address this problem. Expected adverse effects require appropriate diagnosis and intervention to maximize treatment benefits.
In this systematic review, we aimed to provide the researchers and clinicians with an update on the diverse patterns observed in the ECG of patients with COVID-19. We address ECG changes that might be attributed to the injuries imposed by the virus, and those caused by certain medications. As a global concern, COVID-19 requires worldwide collaboration and research towards an ever-growing understanding of the disease.
2. Methodology
2.1. Design
This systematic review aims to explain the ECG changes associated with novel coronavirus infection. This study involves reviewing the currently available evidence on the study objective to provide a better understanding of specific aspects of related knowledge. We conducted a systematic search, and the identified articles evaluated concerning the inclusion and exclusion criteria. We subsequently categorized the included studies and established a synthesis of the review analysis.
2.2. Research question
We aimed to answer the following main question:
-
•
Which available ECG changes associated with novel coronavirus infection have been noted in recent studies?
2.3. Eligibility criteria for the selection of studies
Three researchers independently performed the selection of the studies. We included articles published from December 2019 to May 10, 2020.
The exclusion criteria were as follows:
-
−
Duplicated results in databases.
-
−
Ongoing projects, review articles that included ongoing studies, and papers addressing non-human studies or discussing COVID-19 intervention in general, without reference to ECG changes.
2.4. Search strategy
The systematic search was carried out in May 2020 using the PubMed (Medline), Scopus, and Embase. The search was limited to English-written articles, and published in the January 2020 to May 2020. We searched the keywords of electrocardiography, ECG, EKG, electrocardiogram, electrocardiograph, coronavirus, COVID-19, SARS-CoV-2, Novel Coronavirus, and 2019-nCoV on the following search strategy:
-
A
[electrocardiography] (Title/Abstract) OR [ECG] (Title/Abstract) OR [EKG] (Title/Abstract) OR [electrocardiogram] (Title/Abstract) OR [electrocardiograph] (Title/Abstract).
-
B
[Coronavirus] (Title/Abstract) OR [COVID-19] (Title/Abstract) OR [SARS-CoV-2] (Title/Abstract) OR [Novel Coronavirus] (Title/Abstract) OR [2019-nCoV] (Title/Abstract).
-
C
[A] AND [B].
2.5. Literature selection
We screened the titles and abstracts of retrieved papers to identify studies meeting the eligibility criteria. We included the relevant full-text articles and discussed their results to make the final selection. After studying all eligible papers' full text, the researchers made the final decision for each paper.
3. Results
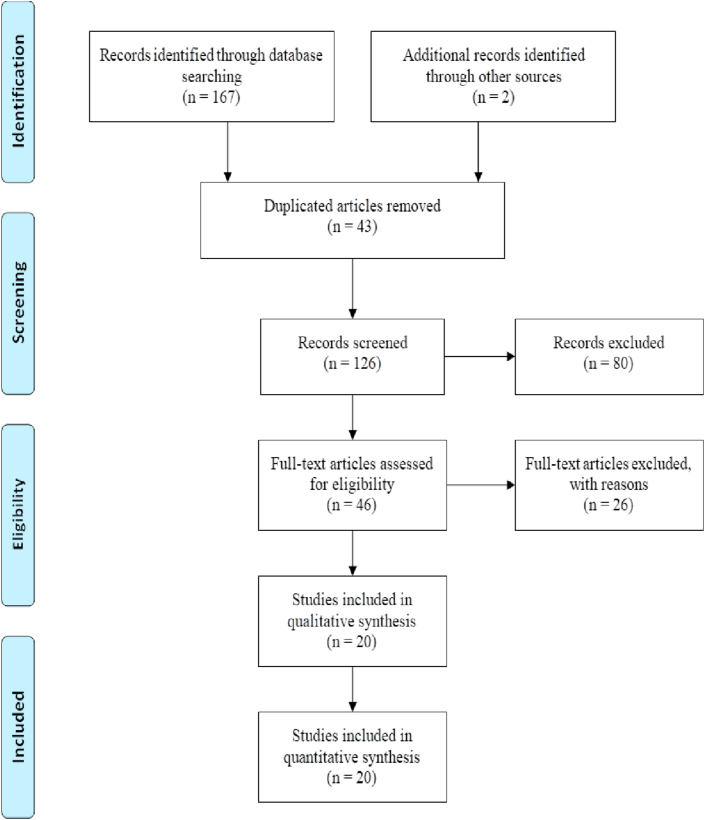
After removing duplicates, abstract, and full-text screening, and adding a few articles manually, 20 studies were encompassed. In the full-text screening process, articles were excluded if their subjects or findings were irrelevant to our study. The PRISMA flow diagram is provided in Fig. 1, which illustrates a thorough presentation of the study selection process. The eligible articles comprised of eleven were case reports, two case series, three retrospective cohorts, two prospective cohorts and observational studies, one consecutive cohort, and one randomized clinical trial (RCT).
Fig. 1.
PRISMA flow diagram of identified articles.
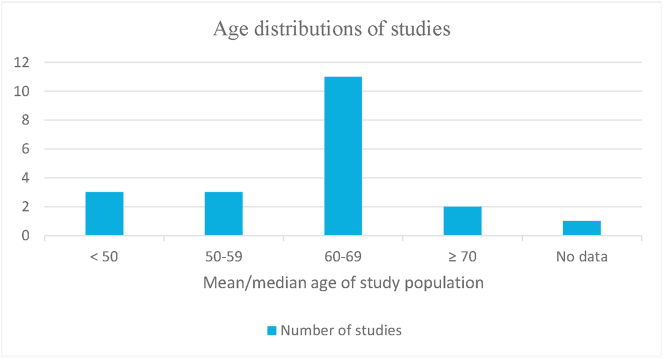
We categorized the studies into two groups based on the etiology of ECG abnormalities: drug-induced and non-drug-induced, where QTc prolongation was the mainstay of all studies reporting drug-induced abnormalities. The drug-induced group accounted for six studies discussed in Table 1, whereas other studies belonged to the non-drug-induced group summarized in Table 2. Fig. 2 describes the age distribution of the selected studies.
Table 1.
Description of the included studies in the drug-induced group.
| ID | First author (reference) | Type of study | Country | Study Population | Study Purpose | ECG findings |
|---|---|---|---|---|---|---|
| 1 | Borba MGS9 | Randomized clinical trial | Brazil | 81 patients (male = 60, female = 21) mean age = 51.1y | To evaluate the efficacy and safety of chloroquine in patients with severe COVID-19. |
|
| 2 | van den Broek MPH16 | Retrospective cohort study | Netherlands | 95 patients (Male = 66%) Age (years): 65 (18–91) |
To evaluate Chloroquine-induced QTc prolongation in COVID-19 patients |
|
| 3 | Mercuro NJ17 | Cohort | USA | 90 COVID-19 positive patients treated with hydroxychloroquine with or without azithromycin (male = 46 (51.1%), female = 44 (48.9%)) | Risk of drug-induced QT interval prolongation |
|
| 4 | Bessiere F18 | Case series | France | 40 COVID-19 positive patients (male = 32 (80%), female = 8 (20%)) | Assessment of QT interval in COVID-19 treated with hydroxychloroquine and +azithromycin |
|
| 5 | Saleh M19 | Prospective observational study | USA | 201 hospitalized patients with COVID-19 (male = 115 (57.2%), female = 86 (42.8%)) | Effects of chloroquine, hydroxychloroquine, and azithromycin on QTc of COVID-19 patients |
|
| 6 | Chorin, E.20 | Consecutive cohort | USA | 84 patients with COVID 19 administered hydroxychloroquine and azithromycin as treatment | Evaluation of hydroxychloroquine and azithromycin effect on QTc prolongation in patients with COVID-19 |
|
∗Abbreviations: TdP (Torsades de pointes),RBBB/LBBB (right/left bundle branch block), PVC (premature ventricular contraction), A-fib (atrial fibrillation).
Table 2.
Description of the included studies in the non-drug-induced group.
| ID | First author (reference) | Type of study | Country | Study Population | Study Purpose | ECG findings |
|---|---|---|---|---|---|---|
| 1 | Shao F21 | Retrospective observational study | China | 136 patients (female = 46, male = 90 | To describe characteristics and outcomes in severe COVID-19 patients with cardiac arrest |
|
| 2 | Deng Q6 | Retrospective | China | 112 hospitalized patients with COVID-19 (male = 57 (50.9%), female = 55 (49.1%)) | Description of findings – suspected myocardial injury |
|
| 3 | Bangalore S22 | Case series | USA | 18 COVID-19 positive patients with ST-elevation on ECG (male = 15 (83%), female = 3 (17%)) | Findings description |
4 diffuse |
| 4 | Cai XQ23 | Case report | China | A 60-year-old male | Clinical manifestations of a COVID 19 patient with a myocardial infection |
|
| 5 | Loghin C24 | Case report | USA | A 29-year-old male | Clinical features of Pseudo acute myocardial infarction in a young COVID-19 patient |
|
| 6 | Dabbagh MF25 | Case report | USA | A 67-year-old female | Development of Cardiac Tamponade and Takotsubo cardiomyopathy in a COVID-19 patient |
|
| 7 | Vidovich MI26 | Case report | USA | A 61-year-old male | Finding Transient Brugada-like ECG pattern in a COVID-19 patient |
|
| 8 | He J27 | Case report | China | Patient 1: A 66-year-old female Patient 2: A 70-year-old male |
ECG and cardiac manifestations in 2 patients diagnosed with COVID-19 | Patient 1 (sequence of events):
|
| 9 | Minhas AS28 | Case report | USA | A 58-year-old female | Clinical manifestations and outcomes of a patient with stress cardiomyopathy or Takotsubo cardiomyopathy in a COVID-19 patient |
|
| 10 | Casey K29 | Case report | USA | A 42-year-old COVID-19 positive male | Case report – acute segmental pulmonary emboli |
|
| 11 | Inciardi RM30 | Case report | Italy | A 53-year-old white woman | Case report – cardiac involvement |
|
| 12 | Chang D31 | Case report | USA | A 49-year-old Bangladeshi man after an episode of syncope | Brugada syndrome in a COVID-19 patient |
|
| 13 | Doyen D32 | Case report | France | A 69-year-old man from Italy presented with ARDS | Myocarditis in a COVID-19 patient |
|
| 14 | Cizgici AY33 | Case report | Turkey | A 78-year-old hypertensive patient | Myopericarditis in a COVID-19 patient |
|
∗Abbreviations: V-fib (ventricular fibrillation), V-tach (ventricular tachycardia), PEA (pulseless electrical activity), IHCA (in-hospital cardiac arrest), ROSC (restoration of spontaneous circulation), LAD/RAD (left/right axis deviation), RBBB (right bundle branch block), A-fib (atrial fibrillation), AV (atrioventricular), LVH (left ventricular hypertrophy).
Fig. 2.
Age distributions of included studies.
4. Discussion
COVID-19 incepted rapidly, affecting many different countries all over the world.16 The beneficiary aspects of this systematic review are extensive, as ECG is a tool utilized vastly worldwide. We discuss some findings that provided physicians do not recognize these patterns, they might erroneously risk their patients’ lives. Major ECG findings are as follows:
4.1. QTc prolongation
Prolonged QTc was mainly due to medication treatment. Six studies were recruited 9,17, 18, 19, 20, 21, and all studies exhibited significant QT-prolongation secondary to drug therapy (p < 0.05) with no prior QTc abnormalities in baseline ECG. The drug of choice was chloroquine/hydroxychloroquine either with (combination therapy) or without (monotherapy) azithromycin.
Two articles merely investigated the effects of chloroquine on QTc interval duration. One17 presented that 23% of patients developed severe and significant QTc interval prolongation (QTc> 500 ms). Similarly, Borba et al9 observed that higher doses of chloroquine resulted in QTc exceeding regular durations (QTc interval >500 ms was recorded in 11.1%, and 18.9% of low-dosage and high-dosage group, respectively).
The other four studies administered both monotherapy (chloroquine/hydroxychloroquine) and combination treatmentfor COVID-19 patients and confirmed that combination therapy is associated with a higher risk of QTc interval prolongation compared with monotherapy.18, 19, 20, 21
A cohort study in Boston18 assessed the effect of hydroxychloroquine with or without azithromycin on QTc prolongation in which QT prolongation was observed in 19% and 21% of monotherapy, and combination therapy cases, respectively. In a similar case series study, Bessiere and colleagues21 reported a broader difference in the two groups' ECG outcomes, indicating 5% and 33% of patients developing QT prolongation in the monotherapy group and combination therapy, respectively.
A retrospective study,19 in which only combination therapy was administered, declared that 11% (nine patients) of COVID 19 patients had a QTc of more than 500 ms when given hydroxychloroquine with azithromycin (five of which had a normal baseline QTc).
Saleh et al20 conducted an observational study of 201 patients receiving either chloroquine or hydroxychloroquine with or without azithromycin. QTc >500 ms was noted in 8.6% of monotherapy-receiving patients versus 9.2% of the combination therapy group.
No records of Torsades de Pointes (TdP) were found in reviewed studies, except for the cohort mentioned above by van den Broek et al,17 which addressed a patient developing TdP three days after his premature discharge. He was later treated with lidocaine.
No arrhythmia-related sudden cardiac death was reported in any of the manuscripts of this group. However, in each study, some of the patients required early treatment termination due to risks of QTc prolongation and ventricular arrhythmia.
Although the articles mentioned above found severe QT prolongation correlating to hydroxychloroquine with or without azithromycin, the absence of TdP and deaths related to arrhythmia observed in most studies raise some hope. However, it must be noted that the degree of QTc prolongation does not linearly correlate with TdP incidence, and TdP might occur even without prolongation of QTc.22 When taking a holistic approach into account, studies should outline the medicines’ overall risks and benefits. Nevertheless, we should discuss these ECG changes to minimize the detrimental outcomes and look after must-not-miss patterns.
4.2. Cardiac arrest
Shao et al23 studied 136 patients with severe COVID-19 who experienced cardiac arrest during hospitalization. Initial ECG patterns and cardiac rhythms were ventricular fibrillation (V-fib) and pulseless V-tach (8, 5.9%), pulseless electrical activity (PEA) (6, 4.4%), and asystole (122, 89.7%). Cardiac arrest occurred after a median of 10 7, 8, 9, 10, 11, 12, 13, 14 days into hospital admission. A total of 18 patients achieved the restoration of spontaneous circulation after resuscitation; six (75%) out of eight V-fib/V-tach patients, one (16.7%) out of six PEA patients, and 11 (9%) out of 122 asystole patients.
In this described setting of cardiac arrest, asystole accounted for the majority of patients. Unfortunately, much more reduced restoration rates of spontaneous circulation were observed in asystole cases than VF or pulseless VT cases. This trend illustrates the importance of developing and implementing careful preventive measures to reduce cardiac arrest risks in hospitalized patients with COVID-19.
4.3. Atrial fibrillation (A-fib)
A-fib existed in baseline ECG of 11% of van den Broek and colleagues’ study population.17 As for new-onset A-fib, Saleh et al20 demonstrated 8.5% of patients developing atrial fibrillation after receiving treatment.
4.4. Ventricular tachycardia
In the mentioned cohort by Saleh et al,20 seven (3.5%) patients showed evidence of monomorphic non-sustained V-tach, and one (0.5%) had sustained hemodynamically stable monomorphic V-tach. Moreover, In Borba et al's study,9 V-tach manifested on ECG records of two patients before their death, although no association between their death and the arrhythmia was proved.
4.5. ST-T abnormalities
In a retrospective cohort in Wuhan, China by Deng and colleagues,6 researchers assessed 112 hospitalized COVID-19 positive patients (67 classified in the severe group) for possible myocardial injury and identified fourteen (12.5%) as suspected myocarditis patients based on American Heart Association's criteria. Abnormal ECG (i.e., ST-T changes) was observed in 22 cases (7 from the non-severe disease group, and 15 with severe disease), including two patients with possible myocarditis. However, ST-T changes were non-specific, considering high age and pre-existing comorbidities of the patients. Also, the study does not state whether the changes were focal or diffused. Based on echocardiography and ECG results, the authors concluded that the patients' myocardial injury is probably due to the disease's systemic effects rather than the COVID-19 virus itself. A case report in Italy24 also studied myocarditis in the setting of COVID-19. ECG changes of the patient included minimal diffuse ST elevations, which were more significant in inferior, and lateral leads, and concurrent ST depression, and T inversion in V1 and aVR. After further evaluation, Lake Louise's criteria for acute myocarditis were fulfilled.
A case series of 18 patients with COVID-19 and ST elevation25 showed local changes in four (22%), and diffuse changes in 14 (78%) cases. ST elevations were either upon presentation (10, 56%) or after a median of 6 days after hospitalization (8, 44%). Myocardial infarction (MI) was observed in 8 (44%) patients, whereas 10 (56%) patients had a non-coronary myocardial injury. Nine (50%) patients underwent angiography, where six showed signs of coronary obstruction. The fatality rate was 72% (13 patients), of which nine died due to non-coronary myocardial damage, and four due to MI. One other study26 also reported ST-elevation myocardial infarction (STEMI) in a COVID-19 infected patient. ST-elevation was observed in leads II, III, and aVF as a result of inferior wall MI. Additionally, ST-segment depression was noted in leads V1–V6.
Loghin et al27 also observed ST elevation in inferior (II, III, and aVF) leads of a COVID-19 positive patient. Furthermore, ST elevation in V6 and marked right axis deviation (RAD) were also detected. However, pretests indicated low MI probability, and imaging showed no signs of coronary calcifications. Therefore, coronary angiography was not performed, and the patient received conservative treatments.
Diffuse concave ST elevation was noted in a patient in Turkey28 who showed healthy coronary arteries on angiography. CT scan revealed ground-glass opacities associated with COVID-19. Therefore, the patient was diagnosed with possible COVID-19 associated myopericarditis.
ST-T changes were also observed in two patients with Takotsubo syndrome,29,30 which included ST elevation in leads I, and aVL, diffuse non-specific ST-T changes, and low voltage limb leads. One of the two patients experienced cardiac tamponade with hemorrhagic effusion before the identification of Takotsubo cardiomyopathy.
T inversion was a pattern observed in some studies. A patient with Takotsubo cardiomyopathy presented deep T inversion in precordial leads of her ECG.29 Moreover, T inversion in leads V1 and aVR has been observed in a patient with acute myocarditis.24 Doyen and colleagues31 observed T inversion in ECGs of a COVID-19 infected patient, which was first limited to anterior leads but was later presented diffusely on a second ECG when the patient was admitted to ICU. Due to the high Global Registry of Acute Coronary Events (GRACE) score, non-ST elevation MI was suspected. However, the diagnosis ruled out with coronary angiography, and myocarditis was suggested based on late gadolinium enhancement imaging.
Various patterns of ST-T abnormalities were the most reported ECG findings, probably not related to medications. Other reported ECG findings might also ensue the potential injuries belong to the virus, but the evidence is still under dispute. These patterns might display the manner of cardiac involvement and alleviate health-care providers in the diagnosis and management of COVID-19 patients. Howbeit, the variety of ECG findings requires strict attention. Nevertheless, larger study populations adjusting their finding for specific confounders, such as cardiac comorbidities, have to establish these findings’ reliability.
4.6. Brugada pattern
Two case reports in the US32,33 reported Brugada patterns in patients with COVID-19.
4.7. S1Q3T3
Two studies34,35 reported the S1Q3T3 pattern in their patients. One was a case of pulmonary embolism in a patient with COVID-19 infection. The other two were more complicated and are discussed in the following sections.
4.8. Other patterns
He et al34 reported two COVID-19 positive cases with two different sequences of ECG changes, which were not limited to the ones mentioned above. The first patient's initial ECG demonstrated first-degree atrioventricular (AV) block, which progressed to developing an S1Q3T3 pattern, followed by Mobitz I second-degree AV block, and AV junctional escape beat. Heart block worsened into an almost complete AV block but reversed into a first-degree AV block within a short period, and the S1Q3T3 pattern was also resolved. The second patient's first ECG abnormality was an incomplete right bundle branch block (RBBB). Inferior and precordial ST elevation was developed later on, which advanced into a triangular QRS-S-T pattern with V-tach episodes during this transition. Unfortunately, the patient deceased within 24 h of the first V-tach occurrence.
One major shortcoming of this systematic review was the limitation in the number of studies, and sample populations. Even in this small number of studies case reports account for a significant proportion of them. Hopefully, the rapidly evolving nature of the research in this field might provide us with increased useful information. We presented these case reports in a different section, as they could produce no firmly accountable evidence.
5. Conclusion
Overall, not only COVID-19 should not be ruled out in the presented ECG findings, but they might also raise clinical suspicions towards it, especially in the situation of an outbreak. Furthermore, due to potential adverse outcomes, recognizing some patterns is even more vital and should be considered, especially in the patients using certain medications.
Funding sources
Not applicable.
Declaration of competing interest
The authors confirm that they have no conflict of interest.
Acknowledgments
The present study was conducted in collaboration with Khalkhal University of Medical Sciences, and the Iranian Institute for Reduction of High-Risk Behaviors, Tehran University of Medical Sciences.
Contributor Information
Esmaeil Mehraeen, Email: es.mehraeen@gmail.com.
Seyed Ahmad Seyed Alinaghi, Email: s_a_alinaghi@yahoo.com.
Ali Nowroozi, Email: nowrooziali77@gmail.com.
Omid Dadras, Email: omiddadras@yahoo.com.
Sanam Alilou, Email: Sanamalilouu@gmail.com.
Parnian Shobeiri, Email: Parnian.shobeiri@gmail.com.
Farzane Behnezhad, Email: farzan1898@gmail.com.
Amirali Karimi, Email: aa-karimi@student.tums.ac.ir.
References
- 1.Organization W.H. 2020. Rolling Updates on Coronavirus Disease (COVID-19)https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen April 24 [Available from: [Google Scholar]
- 2.Organization W.H. 2020. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 March 11 [Available from: [Google Scholar]
- 3.Ghiasvand F., Miandoab S.Z., Harandi H., Golestan F.S, Alinaghi S.A.S. A patient with COVID-19 disease in a referral hospital in Iran: a typical case. Infect Disord Drug Targets. 2020;20(4):559–562. doi: 10.2174/1871526520666200429115535. [DOI] [PubMed] [Google Scholar]
- 4.Mehraeen E.B.F., Saeidi S., Salehi M.A., Noori T., Harandi H., Seyed Alinaghi S. Olfactory and Gustatory dysfunctions of the coronavirus disease (COVID-19): a review of current evidence. Eur Arch Otorhinolaryngol. 2020 Jun 17:1–6. doi: 10.1007/s00405-020-06120-6. [DOI] [PubMed] [Google Scholar]
- 5.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng Q., Hu B., Zhang Y. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J., Wu B., Chen Y. Characteristic ECG manifestations in patients with COVID-19. Can J Cardiol. 2020;36(6):966.E1–966.E4. doi: 10.1016/j.cjca.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santoso A., Pranata R., Wibowo A., Al-Farabi M.J., Huang I., Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.04.052. YAJEM-158923, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borba M.G.S., Val F.F.A., Sampaio V.S. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Network Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berlin D.A., Gulick R.M., Martinez F.J. Severe covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 11.Sekhavati E., Jafari F., SeyedAlinaghi S. Safety and effectiveness of azithromycin in patients with COVID-19: An open-label randomised trial. Int J Antimicrob Agents. 2020 Oct;56(4) doi: 10.1016/j.ijantimicag.2020.106143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandael E., Vandenberk B., Vandenberghe J., Willems R., Foulon V. Risk factors for QTc-prolongation: systematic review of the evidence. Int J Clin Pharm. 2017;39(1):16–25. doi: 10.1007/s11096-016-0414-2. [DOI] [PubMed] [Google Scholar]
- 13.Albert R.K., Schuller J.L., Network C.C.R. Macrolide antibiotics and the risk of cardiac arrhythmias. Am J Respir Crit Care Med. 2014;189(10):1173–1180. doi: 10.1164/rccm.201402-0385CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang B.H., Wu C.H., Hsia C.P., Yin Chen C. Azithromycin-induced torsade de pointes. Pacing Clin Electrophysiol. 2007;30(12):1579–1582. doi: 10.1111/j.1540-8159.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- 15.Morgan N.D., Patel S.V., Dvorkina O. Suspected hydroxychloroquine-associated QT-interval prolongation in a patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19(5):286–288. doi: 10.1097/RHU.0b013e31829d5e50. [DOI] [PubMed] [Google Scholar]
- 16.Adhikari S.P., Meng S., Wu Y.J. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Broek M.P.H., Mohlmann J.E., Abeln B.G.S., Liebregts M., van Dijk V.F., van de Garde E.M.W. Chloroquine-induced QTc prolongation in COVID-19 patients. Neth Heart J. 2020:1–4. doi: 10.1007/s12471-020-01429-7. monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercuro N.J., Yen C.F., Shim D.J. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chorin E., Wadhwani L., Magnani S. QT Interval Prolongation and Torsade De Pointes in Patients with COVID-19 Treated with Hydroxychloroquine/Azithromycin. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saleh M., Gabriels J., Chang D. The effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol. 2020 doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bessiere F., Roccia H., Deliniere A. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020;5(9):1067–1069. doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yap Y.G., Camm A.J. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89(11):1363–1372. doi: 10.1136/heart.89.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao F., Xu S., Ma X. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18–23. doi: 10.1016/j.resuscitation.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bangalore S., Sharma A., Slotwiner A. ST-segment elevation in patients with covid-19 - a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai X.Q., Jiao P.Q., Wu T. Armarium facilitating angina management post myocardial infarction concomitant with coronavirus disease 2019. J Geriatric Cardiol: JGC. 2020;17(4):217–220. doi: 10.11909/j.issn.1671-5411.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loghin C., Chauhan S., Lawless S.M. Pseudo acute myocardial infarction in a young COVID-19 patient. JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cizgici A.Y., Zencirkiran Agus H., Yildiz M. COVID-19 myopericarditis: it should be kept in mind in today’s conditions. AJEM (Am J Emerg Med) 2020;38(7):1547.e5–1547.e6. doi: 10.1016/j.ajem.2020.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dabbagh M.F., Aurora L., D'Souza P., Weinmann A.J., Bhargava P., Basir M.B. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minhas A.S., Scheel P., Garibaldi B. Takotsubo syndrome in the setting of COVID-19 infection. JACC Case reports. 2020 doi: 10.1016/j.jaccas.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395(10235):1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidovich M.I. Transient brugada-like ECG pattern in a patient with coronavirus disease 2019 (COVID-19) JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang D., Saleh M., Garcia-Bengo Y., Choi E., Epstein L., Willner J. COVID-19 infection unmasking Brugada syndrome. HeartRhythm Case Rep. 2020 doi: 10.1016/j.hrcr.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He J., Wu B., Chen Y. Characteristic electrocardiographic manifestations in patients with COVID-19. Can J Cardiol. 2020 doi: 10.1016/j.cjca.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casey K., Iteen A., Nicolini R., Auten J. COVID-19 pneumonia with hemoptysis: acute segmental pulmonary emboli associated with novel coronavirus infection. AJEM (Am J Emerg Med) 2020;38(7):1544.e1–1544.e3. doi: 10.1016/j.ajem.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]